Highlights
-
•
Expression of activator protein-1 (AP-1) family is significantly elevated in triple-negative breast cancer (TNBC), compared with that in other breast cancer subtypes.
-
•
T-5224, an inhibitor of c-Fos/AP-1 resulted in increase of apoptosis and inhibition of proliferation, migration, and invasion of TNBC cells.
-
•
High OLFML2A level is associated with poor prognosis in TNBC patients that were identified from multiple clinical data sets on line.
-
•
AP1-overexpressing TNBC dependent on OLFML2A, and targeting both AP-1 and OLFML2A through T‐5224 may be a synergistic therapeutic strategy for this clinically challenging subset of breast cancer.
Keywords: AP-1, OLFML2A, Triple-negative breast cancer, T-5224
Abbreviation: AP-1, Activator protein-1; BC, Breast cancer; DEGs, Differentially expressed genes; ER, Estrogen receptor; GOBO, Gene expression-based Outcome for Breast cancer Online; GO, Gene Ontology; HER2, Human epidermal growth factor 2; KEGG, Kyoto Encyclopedia of Genes and Genomes; MMPs, Matrix metalloproteinases; OLFML2A, Olfactomedin-like 2A; OLFML2B, Olfactomedin-like 2B; ORR, Overall response rate; PR, Progesterone receptor; PFS, Progression-free survival; TCGA, The Cancer Genome Atlas; TNBC, Triple-negative breast cancer
Abstract
Previous studies have shown that expression of activator protein-1 (AP-1) family is significantly elevated in triple-negative breast cancer (TNBC), compared with that in other breast cancer subtypes. Here we investigated the anti-tumor effect and mechanism of T-5224, an inhibitor of c-Fos/AP-1, on TNBC. We identified that T-5224 inhibited the proliferation, migration, and invasion of TNBC cells and resulted in an increase in apoptosis. Furthermore, we found that OLFML2A is a key regulatory protein acting downstream of AP-1 and is involved in T-5224-targeted AP-1 action. Multiple clinical databases online have identified that high OLFML2A level is associated with poor prognosis in TNBC patients. In summary, our experimental and bioinformatic studies indicated that OLFML2A is necessary for AP-1-overexpressing TNBC. These findings demonstrate that AP-1-overexpressing TNBC dependent on OLFML2A, and targeting both AP-1 and OLFML2A through T‐5224 may be a synergistic therapeutic strategy for this clinically challenging subset of breast cancer.
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer in women, contributing to one of the leading mortalities among women worldwide [1]. There were approximately 2.3 million newly diagnosed BC cases and 0.685 million death worldwide in 2020 [1]. Triple negative breast cancer (TNBC) characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2 (HER2) accounts for 12–17% of all BC [2]. TNBC are usually found in young patients and is associated with high histologic grade, visceral metastasis and distant recurrence [2], [3], [4], [5], [6]. Currently, chemotherapy is the only treatment for TNBC; however, patients with TNBC usually have poor clinical outcomes: the overall response rates (ORRs) is ranged from 10 to 35%, and the progression-free survival (PFS) time is only 2–4 months [7]. Discovering druggable molecular targets is necessary for the treatment of TNBC.
Activator protein-1 (AP-1) transcription factor, consisting of dimeric complexes (either homodimers of Jun family members [c-Jun, JunB, and JunD] or heterodimers of Jun and Fos family members [c-Fos, FosB, Fra-1, and Fra-2]), is a potential therapeutic target for TNBC treatment [8,9]. Compared with ER-positive and HER2-positive cell lines, c-Jun and Fra-1 are overexpressed in TNBC cell lines [10]. Moreover, overexpressed c-Jun and Fra-1 promote the invasion of TNBC cells by repressing the E-cadherin expression via transcriptional upregulation of ZEB2 [10]. The upregulation of Fra-1 is associated with the poor prognosis of TNBC patients [10]. Interestingly, targeting AP-1 by doxycycline-inducible c-Jun dominant negative mutant (Tam67) delays mammary tumor formation in MMTV-erbB2 mice [11], documenting a therapeutic potential of AP-1 in TNBC.
T-5224, an inhibitor of c-Fos/AP-1, is the only selective AP-1 inhibitor used in human clinical trials presently [12]. T-5224 specifically inhibits the DNA binding activity of c-Fos/c-Jun, rather than affects other transcription factors, including C/EBPa and ATF-2, MyoD, Sp-1, and NF-κB/p65 [13]. T-5224 inhibits the development of collagen-induced arthritis through reducing the level of AP-1-mediated inflammatory cytokines (interleukin 1β and matrix-degrading matrix metalloproteinases [MMPs]) [13]. T-5224 markedly suppresses the immune system and prolongs allograft by reducing the nuclear c-Fos and p-c-Jun protein levels and the production of interleukin 2 and interferon-γ in T cells [14]. In addition, T-5224 inhibits the invasion, migration and MMPs of head and neck squamous cell carcinoma (HNSCC) cells in vitro, and prevents lymph node metastasis in HNSCC in an animal model [15]. In this study, we investigated the anti-tumor effect of T-5224 on TNBC and key downstream targets of AP-1 in TNBC pathogenesis.
Materials and methods
Cell culture
MCF-7, T47D, BT549 and Hs578T cells were obtained from FuHeng Cell Center, Shanghai, China. All these cells were authenticated by STR profiling by FuHeng Cell Center. LCC2 cells were derived from MCF-7 cells which were continuously exposed to tamoxifen for six months. MCF-7, LCC2, and Hs578T cells were cultured in Dulbecco's modified Eagle's medium (DMEM, BioInd) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) (BioInd). T47D and BT549 cells were cultured in RPMI-1640 supplemented with 10% FBS and 1% P/S. All these cells were grown in an incubator at 5% CO2 and humidity.
Reagents
The cells were plated and incubated in DMEM or RPMI-1640 supplemented with 10% FBS, and 1% P/S for overnight. The culture media were replaced by DMEM or RPMI-1640 supplemented with 0.5% FBS, and 1% P/S. After 24 h, T-5224 (APExBIO Technology) was supplied in the culture media supplemented with 0.5% FBS, and 1% P/S for 48 h exposure in vitro experiments.
