Abstract
Physiologically-based pharmacokinetic (PBPK) modeling can potentially predict pediatric drug-drug interactions (DDIs) when clinical DDI data are limited. In infants for whom treatment of pulmonary hypertension and prevention or treatment of invasive candidiasis are indicated, sildenafil with fluconazole may be given concurrently. To account for developmental changes in cytochrome P450 (CYP) 3A, we determined and incorporated fluconazole inhibition constants (KI) for CYP3A4, CYP3A5, and CYP3A7 into a PBPK model developed for sildenafil and its active metabolite, N-desmethylsildenafil. Pharmacokinetic (PK) data in preterm infants receiving sildenafil with and without fluconazole were used for model development and evaluation. The simulated PK parameters were comparable to observed values. Following fluconazole co-administration, differences in the fold change for simulated steady-state area under the plasma concentration vs. time curve from 0 to 24 hours (AUCss,0–24) were observed between virtual adults and infants (2.11-fold vs. 2.82-fold change). When given in combination with treatment doses of fluconazole (12 mg/kg i.v. daily), reducing the sildenafil dose by ~ 60% resulted in a geometric mean ratio of 1.01 for simulated AUCss,0–24 relative to virtual infants receiving sildenafil alone. This study highlights the feasibility of PBPK modeling to predict DDIs in infants and the need to include CYP3A7 parameters.
If an investigational drug is suspected to interact with concomitant medications, drug-drug interaction (DDI) studies are performed in healthy adult volunteers during drug development and are communicated in the product label.1 For ethical reasons, pediatric DDI studies are rarely conducted unless children receive the drugs per standard of care. There are also logistic challenges for conducting pediatric DDI studies, such as low enrollment and smaller blood volume, particularly in neonates, available for pharmacokinetic (PK) sampling. Consequently, therapeutic management of pediatric DDIs is based on adult DDI studies, although developmental differences in activity of drug metabolizing enzymes may lead to age-related differences in DDI potential. Within the cytochrome P450 3A (CYP3A) subfamily, CYP3A7 is highly expressed in fetal tissue and neonates and typically has reduced metabolic capacity for drugs compared with CYP3A4, which is primarily expressed in adults.2–7
Physiologically-based pharmacokinetic (PBPK) modeling can predict pediatric DDIs by incorporating drug and system properties, in vitro data, and maturation of drug metabolizing enzymes. PBPK modeling has been applied to characterize CYP3Amediated DDIs in children > 2 years of age.8,9 Using the CYP3Amediated DDI between sildenafil with fluconazole, we leveraged PBPK modeling and sildenafil PK data collected in preterm infants with and without fluconazole to characterize CYP3A DDIs in adults and infants. Sildenafil is a phosphodiesterase type 5 inhibitor used off-label in infants for pulmonary hypertension.10 Hospitalized preterm infants receiving sildenafil are at risk for fungal infection, thus they may also be prescribed the moderate CYP3A inhibitor, fluconazole, for the prophylaxis and treatment of invasive candidiasis.11
Sildenafil is metabolized primarily by CYP3A, with minor contribution by CYP2C9, into 16 metabolites.12–14 Sildenafil has 96% plasma protein binding, preferentially toward alpha-1-acid glycoprotein (AAG), that is concentration independent from 0.1 to 10 μg/mL (0.21–21 μmol/L).15,16 The active metabolite, N-desmethylsildenafil (DMS), has half the phosphodiesterase type 5 inhibitory activity as sildenafil.13,17 One study determined that the in vitro formation kinetic intrinsic clearance (CLint; μL/min/pmol CYP3A) for DMS was similar for CYP3A4 (0.733) and CYP3A5 (0.788), but significantly lower for CYP3A7 (0.079).18 Based on a study in 36 term neonates receiving i.v. sildenafil for persistent pulmonary hypertension of the newborn or hypoxemia, sildenafil clearance (CL) increased 3-fold in the first week of life from 0.84 L/hour on day 1 to 2.58 L/hour at 7 days of age.19 This is likely due to the developmental switch from CYP3A7 to CYP3A4 expression shortly after birth.
