Abstract
Angiomatoid fibrous histiocytoma (AFH) is a rare soft tissue tumor that has only been reported in the central nervous system in case reports. After surgery, patients exhibit tumor recurrence. Pathological diagnosis of AHF remains difficult, especially in sites other than skin. AFH can harbor characteristic translocations implying that the Ewing sarcoma breakpoint region 1 gene (EWSR1) fuses with the transcription factor cyclic AMP response element binding (CREB) family genes. Doxorubicin is a chemotherapy that has previously been used successfully in two metastatic soft tissue AFH cases but never in intracranial AFH. The present report describes a case of an adult with a progressive classical intracranial non-myxoid AFH with ESWR1-CREB1 transcript fusion 4 years after surgery. The patient was treated with doxorubicin as a single agent chemotherapy. This treatment resulted in a prolonged stable disease 15 months after treatment discontinuation. This is the first reported case of a treatment with doxorubicin in an adult with progressive intracranial AFH with ESWR1-CREB1 transcript fusion which was sustained after treatment discontinuation.
Keywords: angiomatoid fibrous histiocytoma, Ewing sarcoma breakpoint region 1 gene, anthracycline, doxorubicin, central nervous system tumor, safety
Introduction
Chromosomal translocations resulting in gene fusions are one mechanism underlying tumorigenesis, and some are more frequent in certain cancer entities. The Ewing sarcoma breakpoint region 1 gene (EWSR1), found on chromosome 22q12.2, has a tendency to fuse with the transcription factor cyclic AMP response element binding (CREB) family genes like CREB1, cAMP response element modulator (CREM), or activating transcription factor 1 (ATF1) (1,2). A group of neoplasms are associated with EWSR1-CREB1 and/or EWSR1-ATF1 gene fusions, including angiomatoid fibrous histiocytoma (AFH) but also, clear cell sarcoma, clear cell sarcoma-like tumor of the gastrointestinal tract, primary pulmonary myxoid sarcoma, hyalinizing clear cell carcinoma of the salivary gland, and soft tissue myoepithelial tumor (3). AFH is a rare soft tissue tumor described initially as ‘angiomatoid malignant fibrous histiocytoma’ by Enzinger in 1979(4). It is now described as an indolent tumor with a favorable prognosis by the 2013 World Health Organization classification (5). It is a rare tumor of soft tissue (<0.5%) and mostly occurs superficially, in the extremities of children and young adults (6). Most AFHs are indolent, with a 15 percent regional recurrence rate and a metastatic rate <5%, most frequently involving regional nodes (7). Pathological diagnostic of AHF remain difficult, especially in other sites than skin. As immunohistochemical phenotype is not specific, molecular analysis is useful to confirm the diagnosis and distinguish this entity from likeness tumors. AFH is associated with 3 characteristic translocations: t(2;22)(q33;q12) EWSR1/CREB1 being the most common, t(12;22)(q13;q12) EWSR1/ATF1, and t(12;16)(q13;p11) FUS/ATF1 (3,8). The intracranial location represents a rare primary site, with six conventional AFH cases reported (9-13) and fifteen myxoid AFH (14-23) described as a novel tumor entity. The EWSR1/CREB1 fusion is reported in the myxoid variant but never in the classical non-myxoid component. None of the patients received chemotherapy for this lesion. Herein we describe a case of a classical intracranial non-myxoid AFH with ESWR1-CREB1 transcript fusion treated with doxorubicin as a single agent chemotherapy, inducing a prolonged stable disease fifteen months after treatment discontinuation.
