Abstract
Background
Implausible false positive results in non-invasive prenatal testing (NIPT) have been occasionally associated with the detection of occult maternal malignancies. Hence, there is a need for approaches allowing accurate prediction of whether the NIPT result is pointing to an underlying malignancy, as well as for organized programs ensuring efficient downstream clinical management of these cases.
Methods
Using a data set of 88,294 NIPT performed at University Hospital Leuven (Belgium) between November 2013 and March 2020, we retrospectively evaluated the positive predictive value (PPV) of our NIPT approach for cancer detection. In this approach, whole-genome cell-free DNA (cfDNA) data from NIPT were scrutinized for the presence of (sub)chromosomal copy number alterations (CNAs) predictive for a malignancy, using an unbiased NIPT analysis pipeline coined GIPSeq. For suspected cases, the presence of a maternal cancer was evaluated via subsequent multidisciplinary clinical follow-up examinations. The cancer-specificity of the identified CNAs in cfDNA was assessed through genetic analyses of a tumor biopsy.
Findings
Fifteen women without a cancer history were identified with a GIPSeq result suggestive of a malignant process. Their cfDNA profiles showed either genome-wide aberrations or a single trisomy 8. Upon clinical examinations, a solid or hematological cancer was identified in 4 and 7 cases, respectively. Three women were identified as having a clonal mosaicism. For one case no underlying condition was found. These numbers add to a PPV of 73%. Based on this experience, we presented a multidisciplinary care path for efficient clinical management of these cases.
Interpretation
The presented approach for analysing NIPT results has a high PPV, yet unknown sensitivity, for detecting asymptomatic malignancies upon routine NIPT. Given the complexity of diagnosing a pregnant woman with cancer, clinical follow-up should occur in a well-designed multidisciplinary setting, such as via the care model that we presented here.
Funding
This work was supported by Research Foundation Flanders and KU Leuven funding.
Keywords: Non-invasive prenatal testing, Cancer detection, Clinical follow-up
Research in context.
Evidence before this study
A PubMed search, using the terms “non-invasive prenatal testing” and “cancer or malignancy”, identified 11 retrospective studies, of which 6 case reports, published between Jan 1, 2013, and June 30, 2020, and reporting on incidental cancer detection following a non-reportable NIPT. Most reported large NIPT series presented scarce details on the identified genomic aberrations or clinical follow-up. Furthermore, no orchestrated guidelines were published on the clinical post-test evaluation of pregnant women suspected of having cancer based on their NIPT result.
Added value of this study
This retrospective study describes one of the largest NIPT series so far, with a unique presentation of detailed clinical work-up information and genome-wide data in cfDNA and tumor DNA of each identified cancer case. Our analytical approach to scrutinize NIPT data for the presence of cfDNA signals suggestive of an occult malignancy, provides an unparalleled PPV of 73%, which is much higher than values reported in previous large-scale NIPT series. Furthermore, based on our experience, we here present a novel comprehensive multidisciplinary care path enabling efficient clinical management in case NIPT suggests a malignancy.
Implications of all the available evidence
Our data show that comprehensive analysis of shallow-sequenced cfDNA allows incidental detection of maternal tumours with relatively high precision. In addition, this work stresses the need for a multidisciplinary approach for downstream management of women suspected of having a cancer based on their NIPT outcome and offers a novel care model for clinical implementation.
Alt-text: Unlabelled box
1. Introduction
Since its implementation in 2011, millions of non-invasive prenatal tests (NIPT) have been conducted across the globe, interrogating the risk of fetal trisomies 13, 18 and 21 [1]. NIPT relies on the analysis of fetal cell-free DNA (cfDNA) that mainly originates from placental trophoblast cells and freely circulates in maternal blood [2]. Because fetal cfDNA fractions exist in a high background of maternal cfDNA, NIPT can also detect maternal chromosomal abnormalities, like those resulting from maternal mosaicisms or malignancies [3]. Indeed, various reports have shown that NIPT detection of tumor-derived cfDNA (ctDNA) in pregnant women with an occult malignancy was causing the aberrant cfDNA signal thereby disturbing the interpretation of fetal trisomies [4], [5], [6], [7], [8], [9]. As the use of NIPT is increasingly expanding to low-risk pregnancies - in some countries it is offered as a first-tier test to all pregnant women - and its scope is being broadened beyond aneuploidy screening, more aberrant results, caused by a malignancy, are expected to emerge. Still, barriers and concerns exist about consenting women prior to NIPT and disclosing NIPT results being suggestive of a maternal cancer [10]. A survey of over 300 genetic counsellors in the United States demonstrated that only 29% of them communicated the possibility of finding a maternal neoplasm in a pre-test setting [11]. A major reason for that was the paucity of available information about the sensitivity, specificity and positive predictive value of NIPT analysis for additional findings, and the lack of clear guidelines on medical follow-up when an abnormal NIPT results suggests a maternal neoplasm. Although some suggestions have been done to guide post-test evaluation [12], no orchestrated guidelines exist for the clinical evaluation of pregnant women suspected of having cancer based on their NIPT result. For example, Belgian guidelines specify which maternal incidental findings (including maternal malignancies) should be reported, yet no direction is given on the clinical management of these findings [13]. This hampers appropriate concerted downstream actions. First, as an aberrant cfDNA profile, being suggestive of a malignancy, might cause parental concern, an effective work-up should be in place to provide the pregnant woman and her family with an accelerated diagnostic flow. Secondly, accumulating evidence indicates that certain cancer therapies can be given relatively safely during pregnancy without major adverse effects on neonatal outcome [14]. As such, a timely cancer diagnosis would allow an early start of the treatment, which may add to a prognosis of the pregnant woman similar to that of non-pregnant women [15], [16], [17].
No prospective large-scale evaluation of incidental cancer detection following routine NIPT has been published, and existing retrospective reports often present scarce details on the identified genomic aberrations and clinical follow-up [4,5]. We here propose a retrospective analysis of a large series of over 85.000 NIPT tests performed in University Hospitals Leuven, between November 2013 and March 2020, in which women with a NIPT that was suggestive for an underlying malignancy were offered multidisciplinary follow-up investigations. NIPT was done using our Genome-wide Imbalance Profile sequencing pipeline (GIPSeq) that enables unbiased genome-wide detection of copy number alterations (CNA) [18]. We show that our analytical approach allows accurate cancer prediction. We also present a clinical work-up plan set up in our hospital for efficient management of pregnant women confronted with a NIPT that suggests an occult maternal malignancy.
2. Methods
2.1. Study design
This study presents the retrospective analysis of all NIPT data collected at the center for Human Genetics, University Hospitals Leuven, Belgium between November 2013 and March 2020, to evaluate the positive predictive value of our unbiased NIPT analysis pipeline in predicting the presence of an occult maternal malignancy and to map the genomic aberrations identified in cfDNA of women with a confirmed cancer diagnosis.
2.2. Participants, blood sampling and ethical consent
NIPT data were collected from a total of 88,294 pregnant women. Genetic counselling was provided prior to blood sampling for NIPT. Pregnant women were informed about potential incidental findings. Contraindications for NIPT were ultrasound anomalies (including a nuchal translucency thickness exceeding 3·5 mm) or a history of organ, tissue or stem cell transplant. Peripheral blood samples were collected from the 10th week of gestation onwards in Cell-Free DNA BCT tubes (Streck) or Cell-Free DNA collection tubes (Roche Diagnostics). In accordance with the Declaration of Helsinki, written informed consent was obtained from all these cases to release information for study purposes beyond trisomy 13, 18 and 21. There was no option to opt-out. This consent was approved by University Hospitals Leuven ethics committee, permitting the use of these data for research purposes. The subset of pregnant women with a NIPT suggestive of an occult maternal malignancy were offered clinical follow-up investigations. All women with a confirmed cancer diagnosis were requested to participate in a parallel research study to evaluate the cancer-specificity of the CNAs detected in cfDNA by GIPSeq. This was done via CNA analysis of a tumor biopsy taken before initiation of the cancer treatment. All eligible women consented to participate to this study (S57197, approved by Ethics Committee Research UZ / KU Leuven). Except for the genetic analyses of tumor biopsy DNA, all results were part of the routine clinical work-up and paid-for-service.