RNA-seq
Whole-transcriptome analysis with total RNA sequencing (RNA-seq) was performed in BioMiao Biological Technology company (Beijing). Illumina Hiseq Xten platform was used to sequence the total RNA samples on the basis of a read length of 2 × 150. HISAT2 version 2.0.0 was used to align raw FASTQ files against the hg19 genome, and utilized feature Counts of Subread with default parameters to count gene expression with transcriptome reference GENCODE v29 version. Fragments per kilobase of transcript per million mapped reads (FPKM) was used to normalize raw counts. Differentially expressed genes (DEGs) were screened by edgeR (R 3.5.2) with P< 0.05 and Fold change > 1.5 (up-regulated gene: the ratio of mean expression level of gene in T-5224 group to the mean expression level of gene in DMSO group > 1.5; down-regulated gene: the ratio of mean expression level of gene in DMSO group to the mean expression level of gene in T-5224 group > 1.5).
Bioinformatic analyses
Volcano plots and heatmaps were performed using ggplot2 (R 3.5.2) and Pheatmap (R 3.5.2), respectively. For genes greater than 3000, Gene Ontology (GO) analysis and Pathway Enrichment analysis (Kyoto Encyclopedia of Genes and Genomes [KEGG] analysis) were carried out using clusterProfiler (R 3.5.2). For genes less than 3000, GO analysis was performed using Functional Annotation Tool from DAVID Bioinformatics Resources 6.8 (https://david.ncifcrf.gov/). The mRNA expression dataset (RNA-seq data) and related clinical characteristics of BC patients were downloaded from The Cancer Genome Atlas (TCGA) (https://cancergenome.nih.gov/) database.
RNA interference
siRNA targeting OLFML2A (si_OLFML2A) and negative control siRNA (si_Ctrl) were purchased from Ambion (Ambion, USA). BT549 and Hs578T cells were transfected with the si_OLFML2A and si_Ctrl at a final concentration of 10 μM/well using the interferin transfection reagent (Polyplus) following the manufacturer's instructions. The culture media were replaced by fresh DMEM or RPMI-1640 media supplemented with 10% FBS, and 1% P/S 24 h after the transfection. The cells were harvested for quantitative real-time PCR analysis after the further culture 48 h or harvested for Western blot after 72 h.
RNA extraction and quantitative real-time PCR
Total RNA was extracted using EasyPure RNA Kit (TransGen Biotech [Beijing] Co., Ltd), and 2 μg of total RNA was reverse-transcribed into cDNA using FastKing gDNA Dispelling RT SuperMix (TianGen Biotech [Beijing] Co., Ltd) in accordance with the manufacturer's instructions. Real-time PCR was performed on Bio-Rad CFX96 Real-Time System using SuperReal PreMix Plus kit (TianGen Biotech [Beijing] Co., Ltd) following the manufacturer's protocol. Quantitation of 36B4 was used as an internal control. The relative expression level was calculated using the comparative Ct method. Differences between two parallel treatment groups were compared using unpaired two-tailed t-test. Primer sequences are listed in supplementary Table 1.
Protein extraction and western blot
Total protein was isolated using the total protein extraction kit (BestBio Science), and the concentrations were measured using BCA Protein Assay Kit (Beyotime Biotechnology). Proteins were separated on the 10% SDS PAGE gels, and subsequently transferred to polyvinylidene fluoride membranes (PALL corporation). The membranes were probed with primary antibodies Fra-1 (R-20) (1:500 dilution) (Santa Cruz Biotechnology), c-Jun (H-79) (1:500 dilution) (Santa Cruz Biotechnology), or OLFML2A (1:500 dilution) (Abcam plc., UK) made from rabbit, and then probed with donkey anti-rabbit IgG antibody (1:10,000 dilution) (GE Healthcare Company). The protein levels were normalized by probing β -actin antibody (1:10,000 dilution) (Sigma-Aldrich LLC.) and sheep anti-mouse IgG antibody (1:10,000 dilution) (GE Healthcare Company). Immunosignals were imaged using a Tanon 5200 Multi Automatic Chemiluminescence / Fluorescence Image Analysis System (China).
Chip-qPCR
Sheared chromatin was prepared by enzymatic shearing using Enzymatic Shearing Kit, and chromatin immunoprecipitation was performed using ChIP-IT Express Enzymatic Magnetic Chromatin Immunoprecipitation Kit (Active Motif [America] Co., Ltd) in accordance with the manufacturer's instructions. Purification of DNA from Chromatin Immunoprecipitation sample was performed using Chromatin IP DNA Purification Kit (Active Motif [America] Co., Ltd) according to the conditions specified by the manufacturer. Real-time PCR was performed on Bio-Rad CFX96 Real-Time System. Primer sequences were as follows: GGGGAGAGCTCACATTCTAAAC (farward); GCAAGTGTTTGGCTGACTCAC (reverse).
Cell proliferation assay
Cell proliferation was measured using WST-1 Kit (Roche Diagnostics) according to the manufacturer's instructions: 8000 cells/well BT549 or 2000 cells/well Hs578T were seeded in a 96-well plate to investigate the effect of T-5224, and 1000 cells/well BT549 or Hs578T were seeded in a 96-well plate to investigate the effect of OLFML2A-konckdown. 10μl of WST-1 reagent was added to the test well and incubated for 2 h when testing cell proliferation. The absorbance was measured at 450 nm using a multilabel plate reader (Thermo scientific).
Cell apoptosis assay
Cell apoptosis was detected by FITC Annexin V Apoptosis Detection Kit I (BD Biosciences) according to the manufacturer's instructions. Cells were harvested, rinsed twice with phosphate‐buffered saline (PBS) and resuspended in 1 × Binding Buffer at 1–5 × 106 cells/mL. 100ul of cells were stained with PI and FITC for 15 min at room temperature and protected from light. Finally, cells apoptosis was analyzed using a FACSCalibur (BD Biosciences) immediately after adding 400ul 1 × Binding Buffer.
Cell migration assay
The wound-healing assay was performed to assess the cell migration in response to the mechanical scratch. When cells reached 90% confluence, a yellow pipette tip was used to make a straight scratch. After the removal of the detached cells, the well was replenished with fresh media containing 0.5% FBS. The recovery of wound was recorded, and the images were captured by a microscope at 12 h, 24 h and 48 h after scratch.
