Abstract
Objectives:
Despite extensive effort, the search for clinically-relevant metabolite biomarkers for early detection, disease monitoring, and outcome prediction in lung cancer remains unfulfilled. Although biofluid evaluation has been explored, the complexity inherent in metabolite data and the dynamic discrepancy between metabolites in biofluids vs. tumor tissue have prevented conclusive results. This proof-of-concept study explored models predictive of staging and chemotherapy response based on metabolomic analysis of fresh, patient-derived non-small cell lung cancer (NSCLC) core biopsies.
Materials and Methods:
Samples (n=36) were evaluated with high-resolution 2DLC-MS/MS and 13C-glucose enrichment, and the data were comprehensively analyzed with machine learning techniques. Patients were categorized as Disease-Control (DC) [encompassing complete-response (CR), partial-response (PR), and stable-disease (SD)] and Progressive-Disease (PD) in terms of first-line chemotherapy. Four major types of learning methods (partial least squares discriminant analysis (PLS-DA), support vector machines (SVM), artificial neural networks, and random forests (RF)) were applied to differentiate between positive (DC and CR/PR) and poor (PD and SD/PD) responses, and between stage I/II/III and stage IV disease. Models were trained with forward feature selection based on variable importance and tested on validation subsets.
Results:
The models predicted patient classifications in the validation subsets with AUC (95%CI): DC vs. PD (SVM), 0.970(0.961–0.979); CR/PR vs. SD/PD (PLS-DA), 0.880(0.865–0.895); stage I/II/III vs. IV (SVM), 0.902(0.880–0.924). Highest performing model was SVM for DC vs. PD (balanced accuracy=0.92; kappa=0.74).
Conclusion:
This study illustrates a comprehensive evaluation of patient tumor-specific metabolic profiles, with the potential to identify disease stage and predict response to first-line chemotherapy.
Keywords: Metabolomics, lung cancer, chemotherapy, machine learning, personalized medicine
INTRODUCTION
Lung cancer is estimated to account for approximately 13% of all new cancer cases and 24% of all cancer deaths in the U.S.(1). For non-small cell lung cancer (NSCLC) patients with advanced disease (stage IIIB-IVB) and performance status ≤2, chemotherapy is the treatment option with best long term outcomes(2). Paclitaxel, gemcitabine, docetaxel, vinorelbine, irinotecan, or pemetrexed are often used in combination with platinum drugs(3). Randomized clinical trials have concluded that no single regimen may be significantly more effective than others(4). Consequently, no reliable method exists to determine which potential first-line therapy would be most effective for a specific patient, who essentially is an experimental subject with drug choice and dosing determined post-hoc first-line therapy based on response and tolerability(2).
This study uses metabolomics to explore the potential to a priori exclude “non-responders” of certain first-line therapy treatment regimens to avoid burdening patients with ineffective treatment. Metabolomics has emerged as a method to potentially resolve the link between genotype and phenotype, giving insight into patient response(5), although no standard methodology has been developed(6,7). One advantage of mass spectrometry (MS) over other techniques such as nuclear magnetic resonance (NMR) spectroscopy is that MS has high sensitivity and peak capacity, especially when coupled with liquid chromatography (LC). Stable isotope resolved metabolomics (SIRM) uses stable isotope tracers (e.g. 13C, 18O and/or 15N) to support studies of biochemical regulation. SIRM follows the fate of the heavy atoms and their incorporation into a multitude of metabolites produced from the labeled primary substrate, and thus quantifies heavy atom-containing metabolites, leading to exact biochemical pathway assignment. Therefore, SIRM allows for a detailed view into cancer metabolism and enables metabolic pathway reconstruction(8). With a sufficiently high resolution, MS can distinguish the m/z differences between 13C labeled molecules and unlabeled molecules, which can lead to insights about glucose metabolism alteration. In particular for NSCLC, an increased capacity for carbon incorporation from glucose into lactate, alanine, citrate, glutamate, succinate, aspartate, and ribosyl moiety of nucleotides has been observed(9).
In contrast to recent lung cancer patient serum metabolomic (6,7) or radiomic(10) profiling, here we report metabolomic analysis of fresh tumor core biopsies, which are typically more difficult to obtain for analysis than biofluids or imaging. Our hypothesis is that a comprehensive machine learning analysis of biopsied tissue metabolomic data is able to differentiate between DC vs. PD and CR/PR vs. SD/PD responses, and between stage I/II/III and stage IV disease. We explore this hypothesis by establishing a workflow that includes patient-derived tissue processing, 13C glucose enrichment, high resolution 2DLC-MS/MS, chemotherapy response assessment and machine learning. Metabolites are categorized for their ability to differentiate between patient responses and disease staging based on model training with forward feature selection based on variable importance. Relative metabolite abundance is evaluated via a correlation analysis. The output of the workflow offers patient-specific metabolomic profiling for potential prediction of response to first-line chemotherapy.
METHODS
Patient sample tissue collection
Informed consent was obtained to participate in this study. All specimens were collected following approved Internal Review Board protocols at University of Louisville Hospital (IRB 05.0523) and Norton Hospital (IRB 18.0264) from patients with known or suspected NSCLC. Demographic information including age, sex, race, smoking history, personal history of malignancy, and relevant family history were recorded. Chemotherapy response was also recorded. Samples were collected by the clinical team, blinded to the research analysis. Patient information was de-identified by the clinical team before evaluation by the research team.
Sex as a biological variable
As lung cancer affects both women and men, samples from both were collected.
Patient sample tissue processing and extraction
Tumor core biopsies were obtained from the University of Louisville and the Norton Hospital interventional radiology departments. Samples were placed in 1.8mL cryovials with either DMEM or RPMI cell culture medium and kept on ice during transport. In laboratory, they were immediately placed into 1mL 13C labeled glucose medium in 24-well cell culture plates and incubated at 5% CO2 and 37°C on a shaker for 24h. After incubation, media and tissue sample were transferred to 1.5mL microcentrifuge tube and centrifuged 180xg for 5min. 13C-glucose media was aspirated and specimen was washed with PBS and centrifuged for 5min, twice. After wash, 500mL acetonitrile was added and tissue was homogenized with pellet mixer. After 2–3min homogenization, 376mL of DNase/RNase free water and 250mL chloroform were added. Contents were vortexed until milky-white color and centrifuged 180xg for 20min. Top (polar) layer was aspirated and frozen at −80°C. As control, a NSCLC tissue biopsy was incubated in unlabeled glucose media for 24h and processed likewise. Sample polar layers were flash frozen in liquid N2, then lyophilized for 24–48h until dried, and transported on ice to CREAM core facility for 2DLC-MS/MS analysis.
2DLC-MS/MS analysis and data pre-processing
Dried samples were dissolved in 100μL 50% acetonitrile and vigorously vortexed for 3min. After centrifugation at 14,000 rpm and 4°C for 20min, 80μL of supernatant was collected. All samples were randomly analyzed on Thermo Q Exactive HF Hybrid Quadrupole-Orbitrap Mass Spectrometer coupled with Thermo DIONEX UltiMate 3000 HPLC system (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a reversed phase chromatography (RPC) column and a hydrophilic interaction chromatography (HILIC) column configured to form a parallel 2DLC-MS system(11). To obtain full MS data, every sample was analyzed by parallel 2DLC-MS in positive (+) and negative (−) modes. For metabolite identification, one unlabeled sample in each group was analyzed by 2DLC-MS/MS in positive and negative modes to acquire MS/MS spectra at 20, 40, and 60 eV collision energies.