2.3. Cell-free plasma and genomic DNA extraction
Plasma was isolated through a standard centrifugation procedure. cfDNA was extracted from 2 to 4 ml plasma, using the QIAamp circulating nucleic acid Kit (Qiagen; manual extraction) or the Maxwell HT ccfDNA kit (Promega; automated procedure). Genomic DNA from blood cells or formalin-fixed paraffin-embedded (FFPE) tumor biopsies was extracted after macrodissection using the Qiagen Blood and Tissue kit. For frozen tumor specimens DNA was extracted using High Pure PCR Template Preparation Kit (Roche). Following sonication (Covaris M220), DNA samples were electrophoretically run on the Agilent 2100 Bioanalyzer system to verify fragmentation. The target DNA fragment size for library preparation was 150–200 bp.
2.4. Genome-wide imbalance profile sequencing (GIPSeq) and NIPT analysis
DNA sequencing libraries of cfDNA or genomic tumor DNA were prepared using the Illumina TruSeq DNA Nano or ChipSeq kit (Illumina) or the KAPA HyperPrep kit (Roche Diagnostics). Whole-genome sequencing was performed on a HiSeq2500, HiSeq4000 or Novaseq sequencer (Illumina) generating 36 or 50 bp reads. For NIPT analysis, our previously described GIPSeq bioinformatics pipeline was applied, using genome-wide parameters (quality score, QS), chromosomal parameters (i.e. z-score, measuring the differential cfDNA chromosomal representation, in standard deviation units, compared to cfDNA from women carrying a euploid fetus; and a zz-score, reflecting the differential representation of a particular chromosome within the sample set of chromosomes) and subchromosomal parameters [18]. In particular, QS was calculated as the standard deviation of all autosomal z-scores following removal of the highest and lowest scoring chromosomes. Routine diagnostic NIPT analysis of these parameters was performed using previously described decision rules [18]. A NIPT result was called ‘interpretable’ when all quality standards were met in combination with an interpretable chromosome call for chromosomes 21, 18 and 13. A NIPT result was classified as ‘non-interpretable’ when no reliable risk estimation of fetal trisomy 21, 18 or 13 was possible, due to low fetal fraction or deviating QS-, z- or zz-scores in the GIPSeq profile [18].
2.5. Classification of GIPSeq results as suggestive of an occult malignancy
For classification of a GIPSeq result as suggestive of an underlying maternal malignancy, we applied the same QS, z- and zz-parameters as being used for routine NIPT analysis, in combination with a visual inspection of the GIPSeq profile. In particular, two types of GIPSeq profiles were flagged as being reminiscent of cancer-related CNAs (Fig. 1). First, since chromosomal instability, including whole chromosome (arm) gains and losses, is a hallmark of tumorigenesis [19], a non-interpretable NIPT result was classified as being suggestive of an underlying maternal malignancy when QS≥2·0 and genome-wide (sub)chromosomal gains and/or losses were present. For cases for whom a technical problem could not be ruled out as a potential cause of the aberrant GIPSeq profile, a second NIPT on an independent blood sample was performed. When GIPseq profiling of the second blood sample reproduced the CNAs found in the first sample, classification of the GIPSeq profile as suggestive of an occult malignancy was reinforced. Second, given that trisomy 8 as a sole change is one of the most frequent numerical aberrations in myeloid malignancies [20], an interpretable NIPT (i.e. a GIPSeq profile with an interpretable call for chromosomes 13, 18 and 21) in combination with a single gain of chromosome 8 (i.e. z- and zz-score≥3·0) was also flagged for follow-up. Where possible, amniocentesis and Fluorescent In Situ Hybridization (FISH) on maternal peripheral blood was done to assess whether the observed trisomy 8 was either from fetal or from maternal origin, respectively. Upon confirmation of a maternal origin of the observed trisomy 8, these GIPSeq profiles were also classified as being suggestive of a maternal cancer. In particular, for these cases a maternal hematological malignancy was suspected. When neither a foetal nor maternal origin of the observed trisomy 8 was found, postpartum analysis of placental tissue was done to examine whether the aberrant GIPSeq result reflected a confined placental mosaicism of trisomy 8.
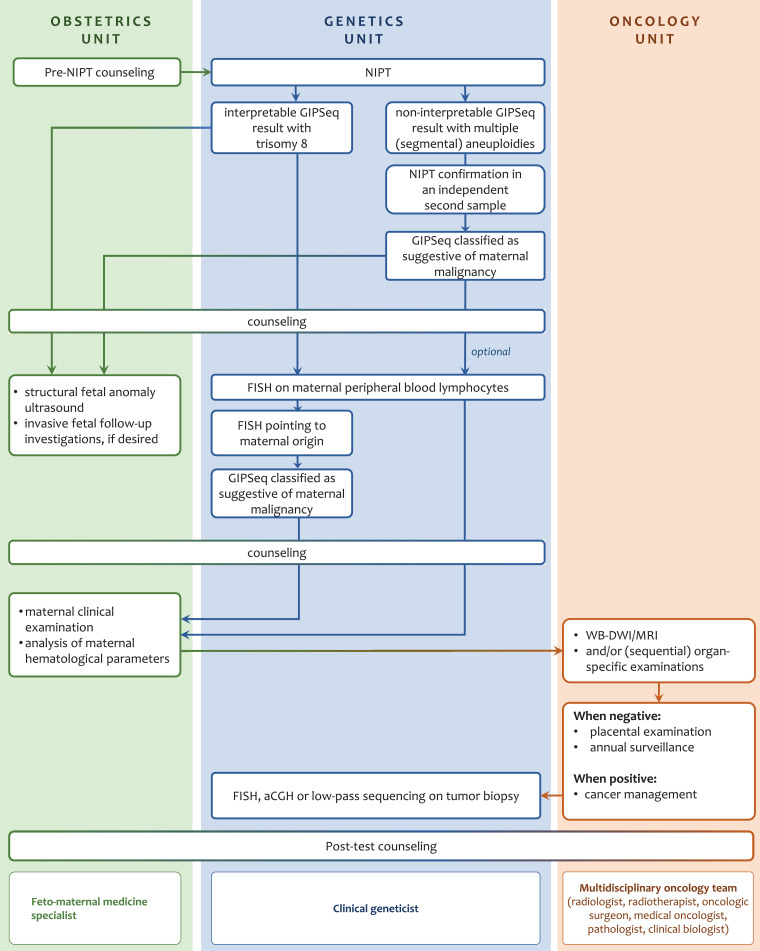
Fig. 1.
Diagram representing the clinical specialties and cross-talk between the different units necessary to ensure efficient management of aberrant NIPT outcomes that are suggestive for an occult maternal malignancy. *interpretable NIPT result refers to a GIPSeq profile where quality standards are met in combination with an interpretable chromosome 21, 18 and 13 call, which is being communicated to the patient. **A non-interpretable NIPT result refers to a GIPSeq profile that does not allow a reliable estimation of the risk of fetal trisomy 13, 18, and 21 due to low fetal fraction or deviating quality parameters (QS-, z- or zz-scores). aCGH, array comparative genomic hybridization; FISH, fluorescent in situ hybridization; WB-DWI/MRI Whole-body Diffusion Weighted MRI.
2.6. Fluorescent in situ hybridization (FISH) and array comparative genomic hybridization (aCGH)
For cases for whom an isolated gain of chromosome 8 was observed in the GIPSeq profile, FISH was applied on maternal peripheral blood to investigate whether this anomaly was present in a fraction of the maternal white blood cells. For cases with a confirmed cancer diagnosis, the tumor origin of CNAs observed in the GIPSeq profile was assessed by subjecting an FFPE or frozen tumor biopsy specimen to FISH or aCGH, respectively, depending on the availability of a biopsy tissue. FISH was performed according to standard procedures using the FISH probes described in Supplementary Table 1. aCGH was performed using the 8 × 60 K CytoSure ISCA v3 microarray and data were analysed with the CytoSure Interpret Software (OGT). In case of amniocentesis, the same aCGH procedure was applied.
