Abstract
Prosthethic Joint Infection (PJI) is a severe complication following joint replacement. Late PJI can occur years after implantation by hematogenous seeding of a microbial agent. Staphylococcus xylosus is a coagulase-negative commensal of the human skin and rarely associated with opportunistic human infections. We report the rare case of a 70-year old Patient suffering from knee pain 18 years after primary Total Knee Arthroplasty. Microbiological sampling detected S. xylosus as causative agent. The patient was successfully treated with a two-stage implant exchange and antibiotic therapy using co-amoxicillin and rifampicin/cotrimoxazol. This case illustrates the ability of S. xylosus to cause late PJI and the importance of not letting coagulase-negative Staphylococci be routinely categorized as contaminants of microbiological samples.
Keywords: Prosthetic joint infection, PJI, Total knee arthroplasty, TKA, Staphylococcus xylosus
Introduction
Staphylococcus xylosus is a gram-positive, coagulase-negative commensal of human and animal skin. It is a ubiquitous bacterium naturally present in food. It is one of the major starter cultures used for meat fermentation [[1], [2], [3]]. S. xylosus can cause bovine mastitis, and hence, it is a clinically relevant pathogen in veterinary medicine [12]. In humans, few reports described S.xylosus infection in hosts with comorbidities [3,10,11].
PJI is a severe complication following joint replacement. No classification system of PJI is unanimously accepted. On the basis of the two major infection routes, PJI can be classified as either exogenous or hematogenous infection. When considering the virulence of bacteria and the host response of immunocompetent patients, PJI can be classified on the basis of symptoms as acute or chronic. The traditional classification differentiates among early (those developing within <3 months after implantation), delayed (3–24 months after surgery), and late (>24 months after implantation) infection. In clinical practice, it is more useful to classify PJI as follows:
1. •Acute hematogenous PJI: Infection with 3 weeks or less of duration of symptoms after an uneventful postoperative period
2. •Early postinterventional PJI: Infection that manifests within 1 month after an invasive procedure (surgery or arthrocentesis)
3. •Chronic PJI: Infection with symptoms that persist for more than 3 weeks and are beyond the early postinterventional period
This simple classification allows the differentiation between PJI which can be potentially cured with débridement and implant retention, and the infection requiring device removal for cure. Acute hematogenous infection is generally caused by virulent bacteria and requires débridement to retain the device. This type of PJI can occur at any time after surgery. The risk is highest within the first 3 months after implantation. However, most hematogenous infections occur late after surgery because the risk for hematogenous seeding persists lifelong. Early postoperative PJI manifests itself by poor wound healing, joint fluid accumulation, or persisting pain. In these cases, early detection by a high degree of suspicion, rapid diagnostic workup, and prompt surgical treatment is required for retention of the implant. In contrast, if these (exogenous) infections are not detected early after surgery (within the first month) or symptoms persist for more than 3 weeks, or both, chronic PJI develops irrespective of the infection route. This form of infection requires removal of all hardware and bone cement for cure. [20]
The cumulative incidence of late PJI ranges between 0.3 % and 0.9 % [7,14].
We present a rare case of a late PJI caused by S. xylosus in a patient who underwent total knee arthroplasty (TKA) 18 years earlier.
Case presentation
A 70-year old male with a history of bilateral TKA and right total hip arthroplasty (THA) presented twice in the emergency department with an over six-week history of new pain in the left knee. Recent trauma was denied. The patient had no clinically relevant comorbid condition. He was physically active. TKA was performed on the left side in 2001 and on the right side in 2004. No complications associated with any of the implants had been reported at any time since implantation. Primary THA on the right side followed in 2015. A spondylodesis and revision of stabilization material in the lumbar spine had been performed without complications in 2018. Approximately 12 weeks prior to first admission in the emergency department he had undergone dental cleaning. The patient exhibited an asymptomatic carious tooth chart and no oral antibiotic prophylaxis was administered. Nine weeks prior to first admission, he had cut his finger while preparing Salami sausage. Recent animal exposure was denied.