Case report
Clinical history and histological findings
A 40-year-old male referred to the emergency department in 2012 for an intracranial hypertension syndrome with headaches and diplopia. Otherwise, his personal and familial medical clinical history was unremarkable. The magnetic resonance imaging (MRI) of the brain revealed a suspicious lesion of the splenium of the corpus callosum and the pineal region, with hypointense T1 signal, hyperintense T2 signal, and with strong enhancement following gadolinium administration. The lesion was associated with bi-parietal edema. Large increase in lipid and choline without apparent necrosis were showed on spectrometry. There was no sign of neoangiogenesis. The original diagnosis was thought to be lymphoma. He had three needle biopsies between 2012 and 2014 but none of them confirmed this diagnosis. He was then started on corticosteroids but the symptoms got worse with seizures, papillary edema, right homonymous hemianopsia, and agnostic alexia requiring a ventriculoperitoneal shunt. Finally, in March 2014, the patient underwent a left parieto-occipital craniotomy and a subtotal (60%) resection (Fig. 1). The surgical approach was decided because of the retrospenial origin of the tumor and the inferior repulse of the deep venous system. Postoperatively, the patient maintained a right homonymous hemianopsia. He was started on radiation therapy for a total planned dose of 36 grays distributed into 20 sessions, but stopped after 12 sessions for his convenience (i.e. radiotherapy risks and consequences). Chemotherapy was not added to the treatment because of the lack of evidence of its benefits in this case.
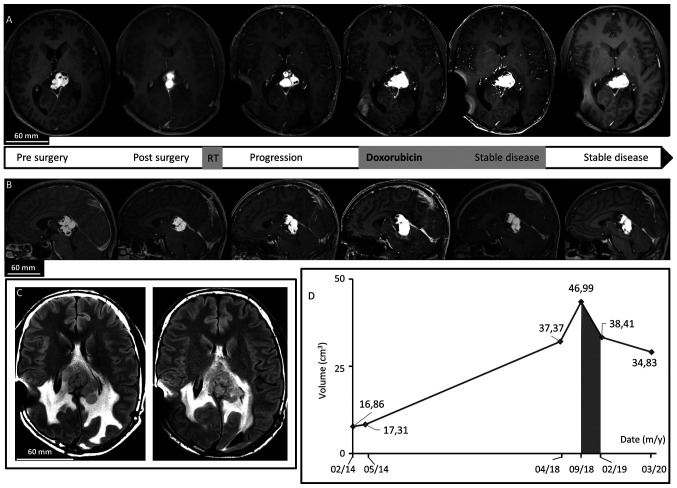
Figure 1.
Brain MRI. (A) Axial after gadolinium injection T1-weighted imaging revealed dominant, patchy intense enhancing lesion of the splenium of the corpus callosum and the pineal region. In chronological order: Before surgery, after surgery showing residual tumor, 4 years after surgery demonstrating progression of the disease, at the beginning of Doxorubicin regimen treatment, just after treatment discontinuation confirming <50% decrease in tumor size, and last follow-up 15 months after treatment discontinuation demonstrating a stable disease. Note the cystic component. (B) Sagittal after gadolinium injection T1-weighted imaging revealed dominant, patchy intense enhancing lesion of the splenium of the corpus callosum and the pineal region before surgery, after surgery, 4 years after surgery, at the beginning of Doxorubicin treatment, after treatment, and at last follow-up. (C) Axial FLAIR T2-weighted images revealing peritumoral edema by large heterogeneous hyperintense signal before treatment with Doxorubicin and at last follow-up. (D) Diagram of evolution of tumor volume on MRI revealed a decrease during and after the Doxorubicin period (grey).
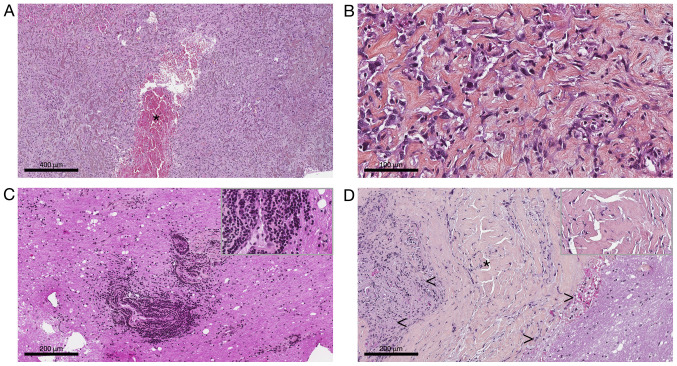
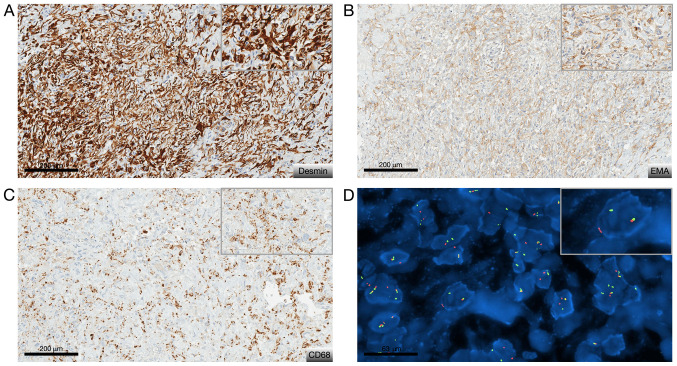