2.7. Clinical follow-up when NIPT suggests an occult maternal malignancy
When a GIPSeq profile was found to be reminiscent of cancer-related CNAs, i.e. when either genome-wide (segmental) aneuploidies were identified or a single gain of chromosome 8 that was found to be of maternal origin, clinical follow-up was offered. The patient was counselled to discuss the possible aetiologies of these findings (Fig. 1). Though cfDNA profiles with multiple abnormalities are considered incompatible with normal fetal development, the presence of large ctDNA fractions in maternal blood may mask the fetal chromosomal profile and prevent a reliable estimation of the risk of fetal trisomy 21, 18 and 13 [9]. Therefore, detailed structural anomaly screening was done via ultrasound. If certainty on fetal chromosomal abnormalities was desired, the possibility of an invasive prenatal test (amniocentesis) was discussed in conferring with the gynecologist, thereby weighing the risks of the invasive test, the anxiety of the pregnant woman and the risk of an abnormal fetal karyotype. In particular, for cases with genome-wide CNAs in cfDNA, suggesting a maternal condition rather than a fetal anomaly, counselling was modulated in the course of our clinical practice thereby informing the patient that the risk of performing an invasive test to interrogate the fetal karyotype might not weigh up to the chances of excluding that these genome-wide imbalances are from fetal origin.
In parallel, the patient was subjected to physical examinations.
Furthermore, as plasma tumor markers show moderate diagnostic sensitivity and are less reliable in pregnancy [21], cases were referred for 3 Tesla Whole-body Diffusion Weighted MRI (WB-DWI/MRI) imaging to screen them for the presence of primary tumours and distant metastases. WB-DWI/MRI was chosen for its safety profile for imaging during pregnancy (due to its absent ionizing radiation), its ability to comprehensively assess the entire body and because of its similar diagnostic performance compared with PET/CT for detecting primary and metastatic malignancies [22]. As WB-DWI/MRI was designed for diagnostic rather than for screening purposes and due to ethical considerations, we applied a low threshold for detecting incidental findings, favouring high sensitivity for lesion detection over specificity. Detected lesions were further examined via dedicated investigations in a second stage.
Additionally, analysis of hematological parameters was done (i.e. peripheral blood cell counts, morphology, clinical biochemistry and protein electrophoresis of peripheral blood) to search for hematological malignancies that might not be detectable via WB-DWI/MRI.
Upon cancer identification, the patient was referred to an oncologist to discuss appropriate therapeutic management [23]. Also, a feto-maternal medicine specialist was monitoring this high-risk pregnancy [23]. For cases with a confirmed cancer diagnosis, the tumor origin of CNAs observed in the GIPSeq profile was assessed by applying FISH or aCGH on a tumor biopsy specimen.
In case no cancer diagnosis was made, the patient was counselled that her risk, particularly for a hematologic malignancy, persists.
2.8. Statistical analysis
The positive predictive value (PPV) of GIPSeq for cancer detection was calculated by determining the ratio of the number of GIPSeq tests that were suggestive of a malignancy in asymptomatic pregnant women and that eventually resulted in a cancer diagnosis over the total number of GIPSeq tests that were suggestive of a malignancy in asymptomatic pregnant women. Women with a known cancer diagnosis before GIPSeq profiling were excluded from these analyses.
2.9. Role of the funding source
The funders of this study had no role in study design, data collection, data analysis, data interpretation or writing of the report. All authors had full access to all of the data. JRV and FA had the final responsibility to submit for publication.
3. Results
3.1. GIPSeq revealed single chromosomal and complex cfDNA aberrations suggestive of cancer
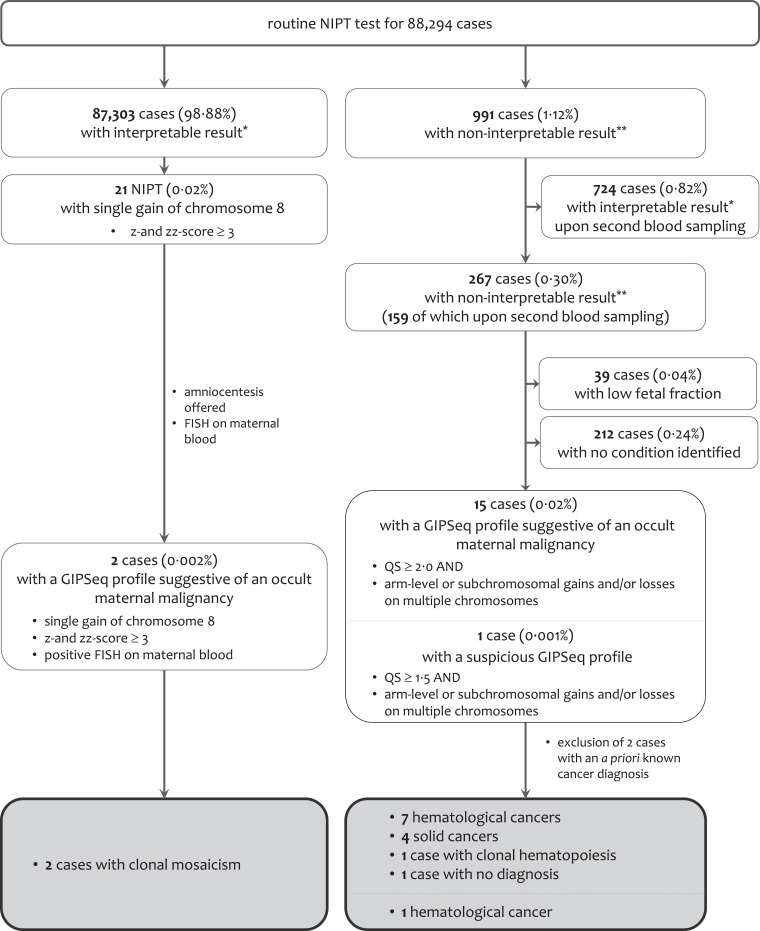
Since the introduction of NIPT in our center in 2013, plasma cfDNA of 88,294 pregnant women was screened using our GIPSeq pipeline. For 991 (1·12%) cases, the result was not interpretable either because of a low fetal fraction or because the overall QS was poor. Where possible, NIPT was done on a second independent blood sample (Fig. 2). This resulted in an interpretable outcome on the fetal karyotype for 724 out of 88.294 cases (0·82%). For 267 pregnant women (0·30%), the risk of fetal trisomy 13, 18 or 21 could not reliably be estimated. For a small subset (0·04%), the non-interpretable GIPSeq result was attributed to a low fetal fraction, whereas for the majority of these cases (0·24%), no underlying disease or condition was identified that could explain the discordant GIPSeq profile. For 15 of the 267 cases, the non-interpretable GIPSeq profile was characterized by an elevated QS (≥2·0) and (segmental) gains and/or losses across multiple chromosomes (Fig. 3A), which were shown to be reproducible for cases for whom two independent blood samples were analysed (Supplemental Figure 1 and Table 1). These GIPSeq profiles were classified as being suggestive of an underlying malignancy. For two of these 15 cases, the presence of the genome-wide (segmental) aneuploidies in the GIPSeq profile could be linked to an underlying malignancy that was already being diagnosed before NIPT screening (Supplemental Figure 2). These women were excluded from further analyses. Within the set of 87,303 cases (98·88%) with an interpretable result on the fetal karyotype, two GIPSeq profiles (0·002%) displayed a single gain of chromosome 8 (z- and zz-score ≥3·0) that was shown to be of maternal origin (Fig. 3A and Supplementary Figure 1A-B). Hence, an occult malignancy was also suspected in these women. Finally, one case was identified with a GIPSeq profile showing (segmental) gains on multiple chromosomes, yet with a QS below 2·0 (case ID-2, Fig. 3A and Table 1). Because of the predominant gain of chromosome 8, being reflected in elevated z- and zz-scores (>3·0), a second NIPT was performed. This confirmed the initial GIPSeq result, hence underscoring a biological rather than a technical cause of the observed cfDNA imbalances. Given the involvement of chromosome 8, this GIPseq profile was found suspicious, yet not classified as suggestive of an occult malignancy. All women with a GIPSeq profile suggesting a malignancy, as well as case ID-2, received genetic counselling. In conferring with the obstetrician, the added value or necessity of performing an invasive prenatal test was discussed. Amniocentesis was finally performed in eight cases, all revealing a normal fetal karyotype (Table 1).
