Abstract
Purpose
We aim to describe the treatment patterns and overall survival (OS) outcomes in patients receiving trastuzumab emtansine (T-DM1) for HER2-positive metastatic breast cancer (HER2+MBC) in routine clinical care.
Methods
Retrospective, whole-of-population cohort study of people initiating T-DM1 for HER2+MBC between October 2015 and May 2019 in Australia. We used dispensing claims to estimate time-to-T-DM1 initiation, duration of treatment, and treatments administered prior to and following T-DM1 therapy. We estimated OS from T-DM1 initiation and stratified results based on whether patients received first- or second-line T-DM1 treatment. We benchmarked outcomes to those reported in the pivotal, EMILIA trial.
Results
345 patients initiated T-DM1: 309 as second-line therapy for HER2+MBC and 36 as first-line therapy. 51% of patients had received endocrine therapy and 98% of second-line patients received pertuzumab prior to starting T-DM1. The median age was 57 years (53 in EMILIA); median time-to-T-DM1 initiation from start of HER2-targeted therapy for HER2+MBC was 11.6 months (IQR: 7.9–16.6); median duration of T-DM1 treatment was 6.5 months (3.1–13.5; 7.6 months in EMILIA), and median OS was 19.3 months (7.9–29.5; 29.9 months in EMILIA).
Conclusions
Our findings highlight differences in patient characteristics (older, more previous pertuzumab therapy) and outcomes (shorter OS) from the T-DM1 pivotal trial and provide real-world estimates that can inform patient, clinician and policy, decisions around the use of HER2-targeted therapies in routine clinical care.
Keywords: Trastuzumab emtansine, T-DM1, HER2-Positive breast cancer, Population-based
Abbreviations: HER2+MBC, HER2-positive metastatic breast cancer; T-DM1, Trastuzumab emtansine; RCT, Randomized controlled trial; EMILIA, Name of pivotal trial for T-DM1; PBS, Pharmaceutical benefits scheme; OS, Overall survival
Highlights
-
•
Real-world T-DM1 recipients are older than trial participants.
-
•
Real-world T-DM1 recipients have more prior pertuzumab exposure than trial participants.
-
•
Median overall survival was 10 months shorter than that reported from the trial.
1. Introduction
HER2-targeted therapies have transformed the treatment and prognosis of patients with HER2-positive metastatic breast cancer (HER2+MBC) [1]. Since the introduction of trastuzumab over twenty years ago, median overall survival for patients treated with HER2-targeted agents has increased markedly [[2], [3], [4], [5]]. In Australia there are currently four HER2-targeted agents publicly subsidized for use in HER2+MBC—trastuzumab, lapatinib, pertuzumab, and trastuzumab emtansine (T-DM1). Newer HER2-targeted agents including tucatinib, neratinib and trastuzumab deruxtecan have shown promising effects in clinical trials and are likely to become available in the near future.
HER2-targeted agents are standard-of-care for HER2-positive breast cancer in early-stage and metastatic treatment settings [[6], [7], [8]]. The evidence supporting their regulation, subsidy, and use, comes primarily from randomized clinical trials (RCTs) conducted in highly selected patient populations treated according to strict protocols [9]. So, the efficacy outcomes reported in RCTs may not directly apply to the more heterogeneous populations treated in routine clinical practice. Further, with the rapid emergence of new and effective HER2-targeted agents, patients treated in routine practice today often have access to more effective therapies than those who were enrolled in the pivotal clinical trials five to 10 years earlier. Access to more effective prior and subsequent therapies may affect the survival outcomes seen when a treatment with proven activity in a clinical trial is introduced to routine practice.
This scenario is illustrated by T-DM1 for HER2+MBC, where, in Australia the medicine is publicly-subsidized for: 1) second-line treatment for HER2+MBC that has progressed following treatment with pertuzumab and trastuzumab; and 2) first-line treatment for HER2+MBC where the cancer has progressed during or within 6 months of completing adjuvant therapy with trastuzumab. At the time of enrolment in the pivotal trial for T-DM1 (EMILIA) [10,11], pertuzumab was only available as an investigational therapy and most patients enrolled in EMILIA would not have received combination trastuzumab and pertuzumab as first-line therapy.
Data from real-world patient cohorts treated in routine clinical care are needed to complement the evidence generated in RCTs and provide information to patients, clinicians, policy makers around expected survival times, treatment durations, and patterns of treatment for patients receiving T-DM1 for HER2+MBC [12]. The aim of this study was to describe the survival outcomes and treatment patterns for a large, population-based cohort of Australian patients accessing publicly-funded T-DM1 for HER2+MBC—both as first- and second-line treatment. We describe patient characteristics; estimate time-to-T-DM1 initiation; detail duration of treatment with T-DM1 and other HER2-targeted therapies; and estimate overall survival (OS) from T-DM1 initiation. We benchmark these outcomes to those reported from the original pivotal trial for T-DM1, EMILIA [10,11].