A population pharmacokinetic (PopPK) model developed in 11 infants (2–121 days old) receiving sildenafil for pulmonary hypertension, of which 3 also received fluconazole, reported that fluconazole decreased sildenafil clearance by 47%.20 Similarly, another PopPK study based on 34 preterm infants receiving sildenafil, with 4 also receiving fluconazole, reported a 59% decrease in sildenafil CL by fluconazole.21 One study reported that the fluconazole inhibitory constant was ninefold higher for CYP3A5 than CYP3A4 (84.6 ± 12.9 μM vs. 9.21 ± 0.51 μM), however, data are not available for CYP3A7.5 Therefore, the goals of this study were to: (i) develop an adult PBPK model for sildenafil and DMS; (ii) to determine and incorporate CYP3A4, CYP3A5, and CYP3A7 inhibitory constants (KI) for fluconazole; (iii) to evaluate CYP3A DDI potential for sildenafil and DMS in adults; (iv) to scale and evaluate the DDI between sildenafil with fluconazole in infants; and (v) to optimize dosing for infants receiving sildenafil with fluconazole.
METHODS
Adult PBPK model development
A whole-body adult PBPK model was developed for sildenafil and DMS in PK-Sim® as part of the open source Open Systems Pharmacology Suite version 8.0 (http://www.open-systems-pharmacology.org) incorporating CYP3A.22 We compared model simulations with and without CYP2C9-mediated metabolism incorporated into the model because it has been postulated to play a minor role in formation of DMS. Adult PK data were digitized from the literature for model development and evaluation (Table S1). A 36-year-old European man with a weight of 73.8 kg and a height of 175.5 cm was used for model development. The relative organ contributions for CYP enzymes were taken from the built-in database query using array levels.23 The reference concentration refers to the highest organ expression per age (whereby the ontogeny factor is 1). The default reference concentration in PK-Sim® was 4.32, 0.04, and 3.84 μmoL/L liver tissue for CYP3A4, CYP3A5, and CYP2C9, respectively.24 CYP3A4 concentrations in pediatrics are calculated as a fraction of 4.32 μmol/L using the CYP3A4 ontogeny function (Supplementary Methods). Because CYP3A7 is greatest at birth, we calculated the liver reference concentration for CYP3A7 in preterm infants as follows: 4 μmol/L for a preterm infant: 158 pmol/mg microsomal protein × 26 mg microsomal protein/g liver × 1 g/mL × 1,000 mL/L × 1*10−6 μmol/pmol.2,25,26 Adult CYP3A7 liver concentrations are therefore calculated as a fraction of 4 μmol/L using the CYP3A7 ontogeny function (Supplementary Methods).
Sildenafil and DMS were comodeled using CYP3A4, CYP3A5, CYP3A7, and CYP2C9 formation kinetics from the literature for DMS.12,18,27 Parameter optimization was performed for sildenafil and DMS lipophilicity using the digitized i.v. adult data incorporating the Monte Carlo algorithm.28 The organ-to-plasma partition coefficients were calculated using the Rodgers and Rowland method.29 Because kinetics for formation of the remaining CYP3A catalyzed sildenafil metabolites are unknown, the sildenafil CYP3A maximal velocity (Vmax) for the remainder of sildenafil metabolites was optimized using the Monte Carlo algorithm (fixing the concentration of half-maximal velocity (KM) to 15 μM18,27; Supplementary Methods). Sildenafil CYP3A7 Vmax was optimized using the preterm infant PK data because CYP3A7 is minimally expressed in adults. After sildenafil CL was optimized, CYP3A4 and CYP3A7 CLint for DMS were manually optimized using adult and preterm infant data, respectively.
Adult PBPK model evaluation
One hundred virtual male adults from 18–58 years of age (healthy male population) or 100 adult patients (50% men) from 46–76 years of age (pulmonary arterial hypertension population) were created based on reported demographics. Population simulations were performed, and the ratio for the mean simulated and observed area under the concentration vs. time curve (AUC) from 0 until the last observed value (AUC0–last) or from 0 to infinity (AUC0–∞) as reported, was compared for each dosing regimen. The mean CL and volume of distribution at steady-state (Vss) were also compared between simulations and observations.