Fig. 2.
Results from routine NIPT testing performed in University Hospitals Leuven between November 2013 and March 2020.
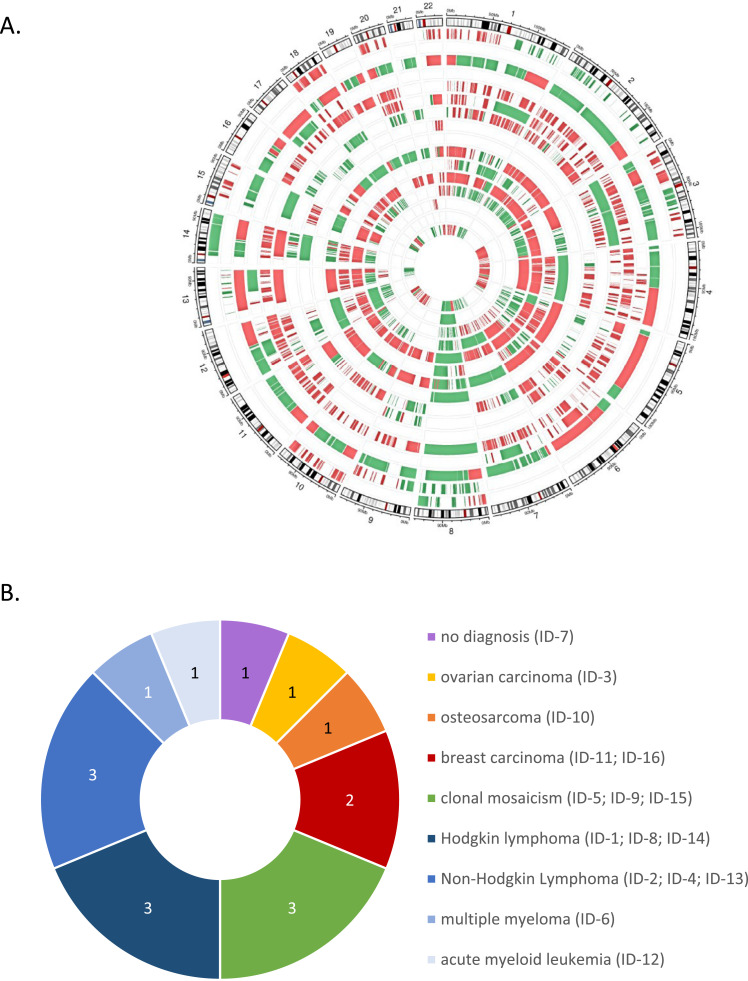
Fig. 3.
A. Chromosomal aberrations observed in plasma cfDNA of asymptomatic pregnant cases with GIPSeq profiles suggestive of cancer. Cases are those listed in Table 1. Where possible, plotting was based on the GIPseq results of the first plasma sample of each case, showing chromosomal anomalies with a z-score≥3.0 (suggesting gain; in green) or ≤3.0 (suggesting loss; in red). Chromosomal regions with clear reproducible gains and losses, resulting in a neutral z-score are displayed as well. For n = 13 cases with no a priori known cancer diagnosis, genome-wide chromosomal aberrations were observed in cfDNA, resulting in QS≥2.0. For some of these cases, high z-scores for almost every chromosome were observed. This indicates that either all chromosomes are indeed affected, or the z-scores of particular individual chromosomes or chromosomal fragments might be skewed due to excessive presentation of other, highly amplified chromosomes or chromosome arms. Two cases presented with a single chromosomal gain of chromosome 8 (z- and zz-scores ≥3•0). Finally, for one case (case ID-2) the GIPSeq profile showed (segmental) gains on multiple chromosomes, but this profile was not classified as suggestive of an occult malignancy because of a QS<2.0. For every case, the genomic representation profile of the autosomal chromosomes is shown in clockwise order, aligned with chromosomal ideograms (outer circle). Cases are shown from the periphery to the center in ascending order from ID-1 to ID-16. B. Pie chart displaying the numbers and types of cancers and premalignant conditions identified in pregnant women undergoing routine NIPT testing in our University Hospital and with a GIPSeq profile suggestive of cancer. Eight hematological malignancies were identified, namely 3 classical Hodgkin lymphomas (type nodular sclerosis Hodgkin lymphoma; stages II, II and IV), 3 non-Hodgkin lymphomas (type primary mediastinal B-cell lymphoma, stage I; follicular lymphoma, stage III; diffuse large B-cell lymphoma, stage II), 1 acute myeloid leukemia (stage M5), 1 multiple myeloma (type light chain lambda). Four pregnant women were diagnosed with a solid cancer type, namely 2 breast cancers (invasive breast carcinoma of no special type, hormone receptor positive, stage II and stage IV), 1 osteosarcoma (conventional high-grade osteosarcoma, stage III) and 1 ovarian cancer (high grade serous ovarian carcinoma, stage IV). Three cases were diagnosed with a clonal mosaicism. Finally, for one case, no disease was identified.
Table 1.
NIPT details and clinical follow-up in cases with cancer-like GIPSEQ profiles.
| Cases with genome-wide CFDNA aberrations | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Maternal age (years) | GA (weeks) | QS NIPT-1 | QS NIPT-2 | Time NIPT-1 to NIPT-2 (days) | Invasive fetal follow-up | Newborn follow-up | Maternal clinical presentation | Hematological analyses | WB-DWI/MRI | Cancer diagnosis | Time NIPT-1 to cancer diagnosis (days) | Confirmatory analyses in tumor or blood DNA (method; reference) | ||||
| ID-1 | 27 | 11 | 2.49 | 3.09 | 15 | normal amniocentesis | no congenital disease | normal | no abnormalities | mass in anterior mediastinum, multiple lymphadenopathies in the left neck | classical Hodgkin lymphoma, nodular sclerosis Hodgkin lymphoma (NSHL); Ann Arbor stage II | 57 | cfDNA aberrations confirmed in tumor DNA (FISH; reported in [6]) | ||||
| ID-3 | 41 | 15 | 10.45 | 2.39 | 11 | no amniocentesis performed | miscarriage | dyspnea and nausea at hospitalization for spontaneous miscarriage | no abnormalities | bilateral ovarian carcinoma, diffuse peritoneal spread, retroperitoneal lymphadenopathies | ovarian carcinoma, high grade serous carcinoma; stage IV | 10 | cfDNA aberrations confirmed in tumor DNA (FISH; reported in [6]) | ||||
| ID-4 | 34 | 14 | 3.11 | 3.72 | 14 | NA | no congenital disease | exhaustion, palpable cervical lymphadenopathies | no abnormalities | multiple supradiaphragmatic and infradiaphragmatic lymphadenopathies | non-Hodgkin lymphoma, follicular; Ann Arbor stage III | 54 | cfDNA aberrations confirmed in tumor DNA (FISH; aCGH; reported in [6]) | ||||
| ID-5 | 27 | 11 | 2.82 | 2.96 | 20 | normal amniocentesis | no congenital disease | normal | karyotyping on bone marrow aspirate confirmed trisomy 8 | none | clonal hematopoiesis; in follow-up | no cancer diagnosis made so far | cfDNA aberrations confirmed in bone marrow DNA (FISH; Suppl. Fig. 1F) | ||||
| ID-6 | 41 | 13 | 8.27 | NA | NA | normal amniocentesis [39] | no congenital disease [39] | normal [39] | elevated Free Light Chain Lambda/Kappa ratio [39] |
normal [39] | multiple myeloma, secreting lambda light chains | 21 | cfDNA aberrations confirmed in tumor DNA (FISH; aCGH; reported in [39]) | ||||
| ID-7 | 30 | 13 | 1.98 | 2.73 | 14 | normal amniocentesis | no congenital disease | normal | no abnormalities | slightly enlarged tonsil (aspecific) | none | no cancer diagnosis made so far | NA | ||||
| ID-8 | 29 | 11 | 3.01 | 2.98 | 12 | no amniocentesis performed | no congenital disease | exhaustion, weight loss since pregnancy | no abnormalities | multiple supradiaphragmatic adenopathies, pathological pelvic bone lesion | classical Hodgkin lymphoma; Ann Arbor stage IV | 33 | cfDNA aberrations confirmed in tumor DNA (FISH; Suppl. Fig. 1D) | ||||
| ID-10 | 29 | 12 | 44.75 | 56.49 | 16 | no amniocentesis performed | termination of pregnancy | back pain | no abnormalities | tumoral mass in left iliac wing, locally advanced | high-grade osteosarcoma; stage III | 30 | cfDNA aberrations confirmed in tumor DNA (aCGH; Suppl. Fig. 1E) | ||||
| ID-11 | 33 | 12 | 3.59 | 4.43 | 13 | no amniocentesis performed | no congenital disease | axillary lymph adenopathy palpable | no abnormalities | breast mass and axillar lymphadenopathies | invasive breast carcinoma of no special type, hormone receptor-positive HER2-negative; stage II | 25 | cfDNA aberrations confirmed in tumor DNA (low-pass sequencing; reported in [35]) | ||||
| ID-12 | 32 | 11 | 11.46 | 17.67 | 18 | normal amniocentesis | termination of pregnancy | normal | bone marrow punction and blood tests suggestive for acute myeloid leukemia | diffuse infiltration of liver, spleen and bone marrow, suggestive for hematological malignancy | acute myeloid leukemia; FAB M5 | 17 | cfDNA aberrations confirmed in tumor DNA (FISH; reported in [40]) | ||||
| ID-13 | 40 | 12 | 23.2 | 10.38 | 12 | NA | no congenital disease | normal | no abnormalities | mass in the spleen, multiple adenopathies retroperitoneal, gastro-hepatic, gastrosplenic, pericardial and pancreatic | non-Hodgkin lymphoma, diffuse large B-cell; Ann-Arbor stage II | 19 | cfDNA aberrations confirmed in tumor DNA (FISH; Fig. 4) | ||||