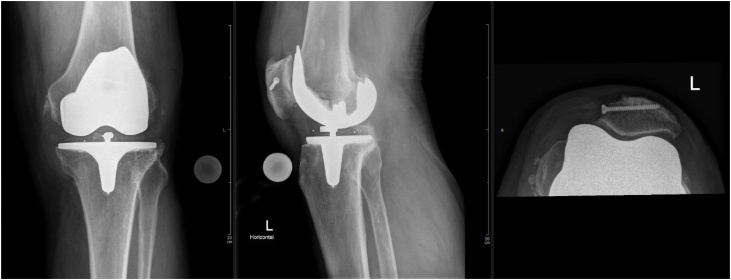
On the first admission, the patient presented slight local erythema, overheating and intra-articular effusion of the left knee. No sinus tract was present. Knee range of motion was painfully limited. Peripheral sensibility, perfusion and motor function was unaffected. Body temperature was 36.4 °C. Laboratory findings showed a C-Reactive Protein (CRP) value of 58.9 mg/L (<5.0 mg/L), white blood cell count 10.5 G/L (3.6–10.5 G/L). Joint puncture of the left knee revealed a synovial fluid WBC count of 8′278/μL (<200/μl). The synovial fluid sample was sent in for microbiological analysis. Conventional radiographs of the left knee showed no sign of implant loosening or fracture (Fig. 1).
Fig. 1.
Primary TKA of patient’s left knee.
The clinical picture together with the synovial WBC cell count indicated a PJI. The time interval between implantation and onset of symptoms pointed towards a late clinical manifestation. Considering the patients six-week history of symptoms, Debridement, Antibiotics and Implant Retention (DAIR) was not an option. Because the microbiological result was pending, the surgical treatment plan was not yet determined (i.e.; 1-stage exchange or 2-stage exchange). The patient was discharged and provisionally planned for an elective 2-stage exchange after results from microbiology laboratory would be certain (species identification and resistance pattern determination).
Four days after first admission the patient returned to the emergency department prior to his planned entry date, because of progressive pain, local erythema and swelling of the left knee. Body temperature was 38.3 °C. Laboratory findings showed an increased CRP value of 96.8 mg/L and an increased WBC count of 12.3 G/L. Meanwhile, the microbiological analysis of the joint puncture showed growth of S. xylosus with susceptibility to amoxicillin/clavulanate. The knee joint was aspirated again for a second microbiological sample. After obtaining blood cultures, an intravenous therapy with amoxicillin/clavulanate was started.
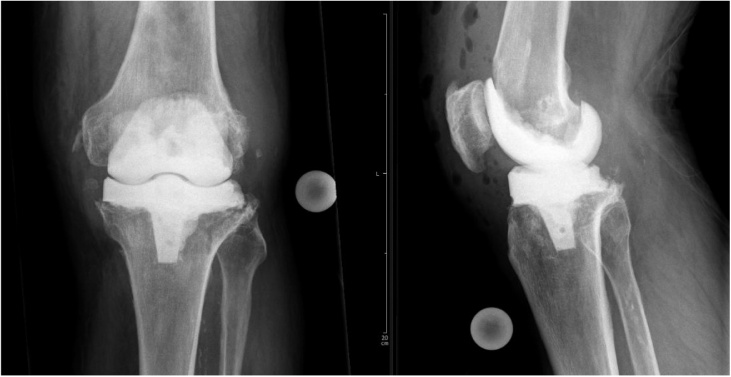
The implant was removed, including a 3.5 mm cortical screw which was situated in the patella after a primary transpatellar approach, and a mobile cement spacer (Heraeus Copal Knee moulds, Refobacin Bone Cement) was implanted (Fig. 2). Biopsy samples were obtained for microbiological and histological analysis. The antibiotic treatment was continued. S. xylosus was detected again in the synovial fluid obtained during the second aspiration. Histological analysis of the biopsies showed chronic detritus synovialitis with prosthesis abrasion and marginally increased neutrophil granulocytes. Histological criteria for infection were not met in the examined material.
Fig. 2.
Temporary cement spacer.