For 2DLC-MS data analysis, (XCMS, RRID:SCR_015538, xcmsonline.scripps.edu) was used for spectrum deconvolution(12) and MetSign software was used for metabolite identification, cross-sample peak list alignment, normalization, and statistical analysis(13). To identify metabolites, 2DLC-MS/MS data of unlabeled sample was first matched to our in-house database that contains parent ion m/z, MS/MS spectra, and retention time of authentic standards (MSI Level 1 identification). Threshold for the spectral similarity of the MS/MS spectra of a metabolite standard and a spectrum of the unlabeled sample were set ≥0.4, while thresholds of retention time difference and m/z variation window were respectively set ≤0.15min and ≤4ppm. 2DLC-MS/MS data without a match (MSI Level 2 identification) were analyzed using Compound Discoverer software v2.0 (Thermo Fisher Scientific, Germany), where MS/MS spectra similarity score threshold was set ≥40 with a maximum score of 100. Identification results of unlabeled metabolites were used for isotopologue assignment in the labeled samples using same parameters for identification of unlabeled metabolites(14).
Organization of MS peak intensity data
2DLC-MS data was presented as an alignment table for each batch with retention time, m/z, signal intensity, stable isotope labeling, name of identified metabolite, and database used for metabolite identification. Building on R package MSCombine(15), a script was employed to combine the dataset into a single data frame by combining metabolites from both positive and negative ion modes. For metabolites with multiple occurrences, the metabolite with lower average peak area across all samples was considered to have lower sensitivity and was left out of the combined dataset. Once metabolites from positive and negative modes were combined after eliminating the low sensitivity redundant metabolites, a preliminary step to handle missing values was performed by removing features which contained more than 50% missing values. This resulted in a dataset of 66 metabolites with approximately 21.8% missing values.
Data normalization and imputation of missing data
The data were normalized by a log transformation, which is commonly applied to biological data, both centering the data and correcting for heteroscedasticity(16). The transformation was applied within each sample independently to prevent data leakage between the training and test sets during cross validation. Multiple imputation is a commonly used multivariate statistical technique to fill in missing data for unlabeled metabolites, particularly in MS-based metabolomics(17). Although it was previously determined that the random forest (RF) method had the highest performance compared to other methods when imputing MS-based metabolomics data(17), we chose to evaluate the performance of various imputation methods on our unique dataset due to their potential influence on the analysis(18). After taking a subset of the original data that only included complete cases (19 metabolic features x 33 samples) and introducing simulated missing values (~10%), the RMSE of various imputation methods were found and used as the measurement for their performance. Probabilistic principal component analysis (PPCA)(19) was found to be the most accurate method of imputation for the complete case subset. Therefore, we applied PPCA to the original data to replace missing values. The R package pcaMethods was used to perform PPCA. The function was seeded with an integer of ‘1234’ to allow for reproducibility and “maxIterations” was set to 1000.
Patient response data
Out of 39 enrolled patients, 23 received chemotherapy and a clinical response assessment while 34 had staging information available. Data collected included subject (age, gender, primary ethnicity, primary race, status, age at death, overall survival, cancer description, histology subtypes, cancer stage and substage, and progression free survival in days), treatment (therapy type, therapy details, days of therapy, chemotherapy agent type, chemotherapy agent repeat units, number of chemotherapy cycles completed, surgery results, overall response to surgery/chemotherapy, days since diagnosis and response assessment types), and other information regarding the specimens. Response assessment was performed clinically or radiologically. The Response Evaluation Criteria in Solid Tumors Group (RECIST)(20) is widely used to categorize target lesions: Complete Response (CR), Partial Response (PR), Progressive Disease (PD), or Stable Disease (SD). Accordingly, clinical assessment was captured by the following classifications: DC (includes CR, PR, and SD), CR/PR, SD/PD, and PD. As a preliminary step for data visualization, principal component analysis (PCA) was performed on imputed MS signal intensity datasets, using prcomp function from stats package in base R.
Data analysis
Model Selection
In a comprehensive study which analyzed the performance of 179 classification methods from 17 method families across 121 datasets(21), the top 3 method families (summarized in Supplement) most likely to be best classifiers were random forest (RF), support vector machines (SVM) and artificial neural networks (ANN). Therefore, we chose to use learning methods from these 3 families in addition to partial least squares discriminant analysis (PLS-DA). Samples were divided into classifications based on either clinical response assessment or staging: DC vs. PD; CR/PR vs. SD/P; Stage I/II/III vs. Stage IV. To prevent overfitting of the models, a rigorous combination of feature selection and cross validation was performed. Test set validation was achieved with 5-fold cross-validation, performed with 100 iterations of random subsampling. Results were reported as the average across all folds and iterations. Methods were performed on imputed unlabeled MS signal intensity datasets, organized with sample ID by row and metabolite by column, using the R package caret (22). For all learning methods implemented with train function, “tuneLength” was set to 10 and “Kappa” was used as the metric for tuning optimal hyperparameter combinations.
Feature Selection and Variable Importance
Three basic approaches were previously evaluated to determine features in metabolomics data(23): PCA loadings, Fisher Discrimination Analysis (FDA) weights, and VIP in PLS-DA, finding that better classification was achieved using features determined from PLS-DA. Therefore, we used PLS-DA variable importance calculated with the generic function “varImp()” from caret package to determine importance of metabolites for classification of patient therapy outcomes and staging, and used these for feature selection, as in(24). The variable importance measure applied to PLS-DA is based on weighted sums of the absolute regression coefficients, where contribution of coefficients is weighted proportionally to reduction in sum of squares. We used these selected features to train PLS-DA, SVM and DNN. For RF method, RF variable importance was used to select features by using “varImp()” from caret package. The variable importance measure applied to RF is based on accuracy for each tree, where out-of-bag prediction accuracy is averaged over all trees after permutations of each predictor variable. For all methods, forward feature selection was performed by training each model independently on a subset of features, incrementing from 5 to 30, chosen by variable importance. The optimal number of features for training each model was chosen based on which subset resulted in the highest average AUROCTest after 100 iterations of 5-fold cross validation.
Measures of Model Performance
Thresholder function within caret package was used to return summary statistics from each model with a range of classification probability thresholds from 0 to 1, incremented by 0.005. For test data, receiver operating characteristics (ROC) curves were found from these summary statistics and area-under-the-ROC (AUROC) was calculated using trapezoid rule by approximating the definite integral. For training data, predictions were found using function extractPredictions from caret package. AUROC of training data was then found using observations and predictions from extractPredictions object with roc function from package pROC. Due to class imbalances (i.e., skewed class distribution) present in dataset, balanced accuracy was reported rather than simply averaging accuracies found on each cross-validation fold(25). Balanced accuracy is simply the average of sensitivity and specificity at a particular classification probability threshold. Another useful measure of model performance is kappa, which measures ‘true agreement’ by taking into account the random chance of a model selecting the correct outcome(26). In our dataset, the concept of optimizing probability thresholds for class imbalances is highly relevant. Therefore we chose the optimum values of balanced accuracy and kappa across a range of probability thresholds.
