Key Points
Question
What are the 3-year efficacy and safety results of a regimen with or without anthracyclines with dual ERBB2 (formerly HER2) blockade for stage II and III ERBB2-positive breast cancer?
Findings
The follow-up results of this randomized multicenter phase 3 trial of 438 patients with stage II and III ERBB2-positive breast cancer showed similar 3-year event-free survival estimates of 93% in patients treated with anthracyclines and 94% in patients treated without anthracyclines. Left ventricular ejection fraction decline was more common in the anthracycline group and 2 patients treated with anthracyclines developed acute leukemia.
Meaning
These results add to the literature on omitting anthracyclines in early-stage ERBB2-positive breast cancer.
Abstract
Importance
Primary analysis of the TRAIN-2 study showed high pathologic complete response rates after neoadjuvant chemotherapy with or without anthracyclines plus dual ERBB2 (formerly HER2) blockade.
Objective
To evaluate 3-year event-free survival (EFS) and overall survival (OS) of an anthracycline-free and anthracycline-containing regimen with dual ERBB2 blockade in patients with stage II and III ERBB2-positive breast cancer.
Design, Setting, and Participants
A total of 438 patients with stage II and III ERBB2-positive breast cancer were enrolled in this randomized, clinical, open-label phase 3 trial across 37 hospitals in the Netherlands from December 9, 2013, until January 14, 2016. Follow-up analyses were performed after a median follow-up of 48.8 months (interquartile range, 44.1-55.2 months). Analysis was performed on an intention-to-treat basis.
Interventions
Participants were randomly assigned on a 1:1 basis, stratified by age, tumor stage, nodal stage, and estrogen receptor status, to receive 3 cycles of fluorouracil (500 mg/m2), epirubicin (90 mg/m2), and cyclophosphamide (500 mg/m2), followed by 6 cycles of paclitaxel and carboplatin or 9 cycles of paclitaxel (80 mg/m2 days 1 and 8) and carboplatin (area under the concentration-time curve, 6 mg/mL/min). Both groups received trastuzumab (6 mg/kg; loading dose 8 mg/kg) and pertuzumab (420 mg intravenously; loading dose 840 mg) every 3 weeks.
Main Outcomes and Measures
Three-year EFS, OS, and safety.
Results
A total of 438 women were randomized, with 219 per group (anthracycline group, median age, 49 years [interquartile range, 43-55 years]; and nonanthracycline group, median age, 48 years [interquartile range, 43-56 years]). A total of 23 EFS events (10.5%) occurred in the anthracycline group and 21 EFS events (9.6%) occurred in the nonanthracycline group (hazard ratio, 0.90; 95% CI, 0.50-1.63; favoring nonanthracyclines). Three-year EFS estimates were 92.7% (95% CI, 89.3%-96.2%) in the anthracycline group and 93.6% (95% CI, 90.4%-96.9%) in the nonanthracycline group and 3-year OS estimates were 97.7% (95% CI, 95.7%-99.7%) in the anthracycline group and 98.2% (95% CI, 96.4%-100%) in the nonanthracycline group. The results were irrespective of hormone receptor and nodal status. A decline in left ventricular ejection fraction of 10% or more from baseline to less than 50% was more common in patients who received anthracyclines than those who did not (17 of 220 [7.7%] vs 7 of 218 [3.2%]; P = .04). Two patients treated with anthracyclines developed acute leukemia.
Conclusions and Relevance
This follow-up analysis of the TRAIN-2 study shows similar 3-year EFS and OS estimates with or without anthracyclines in patients with stage II and III ERBB2-positive breast cancer. Anthracycline use is associated with increased risk of febrile neutropenia, cardiotoxic effects, and secondary malignant neoplasms.
Trial Registration
ClinicalTrials.gov Identifier: NCT01996267
This randomized clinical trial evaluates 3-year event-free survival and overall survival of an anthracycline-free and anthracycline-containing regimen with dual ERBB2 blockade in patients with stage II and III ERBB2-positive breast cancer.
