Summary
Pregnenolone (P5) promotes prostate cancer cell growth, and de novo synthesis of intratumoural P5 is a potential cause of development of castration resistance. Immune cells can also synthesize P5 de novo. Despite its biological importance, little is known about P5's mode of actions, which appears to be context dependent and pleiotropic. A comprehensive proteome-wide spectrum of P5-binding proteins that are involved in its trafficking and functionality remains unknown. Here, we describe an approach that integrates chemical biology for probe synthesis with chemoproteomics to map P5-protein interactions in live prostate cancer cells and murine CD8+ T cells. We subsequently identified P5-binding proteins potentially involved in P5-trafficking and in P5's non-genomic action that may drive the promotion of castrate-resistance prostate cancer and regulate CD8+ T cell function. We envisage that this methodology could be employed for other steroids to map their interactomes directly in a broad range of living cells, tissues, and organisms.
Graphical abstract
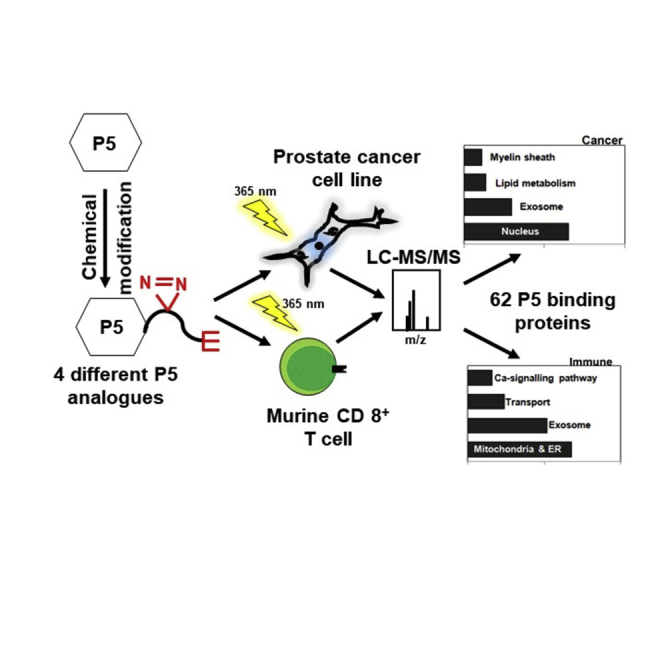
Highlights
-
•
Developed four functional click-enabled analogues of pregnenolone (P5)
-
•
Chemoproteomics prioritizes 62 P5 target proteins in live cancer and immune cells
-
•
These include shared and distinct biochemical role of P5 in cancer and immune cells
-
•
P5 activity in cancer and immune cells is mediated through non-genomic pathways
Introduction
Pregnenolone (P5) is the first bioactive steroid hormone and precursor of all other steroid hormones in steroid biosynthesis (steroidogenesis) pathways. P5 is synthesized from cholesterol by the enzyme CYP11A1 inside the mitochondria of steroidogenic cells (Miller and Auchus, 2011). A high capacity for P5 biosynthesis has been reported in adrenal tissue, gonads, and placenta. Extra-adrenal and extra-gonadal P5 synthesis (also known as local steroidogenesis) has been reported in lymphocytes (Jia et al., 2013; Wang et al., 2013; Mahata et al., 2014), adipocytes (Li et al., 2014), the nervous system (Baulieu, 1998), tumors (Locke et al., 2008), and tumour-infiltrating immune cells (Mahata et al., 2020). The role played by this local P5 synthesis is poorly understood (Miller, 2017), particularly in pathologies such as cancer. In prostate cancer and melanoma P5 promotes tumor growth (Grigoryev et al., 2000; Mahata et al., 2020), whereas in glioma it restricts tumor growth (Xiao et al., 2014). The mode of action of P5 in tumors is incompletely understood.
In the nervous system, P5 is known to regulate synapse formation, promote outgrowth of neurites, enhance myelinization (Mellon, 2007), and improve cognitive and memory function (Mayo et al., 2001). During immune response against helminth parasite infection T helper cells synthesize P5 to restore immune homeostasis (Mahata et al., 2014). Up to now, we do not have a proteome-wide description of the P5-interacting molecules in any living cells.
Traditionally, steroid hormones have been considered to act by regulating transcription (Mazaira et al., 2018). However, rapid activity of steroid hormones can be mediated by non-genomic pathways (Lösel and Wehling, 2003). Non-genomic pathways appear to mediate P5 activity (Weng and Chung, 2016) in a cell-type-specific and context-dependent manner, indicating a need for proteome-wide studies to map the full spectrum of P5 functions.
Here, we have developed a chemical biology method to generate clickable P5-analogues for use in living cells. Exploiting these P5 probes in combination with quantitative mass spectrometry, we profiled global P5-protein interactions directly in two distinct cell types: a steroid-sensitive cell line derived from a metastatic prostate cancer patient (i.e. LNCaP) and de novo P5-producing mouse CD8+ T cells.
Altogether, we identified 62 high-confidence P5-binding proteins and about 387 potential P5-binding proteins localized in the nucleus, mitochondria, and endoplasmic reticulum (ER). These proteins include receptors, channels, transporters, and cytoskeletal proteins such as vimentin and enzymes, of which many represent novel interactions. Overall, we identified P5-binding proteins potentially involved in inter- and intra-cellular P5-trafficking and P5's non-genomic action that drives prostate cancer promotion of castration-resistance and mediates CD8+ T cell regulation.
This study unravels prospective routes for understanding pregnenolone biochemistry in different cellular contexts such as prostate cancer progression and immune cell regulation. We demonstrate a general methodology to decipher P5-biochemistry in de novo steroidogenic and steroid-responsive cells or tissues.
Results
Design of clickable and photoactivatable P5 probes
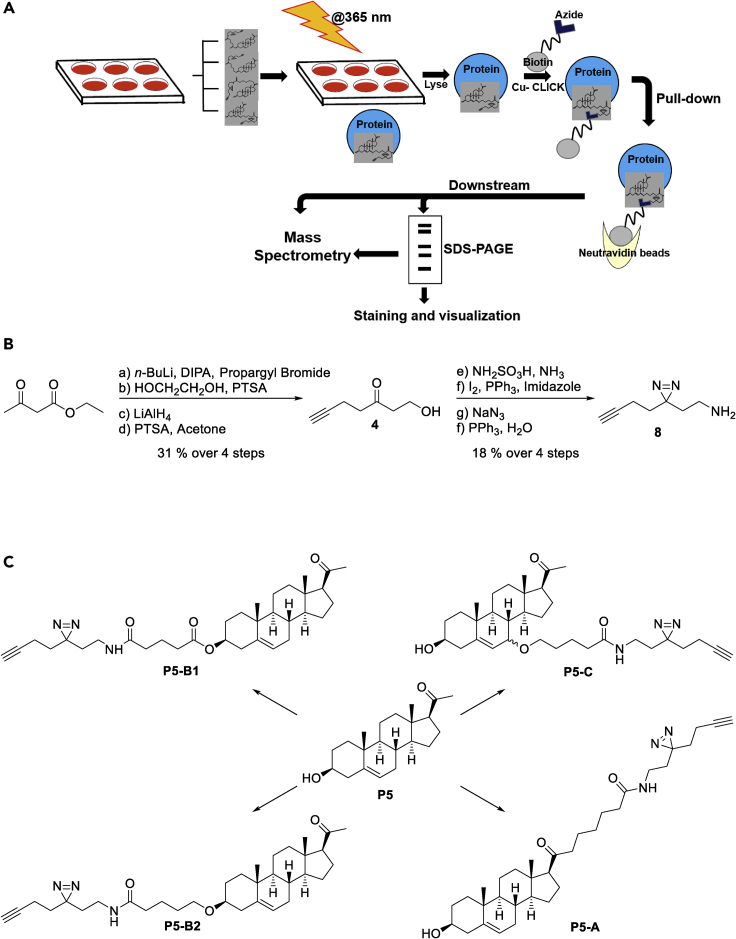
To capture the P5 interactome in living cells, we utilize photoaffinity labeling combined with enrichment of tagged proteins using a bioorthogonal handle. This strategy required modification of the P5 core, while also retaining the primary pharmacology of P5. A minimalist photoaffinity enrichment linker has been introduced to kinase inhibitors to map their interactomes (Li et al., 2013). The linker utilizes a diazirine as the UV-induced cross-linking agent and an alkyne tag, upon which bioorthogonal chemistry could be performed after photoaffinity labeling to enrich tagged proteins using azide-biotin (Figure 1A). The linker was selected due to its small size, reducing the risk of the tag altering the P5 interactome and due to the proven effectiveness of this type of approach (Hulce et al., 2013; Li et al., 2013; Weng et al., 2013). With these considerations in mind, we synthesized molecule 8 (Figure 1B) to conjugate to P5 at three different positions to ensure maximum coverage of molecular space and avoid preclusion of binding by a particular linker. The P5-derived probes were named P5-A, P5-B1, P5-B2, and P5-C (Figures 1C and S1) and tested for bioactivity and cell permeability.