Cell invasion assay
A total of 105 cells were seeded in the upper chamber of 24-well Growth Factor Reduced Corning Matrigel Invasion chamber (Corning) with serum-free media. Media containing 10% FBS and 1% P/S were added to the lower chamber. After 24 h, the cells in the upper chamber were scraped by a cotton swab which wetted by PBS. Cells that invaded to the lower chamber were fixed, stained, and counted under a microscope.
Statistical analysis
Variation analysis was performed using EXCEL and IBM SPSS 24.0 (SPSS Inc., Chicago, IL, USA). Normally distributed data are presented as mean and SD. Abnormally distributed data are presented as median and Q1 to Q3, with Q1 being the 25th percentile and Q3 being the 75th percentile. Correlation analysis and survival analysis were performed using R 3.5.2. P values <0.05 were taken to indicate statistical significance.
Results
Fra-1 is overexpressed in TNBC patients and TNBC cell lines
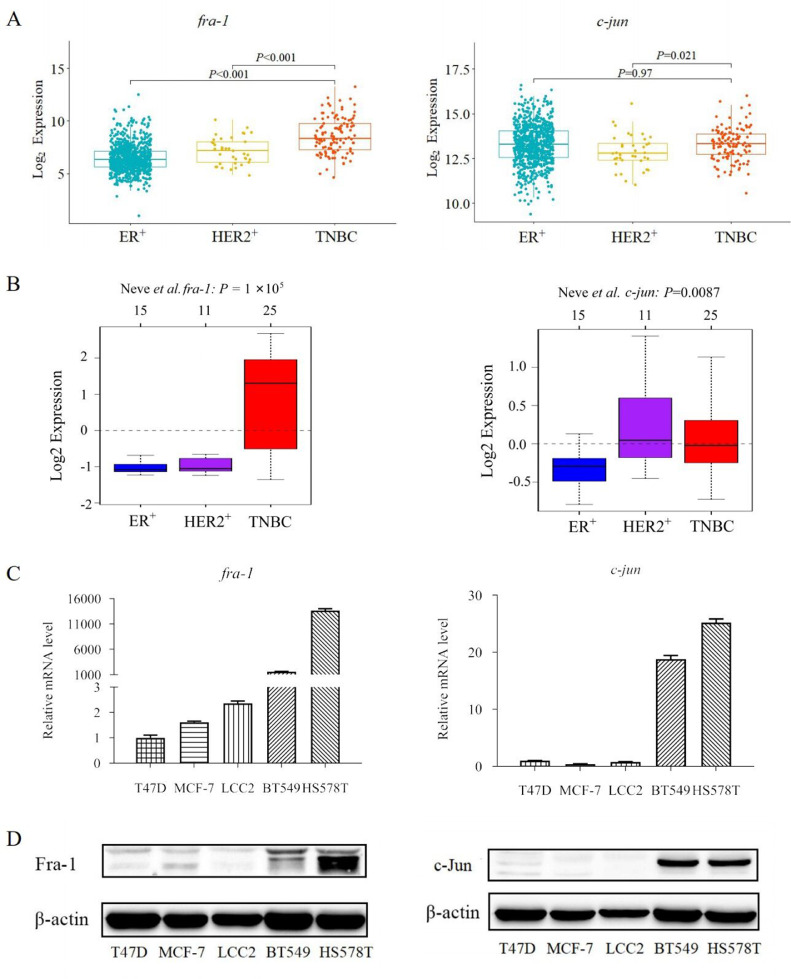
958 BC patients (807 ER-positive, 36 HER2-positive, and 115 TNBC patients) were analyzed with a complete set of immunohistochemical data available in TCGA database, revealing that Fra-1 was significantly overexpressed in TNBC patients (all P < 0.001); however, there was no difference on c-Jun expressions (all P > 0.05) (Fig 1A). We further analyzed GOBO database, identifying that Fra-1 was overexpressed in TNBC cell lines (P < 0.001) and c-Jun was highly expressed in HER2-positive BC cell lines (P < 0.01) (Fig 1B). The expressions of c-Jun, notably, were higher in TNBC cell lines than those in ER-positive BC cell lines. Next, we confirmed that the expressions of Fra-1 and c-Jun were higher in TNBC cell lines than those in non-TNBC cell lines both in mRNA expressions and protein levels using real-time PCR and Western blot respectively (Fig 1C and 1D). In fact, all these results documented that AP-1 upregulation was especially implicated in TNBC pathogenesis, suggesting that inhibition targeting AP-1 may identify key downstream targets of AP-1 in TNBC pathogenesis, enhancing a therapeutic strategy for TNBC.
Fig. 1.
c-Jun and Fra-1 are high expression in TNBC patients and TNBC cell lines. A. Expression analysis of c-Jun and Fra-1 in BC tissues enrolled in TCGA database. B. Expression analysis of c-Jun and Fra-1 in cell lines enrolled in GOBO database. C. mRNA levels of c-Jun and Fra-1 in TNBC cell lines and non-TNBC cell lines. D. Protein levels of c-Jun and Fra-1 in TNBC cell lines and non-TNBC cell lines.