2. Materials and methods
2.1. Study setting and data
The Australian healthcare setting and the datasets used in this study have been described in our research protocol [13]. Briefly, Australia maintains a publicly-funded, universal healthcare system entitling all citizens and permanent residents to subsidized medicines through the Pharmaceutical Benefits Scheme (PBS). A separate funding program, the Herceptin Program, provided subsidized access to trastuzumab for HER2+MBC from December 2001 until July 2015, when the program was ended and trastuzumab for HER2+MBC was listed for subsidy on the PBS. Pertuzumab and T-DM1 also gained subsidy through the PBS in July 2015, while trastuzumab for HER2+EBC and lapatinib were listed on the PBS in October 2006 and May 2008, respectively.
Services Australia—administering body for the PBS and the Herceptin Program—supplied de-identified patient-level data for every person accessing publicly-subsidized trastuzumab, lapatinib, pertuzumab, and T-DM1 for HER2+MBC in Australia between December 3, 2001 and May 31, 2019. The datasets provided by Services Australia were Herceptin Program enrolment data, including year of birth and month/year of death; trastuzumab dispensing records for HER2+MBC, including date and quantity dispensed; and PBS dispensing records detailing other medicines dispensed for all Herceptin Program enrolees between December 3, 2001 and June 30, 2015. Services Australia also provided PBS dispensing records—including all dispensed medicines, not just anti-cancer medicines, dispensing dates, PBS item codes, and PBS authority-to-prescribe codes—for all patients in Australia who accessed PBS-subsidized HER2-targeted therapies from October 1, 2006 until May 31, 2019; and patient information including sex, year of birth, and month/year of death.
2.2. Treatment setting and lines of therapy for HER2+MBC
In Australia, T-DM1, pertuzumab, and lapatinib are only subsidized for use in HER2+MBC. Between December 3, 2001 and June 30, 2015 trastuzumab for HER2+MBC was only available through a separate public program—the Herceptin Program. Following the closing of the Herceptin Program in July 2015, we ascertained treatment settings (EBC/MBC) for trastuzumab by using PBS item codes and PBS authority-to-prescribe codes [14]. Specialized medicines, such as trastuzumab, require prescribers to obtain permission from the PBS before they can prescribe the medicine and this generates an additional code indicating the reason for the prescription (EBC or MBC) [15]. We ascertained treatment lines in the metastatic setting using dispensing records. We considered patients whose first T-DM1 dispensing was also the first dispensing of a HER2-targeted agent in the metastatic setting as first-line T-DM1 patients. We considered patients with dispensings of trastuzumab for HER2+MBC (with and without pertuzumab) preceding T-DM1 dispensings as second-line T-DM1 patients.
The full period of time observed across the datasets is January 1, 2001 through May 31, 2019.
2.3. Study design and participants
Our population-based, retrospective cohort study includes every Australian initiating PBS-subsidized T-DM1 between October 1, 2015 and May 31, 2019, followed until death or May 31, 2019. We commenced our study period three months after T-DM1 was publicly subsidized in July 2015 to ensure our cohort comprised patients initiating T-DM1. Prior to July 2015 patients may have initiated T-DM1 through clinical trials or special access schemes and then continued accessing the medicine through the PBS afterwards. In Australia, once a medicine is PBS-subsidized the government bears the cost of the medicine. Private insurance will not provide reimbursement for medicines already subsidized through public programs, so it is unlikely that patients would access these medicines through other avenues and it would not be necessary for them to pay for the entire cost of the medicine out of their own pocket. As such, our study population likely captures all patients in Australia treated with available HER2-targeted therapies during the study period.
2.4. Outcomes and statistical analysis
We used descriptive statistics to summarize age, sex, and fact of death. We ascertained the number of patients dispensed endocrine therapies during the three years prior to T-DM1 initiation. For each patient we calculated the duration of total time on T-DM1, other HER2-targeted therapies, and all HER2-targeted therapy. For patients previously treated with (neo)adjuvant trastuzumab we also calculated the duration of that treatment. We estimated time on treatment with each agent as the period from first dispensing date until the last dispensing date, plus 30 days or the number of days from last dispensing to date of death, whichever was sooner. We considered a period of more than 90 days between dispensings as a break in treatment, and a dispensing following a break of more than 90 days as a new course of therapy [4].
We used PBS dispensing data to summarize the type and number of other cancer treatments dispensed, as well as their timing in relation to T-DM1 treatment. For patients initiating T-DM1 as second-line therapy we calculated the time-to-T-DM1 initiation as the time from the first dispensing of a HER2-targeted therapy for HER2+MBC until the first T-DM1 dispensing. For patients previously treated with trastuzumab for HER2+EBC, we defined the time-to-T-DM1 initiation as the time from the last trastuzumab for HER2+EBC dispensing until the first T-DM1 dispensing. We calculated OS as the time from first T-DM1 dispensing date until the month of death (set at the last day of the month) or censor. We used Kaplan-Meier methods to estimate duration, time-to-initiation, and OS.