Fluconazole inhibition kinetics
A high-performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) assay was developed for 6β-hydroxytestosterone and the internal standard, 4-androsten-19–1al 3,17-dione (Sigma-Aldrich, St. Louis, MO). The lower limit of quantification was 1 μM and the coefficients of variation for the intraday and interday precision was 11% and 7%, respectively (Supplementary Methods). Nicotinamide adenine dinucleotide phosphate, acetonitrile, fluconazole, and testosterone were purchased from Sigma-Aldrich. Human CYP3A4, CYP3A5, and CYP3A7 + reductase + b5 and 0.5 M phosphate buffer, pH 7.4, were purchased from Corning Life Sciences (Corning, NY). Experiments were performed to determine linear 6β-hydroxytestosterone formation (Supplementary Methods). All experiments were performed in triplicates. To determine fluconazole inhibition, a 4 × 7 matrix of testosterone (15, 50, 150, and 250 μM) and fluconazole concentrations (0, 15, 50, 100, 200, 300, and 400 μM) were evaluated. The reaction volume was 100 μL and contained 20 pmol/mL of CYP3A4/5 or 40 pmol/mL of CYP3A7 in 100 mM potassium phosphate buffer at pH 7.4. The reaction was pre-incubated at 37°C for 5 minutes, and then initiated with nicotinamide adenine dinucleotide phosphate (1 mM final). After 5 minutes (CYP3A4/5) or 30 minutes (CYP3A7) of incubation at 37°C, 50 μL was removed and added to 150 μL of ice cold acetonitrile containing 0.5 μM 4-androsten-19–1al 3,17-dione, centrifuged at 3,700 × g for 15 minutes, and then analyzed by HPLC-MS/MS. Model discrimination was made by visual inspection of Lineweaver-Burk plots, as well as Akaike information criterion comparing reversible unweighted nonlinear regression fits in GraphPad Prism® 8.0 (Supplementary Methods).
Adult DDI evaluation
In order to ensure that CYP3A CL for DMS and sildenafil was accurately parameterized, sildenafil (100 mg oral tablet given on day 1 and day 8) was comodeled with the CYP3A inhibitor ritonavir (Table S2) administered at 300, 400, and 500 mg orally twice daily on days 2, 3, and 4–8, respectively. The ritonavir PBPK model was developed including CYP3A and CYP2D6 metabolism, P-glycoprotein transport and inhibition, and CYP3A4 mixed time-dependent and competitive inhibition plus induction (Supplementary Methods; Table S2). The AUC from 0 to tau (AUC0-τ), AUC0−∞, maximal concentration (Cmax), and the fold increase in AUC and Cmax with and without ritonavir was compared between the observed and simulated data.30
We also evaluated the DDI between sildenafil plus erythromycin based on a study in 26 male volunteers (18–45 years of age) receiving 100 mg sildenafil on days 1 and 6 along with 500 mg oral erythromycin or placebo twice daily on days 2–6.31 The erythromycin PBPK model is available through the Open Systems Pharmacology website: https://github.com/Open-Systems-Pharmacology/Erythromycin-Model. The model includes CYP3A4 N-demethylation and time-dependent inhibition along with total hepatic clearance and organic anion transporting polypeptide 1B1 transport and clearance via glomerular filtration (Supplementary Methods). Finally, the DDI between sildenafil with fluconazole was simulated in healthy adults receiving sildenafil 10 mg i.v. three times daily plus fluconazole 800 mg i.v. followed by 400 mg i.v. daily using a published fluconazole PBPK model.32 The DDI between sildenafil with fluconazole was simulated in adults, although adult DDI data were not available for model evaluation.
Pediatric PBPK model development
PK data for sildenafil and DMS were available from 9 preterm infants (< 32 weeks gestational age (GA) and between 3 and 42 days postnatal age (PNA)) receiving a single dose of sildenafil (0.125 or 0.25 mg/kg i.v.) per standard of care as part of the phase I, multicenter, open label Pediatric Trials Network study (ClinicalTrials.gov Identifier: NCT01670136; Table S1).21 Samples were collected, when possible, within 15 minutes, 1–2, 3–4, 7–8, 12–14, 24–30, and 48–56 hours post the 90-minute infusion and 30-minute flush time. PK data were dose-normalized to 0.25 mg/kg i.v. sildenafil. The four preterm infants receiving sildenafil with fluconazole were administered sildenafil via the i.v. route.
