Key Points
Question
What is the natural course of geographic atrophy (GA) enlargement in the long term and on life expectancy and visual course?
Findings
In this study including 171 participants from 4 cohort studies, more than one-third of patients presented with foveal GA, and three-fourths of eyes with nonfoveal GA developed foveal GA a mean of 5.6 years after presentation. Enlargement rates varied widely but was on average 1.09 mm2 per year, and moderate to severe visual impairment was present in 66% of eyes with GA before death.
Meaning
In this study, enlargement of GA varied widely; intervention studies may have an average of 5 years to inhibit progression and preserve visual acuity.
This cohort study investigates the natural course of geographic atrophy enlargement on long-term life expectancy and visual course.
Abstract
Importance
Treatments for geographic atrophy (GA), a late stage of age-related macular degeneration (AMD), are currently under development. Understanding the natural course is needed for optimal trial design. Although enlargement rates of GA and visual acuity (VA) in the short term are known from clinical studies, knowledge of enlargement in the long term, life expectancy, and visual course is lacking.
Objective
To determine long-term enlargement of GA.
Design, Setting, and Participants
In this study, participant data were collected from 4 population-based cohort studies, with up to 25 years of follow-up and eye examinations at 5-year intervals: the Rotterdam Study cohorts 1, 2, and 3 and the Blue Mountains Eye Study. Data were collected from 1990 to 2015, and data were analyzed from January 2019 to November 2020.
Main Outcomes and Measures
Area of GA was measured pixel by pixel using all available imaging. Area enlargement and enlargement of the square root–transformed area, time until GA reached the central fovea, and time until death were assessed, and best-corrected VA, smoking status, macular lesions according to the Three Continent AMD Consortium classification, a modified version of the Wisconsin age-related maculopathy grading system, and AMD genetic variants were covariates in Spearman, Pearson, or Mann-Whitney analyses.
Results
Of 171 included patients, 106 (62.0%) were female, and the mean (SD) age at inclusion was 82.6 (7.1) years. A total of 147 of 242 eyes with GA (60.7%) were newly diagnosed in our study. The mean area of GA at first presentation was 3.74 mm2 (95% CI, 3.11-4.67). Enlargement rate varied widely between persons (0.02 to 4.05 mm2 per year), with a mean of 1.09 mm2 per year (95% CI, 0.89-1.30). Stage of AMD in the other eye was correlated with GA enlargement (Spearman ρ = 0.34; P = .01). Foveal involvement was already present in incident GA in 55 of 147 eyes (37.4%); 23 of 42 eyes (55%) developed this after a mean (range) period of 5.6 (3-12) years, and foveal involvement did not develop before death in 11 of 42 eyes (26%). After first diagnosis, 121 of 171 patients with GA (70.8%) died after a mean (SD) period of 6.4 (5.4) years. Visual function was visually impaired (less than 20/63) in 47 of 107 patients (43.9%) at last visit before death.
Conclusions and Relevance
In this study, enlargement of GA appeared to be highly variable in the general population. More than one-third of incident GA was foveal at first presentation; those with extrafoveal GA developed foveal GA after a mean of 5.6 years. Future intervention trials should focus on recruiting those patients who have a high chance of severe visual decline within their life expectancy.
Introduction
Geographic atrophy (GA), the dry late stage of age-related macular degeneration (AMD), is a growing problem due to the aging population, especially in the European population. GA is characterized by atrophy of the retinal pigment epithelium (RPE) along with photoreceptor and choriocapillaris loss, which progresses over time. The resulting visual loss can as yet not be prevented,1,2 but promising therapies are underway. More than 50 trials are currently ongoing or have recently been completed, some with very exciting results.3,4,5 The primary end point that most of these trials use is growth rate of the atrophic lesion during a follow-up period varying between 6 months and 6 years.6,7,8,9,10,11 More meaningful for patients and clinicians, however, would be the visual prognosis for the rest of the patients’ lives.12 Enlargement rate over a longer period of time, time until GA reaches the central fovea, and the correlation between enlargement of GA and life expectancy is currently unclear. To improve estimations of GA progression, large studies with long follow-up periods are needed, and combining prospective studies with harmonized data may facilitate this. The current study uses data from 4 population-based cohort studies performed on 2 different continents that have conducted follow-up for up to 3 decades. This provided a unique opportunity to address these questions.
Methods
Study Populations
Participants were included from 4 population-based cohorts; Rotterdam Study (RS) cohorts 1 (n = 7983), 2 (n = 3011), and 3 (n = 3932) and the Blue Mountains Eye Study (BMES; n = 3654).13,14 Persons 55 years and older (RS cohorts 1 and 2) and 45 years and older (RS cohort 3) were recruited from Rotterdam, the Netherlands, and residents 49 years and older (BMES) were recruited from 2 postcode regions west of Sydney, Australia (eMethods 1 in the Supplement). In brief, cohorts had follow-up visits every 5 years from 1990 (RS) and 1992 (BMES) onwards; total follow-up was up to 25 years for Dutch studies and 15 years for the BMES cohort. Both studies received local ethics committee approval. All participants provided written informed consent in accordance with the Declaration of Helsinki to participate in the studies.