We benchmarked patient characteristics, time on treatment, and OS outcomes from our cohort to the results reported from the original pivotal trial, EMILIA, which evaluated second-line T-DM1, following previous treatment with trastuzumab for HER2+MBC, and first-line T-DM1 in patients who relapsed within six months of treatment for early-stage disease [10,11].
All analyses were performed in R version 3.6.1 (R Development Core Team) [16].
3. Results
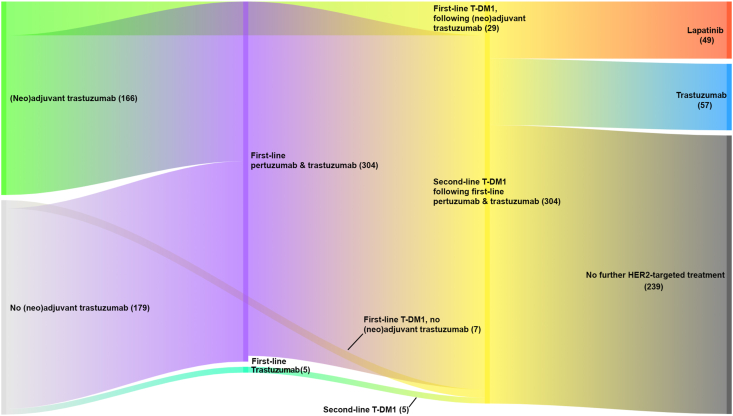
Our cohort comprised 345 patients dispensed T-DM1 at least once during the study period (second-line, n = 309; first-line, n = 36; Fig. 1). Their median age was 57 years (interquartile range [IQR]: 48–67); range: 25–97) and 99% were female (Table 1). The median age of patients initiating T-DM1 as first-line therapy was 52.5 years and 58 years for those initiating T-DM1 as second-line treatment. Patients from our cohort were older than those from EMILIA (median age: 53 years). Forty-eighty percent of our cohort had been treated previously with trastuzumab for HER2+EBC, 81% of those receiving first-line T-DM1 (this proportion is not reported for EMILIA). Just over half of all patients (51%) were treated with endocrine therapies prior to receiving T-DM1, similar to reported proportions of ER/PR-positive patients in EMILIA. By May 31, 2019 39% of the cohort had died.
Fig. 1.
HER2-targeted treatment pathway for patients in our cohort initiating T-DM1. The number of patients in each path is in parentheses.
Table 1.
Characteristics of the entire cohort and according to treatment line for HER2+MBC; and the T-DM1 treatment arm of pivotal trial.
| Overall | Second-line T-DM1 | First-line T-DM1 | EMILIA [10,11] | |
|---|---|---|---|---|
| Patients, n | 345 | 309 | 36 | 495 |
| Baseline measures | ||||
| Age in years at first T-DM1 dispensing, median (IQR) | 57 (48–68) | 58 (49–68) | 52.5 (45.5–65) | 53 (25–84)a |
| <35 | 5 (1%) | – | – | – |
| 35–44 | 53 (16%) | 45 (15%) | 8 (22%) | – |
| 45–54 | 89 (26%) | 76 (25%) | 13 (36%) | – |
| 55–64 | 92 (27%) | 88 (29%) | 4 (11%) | – |
| 65–74 | 66 (19%) | 58 (19%) | 8 (22%) | – |
| ≥75 | 40 (11%) | – | – | – |
| Female, n | 342 (99%) | – | 36 (100%) | a |
| HER2-therapy for MBC prior to T-DM1 initiation, n | 309 (90%) | 309 (100%) | ||
| Pertuzumab & trastuzumab | 304 (89%) | 304 (98%) | – | b |
| Trastuzumab | 5 (1%) | 5 (2%) | – | 495 (100%) |
| Lapatinib | – | – | – | – |
| Duration of HER2-therapy for MBC prior to T-DM1 initiation | ||||
| Pertuzumab & trastuzumab | 10.4 (6.8–16.0) | 10.4 (6.8–16.0) | – | – |
| Trastuzumab | 7.6 (4.3–11.6) | 7.6 (4.3–11.6) | – | – |
| Lapatinib | – | – | – | – |
| Previously treated with trastuzumab for EBC, n | 166 (48%) | 137 (45%) | 29 (81%) | 78 (16%)c |
| Duration of HER2-therapy for EBC prior to T-DM1 initiation (trastuzumab) | 12.0 (12.0–12.3) | 12.0 (11.6–12.9) | 12.0 (10.9–13.0) | – |
| Anti-cancer medicines dispensed any time prior to T-DM1 initiation | ||||
| Taxanes | 292 (84%) | – | – | – |
| Endocrine therapies | 136 (39%) | 130 (42%) | 6 (17%) | 201 (41%)d |
| Anthracyclines | 11 (3%) | – | ||
| Platinum agents | 11 (3%) | 11 (4%) | – | – |
| Capecitabine | 17 (5%) | – | – | – |
| Other non-taxane chemotherapy | 27 (8%) | – | – | – |
|
Post-T-DM1 initiation measures | ||||
| Deaths, n | 135 (39%) | 123 (40%) | 12 (33%) | 303 (61%) |
| Median follow-up time, months (IQR) | 30.9 (20.0–37.6) | 30.7 (20.7–37.6) | 35.5 (9.3–40.3) | 47.8 (41.9–55.5) |
Due to ethical constraints, we cannot report cell counts less < 3 or cells where a count < 3 could be deduced.