T-5224 down-regulates the expression of AP-1 in TNBC cell lines
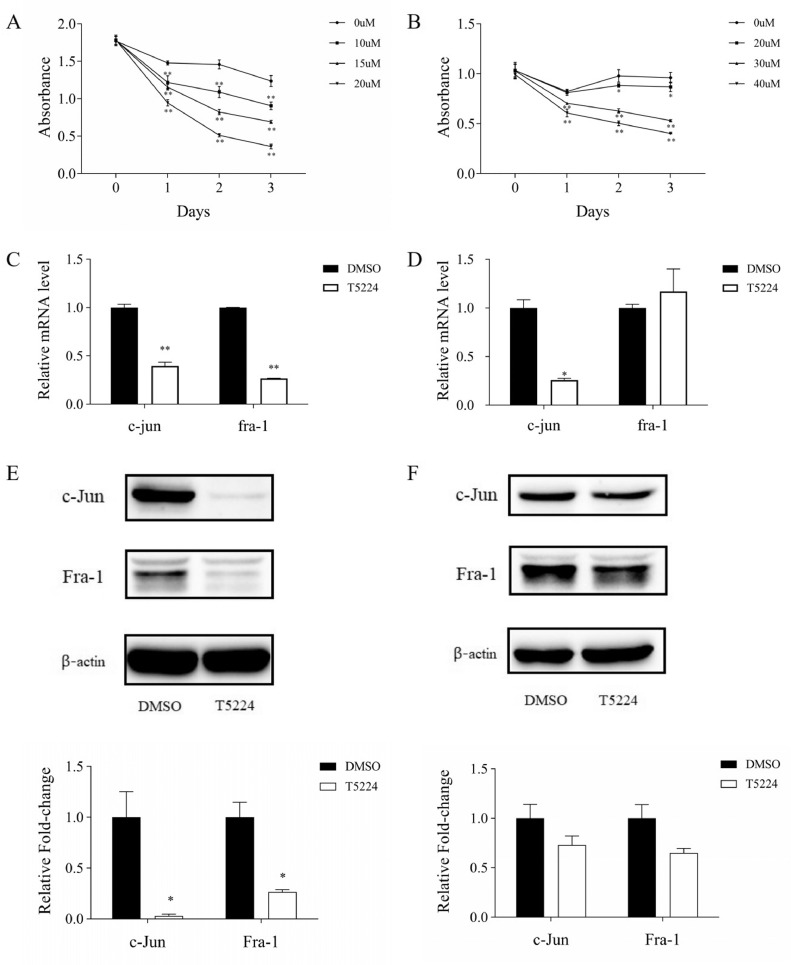
Due to the high expression of AP-1 in TNBC, we postulate that AP-1 plays an important role in TNBC proliferation. Thus, a selective AP-1 inhibitor, T-5224 was used to block AP-1 activation and inhibited TNBC cell growth. T-5224 has been previously investigated in phase II clinical trials for the treatment of arthritis, but the biological effect of T-5224 in BC remains unclear. To test effects of T-5224 on TNBC cells, cell proliferation assay was carried out in TNBC cell lines (BT549 and Hs578T). The results showed that BT549 cells were sensitive to T-5224 at the concentration of 15uM and Hs578T cells were sensitive to T-5224 at the concentration of 40uM (Fig 2A, 2B). Thus, the concentrations of 15uM and 40uM T-5224 were used to specifically block AP-1 activation in present experiments, respectively. Moreover, treatment with T-5224 changed the morphology of TNBC cells: pre-apoptotic cells, shrunk, thinned and stretched cells, and dead cells were observed (supplementary fig s1).
Fig. 2.
T-5224 down-regulates the expression of AP-1 in TNBC cell lines A. T-5224 inhibits the proliferation of BT549 cells; B. T-5224 inhibits the proliferation of Hs578T cells; C. mRNA levels of c-Jun and Fra-1 in BT549 cells treated with DMSO or T-5224; D. mRNA levels of c-Jun and Fra-1 in Hs578T cells treated with DMSO or T-5224; E. Protein levels of c-Jun and Fra-1 in BT549 cells treated with DMSO or T-5224; F. Protein levels of c-Jun and Fra-1 in Hs578T cells treated with DMSO or T-5224. *P < 0.05, **P < 0.01 compared with DMSO.
To examine whether T-5224 efficiently inhibits AP-1 in TNBC cells, we studied expressions of its heterodimeric partners of Fra-1 and c-Jun in BT549 and Hs578T cells following T-5224 treatment. We found that both Fra-1 and c-Jun were significantly downregulated at mRNA expressions and protein expression levels in BT549 cells (Fig 2C, 2E). In Hs578T cells, T-5224 treatment significantly reduced c-Jun mRNA expression at 48 h and c-Jun protein levels at 72 h (Fig 2D, 2F). Similarly, both Fra-1 mRNA and protein expression levels were significantly reduced following T-5224 treatment for 72 h (Fig 2D, 2F and supplementary fig s2). These data suggest that T-5224 treatment in TNBC cell lines selectively inhibits AP-1 and results in the reduction of expression of Fra-1 and c-Jun.
T-5224 inhibits progression of TNBC cells
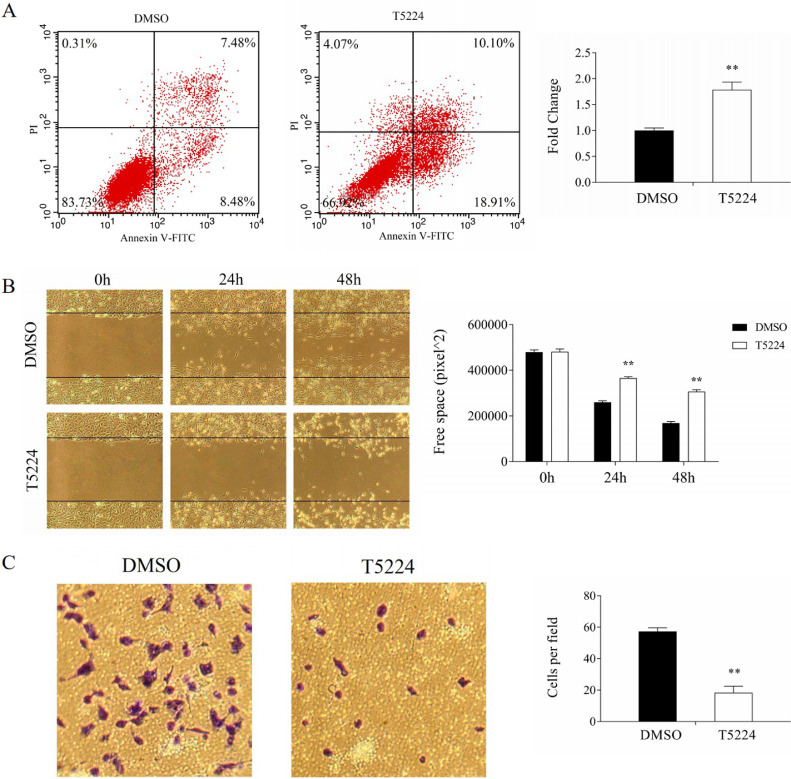
AP-1 is a key regulator of TNBC progression [16], triggering us to investigate the effect of T-5224 on proliferation, migration and invasion of TNBC cells. The proliferation of TNBC cells was significantly inhibited by T-5224 (Fig. 2A, 2B) and the wound-healing was remarkably retarded by T-5224 (Fig. 3B, supplementary Fig. s3B). Furthermore, transwell invasion assay showed that T-5224 decreased the invasions of BT549 cells and Hs578T cells (Fig. 3C, supplementary Fig. s3C). Increased apoptosis contributes to the proliferation, migration and invasion of cancer cells [17]. T-5224 significantly promoted the apoptosis of BT549 cells and Hs578T cells (Fig. 3A, supplementary Fig. s3A). Thus, T-5224 promotes the apoptosis of TNBC cells, reflecting the inhibition of migration and invasion of TNBC cells.