Grading and Definition of GA
The diagnosis of GA was based on grading of multimodal images (color fundus photography [CFP]; autofluorescence; optical coherence tomography), of which presence of CFP was the minimum requirement. GA was defined as RPE atrophy with a minimal diameter of 175 μm and visible choroidal vessels15 within the Early Treatment Diabetic Retinopathy Study (ETDRS) grid on CFP, as a region of hypertransmission and disruption of the RPE on optical coherence tomography,2 and as hypoautofluorescence on autofluorescent imaging.
Detailed information on GA grading and other AMD features can be found in eMethods 2 in the Supplement. Central foveal involvement of GA was considered present when the umbo (foveal dip) showed atrophy of RPE, and perifoveal area involvement was considered present when the edge of GA was within 250 μm15 from the umbo.
Visual Acuity and Study Covariates
Covariates entered the analyses with the outcome at first diagnosis of GA. Smoking status was categorized as current, former, or never. Follow-up time was calculated from the age at baseline visit. Age at death was obtained from death certificates filled out by the family physician and reports from medical specialists.
Best-corrected visual acuity (BCVA) was measured with an ETDRS chart and converted to Snellen measurements. BCVA of the eye with GA entered the analysis; this was the eye with the better BCVA when GA was bilateral. Visual impairment was classified into 4 categories in accordance with the World Health Organization International Classification of Diseases, 11th Revision (ICD-11) (eMethods 2 in the Supplement). AMD status of the other eye was classified by the Three Continent AMD Consortium classification: no AMD, mild early AMD, moderate early AMD, severe early or intermediate AMD, or late AMD.16 Individual genetic and environmental risk scores were calculated as previously described by Buitendijk et al.17 The β’s described in the article by Fritsche et al18 were used to calculate the genetic risk per participant. When single-nucleotide polymorphisms (SNPs) were not available, a proxy was used (eMethods 2 in the Supplement).
Statistical Analysis
Enlargement of the GA area was analyzed as a slope and was calculated as (area of GA at the follow-up visit − area of GA at baseline)/years of follow-up. Enlargement of square root–transformed area was used with the intention to reduce the effect of baseline area on the rate of enlargement and was calculated as (√[area at last visit] − √[baseline area])/years of follow-up. Two-sided McNemar test for paired proportions was used to evaluate which section in the ETDRS grid was most often affected by GA. Correlations of variables with enlargement or with enlargement of the square root–transformed area were calculated using a 2-sided Spearman, Pearson, or Mann-Whitney test where appropriate, using the right eye if a participant had bilateral GA. No corrections were made for multiple testing. Cumulative incidence was calculated from the incidence rate with the formula CIt = 1 − e−IR × t, where CI is the cumulative incidence over a t period of years, e is the constant 2.71828, and IR is the incidence rate. Incidence of new lesions was assumed to have occurred halfway during the follow-up interval. To compare visual impairment between groups, we used Fisher-Freeman-Halton testing because sample sizes in the cross-table were small. A P value less than .05 was considered statistically significant. Calculations were made using SPSS version 25.0 (IBM), and graphical outputs were constructed with GraphPad Prism version 7 (GraphPad Software) (eMethods 2 in the Supplement).
Results
Study Population
Of 171 included patients, 106 (62.0%) were female, and the mean (SD) age at first diagnosis of GA was 82.6 (7.1) years (Table 1). During the entire follow-up of all 4 cohorts, a total of 242 eyes from 171 participants were identified with GA, of which 147 (60.7%) were incident GA (Figure 1). The 10-year cumulative incidence was 2.8% in RS cohort 1 and 3.6% in the BMES cohort; the incidence rates were 2.89 and 3.66 per 1000 person-years, respectively.17 Bilateral GA was present in 71 participants (41.5%). The mean (range) Dice score for delineating GA was 0.83 (0.74-0.99).
Table 1. Characteristics of the 4 Cohort Studies.
| Cohort (start year) | Patients, No. | Age at inclusion, mean (SD), y | No. (%) | Total eyes, No. | Eyes with follow-up data, No. | No. (%; 95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|
| Female | Current smoker | Previous smoker | Prevalent GA | Incident GA | |||||
| BMES (1992) | 26 | 81.6 (7.4) | 19 (73) | 3 (12) | 9 (35) | 44 | 33 | 12 (27; 14.1-40.4) | 32 (73; 59.6-85.9) |
| RS cohort 1 (1990) | 117 | 83.4 (6.7) | 73 (62.4) | 27 (23.1) | 39 (33.3)a | 163 | 50 | 70 (43; 23.6-37.8) | 93 (57; 49.5-64.7) |
| RS cohort 2 (2000) | 20 | 81.0 (7.8) | 8 (40) | 1 (5) | 17 (85) | 25 | 5 | 7 (28; 4.3-35.7) | 18 (72; 4.3-35.7) |
| RS cohort 3 (2006) | 8 | 78.1 (9.7) | 6 (75) | 1 (13) | 3 (38) | 10 | 0 | 6 (60; 26.2-87.8) | 4 (40; 12.2-73.8) |
| Total | 171 | 82.6 (7.1) | 106 (62.0) | 32 (18.7) | 68 (39.8)a | 242 | 88 | 95 (39.3; 33.1-45.4) | 147 (60.7; 54.6-66.9) |
Abbreviations: BMES, Blue Mountains Eye Study; GA, geographic atrophy; RS, Rotterdam Study.