Figure 1.
Design of clickable and photoactivable pregnenolone (P5) probes
(A) Schematic diagram of our chemoproteomic approach to pull down the P5-interacting proteome in live cells.
(B) Synthesis of the photoactivatable and clickable linker with diazirine and alkyne.
(C) Final chemical structures of the four different probes with their linker positions as compared with the mother molecule P5.
P5 probes mimic biological activity of P5 in human cancer cell lines
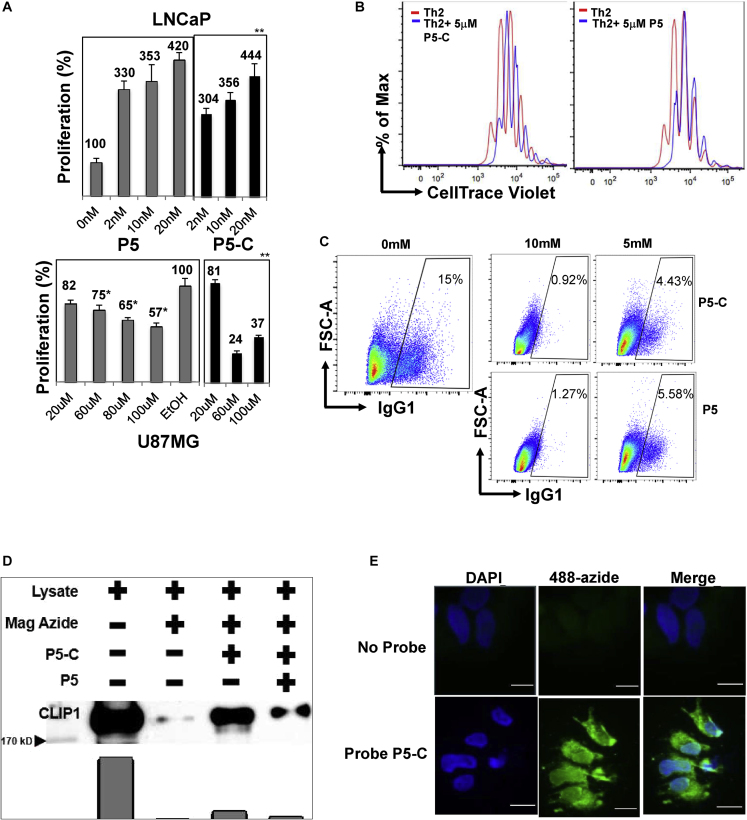
P5 is known to stimulate LNCaP prostate cancer cell growth (Grigoryev et al., 2000) and inhibit U87MG glioma cell growth (Xiao et al., 2014). To test the biological activity of the P5-A, P5-B1, P5-B2, and P5-C we performed cell viability assays using the LNCaP and U87MG cells. Although LNCaP cells show increased cell viability in the presence of P5, it has a cytotoxic effect on U87MG cells (Figure 2A). For LNCaP cells, only two nM of P5-A is sufficient to induce a 4-fold increase in cell proliferation, which corresponds to the effect of 20 nM of P5 (Figure S2A). However, at higher concentration of P5-A, there is decrease in cell viability. For P5-C, cell viability follows exactly the same pattern as for P5. In both cases, cell proliferation gradually increases from 3-fold to 4-fold between 2 nM and 20 nM concentration of P5-C or P5 (Figure 2A top panel). P5-B1 and P5-B2 (although slightly better than P5-B1) can only induce cell growth to 3-fold corresponding to the highest concentration i.e. 20 nM (Figure S2A). In summary, P5-C shows the best retention of the parental pharmacology of P5.
Figure 2.
P5-C mimics biological activity of P5
(A) P5-C mimics biological activity of P5 in human prostate cancer and glioma cell lines
The LNCaP (top panel) and U87MG (bottom panel) cells were incubated equivalent amounts of P5-C (right histograms) and P5 (left histogram). Cell proliferation was evaluated using the XTT cell viability assay in at least five independent experiments. Statistical test were done using Student's t test; ∗p < 0.05, ∗∗p < 0.01. Data are represented as mean ± SEM.
(B) P5-C mimics biological activity of P5 in mouse primary T cells and inhibits T cell proliferation. Naive CD4+ T cells were stained with CellTrace Violet and activated under Th2 differentiation conditions for 72 h in the presence (red histogram) or absence (blue histogram) of 5 μM P5 or P5-C. The cell proliferation profile was captured by a flow-cytometry-based dye decay assay. Data shown are representative of three independent experiments with three mice in each experiment.
(C) P5-C mimics biological activity of P5 in mouse primary B cells and inhibits B cell immunoglobulin class switching. Naive resting B cells were induced with LPS and IL-4 in the presence of different concentrations of P5 and P5-C (0, 5, and 10 μM). Cell-surface expression of IgG1 was analyzed by flow cytometry on the fifth day of stimulation. Data shown are representative of three independent experiments with three mice in each experiment.
(D) The P5-C probe mimics P5 interaction with the previously known P5-binding protein CLIP1 and is competed out by cold P5. HA-tagged CLIP1 was ectopically expressed in HEK cells, and the protein content of the lysate was estimated. About 400 μg of whole-cell lysate was used in two experiments simultaneously, one incubated with 50 nM P5-C and another with 500 nM P5 before 50 nM P5-C. The azide-coated magnetic beads were clicked and pulled down using magnets. The magnetic-azide beads were incubated with cell lysate to capture any background binding of CLIP1 to the beads. SDS-PAGE and western blotting followed by incubation with HA antibody revealed P5-C binding to CLIP 1, and P5 competed out P5-C binding.