Introduction
Combination treatment with anthracyclines and trastuzumab causes cardiac toxic effects in patients with ERBB2 (formerly HER2; OMIM 164870)–positive breast cancer.1 Because of the important antitumor activity of anthracyclines in ERBB2-positive breast cancer, many clinicians are nevertheless reluctant to omit anthracyclines from their patients’ treatment regimens. The noncomparative TRYPHAENA study, the BCIRG (Breast Cancer International Research Group)-006 study, and the TRAIN-2 study evaluated anthracycline-free regimens in patients with ERBB2-positive early breast cancer.1,2,3,4 TRYPHAENA was a neoadjuvant cardiac safety study that reported similar pathologic complete response (pCR) rates with and without anthracyclines in the presence of trastuzumab and pertuzumab.4 The BCIRG-006 study was an adjuvant trial that showed comparable long-term survival for patients treated either with doxorubicin and cyclophosphamide followed by docetaxel plus trastuzumab or with carboplatin, docetaxel, and trastuzumab (TCH).2 The primary analysis of TRAIN-2 showed high pCR rates in breast and axilla (ypT0/is, ypN0) after treatment with and without anthracyclines (67% vs 68%).3 Patients in TRAIN-2 who received anthracyclines experienced more febrile neutropenia, hypokalemia, and left ventricular ejection fraction (LVEF) decline to grade 2 or worse (≥10% or to <50%). Pathologic complete response rates in TRAIN-2 are among the highest in neoadjuvant trials with dual ERBB2 blockade.5,6,7,8,9
Here, we report analyses of the secondary end points of event-free survival (EFS) and overall survival (OS) and updated safety analyses in TRAIN-2. We also report exploratory subgroup analyses for EFS to evaluate if patients with a higher risk of breast cancer recurrence benefit from anthracyclines. Finally, we examine the association between pCR and disease-free survival (DFS).
Methods
Study Design and Participants
The study design and primary outcome results were previously reported.3 TRAIN-2 was a multicenter, open-label, randomized phase 3 trial. Patients were aged 18 years or older with stage II and III ERBB2-positive breast cancer, World Health Organization status of 0 to 1, and LVEF of 50% or more.3 TRAIN-2 was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.10 The medical ethics committee of the Netherlands Cancer Institute approved the study protocol and all amendments. All participants signed written informed consent before any study-related procedure. The trial protocol is available online3 and in Supplement 1. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Randomization and Masking
Patients were centrally randomized on a 1:1 basis to receive the anthracycline-containing regimen or the nonanthracycline-containing regimen. Stratification factors were primary tumor stage (T0-2 vs T3-4), nodal stage (negative vs positive), estrogen receptor status (<10% vs ≥10%), and age (<50 vs ≥50 years).
Procedures
Patients in the nonanthracycline group received nine 3-week cycles of paclitaxel (80 mg/m2 on days 1 and 8) and carboplatin (area under the concentration-time curve [AUC], 6 mg/mL/min on day 1 or AUC, 3 mg/mL/min on days 1 and 8). Patients in the anthracycline group received 3 cycles of fluorouracil (500 mg/m2), epirubicin (90 mg/m2), and cyclophosphamide (500 mg/m2) intravenously for 3 weeks followed by 6 cycles of paclitaxel and carboplatin in the same schedule as in the nonanthracycline group. Trastuzumab (6 mg/kg; loading dose 8 mg/kg) and pertuzumab (420 mg intravenously; loading dose 840 mg) were concurrently administered with chemotherapy every 3 weeks to both groups.3
Breast cancer diagnosis including ERBB2 status and estrogen and progesterone receptor expression was histologically confirmed with core biopsies from the primary tumor before start of treatment. A marker (eg, iodine seed or twist marker) was placed at the primary tumor site in all patients. Baseline nodal status was assessed and screening for distant metastasis was required.3
Evaluation of toxic effects was until 30 days after study treatment and thereafter if considered related to the study. Adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.11 Left ventricular ejection fraction was measured every 3 months during ERBB2 blockade. No measurement after treatment was required, per protocol.3 Breast-conserving or ablative surgery combined with axillary staging (sentinel node procedure, axillary lymph node dissection, or selective removal of initially positive and marked lymph node) was performed within 6 weeks after the last dose of chemotherapy. Adjuvant radiotherapy and endocrine therapy was given according to the Dutch national guidelines.12 Adjuvant trastuzumab was continued to complete 1 year of treatment.
Outcomes
The primary pCR end point of TRAIN-2 was previously reported.3 Here, we report the 3-year EFS and OS estimates as well as updated safety data. Event-free survival is defined as the time from randomization to disease progression resulting in inoperability, recurrence (contralateral ductal carcinoma in situ excluded), secondary primary malignant neoplasms, or death by any cause (equal to progression-free survival). Overall survival was defined as the time from randomization to death from any cause. We performed exploratory subgroup analyses based on hormone receptor status, lymph node status, and disease stage. We also analyzed DFS from surgery to disease recurrence or death from any cause in subgroups based on pathologic response. Other secondary end points were recurrence-free survival and breast cancer–specific survival.