Data for 6 patients missing.
Figure 1. Flowchart of Inclusion and Follow-up.
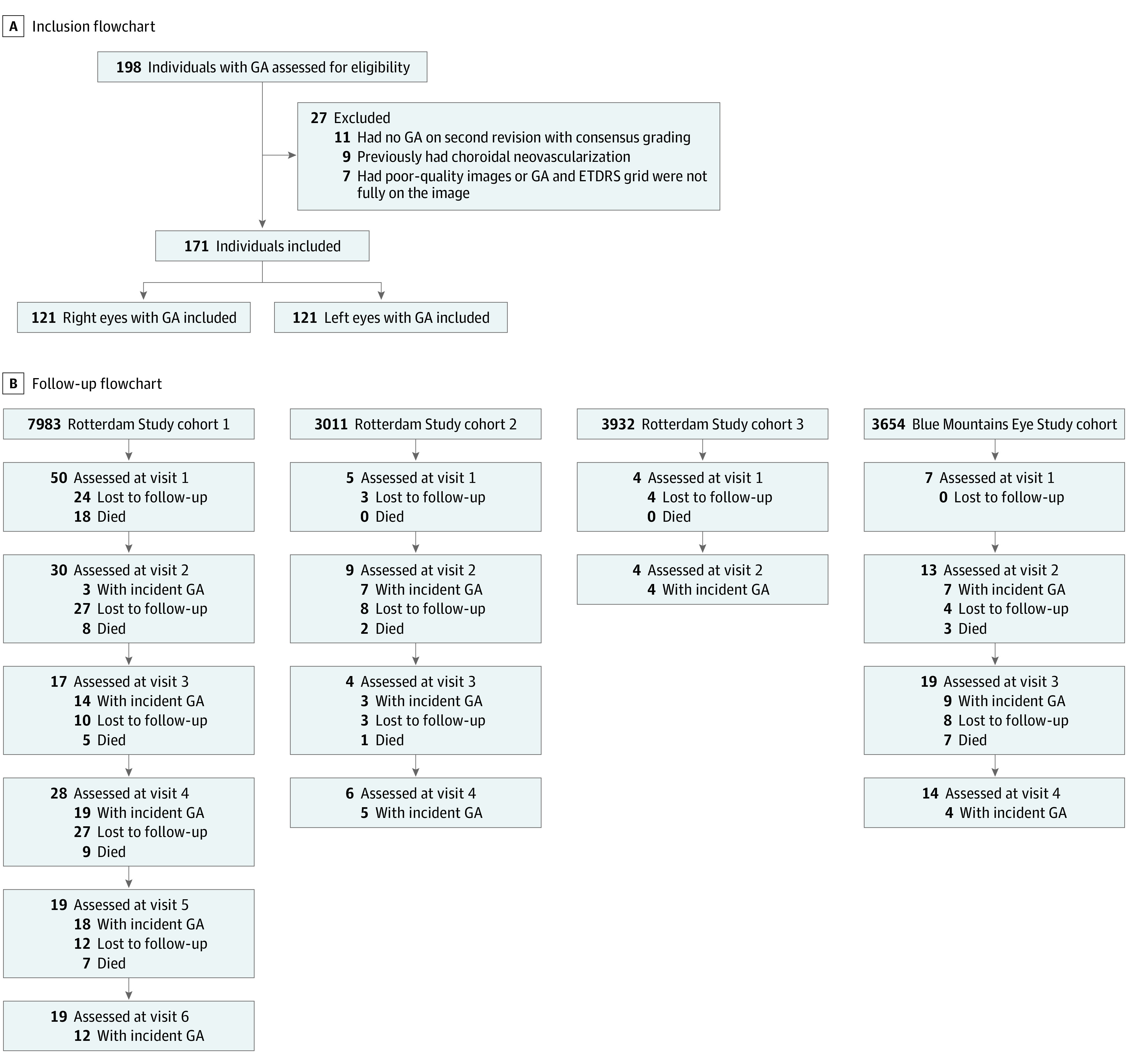
Patients with incident geographic atrophy (GA) are included in the total mentioned in each box. Number of people died is included in the number of people lost to follow-up.