| ID-14 | 22 | 12 | 2.83 | 10.43 | 35 | no amniocentesis performed | no congenital disease | normal | no abnormalities | mass in anterior mediastinum; adenopathies near thoracic outlet and incisura jugularis | classical Hodgkin lymphoma, nodular sclerosis Hodgkin lymphoma (NSHL); Ann-Arbor stage IIA | 57 | cfDNA aberrations confirmed in tumor DNA (FISH; Suppl. Fig. 1C) | ||||
| ID-16 | 39 | 12 | 5.57 | NA | NA | no amniocentesis performed | termination of pregnancy | normal | no abnormalities | mastitis carcinomatosa, multinodular infiltrative tumoral mass | invasive breast carcinoma of no special type cancer, hormone receptor-positive HER2-negative; stage IV | 6 | cfDNA aberrations confirmed in tumor DNA (low-pass sequencing; Fig. 4) | ||||
| Cases with a single trisomy 8 in CFDNA that is of maternal origin | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CASE | Maternal age (years) | GA (weeks) | QS NIPT-1 | QS NIPT-2 | Time NIPT-1 to NIPT-2 (days) | Invasive fetal follow-up | Newborn follow-up | Maternal clinical presentation | Hematological analyses | FISH on maternal peripheral blood | WB-DWI/MRI | Cancer diagnosis | Time NIPT-1 to cancer diagnosis (days) | CfDNA aberrations confirmed in tumor (method; figure) | |||
| ID-9 | 36 | 12 | 0.77 | NA | NA | normal amniocentesis | no congenital disease | normal | normal peripheral blood parameters; bone marrow punction: normal cytology and karyotyping | nuc ish(D8Z2 × 3) [17/200],(RUNX1T1 × 3) [17/200] | none | clonal mosaicism; in follow-up | no cancer diagnosis made so far | cfDNA aberrations confirmed in peripheral blood cell DNA (FISH; Suppl. Fig. 1A) | |||
| ID-15 | 31 | 13 | 1.93 | NA | NA | normal amniocentesis | Transposition of the great arteries | normal | none | nuc ish(D8Z2 × 3) [77/200],(RUNX1T1 × 3) [77/200] | none | clonal mosaicism | no cancer diagnosis made so far | cfDNA aberrations confirmed in peripheral blood cell DNA (FISH; Suppl. Fig. 1B) | |||
| CASES with a GIPSEQ profile showing genome-wide CFDNA aberrations but not classified as suggestive of cancer | |||||||||||||||||
| CASE | Maternal age (years) | GA (weeks) | QS NIPT-1 | QS NIPT-2 | Time NIPT-1 to NIPT-2 (days) | Invasive fetal follow-up | Newborn follow-up | Maternal clinical presentation | Hematological analyses | WB-DWI/MRI | Cancer diagnosis | Time NIPT-1 to cancer diagnosis (days) | CfDNA aberrations confirmed in tumor (method; figure) | ||||
| ID-2 | 36 | 13 | 1.65 | 1.52 | 45 | normal amniocentesis | no congenital disease | normal (at time of NIPT testing); cough and thoracal pain (at time of cancer diagnosis) | no abnormalities | none | non-Hodgkin lymphoma, primary mediastinal B-cell; Ann Arbor stage I | 1393 | cfDNA aberrations confirmed in tumor DNA (FISH; Suppl. Fig. 1G) | ||||
GA, Gestational age; NA, Not available; NIPT-1, -2, NIPT analysis of sample 1 and 2, respectively; QS, Quality scores; WB-DWI/MRI, Whole body diffusion-weighted MRI.
3.2. GIPSeq profiling accurately predicted a (pre)malignant condition
Cases with a GIPSeq result suspicious of cancer were offered further clinical examinations and general hematological analyses (Table 1). One case declined these investigations (case ID-15). In addition, all women with genome-wide cfDNA aberrations that could not be linked to a specific malignancy, were invited to undergo WB-DWI/MRI screening for the presence of malignant lesions. These examinations and downstream organ-specific investigations triggered by the WB-DWI/MRI result, led to the identification of a malignancy in 11 women (Fig. 2 and Fig. 3B). Most diagnoses (66·7%) were of hematological origin, the majority (75·0%) being lymphomas. One-third (33·3%) of cancers were solid tumours. Median time span between initial NIPT testing and cancer diagnosis was 32 days (range 6–57 days). For all confirmed cancer diagnoses, genetic analyses, using aCGH, FISH or low-pass whole genome sequencing (0·1x) of biopsy DNA, confirmed that the CNAs detected in cfDNA originated, at least partially, from tumor DNA (Fig. 4, Supplementary Figure 1C-E and Table 1). For two cases with genome-wide cfDNA aberrations (cases ID-5 and ID-7), no underlying malignancy was identified during clinical follow-up. For case ID-5, the gain of chromosome 8 was the most prominent aberration in cfDNA and was also detectable upon subsequent analysis of a bone marrow biopsy (Supplementary Figure 1F). No morphological signs of a hematological malignancy were observed. Given the patient's history of thrombocytopenia and the identified mosaicism of trisomy 8 in cfDNA and bone marrow cells, the patient was esteemed to have an increased risk of developing a myeloid neoplasm and was advised to have regular follow-up [20]. For case ID-7, the genome-wide cfDNA anomalies had a borderline QS score (QS=1.98, Table 1). The aberrations became more pronounced in a second, independent blood sample, being reflected by an increased QS score (>2.0). Array CGH on maternal leukocyte DNA pointed to a normal karyotype. WB-DWI/MRI showed a lesion in the palatine tonsils (Table 1). Nevertheless, focused clinical examinations did not uncover a condition that could explain the aberrant GIPSeq profile. During the index’ subsequent pregnancy, NIPT testing revealed the very same GIPSeq profile (data not shown). No further examinations were done. Two pregnant women (cases ID-9 and ID-15) were identified with an isolated gain of chromosome 8 in cfDNA, that originated from a low-grade mosaicism in maternal leukocytes (Supplementary Figure 1A-B). Given the link with myeloid malignancies, no WB-DWI/MRI was performed [24]. For case ID-9, bone marrow analysis pointed to a normal morphology and karyotyping (Table 1). Yearly clinical follow-up was advised. Case ID-15 declined further onco-diagnostic investigations due to language barriers. Finally, because of the borderline QS scores (<2·0) in the GIPSeq profile of case ID-2, this woman was not included in our clinical follow-up workflow. Three years following the detection of the aberrant GIPSeq result, this woman was diagnosed with a non-Hodgkin lymphoma (Table 1). Based on the congruency between the detected CNAs in cfDNA and a tumor biopsy (Supplementary Figure 1G), our initial hypothesis about a potential link between the observed imbalances in the GIPSeq profile and an occult maternal malignancy was confirmed. Given the identification of 11 incipient tumours in 15 cases having a GIPSeq profile that was classified as suggestive of a malignancy, our approach resulted in a PPV of 73% for cancer detection.