∗EMILIA reported enrolling men and women but did not report the number of men and women in the trial.
Median age (range).
13 patients enrolled in the EMILIA trial were suspected of having received pertuzumab.
This figure represents patients who received only prior adjuvant trastuzumab. The total number of patients previously treated with adjuvant trastuzumab is not reported.
ER/PR positive.
The median duration of T-DM1 treatment was 6.5 months (IQR: 3.1–14.6), slightly shorter than the duration of treatment reported in EMILIA (median duration: 7.6 months) (Table 2). The median duration of first-line T-DM1 therapy was 13.2 months and 6.5 months for second-line treatment. The vast majority of patients receiving second-line therapy (98%) were treated with pertuzumab and trastuzumab prior to initiating T-DM1 (Fig. 1); 14 (5%) of those receiving the combination stopped pertuzumab and continued on trastuzumab alone before initiating T-DM1, while five patients (1%) received trastuzumab without pertuzumab prior to T-DM1 (Table 2). Most second-line patients (94%) also received a taxane prior to T-DM1 initiation.
Table 2.
Treatment outcomes for the entire cohort and according to treatment line; and the T-DM1 treatment arm of the pivotal trial.
| Overall | Second-line T-DM1 | First-line T-DM1 | EMILIA [10,11] | |
|---|---|---|---|---|
| Time to T-DM1 initiation | ||||
| Median time to second-line T-DM1 initiation from initiation of HER2-targeted therapy for HER2+MBC, months (IQR) | – | 11.6 (7.9–16.6) | – | – |
| Median time to T-DM1 from last adjuvant trastuzumab, months (IQR) |
24.4 (13.6–50.6) |
31.1 (17.0–58.0) |
4.7 (2.0–10.2) |
– |
|
Duration of T-DM1 treatment, months (IQR) |
6.5 (3.1–14.6) |
6.5 (3.1–13.5) |
13.2 (2.6–31.3) |
7.6 (0.0–63.5)a |
|
Treatment following T-DM1 cessation | ||||
| HER2-therapy for MBC following T-DM1 cessation, n | ||||
| Pertuzumab & trastuzumab | – | – | – | 21 (4%) |
| Trastuzumab | 57 (17%) | 51 (17%) | 6 (17%) | 177 (36%) |
| Lapatinib | 49 (14%) | – | – | 241 (49%) |
| Duration of HER2-therapy following T-DM1 cessation | 7.4 (3.5–13.0) | 7.4 (3.5–12.4) | 11.4 (5.0 – NR) | – |
| Anti-cancer medicines dispensed following T-DM1 cessation | ||||
| Taxanes | 13 (4%) | – | – | – |
| Endocrine therapies | 62 (18%) | 53 (17%) | 9 (25%) | – |
| Anthracyclines | 13 (4%) | – | ||
| Platinum agents | 8 (2%) | 8 (3%) | – | – |
| Capecitabine | 86 (25%) | – | – | 254 (51%) |
| Other non-taxane chemotherapy |
56 (16%) |
52 (17%) |
4 (11%) |
– |
| Duration of all HER2-targeted therapy for HER + MBC, months (IQR) | 24.6 (15.1 – NR) | 25.0 (16.1 – NR) | 15.1 (4.3 - NR) | – |
NR = quartile not reached; a median duration (range).
Due to ethical restrictions, we cannot report cell counts less <3 or cells where a count <3 could be deduced.
For patients receiving second-line TDM-1 therapy, the median duration of first-line HER2-targeted therapy for HER2+MBC prior to commencing T-DM1 treatment was 10.4 months (IQR: 6.8–16.0); the median time from the start of HER2+MBC treatment to T-DM1 initiation was 11.6 months (IQR: 7.9–16.6; Table 2); and 85% initiated T-DM1 within one month of their last first-line HER2+MBC treatment. For patients treated with first-line T-DM1 therapy and also treated previously with trastuzumab for HER2+EBC, the median time from last adjuvant trastuzumab dispensing was 4.7 months (IQR: 2.0–10.2).