Statistical Analysis
TRAIN-2 is not powered to detect treatment differences for secondary end points. Results of these analyses are for descriptive purposes. All randomly assigned patients were included in the intention-to-treat analysis for EFS and OS. All patients who underwent surgery are included in the analysis for DFS. We estimated 3-year EFS, DFS, and OS rates using the Kaplan-Meier method. Patients who were lost to follow-up were censored at their last known visit. We used the Cox proportional hazards regression model to estimate hazard ratios (HRs) and corresponding 95% CIs. Subgroup analyses by age, hormone receptor status, disease stage, nodal status and grade, and DFS analysis by pCR were performed using Cox proportional hazards regression models including subgroup, treatment, and subgroup-treatment interaction. We analyzed safety according to the treatment actually received and included all patients who received 1 cycle of study medication. The follow-up period for safety is defined from time of randomization until breast cancer recurrence, distant metastasis, or death. All statistical analyses were performed using R, version 3.5.0 (R Group for Statistical Computing).13 All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
Between December 9, 2013, and January 14, 2016, 438 women were randomly assigned across 37 Dutch hospitals, with 219 per group (anthracycline group, median age, 49 years [interquartile range, 43-55 years]; nonanthracycline group, median age, 48 years [interquartile range, 43-56 years]). Baseline characteristics were balanced between the treatment groups (eTable 1 in Supplement 2). Median follow-up was 48.8 months (interquartile range, 44.1-55.2 months). A total of 255 patients (58.2%) had hormone receptor–positive disease and 147 (33.6%) had stage III disease. Six randomly assigned patients did not meet the eligibility criteria (stage I disease, stage IV disease, ERBB2-negative disease, concurrent second primary tumor, and 2 times concurrent contralateral breast cancer), but were included in the intention-to-treat analyses. Seven patients in the anthracycline group and 13 in the nonanthracycline group were not evaluable for the primary end point. All patients were included in the survival analyses (eFigure 1 in Supplement 2).
Neoadjuvant treatment information was reported previously.3 A total of 195 of 219 patients (89.0%) in the anthracycline group and 213 of 219 patients (97.3%) in the nonanthracycline group completed 1 year of trastuzumab treatment. Reasons for discontinuation of adjuvant trastuzumab in patients treated with anthracyclines were cardiotoxic effects (15 of 216 [6.9%]), patient’s request (4 of 216 [1.9%]) or unknown (1 of 216 [0.5%]). In the nonanthracycline group, 1 patient (0.5%) stopped adjuvant trastuzumab because of peripheral neuropathy and 1 patient (0.5%) stopped adjuvant trastuzumab because the tumor was ERBB2 negative on reevaluation. Three of the 219 patients (1.4%) in the anthracycline group and 4 of 219 patients (1.8%) in the nonanthracycline group received adjuvant chemotherapy. The use of adjuvant endocrine therapy and radiotherapy was similar between the treatment groups (eTable 2 in Supplement 2).
Four years after inclusion of the last patient, 44 of 438 patients (10.0%) had experienced an event. An event occurred in 23 of 219 patients (10.5%) in the anthracycline group vs 21 of 219 (9.6%) in the nonanthracycline group. The estimated 3-year EFS percentages were 92.7% (95% CI, 89.3%-96.2%) in the anthracycline group and 93.6% (95% CI, 90.4%-96.9%) in patients treated without anthracyclines (HR, 0.90 [95% CI, 0.50-1.63], favoring nonanthracycline) (Figure 1A). Nine of 219 patients (4.1%) in the anthracycline group and 8 of 219 patients (3.7%) in the nonanthracycline group had died. One patient treated with anthracyclines died during neoadjuvant treatment owing to a thromboembolic event. Sixteen deaths occurred during follow-up, of which 15 were due to progressive disease. One patient in the nonanthracycline group died of lung cancer. The 3-year OS percentages were 97.7% (95% CI, 95.7%-99.7%) in the anthracycline group and 98.2% (95% CI, 96.4%-100%) in the nonanthracycline group (HR, 0.91 [95% CI, 0.35-2.36]) (Figure 1B). Three-year recurrence-free survival and breast cancer–specific survival rates were consistent with EFS and OS (eFigure 2 in Supplement 2).