Correlations, Statistical Significance and Score Plot Quantification
A Spearman correlation analysis was performed using R package stats and cor.test function. To capture potentially significant metabolites, a 95% confidence level was chosen. Wilcoxon-rank sum test (Mann-Whitney U test), used to test for significant differences between relative abundance of metabolites, was performed using R package stats and wilcox.test function. Differences across survival curves were found using the G-rho family of tests, performed with the R package survival and survdiff function. PCA and PLS-DA score plots were quantified with a version of PCAtoTree software(27). J2 criterion and overlap p-values between classes are reported in all score plots.
Metabolic Network Visualization and Quantitative Enrichment Analysis
Quantitative enrichment analysis (QEA) was performed on unlabeled metabolite dataset using (MetaboAnalyst 4.0, RRID:SCR_015539, www.metaboanalyst.ca/) (28). The log transformed/imputed working dataset was used, so no missing value estimation or normalization was necessary. (Small Molecule Pathway Database (SMPD), RRID:SCR_004844, www.smpdb.ca/) and (KEGG, RRID:SCR_012773, www.kegg.jp/) databases were accessed (Nov. 2020). Metabolic networks were visualized with (Cytoscape 3.7.2, RRID:SCR_003032, cytoscape.org/) and (MetScape 3.1.3, RRID:SCR_014687, metscape.ncibi.org/) using the imputed and log transformed metabolite intensity values for DC vs. PD classification.
RESULTS
Patient characteristics and feature selection
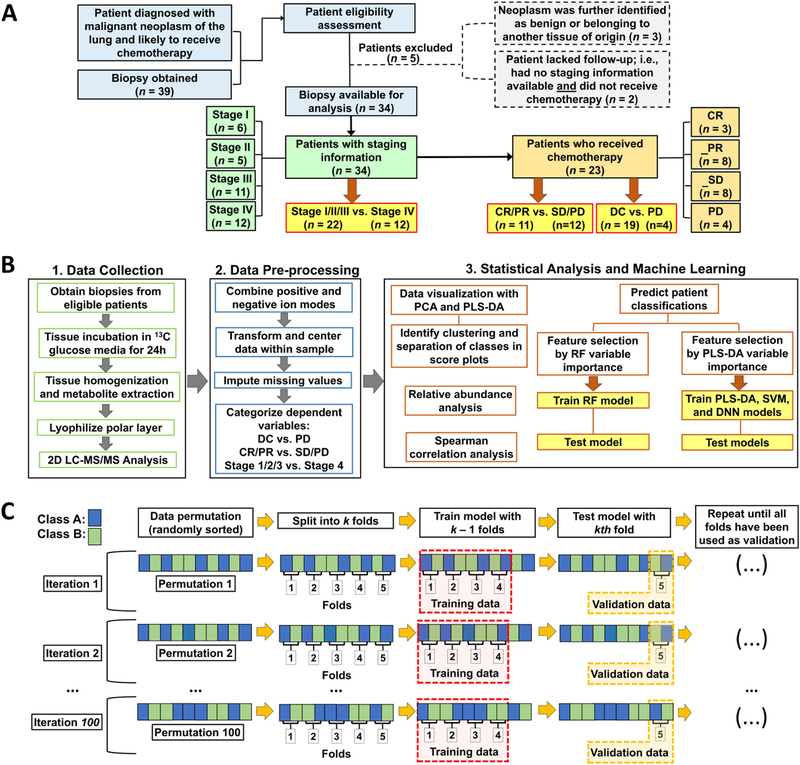
Of 39 patients enrolled in the study and who had metabolomics data generated, 36 had confirmed NSCLC and they were included in working dataset during imputation. Patient characteristics are described in Table_1. Key steps of experimental design from tissue specimen collection to analysis are summarized in Figure_1. Working dataset with metabolites combined from positive and negative ion modes is visualized in Supplementary_Figure_1. Collinearity was high when considering the common 66 metabolites in working dataset. This warranted a feature selection approach to determine important features to differentiate classifications within the dataset.
Table_1.
Patient characteristics.
| Patient # | Sex | Age | Stage | Sub stage | Therapy | RECIST |
|---|---|---|---|---|---|---|
| 1 | F | 50 | 1 | b | Cisplatin; Carboplatin | Adverse Event; NED |
| 2 | F | 53 | 2 | a | Carboplatin and Paclitaxel | PR |
| 3 | M | 42 | 3 | b | Carboplatin; Pemetrexed | SD; SD |
| 4 | M | 64 | 4 | Carboplatin and Pemetrexed | PD | |
| 5 | M | 63 | 3 | a | Cisplatin and Etoposide | SD |
| 6 | M | 60 | 2 | b | Cisplatin and Docetaxel | NED |
| 7 | F | 55 | 1 | a | Carboplatin and Pemetrexed | SD |
| 8 | F | 50 | 3 | N/A | Carboplatin and Paclitaxel | PD |
| 9 | M | 61 | 4 | N/A | ||
| 10 | M | 74 | 4 | Carboplatin and Pemetrexed | PR | |
| 11 | F | 57 | 4 | Carboplatin and Pemetrexed | SD | |
| 12 | M | 68 | 3 | b | N/A | |
| 13 | M | 59 | 4 | Carboplatin, Pemetrexed and Bevacizumab | PD | |
| 14 | F | 70 | 3 | b | Carboplatin and Paclitaxel | SD |
| 15 | M | 60 | 2 | a | N/A | |
| 16 | M | 65 | 4 | Paclitaxel; Pemetrexed; Carboplatin; |
Adverse Event; PD; PD | |
| 17 | F | 67 | 1 | a | N/A | |
| 18 | M | 58 | 3 | b | Cisplatin and Pemetrexed | SD |
| 19 | F | 61 | 4 | N/A | ||
| 20 | F | 65 | 3 | b | N/A | |
| 21 | F | 57 | 3 | a | Carboplatin and Paclitaxel | PR |
| 22 | F | 77 | 4 | Carboplatin, Pemetrexed and Pembrolizumab | SD | |
| 23 | F | 70 | 3 | a | Cisplatin and Pemetrexed | PR |
| 24 | M | 75 | N/A | N/A | N/A | |
| 25 | M | 57 | 4 | Carboplatin, Pemetrexed and Pembrolizumab | PR | |
| 26 | F | 65 | 3 | a | Durvalumab | PR |
| 27 | F | 85 | 1 | a | N/A | |
| 28 | F | 63 | 1 | b | Cisplatin and Docetaxel | NED |
| 29 | M | 63 | 1 | a | N/A | |
| 30 | M | 75 | N/A | N/A | N/A | |
| 31 | F | 74 | 3 | a | Carboplatin and Paclitaxel | SD |
| 32 | F | 68 | 4 | Pembrolizumab | PR | |
| 33 | M | 84 | 2 | b | N/A | |
| 34 | F | 79 | 4 | N/A | ||
| 35 | M | 62 | 4 | Cisplatin and Etoposide | PR | |
| 36 | F | 95 | 2 | b | N/A |
Figure_1. Work-flow of study design.
(A) Study profile. Of the 39 patients who were considered eligible for the study, 34 patients had disease staging determined clinically and 23 patients received chemotherapy. (B) PLS-DA variable importance was used to select feature subsets when training PLS-DA, SVM and DNN models; while RF variable importance was used to select feature subsets when training the RF model. (C) Visual example of model training and validation. The permuted (randomly sorted) dataset is split into k folds (subsets; here, k=5). Model is trained with k-1 folds and validated with the kth fold. This process is repeated until all folds have been used once as the validation set. The next iteration of the cross validation involves another permutation of the complete dataset and repeating the whole process. Final results of each model are the averages of the validations across all folds and all iterations. Color figure online.