Area and Enlargement
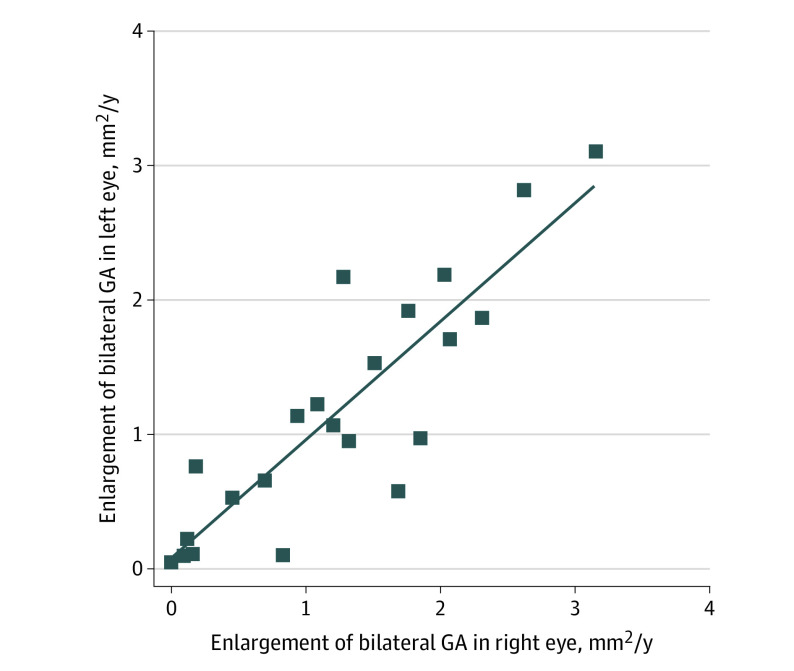
Combining all GA, the mean area at first presentation was 3.74 mm2 (95% CI, 3.11-4.67), with large variations between individuals (range, 0.06-23.25 mm2). RS cohorts 2 and 3 with younger populations presented with smaller mean areas of GA (2.04 mm2 and 2.39 mm2, respectively). The mean starting area in patients with prevalent GA was larger than the starting area in patients with incident GA (mean [range] area, 5.44 [3.34-6.01] mm2 vs 2.64 [0.39-4.97] mm2). Enlargement rate was measured in millimeters squared per year and millimeters per year (eFigure 1 in the Supplement). The mean rate was 1.09 mm2 per year (95% CI, 0.89-1.30), and the median (interquartile range [IQR]) rate was 0.80 (0.33-1.68) mm2 per year (square root transformation, 0.24 mm per year), which was slightly smaller for those with prevalent GA than for those with incident GA (1.03 vs 1.16 mm2 per year) (Table 2). When including the area outside the grid for the 15 eyes where GA extended beyond the grid borders, the mean enlargement rate was 1.31 mm2 per year (95% CI, 1.02-1.60). GA enlargement ranged from 0.53 to 1.28 mm2 per year between the different cohorts. Rates varied more than 200-fold, ranging from 0.02 to 4.05 mm2 per year and up to 6.64 mm2 per year for the 15 eyes with GA growing outside the ETDRS grid. Baseline lesion size was an important determinant of enlargement rate (r = 0.296; P = .02), but this lost statistical significance after square root transformation (r = 0.020; P = .88) (eFigure 1 in the Supplement). We also examined GA enlargement of bilateral GA and observed a high correlation between eyes (r = 0.87; P < .001), even when including the area outside the ETDRS grid (r = 0.71; P < .001) (Figure 2).
Table 2. Area of Geographic Atrophy (GA) at First Presentation and Annual Enlargement.
| Cohort | Starting area (95% CI), mm2 | Starting area (95% CI), mm2 | Mean enlargement (95% CI), mm2 per year | Mean enlargement (95% CI), mm2 per year | ||
|---|---|---|---|---|---|---|
| Prevalent GA | Incident GA | Prevalent GA | Incident GA | |||
| BMES | 4.53 (2.58 to 6.47) | 3.34 (0.41 to 6.27) | 4.97 (2.96 to 6.98) | 1.28 (0.98 to 1.58) | 0.92 (0.48 to 1.35) | 1.49 (1.11 to 1.86) |
| RS cohort 1 | 3.87 (3.10 to 4.65) | 6.01 (4.49 to 7.54) | 2.27 (1.71 to 2.82) | 1.03 (0.73 to 1.32) | 1.13 (0.73 to 1.54) | 0.83 (0.45 to 1.21) |
| RS cohort 2 | 2.04 (0.64 to 3.44) | 4.81 (0.35 to 9.27) | 0.96 (0.52 to 1.40) | 0.53 (0.22 to 0.84) | 0.19 (−0.15 to 0.52) | 0.77 (0.60 to 0.94) |
| RS cohort 3 | 2.39 (0.11 to 4.67) | 3.72 (0.24 to 7.20) | 0.39 (−0.04 to 0.82) | NAa | NAa | NAa |
| Total | 3.74 (3.11 to 4.67) | 5.44 (4.19 to 6.69) | 2.64 (2.05 to 3.24) | 1.09 (0.89 to 1.30) | 1.03 (0.73 to 1.34) | 1.16 (0.90 to 1.43) |
Abbreviations: BMES, Blue Mountains Eye Study; NA, not applicable; RS, Rotterdam Study.
No follow-up data were available.
Figure 2. Correlation of Geographic Atrophy (GA) Between Both Eyes .
Data points indicate patients with bilateral GA. There was high correlation between eyes (r = 0.86; P < .001).