Figure 1. Event-Free Survival and Overall Survival According to Treatment Group.
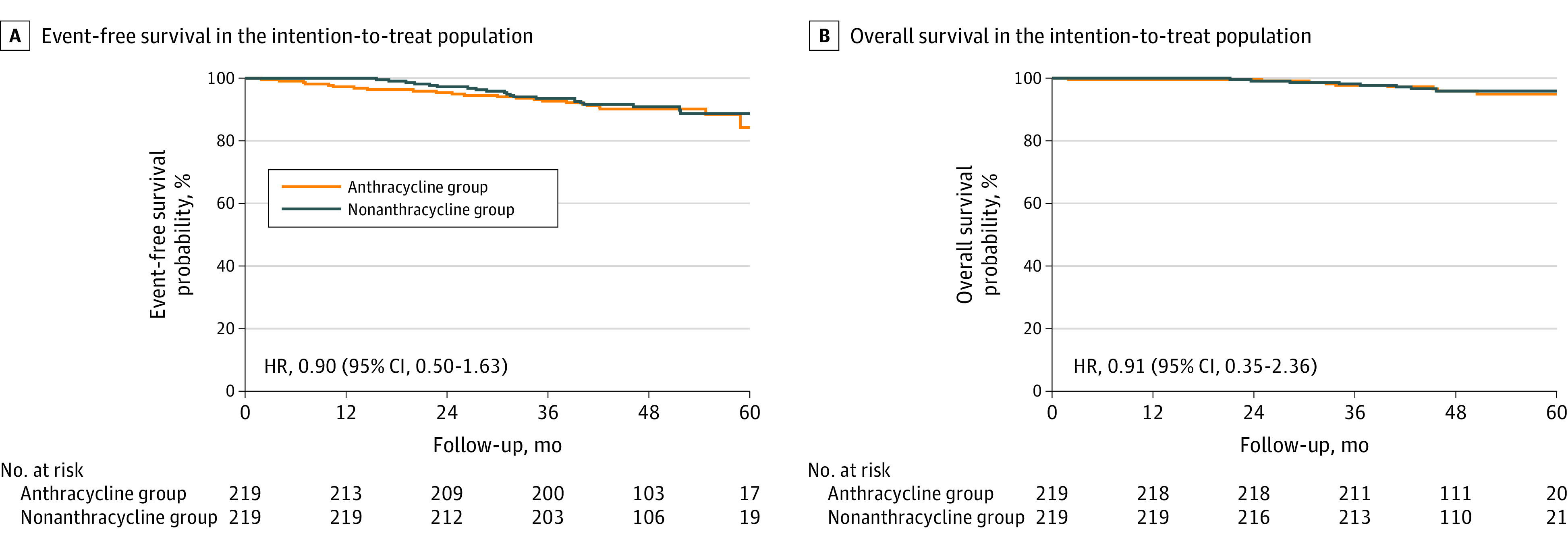
A, Kaplan-Meier estimates of event-free survival in the intention-to-treat population, 4 years after randomization of the last patient. B, Kaplan-Meier estimates of overall survival in the intention-to-treat population, 4 years after randomization of the last patient. HR indicates hazard ratio.
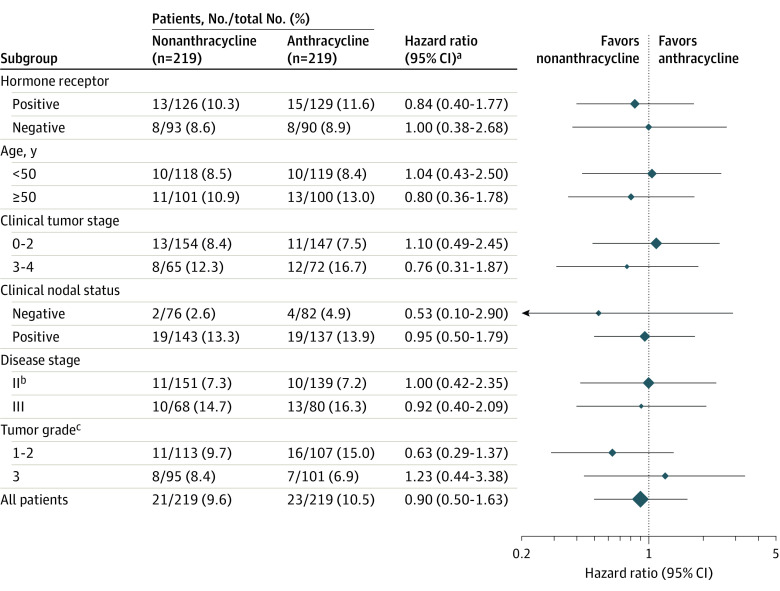
Exploratory subgroup analyses showed a similar risk of an event in patients treated with or without anthracyclines across prespecified and post hoc subgroups including hormone receptor status, lymph node status (N– vs N+ and N0 vs N1 vs N2 or N3), tumor stage (T0-2 vs T3-4), disease stage (II vs III), or grade (1-2 vs 3) (Figure 2).
