Highlights
-
•
BoM was prone to multiple organs metastases and had more complex aberrations.
-
•
BoM was associated with worse prognosis which cannot be salvaged by Osimertinib.
-
•
BoM was an independent prognostic factor for EGFR-TKI treatment in PFS and OS.
Keywords: Bone metastasis, Epidermal growth factor receptor, Tyrosine kinase inhibitor, Non-small-cell-lung-cancer
Abstract
Background
Targeted therapy has been established as the standard-of-care for patients with advanced non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations. Among patients with advanced lung cancer, 30–40% have bone metastases (BoM) at first diagnosis. However, little is known on the clinical characteristics and prognostic factors of BoM in patients with NSCLC harboring EGFR mutations.
Methods
Treatment-naive patients with advanced NSCLC harboring EGFR mutations who were prescribed tyrosine kinase inhibitors (TKIs) were screened and enrolled between June 2009 and April 2019 from West China Hospital. Patients were dichotomized according to whether they had BoM. The demographic characteristics, gene mutation status and therapeutic efficacy, including objective response rate (ORR), progression-free survival (PFS) and overall survival (OS), were collected.
Results
A cohort of 604 patients were enrolled. The BoM group had worse PFS (11.7 vs. 14.0 months, HR = 0.73, p = 0.00013) and OS (32.8 vs. 46.1 months, HR = 0.54, p < 0.0001) compared with the non-BoM group. No significant differences were observed in disease control rate (p = 0.407) or ORR (p = 0.537) between the two groups. The metastatic sites in the two groups exhibited obvious differences. In multivariate analysis, BoM was found to be an independent factor of worse prognosis.
Conclusion
BoM was identified as an independent inferior prognostic factor for EGFR-TKI treatment, and may have complex biological implications.
1. Introduction
Lung cancer is the most common type of cancer and the leading cause of cancer-related mortality worldwide [1]. The 5-year survival rate for metastatic lung cancer is ~ 5%. Compared with traditional chemotherapy, epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have demonstrated superior efficacy in selected patients harbouring sensitive EGFR mutations [2], [3], [4], [5]. Targeted therapy has been the standard first-line treatment for patients with advanced non-small cell lung-cancer (NSCLC) with EGFR mutations. Although the majority of the patients with EGFR-mutant cancer benefit from TKI treatment, clinical response varies and additional factors affecting TKI efficacy must be explored.
Bone is a common site of metastasis of advanced NSCLC. Among patients with advanced lung cancer, 30–40% have bone metastases (BoM) at the time of diagnosis [6]. BoM is closely associated with compromised quality of life and shortened survival of the patients [7], [8]. The median survival of patients with BoM is 6–8 months, even after aggressive treatment [10]. Multidisciplinary treatment of BoM is recommended, including both systemic (targeted therapy, chemotherapy, bisphosphonates, painkillers and psychosocial support) and local therapy (radiotherapy and surgery). NSCLC with EGFR mutations is associated with a higher incidence of BoM [14], [15]. However, little is known on the clinical characteristics and prognostic factors of BoM in NSCLC harboring EGFR mutations. The aim of the present study was to compare the clinical characteristics between NSCLC patients harboring EGFR mutations, with or without BoM (non-BoM).
2. Methods
2.1. Study population
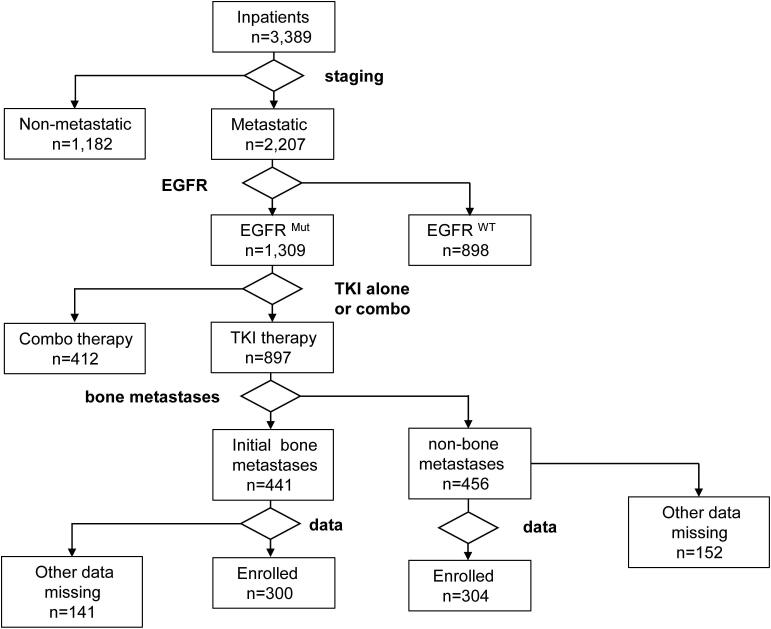
This was a retrospective study where 3,389 patients were screened through the Hospital Information System in West China Hospital between June 2009 and April 2019. A total of 604 enrolled patients had pathologically confirmed, metastatic, treatment-naive NSCLC, harboring EGFR mutations and were prescribed EGFR-TKI as first-line therapy. A total of 300 and 304 patients were grouped into the BoM and non-BoM groups, respectively, according to the presence or absence of BoM at presentation. The median age of the patients in the BoM group was 58.1 years and in the non-BoM group 60.6 years.
The present study was undertaken to explore the relationship between the therapeutic efficacy of EGFR-TKIs and the prognosis of NSCLC patients with BoM harboring EGFR mutations. Patients treated with TKIs combined with chemotherapy, either synchronous or intercalated, or those who had mixed small-cell lung cancer, were excluded from the present study.
