Abstract
Diabetes mellitus type I is an autoimmune disease characterized by insulin deficiency, and patients with diabetes have an increased risk of bone fracture and significantly impaired fracture healing. Pro-inflammatory cytokine tumor necrosis factor-alpha (TNF±) is significantly upregulated in diabetic fractures and is believed to underlie delayed fracture healing commonly observed in diabetes. Our previous genetic screen for the binding partners of progranulin (PGRN), a growth factor-like molecule that induces chondrogenesis, led to the identification of TNFRs as the PGRN-binding receptors. In this study, we employed several in vivo models to ascertain whether PGRN has therapeutic effects in diabetic fracture healing. Herein we report that deletion of PGRN significantly delayed bone fracture healing and aggravated inflammation in the fracture models of mice with type 1 diabetes. In contrast, recombinant PGRN effectively promoted diabetic fracture healing by inhibiting inflammation and by enhancing chondrogenesis. In addition, both TNFR1 pro-inflammatory and TNFR2 anti-inflammatory signaling pathways are involved in PGRN-stimulated diabetic fracture healing. Collectively, these findings illuminate a novel understanding concerning the role of PGRN in diabetic fracture healing and may have application in the development of novel therapeutic intervention strategies for diabetic and other types of impaired fracture healing.
Keywords: progranulin, TNFR1, TNFR2, impaired fracture healing, Type 1 diabetes
1. Introduction
Global projections estimate that the number of individuals with diabetes mellitus (DM) is expected to reach 439 million by 2030, making DM one of the most common metabolic diseases worldwide(1). Diabetic patients have an increased risk of sustaining a fracture as well as impaired fracture healing (2–5). Under physiological conditions, the fracture healing process is initiated with hematoma formation in the inflammatory phase, due to the disruption of the blood vessel upon injury. Subsequently, progenitor cells migrate to the fracture site, proliferate and differentiate into chondrocytes that generate collagenous matrix to form the callus followed by osteoblast-driven endochondral bone formation(6). However, under the diabetic condition, this process is significantly delayed(7). Increasing evidences indicate the pivotal role of growth factors and cytokines in delayed healing(8; 9); microarray and gene set enrichment analysis have uncovered enhanced activation of inflammatory gene sets, particularly components of tumor necrosis factor (TNF) signaling, during the course of diabetic fracture healing(10).
TNFα is a master cytokine with critical functions in the innate immune response underlying fracture healing(11; 12). Studies reveal that in the diabetic fracture healing process, TNFα expression is remarkably increased(13) which contributes to delayed bone repair by accelerating cartilage loss as well as promoting osteoclast formation(10). Given the importance of TNFα as a key inflammatory signaling molecule that is implicated in fracture healing, the development of novel therapies that target TNFα signaling may be of great benefit, with particular importance in conditions that feature impaired fracture healing, including DM.
Progranulin, also known as PGRN, PC-cell-derived growth factor (PCDGF), granulin epithelin precursor (GEP), acrogranin and proepithelin, is a 593-amino-acid autocrine growth factor-like molecule with unique “beads-on-a-string” structure(14). Previous studies indicate that PGRN binds to TNFα receptors and exerts anti-inflammatory and protective effects in various animal models(11; 15–18). In addition to anti-inflammatory properties, PGRN is a downstream molecule of BMP-2 and promotes chondrogenesis through Erk1/2 signaling(19). Further, PGRN promotes bone healing under physiological conditions primarily through TNFR2 signaling(20). Given the importance of TNFα in diabetes and PGRN’s association with TNFRs, we sought to determine whether PGRN has therapeutic effects in impaired diabetic fracture healing. In this study, we employed wild-type C57BL/6 and C57BL/6 background mice genetically modified for absence of PGRN or TNFRs and determined the role of endogenous and recombinant PGRN in diabetic fracture healing as well as the mechanism involved.
2. Methods and Materials
2.1. Media, regents and animals
Dulbecco’s Modified Eagle Medium (DMEM) (catalog# 11965-118) and fetal bovine serum (FBS) (catalog# 16000-044) were purchased from Gibco-BRL (Waltham, EISA). Specific antibodies against NOS-2 (catalog# sc-649) and GAPDH (catalog# 25778) were obtained from Santa Cruz Biotechnology, Inc. (California, CA, USA). Specific antibodies against INK (catalog# 9258S), p-JNK(catalog# 4668S), P38(catalog# 9212S), p-P38(catalog# 9211L), p-P65 (catalog# 3033L) and P65 (catalog# 8242S) were purchased from Cell Signaling Technology (Danvers, MA, USA).
The collagen sponge was obtained as a kind gift from Dr. Gino Bradica (Kensey Nash Corp. Exton, PA). Recombinant PGRN purification has been soundly established in our lab and described in our previous publication(11). Briefly, medium collected from stably transfected 293 EBNA cells expressing recombinant human PGRN was incubated with nickel nitrilotriacetic acid (NiNTA) agarose (Sigma-Aldrich) at 4°C overnight with gentle agitation. After removal of non-specific binding via washing with native binding buffer, the bound PGRN protein was eluted using an imidazole gradient. The purified protein was dialyzed against PBS and endotoxin removal using Pierce Endotoxin removal columns. Final concentration of purified protein was adjusted to 10 ng/ml with 0.2% BSA added to stabilize PGRN during storage at −80°C. All other substances administered to animals were pharmaceutical grade and provided by the New York University’s Association For Assessment and Accreditation Of Laboratory Animal Care International (AAALAC) accredited Division of Comparative Medicine or obtained through commercial vendors.
All animal studies were performed in accordance with institutional guidelines and approval by the Institutional Animal Care and Use Committee of New York University. 12 week old male mice were used for experiments. Each group was randomly assigned and contained 10 mice. Mice were gang housed in a rodent barrier facility. The generation and genotyping of wild type (WT), PGRN-deficient (PGRN−/−), TNFR1-deficient (TNFR1−/−) and TNFR2-deficient (TNFR2−/−) C57BL/6 background mice has been described previously (11).
2.2. Analgesia and anesthesia
0.1 mg/kg body weight of ketamine:xylazine was used for pre-operative analgesia and anesthesia. Isoflurane was used to maintain a surgical plane of anesthesia in the event that ketamine:xylazine did not provide sufficient depth and/or duration. Animals were positioned into a nose cone and isoflurane anesthetic was induced with the flow meter adjusted to 500-1000 ml/min and vaporizer at 5%; for maintenance the flow meter was adjusted to 100-200 ml/min with vaporizer.
2.3. Femoral segmental bone defect model
The femoral segmental bone defect model was established as previously reported(20; 21). The mice were anesthetized, the surgical area depilated and disinfected, and a 1 cm incision at the junction of the knee and lateral femur was established to allow for medial approach and lateral dislocation of the patella. A transverse osteotomy was made through the mid-femoral shaft using sharp surgical scissors; the femur was stabilized via advancement of a 1.5-inch 25-gauge needle inserted between the femoral condyles. A standardized fracture gap of 0.5 mm was maintained by insertion of a 0.5 mm metallic clip into holes generated with a 0.75 mm bit and motorized drill at positions 2mm anterior and posterior from the fracture site (Fig. S1). The wound was irrigated with sterile saline prior to wound closure. The surgical procedure was completed under visualization using a dissecting microscope.
2.4. The femoral drill-hole model
A femoral drill-hole model was generated in WT and PGRN deficient mice with and without diabetes utilizing the procedure already established in the literature(20; 22). Pre-operative preparation of animals followed the same procedure outlined above. A unicortical hole of 0.8mm in diameter was generated through the anterior cortex using a 21-gauge needle. The wound and drill holes were rinsed with sterile saline to remove bone fragments from the cavity prior to wound closure. 10 mice of each grouping for treatment condition and genotype were used in this model.