Foveal Involvement and VA
Of 147 eyes with incident GA, 55 (37.4%) initially presented with central foveal involvement. Of these, 38 of 50 with VA data (76%) presented with a VA less than 20/40, and 8 of 50 (16%) presented with legal blindness (VA less than 20/400). Of 42 eyes with incident GA that were examined at subsequent visits, 8 (19%) already had central foveal involvement at first presentation; 14 eyes (33%) developed central foveal GA within 5 years, 8 (19%) within 10 years, and 1 (2%) within 12 years. Taken together, the mean (range) time for foveal involvement was 5.6 (3-12) years, and the incidence rate was 18.5 per 100 person-years. In 11 eyes (26%), GA never reached the central fovea during a mean (range) of 5.13 (1.5-11) years of follow-up, but GA did reach the perifoveal area in 8 of these eyes.
We examined the predilection site for the first presentation of GA in the ETDRS grid. First presentation occurred most often in the central circle of the ETDRS grid but outside the central fovea (115 lesions of 147 incident lesions [78.2%]), significantly more often than in the superior and temporal inner circle (eFigure 2 in the Supplement). GA was more than twice as likely to develop in the inner or central circle than the outer circle. When incident GA was present in the outer circle, all subfields were equally affected (43 [29.3%] vs 39 [26.5%]).
Almost half of the eyes with incident GA (68 of 140 with VA data [48.6%]) presented with a BCVA of 20/40 or better. Eyes with central foveal involvement had severe visual impairment (BCVA less than 20/200) and blindness (BCVA less than 20/400) significantly more often than eyes without central or perifoveal involvement (9 of 31 eyes vs 0 of 47 eyes; Fisher-Freeman-Halton exact test, P < .001). Patients with central foveal GA had worse BCVA than perifoveal GA (BCVA less than 20/63 in 23 of 31 eyes vs 4 of 17 eyes; Fisher-Freeman-Halton exact test, P = .005).
Factors Related to Enlargement
We investigated determinants of GA enlargement and did not find any differences for age category (younger than 80 years: n = 44 patients; mean [SD], 1.09 [1.09] mm2 per year; median [IQR], 0.69 [0.25-1.72] mm2 per year; mean [SD] for eyes with incident GA, 1.14 [0.86] mm2 per year; 80 years and older: n = 44 patients; mean [SD], 1.05 [0.82] mm2 per year; median [IQR], 0.95 [0.40-1.52] mm2 per year; mean [SD] for eyes with incident GA, 1.19 [0.90] mm2 per year). Sex, presence of hyperpigmentation (n = 37), presence of peripapillary atrophy (n = 37), drusen area (n = 37), smoking (n = 63), genetic risk variants in CFH (rs1061170 [n = 57] and rs1329424 [n = 49]) and ARMS2 (rs10490924 [n = 56]) (eFigure 3 in the Supplement) or a genetic risk score combining CFH (rs1061170 and rs1329424), ARMS2 (rs10490924), and C3 (rs2230199) did not show a significant correlation with enlargement or with the square root–transformed enlargement. Likewise, a combined model of genetic, environmental, and phenotypic factors17 did not reach statistical significance (r = 0.240; P = .10) (eFigure 4 in the Supplement). The only significant correlation with enlargement was AMD status of the fellow eye; GA area grew faster when the fellow eye had a more severe stage (Spearman ρ = 0.34; P = .01).
Survival of Participants With GA
We particularly addressed life expectancy after diagnosis of GA, since this indicates the burden of visual loss and time available for intervention. A total of 121 of 171 participants with GA (70.8%) died during follow-up. Mean (SD) age at death was 90.2 (6.8) years, at a somewhat higher age than persons with choroidal neovascularization or mixed type of late AMD (mean [SD] age, 88.5 [6.4] years) and participants without late AMD (mean [SD] age, 82.9 [8.9] years). Participants with incident GA (n = 59) died a mean (SD) of 6.4 (5.4) years after first presentation, ranging from 6 months to 23 years (eFigure 5 in the Supplement). Among 26 patients who developed incident GA in the other eye, the mean (SD) time from incident GA to death was 5.5 (4.5) years, ranging from 6 months to 18 years.
We investigated the BCVA of eyes with GA at the last visit before death; 108 of 163 (66.3%) had moderate or severe visual impairment (VA less than 20/63) before death. A total of 69 of 89 eyes with prevalent GA (78%) and 39 of 74 eyes with incident GA (53%) had moderate or severe visual impairment (VA less than 20/63) before death. Of those with bilateral GA who died during follow-up, 22 (49%) had moderate or severe visual impairment at the last visit before death; of these, 3 (7%) were blind (Table 3). In RS cohorts 1, 2, and 3, 47 of 107 patients with GA (43.9%) had a BCVA of 20/63 or less at their last visit before death, regardless of if their other eye was affected by AMD.