(E) P5-C is cell permeable. Live LNCaP cells are incubated with P5-C, which is clicked to Alexa Fluor 488 azide (in green) and imaged under a fluorescence microscope. Simultaneously, as a control, cells not incubated with P5-C were clicked and imaged as before. DAPI (in blue) was used in both the experiments to visualize the nucleus. Scale bar: 50
U87MG cells are known to undergo apoptosis in the presence of P5. All four probes induced apoptosis at lower concentrations than the parental P5. P5-B2 showed the highest apoptotic activity with 3-fold reduction in cell viability at only 20 μM concentration; the same concentration of P5 does not induce any significant reduction of cells. In contrast, 100 μM of all the probes produces a significant reduction in cell number that also corresponds to the cell viability at 100 μM in the cells with P5 (Figure 2A bottom panel and Figure S2B).
P5 probes mimic the biological activity of P5 in primary immune cells
P5 inhibits murine T helper cell proliferation and B cell class switching (Mahata et al., 2014). To test whether addition of the linker retains the biological activity of P5 in this context, we performed T helper cell proliferation assays and B cell immunoglobulin class switching assays in the presence of P5-A, P5-B1, P5-B2, and P5-C. The presence of P5 in the in vitro Th2 culture conditions significantly restricts cell proliferation compared with vehicle-only-treated conditions (Figure 2B, right-side panel). We found that P5-C, like P5, inhibits T cell proliferation (Figure 2B, left-side panel). P5-A, P5-B1, and P5-B2 showed similar properties (Figure S3A). In immunoglobulin class-switching experiments we observed that the P5-C and the other P5 probes are equally efficient as P5 (Figures 2C and S3B).
P5 probes mimic P5 protein interactions and are cell permeable
CAP-Gly domain containing linker protein 1 or cytoplasmic linker protein 1 (CLIP1, also known as CLIP170) was previously reported to be a specific P5 binding protein in zebrafish and human (Weng et al., 2013). To test whether our P5 probes mimic P5's binding activity with CLIP1, we expressed CLIP1 ectopically in HEK293 cells, and the lysate was used to analyze the CLIP1 binding affinity of the P5 probes. All the P5 probes were able to pull down the CLIP1 protein from the lysate with similar efficiency (Figures 2D and S4). Unlabeled P5 was able to compete out P5-C binding to the CLIP1 protein, indicating P5-C's ability to capture P5 interactome (Figure 2D) and clearly reflecting its ability to bind known P5-binding proteins.
To confirm the cell permeability of P5-C, we cultured LNCaP cells in the presence or absence of P5-C followed by CLICK reaction with an azide-bearing fluorophore. Subsequent fluorescence microscopy showed that the P5-C could enter live LNCaP cells (Figure 2E).
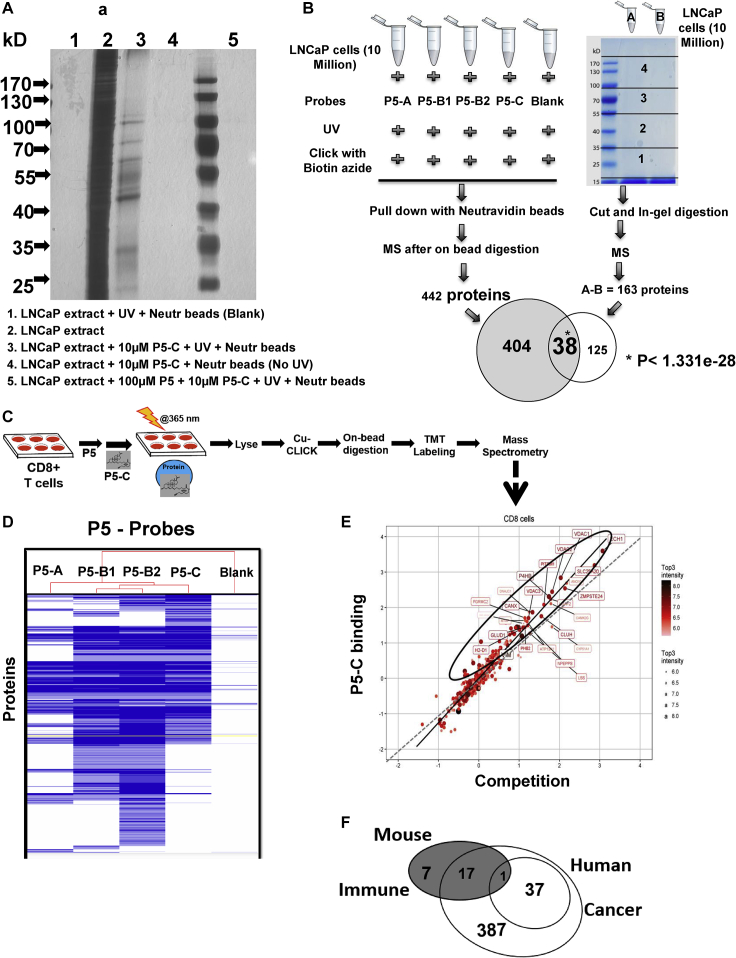
P5-C enriches P5-binding proteins from cell extracts
To ascertain that P5-C can mimic P5's binding ability, LNCaP cell extracts were incubated with P5-C in the presence or absence of competing amounts of P5. In parallel, two other controls were performed, one without UV irradiation and another without P5-C being present. All the samples were loaded onto an SDS-PAGE gel and silver stained (Figure 3A). Ten-fold excess of P5 compared with P5-C was able to effectively compete out the P5-C binding, confirming our earlier observation (with CLIP1). P5-C captures P5-interacting proteins in a complex protein mixture. The samples without UV treatment and the neutravidin beads did not show any significant binding. The above benchmarking and quality control results confirmed the P5-C molecule as true analogue of P5 and an ideal probe to study in vivo P5 interactomes.
Figure 3.
P5 probes enable mass spectrometry profiling of P5-binding proteins in live cells
(A) Gel profiling and specificity of P5-binding proteins with P5-C in LNCaP cell extracts. About 400 μg of LNCaP protein in vitro was mixed either with P5-C alone or in the presence of 10X P5 (competition assay), exposed to UV, and loaded onto lanes 3 and 5, respectively, in a 10% SDS-PAGE. Two control experiments were run in parallel, one without P5-C to check background binding to the beads (lane 1) and another without UV to check the proper activation of the diazirine within the linker (lane 4). Lane 2 has 50 μg of total LNCaP proteins. Lanes 3, 4, and 5 were loaded with the final pulldown from 400 μg of LNCap extracts in laemmli buffer. The gel was stained with silver and imaged.
(B) Experimental design to reveal the identity of P5-interacting proteins in LNCaP cells using two approaches with 10 million live cells. In the right-hand panel gel image, “A” represents pulldown with10 μM P5-C and “B” represents pulldown after competition reaction (100 μM P5+10 μM P5-C). The p value calculations were done as per the hypergeometric probability formula from Chapter 6.1 of Numerical Recipes in C: The Art of Scientific Computing (ISBN 0-521-43108-5) (Press et al., 1992) as found in http://nemates.org/MA/progs/representation.stats.html.
(C) Experimental design to reveal the identity of P5-interacting proteins in live CD8+ T cells.
(D) Hierarchical clustering of all proteins pulled down using all four probes shows the structure-dependent bioactivity of the probes.
(E) Twenty-three P5-interacting proteins in CD8+ T cells. The proteins extracted with P5-C either alone or after competition with P5. P5-C binding proteins either in the presence of P5 (competition assay) or in its absence (experiment) were plotted on a log scale after subtracting the background. The regression equation fitting the two variables is represented by the solid straight line, whereas the dotted line represents the X = Y linear relation showing no variation. The dots in ellipses represent the P5-C binding proteins whose binding can be competed out in the presence of P5.