2.5. Gravity induced Bonnarens and Einhorn bone fracture model
We followed the methodology for the production of a standard closed fracture outlined by Bonnarens and Einhorn as adapted for mice(23; 24). Briefly, WT and PGRN-deficient diabetic mice underwent intramedullary pinning and a standard closed fracture was produced using a specially built fracture apparatus. Specifically, the animal was anesthetized and the knee was flexed. A 1 cm incision was made medial to the patella and the patella was dislocated laterally. A 27 gauge needle was used to establish a hole between the exposed femoral condyles. A steel pin was driven up the shaft of the medullary canal and the exposed advancing end was cut flush with the patellofemoral groove. The clipped end of the pin was buried beneath the condyles so as not to disturb movement. A 1.5 mm long collagen sponge loaded with PGRN or phosphate buffered saline (PBS) was implanted into the planned area of bone defect. After wound closure, the animals were placed supine on the support stage of the fracture apparatus. The femur was extended in abduction over the two support anvils of the fracture apparatus. A weight was dropped from a height sufficient to transfer enough force to drive a blunted guillotine blade into the femur of the animal; creating a bending moment sufficient to produce a fracture. Mice were randomly assigned to groups for sacrifice at Day 10, Day 16 and Day 22 post-surgery. Each group, stratified by genotype, sacrificial time point, and/or treatment included 10 mice.
2.6. Diabetes model
A murine model of Type 1 diabetes was generated as previously described(25). Mice received daily intraperitoneal injections of streptozotocin (STZ) (Sigma, St. Louis, MO) at 50 mg/kg body weight for 5 consecutive days; control littermates received vehicle-only injections. Mice were observed daily until the 16th day after the initial injection. Weight and blood glucose levels were monitored, beginning at 24-hours after the final STZ injection, until sacrifice.
2.7. Soft X-ray photography
Radiographs were taken using a Faxitron X-ray machine (Wheeling, IL) at 5.0-kV over 6.0 seconds(20). The residual gap size was measured by electric caliper on the basis of X-ray photography by two independent, blinded investigators.
2.8. MicroCT analysis
The microCT analysis was performed in accordance with previous publications(26). Briefly, before histological processing, the tissues were scanned using the Scanco vivaCT40 cone-beam scanner (SCANCO Medical, Switzerland) at 55kVp and 145mA current. The tissues were scanned at a resolution of 10.5μm. Specifically, we scanned the entirety of each long bone subjected to an operation. After scanning, thresholding was performed across datasets and 3-dimensional structural reconstruction was carried out.
2.9. Real-time RT-PCR
Total RNA was extracted from primary bone and cartilage tissues and bone marrow stem cells (BMSCs) under direction from instructions accompanying the RNeasy kit (Qiagen, Valencia, CA, USA). Reverse transcription was performed by RT-for-PCR kit (Qiagen, Valencia, CA) in accordance with the manufacturer supplied protocol. We performed the PCR reaction using the 20-ml SYBR Green system in a 96-well optical reaction plate formatted in the 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The generation of a single specific PCR product was evaluated by melt curve analysis. Each experiment was repeated three times. The primers are designed as listed in the following table.
| Primer | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| TNFα | CACAGAAAGCATGATCCGCGACGT | CGGCAGAGAGGAGGTTGACTTTCT |
| IL-1β | AATCTCACAGCAGCACATCA | AAGGTGCTCATGTCCTCATC |
| NOS-2 | ACAGGAGGGGTTAAAGCTGC | TTGTCTCCAAGGGACCAGG |
| COX-2 | TGTGACTGTACCCGGACTGG | TGCACATTGTAAGTAGGTGGAC |
| GAPDH | AGAACATCATCCCTGCATCC | AGTTGCTGTTGAAGTCGC |
2.10. Western blotting
We collected total protein from cartilaginous callus tissues or primary cultured BMSCs via tissue lysis in RIPA lysis buffer with protease inhibitors (Santa Cruz Biotechnology, Inc.). Extracted proteins were separated on 10% SDS-polyacrylamide gels, followed by electro-transfer onto a nitrocellulose membrane. Following transfer, the membrane was blocked with 3% BSA in Tris buffer-saline-Tween 20 (10 mM Tris-HCl, pH 8.0; 150 mM NaCl; and 0.5% Tween 20) for 1 hour at room temperature. After 3 times’ washing with TBST, the membrane was incubated at 4°C overnight with polyclonal anti-p-P38 (diluted 1:1000, Cell Signaling Technology, Catalog Number 4511) or polyclonal anti-P38 (diluted 1:1000, Cell Signaling Technology, Catalog Number 8690) or polyclonal anti-p-P65 (diluted 1:1000, Cell Signaling Technology, Catalog Number 3033) or polyclonal anti-p-JNK (diluted 1:1000, Cell Signaling Technology, Catalog Number 4688) or polyclonal anti-JNK(diluted 1:1000, Cell Signaling Technology, Catalog Number 9252) or polyclonal anti-p-IKB(diluted 1:1000, Cell Signaling Technology, Catalog Number 2859) or anti-IKB(diluted 1:1000, Cell Signaling Technology, Catalog Number 4814) or polyclonal anti-NOS-2 (diluted 1:1000, Santa Cruz Biotechnology Inc., Catalog Number SC651) or polyclonal anti-GAPDH (diluted 1:1000, Santa Cruz Biotechnology Inc., Catalog Number SC25778) or polyclonal anti-lamin B (diluted 1:1000, Santa Cruz Biotechnology Inc., Catalog Number SC6217). After 3 washes with TBST, the secondary antibody (horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin; Jackson Immunology Research, 1:10000 dilution) was added and the membrane was incubated for 1 hour at room temperature with agitation. Bound antibody was visualized by enhanced chemiluminescence system (Amersham Life Science, Arlington Heights, IL, USA). Image J program was used to analyze the band intensity.
2.11. Histology
Immediately after sacrifice, or directly following microCT scanning, mouse tissues were processed for histology. The tissues were fixed in 4% PFA for 2 days at room temperature. After fully washing 3 times in phosphate buffered saline, the tissues were decalcified in 10% w/v EDTA over the course of 2 weeks with agitation at 4°C prior to paraffin infiltration and embedding. 5 μm thick serial tissue sections underwent Safranin-O/fast green and hematoxylin-eosin (HE) staining following standard protocols.
2.12. Bony bridging analysis
Bony bridging analysis was undertaken using histological staining as previously reported(27). Histological scoring of Safranin O stained fracture callus was defined as following: fibrous tissue, score 1; predominantly fibrous tissue with small amount of cartilage, score 2; equal mixture of fibrous and cartilaginous tissue, score 3; predominantly cartilage with small amount of fibrous tissue, score 4; cartilage alone , score 5; predominantly cartilage with small amount of immature bone, score 6; equal mixture of cartilage and immature bone, score 7; predominantly immature bone with small amount of cartilage, score 8; union of fracture fragments by immature bone, score 9; union of fracture fragments by mature bone, score 10.
2.13. Statistical analysis
The Statistical Package for Social Sciences version 17.0 (SPSS Inc, Chicago, IL) was used for standard statistical analysis including one-way ANOVA and Student’s t-test. Statistical significance was achieved when p < 0.05.
3. Results
3.1. PGRN deficiency causes further delay in diabetic fracture healing
To investigate the function of endogenous PGRN in diabetic fracture healing, we generated a femoral segmental nonunion fracture model using streptozotocin (STZ) induced type 1 diabetes with C57BL/6J (WT), PGRN deficient backgrounds and healthy control mice(28). As shown in Fig. 1A&1B, diabetic WT, non-diabetic PGRN deficient and diabetic PGRN deicient mice exhibit diminished fracture healing as compared to non-diabetic WT mice; however, this reduced fracture healing was more pronounced in diabetic PGRN-deficient mice relative to non-diabetic PGRN deficient or WT diabetic mice. Similar to previous report(20), PGRN deficiency delayed fracture healing in the non-diabetic group. A cortical drill-hole model was also established; 10 days after surgery, healing was evaluated by micro CT analyses. MicroCT revealed that the callus fully shrouded the drill-hole in WT mice without diabetes and partially covered the hole in WT mice with diabetes and PGRN-deficient mice without diabetes. However, there was little appreciable callus development in PGRN deficient mice with diabetes (Fig. 1C). Moreover, deficiency of PGRN led to meaningfully reduced new bone formation indicated by analysis of the microCT parameter BV/TV (Fig.1D).
Fig.1. Deletion of PGRN further delays diabetic bone fracture healing.