Fig. 3.
Effect of T-5224 on BT549 cells. A. T-5224 promoted apoptosis of BT549 cells; B. T-5224 inhibited migration of BT549 cells; C. T-5224 inhibited invasion of BT549 cells. **P < 0.01 compared with DMSO (n = 3).
OLFML2A is necessary for T-5224-targeted AP-1 action
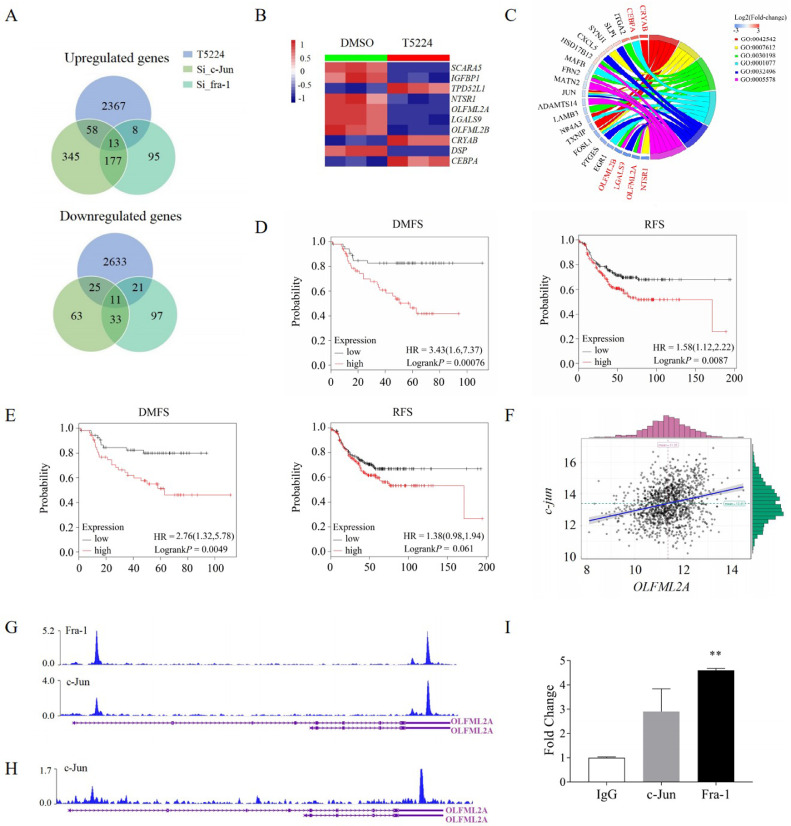
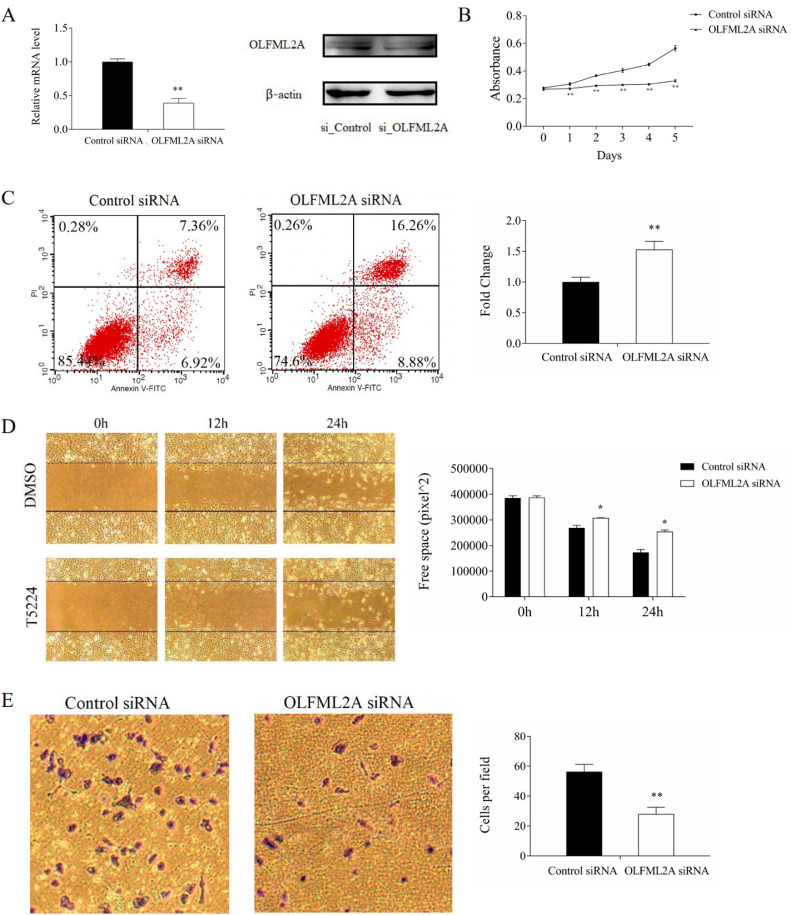
2446 (47.6%) up-regulated genes and 2690 (52.4%) down-regulated genes were observed after the validation of RNA-Seq (supplementary Fig. s4). To determine the target genes of AP-1 transcription factor, we overlapped the genome-wide DEGs after c-Jun knockdown, fra-1 knockdown, and T-5224 treatment in the BT549 cells and identified 79 genes upregulated and 57 genes downregulated (Fig 4A). Among the top ten DEGs (Fig 4B; supplementary Table 2) of these 136 genes, six genes (NTSR1, OLFML2A, LGALS9, OLFML2B, CRYAB, and CEBPA) were involved in biological processes, cellular components and molecular functions (Fig 4C). To find out relationships between the expressions of these genes and the prognosis of ER-negative (ER-negative and PR-negative) patients, we explored the Kaplan-Meier plotter database and revealed that ER-negative patients with either higher olfactomedin-like 2A (OLFML2A) expression or higher olfactomedin-like 2B (OLFML2B) expression had poorer recurrence-free survivals and distance metastasis free survivals, compared to either lower OLFML2A expressions or lower OLFML2B expressions (Fig 4D, 4E). Interestingly, strong correlations were found between OLFML2A and c-Jun gene expressions, suggesting that OLFML2A is a potential target gene of T-5224-targeted AP-1 (Fig 4F, supplementary Fig s5). We further found that c-Jun was bound to OLFML2A from the ChIP-Seq data in the Cistrome Data Browser database (Fig 4G, 4H). ChIP-PCR analysis clearly confirmed the recruitment of Fra-1 and c-Jun to OLFML2A (Fig 4I). Those results highlighted that OLFML2A was a target gene of AP-1 transcription factor, further confirmed by low expressions of OLFML2A in both mRNA and protein expression levels when treated with T-5224 (supplementary Fig s6). The knockdown of OLFML2A significantly inhibited proliferation, wound healing, and invasion of TNBC cells and promoted apoptosis of TNBC cells (Fig 5A–5E, supplementary Fig s7A–s7E). Therefore, OLFML2A is necessary for T-5224-targeted AP-1 action.