Table 3. Visual Impairment (VI) at Last Visit in Deceased Participants With Geographic Atrophy (GA).
| GA type | No. (%; 95% CI) | |||
|---|---|---|---|---|
| Blind (VA less than 20/400) | Severe VI (VA less than 20/200) | Moderate VI (VA less than 20/63) | Mild VI (VA less than 20/40) | |
| Incidenta | 7 (9; 2.79-16.13) | 7 (9; 2.79-16.13) | 25 (32; 23.01-44.56) | 8 (10; 3.74-17.89) |
| Prevalentb | 16 (18; 10.00-25.96) | 14 (16; 8.17-23.29) | 39 (43; 33.51-54.13) | 5 (6; 0.83-10.40) |
| Bilateral | 3 (7; 1.37-17.90) | 3 (7; 1.37-17.90) | 16 (35; 21.02-48.55) | 7 (15; 4.84-25.60) |
Abbreviation: VA, visual acuity.
Data missing for 3 patients.
Data missing for 1 patient.
Discussion
This study investigates the occurrence and natural course of GA in 2 populations across 2 continents. Age-specific prevalence and incidence of GA were similar in these cohorts.17 We found that GA increased in size, with a mean of 1.09 mm2 per year (95% CI, 0.89-1.30) and ranging from 0.53 to 1.28 mm2 per year between the different cohorts. The mean square root transformation of enlargement within the ETDRS grid area was 0.24 mm per year. The mean rate of enlargement was 1.16 mm2 per year for eyes with incident GA compared with 1.03 mm2 per year for eyes with prevalent GA. First presentation of GA occurred most often in the central circle of the ETDRS grid but outside the fovea, and one-third presented with atrophy involving the ETDRS outer circle (more than 1500 μm from the fovea). The incidence rate of GA to reach the central fovea was 18.5 per 100 person-years (ie, 60.4% reached the fovea in 5 years). The mean (SD) life expectancy of those affected was 6.4 (5.4) years after the initial presentation; the mean (SD) age at death 90.2 (6.8) years, and 47 of 107 (43.9%) died with moderate visual impairment or worse.
Most studies on growth of GA have focused on patients from clinics. In these studies, enlargement of GA ranged from 1.14 mm2 per year to 3.1 mm2 per year, as summarized by Fleckenstein et al.19 The enlargement rate of GA in our study was somewhat lower, but this can be explained by patients with relatively small lesion areas at first presentation, who were not necessarily symptomatic. When comparing the square root of GA enlargement, enlargement rates were more in line with growth rates reported in the literature. Our results were also very similar to those of Holmen et al,20 who also studied incident GA and found an enlargement rate of 0.97 mm2 per year. With respect to time to foveal involvement, Age-Related Eye Disease Study (AREDS) investigators reported that 57% developed this after 4 years.7 This is very similar to our 5-year cumulative incidence rate of 60.4%. Central fovea as the initiation site for GA was also very similar between our studies (33% in AREDS7 vs 37.4% in our study). Mauschitz et al21 focused on the location of GA within the ETDRS grid and found the center and inner zones to be most frequently involved in the atrophic lesion. The atrophy extended more rapidly toward the periphery than toward the fovea.22 Our study also demonstrated extrafoveal involvement at initiation but found progression to the fovea in a significant proportion at long term.
The highly correlated enlargement (r = 0.87) we observed between the 2 eyes among patients with bilateral GA was previously noticed by other research groups, who found a correlation coefficient ranging from 0.74 to 0.88.8,23,24,25,26,27 This high correlation suggests a common mechanism, which may be driven by genetics. Only few studies investigated AMD genetic risk variants for GA enlargement; they predominantly examined the important SNPs in ARMS2 (rs10490924) and CHF (rs1061170).7,28,29,30 Two studies found an increase in GA enlargement for the CFH SNP and 2 studies for the AMRS2 SNP, but other studies did not find any statistically significant results.28,29,31,32 We analyzed AMD risk variants as part of a risk score, as well as separate SNPs. We did not find a significant association with GA enlargement at the univariate level or combined in a genetic and environmental risk score. A consideration is the homogeneous genetic background of GA cases; more than 95% (104 of 109) had at least 1 of the 4 major risk SNPs, hampering detailed analysis of the genetic effect. A 2019 genome-wide association study found novel SNPs specifically associated with GA enlargement: variants in PRMT6 (rs11184959) and LSS (rs2839127).33 Jointly, these SNPs explained only 6% of the variation in GA enlargement.
In this study, we showed that participants with GA generally have functional VA at first presentation but develop a progressive decline thereafter. GA in and close to the central fovea had the largest impact on VA, as expected and found before.12,25 Although our examinations at 5-year intervals enabled only a rough estimate of the proportion of participants with GA who became blind before death, we observed that a high proportion of eyes with GA (66.3%) were severely visually impaired or blind before death; 49% of patients had bilateral low vision and 7% were blind. Our data were very much in line with those of Chakravarthy et al,34 who observed 7.1% of people with bilateral GA with a VA less than 20/400 at first presentation in clinic and 71.1% with a VA less than 20/40.
The time between diagnosis and death varied widely (6 months to 23 years) among patients with GA, with a median of 5 years. This period is important as it is the time available for potential interventions. Based on our data, early screening for GA before visual symptoms occur seems most promising to find eligible candidates for trials or future interventions. This improves treatment potential enormously as it increases the chance of preserving functional vision for the longer term.