2.2. Diagnosis of BoM
BoM were diagnosed using methods according to the treating physicians’ discretion. Patients were screened by bone scanning for possible metastases, and those with suspected BoM were confirmed by CT scan of the involved sites. When CT scans were deemed insufficient to establish the presence of BoM, magnetic resonance imaging (MRI) was prescribed as a supplementary diagnostic method. For a proportion of patients, 18FDG PET-scan was employed. Patients had different diagnostic approaches, but all were based on the current recommendations and guidelines, and were consistent during the period of observation.
2.3. Treatments
Each patient was treated as per the treating physicians’ discretion. Either gefitinib (250 mg once a day, AstraZeneca, UK), or erlotinib (150 mg once a day, Roche, Switzerland), or icotinib (125 mg 3 times per day, Beta, China) was administered. Treatment continued until disease progression, or unacceptable toxicity, or death from any cause. In treatment-resistant patients harboring T790M mutation, osimertinib (80 mg once a day, AstraZeneca, UK) was prescribed as salvage therapy.
2.4. Genetic testing
Genetic testing was performed on tumor tissues. EGFR mutation was detected by ARMS using a commercially available kit (AmoyDx, Shameng, China) at the College of American Pathologists-certified lab of West China Hospital. Tumor content was assessed by board-certified pathologists using hematoxylin and eosin staining. All specimens contained > 10% of tumor content. DNA was extracted using the QIAamp DNA mini kit (Qiagen). In some patients, comprehensive genomic profiling was performed by next generation sequencing (NGS) with 56 cancer-related gene panels covering the whole exons of the EGFR gene at a mean coverage depth of > 800X. The genomic alterations, including single base substitutions, insertions/deletions, copy number variations, as well as gene rearrangement and fusions, were assessed.
2.5. Outcome measures
Tumors were assessed every 2 months radiographically, including CT of the chest and upper abdomen, MRI of the head and bone scintigraphy. Tumor response was evaluated as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD), according to RECIST 1.1. The PFS was defined as the time interval from the initiation of therapy to the date of disease progression, intolerable side effects, or death from any cause. The OS was defined as the time interval from the initiation of the therapy to the date of death from any cause.
2.6. Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (IBM Corp., Chicago, IL) and R version 4.0.3. The quantitative data were compared using Chi-squared test and Fischer’s exact test according to Cochran’s rule. Kaplan-Meier curves WERE used to compare survival. Multivariate analysis was performed by using a Cox proportional hazard model. All P-values were based on a two-tailed hypothesis, and statistical significance was assumed if p < 0.05.
3. Ethics statement
The Ethics Committee of Sichuan University reviewed and approved the study protocol and the study was performed in accordance with the principles outlined in the Declaration of Helsinki. Since this was a retrospective study of an anonymized database and had no influence on patient care, patient consent was not required. The study was performed with respect to the confidentiality of individual patient data.
4. Results
4.1. Baseline characteristics
In this study, a total of 3,389 patients were screened, and 604 patients were enrolled, of whom 300 and 304 patients were grouped into the BoM and non-BoM groups, respectively (Fig. 1). Both groups had comparable demographic characteristics, other than age and EGFR mutations. The patients in the BoM group (median age, 58.1 years) were younger compared with those in the non-BoM group (median age, 60.6 years), although the difference was non-significant. Meanwhile, fewer patients aged ≥ 60 years were in the BoM group (n = 131, 43.7%) compared with the non-BoM group (n = 171, 56.3%, p = 0.003). L858R point mutation was more commonly found in the BoM group, while other mutations (mostly exon 19 deletion) occurred at higher frequencies in the non-BoM group (p = 0.022). Finally, the presence of ≥ 3 gene mutations in patients with BoM for whom such data were available was more common than in the non-BoM group (p = 0.006, Table 1).
Fig. 1.
The flow chart of patients screening.
Table 1.
The demographic features of population.