Figure 2. Subgroup Analysis Event-Free Survival According to Treatment Arm.
Hazard ratios are for event-free survival in Cox proportional hazards regression models (including subgroup, treatment, and interaction) including 95% CI (P value for interaction not given because the analysis is descriptive). The sizes of the data markers are proportional to the number of patients.
aHazard ratio less than 1 favors nonanthracyclines.
bDisease stage II includes 1 patient with stage I disease (nonanthracycline group) and disease stage III includes 1 patient with stage IV disease (anthracycline group).
cTumor grade was missing for 11 patients in both groups.
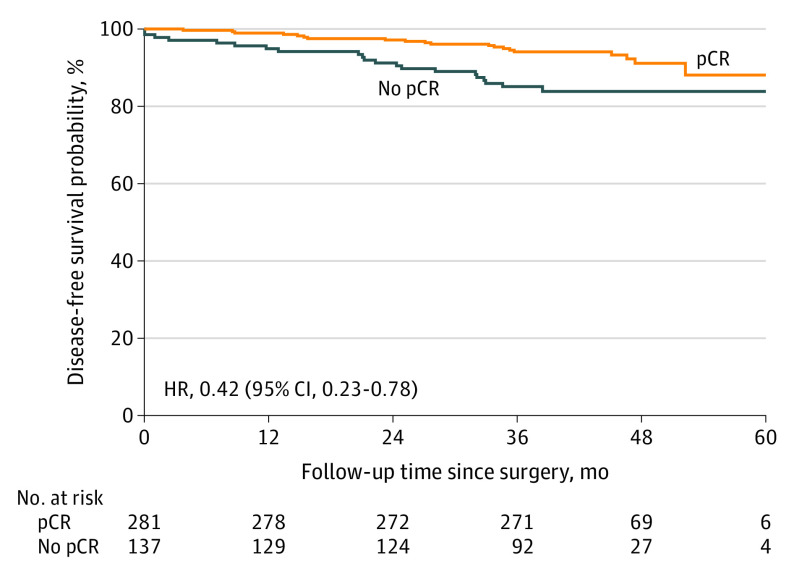
Pathologic complete response was associated with DFS. In patients who did not reach a pCR of the breast and axillary lymph nodes, 21 of 137 (15.3%) progressed or died, vs 20 of 281 patients (7.1%) who had a pCR (HR, 0.42 [95% CI, 0.23-0.78]; P = .006) (Figure 3). This association seemed more pronounced in hormone receptor–negative patients (HR, 0.25 [95% CI, 0.08-0.75]) than in hormone receptor–positive patients (HR, 0.56 [95% CI, 0.26-1.20]; P = .23), but did not differ within the treatment groups (anthracycline group: HR, 0.44 [95% CI, 0.19-1.03] vs nonanthracycline group: HR, 0.40 [95% CI, 0.16-0.98]; P = .87) (eFigure 3 in Supplement 2).
Figure 3. Disease-Free Survival According to Response.
Kaplan-Meier estimates of disease-free survival, 4 years after randomization of the last patient by pathologic complete response (pCR). HR indicates hazard ratio.