Strengths and Limitations
Our results need to be viewed in light of the strengths and limitations of the study. The long follow-up is a major strength of the study; to our knowledge, thus far, follow-up was 5 to 6 years in most studies.19 Only the BMES study showed results of 15 years of follow-up previously.28 The long follow-up also enabled us to view GA in the context of life expectancy. The population-based cohort design enabled identification of patients with GA who were not selected by clinical symptoms and who reflected the entire spectrum of GA. The still-functional vision at first presentation makes these participants interesting for interventions and suggests that screening for GA in an elderly population may facilitate identification of participants eligible for sight-saving therapies. Another strength was the usage of all available imaging per eye to trace the borders of GA simultaneously with software specifically designed for this project, allowing more precise delineation.
Among this study’s limitations was the availability of only CFP in the earlier visits of RS cohorts 1, 2, and 3 and the BMES cohort. Another limitation was the low number of participants with incident GA; this hampered the statistical power of the risk associations, particularly in individual studies. The pooled studies improved estimations but may have increased heterogenesis of data. In addition, the period between follow-up visits was relatively long, jeopardizing the accuracy of time estimates.
Conclusions
In conclusion, our natural history study of GA ascertained from a general population showed that 37% of patients with newly diagnosed GA already had atrophy in the fovea, suggesting that a relatively high proportion of patients with GA are not suitable for sight-saving therapy at an early stage. Nonfoveal GA reached the fovea on average within 5.6 years, which implies that this is the mean time window for treatment. However, this is highly variable and best predicted by the other eye. Almost half of the patients lost functional vision during their life, making a strong case that the need for successful therapy is high.
eMethods 1. Cohort descriptions.
eMethods 2. Grading of geographic atrophy, classification of visual impairment, software used for calculations, and genetic risk score.
eFigure 1. Distribution of geographic atrophy enlargement.
eFigure 2. Geographic atrophy in ETDRS grid.
eFigure 3. Geographic atrophy enlargement related to single-nucleotide polymorphisms.
eFigure 4. Geographic atrophy enlargement and age-related macular degeneration risk score.
eFigure 5. Years with geographic atrophy before death.
References
- 1.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982-4991. doi: 10.1167/iovs.09-3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: Classification of Atrophy report 3. Ophthalmology. 2018;125(4):537-548. doi: 10.1016/j.ophtha.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng QE, Gao J, Kim BJ, Ying GS. Design characteristics of geographic atrophy treatment trials: systematic review of registered trials in ClinicalTrials.gov. Ophthalmol Retina. 2018;2(6):518-525. doi: 10.1016/j.oret.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 4.Liao DS, Grossi FV, El Mehdi D, et al. Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age-related macular degeneration: a randomized phase 2 trial. Ophthalmology. 2020;127(2):186-195. doi: 10.1016/j.ophtha.2019.07.011 [DOI] [PubMed] [Google Scholar]
- 5.Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128(4):576-586. [DOI] [PubMed] [Google Scholar]
- 6.Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S; FAM-Study Group . Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143(3):463-472. doi: 10.1016/j.ajo.2006.11.041 [DOI] [PubMed] [Google Scholar]
- 7.Keenan TD, Agrón E, Domalpally A, et al. ; AREDS2 Research Group . Progression of geographic atrophy in age-related macular degeneration: AREDS2 report number 16. Ophthalmology. 2018;125(12):1913-1928. doi: 10.1016/j.ophtha.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106(9):1768-1779. doi: 10.1016/S0161-6420(99)90340-8 [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Meuer SM, Knudtson MD, Klein BE. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye study. Am J Ophthalmol. 2008;146(5):692-699. doi: 10.1016/j.ajo.2008.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz-Valckenberg S, Sahel JA, Danis R, et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression study). Ophthalmology. 2016;123(2):361-368. doi: 10.1016/j.ophtha.2015.09.036 [DOI] [PubMed] [Google Scholar]
- 11.Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079-1091. doi: 10.1016/j.ophtha.2013.11.023 [DOI] [PubMed] [Google Scholar]
- 12.Schmitz-Valckenberg S, Nadal J, Fimmers R, et al. ; FAM Study Group . Modeling visual acuity in geographic atrophy secondary to age-related macular degeneration. Ophthalmologica. 2016;235(4):215-224. doi: 10.1159/000445217 [DOI] [PubMed] [Google Scholar]
- 13.Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32(9):807-850. doi: 10.1007/s10654-017-0321-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. the Blue Mountains Eye study. Ophthalmology. 1995;102(10):1450-1460. doi: 10.1016/S0161-6420(95)30846-9 [DOI] [PubMed] [Google Scholar]