| Characteristic | Bone metastasis | Non-bone metastasis | P value |
|---|---|---|---|
| Total N (%) | 300(49.7%) | 304(50.3%) | |
| Gender | |||
| Female | 183(61.0%) | 173(56.9%) | |
| Male | 117(39.0%) | 131(43.1%) | 0.348 |
| Age group | |||
| <60 year | 169(56.3%) | 133(43.7%) | |
| ≥60 year | 131(43.7%) | 171(56.3%) | 0.003 |
| Smoking history | |||
| Yes | 75(25.0%) | 80(26.3%) | |
| No | 225(75.0%) | 224(73.7%) | 0.782 |
| ECOG | |||
| 0 | 163(54.3%) | 175(57.6%) | |
| ≥1 | 137(45.7%) | 129(42.4%) | 0.473 |
| EGFR mutation | |||
| Exon 19 deletion | 129(43.0%) | 140(46.1%) | |
| L858R mutation | 138(46.0%) | 110(36.2%) | |
| Other | 33(11.0%) | 54(17.7%) | 0.022 |
| Histological type | |||
| Adenocarcinoma | 289(96.3%) | 291(95.7%) | |
| Non-adenocarcinoma | 11(3.7%) | 13(4.3%) | 0.891 |
| Targeted drugs | |||
| Icotinib | 82(27.3%) | 86(28.3%) | |
| Gefitinib | 169(56.3%) | 153(50.3%) | |
| Erlotinib | 49(16.4%) | 65(21.4%) | 0.210 |
| ≥3 mutations(available) | |||
| Yes | 29(58%) | 13(29.5%) | |
| No | 21(42%) | 31(70.5%) | 0.006 |
4.2. The BoM group was prone to multiple organ metastases
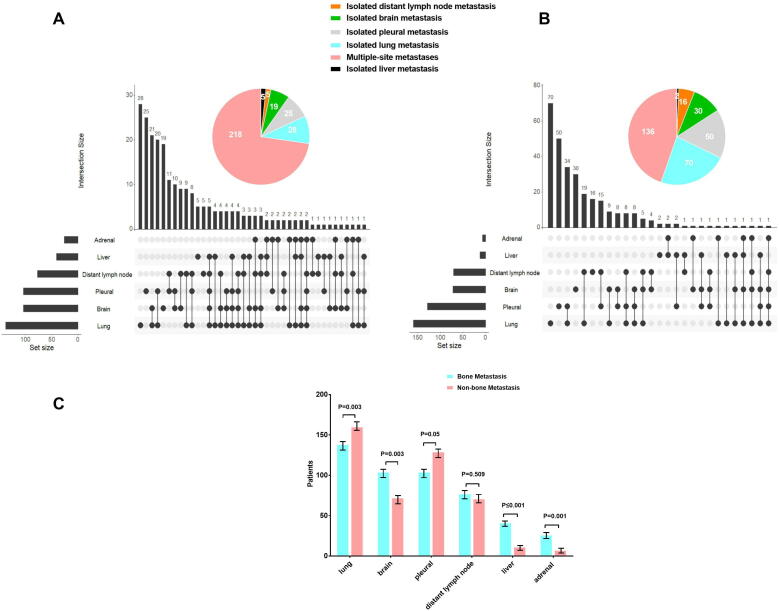
The data on organ metastasis in this cohort of patients were collected and analyzed. In the BoM group, the most common metastatic organs were the lung (46%), brain (34.7%), pleura (34.7%), distant lymph nodes (25.7%), liver (13.7%) and adrenal gland (8.7%). The brain (p = 0.003), liver (p ≤ 0.001) and adrenal gland (p = 0.001) were more commonly involved in the BoM group, while lung (p = 0.003) and pleural (p = 0.05) involvement was more common in the non-BoM group. Distant lymph node metastasis occurred in similar frequency in the two groups (p = 0.509). Of note, multi-organ metastases (≥3) were more common in the BoM group (p ≤ 0.001; Fig. 2).
Fig. 2.
Summary of metastatic sites in BoM (A) and Non-BoM group (B). Multi-organ metastases (≥3) were more common in BoM group (p ≤ 0.001). In BoM group, brain (p = 0.003), liver (p ≤ 0.001), and adrenal gland (p = 0.001) were more common, while lung (p = 0.003) and pleural (p = 0.05) were more significant in Non-BoM group. Distant lymph node metastases were similar in 2 groups (p = 0.509, C).
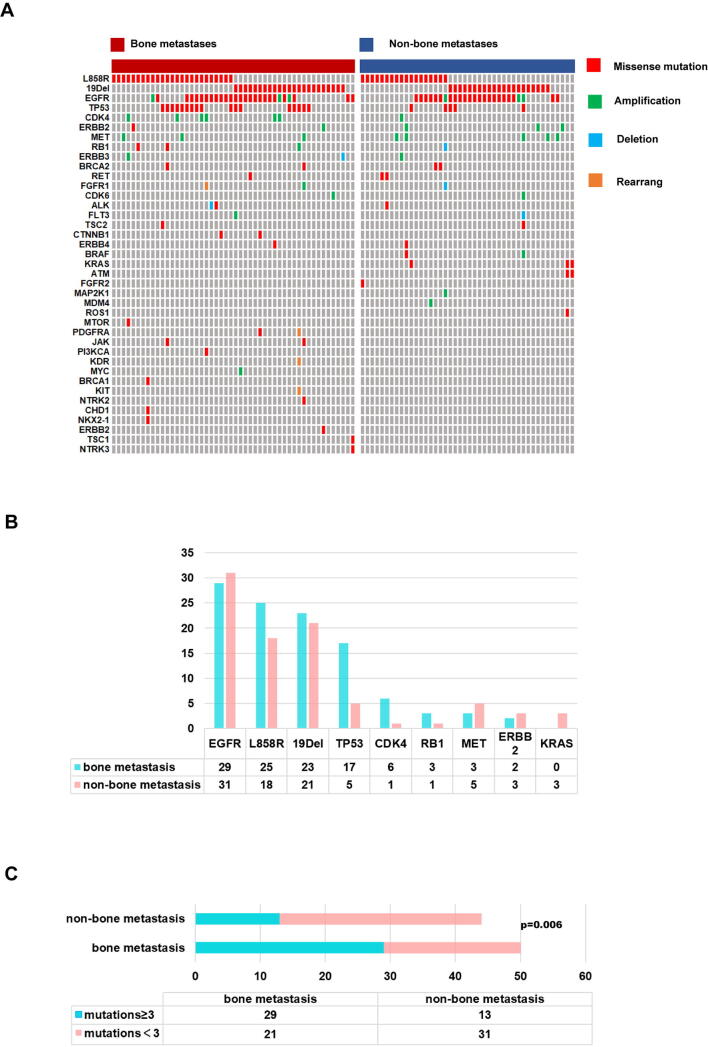
4.3. Genetic aberrations in the two groups
Targeted sequencing results were collected for our patients. Data were available for 50 and 29 patients in the BoM and non-BoM groups, respectively (Fig. S2A). The L858R, TP53 and CDK4 mutations were more frequent in the BoM group. In addition, re-occurring genetic aberrations (defined as occurrence ≥ 3 in this cohort) were more commonly seen in the BoM group (Fig. S2B). Complex aberrations (defined as ≥ 3 parallel aberrations in 1 patient) were more common in the BoM group (Fig. S2C).