The healthy male virtual subject was scaled to a male virtual preterm infant based on mean observed demographics (22 days PNA, 25 weeks GA, and 849 g weight). The preterm infant (24–40 weeks GA) virtual population within PK-Sim® includes physiological parameters (body weight, height, organ volumes and blood flow rates, and tissue composition; Supplementary Methods).33 CL was scaled using PK-Sim® ontogeny functions (Supplementary Methods). Protein binding to AAG was scaled using a Hill-function-like increase and decrease during the maturation and aging phases, respectively.34 Simulations in term infants were performed using a virtual population of 100 term infants (36–40 weeks GA, and 0–3 days PNA). CL and Vss were compared against published values from two PopPK models developed in preterm and term infants.19,21
Sensitivity analysis
Sensitivity analyses were performed for a healthy adult, preterm infant, term infant at birth, term infant at 2 weeks of age, 1-month-old, 2-month-old, 3-month-old, 6-month-old, 1-year-old, 2-year-old, 5-year-old, and a 12-year-old. The virtual preterm infant received i.v. sildenafil, whereas the other virtual subjects received sildenafil orally. Parameters with sensitivity values < −1 or > 1 were reported for Cmax and AUC0−∞ (Supplementary Methods). Sensitivity values for CYP3A relative expression were compared among these age groups.
Pediatric DDI dosing evaluation and recommendations
There were four preterm infants with PK data who received sildenafil with fluconazole. We comodeled sildenafil with fluconazole in preterm infants leveraging a previously published fluconazole PBPK model in adults and infants, including renal clearance and uridine 5′-disphospho-glucuronosyltransferase family 2 member B7 (UGT2B7) metabolism.32,35 Only the indication (prophylaxis vs. treatment) for fluconazole was recorded for these preterm infants. Without specific details on the exact dosing or route of fluconazole administration, we assumed fluconazole dosing based on the 2016 Infectious Diseases Society of America recommended guidelines for neonatal candidiasis: 12 mg/kg daily administered i.v. for treatment and 6 mg/kg administered i.v. every 72 hours for prophylaxis.36 We simulated prophylaxis and treatment dosing for fluconazole in combination with sildenafil and compared with data in preterm infants receiving fluconazole for prophylaxis or treatment, respectively.
To simulate optimal dosing for this combination in neonates, we created a virtual population of 1,000 preterm and term infants (50% girls) ranging from 24–40 weeks GA and 0–14 days PNA. Following the US Food and Drug Administration (FDA) guidance, we targeted dosing that would achieve simulated geometric mean ratios of Cmax and AUC from 0–24 hours (AUC0–24) for sildenafil with fluconazole relative to sildenafil alone within the 80–125% equivalence range. Cmax and AUC0–24 included both sildenafil and DMS, taking into account differences in relative phosphodiesterase type 5 inhibitory activity and free fraction (Eq. 1).1
| (1) |
where 0.5 and 1.25 refers to relative differences in phosphodiesterase type 5 inhibitory activity and protein binding between DMS and sildenafil, respectively.
Exposure ratios were stratified by postmenstrual age using the World Health Organization categories of preterm birth: GA < 28 (extremely preterm), ≥ 28 to < 32 (very preterm, ≥ 32 to < 37 (moderate to late preterm), and ≥ 37 weeks (term). The reference dose for sildenafil was 0.25, 0.5, and 1 mg/kg i.v. every 8 hours (90-minute infusion). Sildenafil (0.09, 0.13, 0.18, 0.26, 0.36, and 0.52 mg/kg every 8 hours, administered by a 30-minute and 90-minute infusion) plus fluconazole (12 mg/kg i.v. daily, administered by a 60-minute infusion) were simulated and compared against reference doses of sildenafil.
RESULTS
Adult PBPK model
We developed a whole-body PBPK model for sildenafil and DMS incorporating Michaelis–Menten kinetics by CYP3A4, CYP3A5, and CYP3A7 for sildenafil CL and first-order CLint by CYP3A4 and CYP3A7 for elimination of the DMS metabolite (Table 1; Figure S1). The role of CYP2C9 was insignificant, for example, the AUC0–∞ for the 100 mg dose was 1,580 vs. 1,581 ng*hour/mL for sildenafil and was 661 vs. 659 ng*hour/mL for DMS with and without CYP2C9, respectively. Therefore, unless stated otherwise, all results are without CYP2C9 contribution for parsimony. The adult sildenafil PBPK model adequately captured observed data digitized from the literature (Figures S2–S6; Tables 2 and 3).
Table 1.