(F) Common and specific P5-interacting proteins in human prostate cancer and mouse immune cells. P5-interactomes from human prostate cancer (two concentric empty circles) and murine CD8+ T cells (filled circle) have 16 proteins in common. Among them, only one protein was common to CD8+ T cells and core “P5-binding proteins’ from LNCaP. Another 17 CD8+ T cell proteins were found in the 404 “potential P5-binding proteins” from LNCaP. Seven and four hundred twenty-four (37 + 387) proteins are specific to CD8+ T cells and LNCaP, respectively.
LNCaP cells are steroid-sensitive prostate cancer cell line, which has been used widely as a model of human prostate cancer and was previously shown to respond to P5 (Grigoryev et al., 2000). Prostate cancer cells and tumour-infiltrating T cells produce P5 de novo, which can cause autocrine and paracrine responses in the tumor microenvironment (Locke et al., 2008; Mahata et al., 2020). Therefore, we proceeded to use these two cell types to reveal the proteome-wide map of P5-interacting proteins. We also used a published cholesterol interactome dataset (Hulce et al., 2013) to show the overlap with sterol-binding proteins in our P5 interactome (Table 1 and Figure S9).
Table 1.
The details of 62 “P5-binding proteins”
| Gene names | Organism | Protein names |
|---|---|---|
| ACSL1a | Homo sapiens (Human) | Long-chain-fatty-acid--CoA ligase 1 |
| ACTN4 | Homo sapiens (Human) | Actinin alpha 4 isoform 3 |
| AIFM1a | Homo sapiens (Human) | Apoptosis-inducing factor 1, mitochondrial |
| ALDH3A2a | Homo sapiens (Human) | Fatty aldehyde dehydrogenase |
| ATL3 | Homo sapiens (Human) | Atlastin-3 |
| CAND1 | Homo sapiens (Human) | Cullin-associated NEDD8-dissociated protein1 |
| CERS2 | Homo sapiens (Human) | Ceramide synthase 2 |
| CKAP4a | Homo sapiens (Human) | Cytoskeleton-associated protein 4 |
| CPT1Aa | Homo sapiens (Human) | Carnitine palmitoyltransferase 1A |
| DDX21 | Homo sapiens (Human) | Nucleolar RNA helicase 2 |
| DHCR24a | Homo sapiens (Human) | Delta(24)-sterol reductase |
| DHX9 | Homo sapiens (Human) | ATP-dependent RNA helicase A |
| EMDa | Homo sapiens (Human) | Emerin |
| GANAB | Homo sapiens (Human) | Neutral alpha-glucosidase AB |
| ILF3 | Homo sapiens (Human) | Interleukin enhancer-binding factor 3 |
| IMMTa | Homo sapiens (Human) | MICOS complex subunit MIC60 |
| LMNAa | Homo sapiens (Human) | Prelamin-A/C |
| LMNB1 | Homo sapiens (Human) | Lamin-B1 |
| MATR3 | Homo sapiens (Human) | Matrin-3 |
| MYH9 | Homo sapiens (Human) | Myosin-9 |
| NDC1 | Homo sapiens (Human) | Nucleoporin |
| NDUFS1 | Homo sapiens (Human) | NADH-ubiquinone oxidoreductase |
| NOP58 | Homo sapiens (Human) | Nucleolar protein 58 |
| NPEPPS# | Homo sapiens (Human) | Puromycin-sensitive aminopeptidase |
| NUP93 | Homo sapiens (Human) | Nuclear pore complex protein Nup93 |
| PARP1 | Homo sapiens (Human) | Poly [ADP-ribose] polymerase 1 |
| PEBP1 | Homo sapiens (Human) | Phosphatidylethanolamine-binding protein |
| PHBa | Homo sapiens (Human) | Prohibitin |
| RPN1a | Homo sapiens (Human) | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 1 |
| SURF4a | Homo sapiens (Human) | Surfeit 4 |
| TRAP1 | Homo sapiens (Human) | Heat shock protein 75 kDa, mitochondrial |
| UBA1 | Homo sapiens (Human) | Ubiquitin-like modifier-activating enzyme 1 |
| XRCC6 | Homo sapiens (Human) | X-ray repair complementing defective repair in Chinese hamster cells 6 |
| CPT2a | Homo sapiens (Human) | Carnitine O-palmitoyltransferase 2 |
| SF3B3 | Homo sapiens (Human) | Splicing factor 3B subunit 3 |
| SFPQ | Homo sapiens (Human) | Splicing factor, proline- and glutamine-rich |
| SRPRBa | Homo sapiens (Human) | Signal recognition particle receptor subunit beta |
| TMX1 | Homo sapiens (Human) | Thioredoxin-related transmembrane protein 1 |
| GLUD1 | Mus musculus | Glutamate dehydrogenase 1 |
| P4HB | Mus musculus | Prolyl 4-hydroxylase, beta polypeptide |
| PITRM1 | Mus musculus | Pitrilysin metallepetidase 1 |
| RAB11Aa | Mus musculus | RAS oncogene family |
| CAMK2G | Mus musculus | Calcium/calmodulin-dependent protein kinase |
| H2-D1a | Mus musculus | Histocompatibility 2, D region locus 1 |
| PGRMC2a | Mus musculus | P4 receptor membrane component 2 |
| VDAC1a | Mus musculus | Voltage-dependent anion channel 1 |
| VDAC2a | Mus musculus | Voltage-dependent anion channel 2 |
| VDAC3a | Mus musculus | Voltage-dependent anion channel 3 |
| DNAJC1a | Mus musculus | DnaJ heat shock protein family |
| ATP13A1a | Mus musculus | ATPase type 13A1 |
| CANXa | Mus musculus | Calnexin |
| U2AF | Mus musculus | U2 small nuclear ribonucleoprotein auxiliary factor |
| ECH1 | Mus musculus | Enoyl coenzyme A hydratase 1 |
| VIM | Mus musculus | Vimentin |
| PHB2 | Mus musculus | Prohibitin 2 |
| CLUH | Mus musculus | Clustered mitochondria (cluA/CLU1) homolog |
| NPEPPS# | Mus musculus | Aminopeptidase puromycin sensitive |
| CYP51a | Mus musculus | Cytochrome P450, family 51 |
| ANO10 | Mus musculus | Anoctamin 10 |
| SLC25A20a | Mus musculus | Mitochondrial carnitine/acylcarnitine translocase, member |
| ZMPSTE24a | Mus musculus | Zinc metallopeptidase |
| MTAP | Mus musculus | Methylthioadenosine phosphorylase |
| LSSa | Mus musculus | Lanosterol synthase |
Also known to interact with sterol in a different cell type, # present in both human cancer and mouse immune cells. 38 P5 target proteins in LNCaP prostate cell line from Homo sapiens and 25 P5 target proteins from CD8+ immune cell from Mus musculus.
P5 probes enable mass spectrometry profiling of P5-binding proteins in LNCaP and murine CD8+ T cells
P5-interactomes in LNCaP and CD8+ T cells were captured as per the schematics (Figures 3B and 3C). Hierarchical clustering showed the binding potential of the probes to be distinct, which might be associated with the availability of molecular space due to the distinct pattern of linker substitution (Figure 3D). P5-B1 and P5-B2 showed the closest correlation (Tyanova and Cox, 2018) (Figure S10), which was expected as they were both substituted at the hydroxyl – position of P5. The slight differences might be due to the addition of a carbonyl group in P5-B2. The protein pull-down efficiency of P5-A was lowest as compared with the others.