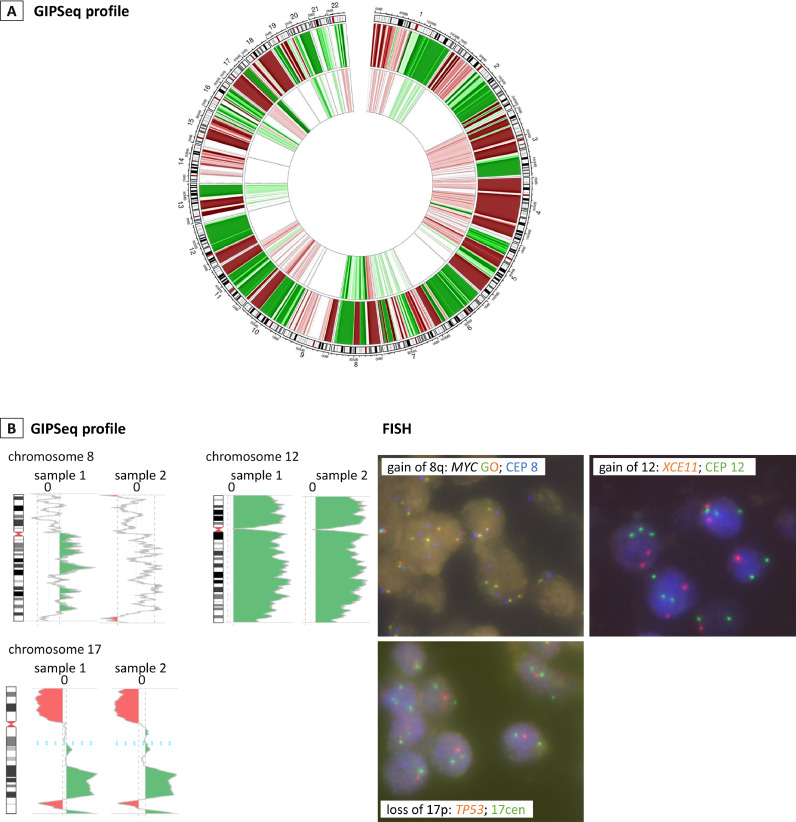
Fig. 4.
Molecular analyses in tumor biopsies of two pregnant women for whom a cancer diagnosis was made upon aberrant routine NIPT testing. A, Circos plots of matched cfDNA:tumor DNA samples of pregnant case ID-16 who was diagnosed with a stage IV breast cancer. Plotting was done similarly as for Fig. 2. The outer circle shows the copy number profile of genomic tumor DNA extracted from tumor biopsy (whole-genome low-pass sequencing, 0.1x coverage). The inner circle depicts the matched genome-wide GIPSeq profile in plasma cfDNA (NIPT sample), showing high congruency with aberrancies observed in most of the chromosomes in tumor DNA. Inconsistencies, noticed on some chromosomes, might be explained by the metastatic status of the tumor with potential presence of additional circulating subclones. B, Pregnant woman ID-13 was diagnosed with a diffuse large B-cell non-Hodgkin lymphoma upon aberrant GIPSeq profiling. FISH, performed on a lymph node biopsy, confirmed the tumor origin of specific copy number gains and losses observed in cfDNA, namely tri-/tetrasomy of the region 8q24/MYC and of the centromeric region of chromosome 8 in 20% of nuclei {LSI MYC (spectrum orange/green) [8q24,Vysis]/ CEP 8 (spectrum aqua) [8p11.1-q11.1,Vysis]}, trisomy 12 in 60% of nuclei {XCE11 (spectrum orange) [Metasystems] + LSI CEP 12 (spectrum green) [12p11.1-q11, Vysis]} and monoallelic loss of the region 17p13/TP53 with disomy of the centromeric region of chromosome 17 in 80% of nuclei {XL TP53 (spectrum orange) /17cen (spectrum green) [17p13/17cen, Metasystems]}.
4. Discussion
Incidental detection of an occult maternal malignancy during pregnancy discloses a medical-ethical dilemma between ensuring the best treatment options for the patient and safeguarding fetal health. It has now been shown that oncological treatment in pregnancy can be possible without affecting short-term neonate outcomes [25,26]. Detecting a malignancy during pregnancy also allows the identification of possible obstetric and neonatal risks. This should not be underestimated, as cancer in pregnancy is related to maternal and fetal morbidity [23]. Yet, the added value of disclosing incidental NIPT findings suggesting a maternal malignancy only holds true when high specificities are achieved and unnecessary (invasive) diagnostic procedures and concurrent parental anxiety can be avoided. Hence, there is a need for methods that allow accurate prediction of the etiology of aberrant cfDNA signals as well as for organized programs ensuring efficient downstream clinical management when NIPT suggest a malignancy. The analytical approach we presented, provides a PPV of 73% and relies on a combinatory bioinformatics analysis of genome-wide and chromosomal cfDNA parameters together with visually scrutinizing the cfDNA profile. This PPV is much higher than values reported in previous large NIPT series [5,8]. Ji et al. also reported a PPV of 75%, yet this was only achieved by combining the analysis of NIPT cfDNA data and serum protein levels [4]. Similarly as previously reported, hematological malignancies were most frequently identified, followed by detection of breast cancers [5,7,8]. This is in line with cancer types being most prevalent during pregnancy [14]. The preponderance of hematological diagnoses via NIPT might be plausible when assuming that cfDNA is largely derived from hematopoietic cells. Some of the pregnant cases in our cohort that were diagnosed with cancer following NIPT screening were not completely asymptomatic and presented with adenopathy, nausea or exhaustion. Yet, it should be noted that cancer symptoms are often misinterpreted as physiologic gestational symptoms, conferring pregnant women a higher risk of not being diagnosed at early cancer stages [14,27]. For all identified cancer cases, the cfDNA signal was shown to truly originate from the tumor. Similarly as reported previously, cfDNA aberrations associated with a maternal cancer were most often of complex nature [8,28]. Though benign uterine leiomyomas have also been described as a potential source of genome-wide chromosomal CNAs in NIPT results [5], no such diagnosis was made in our cohort. For one case in our cohort with genome-wide cfDNA aberrations, the anomalies were shown to originate from aberrant bone marrow clones, thereby pointing to a premalignant condition [29]. At the same time, our approach led to the identification of a clonal mosaicism in women with an isolated trisomy 8 in cfDNA. Similarly, in a Dutch study on NIPT implementation, the investigators found single 5q and 20q deletions - both being recurrently associated with myeloid neoplasms - to originate from the maternal hematopoietic system but without clinical signs of a malignancy [8]. We made similar observations when plasma cfDNA of asymptomatic elderly people was screened for the presence of cancer-like signals using GIPSeq profiling [30]. Yet, the prevalence of such clonal haematopoiesis-related chromosomal alterations in women at childbearing age is more rare [31]. First, our findings underscore that the potential presence of an occult maternal malignancy should come to mind when NIPT yields an unusual result despite subsequent normal invasive fetal karyotyping. Secondly, given that most malignancies identified via NIPT are of hematologic nature (own data and [4,5,8,32]), and that chromosomal abnormalities might be present years before a hematological malignancy becomes evident [31], increased surveillance should be offered to pregnant women for whom no clinical evidence of a malignancy is found. This is highlighted by one case in our cohort for which the CNAs detectable in cfDNA had borderline scoring parameters, but were found to be a prelude to a lymphoma diagnosis 3 years after NIPT. We recommend a multidisciplinary approach for optimal management of NIPT results suggestive of an occult malignancy. Based on our vast experience, we presented a unique comprehensive model to serve this goal. Combining WB-DWI/MRI screening with hematological laboratory analyses, an underlying (pre)malignant cause was identified in all but one pregnant