During the study period, 137 patients received T-DM1 treatment until death or the study censor date. Of the remaining 208 patients who we observed stopping T-DM1 treatment, 118 (57%) did not receive further HER2-targeted therapy, 57 (27%) received subsequent trastuzumab (36% in EMILIA), and 49 (24%) received subsequent lapatinib (49% in EMILIA; Fig. 1). The median duration of post-T-DM1 HER2-targeted treatment with these agents was 7.4 months (IQR: 3.5–13.0; Table 2). The most commonly dispensed chemotherapy agents following T-DM1 cessation were capecitabine and non-taxane chemotherapy (including vinorelbine, gemcitabine, and eribulin). The median duration of all HER2-targeted therapy for HER2+MBC, excluding any breaks in treatment, was 24.6 months (IQR: 15.1–75th percentile not reached [NR]).
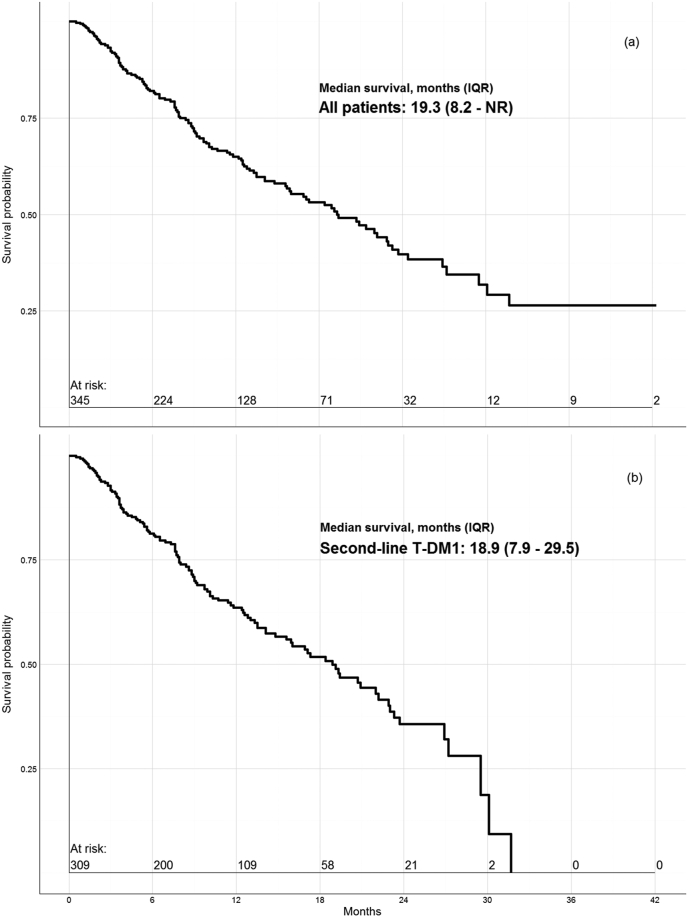
Median OS was 19.3 months (IQR: 8.2 – NR) for the entire cohort; 18.9 months (IQR: 7.9–29.5) for second-line T-DM1 initiators (Fig. 2); and not reached for first-line T-DM1 patients (their three-year survival rate was 53%). These figures compare to median survival times reported in the original and final published results, respectively of: 30.9 months [11] and 29.9 months [10] from the EMILIA trial.
Fig. 2.
Survival probability plot for (a) all patients initiating T-DM1 and (b) those treated with second-line T-DM1.
4. Discussion
In our population-based cohort study we report treatment patterns and survival outcomes for every patient in Australia accessing publicly-funded T-DM1 for HER2+MBC since 2015. Our findings highlight some differences in patient characteristics and outcomes from the T-DM1 pivotal trial and provide real-world estimates that can inform patient, clinician and policy, decisions around the use of HER2-targeted therapies in routine clinical care.
Similar to our previous research on the real-world use and outcomes of trastuzumab for HER2+MBC [4], our cohort was generally older than patients enrolled in the pivotal T-DM1 trial. The median age of participants in the EMILIA trial was 53 years with 14% of all patients enrolled being 65 years or older and only 3% 75 years or older [10,11]. The proportions of patients in our cohort 65 years and older (31%) and 75 years and older (11%) were notably larger than that reported from EMILIA. Older patients represent a growing proportion of patients treated with HER2-targeted agents [17] and recent Canadian real-world cohorts (of similar size to ours) from Ontario and Alberta reported median ages closer to our cohort (56 and 58 years, respectively [18,19]).
The proportion of our cohort with previous (neo)adjuvant treatment with trastuzumab (48%) was also similar to that reported in other observational studies [[19], [20], [21]]. The high proportion of patients treated with first-line T-DM1 who received (neo)adjuvant trastuzumab in our cohort (81%) likely reflects Australian prescribing restrictions for T-DM1 that require patients to relapse on or within 6 months of completing adjuvant trastuzumab in order to access first-line T-DM1, and overall, T-DM1 appears to have been used largely within Australian prescribing restrictions [22]. This finding is an interesting contrast to our previous research examining adherence to the prescribing restrictions around trastuzumab during the Herceptin Program, where we found a large proportion of treatment outside the prescribing restrictions [23].