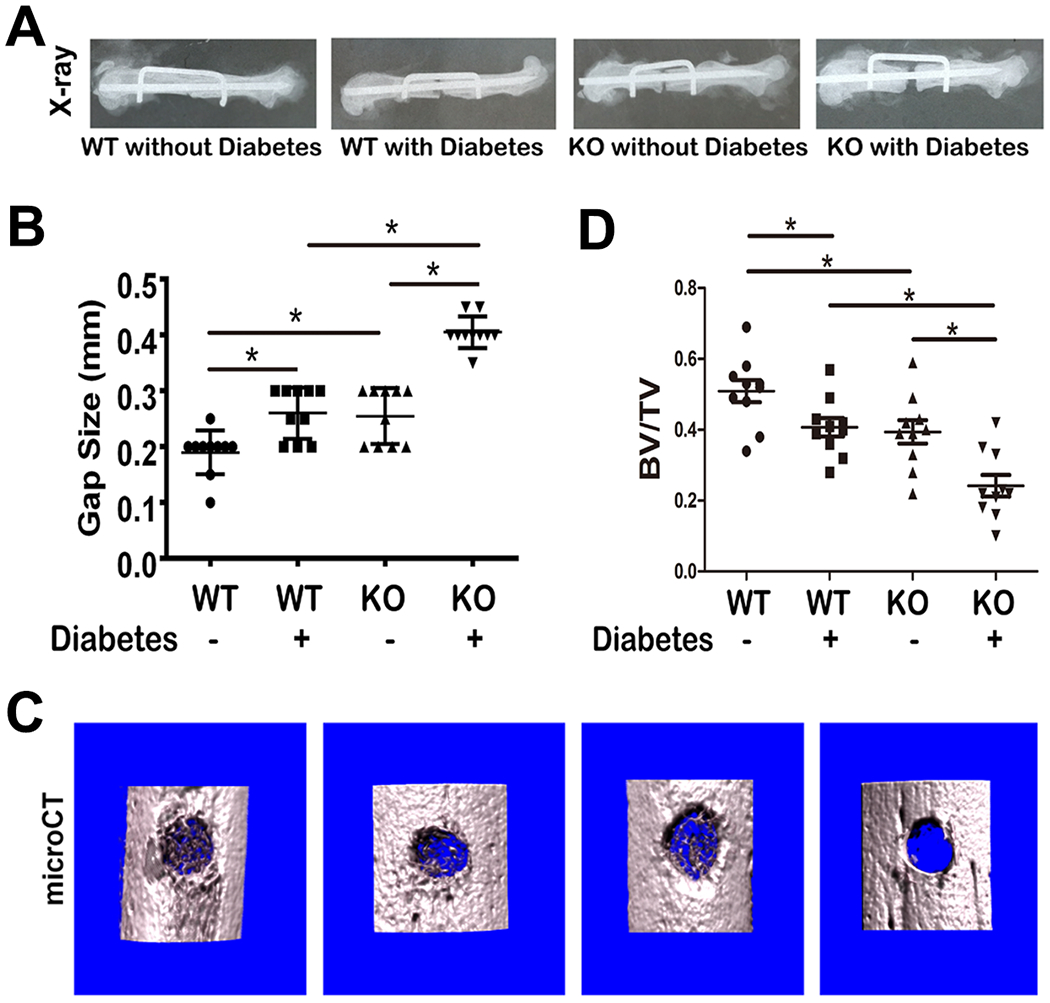
(A) Bone healing of WT with or without diabetes, and PGRN−/− mice with or without diabetes, as indicated, after 4 weeks of establishment of femoral segmental bone defect model, assayed by X-ray. (B) Residual gap size based on radiology results in panel A. (C) Representative microCT image of drill-hole model. (D) Quantification of BV/TV, based on microCT. Each group contained 10 mice. Student’s t-test was used for the statistical analysis.
3.2. Deletion of PGRN accelerates inflammation in diabetic fracture healing
Total RNA isolated from the callus from mice subjected to the drill-hole model was utilized in performance of real-time PCR to establish whether PGRN-deficiency mediated the pathological delay in the diabetic bone fracture healing process. As shown in Fig. 2A–2D, the transcriptional levels of several pro-inflammatory cytokines, including TNFα, IL-1β, NOS-2 and COX-2, were up-regulated in diabetic WT mice and non-diabetic PGRN knockout mice. Additionally, the upregulation of these molecules was further pronounced in diabetic PGRN-deficient mice. Given that NOS-2 is regarded as a marker of inflammation severity(29), we further examined NOS-2 protein expression by immunoblotting. As demonstrated in Fig.2E&F, in WT group, diabetic condition increased NOS-2 expression. Additionally, loss-of-PGRN lead to increased expression of NOS-2 in non-diabetic mice. Notably, the absence of PGRN under diabetic condition is associated with a more robust upregulation of NOS-2. Together, these data suggest that loss of PGRN leads to enhanced inflammation in the diabetic fracture healing process.
Fig.2. Deletion of PGRN accelerates inflammation in diabetic fracture healing.
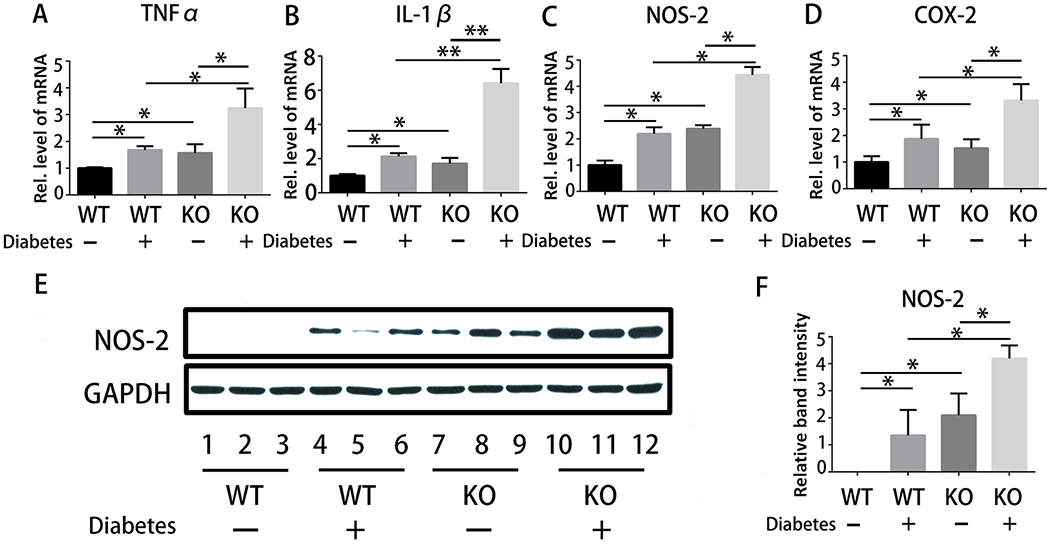
(A-D) Real-time PCR assay for transcriptional levels of pro-inflammatory cytokines, including TNFα, IL-1β, COX-2 and NOS-2, in the drill-hole model; n=10. (E) Expression of NOS-2 in WT without diabetes, WT with diabetes, PGRN KO without diabetes and PGRN KO with diabetes in the drill-hole model, assayed by immunoblotting. (F) Quantitative analysis of immunoblotting in (E), assayed by ImageJ program. Student’s t-test analysis was used for the statistical analysis. The values are the mean ±SD . *p < 0.05 vs. control group.
3.3. Recombinant PGRN promotes diabetic fracture healing by inhibiting inflammation
We first tested the therapeutic effect of recombinant PGRN through establishment of a femoral segmental nonunion fracture model in WT mice. In these experiments, local delivery of 10ug of PGRN or PBS was accomplished via placement of a loaded collagen sponge into the fracture site(28). As shown in Fig. 3A, PGRN significantly enhanced diabetic bone healing; histological analysis disclosed more bone formation and less residual bone gap in the PGRN-treated group as compared to PBS-treated mice model (Fig. 3B). Given that PGRN exhibits an anti-inflammatory effect, we next determined whether PGRN-mediated diabetic fracture healing associated with the inhibition of TNFα-induced inflammation. To address this issue, we collected callus from mice subjected to a femoral segmental nonunion fracture and extracted total protein and RNA to measure the inflammation-related cytokines. We show thatNOS-2 protein levels were significantly reduced following application of recombinant PGRN (Fig.3C). Additionally, recombinant PGRN significantly diminished the transcriptional levels of pro-inflammatory cytokines, including IL-1β, NOS-2 and COX-2 (Fig.3D–3F).