Classification results
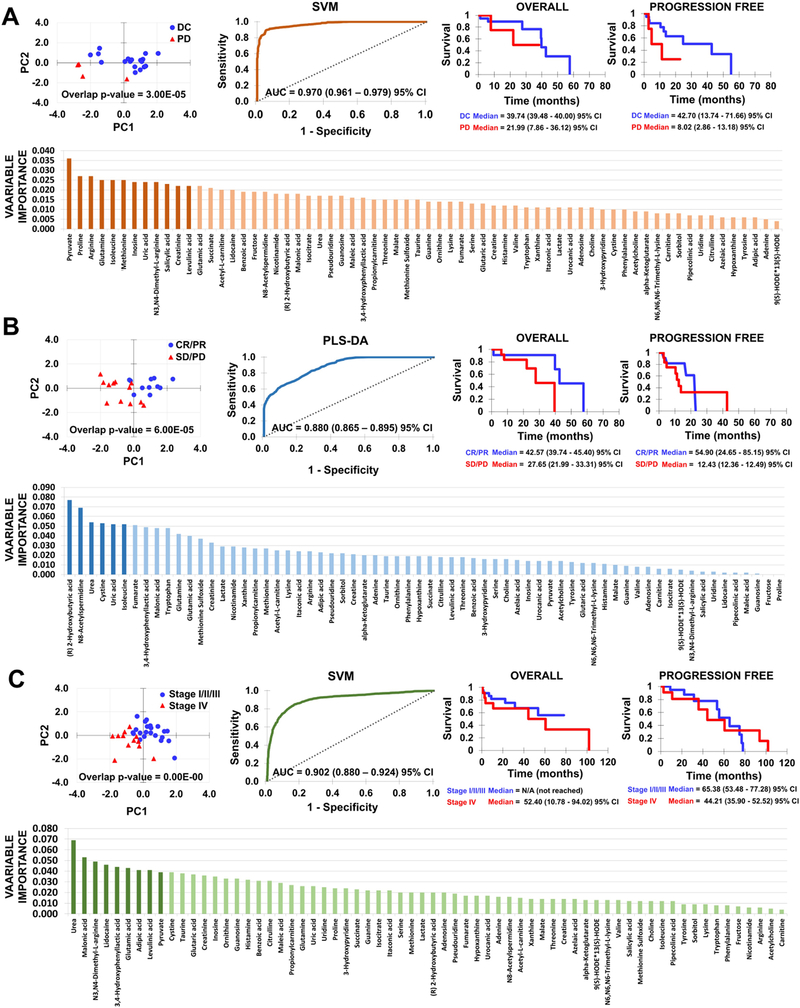
For each patient classification, Figure_2 shows PLS-DA score plot with distribution of samples on the first two components using the optimum subset of features after forward feature selection. Score plots, illustrating separation between classes and within samples of same class after selecting optimal number of features with PLS-DA, were quantified with the J2 criterion and overlap p-values, showing that statistically significant (p≤0.05) separation was achieved for all classes. Comparison of score plots for classifications before and after feature selection (Supplementary_Figure_2) shows that whereas PLS-DA is able to separate the classes, PCA would have been insufficient. Figure_2 further shows the highest-performing model classifications: DC vs. PD with SVM (AUC = 0.970 (0.961 – 0.979) 95% CI), CR/PR vs. SD/PD with PLS-DA (AUC = 0.880 (0.865 – 0.895) 95% CI), and stage I/II/III vs. stage IV with SVM (AUC = 0.902 (0.880 – 0.924) 95% CI). Supplemental_Figure_3 illustrates performance measures (AUC, balanced accuracy, and kappa) of all learning methods as a function of features retained for each classification. While SVM performed well with DC vs. PD and stage I/II/III vs. stage IV, PLS-DA and DNN performed better with CR/PR vs. SD/PD. Generally, RF had the lowest performance. AUROC curves for all learning methods using optimal subset of features are shown with corresponding performance measures in Supplementary_Figure_4. Highest balanced accuracy (0.92) was achieved for DC vs. PD with SVM. Figure_ 2 further shows Kaplan–Meier survival curves for progression-free survival (PFS) and overall survival (OS).
Figure_2. Classification model results:
(A) DC vs. PD; (B) CR/PR vs. SD/PD; (C) Stage I/II/III vs. Stage IV. For each classification (left to right): PLS-DA score plot with distribution of samples on the first two components using optimum subset of features after forward feature selection; AUROC curve for highest performing model; Kaplan–Meier curves for overall (OS) and progression-free (PFS) survival; PLS-DA variable importance ranking for all 66 features (darker bars: features used to train highest-performing model).
Key metabolites identified by variable importance
Ranking of PLS-DA variable importance for the three patient comparisons is shown in Figure_2. For DC vs. PD, the top 12 metabolites trained the SVM model: pyruvate, proline, arginine, glutamine, isoleucine, methionine, inosine, uric acid, N3,N4-dimethyl-L-arginine, salicylic acid, creatine, and levulinic acid (Supplementary_Table_1). Isoleucine, creatinine, and serine correlated with DC, while pyruvate and uric acid correlated with PD. For CR/PR vs. SD/PD, the top 6 metabolites trained the PLS-DA model: (R) 2-Hydroxybutyric acid, N8-acetylspermidine, urea, cystine, uric acid, and isoleucine (Supplementary_Table_2). Fumarate, tryptophan, methionine sulfoxide, and creatinine correlated with CR/PR, while N8-acetylspermidine, uric acid, and malonic acid correlated with SD/PD. For Stage I/II/III vs. Stage IV, the top 9 metabolites trained the SVM model: urea, malonic acid, N3,N4-dimethyl-L-arginine, lidocaine, 3,4-hydroxyphenyllactic acid, glutamic acid, adipic acid, levulinic acid, and pyruvate (Supplementary_Table_3). Glutaric acid correlated with stage 4. Key metabolites identified by RF are summarized in Supplementary_Tables_ 4–6.