To generate consensus LNCaP P5 interactome, we considered the four different probes as four replicates and only accepted those proteins with >2 unique peptides in at least 3 replicates (or probe pull downs) as “potential P5 binding” (described in detail in the transparent methods section under the heading “Analysis of the MS results”). This allowed us to obtain a list of 442 LNCaP proteins that has representation in at least 3 P5 analogue interactome dataset with >2 unique peptides.
In a parallel approach, we used P5-C with or without competition with P5. After SDS-PAGE gel analysis, the gel lanes were cut out and subsequently digested in-gel followed by LC-MS analysis to identify proteins (Figures 3B and S5). We then selected only those proteins that could be effectively competed by P5, thereby restricting to proteins with high specificity for P5. We captured 163 P5-interacting proteins. Among those, 38 were common to both approaches described above and therefore termed as “P5-binding proteins” (Table 1). The rest of the 404 out of the 442 proteins obtained from the on-bead digestion experiment were simultaneously analyzed and named as “potential P5 binding proteins” (Figure 3B). (125 out of the 163 proteins from the in-gel digestion experiment were excluded from further analysis). These 38 proteins (P < 1.331 × 10−28) (Press et al., 1992) were P5-C binding that has representation in both the gel and the on-bead digestion experiment.
To identify proteins enriched with P5-C in murine immune cells, we carried out proteomics using the tandem mass tag (TMT) for relative quantification. The schematic in Figure 3C outlines the protocol. Analysis revealed 25 proteins in the P5-interactome in CD8+ T cells (Figure 3E). Overall, 18 of these 25 murine proteins were also found in human LNCaP. The remaining seven are CD8+ T-cell-specific proteins (Figure 3F).
In total, we enriched a catalog of 62 “P5 binding proteins” and about 387 “potential P5 binding” proteins from two different cell types. Twenty-seven of these sixty-two proteins also have sterol sensitivity; after filtering them we report 35 proteins that specifically bind to P5 (Table 1 and Figure S9).
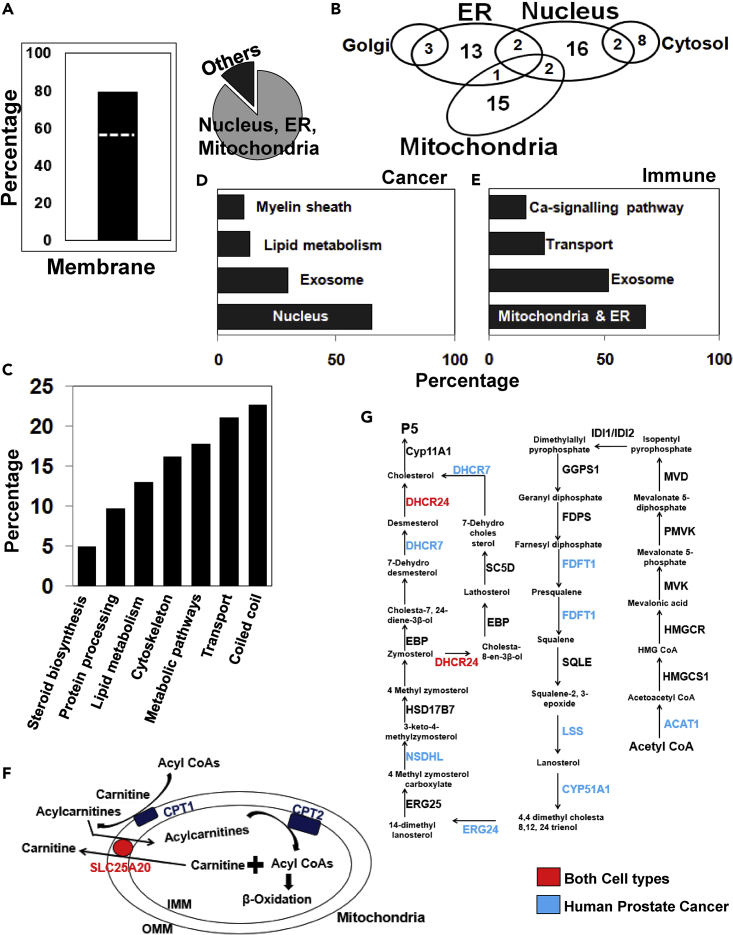
P5-interacting proteins in cancer and immune cells are predominantly localized in organellar membranes
Quantitative mass spectrometry experiments successfully allowed us to profile and categorize P5-interacting proteins. Overall in the two cell types, 49 out of 62 (80%) are membrane proteins, with 27 of the 49 membrane proteins (∼55%) being transmembrane (Figure 4A left panel). In total nuclear, mitochondrial and ER-localized proteins represent 87% of the P5-binding proteins (Figure 4A right panel). Overall, 54 organellar proteins are almost equally distributed among nucleus, mitochondria, and ER (Figure 4B). Interestingly, a majority of the P5-enriched proteins in prostate cancer are annotated as nuclear (65%), whereas ER and mitochondrial (68%) proteins form the majority in CD8+ T cells (Figures 4D and 4E).
Figure 4.
General and distinct P-5-interacting protein annotation in cancer and immune cells
(A) (Left panel) P5-binding protein activity is mediated via membrane proteins, and the majority (55%) of membrane proteins in P5-interactome are transmembrane (dotted line). (Right panel) The P5-interactome acts through the nucleus, mitochondria, and ER.
(B) Compartmentalization of the 54 organellar proteins enriched from the two cell types.
(C) Annotation of the 62 protein high-confidence P5-interactome.
(D) Functional classification of P5-binding proteins from prostate cancer cells.
(E) Functional categorization of P5-binding proteins from CD8+ T cells.
(F) The main carnitine shuttle proteins that regulate β-oxidation are P5 targets. Color codes are cell-type-specific; (red) for both cell types and (blue) for only cancer cells.
(G) Many cholesterol biosynthesis enzymes are P5 targets. The enzymes from each step of the pathway are color coded (same as above), showing common and distinct cell-type-specific P5 targets.
Consistent with a non-genomic mode of action of P5, none of the nuclear-localized proteins are annotated as putative steroid receptors, but we did retrieve the progesterone receptor membrane component 2 (PGRMC2) in the P5 interactome from both cell types. The PGRMC2 family belongs to the unconventional membrane receptor family of the hormone progesterone (P4) (Wendler and Wehling, 2013), a direct product of pregnenolone.
De novo synthesis of P5 in CD8+ T cells indicated P5's role in modulating immune cell plasticity and differentiation (Jia et al., 2013). Seventeen out of twenty-five (70%) of the P5-interacting proteins in CD8+ T cells are localized in membranes, which were annotated to be either mitochondrial or ER.
P5's activity is mediated by metabolic pathways
In common across both cell types, about 18% of the P5-binding proteins represent metabolic enzymes, and 13% belong to lipid metabolism (Figure 4C). Three proteins (5%) are key enzymes that regulate lanosterol and cholesterol biosynthesis. Specific to the prostate cancer cells, 13% of the “P5-binding proteins” belong to lipid metabolism pathways (Figure 4D), which are mostly absent in CD8+ T cells. The list contains key regulators of the fatty acid metabolism pathway, such as the carnitine palmitoyltransferase family proteins CPT1A and CPT2. These enzymes are from the outer and inner mitochondrial membrane, respectively and control the β-oxidation pathway in mitochondria (Figure 4F).