Fig.3. Recombinant PGRN promotes diabetic bone fracture healing by inhibiting inflammation.
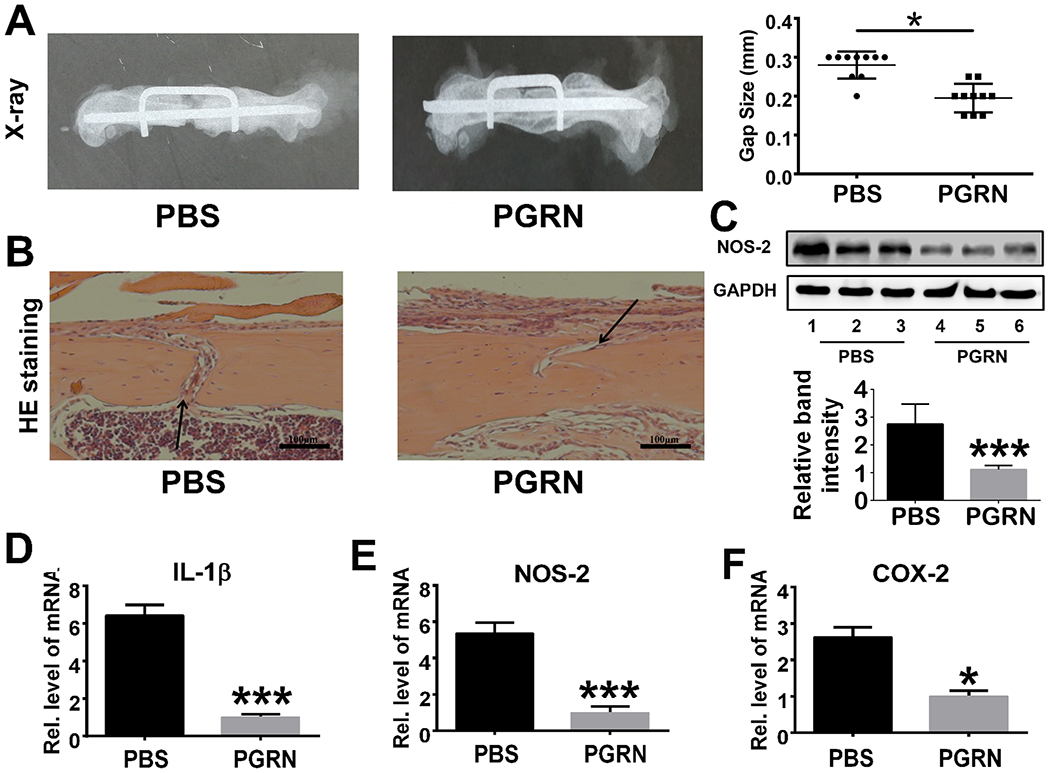
(A) Recombinant PGRN significantly enhanced diabetic bone regeneration in WT mice subjected to the femoral segmental bone defect model, assayed by X-ray. Residual gap size was quantified based on radiology results. (B) HE staining of PBS- and PGRN-treated diabetic drill-hole model. Black arrows indicate bone gap. (C) Expression and quantification of NOS-2 in callus of PBS-/PGRN-treated diabetic drill-hole model mice assayed by Western blot and ImageJ program. (D-F) Transcriptional levels of IL-1β, NOS-2 and COX-2 from the callus of drill-hole model. Each group contained 10 mice. Student’s t-test was used for the statistical analysis. The values are the mean ±SD. *p < 0.05 and ***p < 0.001 vs. control group.
3.4. Recombinant PGRN promotes diabetic fracture healing by accelerating chondrogenesis
PGRN is known to promote chondrogenesis under physiological conditions (30). To determine whether PGRN-mediated chondrogenesis contributes to PGRN-stimulated diabetic fracture healing, we established a closed fracture mouse model (also called the Bonnarens and Einhorn model)(13), in diabetic WT mice, followed by local delivery of collagen sponge containing PGRN or PBS. We collected the tissues on Day 10, Day 16 and Day 22 post-surgery according to previous findings(31). In accord with previous publications, safranin O staining indicated that callus started to appear on Day 16 in the PBS-treated diabetic bone fracture model(32). Excitingly, local application of recombinant PGRN accelerated this process. Callus appeared on Day 10 in PGRN-treated diabetic mice and the bone was nearly healed on Day 22 (Fig.4A). Additionally, bony bridging analysis also demonstrated that local delivery of recombinant PGRN promoted the diabetic fracture healing process (Fig.4B). To further determine the effects of PGRN-treatment potentially contributory to promotion of diabetic bone fracture healing, we collected the tissues around the fracture site, followed by total RNA extraction. Real-time PCR indicated that chondrogenic markers, such as type II collagen (Col II) and aggrecan (ACN), were significantly up-regulated in PGRN-treated group relative to the PBS-treated group (Fig.4C&D). Collectively, these results suggest that PGRN-stimulated chondrogenesis might contribute to its therapeutic effect in diabetic bone fracture healing.
Fig.4. Recombinant PGRN promotes diabetic bone fracture healing by accelerating chondrogenesis.
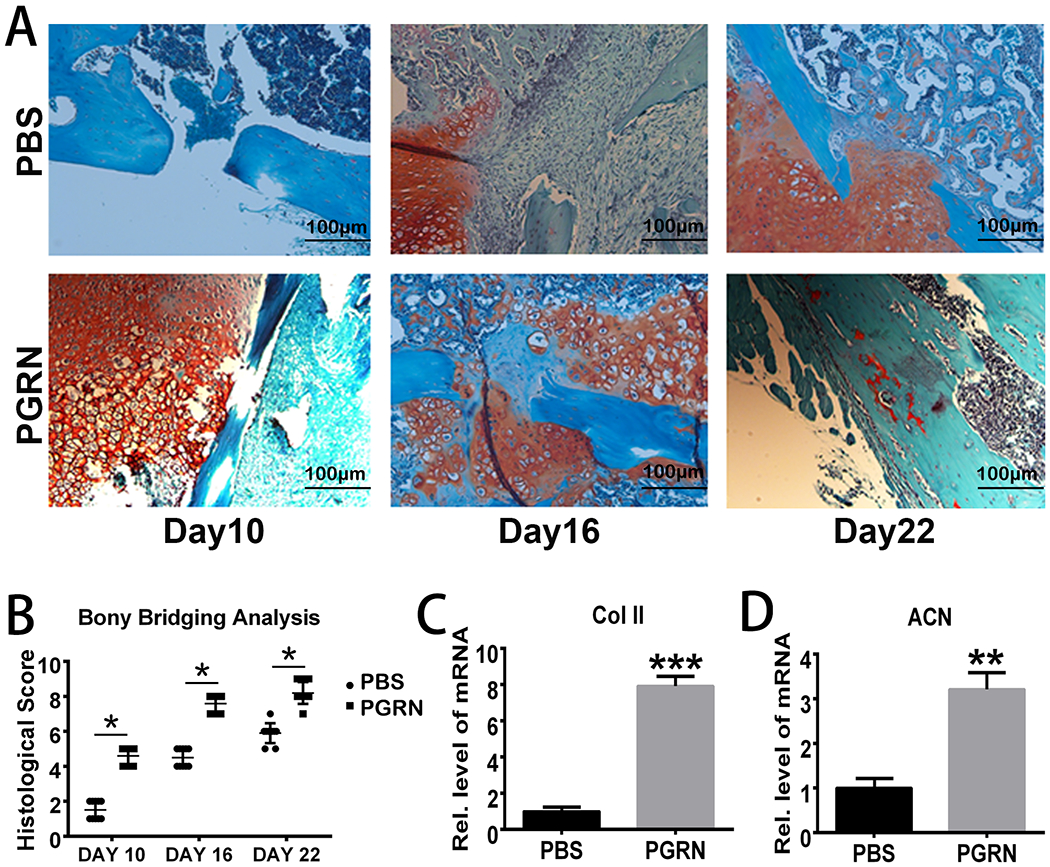
(A) Safranin O staining of Bonnarens and Einhorn model in diabetic bone fracture healing at various time points. (B) Quantification of bony bridging analysis based on the Safranin O staining. Each group contained 10 mice. Two way ANOVA was used for the statistical analysis. (C-D) Transcriptional levels of Collagen II (Col II) and aggrecan (ACN) in PBS-/PGRN- treated diabetic callus on Day 10. Student’s t-test was used for the statistical analysis. The values are the mean ±SD. **p < 0.01 and ***p < 0.001 vs. control group.