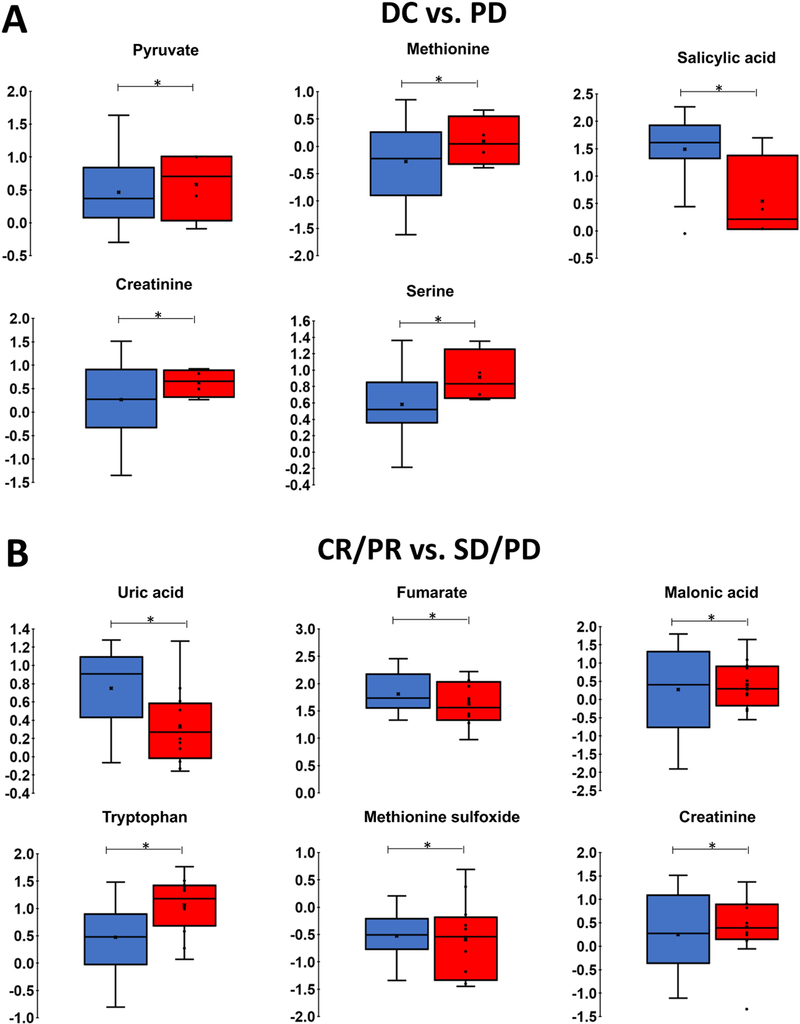
Figure_3 shows metabolites with a significant effect of group in terms of relative abundance (Wilcoxon rank-sum test, p≤0.05). Pyruvate, methionine, salicylic acid, creatinine, and serine were associated with DC vs. PD, while uric acid, fumarate, malonic acid, tryptophan, methionine sulfoxide, and creatinine were associated with CR/PR vs. SD/PD. Supplementary_Figure_ 5 shows the set of metabolites not reaching significance with p≤0.1.
Figure_3. Relative abundance of key metabolites.
Each box represents 1st and 3rd quartiles. Bands within represent the median and x is the mean. Ends of whiskers are maximum and minimum, with points outside being outliers. P-values found by Wilcoxon rank-sum test (*p≤0.05). (A) DC (blue) vs. PD (red); (B) CR/PR (blue) vs. SD/PD (red). Color figure online.
Key metabolites for all patient classifications are summarized in Table_2.
Table_2. Metabolites as a function of patient classification.
Subset of metabolites identified by variable importance correlated to chemotherapy response and staging (Spearman correlation; p≤0.05). Medium gray: favorable response/staging; dark gray: poor response or Stage IV; light gray: not statistically significant with correlation p≤0.1. Checkmarks: significant effect of group in terms of relative abundance (Wilcoxon rank-sum test, p≤0.05; unchecked: p≤0.1).
| METABOLITE | DC | PD | CR/PR | SD/PD | Stage I/II/III | Stage IV |
|---|---|---|---|---|---|---|
| Pyruvate | ✓ | |||||
| Isoleucine | ||||||
| Uric Acid | ✓ | |||||
| Creatinine | ✓ | ✓ | ||||
| Serine | ✓ | |||||
| N8-Acetylspermidine | ||||||
| Fumarate | ✓ | |||||
| Malonic Acid | ✓ | |||||
| Tryptophan | ✓ | |||||
| Methionine Sulfoxide | ✓ | |||||
| Glutaric Acid | ||||||
| Methionine | ✓ | |||||
| Salicylic Acid | ✓ | |||||
| Threonine | ||||||
| Valine | ||||||
| Phenylalanine | ||||||
| (R) 2-Hydroxybutyric acid | ||||||
| Cystine | ||||||
| 3,4-Hydroxyphenyllactic Acid | ||||||
| Lysine |
13C stable-isotope resolved metabolomics
Log transformed relative abundances and labeled fraction relative abundances of lactate for patient classifications are in Supplementary_Figure_6. Labeled fractions were calculated as (13C -Labeled metabolite x peak area)/(Unlabeled metabolite x peak area). Due to the number of missing values in 13C labeled metabolite dataset, we relied on complete case analysis, as imputation would be inaccurate. Although results were not statistically significant, interesting trends emerge. 13C -labeled lactate was higher in PD, SD/PD and stage IV classifications by median, and DC and stage I/II/III had a larger range of values in log transformed set. Medians between DC and PD, and between stage I/II/III and IV, were similar in the labeled fraction set.
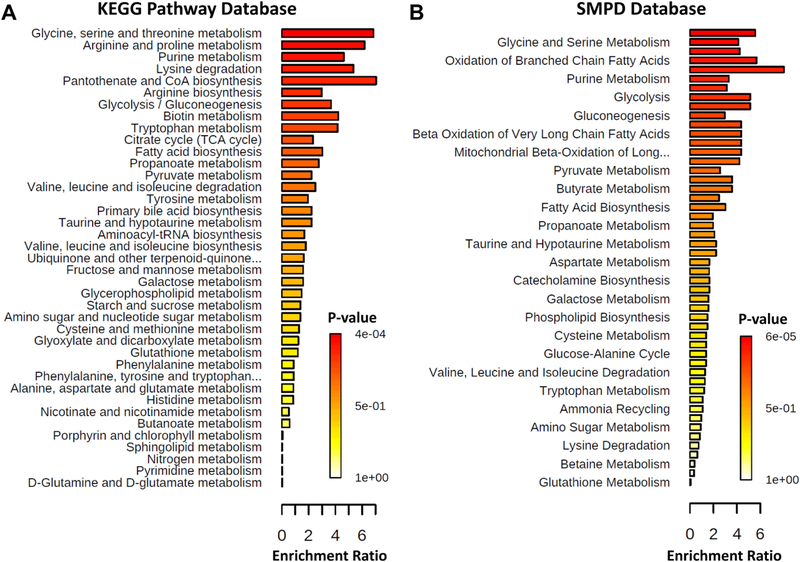
Metabolic pathway enrichment and network analysis
Top metabolic pathways identified in MetaboAnalyst 4.0 by the chosen databases are shown in Figure_4 and listed with associated statistics in Supplementary_Table_7. Top 5 metabolic pathways identified by KEGG database by p-value were: glycine, serine and threonine metabolism; arginine and proline metabolism; purine metabolism; lysine degradation; pantothenate and CoA biosynthesis. Top 5 metabolic pathways identified by SMPD database by p-value were: arginine and proline metabolism; glycine and serine metabolism; carnitine synthesis; oxidation of branched chain fatty acids; D-arginine and D-ornithine metabolism. Visualization of an integrated metabolic network of serine, methionine, and isoluecine related to DC vs. PD is shown in Supplementary Figure 7.
Figure_4. Quantitative Enrichment Analysis.
Enriched metabolic pathways (see Supplementary Table 7) were found with MetaboAnalyst 4.0 using (A) KEGG pathway database and (B) SMPD database.
DISCUSSION
This proof-of-concept study explored the hypothesis that a comprehensive machine learning analysis of biopsied tissue metabolomic data would be able to differentiate between DC vs. PD and CR/PR vs. SD/PD responses, and between stage I/II/III and stage IV NSCLC disease. The study adhered to CHARMS checklist(29) and REMARK criteria(30) to ensure the integrity of the prediction models. A metabolite set of 66 common metabolites was selected among 36 total samples. Potential sets of metabolites associated with disease staging and patient response to chemotherapy were identified in terms of PLS-DA variable importance (Figure_2, Supplementary_Tables_1–3), and to train PLS-DA, SVM and DNN learning models. A rigorous combination of feature selection and k-fold cross validation was performed to minimize overfitting of the models. While for all three models AUROCTrain=1.0, SVM performed the best with DC vs. PD (AUROCTest=0.970) and Stage I/II/III vs. Stage IV (AUROCTest=0.902) while PLS-DA performed best with CR/PR vs. SD/PD (AUROCTest=0.880) (Supplementary_Figure_4). Although RF as a learning model did not perform as well, the ranking of metabolites in terms of RF variable importance was generally consistent with those of PLS-DA variable importance (Supplementary_Tables_4–6).