In addition, P5 binds to carnitine acyl carnitine translocase (SLC25A20), the carnitine and acylcarnitine transporter in both LNCaP and CD8+ T cells (Figure 4F). Acyl CoA synthetase ACSL1, which regulates the metabolism of fatty acids through its conversion to respective acyl CoAs, also binds to P5. P5-binding proteins were also found to be involved in ceramide and N-glycan biosynthesis pathways. These metabolic pathways are known to play a key role in prostate cancer progression (Engedal and Saatcioglu, 2001; Melone et al., 2018; Pinho and Reis, 2015). Many enzymes in the cholesterol biosynthesis pathway are found in LNCaP cells or both cell types (Figure 4G). A similar binding pattern has been noted for cholesterol (Hulce et al., 2013).
Considering all 442 P5-binding proteins in LNCaP cells, we also note a significant overrepresentation of other metabolic pathways (Figure S6).
Click-proteomics reveals P5 interactions with cellular transport and cytoskeletal proteins
The classical model of diffusion-mediated steroid transport is not sufficient to explain the steroid trafficking within and across cells, and therefore, specific receptor-mediated transport and signaling mechanisms are gaining importance (Chanphai et al., 2016; Okamoto et al., 2018). To date, the data regarding P5 inter- and intra-cellular transport are very limited. About a fifth (13 out of 62) of the P5 interactome proteins are related to transport (Figure 4C). The voltage-dependent ion channels, VDAC1, 2, and 3 are the primary regulators of metabolite exchange between the mitochondria and the rest of the cell. We retrieved VDAC1, 2, and 3 from both cell types.
In CD8+ T cells, 24% (6 of 25) of the P-interactome are related to transport (Figure 4E). All of these proteins belong to the key ion transport family, and about 16% are involved in calcium signaling (Figure 4E). Calcium signaling is important for ER-mediated stress response and protein folding (Carreras-Sureda et al., 2018).
Twenty-five percent of the 442 LNCaP P5-interactome are related to transport of different classes (Figure S7). This includes three nuclear pore complex proteins, NUP93, NUP210, and NDC1, relevant to nuclear transportation, as well as TMED10, TMED9, and SEC22B, which belong to the ER and Golgi cisternae involved in vesicle trafficking.
We found enrichment of exosome-related proteins in both cell types. Exosomes are membrane-bound extracellular vesicles with key roles in intercellular communication and transport. Exosome exchange can regulate CD8+ T cell function and communication with other immune and tumor cells in the tumor microenvironment (Anel et al., 2019; Li et al., 2006).
NPC1 and SCP2 are cholesterol-binding membrane proteins essential for intercellular cholesterol trafficking in mammals. STEAP1, a highly expressed protein at prostate cancer cell-cell junctions also features in the P5 interactome. This protein is known to function as a channel or transporter at cell-cell junctions.
P5 modulates cytoskeleton by binding to cytoskeletal proteins (Murakami et al., 2000; Weng et al., 2013). Overall, about 16% of the P5-binding proteins are cytoskeletal (Figure 4C), which is consistent with P5's reported role in cytoskeleton organization (Murakami et al., 2000; Weng et al., 2013). Our P5-interactome capture revealed novel proteins such as Vimentin, an intermediate filament protein with a critical role in CD8+ T cell immune response (Li et al., 2015). We also found nuclear skeleton proteins associated with P5, including the lamins LMNA and LMNB1, which play a role in prostate cancer progression (Saarinen et al., 2015).
Discussion
Historically, P5 has been considered as an important molecule because it is the precursor of all functional steroid hormones, but the biological role of P5 itself is still emerging. Its immense importance as a bioactive molecule has been documented in many studies (Mahata et al., 2014; Murugan et al., 2019; Vallée et al., 2014; Weng et al., 2013), yet its full interactome-map is unknown. Therefore, in this study we not only developed a method to profile P5-interacting proteins in live cells but also utilized it to reveal P5's biochemistry in P5-sensitive cancer and P5-producing immune cells. This approach can be applied in any biological context where P5 has a role.
We chose prostate cancer cells as a model because prostate cancer is a major health concern globally (Center et al., 2012; Ferlay et al., 2010), and previously it has been demonstrated definitively that P5 (and not P5 derivatives or P5-derived steroid hormones) drives LNCaP cell growth (Grigoryev et al., 2000). Moreover, intra-tumoural de novo steroidogenesis, either by neoplastic cancer cells (Armandari et al., 2014; Locke et al., 2008), tumour-infiltrating immune cells (Mahata et al., 2020), or osteoblasts (Hagberg Thulin et al., 2016), has been proposed as a driver of castrate-resistant prostate cancer. Therefore, we also studied P5-producing CD8+ T cells, which are primary anti-tumour effector cells in the tumor microenvironment.
Here, we developed biologically active P5 probes that mimic P5's molecular, cellular, and biological activities (Figure 2, S3, and S4). We exploited these probes to identify P5-binding proteins providing detailed insights of P5 function in prostate cancer and immune cells.
Among the three probes we synthesized, P5-C showed the most promising results, and structurally it is the best-suited probe for functional interactions, with both the hydroxyl and carbonyl groups available for reaction with protein molecules in the lysate. The probes enabled us to profile P5-binding proteins, which shed light on two major areas: (1) proteins that are involved in intra- and inter-cellular P5 trafficking and (2) how P5 may exert a biological effect via enzymatic activity as well as the cytoskeleton and membrane protein function.
The detailed mechanisms of how P5 is trafficked within and between cells has hitherto remained obscure. About 20% of the P5-interactome are related to transport (Figures 4C and 4E), which are relevant not only for P5's own transport but also for affecting transport of other biomolecules (Figure S6). VDACs are mitochondrial membrane proteins mediating metabolite exchange between the mitochondria and cytosol. Cholesterol and neurosteroids, including P5 and its derivatives, are known to bind VDACs (Hiller et al., 2008; Hulce et al., 2013) and regulate their activity (Figure S9). At the same time, we note that VDACs have also been reported as common off-targets for the diazirine photoactivable linker (Kleiner et al., 2017).
P5's interaction with cytoskeletal proteins is well established (Murakami et al., 2000; Weng et al., 2013). However, we do not find CLIP 1 in LNCaP or CD8+ T cell pull-down datasets. Data from the Human Protein Atlas (http://www.proteinatlas.org) (Uhlen et al., 2017, 2017, 2017) suggests that prostate cancer and immune cells have very low CLIP1 protein expression. Thus we may not be able to detect CLIP1 in our pulldowns due to the low protein abundance of CLIP1 in these cells. The most interesting among the new cytoskeletal proteins we found is the intermediary filament protein Vimentin. This was found specifically in CD8+ T cells and has a key regulatory role in the immune response during T cell activation (Li et al., 2015). The interaction of Vimentin with P5 is yet to be characterized.
Our data suggest that P5's mode of action seems to be mainly non-genomic, as reported previously for steroid hormones (Davis et al., 2002; Lösel and Wehling, 2003; Weng and Chung, 2016). This is despite a radioactive P5 pull-down experiment that produced a signal around 110 kD (Grigoryev et al., 2000), a molecular weight corresponding to that of the androgen receptor (AR). We did not find evidence of any binding to transcription factors. Our detection of the unconventional P4 membrane receptors progesterone receptor membrane component (PGRMC) 1 and PGMRC2 (Table 1 and ST1) is intriguing, suggesting a possible regulation of P4 non-canonical membrane components by P5.