4.4. Worse outcomes in the BoM group
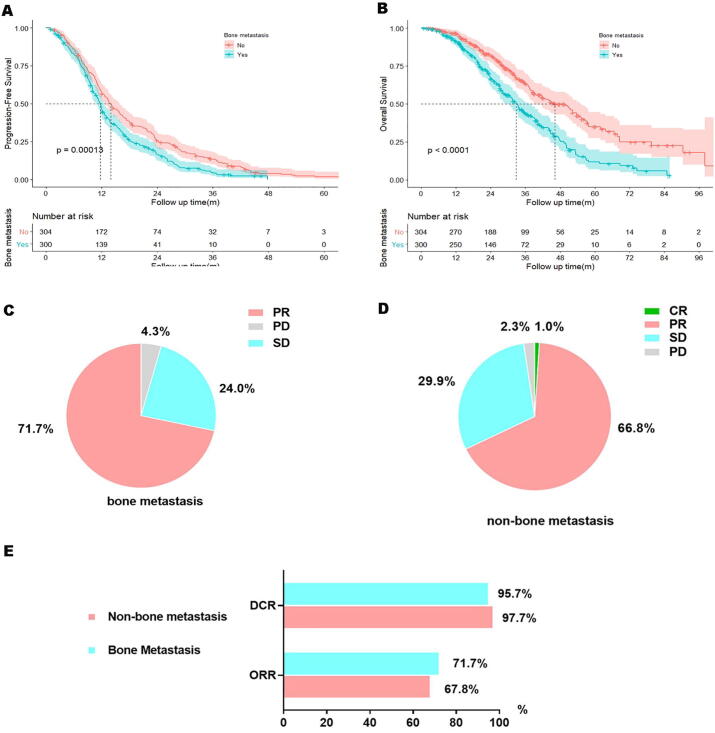
To explore the effect of BoM on TKI therapeutic efficacy, PFS and OS curves of the two groups were constructed and compared. The BoM group exhibited a shorter PFS (11.7 months, 95% CI: 10.9–12.5 months) and OS (32.8 months, 95% CI: 29.8–35.8 months) compared with the non-BoM group (PFS:14.0 months, HR = 0.73, 95% CI: 0.61–0.86, p = 0.00013; OS: 46.1 months, HR = 0.54, 95% CI:0.43–0.67, p < 0.0001; Fig. 3A and B).
Fig. 3.
Shorter PFS (A) or OS (B) in BoM group compared to Non-BoM group. Little difference in ORR (C bone metastasis, D non-bone metastasis, p = 0.537, E) was observed.
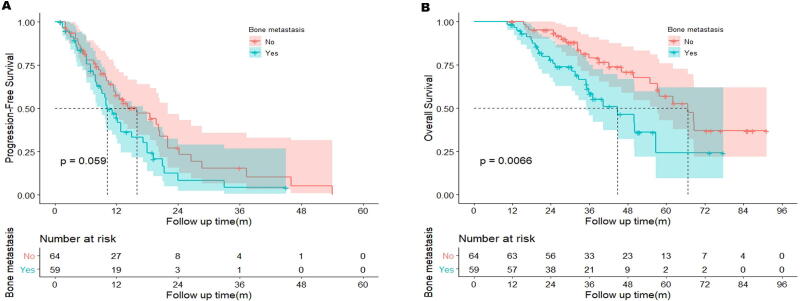
To test whether the negative impact of BoM could be improved by osimertinib, information of salvage therapy with osimertinib after initial treatment failure was collected. In the BoM group, a total of 59 patients received subsequent osimertinib, compared with 64 patients in the non-BoM group. In this subgroup, unfavorable PFS and OS were still observed in the BoM group (PFS: 10.2 vs. 16.0 months, HR = 0.66, 95% CI: 0.42–1.04, p = 0.059; OS: 44.8 vs. 66.6 months, HR = 0.47, 95% CI: 0.26–0.85, p = 0.0066, Fig. S1A and B). Hence, the worse treatment efficacy with TKIs could not be rescued by osimertinib.
No CR was observed in the BoM group, while 1% CR was achieved in the non-BoM group. The BoM group achieved a similar DCR (95.7 vs. 97.7%, respectively) and ORR (71.7 vs. 67.8%) compared with the non-BoM group (Fig. 3C-E).