Isoleucine was a key metabolite identified by both PLS-DA and RF variable importance and correlated to disease control. Branched-chain amino acids (BCAA’s: isoleucine, leucine, and valine) are preferentially uptaken by tumor cells and used for protein synthesis or oxidized for energy production(31). Enzymes BCAT1 and BCAT2 catalyze the first step in BCAA degradation and are overexpressed in many cancers(32). Isoleucine can be converted into acetyl-coA through BCAA metabolism(33), which can then fuel the TCA cycle. High levels of isoleucine as well as valine may imply lower activity of BCAA metabolism-related enzymes, thereby limiting acetyl-coA available for the TCA cycle and restraining the cancer aggressiveness. Creatinine correlated with improved response (DC and CR/PR), while serine correlated with DC. These metabolites are shown to support an anti-tumor immune response(34,35). In contrast, pyruvate correlated with PD, as it promotes tumor proliferation(36), angiogenesis(37), and immune response downstaging(38). Malonic acid, associated with epithelial-mesenchymal transition indicative of aggressive cancer(39), correlated with SD/PD, while uric acid correlated with poor response (PD and SD/PD). N8-Acetylspermidine, known to induce cell differentiation(40), also correlated with SD/PD. Interestingly, fumarate correlated with CR/PR, although fumarate accumulation could confer a proliferative signal(41) and prevent HIF degradation(42). Methionine sulfoxide and tryptophan correlated with CR/PR, with the former known to relieve acidity-induced cellular stress(43) while the latter supports immune system activity(44). Lastly, glutaric acid correlated with Stage IV.
Although it is no surprise that glycolysis, purine metabolism and several amino acid metabolism pathways were considered significant (Figure_4 & Supplementary_Table_7), the detection of gluconeogenesis is interesting and may warrant further investigation. The only organ tissues normally expressing genes necessary for gluconeogenic pathway are kidneys and liver(45). In cancer, upregulated phosphoenolpyruvate carboxykinase (PEPCK) transcription is a known marker of tumorigenic cells, enhancing gluconeogenesis(45). In non-gluconeogenic tissues (such as lung), PEPCK plays a protumorigenic role by expanding the source of nutrition rather than increasing glucose production(45). Additionally, pathways related to fatty acid oxidation were found to be significant, implying an important role of fatty acid (FA) metabolism in NSCLC. Although FA metabolism in cancer is poorly understood, it is thought to be complex and dependent on specific tissue types(46). Metastasis can only occur when cell membranes have high fluidity by incorporation of high amounts of unsaturated FAs relative to saturated FAs(47). In contrast, cancer cells with high lipid saturation and low membrane fluidity show resistance to chemotherapeutics (48), possibly linking FA synthesis and drug resistance. Lung cancer patients with high plasma membrane fluidity tend to have worse prognosis(49). Fatty acid oxidation is also an important source of ATP production, especially after loss of attachment to the extracellular matrix(46). Several studies have examined the role of fatty acid metabolism related genes in relation to various cancer types(46), finding that FA metabolism genes may be tumor-suppressive in some cancers while being pro-oncogenic in others.
While most patients in this study received combination chemo- and immunotherapy, a few received either chemo- or immunotherapy only (Table 1). Differences expected in metabolic profiles with immunotherapy vs. chemotherapy could be explored with a larger patient set in the future. Further, investigation of interactions of drug action mechanisms with key metabolites could yield insight into treatment response beyond potential variations in tumor types. Chemotherapeutics included cisplatin and carboplatin, which exert cytotoxic effects by generating ROS and inhibiting cell replication via DNA cross-linking; paclitaxel and docetaxel, which inhibit microtubule depolymerization; etoposide, which damages DNA by targeting topoisomerase II; pemetrexed, which inhibits enzyme targets related to folate metabolism and pyrimidine and purine synthesis. Immunotherapeutics included pembrolizumab, which binds to PD-1 receptor and blocks PD-L1 and PD-L2; durvalumab, which blocks PD-L1 interaction with PD-1 and CD80.
As depicted in Supplementary Figure 7, serine gives rise to cystathionine, a precursor for glutathione, which is known to chelate cisplatin and play a role in copper-transporter-mediated cisplatin efflux(50). Glutathione can also act protect against platinum induced oxidative stress(50), implying that high serine levels may lead to cisplatin/carboplatin resistance. This could explain elevated serine in PD patients, since in this study all PD patients received carboplatin. It is unsurprising that pyruvate was a strong predictor of chemotherapy response, since it has a central role is fundamental metabolic pathways such as glycolysis, citric acid cycle, urea cycle, and amino acid metabolism. Increased pyruvate levels in PD patients may indicate a general increase in metabolic rate, resulting in a more aggressive NSCLC phenotype. Additionally, it has been hypothesized that pyruvate is increased in mitochondria of cisplatin-resistant cells, due to less dependence on glucose and lower lactate production, where it helps TCA cycle replenishment(51).
Recently, lung cancer chemotherapy prediction models have been proposed using metabolomics data derived from serum or plasma samples. Stage III and IV SCLC and NSCLC patient samples were analyzed in terms of PR vs. PD response to platinum-based chemotherapy(6), finding eight out of 21 key metabolites (PLS-DA VIP scores ≥1) to be significant based on Spearman correlation analysis: phenylalanine, tyrosine, tryptophan, citric acid/isocitric acid, α-ketoglutarate, succinate, d/l-2-hydroxyglutaric acid, and pyroglutamic acid. Top metabolic pathways found during QEA in (6) matching those in our study included tryptophan metabolism and gluconeogenesis (Figure_4). Stage IIIB and IV nonsquamous NSCLC patient samples were analyzed in terms of DC vs. PD response to pemetrexed plus platinum chemotherapy(7). Out of 32 metabolites with PLS-DA VIP scores ≥1, eight key metabolites had lower levels in DC: choline, taurine, hypotaurine, uridine, betaine, dimethylglycine, dodecanoylcarnitine and L-palmitoylcarnitine, and three metabolites elevated in DC: palmitic amide, imidazole-4-acetaldehyde, and niacinamide. Although several metabolites and metabolite derivatives (structural analogues) identified in these studies were also detected in our samples, most key metabolites in this study are different. Regarding chemotherapy response classification, succinate and phenylalanine were in common with previous optimal metabolic feature subsets, while tryptophan was in common in terms of correlation p-value. Phenylalanine was potentially elevated in PD patients in this study (Supplementary Figure 5), in contrast with (6), which found phenylalanine to be lowered in PD compared to PR. Elevated phenylalanine has been noted in hepatocellular carcinoma tissue(52), possibly due to increased amino acid consumption, and in sera of ovarian carcinoma patients(53), for which cancer-associated inflammation and immune activation may impair phenylalanine (4)-hydroxylase (PAH) activity, leading to accumulation. Interestingly, neither of the previous studies(6,7) nor our study found lactate to be important for determining chemotherapy response. Lactate has been shown to have multiple roles during tumor growth(54), with production controlled by tumor cells to maintain pericellular pH homeostasis(55) and regulate the microenvironment(56). Lactate promotes M2-like macrophage polarization and induces epigenetic regulation via lactate-derived histone lactylation(57). Lactate was considered for statistical analysis via SIRM, with 13C labeled lactate detected in 31 of 36 samples. However, we were unable to discern any predictive capacity of 13C labeled lactate (Supplementary_ Figure_ 6) from tissue incubated immediately after excision. 13C labeled lactate has previously detected altered regulation of lung cancer metabolic pathways when infused into patients immediately prior to excision(9). These results indicate that the amount of 13C labeled lactate present in any given sample, which would be strictly due to glycolysis, may not necessarily reflect staging or chemotherapy response.