Fig. 4.
OLFML2A is a target gene of AP-1 transcription factor A. Venn diagram showing the overlap between c-Jun knockdown and transfection with T-5224 or fra-1 knockdown and transfection with T-5224 in the BT549 cells; B. Heatmap showing the top 10 differentially expressed genes in 136 overlapped genes; C. GO analysis of 136 overlapped genes including the top 10 differentially expressed genes; D. Expression level of OLFML2A was associated with prognosis of ER-negative breast cancer patients; E. Expression level of OLFML2B was associated with prognosis of ER-negative breast cancer patients; F. Correlation between the expression of OLFML2A and c-Jun; G. Binding region of c-Jun or Fra-1 in the OLFML2A gene of CHIP-seq from research of Zhao et al. in the Cistrome Data Browser database; H. Binding region of c-Jun in the OLFML2A gene of CHIP-seq from research of Qiao et al. in the Cistrome Data Browser database; I: ChIP-PCR analysis confirms the recruitment of Fra-1 and c-Jun to OLFML2A.
Fig. 5.
Effect of OLFML2A knockdown on BT549 cells. A. OLFML2A was successfully inhibited both in mRNA and protein level by si_OLFML2A; B. OLFML2A knockdown inhibited proliferation of BT549 cells; C. OLFML2A knockdown promoted apoptosis of BT549 cells; D. OLFML2A knockdown inhibited migration of BT549 cells; E. OLFML2A knockdown inhibited invasion of BT549 cells. **P < 0.01 * P < 0.05 compared with DMSO.
High OLFML2A level is associated with poor prognosis in TNBC patients
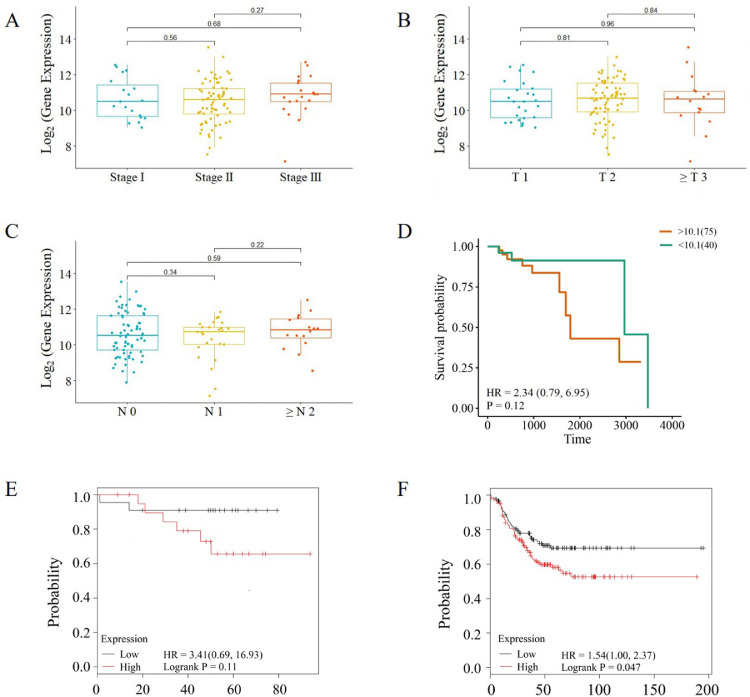
We further selected 115 TNBC patients with a complete set of survival data from TCGA database to validate relationships between OLFML2A expressions and the prognosis of patients with TNBC. The results showed that TNBC patients with higher OLFML2A expression had higher AJCC stage, T stage, M stage of tumor, and poorer overall survival outcomes than those with lower OLFML2A expressions (Fig 6A-6D). Analyzing the Kaplan-Meier plotter database revealed a same result that TNBC patients with higher OLFML2A expression had poorer recurrence-free survivals and distance metastasis free survivals than those with lower OLFML2A expressions (Fig 6E, 6F).
Fig. 6.
Expression level of OLFML2A is associated with tumor stage and prognosis of TNBC patients. A: TNBC patients with higher OLFML2A expression had higher AJCC stage tumor; B: TNBC patients with higher OLFML2A expression had higher T stage tumor; C: TNBC patients with higher OLFML2A expression had higher M stage of tumor; D. Expression level of OLFML2A was associated with prognosis of TNBC patients; E. TNBC patients with high OLFML2A expression have poorer distance metastasis free survival outcomes than those with low OLFML2A expression; F. TNBC patients with high OLFML2A expression have poorer recurrence-free survival outcomes than those with low OLFML2A expression.
Discussion
Due to the poor clinical outcome and the lack of therapeutic options, identifying druggable molecular targets is necessary for the treatment of TNBC. Previous studies have shown that AP-1 upregulation was vitally important in TNBC pathogenesis, providing a potential therapeutic target for TNBC. RNA-based therapeutics, such as siRNAs, microRNAs (miRNAs), antisense oligonucleotides, and CRISPR–Cas9, have shown great potentials in targeting AP-1 and created entirely new therapeutic paradigms in TNBC. However, overcoming the lipid bilayer to deliver RNAs into cells has remained in a major problem in the development of RNA therapeutics [18]. T-5224, a selective AP-1 inhibitor, has been previously investigated in phase II clinical trials for treating arthritis. On the basis of those observations, we hypothesized that T-5224 could be used to inhibit AP-1 activation and inhibit the TNBC progression. In the present study, we investigated the anti-tumor effect of T-5224 along with its molecular mechanisms. Our results demonstrated that T‐5224 inhibits the proliferation, migration, and invasion of TNBC cells via AP-1 OLFML2A axis, suggesting OLFML2A a key regulatory protein involved in T-5224-targeted AP-1 against TNBC.