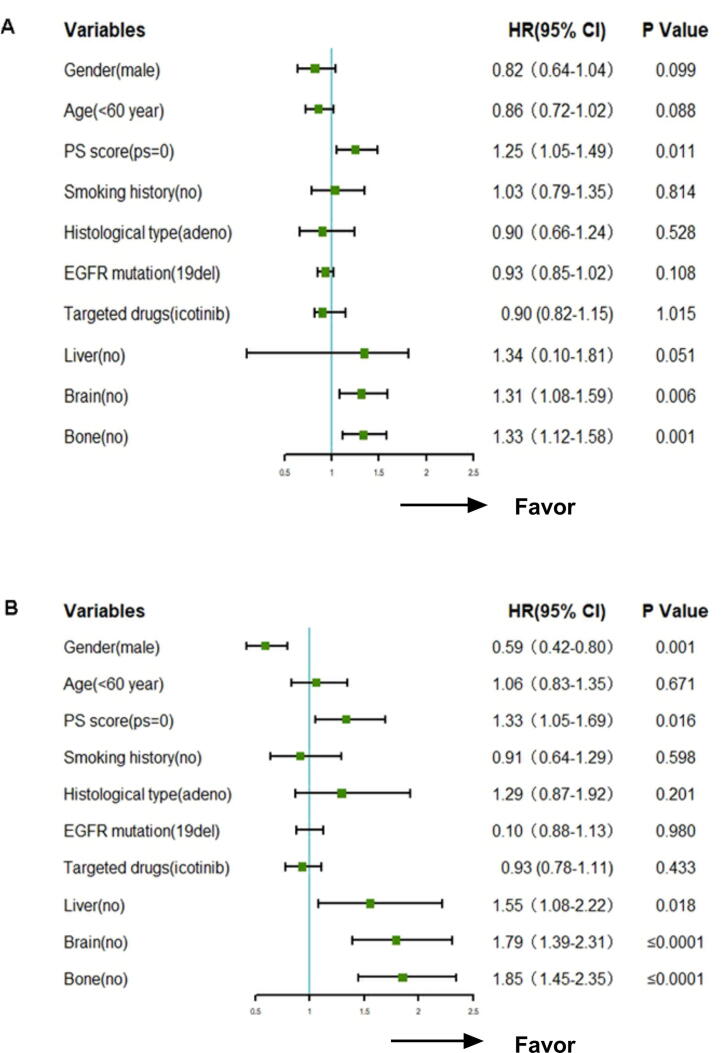
4.5. Multivariate cox regression analysis
Multivariate analysis indicated that bone and brain metastases and PS score influenced PFS independently (Fig. 4A), while bone, liver and brain metastases, PS score and sex influenced OS independently (Fig. 4B). Bone and brain metastases, as well as PS score, were proven to be independent factors for both PFS and OS (p = 0.001).
Fig. 4.
Multivariate cox regression analysis (A: PFS, B: OS).
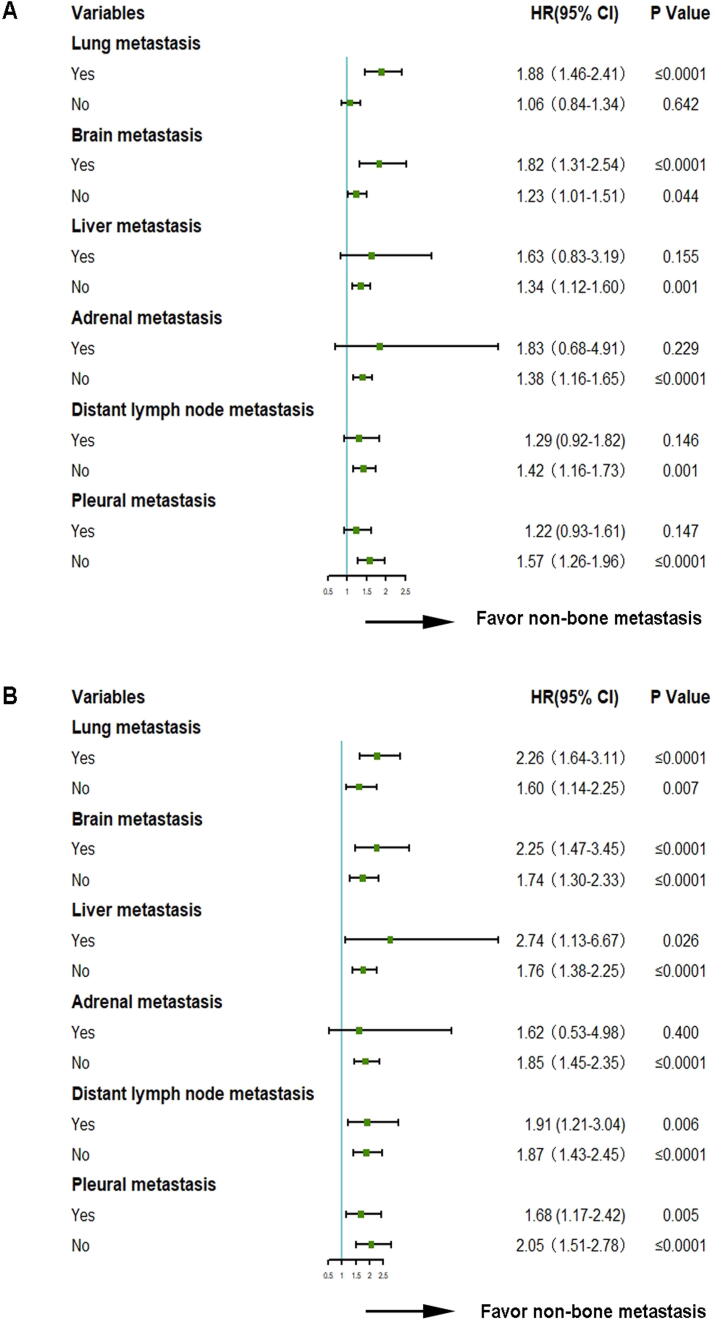
4.6. Subgroup analysis of metastatic organs
Subgroup analysis indicated that certain factors, including lung, brain, liver, adrenal gland, distant lymph node and pleural metastases, all influenced PFS and OS. The non-BoM group was always superior to BoM group, regardless of the presence of organ metastases (Fig. 5).
Fig. 5.
Different metastatic organs were associated with PFS (A) or OS (B).