3.5. PGRN promotes diabetic fracture healing by inhibiting TNFα-mediated catabolic responses
Given the importance of TNFα in the diabetic fracture healing process and PGRN’s anti-inflammatory effect through the TNFR pathway (14; 33–42), we determined whether PGRN accelerated diabetic fracture healing by inhibiting TNFα-induced catabolic responses. To elucidate molecular mechanisms involved, we isolated primary bone marrow cells from normal and diabetic mice, cultured in the absence or presence of TNFα and without or with PGRN. Interestingly, in normal bone marrow cells, TNFα induced phosphorylation of p38, JNK and NF-κB signaling, wherease this activation was inhibited by the presence of recombinant PGRN, which is in line with previous findings(11). Additionally, diabetic bone marrow cells exhibited over-activation of pro-inflammatory signaling pathways compared to normal bone marrow cells, and this activation was further enhanced in the presence of TNFα. Interestingly, recombinant PGRN inhibited TNFα-induced signaling in both normal and diabetic cells (Fig.5A–5D). Notably, the activation of these pro-inflammatory pathways remained relatively elevated level in diabetic cells compared to normal cells even in presence of PGRN (Fig.5A–5D). Given the importance of TNFα/NF-κB signaling, we investigated the phorsphorylation of p65. As indicated in Fig.5E&G, diabetic bone marrow cells exhibited overactivation of p65 in comparision to normal bone marrow cells. In both normal and diabetic bone marrow cells, PGRN effectively inhibited the activation of NF-κB signaling, however, PGRN administration did not resolve the heightened p65 activation observed in diabetic bone marrow cells to the level exhibited by normal bone marrow cells (Fig.5E&G). Additionally, NOS-2 protein expression was increased in the presence of TNFα in both normal and diabetic cells. However, this upregulation was largely diminished with additional use of recombinant PGRN (Fig.5F&H). After sitimulation with TNFα with or without PGRN, we extracted total RNA for real-time PCR. As illustrated in Fig.5I–5K, in diabetic bone marrow cells, PGRN effectively inhibited TNF-induced transcriptional levels of pro-inflammatory cytokines, including IL-1β, COX-2 and NOS-2.
Fig.5. PGRN promotes diabetic fracture healing by inhibiting TNFα-mediated catabolic responses.
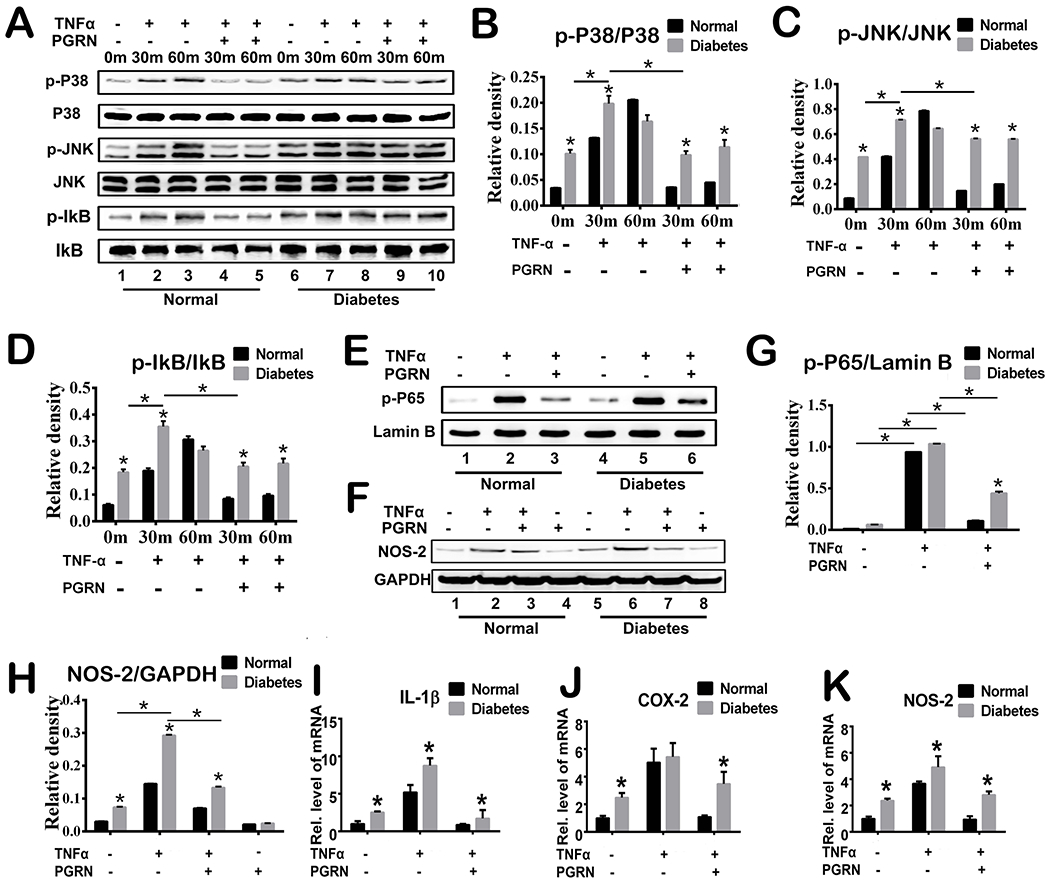
(A) Primary mouse bone marrow cells were incubated with or without TNFα (10ng/ml) in the presence or absence of PGRN (200 ng/ml) for 48 hours, and phosphorylation and expression of the indicated signaling molecules were determined by Western Blotting. (B-D) Relative band density of p-P38/P38, p-JNK/JNK and p-IkB/IkB based on Western Blotting. (E) Phosphorylation and expression of p-65 was determined by Western Blotting assay. Lamin B is employed as loading control. (F) Expression of NOS-2 and GAPDH (serving as a loading control) was determined by Western Blotting assay. (G-H) Relative band density of p-P65/lamin B and NOS-2 based on Western Blotting. (I-K) Primary mouse bone marrow cells were cultured without or with TNFα in absence or presence of PGRN for 6 hours. Transcriptional levels of IL-1β, COX-2 and NOS-2 were determined by Real-time PCR assay. Two-way ANOVA was used for the statistical analysis. The values are the mean ±SD. *p < 0.05 vs. control group.
3.6. PGRN promotes diabetic fracture healing through TNFR2-Akt/Erk1/2/mTOR signaling
TNFR2 signaling is known to mediate cartilage anabolism(43; 44). Additionally, we previously reported that recombinant PGRN effectively promoted chondrocyte differentiation and proliferation through Akt and Erk1/2 signaling(19; 45). Taking into consideration the importance of chondrogenesis in the bone fracture healing process, we also explored the potential impact of PGRN-mediated anabolism and its dependence on TNFRs in diabetic fracture healing. For this purpose, we isolated diabetic bone marrow cells from WT, TNFR1−/− and TNFR2−/− mice, and cultured the cells in the absence or presence of recombinant PGRN. As shown in Fig.6A&B, the transcriptional levels of Col II and ACN were significantly induced by PGRN in WT and TNFR1−/− groups. However, PGRN-mediated induction of these chondrogenic markers was almost abolished in the TNFR2−/− group. These data suggest that PGRN-mediated chondrogenesis in diabetic fracture healing largely relies on the TNFR2 pathway. Furthermore, we implemented the PathScan Multiplex Western Cocktail in examining the effects of PGRN-activated signaling on the MAPK signal. As shown in Fig.6C, PGRN activated Akt and mTOR signaling, and slightly activated Erk1/2 signaling.
Fig.6. PGRN promotes chondrogenesis through TNFR2-Akt/Erk1/2/mTOR signaling.