From tumor specimens obtained after fine needle biopsy and after surgery, several formalin-zinc fixed paraffin-embedded (FFPE) blocks and Hematoxylin-Eosin-Saffron stained slides were submitted to histological examination after obtaining written informed and signed consent. It revealed a pathological tissue dominated by thick organized collagen fibers mixed with spindle or epithelioid cells. The nuclei were bland with open chromatin resembling those of macrophages or histiocytes (Fig. 2A and B). There was no pseudo syncytial growth pattern. The tumor exhibit large blood-filled pseudo-vascular spaces (Fig. 2A). Lympho-plasmocytic cuffs as well as thick fibrous pseudocapsule were not seen anymore compared to the second and third biopsy where they were respectively present (Fig. 2C and D). There was no myxoid feature nor necrosis, and no mitosis. Immunohistochemically was carried out on 4-µm-thick FFPE tissue section using UltraView Ventana Universal DAB Detection Kit® (F. Hoffmann-La Roche AG, Switzerland). There was only cytoplastic diffuse staining for desmin, patchy staining for EMA and CD68. The Ki-67 labeling index was low (<7%) (Fig. 3A-C). Fluorescence in situ hybridization (FISH) analysis was performed on tumoral nuclei of paraffin embedded 4-µm-sections using ZytoLight FISH-Tissue Implementation Kit with EWSR1 Dual Color Break Apart Probe (Z-2096-50; ZytoVision), specific for EWSR1 at 22q12.2. The number of orange and green dots were then counted (centromeric probe in 5' to the breakpoint and telomeric probe in 3' to the breakpoint, respectively), both into intron 4 of EWSR1, after DNA were counterstained with DAPI, using a fluorescence microscope. Fifty non-overlapping intact nuclei were examined for EWSR1 rearrangement. Eighty percent of them presented in this case a split signal also called break apart signal meaning that separated orange and green dots or single orange dots were seen consistent with a EWSR1 rearrangement (>20% of rearranged nuclei) (Fig. 3D). To look for its fusion partner, a retrotranscriptase-quantitative polymerase chain reaction was then performed in two different molecular departments on FFPE and frozen tissue and confirmed the presence of EWSR1/CREB1 fusion transcript. Diagnosis of classical non-myxoid angiomatoid fibrous histiocytoma with the fusion transcript EWSR1/CREB1 was made by the association of morphological, immunohistochemical and molecular data (5).
Figure 2.
Pathological findings of tumor biopsy and resection. Characteristic histological features of angiomatoid fibrous histiocytoma from (A and B) both surgical specimen and (C and D) the biopsy sample. (A and B) Hematoxylin-Eosin-Saffron staining of surgical specimen. Original magnification, (A) x5 and (B) x40. Spindle-cell or epithelioid proliferation dispersed in thick organized collagen fibers with bland open chromatin nuclei. Note the blood-filled pseudo-vascular spaces (*), which were lined by tumoral cells. (C and D) Hematoxylin-Eosin-Saffron staining of biopsy sample. Original magnification, x20. (C) Peripheral lymphocytic infiltrate corresponding to perivascular cuffs. (D) Thick pseudo-capsule (*) lining tumor (<) and surrounding nervous tissue (>). The top right corner frame shows a 1.5X higher magnification (C and D).
Figure 3.
Immunohistochemical staining and FISH analysis results. Characteristic (A to C) phenotypical and (D) molecular features of angiomatoid fibrous histiocytoma. (A) Immunohistochemical view revealing diffusely positive staining for desmin (D33 clone; magnification, x20), (B) patchy staining for EMA (E29 clone; magnification, x20), and (C) CD68 (KP1 clone; magnification, x20). (D) FISH view revealing EWSR1 rearrangement. Original magnification, x63. The top right corner frame shows a 1.5X higher magnification. EMA, epithelial membrane antigen; FISH, fluorescence in situ hybridization.