4.7. Characteristics of patients with BoM
In the present study, the majority of the patients received treatment with bisphosphonates (n = 214, 71.3%). This treatment was not significantly associated with PFS (12.0 vs. 11.7 months, HR = 1.08, p = 0.538) or OS (31.7 vs. 33.2 months, HR = 0.86, p = 0.393). Only 3 patients received denosumab due to inaccessibility. A total of 67 (22.3%) and 19 (6.3%) patients received radiotherapy and surgery, respectively. Only a small number of patients experienced skeletal-related events (SRE, n = 35, 11.7%), including pathological fractures (n = 24, 8.0%), spinal cord compression (n = 6, 2.0%) and hypercalcemia (n = 5, 1.7%). The onset of SRE was associated with worse OS (33.5 vs. 26.1 months, HR = 1.84, p = 0.005).
5. Discussion
The present study described the association between BoM and therapeutic efficacy of TKIs in patients with advanced NSCLC. It was demonstrated that BoM was associated with poor PFS and OS, and the inferior treatment efficacy could not be rescued by osimertinib as salvage therapy. In addition, the BoM group was prone to multi-organ metastases, and had more frequent re-occurring mutations and more complex aberrations. Finally, BoM was identified as an independent prognostic factor for both PFS and OS.
The mechanism of tumor metastasis to specific organs remains unknown. Chen et al. discovered that osteopontin (a SIBLING glycoprotein) was a predictive biomarker that was significantly associated with prognosis of NSCLC patients with BoM [9], while Hsiao et al. reported that lumican (a protein of the leucin-rich proteoglycan family and a main proteoglycan component of bone matrix) can promoted lung cancer cell migration to the bone via an autocrine regulatory mechanism [10]. The results of the present study also indicated the metastasis to the bone was not random, but a highly regulated event.
The fact that BoM is associated with worse outcome in lung cancer has been well established [11], [12], [13], [14], [15]. Sugiura et al. reported that EGFR-TKIs may improve treatment efficacy in patients with BoM compared with other therapies [16]. Subsequent studies also confirmed that patients with BoM could benefit from treatment with TKIs [17], [18]. However, no studies have described the prognosis in NSCLC patients with BoM at the time of diagnosis and TKIs administered as initial therapy. To the best of our knowledge, our study was the first to demonstrate that BoM was linked to poor prognosis in patients receiving TKIs. Although osimertinib was recommended as salvage therapy for patients with T790M mutation after treatment with TKIs [19], [20], the inferior efficacy of BoM could not be improved by osimertinib as second-line therapy in the present study. These results again validated that BoM was an independent factor for unfavorable outcome.
At present, the precise mechanism underlying the negative prognostic effect of BoM in NSCLC remains unclear. In this study, it was found that BoM was associated with propensity for multi-organ metastases, including brain and liver metastases, both of which are well-known factors associated with dismal prognosis [21], [22]. A recent study based on the SEER database demonstrated that the prognosis of lung cancer worsened with increasing number of metastatic sites [23]. Our study revealed that multi-organ involvement was more commonly observed in the BoM group.
In addition, several studies demonstrated that NSCLC patients with more gene mutations had a higher rate of organ metastases [24], [25], [26], [27], [28], [29], [30], [31]. In the present study, the BoM group exhibited a higher frequency of mutations, which may be related to cancer cell transfer to other sites. Whether the genetic aberrations contributed to the selection of the organ metastasis pattern remains largely unknown, but there is evidence the genomic heterogeneity is associated with the clinical unresponsiveness to treatment [32], [33], [34].
There were certain limitations to the present study. First, due to the retrospective design of the study, potential biases were inevitable. The treatments were performed as per the physicians’ discretion, and were not based on randomization, which may lead to selection bias. In addition, subsequent therapies varied, which may affect survival. Furthermore, the presence of BoM was based on different radiographic examinations, each with different sensitivity and specificity. Second, genetic profiles were available in only a small fraction of our patients. This was because an NGS targeted sequencing platform was not available prior to 2017. Those without information on their genetic mutation landscape may possibly confound the present analysis. Third, the comparison of the BoM and non-BoM groups was not based on randomization.
In summary, the present study demonstrated that BoM was an independent poor prognostic factor for patients with advanced NSCLC harboring EGFR mutations after TKI treatment, and the outcome could not be improved by subsequent osimertinib therapy. Such patients were more likely to develop multiple organ metastasis and exhibited a higher frequency of gene mutations, which may be one of the reasons for the worse outcome observed among NSCL patients with BoM. These findings may help to further improve our current understanding of the clinical and prognostic characteristics of BoM, and they may also guide the design of more aggressive treatment for these patients.