- 15.Bird AC, Bressler NM, Bressler SB, et al. ; The International ARM Epidemiological Study Group . An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39(5):367-374. doi: 10.1016/S0039-6257(05)80092-X [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Meuer SM, Myers CE, et al. Harmonizing the classification of age-related macular degeneration in the Three-Continent AMD Consortium. Ophthalmic Epidemiol. 2014;21(1):14-23. doi: 10.3109/09286586.2013.867512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buitendijk GHS, Rochtchina E, Myers C, et al. Prediction of age-related macular degeneration in the general population: the Three Continent AMD Consortium. Ophthalmology. 2013;120(12):2644-2655. doi: 10.1016/j.ophtha.2013.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritsche LG, Igl W, Bailey JN, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134-143. doi: 10.1038/ng.3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369-390. doi: 10.1016/j.ophtha.2017.08.038 [DOI] [PubMed] [Google Scholar]
- 20.Holmen IC, Aul B, Pak JW, et al. ; Age-Related Eye Disease Study 2 Research Group . Precursors and development of geographic atrophy with autofluorescence imaging: Age-Related Eye Disease study 2 report number 18. Ophthalmol Retina. 2019;3(9):724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauschitz MM, Fonseca S, Chang P, et al. ; GAP Study Group . Topography of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(8):4932-4939. doi: 10.1167/iovs.12-9711 [DOI] [PubMed] [Google Scholar]
- 22.Lindner M, Böker A, Mauschitz MM, et al. ; Fundus Autofluorescence in Age-Related Macular Degeneration Study Group . Directional kinetics of geographic atrophy progression in age-related macular degeneration with foveal sparing. Ophthalmology. 2015;122(7):1356-1365. doi: 10.1016/j.ophtha.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 23.Fleckenstein M, Adrion C, Schmitz-Valckenberg S, et al. ; FAM Study Group . Concordance of disease progression in bilateral geographic atrophy due to AMD. Invest Ophthalmol Vis Sci. 2010;51(2):637-642. doi: 10.1167/iovs.09-3547 [DOI] [PubMed] [Google Scholar]
- 24.Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114(2):271-277. doi: 10.1016/j.ophtha.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindblad AS, Lloyd PC, Clemons TE, et al. ; Age-Related Eye Disease Study Research Group . Change in area of geographic atrophy in the Age-Related Eye Disease study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168-1174. doi: 10.1001/archophthalmol.2009.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleckenstein M, Schmitz-Valckenberg S, Adrion C, et al. ; FAM Study Group . Progression of age-related geographic atrophy: role of the fellow eye. Invest Ophthalmol Vis Sci. 2011;52(9):6552-6557. doi: 10.1167/iovs.11-7298 [DOI] [PubMed] [Google Scholar]
- 27.Bellmann C, Jorzik J, Spital G, Unnebrink K, Pauleikhoff D, Holz FG. Symmetry of bilateral lesions in geographic atrophy in patients with age-related macular degeneration. Arch Ophthalmol. 2002;120(5):579-584. doi: 10.1001/archopht.120.5.579 [DOI] [PubMed] [Google Scholar]
- 28.Joachim N, Mitchell P, Kifley A, Rochtchina E, Hong T, Wang JJ. Incidence and progression of geographic atrophy: observations from a population-based cohort. Ophthalmology. 2013;120(10):2042-2050. doi: 10.1016/j.ophtha.2013.03.029 [DOI] [PubMed] [Google Scholar]
- 29.Klein ML, Ferris FL III, Francis PJ, et al. Progression of geographic atrophy and genotype in age-related macular degeneration. Ophthalmology. 2010;117(8):1554-1559, 1559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleckenstein M, Grassmann F, Lindner M, et al. Distinct genetic risk profile of the rapidly progressing diffuse-trickling subtype of geographic atrophy in age-related macular degeneration (AMD). Invest Ophthalmol Vis Sci. 2016;57(6):2463-2471. doi: 10.1167/iovs.15-18593 [DOI] [PubMed] [Google Scholar]
- 31.Grassmann F, Fleckenstein M, Chew EY, et al. Clinical and genetic factors associated with progression of geographic atrophy lesions in age-related macular degeneration. PLoS One. 2015;10(5):e0126636. doi: 10.1371/journal.pone.0126636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caire J, Recalde S, Velazquez-Villoria A, et al. ; Spanish Multicenter Group on AMD . Growth of geographic atrophy on fundus autofluorescence and polymorphisms of CFH, CFB, C3, FHR1-3, and ARMS2 in age-related macular degeneration. JAMA Ophthalmol. 2014;132(5):528-534. doi: 10.1001/jamaophthalmol.2013.8175 [DOI] [PubMed] [Google Scholar]
- 33.Grassmann F, Harsch S, Brandl C, et al. Assessment of novel genome-wide significant gene loci and lesion growth in geographic atrophy secondary to age-related macular degeneration. JAMA Ophthalmol. 2019;137(8):867-876. doi: 10.1001/jamaophthalmol.2019.1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakravarthy U, Bailey CC, Johnston RL, et al. Characterizing disease burden and progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(6):842-849. doi: 10.1016/j.ophtha.2017.11.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Cohort descriptions.
eMethods 2. Grading of geographic atrophy, classification of visual impairment, software used for calculations, and genetic risk score.
eFigure 1. Distribution of geographic atrophy enlargement.
eFigure 2. Geographic atrophy in ETDRS grid.
eFigure 3. Geographic atrophy enlargement related to single-nucleotide polymorphisms.
eFigure 4. Geographic atrophy enlargement and age-related macular degeneration risk score.
eFigure 5. Years with geographic atrophy before death.