One patient allocated to the nonanthracycline treatment group received anthracyclines and was therefore assessed accordingly for safety analysis. Most grade 3 or higher adverse events and serious adverse events are updated for the overall study period (eTables 3-5 in Supplement 2), but most occurred during neoadjuvant chemotherapy.3 Adverse events reported after surgery were mostly wound infections and complications after surgery and cardiac events. Decline of LVEF of 10% or more from baseline and LVEF of less than 50% at any time was reported more frequently in the anthracycline group (17 of 220 [7.7%]) than in the nonanthracycline group (7 of 218 [3.2%]; P = .04). Grade 2 or worse LVEF decline according to CTCAE, version 4.3 (LVEF decline ≥10% or LVEF<50%) was reported in 80 of 220 patients (36.4%) in the anthracycline group vs 49 of 218 patients (22.5%) in the group treated without anthracyclines. A total of 36 of 78 patients (46.2%) treated with anthracyclines and 16 of 48 patients (33.3%) treated without anthracyclines had grade 3 LVEF decline at the last visit. Follow-up LVEF measurement was missing for 3 of 129 patients with a grade 2 or worse LVEF decline. Two patients treated with anthracyclines developed secondary acute leukemia, 111 days and 1067 days after the last chemotherapy administration. Both events were considered chemotherapy related.
Discussion
The 3-year follow-up of the TRAIN-2 study showed high EFS (92.7% vs 93.6%) and OS (97.7% vs 98.2%) in patients with stage II and III ERBB2-positive breast cancer treated with or without anthracyclines. These results are in line with the outcome of the primary end point that showed similar pCR rates in the 2 treatment groups. In addition, we found no indication that patients with a higher risk of breast cancer recurrence derive benefit from anthracyclines. Anthracycline use was associated with higher incidence of febrile neutropenia and cardiotoxic effects, leading to premature discontinuation of trastuzumab. In addition, 2 patients developed chemotherapy-related acute leukemia after receiving anthracyclines.
ERBB2-Positive breast cancer is historically considered to be specifically sensitive to anthracyclines owing to TOP2A gene (OMIM 126430) coamplification or deletion.14 However, clinical studies evaluating TOP2A alterations as a predictive biomarker for anthracycline benefit show conflicting results.15 TOP2A alterations and ERBB2 amplification and CEP17 duplication may be markers of increased chemotherapy sensitivity in general, rather than a predictor of anthracycline benefit.
Various clinical trials indicate that patients with ERBB2-positive breast cancer may not require anthracyclines. Tolaney and colleagues16,17 showed excellent long-term follow-up results in patients with small node–negative disease treated with trastuzumab and paclitaxel. The 10-year follow-up of the BCIRG-006 showed similar DFS and OS and worse toxic effects with an anthracycline-based regimen compared with TCH, although publication of these long-term results is awaited.2 TRYPHAENA was a noncomparative trial and reported a 3-year progression-free survival rate of 89% in the anthracycline group vs 87% in the anthracycline-free group in the presence of dual ERBB2 blockade.18 The follow-up results of TRAIN-2 are in line with those of other trials that used dual ERBB2 blockade.9,19,20,21 The publication of the KATHERINE trial led to a change in practice for many patients with residual invasive disease after neoadjuvant therapy.22 Adjuvant trastuzumab emtansine was not available for patients in TRAIN-2 and different long-term outcomes might have been observed. However, the KATHERINE trial did not report a differential effect of trastuzumab emtansine for patients with or without prior use of anthracyclines.22
The mechanism of synergistic cardiotoxic effects with anthracycline and trastuzumab is not completely understood, but the interference of trastuzumab with myocyte repair and anthracycline-induced direct myocyte damage jointly appear to increase the risk of presymptomatic and clinically relevant heart failure. Changes in the neureguline 1 pathway after the binding of trastuzumab to the ERBB2 receptor on cardiomyocytes increase the susceptibility of myofilaments to anthracyclines.23,24 In BCIRG-006, 2% of the patients treated with doxorubicin plus cyclophosphamide followed by docetaxel plus trastuzumab vs 0.4% of the patients treated with the TCH regimen developed grade 3 or 4 congestive heart failure.25 Also, 18.6% of the patients developed an LVEF decline of 10% or more in the group treated with anthracyclines, similar to what we found in TRAIN-2. The TRYPHAENA study showed data only for an LVEF decline of 10% to less than 50% and symptomatic left ventricular systolic dysfunction and found no substantial difference in cardiac toxic effects with or without anthracyclines.4,18 Although LVEF decline is often (partially) reversible, long-term effects of asymptomatic LVEF decline are largely unknown and could be important, especially in younger women. Therefore, it remains important to study the long-term clinical relevance of asymptomatic LVEF decline.4,26,27
Given the improvements in survival for patients with ERBB2-positive breast cancer, de-escalation of chemotherapy is an attractive approach to decrease toxic effects. In the NEOSPHERE study, 17% of the patients treated with 4 cycles of trastuzumab and pertuzumab achieved a pCR, suggesting that chemotherapy could be omitted in some patients.6 The KRISTINE trial, however, showed that patients randomly assigned to receive trastuzumab emtansine plus pertuzumab for 1 year had a higher risk of progression or recurrence than patients treated with TCH plus pertuzumab followed by 1 year of dual ERBB2 blockade.21 The PHERGain study showed a 37.9% pCR rate in patients treated with 8 cycles of trastuzumab and pertuzumab (plus endocrine therapy if they had hormone receptor–positive cancer) and who were positron emission tomography responders after 2 cycles.28 Considering the results of the TRAIN-2 study, we started the TRAIN-3 study (NCT03820063). TRAIN-3 is a multicenter phase 2 trial that investigates the hypothesis that patients who achieve complete radiologic remission after 3 or 6 cycles of anthracycline-free chemotherapy combined with dual ERBB2 blockade are candidates for early surgery without compromising long-term outcome.