6. Author Statement
None.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2021.100369.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R., Torre L., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Scagliotti G.V., Parikh P., von Pawel J., Biesma B., Vansteenkiste J., Manegold C., Serwatowski P., Gatzemeier U., Digumarti R., Zukin M., Lee J.S., Mellemgaard A., Park K., Patil S., Rolski J., Goksel T., de Marinis F., Simms L., Sugarman K.P., Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 3.Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., Gemma A., Harada M., Yoshizawa H., Kinoshita I., Fujita Y., Okinaga S., Hirano H., Yoshimori K., Harada T., Ogura T., Ando M., Miyazawa H., Tanaka T., Saijo Y., Hagiwara K., Morita S., Nukiwa T. Gefitinib or Chemotherapy for Non–SmallCell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C., Wu Y.-L., Chen G., Feng J., Liu X.-Q., Wang C., Zhang S., Wang J., Zhou S., Ren S., Lu S., Zhang L., Hu C., Hu C., Luo Y., Chen L., Ye M., Huang J., Zhi X., Zhang Y., Xiu Q., Ma J., Zhang L., You C. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y., Zhang L., Liu X., Zhou C., Zhang L., Zhang S., Wang D., Li Q., Qin S., Hu C., Zhang Y., Chen J., Cheng Y., Feng J., Zhang H., Song Y., Wu Y.-L., Xu N., Zhou J., Luo R., Bai C., Jin Y., Liu W., Wei Z., Tan F., Wang Y., Ding L., Dai H., Jiao S., Wang J., Liang L., Zhang W., Sun Y. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953–961. doi: 10.1016/S1470-2045(13)70355-3. [DOI] [PubMed] [Google Scholar]
- 6.Santini D., Barni S., Intagliata S., Falcone A., Ferraù F., Galetta D., Moscetti L., La Verde N., Ibrahim T., Petrelli F., Vasile E., Ginocchi L., Ottaviani D., Longo F., Ortega C., Russo A., Badalamenti G., Collovà E., Lanzetta G., Mansueto G., Adamo V., De Marinis F., Satolli M.A., Cantile F., Mancuso A., Tanca F.M., Addeo R., Russano M., Sterpi M., Pantano F., Vincenzi B., Tonini G. Natural History of Non-Small-Cell Lung Cancer with Bone Metastases. Sci. Rep. 2015;5(1) doi: 10.1038/srep18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuchuk M., Kuchuk I., Sabri E., Hutton B., Clemons M., Wheatley-Price P. The incidence and clinical impact of bone metastases in non-small cell lung cancer. Lung Cancer. 2015;89(2):197–202. doi: 10.1016/j.lungcan.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Kuchuk M., Addison C.L., Clemons M., Kuchuk I., Wheatley-Price P. Incidence and consequences of bone metastases in lung cancer patients. J Bone Oncol. 2013;2(1):22–29. doi: 10.1016/j.jbo.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Liu H., Wu W., Li Y., Li J. Osteopontin genetic variants are associated with overall survival in advanced non-small-cell lung cancer patients and bone metastasis. J. Exp. Clin. Cancer Res. 2013;32:45. doi: 10.1186/1756-9966-32-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsiao K.-C., Chu P.-Y., Chang G.-C., Liu K.-J. Elevated Expression of Lumican in Lung Cancer Cells Promotes Bone Metastasis through an Autocrine Regulatory Mechanism. Cancers. 2020;12:233. doi: 10.3390/cancers12010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 12.Hong S.-H., Kim Y.-S., Lee J.E., Kim I.-H., Kim S.J., Han D., Yoo I.R., Chung Y.-G., Kim Y.-H., Lee K.-Y., Kang J.-H. Clinical characteristics and continued epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor administration in EGFR-mutated non-small cell lung cancer with skeletal metastasis. Cancer Res. Treat. 2016;48:1110–1119. doi: 10.4143/crt.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang J.A., Lee J.Y., Kim W.S., Song J.S., Rho J.K., Choi C.-M., Lee J.C. Clinical implications of isolated bone failure without systemic disease progression during EGFR-TKI treatment. Clin. Lung Cancer. 2016;17(6):573–580.e1. doi: 10.1016/j.cllc.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Lin J.J., Cardarella S., Lydon C.A., Dahlberg S.E., Jackman D.M., Jänne P.A., Johnson B.E. Five-Year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J. Thorac. Oncol. 2016;11(4):556–565. doi: 10.1016/j.jtho.2015.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willeumier J.J., van der Hoeven N.M.A., Bollen L., Willems L.N.A., Fiocco M., van der Linden Y.M., Dijkstra P.D.S. Epidermal growth factor receptor mutations should be considered as a prognostic factor for survival of patients with pathological fractures or painful bone metastases from non-small cell lung cancer. Bone Joint J. 2017;99-B(4):516–521. doi: 10.1302/0301-620X.99B4.BJJ-2016-0872.R1. [DOI] [PubMed] [Google Scholar]
- 16.Sugiura H., Yamada K., Sugiura T., Hida T., Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin. Orthop. Relat. Res. 2008;466:729–736. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae H.-M., Lee S.-H., Kim T.M., Kim D.-W., Yang S.-C., Wu H.G., Kim Y.W., Heo D.S. Prognostic factors for non-small cell lung cancer with bone metastasis at the time of diagnosis. Lung Cancer. 2012;77(3):572–577. doi: 10.1016/j.lungcan.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 18.Cetin K., Christiansen C.F., Jacobsen J.B., Nørgaard M., Sørensen H.T. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: A Danish population-based cohort study. Lung Cancer. 2014;86:247–254. doi: 10.1016/j.lungcan.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., Okamoto I., Zhou C., Cho B.C., Cheng Y., Cho E.K., Voon P.J., Planchard D., Su W.C., Gray J.E., Lee S.M., Hodge R., Marotti M., Rukazenkov Y., Ramalingam S.S., Investigators F. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 20.Ramalingam S.S., Vansteenkiste J., Planchard D., Cho B.C., Gray J.E., Ohe Y., Zhou C., Reungwetwattana T., Cheng Y., Chewaskulyong B., Shah R., Cobo M., Lee K.H., Cheema P., Tiseo M., John T., Lin M.C., Imamura F., Kurata T., Todd A., Hodge R., Saggese M., Rukazenkov Y., Soria J.C., Investigators F. Overall survival with osimertinib in untreated EGFR-mutated advanced NSCLC. N. Engl. J. Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 21.Sperduto P.W., Kased N., Roberge D., Xu Z., Shanley R., Luo X., Sneed P.K., Chao S.T., Weil R.J., Suh J., Bhatt A., Jensen A.W., Brown P.D., Shih H.A., Kirkpatrick J., Gaspar L.E., Fiveash J.B., Chiang V., Knisely J.P., Sperduto C.M., Lin N., Mehta M. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura T., Kurishima K., Nakazawa K., Kagohashi K., Ishikawa H., Satoh H., Hizawa N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol. Clin. Oncol. 2015;3:217–221. doi: 10.3892/mco.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C., Mao M., Guo X., Cui P., Zhang L., Xu Y., Li L., Han X., Peltzer K., Xiong S., Baklaushev V.P., Wang X., Wang G. Nomogram based on homogeneous and heterogeneous associated factors for predicting bone metastases in patients with different histological types of lung cancer. BMC Cancer. 2019;19:238. doi: 10.1186/s12885-019-5445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Confavreux C.B., Girard N., Pialat J.B., Bringuier P.P., Devouassoux-Shisheboran M., Rousseau J.C., Isaac S., Thivolet-Bejui F., Clezardin P., Brevet M. Mutational profiling of bone metastases from lung adenocarcinoma: results of a prospective study (POUMOS-TEC) Bonekey Rep. 2014;3:580. doi: 10.1038/bonekey.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto D., Ueda H., Shimizu R., Kato R., Otoshi T., Kawamura T., Tamai K., Shibata Y., Matsumoto T., Nagata K., Otsuka K., Nakagawa A., Otsuka K., Katakami N., Tomii K. Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: importance of bone metastasis. Clin. Exp. Metastasis. 2014;31(5):543–551. doi: 10.1007/s10585-014-9648-3. [DOI] [PubMed] [Google Scholar]
- 26.Hendriks L.E.L., Smit E.F., Vosse B.A.H., Mellema W.W., Heideman D.A.M., Bootsma G.P., Westenend M., Pitz C., de Vries G.J., Houben R., Grünberg K., Bendek M., Speel E.J.M., Dingemans A.M.C. EGFR mutated non-small cell lung cancer patients: More prone to development of bone and brain metastases? Lung Cancer. 2014;84:86–91. doi: 10.1016/j.lungcan.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Guan J., Chen M., Xiao N., Li L.u., Zhang Y., Li Q., Yang M.i., Liu L., Chen L. EGFR mutations are associated with higher incidence of distant metastases and smaller tumor size in patients with non-small-cell lung cancer based on PET/CT scan. Med. Oncol. 2016;33(1) doi: 10.1007/s12032-015-0714-8. [DOI] [PubMed] [Google Scholar]
- 28.Li H., Cao J., Zhang X., Song X., Wang W., Jia S., Li Z., Jia H., Cao X., Zhou W., Lian J., Han S., Yang W., Xi Y., Lian S., Jing H. Correlation between status of epidermal growth factor receptor mutation and distant metastases of lung adenocarcinoma upon initial diagnosis based on 1063 patients in China. Clin. Exp. Metastasis. 2017;34(1):63–71. doi: 10.1007/s10585-016-9822-x. [DOI] [PubMed] [Google Scholar]
- 29.Kuijpers C.C.H.J., Hendriks L.E.L., Derks J.L., Dingemans A.-M.-C., van Lindert A.S.R., van den Heuvel M.M., Damhuis R.A., Willems S.M. Association of molecular status and metastatic organs at diagnosis in patients with stage IV non-squamous non-small cell lung cancer. Lung Cancer. 2018;121:76–81. doi: 10.1016/j.lungcan.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Krpina K., Budimir B., Kukulj S., Marušić A., Jakopović M., Samaržija M. Correlation of molecular status and anatomic sites of metastases at diagnosis of EGFR positive non-small cell lung cancer (NSCLC), A single institution experience. Ann. Oncol. 2019;30:ii52. doi: 10.1093/annonc/mdz063.034. [DOI] [Google Scholar]
- 31.Digumarthy S.R., Mendoza D.P., Lin J.J., Rooney M., Do A., Chin E., Yeap B.Y., Shaw A.T., Gainor J.F. Imaging features and patterns of metastasis in non-small cell lung cancer with RET rearrangements. Cancers (Basel) 2020;12(3):693. doi: 10.3390/cancers12030693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee C.K., Man J., Lord S., Links M., Gebski V., Mok T., Yang J.-H. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-A meta-analysis. J Thorac Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Lisberg A., Cummings A., Goldman J.W., Bornazyan K., Reese N., Wang T., Coluzzi P., Ledezma B., Mendenhall M., Hunt J., Wolf B., Jones B., Madrigal J., Horton J., Spiegel M., Carroll J., Gukasyan J., Williams T., Sauer L., Wells C., Hardy A., Linares P., Lim C., Ma L., Adame C., Garon E.B. A phase II study of pembrolizumab in EGFR-Mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J. Thorac. Oncol. 2018;13(8):1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai G.G.Y., Lim T.H., Lim J., Liew P.J.R., Kwang X.L., Nahar R., Aung Z.W., Takano A., Lee Y.Y., Lau D.P.X., Tan G.S., Tan S.H., Tan W.L., Ang M.-K., Toh C.K., Tan B.S., Devanand A., Too C.W., Gogna A., Ong B.H., Koh T.P.T., Kanesvaran R., Ng Q.S., Jain A., Rajasekaran T., Yuan J.u., Lim T.K.H., Lim A.S.T., Hillmer A.M., Lim W.T., Iyer N.G., Tam W.L., Zhai W., Tan E.-H., Tan D.S.W. Clonal MET amplification as a determinant of tyrosine kinase inhibitor resistance in epidermal growth factor receptor-mutant non–small-cell lung cancer. J. Clin. Oncol. 2019;37(11):876–884. doi: 10.1200/JCO.18.00177. [DOI] [PubMed] [Google Scholar]