Patient management and outcomes
The patient was monitored for four years until MRI demonstrated tumor progression. The last MRI before progression in August 2017 showed a lesion of 35x28x29 mm.
On the MRI of April 2018, the lesion of the pineal region was heterogeneous and measure 40 mm of height, 27 mm of anteroposterior length, and 38 mm of width. It had a hypointense T1 signal, heterogeneous hyperintense T2 signal, and strong patchy enhancement following gadolinium administration. There were some cystic components, with the largest in the right anterior superior part with a diameter of 26 mm (Fig. 1). There was also peritumoral edema. At this time, surgical resection was considered too risky without possibility of complete removal and the patient could not be re-irradiated. The interdisciplinary tumor board decided to treat him as if he had a sarcoma-like tumor. In September 2018, as the tumor was still on progression (Fig. 1), he was started on intravenously doxorubicin 60 mg/m² every three weeks for a total of seven injections. Less than a month after treatment cessation, the patient was neurologically stable and brain MRI showed a <50% decrease in tumor size, considered as stable disease by the Response Assessment in Neuro-Oncology criteria (Fig. 1). Toxicities were measured by the Common Terminology Criteria for Adverse Events v5.0. The patient had a grade III constipation requiring a short-term hospitalization and a treatment with osmotic laxatives and mechanical removal. Otherwise, treatment was well tolerated; by the end, he had grade II alopecia, grade I asthenia, anorexia, and oral mycosis treated with oral bicarbonate. He had no feared anthracycline complication, meaning neither cardiac failure nor hepatotoxicity. Six months after stopping doxorubicin, he recovered from toxicities and MRI showed no signs of progression. Fourteen months after doxorubicin discontinuation in March 2020, MRI and neurological examination showed stable disease (Fig. 1). He still had some mild blurred vision and alexia. Although he was not cured from the disease, the tumor's progression was stopped and both his neuro-cognitive functions and quality of life were preserved.
Discussion
To our knowledge, this is the first case of a patient with classical non-myxoid intracranial AFH treated with single chemotherapy inducing prolonged stable disease. Even if the location is extremely rare, AFH was here confirmed by the integration of radiological, morphological and immunohistochemical data with the molecular analysis. The latter demonstrated the original EWSR1-CREB1 fusion, heretofore only described in intracranial myxoid mesenchymal variant. Indeed, even if EWSR1-CREB1 is the most frequently described fusion transcript in this entity (24), it is the first reported description in intracranial classical AFH. Most soft tissue AFH are indolent with 15 percent risk of local recurrence and less than 5 percent risk of metastases, predominantly to regional lymph nodes (7,25,26). It accounts for 0.3% of all soft tissue tumors and usually occurs in children and young adult (6). The intracranial location is extremely rare and only cases reports are described. This tumor has been reported in twenty-one previous instances: Six conventional AFH (9-13) and fifteen myxoid AFH (14-23). Characteristics of these tumors and their outcomes are listed in Table I. Unlike the present case, medium reported age at diagnosis is 26-year-old with a female predominance. Long-term outcomes (<1 year) are not available in 11 cases but the recurrence rate for the others is 60% (9-19,23). The scarcity of this location makes the diagnosis difficult. When utilizing imaging results, the most frequently established diagnosis is meningioma or lymphoma (12,18,23). Histologically, the diagnostic of intracranial AFH is difficult: The tumor is well delimited with lobulated or multinodular borders and thick fibrous pseudocapsule. In up to 80% of tumors, dense lymphoplasmacytic infiltrate or cuffs can be seen, resembling those of schwannoma. Multifocal hemorrhage is seen in most cases, forming blood-filled cystic spaces of variable size. Mostly half of the AFHs express desmin, without positivity for myogenin or MyoD1. Many express EMA and CD68(3). In our case, diagnosis was complicated due to repeated intracranial biopsy sampling of the tumor that not only gives little insights of its morphological characteristics but also induces changes in morphological features (like fibrosis, hemorrhage or tissue distortion). This tumor expressed desmin, EMA and CD68, which finally made us suspect AFH diagnosis and ask for the molecular analyses that confirmed it (25).