P5's non-genomic role is evident from its interactome, which contains key proteins playing important roles in reprogramming the metabolic output of the cells. Cancer cells acquire metabolic flexibility, and one point of regulation is through a carnitine shuttle within the mitochondria (Melone et al., 2018). The importance of lipid metabolism in prostate cancer has been well established (Wu et al., 2014). In this context, the presence of carnitine palmitoyltransferase 1A and 2 (CPT1A and CPT2); vital enzymes of the fatty acid oxidation pathway (Bonnefont et al., 2004); and SLC25A20, the carnitine and acylcarnitine mitochondrial transporter (Figure 4F), is intriguing. The presence of several key enzymes of cholesterol biosynthesis (Figure 4G) and transport in P5-interactome indicates the allosteric role of P5 and feedback to its own synthesis pathway.
Consistent with the recognized role of P5 as a neurosteroid (Agís-Balboa et al., 2006), our study retrieved neurodegenerative pathway proteins (Figures 3F and S7). Thus our results provide biochemical targets of P5 that are important to understand not only its function as a neurosteroid but also its regulatory role in neurodegenerative diseases.
Taken together, we have successfully mapped the P5 interactome and captured the general as well as cell-type-specific binding of P5. Apart from P5's emerging role in regulating cytoskeleton organization, we found evidence of its potential role in key metabolic and transport pathways. This could lead to the understanding of steroid hormone regulation of prostate cancer progression and immune cell function. Our study demonstrates that a chemoproteomic approach can be extended to other de novo steroidogenic cell types (e.g. adipocytes, thymic epithelial cells, glial cells and neurons).
Limitations of the study
Functional validation of P5 target proteins is required to dissect their modes of action as mediators of P5 biochemistry. To gain deeper insights into P5's biology in cancer and immune cells, future follow-up studies will be required to experimentally validate the individual P5-protein interactions reported here.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Sarah A. Teichmann (st9@sanger.ac.uk).
Materials availability
This study generated four pregnenolone analogues. The probe backbone was synthesized by a company, and the details for this are in the “transparent methods” file. The linker was generated in the Ley lab on a very limited scale sufficient for the biochemical experiments. All the details of the synthesis and analysis are included in the “transparent methods.”
Data and code availability
Supplementary information and chemical compound information are provided in the section transparent methods. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2019) partner repository with the dataset identifier PXD025574. Database: PXD025574.
Methods
All methods can be found in the accompanying transparent methods supplemental file.
Acknowledgments
We thank Mathew Garnett for providing the LNCaP and U87MG cell lines; Fengtang Yang and the molecular cytogenetics group for their technical help with fluorescence microscopy; and Jyoti Choudhary and Lu Yu, formerly of the proteomics mass spectrometry group of the Wellcome Sanger Institute. We acknowledge the EMBL Proteomics Core Facility for expert help. ACG acknowledge the financial support of the Louis-Jeantet Foundation, Switzerland. The ERC consolidator grant (ThDEFINE, Project ID: 646794) supported this study. SR was supported by a fellowship from the EMBL Interdisciplinary Postdoc (EI3POD) programme under Marie Skłodowska-Curie Actions COFUND (grant number 229597) and Ashoka University, India Individual Research grant for research visits. BM was supported by a CRUK Cancer Immunology grant, UK (Ref. 20193).
Author contributions
SR and SAT designed the experiments. SVL, SAT, SR, and JS designed the experiments related to chemical synthesis of the probes. JS helped SR synthesize the P5 probes, and JS did the analysis of the NMR, IR, and figures of the synthesized probes. SR performed the experiments. BM and JP designed and performed the Th2 and immunoglobulin class switching experiments and helped SR culturing LNCaP cells occasionally. SR wrote the manuscript with help from BM. MLH did the on-bead digestion and mass spectrometry of CD8+ T cells. SAT, SVL, and A-CG supervised the study. All authors commented on and approved the draft manuscript before submission.
Declaration of interests
The authors declare no competing financial interests.
Published: May 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102485.
Contributor Information
Sougata Roy, Email: sougata.roy@ashoka.edu.in.
Sarah A. Teichmann, Email: st9@sanger.ac.uk.
Supplemental information
References
- Agís-Balboa R.C., Pinna G., Zhubi A., Maloku E., Veldic M., Costa E., Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. PNAS. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anel A., Gallego-Lleyda A., de Miguel D., Naval J., Martínez-Lostao L. Role of exosomes in the regulation of T-cell mediated immune responses and in autoimmune disease. Cells. 2019;8:154. doi: 10.3390/cells8020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armandari I., Hamid A.R., Verhaegh G., Schalken J. Intratumoral steroidogenesis in castration-resistant prostate cancer: a target for therapy. Prostate Int. 2014;2:105–113. doi: 10.12954/PI.14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E.E. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Bonnefont J.-P., Djouadi F., Prip-Buus C., Gobin S., Munnich A., Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol. Aspects Med. Carnitine. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Carreras-Sureda A., Pihán P., Hetz C. Calcium signaling at the endoplasmic reticulum: fine-tuning stress responses. Cell Calcium, the role of Ca2+ signals in the regulation of cell death & survival processes in health. Dis. Ther. 2018;70:24–31. doi: 10.1016/j.ceca.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Center M.M., Jemal A., Lortet-Tieulent J., Ward E., Ferlay J., Brawley O., Bray F. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- Chanphai P., Vesper A.R., Bariyanga J., Bérubé G., Tajmir-Riahi H.A. Review on the delivery of steroids by carrier proteins. J. Photochem. Photobiol. B: Biol. 2016;161:184–191. doi: 10.1016/j.jphotobiol.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Davis P.J., Tillmann H.C., Davis F.B., Wehling M. Comparison of the mechanisms of nongenomic actions of thyroid hormone and steroid hormones. J. Endocrinol. Invest. 2002;25:377–388. doi: 10.1007/BF03344022. [DOI] [PubMed] [Google Scholar]
- Engedal N., Saatcioglu F. Ceramide-induced cell death in the prostate cancer cell line LNCaP has both necrotic and apoptotic features. Prostate. 2001;46:289–297. doi: 10.1002/1097-0045(20010301)46:4<289::aid-pros1035>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H.-R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Grigoryev D.N., Long B.J., Njar V.C., Brodie A.H. Pregnenolone stimulates LNCaP prostate cancer cell growth via the mutated androgen receptor. J. Steroid Biochem. Mol. Biol. 2000;75:1–10. doi: 10.1016/s0960-0760(00)00131-x. [DOI] [PubMed] [Google Scholar]