A major difference with previous studies is that this study utilized fresh tumor tissue samples, circumventing the assumption that serum metabolic profiles are directly linked to tumor tissue behavior. This assumption may not hold, especially for amino acids since low-mass intracellular proteins may have difficulty shedding into circulation(58). Another difference is that this study included NSCLC patients at all stages and various chemotherapy regimens (Tables 1). Further, cancer metabolomics studies often exclude metabolites with missing values. The simplest approach for handling missing data is the ad hoc method of complete case analysis (CCA)(59), as in(6,7), for which features with missing data are excluded from the dataset being analyzed. CCA has the potential for excluding metabolites from the prediction model that may be influential on the response. Working under the assumption that missing values were missing at random (MAR), we chose to perform imputations using PPCA, after concluding it was the most accurate imputation method on a complete subset of data taken from the original data. We chose CCA as appropriate for the 13C labeled metabolite set, as it was missing ~45% in total.
Altogether, these caveats and methodology differences may explain discrepancies between our results and those from previous studies. We have presented a proof-of-concept study with a relatively small patient number, laying a foundation for systematically examining metabolic characteristics of patient-derived NSCLC tissue with the longer term objective of predicting patient-specific response to established first-line chemotherapy regimens. With a sufficiently large longitudinal dataset, the prognostic value of predictive metabolomic biomarkers could also be determined. For example, high levels of uric acid may imply enhanced purine metabolism, where a therapeutic such as pemetrexed, which inhibits purine synthesis, might be more efficacious. Although metabolomics is a promising emerging field when applied for biomarker discovery, it faces limitations which have largely prevented clinical translation. The complexity of the data lends itself to mathematical and computational analyses, which would allow tissue-scale simulation of a variety of tumor characteristics and chemotherapeutic responses to a range of cytotoxic agents while providing further validation to statistical analyses. Longer term, a higher dimensional framework integrating comprehensive omics data could lead to enhanced understanding of how cancer metabolism influences NSCLC progression and response to therapy.
Supplementary Material
Highlights.
Designs workflow for personalized prediction of response to first-line chemotherapy
Performs systematic metabolomic analysis of lung cancer fresh tumor core biopsies
Machine learning analyses differentiate between treatment responses and disease stage
Predicts response and identifies metabolites associated with patient classifications
ACKNOWLEDGEMENTS
Authors acknowledge contribution of Grace Mahlbacher, Dr. Sanaya Stocke, and Dr. Brian Clem to processing of patient samples; Melissa Hall, Andrei Smolenkov, and Danyelle Clark for James Graham Brown Cancer Center Biorepository support; Andrea Spencer, Lauren Whelan, Dr. John Hamm, and Dr. Stephen Wyatt for collection of samples at Norton Hospital.
RESEARCH SUPPORT: DMM, VvB, HBF acknowledge support by National Cancer Institute grant R15CA203605 (Frieboes).
ABBREVIATIONS
- ANN
artificial neural network
- CCA
complete case analysis
- CR
complete response
- CT
computed tomography
- DC
disease control
- LC
liquid chromatography
- MRI
resonance magnetic imaging
- MSI
Metabolomics Standards Initiative
- NMR
nuclear magnetic resonance spectroscopy
- NSCLC
non-small cell lung cancer
- MS
mass spectrometry
- PCA
principal component analysis
- PD
progressive disease
- PET
positron emission tomography
- PLS-DA
partial least squares discriminant analysis
- PR
partial response
- RF
random forests
- SCLC
small cell lung cancer
- SD
stable disease
- SIRM
stable isotope resolved metabolomics
- SVM
support vector machines
- TCA
tricarboxylic acid cycle
Footnotes
CONFLICTS OF INTEREST:
The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY
Datasets have been deposited in (Metabolomics Workbench (MetWB), RRID:SCR_013794, www.metabolomicsworkbench.org) (Study ST001527).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34 [DOI] [PubMed] [Google Scholar]
- 2.Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S Jr., Brahmer JR, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015;33:3488–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–8 [DOI] [PubMed] [Google Scholar]
- 5.Bamji-Stocke S, van Berkel V, Miller DM, Frieboes HB. A review of metabolism-associated biomarkers in lung cancer diagnosis and treatment. Metabolomics 2018;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng F, Liu Y, He C, Kong Y, Ouyang Q, Xie X, et al. Prediction of platinum-based chemotherapy efficacy in lung cancer based on LC-MS metabolomics approach. J Pharm Biomed Anal 2018;154:95–101 [DOI] [PubMed] [Google Scholar]
- 7.Tian Y, Wang Z, Liu X, Duan J, Feng G, Yin Y, et al. Prediction of Chemotherapeutic Efficacy in Non-Small Cell Lung Cancer by Serum Metabolomic Profiling. Clin Cancer Res 2018;24:2100–9 [DOI] [PubMed] [Google Scholar]
- 8.Bruntz RC, Lane AN, Higashi RM, Fan TW. Exploring cancer metabolism using stable isotope-resolved metabolomics (SIRM). J Biol Chem 2017;292:11601–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, et al. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM). Mol Cancer 2009;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dercle L, Fronheiser M, Lu L, Du S, Hayes W, Leung DK, et al. Identification of Non-Small Cell Lung Cancer Sensitive to Systemic Cancer Therapies Using Radiomics. Clin Cancer Res 2020;26:2151–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klavins K, Drexler H, Hann S, Koellensperger G. Quantitative metabolite profiling utilizing parallel column analysis for simultaneous reversed-phase and hydrophilic interaction liquid chromatography separations combined with tandem mass spectrometry. Analytical chemistry 2014;86:4145–50 [DOI] [PubMed] [Google Scholar]
- 12.Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS Online: A Web-Based Platform to Process Untargeted Metabolomic Data. Analytical chemistry 2012;84:5035–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X, Sun W, Shi X, Koo I, Wang B, Zhang J, et al. MetSign: a computational platform for high-resolution mass spectrometry-based metabolomics. Analytical chemistry 2011;83:7668–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei X, Lorkiewicz PK, Shi B, Salabei JK, Hill BG, Kim S, et al. Analysis of stable isotope assisted metabolomics data acquired by high resolution mass spectrometry. Anal Methods-Uk 2017;9:2275–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mónica Calderón-Santiago MAF-P, Feliciano Priego-Capote & María D. Luque de Castro MSCombine: a tool for merging untargeted metabolomic data from high-resolution mass spectrometry in the positive and negative ionization modes. Metabolomics 2016;12 [Google Scholar]