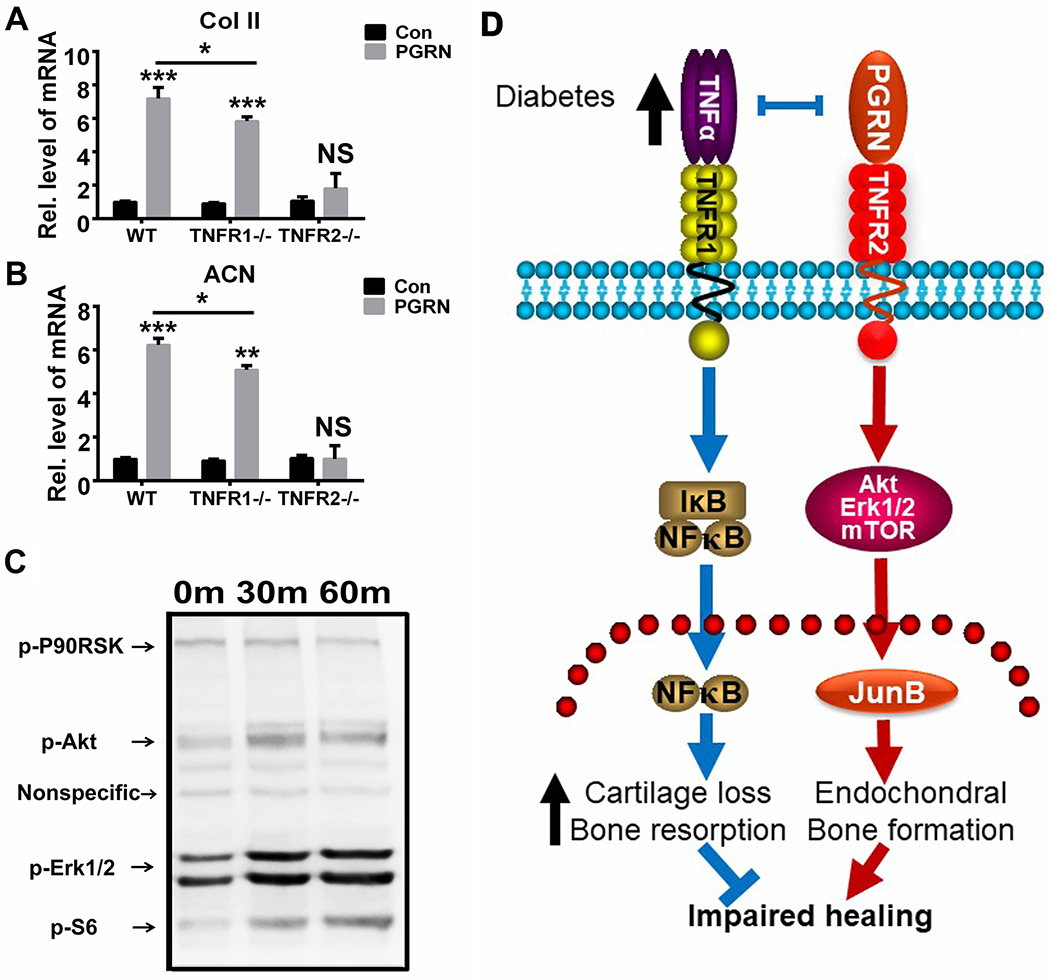
(A&B) Primary mouse bone marrow cells of WT, TNFR1−/− and TNFR2−/− mouse were cultured with or without PGRN (200 ng/ml) for 6 hours, transcriptional levels of type II collagen (Col II) and aggrecan (ACN) were determined by Real-time PCR assay. The values are the mean ±SD. **p < 0.01 and ***p < 0.001 vs. control group. Two-way ANOVA was used for the statistical analysis. (C) Primary mouse bone marrow cells of WT mice were incubated in presence of PGRN, and phosphorylation of the indicated signaling molecules were determined by cocktail scanning. (D) A proposed model for the role of PGRN in diabetic bone fracture healing process.
4. Discussion
Diabetes mellitus is the most common metabolic disorder in the world; future prevalence estimates anticipate steady growth in the number of affected individuals(1). Reduced bone mineral density is a prominent complication of diabetes thought to contribute to enhanced fracture risk evidenced by increased long bone fractures in diabetics(46–49). There are several mechanisms responsible for osteopenia in diabetes, including reduced bone formation, increased bone resorption, and decreased production of extracellular matrix(50–52). Furthermore, patients and animals with diabetes exhibit an increased risk of fracture as well as delayed fracture healing; clinical studies have shown that diabetes may prolong fracture healing and fractures are associated with delayed union, non-union, or pseudoarthrosis(53; 54). Animal studies revealed that new bone formation was significantly decreased in diabetic animals compared to their control littermates(55). More importantly, under diabetic condition, the process of transition from cartilage to bone was remarkably delayed, resulting in reduced endochondral ossification(56). Under physiological conditions, the reparative phase of fracture healing is initiated by proliferation and chondroblastic differentiation of periosteal precursor cells at the fracture site, which becomes a hyaline cartilage callus. The cartilage callus then calcifies in the process of endochondral ossification(7; 57). However, under diabetic conditions, dysregulated chondrocyte apoptosis and osteoblast differentiation affect this transition from cartilage to bone. Specifically, excessive osteoclast activity disturbs remodeling of the osseous callus(58). Insulin insufficiency, hyperglycemia, and oxidative stress all act in concert to reduce osteoblast differentiation, increase osteoclast activity, and increase apoptosis of chondrocytes and osteoblasts in diabetic fracture healing(59).
Inflammatory responses coincide with fracture healing. Large-scale transcriptional profiling analysis performed on RNA isolated from normal and diabetic callus tissue demonstrates involvement of TNFα and a key role for this cytokine in pathogenic bone healing(10). Interestingly, TNFα up-regulation is prominent on day 16 post-injury, at the phase of the transition from cartilage to bone(10). Furthermore, studies indicate TNFα may impair fracture healing by driving chondrocyte apoptosis. Recently, by applying TNFα inhibitors, diabetic mice demonstrated a significant reduction in chondrocyte apoptosis compared to normoglycemic mice(32). Additionally, TNFα appears to play a more prominent role in fracture healing in diabetes than in normoglycemic animals. Given the prominence of TNFα at the apex of the proinflammatory cytokine cascade and its predominance in the pathogenesis of diabetic fracture, targeted modulation of TNF signaling with novel molecules may offer an interesting avenue for the development of biologies for accelerating delayed diabetic fracture healing.
Indeed, PGRN was isolated as a binding molecule of TNFRs and a modulator of TNF signaling(11; 33; 60–62). PGRN is expressed in both chondrocytes and cartilage, and acts as a downstream molecule of BMP-2 to stimulate chondrocyte differentiation(19; 63). In this study, we employed multiple models of diabetic fracture, most importantly the Bonnarens and Einhorn model which comprises both inflammation and chondrogenesis after fracture, to evaluate the role of PGRN in diabetic fracture healing. We report that deletion of PGRN in genetically modified mice further impaired the diabetic bone healing process. Additionally, under diabetic condition, the expression of pro-inflammatory cytokines was remarkably increased in the callus tissues. Moreover, loss of PGRN further enhanced expression of these molecules. However, we did not observe the significant differences in inflammatory cell populations, including macrophage cells and neutrophils, in fracture hematoma on day 1 after diabetic fracture of WT and PGRN−/− mice (data not shown). Whether PGRN affects the macrophage polarization and the ratio of inflammatory Ml macrophages to anti-inflammatory M2 macrophages, as well as whether PGRN’s effects on inflammatory cells in diabetic fracture healing is time point-dependent, warrant further investigations.
The treatment of fractures and large bone defects are a significant challenge in the field of orthopaedics(64). Chondrogenesis and endochondral ossification play a critical role in the healing of these defects, and certain chondrogenic molecules can help to promote bone repair(65). Interestingly, local delivery of PGRN was shown to markedly enhance bone regeneration under physical condition. Additionally, BMP-2-induced healing has been shown to be markedly inhibited in PGRN-deficient mice, and treatment with recombinant PGRN reversed this impairment(20). These findings suggest that PGRN is a potential regulator of cartilage callus formation in the bone regeneration process, and that PGRN may act in part by participating in BMP-2-induced osteoblastogenesis. To investigate whether recombinant PGRN could promote diabetic bone fracture healing, we locally delivered recombinant PGRN and observed effective promotion of diabetic fracture healing through accelerating callus formation and the transition from cartilage to bone.