Limitations
Some limitations of the present analyses must be acknowledged. First, the study was not powered to detect differences in EFS and OS; these results are therefore descriptive. Second, the neoadjuvant treatment regimen included 9 cycles, while most commonly used anthracycline-free regimens have 6 cycles. We designed this regimen to match the duration of common anthracycline-based regimens. Third, the dosing of epirubicin was 270 mg/m2, while 360 mg/m2 would have been more equivalent to commonly used regimens. This lower dose may have resulted in suboptimal efficacy. In addition, a dose-dense schedule could have been a more effective way to administer anthracyclines. Follow-up data of the BERENICE study will provide more data to discuss the safety and toxic effects of dose-dense schedules in the context of ERBB2-positive breast cancer.8 Fourth, the follow-up of the TRAIN-2 study is too short for final evaluation, especially in hormone receptor–positive patients.
Conclusions
This follow-up analysis of the TRAIN-2 study shows similar 3-year EFS and OS with or without anthracyclines in patients with stage II and III ERBB2-positive breast cancer. Anthracycline use was associated with an increased risk of febrile neutropenia, cardiotoxic effects, and secondary malignant neoplasms. These results add to the literature on omitting anthracyclines in patients with early-stage ERBB2-positive breast cancer.
Trial Protocol
eFigure 1. CONSORT Diagram
eTable 1. Baseline Characteristics According to Treatment Group
eTable 2. Adjuvant Treatment
eTable 3. List of First Events According to Treatment Allocation
eFigure 2. Recurrence-Free Survival and Breast Cancer–Specific Survival
eFigure 3. Disease-Free Survival
eTable 4. Most Common Grade 3 and Worse Adverse Events in the Overall Study Period
eTable 5. Most Common Serious Adverse Events in the Overall Study Period
eTable 6. Any Cause Adverse Events in Safety Population in the Overall Treatment Period
Data Sharing Statement
References
- 1.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-792. doi: 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 2.Slamon D, Eiermann W, Robert N, et al. Abstract S5-04: ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Cancer Res. 2016;76 (suppl 4):S5-04. doi: 10.1158/1538-7445.SABCS15-S5-04 [DOI] [Google Scholar]
- 3.van Ramshorst MS, van der Voort A, van Werkhoven ED, et al. ; Dutch Breast Cancer Research Group (BOOG) . Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(12):1630-1640. doi: 10.1016/S1470-2045(18)30570-9 [DOI] [PubMed] [Google Scholar]
- 4.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24(9):2278-2284. doi: 10.1093/annonc/mdt182 [DOI] [PubMed] [Google Scholar]
- 5.Baselga J, Bradbury I, Eidtmann H, et al. ; NeoALTTO Study Team . Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633-640. doi: 10.1016/S0140-6736(11)61847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25-32. doi: 10.1016/S1470-2045(11)70336-9 [DOI] [PubMed] [Google Scholar]
- 7.Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19(1):115-126. doi: 10.1016/S1470-2045(17)30716-7 [DOI] [PubMed] [Google Scholar]
- 8.Swain SM, Ewer MS, Viale G, et al. ; BERENICE Study Group . Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29(3):646-653. doi: 10.1093/annonc/mdx773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Martinez A, Krop IE, Hillman DW, et al. Survival, pathologic response, and genomics in CALGB 40601 (Alliance), a neoadjuvant phase III trial of paclitaxel-trastuzumab with or without lapatinib in HER2-positive breast cancer. J Clin Oncol. 2020;38(35):4184-4193. doi: 10.1200/JCO.20.01276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute, Division of Cancer Treatment and Diagnosis. Common Terminology Criteria for Adverse Events (CTCAE). Published June 14, 2010. Accessed January 5, 2016. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- 12.Federatie Medisch Specialisten. Richtlijnendatabase. Accessed April 12, 2021. https://richtlijnendatabase.nl/richtlijn/borstkanker/algemeen.html