AP-1 (Jun and Fos family members) has been implicated in the pathogenesis of lung cancer [19,20], colorectal cancer [21], prostate cancer [22], ovarian cancer [23], and BC [24,25]. The mRNA levels of AP-1 family members (Fra-1, Fra-2, Jun-B, and Jun-D) are significantly higher in BC tissues than those in adjacent non-neoplastic tissues [24]. Moreover, c-Jun overexpression in MCF-7 cells is associated with low expression of ER, reflecting tamoxifen resistance [26]. Overexpression of c-Jun and Fra-1 promote invasion of BT549 cells and Hs578T cells by repressing the expression of E-cadherin via transcriptional upregulation of ZEB2 [10]. Targeting AP-1 by Tam67 inhibits BC cell growth in vitro and in vivo [11]. Similarly, we revealed that T-5224, an inhibitor of the c-Fos/AP-1, significantly inhibited the proliferation, migration and invasion of TNBC cells and promoted apoptosis of TNBC cells. However, Sunao Tanaka et al. found that T-5224 alone exhibited no anticancer effect but could enhance the anticancer effect of eribulin [27]. The discrepancy between ours and Sunao's may be due to the dose difference of T-5224. Sunao Tanaka et al. used 10 nm T-5224 to investigate the effect of T-5224 on TNBC cell growth whereas in our study, BT549 cells were found to be sensitive to T-5224 at the concentration of 15uM and Hs578T cells were sensitive to T-5224 at the concentration of 40uM. Overall, these results document a critical role of AP-1 in BC.
AP-1 is an oncoprotein involved in the regulations of transcription, cellular proliferation, transformation, and invasion [12]. Comprehensive characterization of AP-1 signaling in TNBC is of pivotal importance in understanding how AP-1 contributes to the proliferative and invasive phenotypes of TNBC. Using microarray, our studies previously identified differential expressions of 419 and 690 genes upon Fra-1 and c-Jun knockdown respectively [10]. Microarrays represented the most comprehensive approach in measuring gene expression and has been used to derive a series of gene expression signatures aimed at characterizing the biology of the disease and helping clinicians predict relapse and treatment response [28]. In this study, we investigated key downstream targets of AP-1 in TNBC following T-5224 treatments using Next Generation RNA-seq. We identified 2446 up-regulated genes and 2690 down-regulated genes after T-5224 treatments. We also searched common target genes of the AP-1 transcription factor by overlapping the genome-wide DEGs data after c-Jun knockdown, fra-1 knockdown, and T-5224 treated BT549 cells. The results demonstrated that OLFML2A is necessary for T-5224-targeted AP-1 action, and the high OLFML2A level is associated with poor prognosis in TNBC patients.
Olfactomedin domain-containing proteins are involved in cell-cell adhesion, cell cycle regulation and tumorigenesis [29]. OLFML2A and OLFML2B encode for secreted glycoproteins which are closely related to each other [30]. Genomic and GeneChip expression profiling revealed that Amorphophalli Rhizoma inhibited TNBC cells by attenuating OLFML2A [31]. We found that OLFML2A knockdown significantly inhibited the proliferation, migration, and invasion of TNBC cells and promoted the apoptosis of TNBC cells. These results together indicated that OLFML2A may be a key therapeutic target for TNBC.
In summary, we provide the first genome-wide analysis of the effect of T-5224 on TNBC and demonstrate the anti-cancer effect of T-5224 in TNBC. Our results revealed a novel mechanism underlying AP-1–mediated regulation of progression of TNBC cells. OLFML2A, as a key downstream target of AP-1 in TNBC pathogenesis, is necessary for anti-TNBC effect of T-5224, suggesting that targeting both AP-1 and OLFML2A through T-5224 may be a synergistic therapeutic strategy for TNBC.
Declaration of Competing Interest
The author(s) declare no competing interests.
Acknowledgments
Author contributions
Zhao Qian: Investigation, Writing - Original Draft, Validation, Formal analysis. Zhang Kaixin: Investigation. Li Yong: Resources. Ren Yaxuan: Formal analysis. Shi Jikang: Formal analysis. Gu Yulu: Data Curation. Qiu Shuang: Visualization. Liu Sainan: Visualization. Cheng Yi: Writing - Review & Editing, Supervision. Qiao Yichun: Conceptualization, Funding acquisition, Methodology. Liu Yawen: Funding acquisition, Supervision, Project administration.
Data availability
The datasets generated and/or analyzed during the current study are available from the correspondence author on a reasonable request.
Funding
This work was funded by the National Natural Science Foundation of China (NSFC) (grant number: 81702606), the Science Technology Department of Jilin Province (grant number: 20170520007JH, 20180414086GH and 20140413016GH), the Department of Health and Family Planning Commission of Jilin Province (grant number: 2017Q037), and the Education Department of Jilin Province (grant number: JJKH20180238KJ).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101100.
Contributor Information
Yichun Qiao, Email: qiaoyichun@jlu.edu.cn.
Yawen Liu, Email: ywliu@jlu.edu.cn.