Given that PGRN promoted diabetic fracture healing, we attempted to elucidate the mechanism involved. TNFα is an inflammatory molecule and acts to enhance bone resorption and inhibit osteogenesis when it is overexpressed, an effect that is largely mediated through its interaction with TNFR1. On the contrary, TNFR2 is believed to have a protective effect, and plays an important role in bone and cartilage regeneration under physiological conditions. PGRN could bind to TNFR1 and TNFR2(36–40; 60; 63; 66–68). Besides the receptors involved in PGRN’s effect, we examined the intracellular signaling pathways implicated. We found that recombinant PGRN effectively inhibited TNFα mediated p38, INK and NF-κB phosphorylation. Consequently, the transcriptional levels of pro-inflammatory makers, including IL-1β, NOS-2 and COX-2 were reduced in presence of PGRN under diabetic condition. On the other hand, PGRN significantly increased the expressions of chondrogenic marker genes, including type II collagen and aggrecan. Interestingly, PGRN-stimulated chondrogenesis was slightly reduced in TNFRl-defeicient cells and was almost abolished in TNFR2-deficient cells. The reason why PGRN-mediated chondrogenesis was also slightly reduced in TNFR1-deficient mice may due to the fact that recombinant PGRN needs to overcome the elevated TNFα under diabetic condition. Intriguingly, we found that Akt, Erk1/2 and mTOR signaling were activated by PGRN treatment in the course of PGRN-stimulated chondrogenesis.
Collectively, PGRN, a growth factor-like molecule known to induce chondrogenesis and to inhibit TNFα-induced inflammation, effectively promotes impaired fracture healing in mouse diabetic fracture models. PGRN exerts its effect in the diabetic fracture healing process probably through two pathways: 1) PGRN directly binds to TNFR1 and inhibits TNFα-mediated inflammatory and bone erosion; 2) PGRN binds to TNFR2 and activates the Akt, Erk1/2 and mTOR signaling, resulting in accelerated callus formation and promotion of transition from callus to new bone (Fig. 6D). These findings not only provide novel insights into the role of PGRN in diabetic bone fracture healing, but may also lead to the development of novel therapeutic intervention strategies for various impaired fracture healing, in particular diabetic bone fracture healing.
Supplementary Material
Acknowledgments
This work was supported by NIH research grants R01AR062207, R01AR061484, 1R01NS103931, and a DOD research grant W81XWH-16-1-0482 (to C. J. Liu).
Footnotes
Conflict of interest
We herein declare that we have no conflict of interest.
Reference
- 1.Shaw JE, Sicree RA, Zimmet PZ. 2010. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice 87:4–14 [DOI] [PubMed] [Google Scholar]
- 2.Sellmeyer DE, Civitelli R, Hofbauer LC, Khosla S, Lecka-Czernik B, Schwartz AV. 2016. Skeletal Metabolism, Fracture Risk, and Fracture Outcomes in Type 1 and Type 2 Diabetes. Diabetes 65:1757–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, et al. 2018. Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int 29:2585–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strotmeyer ES, Cauley JA. 2007. Diabetes mellitus, bone mineral density, and fracture risk. Current opinion in endocrinology, diabetes, and obesity 14:429–35 [DOI] [PubMed] [Google Scholar]
- 5.Vestergaard P 2007. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporosis International 18:427–44 [DOI] [PubMed] [Google Scholar]
- 6.Einhorn TA. 1998. The Cell and Molecular Biology of Fracture Healing. Clinical Orthopaedics and Related Research® 355:S7–S21 [DOI] [PubMed] [Google Scholar]
- 7.Kayal RA, Alblowi J, McKenzie E, Krothapalli N, Silkman L, et al. 2009. Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone 44:357–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martino MM, Briquez PS, Guc E, Tortelli F, Kilarski WW, et al. 2014. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343:885–8 [DOI] [PubMed] [Google Scholar]
- 9.Wei J, Hettinghouse A, Liu C. 2016. The role of progranulin in arthritis. Annals of the New York Academy of Sciences 1383:5–20 [DOI] [PubMed] [Google Scholar]
- 10.Alblowi J, Kayal RA, Siqueira M, McKenzie E, Krothapalli N, et al. 2009. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol 175:1574–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, et al. 2011. The Growth Factor Progranulin Binds to TNF Receptors and Is Therapeutic Against Inflammatory Arthritis in Mice. Science 332:478–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, et al. 2001. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res 16:1004–14 [DOI] [PubMed] [Google Scholar]
- 13.Alblowi J, Tian C, Siqueira MF, Kayal RA, McKenzie E, et al. 2013. Chemokine expression is upregulated in chondrocytes in diabetic fracture healing. Bone 53:294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J, Hettinghouse A, Liu C. 2016. The role of progranulin in arthritis. Annals of the New York Academy of Sciences 1383:5–20 [DOI] [PubMed] [Google Scholar]
- 15.Williams A, Wang EC, Thurner L, Liu CJ. 2016. Review: Novel Insights Into Tumor Necrosis Factor Receptor, Death Receptor 3, and Progranulin Pathways in Arthritis and Bone Remodeling. Arthritis & rheumatology 68:2845–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao YP, Wei JL, Tian QY, Liu AT, Yi YS, et al. 2016. Progranulin suppresses titanium particle induced inflammatory osteolysis by targeting TNFalpha signaling. Scientific reports 6:20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi T, Ebina K, Hirao M, Kawase R, Ohama T, et al. 2015. Progranulin plays crucial roles in preserving bone mass by inhibiting TNF-alpha-induced osteoclastogenesis and promoting osteoblastic differentiation in mice. Biochemical and biophysical research communications 465:638–43 [DOI] [PubMed] [Google Scholar]
- 18.Zhao YP, Liu B, Tian QY, Wei JL, Richbourgh B, Liu CJ. 2015. Progranulin protects against osteoarthritis through interacting with TNF-alpha and beta-Catenin signalling. Ann Rheum Dis 74:2244–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng JQ, Guo FJ, Jiang BC, Zhang Y, Frenkel S, et al. 2010. Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB J 24:1879–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao YP, Tian QY, Frenkel S, Liu CJ. 2013. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials 34:6412–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia P, Holstein JH, Maier S, Schaumloffel H, Al-Marrawi F, et al. 2008. Development of a reliable non-union model in mice. The Journal of surgical research 147:84–91 [DOI] [PubMed] [Google Scholar]
- 22.He YX, Zhang G, Pan XH, Liu Z, Zheng LZ, et al. 2011. Impaired bone healing pattern in mice with ovariectomy-induced osteoporosis: A drill-hole defect model. Bone 48:1388–400 [DOI] [PubMed] [Google Scholar]
- 23.Bonnarens F, Einhorn TA. 1984. Production of a standard closed fracture in laboratory animal bone. J Orthop Res 2:97–101 [DOI] [PubMed] [Google Scholar]
- 24.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, et al. 2003. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 18:1584–92 [DOI] [PubMed] [Google Scholar]
- 25.Hu J, Dziumbla S, Lin J, Bibli SI, Zukunft S, et al. 2017. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature 552:248–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lietman CD, Lim J, Grafe I, Chen Y, Ding H, et al. 2017. Fkbp10 Deletion in Osteoblasts Leads to Qualitative Defects in Bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 32:1354–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oetgen ME, Merrell GA, Troiano NW, Horowitz MC, Kacena MA. 2008. Development of a femoral non-union model in the mouse. Injury 39:1119–26 [DOI] [PubMed] [Google Scholar]
- 28.Ding Y, Wei J, Hettinghouse A, Guo Y, Tian Q, et al. 2017. Progranulin Growth Factor Promotes Diabetic Bone Healing. In ORS 2017 Annual Meeting. San Diego Convention Center [Google Scholar]
- 29.Nathan C 2006. Role of iNOS in human host defense. Science 312:1874–5; author reply-5 [DOI] [PubMed] [Google Scholar]