The majority (98%) of patients treated with second-line T-DM1 in our study received pertuzumab plus trastuzumab prior to T-DM1, different to the EMILIA trial where very few participants would have had access to pertuzumab. Our estimate of the median time from initiation of HER2+MBC treatment to T-DM1 initiation (11.6 months) was six months shorter than the median progression-free survival estimate reported for the group receiving pertuzumab (18.7 months) in the CLEOPATRA trial [24], suggesting that our cohort of patients receiving second-line T-DM1 experienced faster disease progression on first-line therapy. Duration of T-DM1 treatment was similar between our cohort (6.5 months) and the EMILIA trial (7.6 months).
The median survival estimate of 19.3 months in patients receiving T-DM1 was 11 months shorter than the original EMILIA estimate (30.9 months) [11]—which was the survival estimate used to support the public subsidy of T-DM1 in Australia [25]— and 10 months shorter than the final EMILIA median OS estimate of 29.9 months [10]. These differences in OS may be due to the older age of our population and the large proportion of our cohort receiving (neo)adjuvant trastuzumab therapy. Previous research, including our own, has shown shorter OS for patients 65 years and older [17], as well as worse outcomes in the metastatic setting associated with HER2-targeted therapy in patients previously treated with (neo)adjuvant trastuzumab [26,27].
That most of our cohort treated with second-line T-DM1 therapy had received prior pertuzumab treatment is another potential factor explaining the survival differences between our study and the EMILIA trial. The recent Canadian studies have reported similar OS estimates (15.4 months and 19.0 months) from their real-world cohorts to ours (18.9 months) [18,19]. Both of these studies also reported worse OS outcomes for patients who received T-DM1 following treatment with pertuzumab [18,19]. More research from patients treated in routine clinical care is needed to better understand the impact of prior pertuzumab treatment on the outcomes observed with T-DM1 therapy, but our OS estimate for second-line T-DM1 patients was more similar to that from the third- and later-line T-DM1-treated patients enrolled in the TH3RESA study (final estimate: 23 months) [28].
Median survival was not reached for our first-line patients. The same result was reported in the original results from the MARIANNE trial—which also examined first-line T-DM1 therapy for HER2+MBC—with the same median follow-up time (35 months) as our first-line T-DM1 patients [29]. However, due to Australia prescribing restrictions for first-line T-DM1 our cohort differs from the patients enrolled in the MARIANNE trial. Our first-line T-DM1 patients were required to have relapsed within 6 months of completing trastuzumab for HER2+EBC, and this restriction likely selected patients with more aggressive, early-relapsing disease. The aim of the MARIANNE trial was to assess T-DM1 as a first-line option on par with the current recommended first-line treatment, trastuzumab plus pertuzumab and taxane—regardless of previous (neo)adjuvant treatment. Nonetheless, our cohort was of similar age and had a similar median duration of treatment with T-DM1 to MARIANNE patients. We require more data to fully evaluate the use and outcomes of first-line T-DM1 in Australia, though we note our preliminary findings here are not dissimilar from our previous results for patient initiating first-line trastuzumab for HER2+MBC previously treated with (neo)adjuvant trastuzumab [27].
The strengths of our study include its national, whole-of-population data capture—while Australians can access T-DM1 independent of the PBS, this is unlikely given the high cost of the medicine. As such, our cohort likely represents nearly all Australians treated with T-DM1 in routine clinical practice during the study period. Our study also has a number of limitations inherent to using administrative data for observational research. Our data were collected for the purpose of reimbursement and they lack many important clinical measures including performance status, diagnoses dates, hormone receptor status, dates of disease progression, and patient comorbidities. We were only able to observe people treated with publicly-subsidized HER2-targeted therapies, not patients who may have been diagnosed with HER2+MBC but did not receive a HER2-targeted agent. The PBS subsidizes medicines for outpatients and private hospital patients, who make up the overwhelming majority of people receiving HER2-targeted therapies in Australia. The vast majority of oncology protocols are administered in the outpatient setting or to private hospital inpatients (both captured in our PBS data) [13]. Similarly, we do not have data around cardiac or other adverse events and were unable to examine safety issues around the use of T-DM1 in a real world setting with an older population. Finally, our study period covered 44 months, from October 2015 through May 2019, and a longer data series will allow for a more robust examination of first-line T-DM1 outcomes.
5. Conclusion
The HER2-targeted treatment pathway is complex with multiple agents being used across treatment settings and treatment lines. This results in uncertainty and evidence gaps for patients, treating clinicians and policy makers as trial data do not always provide the necessary evidence to reflect how practice will play out in the real-world. We found patients from our real-world cohort were older and more heavily pre-treated with HER2-targeted therapies ((neo)adjuvant trastuzumab and pertuzumab) than those enrolled in the pivotal trial. Our survival estimates were shorter than those from the pivotal trial, though duration of treatment was similar. Policies governing the use of expensive new cancer treatments must often strike a balance between providing access to potentially life-saving treatments while preserving the sustainability of public funding. To that end the PBS aligns access restrictions with trial evidence to ensure medicines are used in the fashion by which their benefit was demonstrated. As the evidence base supporting the use of HER2-targeted therapies is always evolving, our results provide useful feedback for Australian medicines policy makers. Moreover, our study provides valuable data around the real-world use and outcomes of T-DM1 to inform patients and clinicians around expected treatment and survival in routine clinical care.