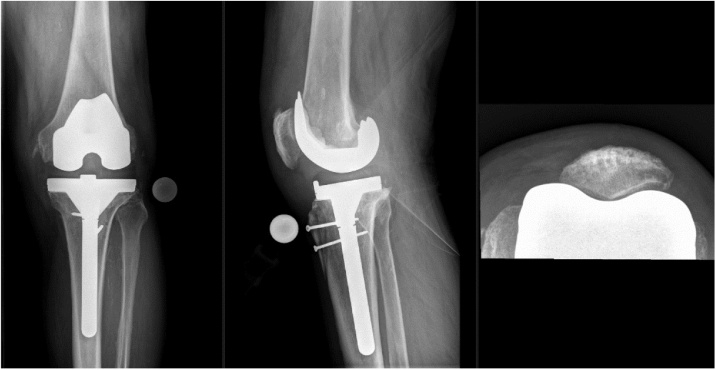
20 days later, a new total knee prosthesis was implanted while intravenous antibiotic therapy continued (Fig. 3). The samples taken in both operations did not show any microbiological growth. Intravenous amoxicillin/clavulanate was administered for a total of 25 days, followed by oral rifampicin and cotrimoxazol for another nine weeks.
Fig. 3.
Defintive revision TKA.
During outpatient follow-up exams at two months, four months and one year after the revision TKA, the left knee showed no effusion, intact stability and a range of motion of 110/0/0 for flexion/extension and the patient was satisfied with the function of the knee prosthesis.
Discussion
To our knowledge, only one case of Prosthetic Joint Infection (PJI) associated with S. xylosus is reported in current literature [13]. In our reported case, the time interval from implantation to onset of symptoms plus the lack of a sinus tract indicated a haematogenous pathogenesis. The monoculture growth of the microorganism in two different samples obtained at two different time points strongly indicates that S. xylosus is the causative pathogen.
The source of haematogenous seeding remains unknown and is speculative.
One possible source could be the Salami consumption while suffering from a carious tooth chart, cutting his finger, especially while preparing Salami, or gingival microtraumata during dental care.
The most common microbiological agents in haematogenous PJIs are Staphylococcus aureus and Streptococcus spp. [17]. Coagulase-negative staphylococci (CoNS) rarely cause haematogenous infections. Among CoNS, S.lugdunensis is typically associated with late infections [18], a species which resembles S. aureus in some regards.
CoNS are frequently summarized and referred to as one group to distinguish them from Staphylococcus aureus (and other coagulase-positive staphylococcal species). CoNS in fact comprise a heterogeneous group, ranging from true nonpathogenic to facultative pathogenic species with low, medium or even high virulence potential [19]. However, detailed species identification may help to allocate data with respect to host (e.g.; exchange between humans and animals) or anatomical colonization site of the human body. Considering the increasing application of sophisticated molecular methods for species identification in routine clinical microbiology, it is possible that the group of CoNS causing PJIs will be divided in more detail. The aforementioned taxonomy grouping may explain why, to the best of our knowledge, PJI due to S. xylosus has only been reported once previously [13].
Samples showing CoNS growth lead to challenging situations. CoNS are frequent contaminants of blood samples and therefore CoNS may mistakenly be disregarded as the causative agent for infections of any kind. [15]. In suspected PJIs, where only aspirated joint fluid is positive for CoNS (and no microbiological growth in tissue samples, negative sonication and normal histology) it therefore is difficult to define whether there effectively is PJI or a contamination. Considering the patients clinical symptomps and due to the patient history with fever and a second arthrocentesis again showing S. xylosus there was little doubt about the presence of PJI and that the causative agent was S. xylosus.
Time interval from implantation and onset of symptoms to certain diagnosis of PJI is an important variable. It helps to estimate the possible pathogenesis and the duration of PJI, and hence, the surgical and antibiotic therapy regime. It also underlines the importance of life long oral hygiene following arthroplasty implantation [16].
Conclusion
-
-
S. xylosus can cause PJI.
-
-
If S. xylosus is found in microbiological analysis of samples taken from a joint with suspected PJI, it must not be routinely regarded as contamination.
Conflicts of interest
There are no conflicts of interest of any kind.
Sources of funding
There are no sources of funding of any kind.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
The patient has agreed to collection and publication of data surrounding his case (see “Consent”). The data has been fully anonymized and the patient will not suffer consequences of any kind by writing and publication of this case report. The process of gaining an official ethical approval in Switzerland is associated with a massive amount of time and financing and would not be appropriate in this case.
Author contribution
Mr Yves Etienne Brand: Data Collection, Data Analysis, Writing.
Dr Benjamin Rufer: Writing, Supervision.