Appendix. Supplementary materials
Reference
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [J] CA Cancer J Clin. 2021 Feb 4 doi: 10.3322/caac.21660. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Foulkes W.D., Smith I.E., Reis J.S. Triple-Negative Breast Cancer [J] N. Engl. J. Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 3.Malorni L., Shetty P.B., De Angelis C. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up [J] Breast Cancer Res. Treat. 2012;136(3):795–804. doi: 10.1007/s10549-012-2315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liedtke C., Mazouni C., Hess K.R. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer [J] J. Clin. Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 5.Dent R., Hanna W.M., Trudeau M. Pattern of metastatic spread in triple-negative breast cancer [J] Breast Cancer Res. Treat. 2009;115(2):423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Pinilla S.M., Sarrio D., Honrado E. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas [J] Clin. Cancer Res. 2006;12(5):1533–1539. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- 7.Rugo H.S., Roche H., Thomas E. Efficacy and Safety of Ixabepilone and Capecitabine in Patients With Advanced Triple-negative Breast Cancer: a Pooled Analysis From Two Large Phase III, Randomized Clinical Trials [J] Clin. Breast Cancer. 2018;18(6):489–497. doi: 10.1016/j.clbc.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation [J] Biochim. Biophys. Acta. 1991;1072(2–3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 9.Hai T.W., Liu F., Allegretto E.A. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1 [J] Genes Dev. 1988;2(10):1216–1226. doi: 10.1101/gad.2.10.1216. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C., Qiao Y., Jonsson P. Genome-wide profiling of AP-1-regulated transcription provides insights into the invasiveness of triple-negative breast cancer [J] Cancer Res. 2014;74(14):3983–3994. doi: 10.1158/0008-5472.CAN-13-3396. [DOI] [PubMed] [Google Scholar]
- 11.Shen Q., Uray I.P., Li Y. Targeting the activator protein 1 transcription factor for the prevention of estrogen receptor-negative mammary tumors [J] Cancer Prev. Res. (Phila.) 2008;1(1):45–55. doi: 10.1158/1940-6207.CAPR-08-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye N., Ding Y., Wild C. Small molecule inhibitors targeting activator protein 1 (AP-1) [J] J. Med. Chem. 2014;57(16):6930–6948. doi: 10.1021/jm5004733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aikawa Y., Morimoto K., Yamamoto T. Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1 [J] Nat. Biotechnol. 2008;26(7):817–823. doi: 10.1038/nbt1412. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida T., Yamashita K., Watanabe M. The Impact of c-Fos/Activator Protein-1 Inhibition on Allogeneic Pancreatic Islet Transplantation [J] Am. J. Transplant. 2015;15(10):2565–2575. doi: 10.1111/ajt.13338. [DOI] [PubMed] [Google Scholar]
- 15.Kamide D., Yamashita T., Araki K. Selective activator protein-1 inhibitor T-5224 prevents lymph node metastasis in an oral cancer model [J] Cancer Sci. 2016;107(5):666–673. doi: 10.1111/cas.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao Y., He H., Jonsson P. AP-1 Is a Key Regulator of Proinflammatory Cytokine TNFalpha-mediated Triple-negative Breast Cancer Progression [J] J. Biol. Chem. 2016;291(10):5068–5079. doi: 10.1074/jbc.M115.702571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryoo H.D., Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer [J] Cold Spring Harb. Perspect. Biol. 2012;4(8) doi: 10.1101/cshperspect.a008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowdy S.F. Overcoming cellular barriers for RNA therapeutics [J] Nat. Biotechnol. 2017;35(3):222–229. doi: 10.1038/nbt.3802. [DOI] [PubMed] [Google Scholar]
- 19.Ming J., Jiang G., Zhang Q. Interleukin-7 up-regulates cyclin D1 via activator protein-1 to promote proliferation of cell in lung cancer [J] Cancer Immunol. Immunother. 2012;61(1):79–88. doi: 10.1007/s00262-011-1078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu Y., Kinoshita I., Kikuchi J. Growth inhibition of non-small cell lung cancer cells by AP-1 blockade using a cJun dominant-negative mutant [J] Br. J. Cancer. 2008;98(5):915–922. doi: 10.1038/sj.bjc.6604267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H., Ren G., Wang T. Aberrantly expressed Fra-1 by IL-6/STAT3 transactivation promotes colorectal cancer aggressiveness through epithelial-mesenchymal transition [J] Carcinogenesis. 2015;36(4):459–468. doi: 10.1093/carcin/bgv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang X., Jessen W.J., Al-Ahmadie H. Activator protein-1 transcription factors are associated with progression and recurrence of prostate cancer [J] Cancer Res. 2008;68(7):2132–2144. doi: 10.1158/0008-5472.CAN-07-6055. [DOI] [PubMed] [Google Scholar]
- 23.Hein S., Mahner S., Kanowski C. Expression of Jun and Fos proteins in ovarian tumors of different malignant potential and in ovarian cancer cell lines [J] Oncol. Rep. 2009;22(1):177–183. doi: 10.3892/or_00000422. [DOI] [PubMed] [Google Scholar]
- 24.Kharman-Biz A., Gao H., Ghiasvand R. Expression of activator protein-1 (AP-1) family members in breast cancer [J] BMC Cancer. 2013;13:441–451. doi: 10.1186/1471-2407-13-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X., Ma Y., Yang W. Identification of therapeutic targets for breast cancer using biological informatics methods [J] Mol Med Rep. 2015;12(2):1789–1795. doi: 10.3892/mmr.2015.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith L.M., Wise S.C., Hendricks D.T. cJun overexpression in MCF- 7 breast cancer cells produces a tumorigenic, invasive and hormone resistant phenotype [J] Oncogene. 1999;18(44):6063–6070. doi: 10.1038/sj.onc.1202989. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S., Ishii T., Sato F. Eribulin mesylate-induced c-Fos upregulation enhances cell survival in breast cancer cell lines [J] Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.03.042. [DOI] [PubMed] [Google Scholar]
- 28.Fumagalli D., Blanchet-Cohen A., Brown D. Transfer of clinically relevant gene expression signatures in breast cancer: from Affymetrix microarray to Illumina RNA-Sequencing technology [J] BMC Genomics. 2014;15:1008. doi: 10.1186/1471-2164-15-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomarev S.I., Nakaya N. Olfactomedin domain-containing proteins: possible mechanisms of action and functions in normal development and pathology [J] Mol. Neurobiol. 2009;40(2):122–138. doi: 10.1007/s12035-009-8076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng L.C., Han Z.G., Ma W.J. Elucidation of subfamily segregation and intramolecular coevolution of the olfactomedin-like proteins by comprehensive phylogenetic analysis and gene expression pattern assessment [J] FEBS Lett. 2005;579(25):5443–5453. doi: 10.1016/j.febslet.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 31.Wu C., Chen M., Zhang Q. Genomic and GeneChip expression profiling reveals the inhibitory effects of Amorphophalli Rhizoma in TNBC cells [J] J. Ethnopharmacol. 2019;235:206–218. doi: 10.1016/j.jep.2019.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the correspondence author on a reasonable request.