- 13.The R Project for Statistical Computing. Accessed April 9, 2021. http://www.r-project.org/ [Google Scholar]
- 14.Muss HB, Thor AD, Berry DA, et al. c-erbB-2 Expression and response to adjuvant therapy in women with node-positive early breast cancer. N Engl J Med. 1994;330(18):1260-1266. doi: 10.1056/NEJM199405053301802 [DOI] [PubMed] [Google Scholar]
- 15.Konecny GE, Pauletti G, Untch M, et al. Association between HER2, TOP2A, and response to anthracycline-based preoperative chemotherapy in high-risk primary breast cancer. Breast Cancer Res Treat. 2010;120(2):481-489. doi: 10.1007/s10549-010-0744-z [DOI] [PubMed] [Google Scholar]
- 16.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372(2):134-141. doi: 10.1056/NEJMoa1406281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolaney SM, Barry WT, Guo H, et al. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC). J Clin Oncol. 2017;35(suppl):511. doi: 10.1200/JCO.2017.35.15_suppl.511 [DOI] [Google Scholar]
- 18.Schneeweiss A, Chia S, Hickish T, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Cancer. 2018;89:27-35. doi: 10.1016/j.ejca.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 19.Gianni L, Pienkowski T, Im YH, et al. 5-Year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791-800. doi: 10.1016/S1470-2045(16)00163-7 [DOI] [PubMed] [Google Scholar]
- 20.de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15(10):1137-1146. doi: 10.1016/S1470-2045(14)70320-1 [DOI] [PubMed] [Google Scholar]
- 21.Hurvitz SA, Martin M, Jung KH, et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2–positive breast cancer: three-year outcomes from the phase III KRISTINE Study. J Clin Oncol. 2019;37(25):2206-2216. doi: 10.1200/JCO.19.00882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Minckwitz G, Huang CS, Mano MS, et al. ; KATHERINE Investigators . Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi: 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 23.Guglin M, Cutro R, Mishkin JD. Trastuzumab-induced cardiomyopathy. J Card Fail. 2008;14(5):437-444. doi: 10.1016/j.cardfail.2008.02.002 [DOI] [PubMed] [Google Scholar]
- 24.Nicolazzi MA, Carnicelli A, Fuorlo M, et al. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur Rev Med Pharmacol Sci. 2018;22(7):2175-2185. [DOI] [PubMed] [Google Scholar]
- 25.Slamon D, Eiermann W, Robert N, et al. ; Breast Cancer International Research Group . Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-1283. doi: 10.1056/NEJMoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantarro S, Rossi M, Bonifazi M, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med. 2016;11(1):123-140. doi: 10.1007/s11739-015-1362-x [DOI] [PubMed] [Google Scholar]
- 27.Bonneterre J, Roché H, Kerbrat P, et al. Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French adjuvant study group. J Clin Oncol. 2004;22(15):3070-3079. doi: 10.1200/JCO.2004.03.098 [DOI] [PubMed] [Google Scholar]
- 28.Cortes J, Gebhart G, Borrego MR, et al. Chemotherapy (CT) de-escalation using an FDG-PET/CT (F-PET) and pathological response-adapted strategy in HER2[+] early breast cancer (EBC): PHERGain Trial. J Clin Oncol. 2020;38(suppl 15):503. doi: 10.1200/JCO.2020.38.15_suppl.503 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. CONSORT Diagram
eTable 1. Baseline Characteristics According to Treatment Group
eTable 2. Adjuvant Treatment
eTable 3. List of First Events According to Treatment Allocation
eFigure 2. Recurrence-Free Survival and Breast Cancer–Specific Survival
eFigure 3. Disease-Free Survival
eTable 4. Most Common Grade 3 and Worse Adverse Events in the Overall Study Period
eTable 5. Most Common Serious Adverse Events in the Overall Study Period
eTable 6. Any Cause Adverse Events in Safety Population in the Overall Treatment Period
Data Sharing Statement