Acknowledgement
We would like to thank Prof. Parham Sendi for reviewing our report and the collegial exchange of ideas during the process of writing.
Contributor Information
Yves E. Brand, Email: yvesbrand@sonnenhof.ch.
Benjamin Rufer, Email: benjaminrufer@sonnenhof.ch.
References
- 1.Dordet-Frisoni E., Dorchies G., De Araujo C., Talon R., Leroy S. Genomic diversity in Staphylococcus xylosus. Appl Environ Microbiol. 2007;73(22):7199–7209. doi: 10.1128/AEM.01629-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schleifer K.H., Kloos W.E. Isolation and characterization of Staphylococci from human skin I. Amended descriptions of Staphylococcus epidermidis and Staphylococcus saprophyticus and descriptions of three new species: Staphylococcus cohnii, Staphylococcus haemolyticus, and Staphylococcus xylosus. Int J Syst Evol Microbiol. 1975;25(1):50–61. [Google Scholar]
- 3.Conrad S.A., West B.C. Endocarditis caused by Staphylococcus xylosus associated with intravenous drug abuse. J Infect Dis. 1984;149(5):826–827. doi: 10.1093/infdis/149.5.826. [DOI] [PubMed] [Google Scholar]
- 7.Huotari K., Peltola M., Jämsen E. The incidence of late prosthetic joint infections: a registry-based study of 112,708 primary hip and knee replacements. Acta Orthop. 2015;86(3):321–325. doi: 10.3109/17453674.2015.1035173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tselenis-Kotsowilis A.D., Koliomichalis M.P., Papavassiliou J.T. Acute pyelonephritis caused by Staphylococcus xylosus. J Clin Microbiol. 1982;16(3):593–594. doi: 10.1128/jcm.16.3.593-594.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhaddar A., Elouennass M., Naama O., Boucetta M. Staphylococcus xylosus isolated from an otogenic brain abscess in an adolescent. Surg Infect. 2010;11(6):559–561. doi: 10.1089/sur.2010.010. [DOI] [PubMed] [Google Scholar]
- 12.Thorberg B.M., Danielsson-Tham M.L., Emanuelson U., Waller K.P. Bovine subclinical mastitis caused by different types of coagulase-negative staphylococci. J Dairy Sci. 2009;92(10):4962–4970. doi: 10.3168/jds.2009-2184. [DOI] [PubMed] [Google Scholar]
- 13.Scorzolini L., Lichtner M., Iannetta M., Mengoni F., Russo G., Panni A.S. Sonication technique improves microbiological diagnosis in patients treated with antibiotics before surgery for prosthetic joint infections. New Microbiol. 2014;37(3):321–328. [PubMed] [Google Scholar]
- 14.Namba R.S., Inacio M.C., Paxton E.W. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. JBJS. 2013;95(9):775–782. doi: 10.2106/JBJS.L.00211. [DOI] [PubMed] [Google Scholar]
- 15.Thylefors J.D., Harbarth S., Pittet D. Increasing bacteremia due to coagulase-negative staphylococci: fiction or reality? Infect Control Hosp Epidemiol. 1998;19(8):581–589. doi: 10.1086/647878. [DOI] [PubMed] [Google Scholar]
- 16.Sendi Parham. Antibiotic prophylaxis during dental procedures in patients with prosthetic joints. J Bone Jt Infect. 2016;1:42. doi: 10.7150/jbji.16318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rakow A., Perka C., Trampuz A., Renz N. Origin and characteristics of haematogenous periprosthetic joint infection. Clin Microbiol Infect. 2019;25(7):845–850. doi: 10.1016/j.cmi.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Sampathkumar P., Osmon D.R., Cockerill F.R., III Prosthetic joint infection due to Staphylococcus lugdunensis. Mayo Clinic Proceedings (Vol. 75, No. 5, pp. 511-512); May, Elsevier; 2000. [DOI] [PubMed] [Google Scholar]
- 19.Heilmann C., Ziebuhr W., Becker K. Are coagulase-negative staphylococci virulent? Clin Microbiol Infect. 2019;25(9):1071–1080. doi: 10.1016/j.cmi.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Bennet J.E., Blaser M.J., Dolin R. Elsevier Saunders; 2015. Principles and practice of infectious diseases. [Google Scholar]