case with a NIPT profile suggestive of an occult maternal malignancy. Alternatively to the WB-DWI/MRI method to screen the patient for the presence of cancer-like lesions, sequential organ-specific examinations may be applied when NIPT points to a maternal malignancy. Would the oncological investigations be negative, a postpartum follow-up GIPSeq analysis could be performed to evaluate the evolution of the cfDNA profile. For the woman in our cohort that had consistent aberrant GIPSeq profiles during two subsequent pregnancies, such follow-up investigations of the cfDNA profile could be informative about any further progression of the observed aberrancies. Furthermore, it should be noted that an isolated anomaly in cfDNA, such as trisomy 8, from which the origin cannot be traced back to a mosaicism in maternal cells, may also originate from a demised co-twin or confined placental mosaicism [33,34]. Evaluation of a placental biopsy allows examining the latter possibility. When comparing our detection rate with the reported incidence rates of cancer in pregnancy (being 1 cancer diagnosis in 1000 to 2000 pregnancies), it can be assumed that a substantial number of cancers cases are missed. First, our approach relies on stringent scoring parameters, potentially resulting in a number of false negative cases. This is underscored by the case with a borderline GIPSeq profile that was not classified as being suggestive of a malignancy but that could be finally linked to a cancer diagnosis, and by our data evaluating GIPSeq profiling in pregnant cancer patients [35]. Second, current NIPT-based algorithms are restricted to the detection of copy variable tumours and will miss copy neutral tumours. Additionally, other tumor characteristics, such as tumor type, size and proliferation status, might determine the degree of tumor cfDNA shedding into the circulation and hence have an impact on the sensitivity of tumor detection. To confirm and validate our PPV, large-scale research, preferably in a multicentre setting, is needed. Such studies can deliver new information that can stimulate further refinements of the bioinformatics cfDNA analysis pipeline and the clinical workflow. Whereas we demonstrate that comprehensive analysis of shallow-sequenced cfDNA allows the incidental detection of maternal tumors with relatively high precision, it should be emphasized that NIPT should not be considered as a cancer screening test. At present, we have no long-term outcome data of women in our cohort with a normal interpretable NIPT or a non-interpretable NIPT not classified as cancer-like. Hence, the overall sensitivity of our approach to detect cancers as well as the specificity for different cancer types remain unknown. Current investigations in the liquid biopsy field of cancer screening are exploiting supramolecular information contained in cfDNA to identify the tissue of origin and subsequently guide oncological investigations [36,37]. In future, such strategies might further studied and implemented in the obstetrics field [38]. With the presented analytical approach and post-test patient management model, we hope to stimulate such advancements as well as the development of institutional or national orchestrated guidelines for the clinical evaluation of pregnant women suspected of having a cancer based on their NIPT outcome.
Declaration of Competing Interest
EL reports personal fees from Springworks Therapeutics outside the submitted work. All other authors declare no competing interests.
Acknowledgments
Author contribution
LL designed the study, assembled and integrated sequencing and clinical data, designed the figures and developed the main manuscript. NB designed the study, analysed and collected NIPT profiles and contributed to the writing process. CM assisted in patient management and collected and interpreted clinical follow-up data. LVC analysed NIPT profiles. HC and LD performed bio-informatics analyses. PVB supervised hemato-oncological consultations and interpreted NIPT cfDNA profiles. DD supervised hemato-oncological consultations. LM interpreted hematological analyses and supervised and interpreted FISH analyses in biopsy tissues. BD designed and interpreted FISH analyses in tumor tissues. PN supervised clinical management of breast cancer patients. GF was responsible for analysis of breast cancer biopsies. TT was responsible for analysis of biopsies from hematological malignancies. LLannoo and KVC were responsible for the obstetrics follow-up of the patients. TJ contributed to analysis of low-pass sequencing data and figure design. IVB supervised and interpreted aCGH analyses in tumor biopsies. VV supervised WB-DWI MRI imaging. KD and EL clinically interpreted NIPT results and took care of genetic counselling. KVDB supervised the analysis of NIPT profiles and FISH analyses in blood cells. JRV designed the study, supervised the analysis and interpretation of NIPT data, contributed to the design of the manuscript and acquired the grant funds. FA designed the study, supervised the interpretation of oncological data, contributed to the design of the manuscript and acquired the grant funds. LL, NB, CM, LVC, KVDB, PV and LM verified the underlying data. All authors critically revised and approved the report.
Data sharing statement
The anonymized data that support the findings of this study, as well as related documents, are available from the corresponding author upon reasonable request.
Funding
This work was supported by FWO (G080217N to FA and JRV) and KU Leuven funding (no. C1/018 to JRV).
Acknowledgements
Frédéric Amant is a senior clinical researcher for the Research Foundation Flanders (FWO).
We thank Katrien Van Tornout for administrative help and taking patient blood samples for confirmatory analyses and Geneviève Ameye for her excellent technical assistance for confirmatory FISH analyses.
Footnotes
Eric Legius has received occasional consulting fees from Springwork Therapeutics
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100856.
Contributor Information
Joris Robert Vermeesch, Email: joris.vermeesch@kuleuven.be.
Frédéric Amant, Email: frederic.amant@uzleuven.be.