Table I.
Comprehensive list of reported cases of angiomatoid fibrous histiocytoma (with myxoid component or not) and their treatment with reported outcomes.
| AFH | Authors | Age (years)/sex | Location | Symptoms/signs (clinical features) | MRI | Surgery | IHC | Genetic marker | Treatment post-surgery | Time to recurrence | Total follow up/recurrence | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional AFH | Dunham et al, 2008 | 25/M | Occipital lobe | Visual problems, headaches | Cystic component, heterogeneously enhancement | GTR | EMA+, desmin+ | EWSR1/ATF1 | None | NM | NM | (9) |
| Ochalski et al, 2010 | 35/M | Temporal lobe | Headaches, facial weakness | Minimal enhancement | GTR | Desmin+, EMA NM | Rearranged EWSR1 gene | GKSa | 0.8 months | 49 months/multipleb | (10) | |
| Hansen et al, 2015 | 17/F | Parieto-occipital lobe | Headaches | Heterogeneously enhancement, edema | GTR | EMA+, desmin+ | Negative | None | NA | 3 months/none | (11) | |
| Alshareef et al, 2016 | 58/F | Porous trigeminus | Facial weakness | Heterogeneously enhancement, edema | GTR | NM | Rearranged EWSR1 gene | None | NA | 6 months/none | (12) | |
| Konstantinidis et al, 2019 | 13/F | Frontal lobe | Headaches | Cystic component, enhancement, edema | GTR | Desmin+, EMA NM | EWSR1/ATF1 | None | 5 years (surgery) | 11 years/yes | (13) | |
| Konstantinidis et al, 2019 | 12/F | Frontal lobe | Visual problems, headaches | Cystic component, | STR | EMA+, desmin+ | EWSR1/CREM | None | 28 months | 28 months/yes | (13) | |
| Myxoid mesenchymal | Kao et al, 2017 | 15/F | Meninges | NM | NM | NM | EMA+, desmin+ | EWSR1/CREM | None | NA | 17 months/none | (14) |
| AFH | Kao et al, 2017 | 23/F | Meninges (occipital) | NM | NM | NM | EMA+, desmin+ | EWSR1/CREB1 | None | NM | NM | (14) |
| Kao et al, 2017 | 20/M | Frontal lobe | NM | NM | NM | EMA+, desmin+ | EWSR1/CREB1 | None | NM | NM | (14) | |
| Kao et al, 2017 | 12/M | Frontal lobe | Seizures | NM | NM | EMA+, desmin- | EWSR1/ATF1 | None | NM | NM | (14) | |
| Bale et al, 2018 | 12/M | Posterior cerebellar fossa | Headaches | Heterogeneously enhancement | STR | EMA+, desmin+ | EWSR1/CREB1 | None | NA | 12 months/none | (17) | |
| Bale et al, 2018 | 14/F | Intraventricular | Headaches, visual problems | Heterogeneously enhancement, edema | STR | EMA+, desmin+ | EWSR1/CREB1 | None | NA | 12 months/none | (17) | |
| Bale et al, 2018 | 18/M | Frontal lobe | Seizures | Enhancement, edema | STR | EMA+, desmin+ | EWSR1/CREM | None | NA | 12 months/none | (17) | |
| Sciot et al, 2018 | 17/F | Frontal lobe | Hemiparesis, seizures | Cystic component, minimal enhancement | GTR | EMA+, desmin- | EWSR1/ATF1 | RT after 2nd surgery | 3 months (surgery and RT) | 7 years/two | (16) | |
| Gareton et al, 2018 | 19/M | Tentorium cerebelli | Seizures | NM | GTR | EMA+, desmin- | EWSR1/CREM | 6 API/AI and RT | 10 years | 10 years/yes | (15) | |
| Spatz et al, 2018 | 22/F | Occipital lobe | Visual problems, headaches, seizure | Heterogeneously enhancement, edema | STR | EMA+, desmin+ | NM | None | NA | 3 months/none | (23) | |
| Ghanbari et al, 2019 | 58/F | Parafalcine | Seizure | Homogenous enhancement, edema | STR | EMA+, desmin+ | EWSR1/CREB1 | None | NA | 3 months/none | (18) | |
| Gunness et al, 2019 | 32/F | Temporal lobe | Headaches | Cystic component, heterogeneously enhancement | STR | NM | NM | None | 1 year (surgery) | 2 years/yes | (19) | |
| White et al, 2019 | 9/M | Frontal lobe | Fatigue, abulia | Cystic component, enhancement | GTR | Desmin+/-, EMA NM | EWSR1/CREM | None | 6 months (surgery and RT) | 6 months/yes | (20) | |
| Ballester et al, 2020 | 67/M | Temporal lobe | Aphasia, confusion | Cystic component, enhancement, edema | STR | EMA+, desmin+ | EWSR1/ATF1 | None | NA | 3.5 months/none | (21) | |