- Hagberg Thulin M., Nilsson M.E., Thulin P., Céraline J., Ohlsson C., Damber J.-E., Welén K. Osteoblasts promote castration-resistant prostate cancer by altering intratumoral steroidogenesis. Mol. Cell Endocrinol. 2016;422:182–191. doi: 10.1016/j.mce.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Hiller S., Garces R.G., Malia T.J., Orekhov V.Y., Colombini M., Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulce J.J., Cognetta A.B., Niphakis M.J., Tully S.E., Cravatt B.F. Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nat. Methods. 2013;10:259–264. doi: 10.1038/nmeth.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Domenico J., Takeda K., Han J., Wang M., Armstrong M., Reisdorph N., O’Connor B.P., Lucas J.J., Gelfand E.W. Steroidogenic enzyme Cyp11a1 regulates Type 2 CD8+ T cell skewing in allergic lung disease. PNAS. 2013;110:8152–8157. doi: 10.1073/pnas.1216671110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner P., Heydenreuter W., Stahl M., Korotkov V.S., Sieber S.A. A whole proteome inventory of background photocrosslinker binding. Angew. Chem. Int. Edition. 2017;56:1396–1401. doi: 10.1002/anie.201605993. [DOI] [PubMed] [Google Scholar]
- Li D., Rebecca P., Cruz M.A., Molldrem J.J., Champlin R.E., Ma Q. Intermediate filament (IF) protein vimentin regulates T cell mediated immune response in Gvhd. Blood. 2015;126:3073. [Google Scholar]
- Li J., Daly E., Campioli E., Wabitsch M., Papadopoulos V. De novo synthesis of steroids and oxysterols in adipocytes. J. Biol. Chem. 2014;289:747–764. doi: 10.1074/jbc.M113.534172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-B., Zhang Z.-R., Schluesener H.J., Xu S.-Q. Role of exosomes in immune regulation. J. Cell Mol. Med. 2006;10:364–375. doi: 10.1111/j.1582-4934.2006.tb00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Hao P., Li L., Tan C.Y.J., Cheng X., Chen G.Y.J., Sze S.K., Shen H.-M., Yao S.Q. Design and synthesis of minimalist terminal alkyne-containing diazirine photo-crosslinkers and their incorporation into kinase inhibitors for cell- and tissue-based proteome profiling. Angew. Chem. Int. Edition. 2013;52:8551–8556. doi: 10.1002/anie.201300683. [DOI] [PubMed] [Google Scholar]
- Locke J.A., Guns E.S., Lubik A.A., Adomat H.H., Hendy S.C., Wood C.A., Ettinger S.L., Gleave M.E., Nelson C.C. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- Lösel R., Wehling M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell Biol. 2003;4:46–55. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- Mahata B., Pramanik J., van der Weyden L., Polanski K., Kar G., Riedel A., Chen X., Fonseca N.A., Kundu K., Campos L.S. Tumors induce de novo steroid biosynthesis in T cells to evade immunity. Nat. Commun. 2020;11:3588. doi: 10.1038/s41467-020-17339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata B., Zhang X., Kolodziejczyk A.A., Proserpio V., Haim-Vilmovsky L., Taylor A.E., Hebenstreit D., Dingler F.A., Moignard V., Göttgens B. Single-cell RNA sequencing reveals T helper cells synthesizing steroids de novo to contribute to immune homeostasis. Cell Rep. 2014;7:1130–1142. doi: 10.1016/j.celrep.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo W., Le Moal M., Abrous D.N. Pregnenolone sulfate and aging of cognitive functions: behavioral, neurochemical, and morphological investigations. Horm. Behav. 2001;40:215–217. doi: 10.1006/hbeh.2001.1677. [DOI] [PubMed] [Google Scholar]
- Mazaira G.I., Zgajnar N.R., Lotufo C.M., Daneri-Becerra C., Sivils J.C., Soto O.B., Cox M.B., Galigniana M.D. The nuclear receptor field: a historical overview and future challenges. Nucl. Receptor Res. 2018;5 doi: 10.11131/2018/101320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon S.H. Neurosteroid regulation of central nervous system development. Pharmacol. Ther. Neurosteroids Spec. Issue. 2007;116:107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone M.A.B., Valentino A., Margarucci S., Galderisi U., Giordano A., Peluso G. The carnitine system and cancer metabolic plasticity. Cell Death Dis. 2018;9:1–12. doi: 10.1038/s41419-018-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.L. Steroidogenesis: Unanswered questions. Trends Endocrinol. Metab. 2017;28:771–793. doi: 10.1016/j.tem.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Miller W.L., Auchus R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Fellous A., Baulieu E.E., Robel P. Pregnenolone binds to microtubule-associated protein 2 and stimulates microtubule assembly. Proc. Natl. Acad. Sci. U S A. 2000;97:3579–3584. doi: 10.1073/pnas.97.7.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan S., Jakka P., Namani S., Mujumdar V., Radhakrishnan G. The neurosteroid pregnenolone promotes degradation of key proteins in the innate immune signaling to suppress inflammation. J. Biol. Chem. 2019;294:4596–4607. doi: 10.1074/jbc.RA118.005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N., Viswanatha R., Bittar R., Li Z., Haga-Yamanaka S., Perrimon N., Yamanaka N. A membrane transporter is required for steroid hormone uptake in Drosophila. Developmental Cell. 2018;47:294–305.e7. doi: 10.1016/j.devcel.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D.J., Inuganti A., Griss J., Mayer G., Eisenacher M. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S.S., Reis C.A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- Press W.H., Teukolsky S.A., Vetterling W.T., Flannery B.P. Second Edition. Cambridge University Press; 1992. Numerical Recipes in C; the Art of Scientific Computing. [Google Scholar]
- Saarinen I., Mirtti T., Seikkula H., Boström P.J., Taimen P. Differential predictive roles of A- and B-type nuclear lamins in prostate cancer progression. PLoS One. 2015;10:e0140671. doi: 10.1371/journal.pone.0140671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyanova S., Cox J. Perseus: a bioinformatics platform for integrative analysis of proteomics data in cancer research. In: von Stechow L., editor. Cancer Systems Biology: Methods and Protocols, Methods in Molecular Biology. Springer; 2018. pp. 133–148. [DOI] [PubMed] [Google Scholar]
- Uhlen M., Zhang C., Lee S., Sjöstedt E., Fagerberg L., Bidkhori G., Benfeitas R., Arif M., Liu Z., Edfors F. A pathology atlas of the human cancer transcriptome. Science. 2017;357 doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- Vallée M., Vitiello S., Bellocchio L., Hébert-Chatelain E., Monlezun S., Martin-Garcia E., Kasanetz F., Baillie G.L., Panin F., Cathala A. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Ramirez J., Han J., Jia Y., Domenico J., Seibold M.A., Hagman J.R., Gelfand E.W. The steroidogenic enzyme Cyp11a1 is essential for development of peanut-induced intestinal anaphylaxis. J. Allergy Clin. Immunol. 2013;132:1174–1183.e8. doi: 10.1016/j.jaci.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler A., Wehling M. PGRMC2, a yet uncharacterized protein with potential as tumor suppressor, migration inhibitor, and regulator of cytochrome P450 enzyme activity. Steroids, FASEB SRC - Steroid Signaling. 2013;78:555–558. doi: 10.1016/j.steroids.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Weng J.-H., Chung B. Nongenomic actions of neurosteroid pregnenolone and its metabolites. Steroids Proc. 9th Int. Meet. Rapid Responses Steroid Horm. (RRSH 2015) 2016;111:54–59. doi: 10.1016/j.steroids.2016.01.017. [DOI] [PubMed] [Google Scholar]
- Weng J.-H., Liang M.-R., Chen C.-H., Tong S.-K., Huang T.-C., Lee S.-P., Chen Y.-R., Chen C.-T., Chung B. Pregnenolone activates CLIP-170 to promote microtubule growth and cell migration. Nat. Chem. Biol. 2013;9:636–642. doi: 10.1038/nchembio.1321. [DOI] [PubMed] [Google Scholar]
- Wu X., Daniels G., Lee P., Monaco M.E. Lipid metabolism in prostate cancer. Am. J. Clin. Exp. Urol. 2014;2:111–120. [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Chen L., Ouyang Y., Zhu W., Qiu P., Su X., Dou Y., Tang L., Yan M., Zhang H. Pregnenolone, a cholesterol metabolite, induces glioma cell apoptosis via activating extrinsic and intrinsic apoptotic pathways. Oncol. Lett. 2014;8:645–650. doi: 10.3892/ol.2014.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary information and chemical compound information are provided in the section transparent methods. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2019) partner repository with the dataset identifier PXD025574. Database: PXD025574.