Appendix. Supplementary materials
References
- 1.Samura O. Update on noninvasive prenatal testing: a review based on current worldwide research. J Obstet Gynaecol Res. 2020;46:1246–1254. doi: 10.1111/jog.14268. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi D.W., Chiu R.W.K. Sequencing of circulating cell-free DNA during pregnancy. N Engl J Med. 2018;379:464–473. doi: 10.1056/NEJMra1705345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady P., Brison N., Van Den Bogaert K. Clinical implementation of NIPT - technical and biological challenges. Clin Genet. 2016;89:523–530. doi: 10.1111/cge.12598. [DOI] [PubMed] [Google Scholar]
- 4.Ji X., Li J., Huang Y. Identifying occult maternal malignancies from 1.93 million pregnant women undergoing noninvasive prenatal screening tests. Genet Med. 2019;21:2293–2302. doi: 10.1038/s41436-019-0510-5. [DOI] [PubMed] [Google Scholar]
- 5.Dharajiya N.G., Grosu D.S., Farkas D.H. Incidental detection of maternal neoplasia in noninvasive prenatal testing. Clin Chem. 2018;64:329–335. doi: 10.1373/clinchem.2017.277517. [DOI] [PubMed] [Google Scholar]
- 6.Amant F., Verheecke M., Wlodarska I. Presymptomatic identification of cancers in pregnant women during noninvasive prenatal testing. JAMA Oncol. 2015;1:814–819. doi: 10.1001/jamaoncol.2015.1883. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi D.W., Chudova D., Sehnert A.J. Noninvasive prenatal testing and incidental detection of occult maternal malignancies. JAMA - J Am Med Assoc. 2015;314:162–169. doi: 10.1001/jama.2015.7120. [DOI] [PubMed] [Google Scholar]
- 8.van der Meij K.R.M., Sistermans E.A., Macville M.V.E. TRIDENT-2: national Implementation of genome-wide non-invasive prenatal testing as a first-tier screening test in the Netherlands. Am J Hum Genet. 2019;105:1091–1101. doi: 10.1016/j.ajhg.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenaerts L., Van Calsteren K., Che H., Vermeesch J.R., Amant F. Pregnant women with confirmed neoplasms should not have noninvasive prenatal testing. Prenat Diagn. 2019;39 doi: 10.1002/pd.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benn P., Plon S.E., Bianchi D.W. Current controversies in prenatal diagnosis 2: NIPT results suggesting maternal cancer should always be disclosed. Prenat Diagn. 2019;39:339–343. doi: 10.1002/pd.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giles M.E., Murphy L., Krstić N., Sullivan C., Hashmi S.S., Stevens B. Prenatal cfDNA screening results indicative of maternal neoplasm: survey of current practice and management needs. Prenat Diagn. 2017;37:126–132. doi: 10.1002/pd.4973. [DOI] [PubMed] [Google Scholar]
- 12.Carlson L.M., Hardisty E., Coombs C.C., Vora N.L. Maternal malignancy evaluation after discordant cell-free DNA results. Obstet Gynecol. 2018;131:464–468. doi: 10.1097/AOG.0000000000002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belgian Society of Human Genetics B. Belgian guidelines for managing incidental findings detected by NIPT. 2019. https://www.college-genetics.be/assets/recommendations/fr/guidelines/BELGIAN GUIDELINES FOR MANAGING INCIDENTAL FINDINGS DETECTED BY NIPT (2019).pdf
- 14.de Haan J., Verheecke M., Van Calsteren K. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018;19:337–346. doi: 10.1016/S1470-2045(18)30059-7. [DOI] [PubMed] [Google Scholar]
- 15.Maggen C., Dierickx D., Lugtenburg P. Obstetric and maternal outcomes in patients diagnosed with Hodgkin lymphoma during pregnancy: a multicentre, retrospective, cohort study. Lancet Haematol. 2019;6:e551–e561. doi: 10.1016/S2352-3026(19)30195-4. [DOI] [PubMed] [Google Scholar]
- 16.Amant F., Von Minckwitz G., Han S.N. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol. 2013;31:2532–2539. doi: 10.1200/JCO.2012.45.6335. [DOI] [PubMed] [Google Scholar]
- 17.Halaska M.J., Uzan C., Han S.N. Characteristics of patients with cervical cancer during pregnancy: a multicenter matched cohort study. An initiative from the International Network on Cancer, Infertility and Pregnancy. Int J Gynecol Cancer. 2019;29:676–682. doi: 10.1136/ijgc-2018-000103. [DOI] [PubMed] [Google Scholar]
- 18.Bayindir B., Dehaspe L., Brison N. Noninvasive prenatal testing using a novel analysis pipeline to screen for all autosomal fetal aneuploidies improves pregnancy management. Eur J Hum Genet. 2015;23:1286–1293. doi: 10.1038/ejhg.2014.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negrini S., Gorgoulis V.G., Halazonetis T.D. Genomic instability an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 20.Paulsson K., Säll T., Fioretos T., Mitelman F., Johansson B. The incidence of trisomy 8 as a sole chromosomal aberration in myeloid malignancies varies in relation to gender, age, prior iatrogenic genotoxic exposure, and morphology. Cancer Genet Cytogenet. 2001;130:160–165. doi: 10.1016/s0165-4608(01)00486-1. [DOI] [PubMed] [Google Scholar]
- 21.Han S.N., Lotgerink A., Gziri M.M., Van Calsteren K., Hanssens M., Amant F. Physiologic variations of serum tumor markers in gynecological malignancies during pregnancy: a systematic review. BMC Med. 2012;10 doi: 10.1186/1741-7015-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han S.N., Amant F., Michielsen K. Feasibility of whole-body diffusion-weighted MRI for detection of primary tumour, nodal and distant metastases in women with cancer during pregnancy: a pilot study. Eur Radiol. 2018;28:1862–1874. doi: 10.1007/s00330-017-5126-z. [DOI] [PubMed] [Google Scholar]
- 23.Maggen C., van Gerwen M., Van Calsteren K., Vandenbroucke T., Amant F.F. BMJ Publishing Group; 2019. Management of cancer during pregnancy and current evidence of obstetric, neonatal and pediatric outcome: a review article. [DOI] [PubMed] [Google Scholar]
- 24.Paulsson K., Johansson B. Trisomy 8 as the sole chromosomal aberration in acute myeloid leukemia and myelodysplastic syndromes. Pathol Biol. 2007;55:37–48. doi: 10.1016/j.patbio.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Amant F., Vandenbroucke T., Verheecke M. Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med. 2015;373:1824–1834. doi: 10.1056/NEJMoa1508913. [DOI] [PubMed] [Google Scholar]
- 26.Vandenbroucke T., Verheecke M., Fumagalli M., Lok C., Amant F. Effects of cancer treatment during pregnancy on fetal and child development. Lancet Child Adolesc Heal. 2017;1:302–310. doi: 10.1016/S2352-4642(17)30091-3. [DOI] [PubMed] [Google Scholar]
- 27.Voulgaris E., Pentheroudakis G., Pavlidis N. Cancer and pregnancy: a comprehensive review. Surg Oncol. 2011;20:e175–e185. doi: 10.1016/j.suronc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Snyder H.L., Curnow K.J., Bhatt S., Bianchi D.W. Follow-up of multiple aneuploidies and single monosomies detected by noninvasive prenatal testing: implications for management and counseling. Prenat Diagn. 2016;36:203–209. doi: 10.1002/pd.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartmann L., Metzeler K.H. Clonal hematopoiesis and preleukemia—Genetics, biology, and clinical implications. Genes Chromosom Cancer. 2019;58:828–838. doi: 10.1002/gcc.22756. [DOI] [PubMed] [Google Scholar]
- 30.Lenaerts L., Vandenberghe P., Brison N. Genomewide copy number alteration screening of circulating plasma DNA: potential for the detection of incipient tumors. Ann Oncol. 2019;30 doi: 10.1093/annonc/mdy476. [DOI] [PubMed] [Google Scholar]
- 31.Laurie C.C., Laurie C.A., Rice K. Detectable clonal mosaicism from birth to old age and its relationship to cancer. Nat Genet. 2012;44:642–650. doi: 10.1038/ng.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bianchi D.W. Circulating fetal DNA: its origin and diagnostic potential - a review. Placenta. 2004 doi: 10.1016/j.placenta.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Hartwig T.S., Ambye L., Sørensen S., Jørgensen F.S. Discordant non-invasive prenatal testing (NIPT) – a systematic review. Prenat Diagn. 2017;37:527–539. doi: 10.1002/pd.5049. [DOI] [PubMed] [Google Scholar]
- 34.Van Den Bogaert K., Lannoo L., Brison N., et al. Outcome of publicly funded nationwide first-tier noninvasive prenatal screening. doi: 10.1038/s41436-021-01101-4. [DOI] [PubMed]
- 35.Lenaerts L., Che H., Brison N. Breast cancer detection and treatment monitoring using a noninvasive prenatal testing platform: utility in pregnant and nonpregnant populations. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa196. [DOI] [PubMed] [Google Scholar]
- 36.Liu M.C., Oxnard G.R., Klein E.A. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31:745–759. doi: 10.1016/j.annonc.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder M.W., Kircher M., Hill A.J., Daza R.M., Shendure J. Cell-free DNA Comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell. 2016;164:57–68. doi: 10.1016/j.cell.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng X., Li H.-.D., Wu F.-.X., Wang J. Identifying the tissues-of-origin of circulating cell-free DNAs is a promising way in noninvasive diagnostics. Brief Bioinform. 2020 doi: 10.1093/bib/bbaa060. [DOI] [PubMed] [Google Scholar]
- 39.Imbert-Bouteille M., Chiesa J., Gaillard J.B. An incidental finding of maternal multiple myeloma by non invasive prenatal testing. Prenat Diagn. 2017;37:1257–1260. doi: 10.1002/pd.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rengifo L.Y., Michaux L., Maertens J. Noninvasive prenatal testing detected acute myeloid leukemia in paucisymptomatic pregnant patient. Clin Case Reports. 2020 doi: 10.1002/ccr3.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.