| Komatsu et al, 2020 | 53/F | Third ventricle | Headache, dizziness | Homogenous enhancement | STR | EMA+, desmin+ | EWSR1/CREB1 | GKS | NA | 3 months/none | (22) |
aGKS after 4 surgeries and then after 3 other surgeries;
bdied after 9 surgeries and 2 GKS. API, doxorubicin-ifosfamide-cisplatin-based regimen; AI, doxorubicin-ifosfamide-based regimen; AFH, angiomatoid fibrous histiocytoma; EMA, epithelial membrane antigen; F, female; GKS, gamma knife surgery; GTR, gross total resection; IHC, immunohistochemistry; M, male; NA, not applicable; NM, not mentioned; RT, radiotherapy; STR, subtotal resection.
All the reports support gross total resection as the gold standard treatment at presentation and recurrence. Indeed there is a lack of proof regarding radiotherapy and chemotherapy. In metastatic soft tissue AFH, anthracycline based chemotherapy has previously been used successfully in two cases suggesting the likely usefulness of such treatment in patient with unresectable and/or metastatic disease (26,27). Also, one of the latest case reports of intracranial AFH suggests that use of chemotherapy as an adjuvant therapy could be considered if surgical resection was vain or deleterious to the patient (13). Actually, one patient was treated with 6 cycles of API/AI type chemotherapy but it was for a myxoid-variant AFH and after complete tumor removal. None of the patients presented in the cases was challenged with chemotherapy at tumor progression.
This case report confirms that the diagnostic of intracranial AFH can be problematic and a gross total resection, when possible, must be considered. Besides, an integrated approach using morphology, immunohistochemistry and molecular analysis is recommended to support the diagnosis of this rare entity. The potential relation with the myxoid component needs to be further studied. Treatments options after surgery for recurrent or progressive intracranial AFH, myxoid or not, are scarce and the optimal treatment sequence is unknown. Here we present the first case of intracranial conventional AFH with EWSR1/CREB1 fusion transcript, treated with doxorubicin at progression, inducing prolonged stable disease fourteen months after treatment discontinuation.
In conclusion, in the absence of gold standard management for such cases, the present case suggests that chemotherapy should be considered in intracranial AFH when surgery is not an option. Desmin staining and EWSR1 gene fusions should be searched for in all cases possibly compatible with intracranial AFH especially in EMA positive spindle cell tumors without typical meningioma features. A single institute observational study is currently ongoing in Italy. All the medical records, radiological imaging, and histological slides are being reviewed to identify the best therapeutic approach (NCT03759327). Moreover, radiation therapy with or without chemotherapy combination (including doxorubicin) or targeted therapy before surgery are currently being explored for patients with newly diagnosed non-rhabdomyosarcoma soft tissue sarcomas (comprising AFH) (NCT02180867), which could give us clues to the best treatment for intracranial AFH patients.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LG instructed and participated in the treatment of the patient, performed the literature search, and mainly wrote the manuscript. LG and TF created the figures and tables. JH and FD instructed and participated in the treatment of the patient. TF, JH and FD provided critical revisions of the manuscript for important intellectual content. TF, DM and DP carefully reviewed the pathological findings. RA carefully reviewed the radiology findings. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The patient provided written informed consent prior to treatment.