- 30.Kong L, Zhao YP, Tian QY, Feng JQ, Kobayashi T, et al. 2016. Extracellular matrix protein 1, a direct targeting molecule of parathyroid hormone-related peptide, negatively regulates chondrogenesis and endochondral ossification via associating with progranulin growth factor. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 30:2741–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim JC, Ko KI, Mattos M, Fang M, Zhang C, et al. 2017. TNFalpha contributes to diabetes impaired angiogenesis in fracture healing. Bone 99:26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kayal RA, Siqueira M, Alblowi J, McLean J, Krothapalli N, et al. 2010. TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO1. J Bone Miner Res 25:1604–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Q, Zhao S, Liu C. 2014. A solid-phase assay for studying direct binding of progranulin to TNFR and progranulin antagonism of TNF/TNFR interactions. Methods Mol Biol 1155:163–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jian J, Konopka J, Liu C. 2013. Insights into the role of progranulin in immunity, infection, and inflammation. J Leukoc Biol 93:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mundra JJ, Jian J, Bhagat P, Liu CJ. 2016. Progranulin inhibits expression and release of chemokines CXCL9 and CXCL10 in a TNFR1 dependent manner. Scientific reports 6:21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abella V, Scotece M, Conde J, Lopez V, Pirozzi C, et al. 2016. The novel adipokine progranulin counteracts IL-1 and TLR4-driven inflammatory response in human and murine chondrocytes via TNFR1. Scientific reports 6:20356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou B, Li H, Liu J, Xu L, Guo Q, et al. 2015. Progranulin induces adipose insulin resistance and autophagic imbalance via TNFR1 in mice. Journal of molecular endocrinology 55:231–43 [DOI] [PubMed] [Google Scholar]
- 38.Briot K, Cortet B, Roux C, Fardet L, Abitbol V, et al. 2014. 2014 update of recommendations on the prevention and treatment of glucocorticoid-induced osteoporosis. Joint, bone, spine : revue du rhumatisme 81:493–501 [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Yang D, Tian J, Gao A, Shen Y, et al. 2017. Tumor necrosis factor receptor 2/AKT and ERK signaling pathways contribute to the switch from fibroblasts to CAFs by progranulin in microenvironment of colorectal cancer. Oncotarget 8:26323–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang D, Wang LL, Dong TT, Shen YH, Guo XS, et al. 2015. Progranulin promotes colorectal cancer proliferation and angiogenesis through TNFR2/Akt and ERK signaling pathways. American journal of cancer research 5:3085–97 [PMC free article] [PubMed] [Google Scholar]
- 41.Wei F, Zhang Y, Jian J, Mundra JJ, Tian Q, et al. 2014. PGRN protects against colitis progression in mice in an IL-10 and TNFR2 dependent manner. Scientific reports 4:7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang BC, Liu H, Talwar A, Jian J. 2015. New discovery rarely runs smooth: an update on progranulin/TNFR interactions. Protein & cell 6:792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCann FE, Perocheau DP, Ruspi G, Blazek K, Davies ML, et al. 2014. Selective tumor necrosis factor receptor I blockade is antiinflammatory and reveals immunoregulatory role of tumor necrosis factor receptor II in collagen-induced arthritis. Arthritis & rheumatology 66:2728–38 [DOI] [PubMed] [Google Scholar]
- 44.Higuchi Y, McTiernan CF, Frye CB, McGowan BS, Chan TO, Feldman AM. 2004. Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation 109:1892–7 [DOI] [PubMed] [Google Scholar]
- 45.Guo FJ, Xiong Z, Han X, Liu C, Liu Y, et al. 2014. XBP1S, a BMP2-inducible transcription factor, accelerates endochondral bone growth by activating GEP growth factor. Journal of cellular and molecular medicine 18:1157–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farr JN, Khosla S. 2016. Determinants of bone strength and quality in diabetes mellitus in humans. Bone 82:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gehling DJ, Lecka-Czernik B, Ebraheim NA. 2016. Orthopedic complications in diabetes. Bone 82:79–92 [DOI] [PubMed] [Google Scholar]
- 48.Sun M, Yang J, Wang J, Hao T, Jiang D, et al. 2016. TNF-α is upregulated in T2DM patients with fracture and promotes the apoptosis of osteoblast cells in vitro in the presence of high glucose. Cytokine 80:35–42 [DOI] [PubMed] [Google Scholar]
- 49.Alblowi J, Kayal RA, Siqueira M, McKenzie E, Krothapalli N, et al. 2009. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. The American journal of pathology 175:1574–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. 2000. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. Journal of endocrinological investigation 23:295–303 [DOI] [PubMed] [Google Scholar]
- 51.Thrailkill KM, Liu L, Wahl EC, Bunn RC, Perrien DS, et al. 2005. Bone formation is impaired in a model of type 1 diabetes. Diabetes 54:2875–81 [DOI] [PubMed] [Google Scholar]
- 52.Hiebert LM. 2017. Proteoglycans and Diabetes. Current pharmaceutical design 23:1500–9 [DOI] [PubMed] [Google Scholar]
- 53.Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. 2015. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN). Diabetes Care 38:1913–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norris R, Parker M. 2011. Diabetes mellitus and hip fracture: a study of 5966 cases. Injury 42:1313–6 [DOI] [PubMed] [Google Scholar]
- 55.Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, et al. 2007. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 22:560–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown ML, Yukata K, Farnsworth CW, Chen DG, Awad H, et al. 2014. Delayed fracture healing and increased callus adiposity in a C57BL/6J murine model of obesity-associated type 2 diabetes mellitus. PloS one 9:e99656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, et al. 2007. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res 22:560–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiao H, Xiao E, Graves DT. 2015. Diabetes and Its Effect on Bone and Fracture Healing. Current osteoporosis reports 13:327–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamada Y, Fujii H, Fukagawa M. 2009. Role of oxidative stress in diabetic bone disorder. Bone 45 Suppl 1: S35–8 [DOI] [PubMed] [Google Scholar]
- 60.Liu C, Li XX, Gao W, Liu W, Liu DS. 2014. Progranulin-derived Atsttrin directly binds to TNFRSF25 (DR3) and inhibits TNF-like ligand 1A (TL1A) activity. PloS one 9:e92743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jian J, Zhao S, Tian Q, Gonzalez-Gugel E, Mundra JJ, et al. 2013. Progranulin directly binds to the CRD2 and CRD3 of TNFR extracellular domains. FEBS Lett 587:3428–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu CJ, Bosch X. 2012. Progranulin: A growth factor, a novel TNFR ligand and a drug target. Pharmacology & therapeutics 133:124–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Q, Xia Q, Wu Y, Zhang X, Wen F, et al. 2015. 3D-Printed Atsttrin-Incorporated Alginate/Hydroxyapatite Scaffold Promotes Bone Defect Regeneration with TNF/TNFR Signaling Involvement. Advanced healthcare materials 4:1701–8 [DOI] [PubMed] [Google Scholar]
- 64.Jakus AE, Rutz AL, Jordan SW, Kannan A, Mitchell SM, et al. 2016. Hyperelastic ″bone″: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Science translational medicine 8:358ra127. [DOI] [PubMed] [Google Scholar]
- 65.Reichert JC, Cipitria A, Epari DR, Saifzadeh S, Krishnakanth P, et al. 2012. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Science translational medicine 4:141ra93. [DOI] [PubMed] [Google Scholar]
- 66.Wei JL, Fu W, Ding YJ, Hettinghouse A, Lendhey M, et al. 2017. Progranulin derivative Atsttrin protects against early osteoarthritis in mouse and rat models. Arthritis research & therapy 19:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan W, Ding A, Kim HJ, Zheng H, Wei F, Ma X. 2016. Progranulin Controls Sepsis via C/EBPalpha-Regulated 1110 Transcription and Ubiquitin Ligase/Proteasome-Mediated Protein Degradation. Journal of immunology 197:3393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alquezar C, de la Encarnacion A, Moreno F, Lopez de Munain A, Martin-Requero A. 2016. Progranulin deficiency induces overactivation of WNT5A expression via TNF-alpha/NF-kappaB pathway in peripheral cells from frontotemporal dementia-linked granulin mutation carriers. Journal of psychiatry & neuroscience : JPN 41:225–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