Ethics approval, consent to participate, and data availability
Our study was approved by the NSW Population and Health Services Research Ethics Committee (Approval Number: 2010/02/213) and data access was granted by the Australian.
Department of Human Services (DHS) External Request Evaluation Committee (Approval Numbers: MI1474, MI1475, MI1477, MI5858, MI10868). Individual consent for the release of these data has been waived according to the Australian Privacy Act of 1988 [13]. Direct access to the data and analytical files for other individuals or authorities is not permitted without the express permission of the approving human research ethics committees and data custodians.
Author contributions
BD, BEK, MT, NH, SJL, and SAP significantly contributed to the conceptualization of the study and its design. BD and SAP contributed to data acquisition, BD performed data analyses, and BD, BEK, MT, NH, SJL, and SAP interpreted the results. The manuscript was drafted by BD, and BD, BEK, MT, NH, SJL, and SAP revised the manuscript drafts critically and approved the final version of the manuscript.
Funding
This work was supported by a Cooperative Research Centre Project (CRC-P) grant from the Australian Government Department of Industry, Innovation, and Science (ID: CRC-P-439). MT is supported by an Australian Government Research Training Program Scholarship, an NHMRC Postgraduate Research Scholarship (ID: 1151479), a National Breast Cancer Foundation Postgraduate Scholarship Top-Up (ID: DS-18-01), and a Translational Cancer Research Network Clinical PhD Scholarship Top-Up award, supported by the Cancer Institute NSW (no grant number). NH is supported by a National Breast Cancer Foundation (Australia) Cancer Research Leadership Fellowship. The funding bodies had no role in the design of the study, analysis of the data, interpretation of the findings, or the writing of the manuscript.
Disclosures
BEK has received conference support and speaker’s honorariums from Roche and Novartis and advisory board honorariums from Roche, Novartis and Teva. SAP is a member of the Drug Utilisation Sub Committee of the Pharmaceutical Benefits Advisory Committee. The views expressed in this paper do not represent those of the either committee. The remaining authors declare no conflicts of interest.
Declaration of competing interest
BEK has received conference support and speaker's honorariums from Roche and Novartis and advisory board honorariums from Roche, Novartis and Teva. SAP is a member of the Drug Utilisation Sub Committee of the Pharmaceutical Benefits Advisory Committee. The views expressed in this paper do not represent those of the either committee. The remaining authors declare no conflicts of interest.
Acknowledgements
We acknowledge the contribution of Sally Crossing (AM) (1946–2016) as the Health Consumer Advocate on this research program. We thank Services Australia for providing the data for this research.
References
- 1.Balduzzi S., Mantarro S., Guarneri V., Tagliabue L., Pistotti V., Moja L. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;(6) doi: 10.1002/14651858.CD006242.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Swain S.M., Miles D., Kim S.B., Im Y.H., Im S.A., Semiglazov V. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 4.Daniels B., Kiely B.E., Lord S.J., Houssami N., Lu C.Y., Ward R.L. Trastuzumab for metastatic breast cancer: real world outcomes from an Australian whole-of-population cohort (2001-2016) Breast. 2018;38:7–13. doi: 10.1016/j.breast.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Daniels B., Kiely B.E., Lord S.J., Houssami N., Lu C.Y., Ward R.L. Long-term survival in trastuzumab-treated patients with HER2-positive metastatic breast cancer: real-world outcomes and treatment patterns in a whole-of-population Australian cohort (2001-2016) Breast Canc Res Treat. 2018;171(1):151–159. doi: 10.1007/s10549-018-4804-0. [DOI] [PubMed] [Google Scholar]
- 6.Giordano S.H., Temin S., Chandarlapaty S., Crews J.R., Esteva F.J., Kirshner J.J. Systemic therapy for patients with advanced human epidermal growth factor receptor 2–positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(26):2736–2740. doi: 10.1200/JCO.2018.79.2697. [DOI] [PubMed] [Google Scholar]
- 7.Denduluri N., Chavez-MacGregor M., Telli M.L., Eisen A., Graff S.L., Hassett M.J. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2018;36(23):2433–2443. doi: 10.1200/JCO.2018.78.8604. [DOI] [PubMed] [Google Scholar]
- 8.von Minckwitz G., Huang C.-S., Mano M.S., Loibl S., Mamounas E.P., Untch M. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2018;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 9.Booth C.M., Tannock I.F. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Canc. 2014;110(3):551–555. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diéras V., Miles D., Verma S., Pegram M., Welslau M., Baselga J. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visvanathan K., Levit L.A., Raghavan D., Hudis C.A., Wong S., Dueck A. Untapped potential of observational research to inform clinical decision making: American society of clinical oncology research statement. J Clin Oncol. 2017;35(16):1845–1854. doi: 10.1200/JCO.2017.72.6414. [DOI] [PubMed] [Google Scholar]