Patient consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lemaigre FP, Ace CI, Green MR. The cAMP response element binding protein, CREB, is a potent inhibitor of diverse transcriptional activators. Nucleic Acids Res. 1993;21:2907–2911. doi: 10.1093/nar/21.12.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 3.Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: The current status. Am J Surg Pathol. 2012;36(11) doi: 10.1097/PAS.0b013e31825485c5. [DOI] [PubMed] [Google Scholar]
- 4.Enzinger FM. Angiomatoid malignant fibrous histiocytoma. A distinct fibrohistiocytic tumor of children and young adults simulating a vascular neoplasm. Cancer. 1979;44:2147–2157. doi: 10.1002/1097-0142(197912)44:6<2147::aid-cncr2820440627>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Antonescu CR, Rossi S. WHO Classification of Tumours of Soft Tissue and Bone. Vol 5. 4th edition. Fletcher CD, Bridge JA, Hogendoorn PC and Mertens F (eds). IARC, Lyon, pp204-205, 2013. [Google Scholar]
- 6.Saito K, Kobayashi E, Yoshida A, Araki Y, Kubota D, Tanzawa Y, Kawai A, Yanagawa T, Takagishi K, Chuman H. Angiomatoid fibrous histiocytoma: A series of seven cases including genetically confirmed aggressive cases and a literature review. BMC Musculoskelet Disord. 2017;18(31) doi: 10.1186/s12891-017-1390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanburg-Smith JC, Miettinen M. Angiomatoid ‘malignant’ fibrous histiocytoma: A clinicopathologic study of 158 cases and further exploration of the myoid phenotype. Hum Pathol. 1999;30:1336–1343. doi: 10.1016/s0046-8177(99)90065-5. [DOI] [PubMed] [Google Scholar]
- 8.Huret JL. EWSR1 (Ewing sarcoma breakpoint region 1) Atlas Genet Cytogenet Oncol Haematol. 2011;15:395–407. [Google Scholar]
- 9.Dunham C, Hussong J, Seiff M, Pfeifer J, Perry A. Primary Intracerebral angiomatoid fibrous histiocytoma: Report of a case with a t(12;22)(q13;q12) causing type 1 fusion of the EWS and ATF-1 genes. Am J Surg Pathol. 2008;32:478–484. doi: 10.1097/PAS.0b013e3181453451. [DOI] [PubMed] [Google Scholar]
- 10.Ochalski PG, Edinger JT, Horowitz MB, Stetler WR, Murdoch GH, Kassam AB, Engh JA. Intracranial angiomatoid fibrous histiocytoma presenting as recurrent multifocal intraparenchymal hemorrhage. J Neurosurg. 2010;112:978–982. doi: 10.3171/2009.8.JNS081518. [DOI] [PubMed] [Google Scholar]
- 11.Hansen JM, Larsen VA, Scheie D, Perry A, Skjøth-Rasmussen J. Primary intracranial angiomatoid fibrous histiocytoma presenting with anaemia and migraine-like headaches and aura as early clinical features. Cephalalgia. 2015;35:1334–1336. doi: 10.1177/0333102415583988. [DOI] [PubMed] [Google Scholar]
- 12.Alshareef MA, Almadidy Z, Baker T, Perry A, Welsh CT, Vandergrift WA III. Intracranial angiomatoid fibrous histiocytoma: Case report and literature review. World Neurosurg. 2016;96:403–409. doi: 10.1016/j.wneu.2016.09.059. [DOI] [PubMed] [Google Scholar]
- 13.Konstantinidis A, Cheesman E, O'Sullivan J, Pavaine J, Avula S, Pizer B, Kilday JP. Intracranial angiomatoid fibrous histiocytoma with EWSR1-CREB family fusions: A report of 2 pediatric cases. World Neurosurg. 2019;126:113–119. doi: 10.1016/j.wneu.2019.02.107. [DOI] [PubMed] [Google Scholar]
- 14.Kao YC, Sung YS, Zhang L, Chen CL, Vaiyapuri S, Rosenblum MK, Antonescu CR. EWSR1 fusions with CREB family transcription factors define a novel myxoid mesenchymal tumor with predilection for intracranial location. Am J Surg Pathol. 2017;41:482–490. doi: 10.1097/PAS.0000000000000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gareton A, Pierron G, Mokhtari K, Tran S, Tauziède-Espariat A, Pallud J, Louvel G, Meary E, Capelle L, Chrétien F, Varlet P. ESWR1-CREM fusion in an intracranial myxoid angiomatoid fibrous histiocytoma-like tumor: A case report and literature review. J Neuropathol Exp Neurol. 2018;77:537–541. doi: 10.1093/jnen/nly039. [DOI] [PubMed] [Google Scholar]