- 16.van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics 2006;7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei R, Wang J, Su M, Jia E, Chen S, Chen T, et al. Missing Value Imputation Approach for Mass Spectrometry-based Metabolomics Data. Sci Rep 2018;8:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller HA, Emam R, Lynch CM, Bockhorst S, Frieboes HB. Discrepancies in metabolomic biomarker identification from patient-derived lung cancer revealed by combined variation in data pre-treatment and imputation methods. Metabolomics 2021;17:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tipping MB, CM.. Probabilistic principal component analysis. JR Statist Soc 1999;61:611–22 [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47 [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Delgado MC E; Barro S Do we Need Hundreds of Classifiers to Solve Real World Classification Problems? Journal of Machine Learning Research 2014;15:3133–81 [Google Scholar]
- 22.Kuhn M Building predictive models in R using the caret package. Journal of Statistical Software 2008;28:1–2627774042 [Google Scholar]
- 23.Cho HW, Kim SB, Jeong MK, Park Y, Miller NG, Ziegler TR, et al. Discovery of metabolite features for the modelling and analysis of high-resolution NMR spectra. Int J Data Min Bioinform 2008;2:176–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gromski PS, Xu Y, Correa E, Ellis DI, Turner ML, Goodacre R. A comparative investigation of modern feature selection and classification approaches for the analysis of mass spectrometry data. Anal Chim Acta 2014;829:1–8 [DOI] [PubMed] [Google Scholar]
- 25.Buhmann; KHBCSOKESJM. The Balanced Accuracy and Its Posterior Distribution. 2010 20th International Conference on Pattern Recognition 2010:3121–4 [Google Scholar]
- 26.Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther 2005;85:257–68 [PubMed] [Google Scholar]
- 27.Worley B, Halouska S, Powers R. Utilities for quantifying separation in PCA/PLS-DA scores plots. Anal Biochem 2013;433:102–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 2018;46:W486–W94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med 2014;11:e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Urol 2005;2:416–22 [PubMed] [Google Scholar]
- 31.Ananieva E Targeting amino acid metabolism in cancer growth and anti-tumor immune response. World J Biol Chem 2015;6:281–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayers JR, Torrence ME, Danai LV, Papagiannakopoulos T, Davidson SM, Bauer MR, et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 2016;353:1161–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adeva-Andany MM, Lopez-Maside L, Donapetry-Garcia C, Fernandez-Fernandez C, Sixto-Leal C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 2017;49:1005–28 [DOI] [PubMed] [Google Scholar]
- 34.Salazar JH. Overview of Urea and Creatinine. Laboratory Medicine 2014;45:e19–e20 [Google Scholar]
- 35.Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, Samborska B, et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab 2017;25:345–57 [DOI] [PubMed] [Google Scholar]
- 36.Sellers K, Fox MP, Bousamra M 2nd, Slone SP, Higashi RM, Miller DM, et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest 2015;125:687–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung SY, Song HS, Park SY, Chung SH, Kim YJ. Pyruvate promotes tumor angiogenesis through HIF-1-dependent PAI-1 expression. Int J Oncol 2011;38:571–6 [DOI] [PubMed] [Google Scholar]
- 38.Abusalamah H, Reel JM, Lupfer CR. Pyruvate affects inflammatory responses of macrophages during influenza A virus infection. Virus Res 2020;286:198088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aspuria PP, Lunt SY, Varemo L, Vergnes L, Gozo M, Beach JA, et al. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab 2014;2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mudumba S, Menezes A, Fries D, Blankenship J. Differentiation of PC12 cells induced by N8-acetylspermidine and by N8-acetylspermidine deacetylase inhibition. Biochem Pharmacol 2002;63:2011–8 [DOI] [PubMed] [Google Scholar]
- 41.Kerins MJ, Vashisht AA, Liang BX, Duckworth SJ, Praslicka BJ, Wohlschlegel JA, et al. Fumarate Mediates a Chronic Proliferative Signal in Fumarate Hydratase-Inactivated Cancer Cells by Increasing Transcription and Translation of Ferritin Genes. Mol Cell Biol 2017;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 2005;8:143–53 [DOI] [PubMed] [Google Scholar]
- 43.Lim JM, Kim G, Levine RL. Methionine in Proteins: It’s Not Just for Protein Initiation Anymore. Neurochem Res 2019;44:247–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tantawy AA, Naguib DM. Arginine, histidine and tryptophan: A new hope for cancer immunotherapy. PharmaNutrition 2019;8:100148 [Google Scholar]
- 45.Wang Z, Dong C. Gluconeogenesis in Cancer: Function and Regulation of PEPCK, FBPase, and G6Pase. Trends Cancer 2019;5:30–45 [DOI] [PubMed] [Google Scholar]
- 46.Chen M, Huang J. The expanded role of fatty acid metabolism in cancer: new aspects and targets. Precis Clin Med 2019;2:183–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao W, Prijic S, Urban BC, Tisza MJ, Zuo Y, Li L, et al. Candidate Antimetastasis Drugs Suppress the Metastatic Capacity of Breast Cancer Cells by Reducing Membrane Fluidity. Cancer Res 2016;76:2037–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res 2010;70:8117–26 [DOI] [PubMed] [Google Scholar]
- 49.Sok M, Sentjurc M, Schara M, Stare J, Rott T. Cell membrane fluidity and prognosis of lung cancer. Ann Thorac Surg 2002;73:1567–71 [DOI] [PubMed] [Google Scholar]
- 50.Chen HH, Kuo MT. Role of glutathione in the regulation of Cisplatin resistance in cancer chemotherapy. Met Based Drugs 2010;2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wangpaichitr M, Wu C, Li YY, Nguyen DJM, Kandemir H, Shah S, et al. Exploiting ROS and metabolic differences to kill cisplatin resistant lung cancer. Oncotarget 2017;8:49275–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe A, Higashi T, Sakata T, Nagashima H. Serum amino acid levels in patients with hepatocellular carcinoma. Cancer 1984;54:1875–82 [DOI] [PubMed] [Google Scholar]
- 53.Neurauter G, Grahmann AV, Klieber M, Zeimet A, Ledochowski M, Sperner-Unterweger B, et al. Serum phenylalanine concentrations in patients with ovarian carcinoma correlate with concentrations of immune activation markers and of isoprostane-8. Cancer Lett 2008;272:141–7 [DOI] [PubMed] [Google Scholar]
- 54.Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des 2012;18:1319–30 [DOI] [PubMed] [Google Scholar]
- 55.Mazzio EA, Boukli N, Rivera N, Soliman KF. Pericellular pH homeostasis is a primary function of the Warburg effect: inversion of metabolic systems to control lactate steady state in tumor cells. Cancer Sci 2012;103:422–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de la Cruz-Lopez KG, Castro-Munoz LJ, Reyes-Hernandez DO, Garcia-Carranca A, Manzo-Merino J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front Oncol 2019;9:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019;574:575–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frieboes HB, Curtis LT, Wu M, Kani K, Mallick P. Simulation of the Protein-Shedding Kinetics of a Fully Vascularized Tumor. Cancer Inform 2015;14:163–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Do KT, Wahl S, Raffler J, Molnos S, Laimighofer M, Adamski J, et al. Characterization of missing values in untargeted MS-based metabolomics data and evaluation of missing data handling strategies. Metabolomics 2018;14:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets have been deposited in (Metabolomics Workbench (MetWB), RRID:SCR_013794, www.metabolomicsworkbench.org) (Study ST001527).