- 13.Daniels B., Lord S.J., Kiely B.E., Houssami N., Haywood P., Lu C.Y. Use and outcomes of targeted therapies in early and metastatic HER2-positive breast cancer in Australia: protocol detailing observations in a whole of population cohort. BMJ Open. 2017;7(1) doi: 10.1136/bmjopen-2016-014439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Australian government department of Health. Trastuzumab: PBS listing. 2020. https://www.pbs.gov.au/medicine/item/10383L-10391X-10401K-10402L-10581X-10588G-10589H-10597R-10682F-10743K-10798H-10803N-10811B-10817H-4632T-4639E-4650R-4703M-7264H-7265J-7266K-7267L Available from: [Google Scholar]
- 15.Mellish L., Karanges E.A., Litchfield M.J., Schaffer A.L., Blanch B., Daniels B.J. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes. 2015;8(1):634. doi: 10.1186/s13104-015-1616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team. R . R Foundation for Statistical Computing; Vienna, Austria: 2019. A language and environment for statistical computing. [Google Scholar]
- 17.Daniels B., Kiely B.E., Tang M., Tervonen H., Pearson S.-A. Trastuzumab use in older patients with HER2-positive metastatic breast cancer: outcomes and treatment patterns in a whole-of-population Australian cohort (2003–2015) BMC Canc. 2019;19(1):909. doi: 10.1186/s12885-019-6126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong I.Y., Yan A.T., Earle C.C., Trudeau M.E., Eisen A., Chan K.K.W. Comparison of outcomes in a population-based cohort of metastatic breast cancer patients receiving anti-HER2 therapy with clinical trial outcomes. Breast Canc Res Treat. 2020;181(1):155–165. doi: 10.1007/s10549-020-05614-5. [DOI] [PubMed] [Google Scholar]
- 19.Lupichuk S., Cheung W.Y., Stewart D. Pertuzumab and trastuzumab emtansine for human epidermal growth factor receptor-2-positive metastatic breast cancer: contemporary population-based outcomes. Breast Canc Basic Clin Res. 2019;13 doi: 10.1177/1178223419879429. 1178223419879429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conte B., Fabi A., Poggio F., Blondeaux E., Dellepiane C., D'Alonzo A. T-DM1 efficacy in patients with HER2-positive metastatic breast cancer progressing after a taxane plus pertuzumab and trastuzumab: an Italian multicenter observational study. Clin Breast Canc. 2020;20(2):e181–e187. doi: 10.1016/j.clbc.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Prete S.D., Montella L., Arpino G., Buono G., Buonerba C., Dolce P. Second line trastuzumab emtansine following horizontal dual blockade in a real-life setting. Oncotarget. 2020;11(22):2083–2091. doi: 10.18632/oncotarget.27603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Australian government department of Health. Trastuzumab emtansine: PBS listing. 2020. https://www.pbs.gov.au/medicine/item/10281D-10282E Available from: [Google Scholar]
- 23.Daniels B., Girosi F., Tervonen H., Kiely B.E., Lord S.J., Houssami N. Adherence to prescribing restrictions for HER2-positive metastatic breast cancer in Australia: a national population-based observational study (2001-2016) PloS One. 2018;13(7) doi: 10.1371/journal.pone.0198152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swain S.M., Baselga J., Kim S.-B., Ro J., Semiglazov V., Campone M. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pharmaceutical Benefits Advisory Committee Recommendations made by the PBAC - november 2014 Canberra 2014. https://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/pbac-outcomes/2014-11 [Available from:
- 26.Murthy R.K., Varma A., Mishra P., Hess K.R., Young E., Murray J.L. Effect of adjuvant/neoadjuvant trastuzumab on clinical outcomes in patients with HER2-positive metastatic breast cancer. Cancer. 2014;120(13):1932–1938. doi: 10.1002/cncr.28689. [DOI] [PubMed] [Google Scholar]
- 27.Daniels B., Kiely B.E., Houssami N., Lord S.J., Dobbins T., Lu C.Y. Survival outcomes for Australian women receiving trastuzumab for HER2-positive metastatic breast cancer following (neo)adjuvant trastuzumab: a national population-based observational study (2006–2014) Br J Canc. 2018;118(3):441–447. doi: 10.1038/bjc.2017.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krop I.E., Kim S.B., Martin A.G., LoRusso P.M., Ferrero J.M., Badovinac-Crnjevic T. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743–754. doi: 10.1016/S1470-2045(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 29.Perez E.A., Barrios C., Eiermann W., Toi M., Im Y.-H., Conte P. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2–positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2016;35(2):141–148. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]