- 16.Sciot R, Jacobs S, Calenbergh FV, Demaerel P, Wozniak A, Debiec-Rychter M. Primary myxoid mesenchymal tumour with intracranial location: Report of a case with a EWSR1-ATF1 fusion. Histopathology. 2018;72:880–883. doi: 10.1111/his.13437. [DOI] [PubMed] [Google Scholar]
- 17.Bale TA, Oviedo A, Kozakewich H, Giannini C, Davineni PK, Ligon K, Alexandrescu S. Intracranial myxoid mesenchymal tumors with EWSR1-CREB family gene fusions: Myxoid variant of angiomatoid fibrous histiocytoma or novel entity? Brain Pathol. 2018;28:183–191. doi: 10.1111/bpa.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghanbari N, Lam A, Wycoco V, Lee G. Intracranial myxoid variant of angiomatoid fibrous histiocytoma: A case report and literature review. Cureus. 2019;11(e4261) doi: 10.7759/cureus.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunness VR, Munoz I, González-López P, Alshafai N, Mikalkova A, Spears J. Intracranial angiomatoid fibrous histiocytoma with Hodgkin lymphoma. Med J Malaysia. 2019;74:234–236. [PubMed] [Google Scholar]
- 20.White MD, McDowell MM, Pearce TM, Bukowinski AJ, Greene S. Intracranial Myxoid mesenchymal tumor with rare EWSR1-CREM translocation. Pediatr Neurosurg. 2019;54:347–353. doi: 10.1159/000501695. [DOI] [PubMed] [Google Scholar]
- 21.Ballester LY, Meis JM, Lazar AJ, Prabhu SS, Hoang KB, Leeds NE, Fuller GN. Intracranial myxoid mesenchymal tumor with EWSR1-ATF1 Fusion. J Neuropathol Exp Neurol. 2020;79:347–351. doi: 10.1093/jnen/nlz140. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu M, Yoshida A, Tanaka K, Matsuo K, Sasayama T, Kojita Y, Kanda T, Kodama Y, Itoh T, Hirose T. Intracranial myxoid mesenchymal tumor with EWSR1-CREB1 gene fusion: A case report and literature review. Brain Tumor Pathol. 2020;37:76–80. doi: 10.1007/s10014-020-00359-x. [DOI] [PubMed] [Google Scholar]
- 23.Spatz M, Nussbaum ES, Lyons L, Greenberg S, Kallmes KM, Nussbaum LA. doi: 10.1080/02688697.2018.1451823. Primary intracranial angiomatoid fibrous histiocytoma: A case report and literature review. Br J Neurosurg: Mar 15, 2018 (Epub ahead of print). doi: 10.1080/02688697.2018.1451823. [DOI] [PubMed] [Google Scholar]
- 24.Antonescu CR, Dal Cin P, Nafa K, Teot LA, Surti U, Fletcher CD, Ladanyi M. EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer. 2007;46:1051–1060. doi: 10.1002/gcc.20491. [DOI] [PubMed] [Google Scholar]
- 25.Thway K, Fisher C. Angiomatoid fibrous histiocytoma: The current status of pathology and genetics. Arch Pathol Lab Med. 2015;139:674–682. doi: 10.5858/arpa.2014-0234-RA. [DOI] [PubMed] [Google Scholar]
- 26.Ogden S, Harave S, McPartland J, Brennan B, Jeys L, Losty P, Pizer B. doi: 10.1002/pbc.26376. Angiomatoid fibrous histiocytoma: A case of local recurrence and metastases to loco-regional lymph nodes that responded to chemotherapy. Pediatr Blood Cancer: 64, 2017 doi: 10.1002/pbc.26376. [DOI] [PubMed] [Google Scholar]
- 27.Bernini JC, Fort DW, Pritchard M, Rogers BB, Winick NJ. Adjuvant chemotherapy for treatment of unresectable and metastatic angiomatoid malignant fibrous histiocytoma. Cancer. 1994;74:962–964. doi: 10.1002/1097-0142(19940801)74:3<962::aid-cncr2820740327>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.