Final PBPK model parameters
| Parameter | Sildenafil | Source | DMS | Source |
|---|---|---|---|---|
| Molecular weight, g/mol | 474.58 | 46 | 460.55 | 46 |
| Log P | 3.02 | Optimized | 2.29 | Optimized |
| pKa | 5.97 | 46 | 7.16 | 46 |
| Water solubility, mg/mL | 3.50 | 46 | 0.419 | 46 |
| Compound type | Base | 46 | Base | 46 |
| fu, predominant plasma binding protein | 0.04 (AAG) | 15,16 | 0.04 (AAG) | 15,16 |
| Intestinal permeability, cm/min | 8.31 × 10−6 | PK-Sim® predicteda | 1.34 × 10−6 | PK-Sim® predicteda |
| Vmax CYP3A4 sink,b,c pmol/min/pmol | 10.0 | Optimized | _ | _ |
| KM CYP3A4/5/7 sink,b,c μM | 15 | 27 | – | – |
| Vmax CYP3A5 sink,b,c pmol/min/pmol | 10.8 | Optimized | – | – |
| Vmax CYP3A7 sink,b,c pmol/min/pmol | 3.86 | Optimized | – | – |
| Vmax CYP3A4 DMS, pmol/min/pmol | 1.00 | 27 | – | – |
| KM CYP3A4 DMS, μM | 15 | 27 | – | – |
| Vmax CYP3A5 DMS, pmol/min/pmol | 1.38 | 27 | – | – |
| KM CYP3A5 DMS, μM | 14.7 | 27 | – | – |
| Vmax CYP3A7 DMS, pmol/min/pmol | 0.05 | Optimized | – | – |
| KM CYP3A7 DMS, μM | 5.71 | 18 | – | – |
| Vmax CYP2C9 DMS, pmol/min/mg | 78 | 12 | – | – |
| KM CYP2C9 DMS, μM | 27 | 12 | – | – |
| Intrinsic clearance, L/min | ||||
| CYP3A7 | – | – | 0.09 | Optimized |
| CYP3A4 | – | – | 0.32 | Optimized |
AAG, alpha-1-acid glycoprotein; DMS, N-desmethylsildenafil; fu, unbound fraction in plasma; KM, Michaelis–Menten constant, which describes the interaction of substrate and enzyme in the absence of inhibitor; Log P, logarithmic of octanol-water partition coefficient; PBPK, physiologically-based pharmacokinetic; pKa, negative logarithmic of the acid dissociation constant; Vmax, maximal rate of metabolism.
Intestinal permeability via transcellular route calculated as 266 * (molecular weight * 109) ^ (−4.5) * 10^ log (molecular weight) * 60 * 10−1.
Sink compartment refers to the remainder of sildenafil for all other metabolites besides DMS.
Sildenafil clearance was parameterized by CYP: cytochrome P450 3A4, CYP3A5, and CYP3A7 Vmax/KM, whereas DMS was parameterized by CYP3A4 and CYP3A7 intrinsic hepatic clearance.
Table 2.
Comparison of the observed and simulated AUC for the adult PBPK model
| Sildenafil AUC (ng*hr/mL) | DMS AUC (ng*hr/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Dosing Regimen | Simulateda | Observedb | Ratio | Simulateda | Observedb | Ratio | Reference for Observed Data |
| Single oral dose | |||||||
| 25 mgb | 320 | 361 | 0.89 | 149 | 147 | 1.01 | 47 |
| 50 mgb | 693 | 738 | 0.94 | 309 | 328 | 0.94 | 47 |
| 100 mgb | 1581 | 1685 | 0.94 | 659 | 776 | 0.85 | 47 |
| 200 mgb | 3807 | 3755 | 1.01 | 1446 | 1822 | 0.79 | 47 |
| Multiple oral dose | |||||||
| 80 mg p.o. t.i.d.c | 1209 | 1720 | 0.70 | - | - | - | 48 |
| Single i.v. dose | |||||||
| 10 mg i.v. bolusd | 402 | 330 | 1.22 | - | - | - | 49 |
| 25 mg/25-minute infusion i.v.e | 999 | 971 | 1.03 | 275 | 147 | 1.87 | 13 |
| 50 mg/50-minute infusion i.v.b | 2150 | 1291 | 1.67 | - | - | - | 47 |
AUC, area under the plasma concentration vs. time curve; AUC0–∞, area under the plasma concentration vs. time curve from 0 to infinity; AUC0–τ, area under the plasma concentration vs. time curve from 0 to tau; DMS, N-desmethylsildenafil; PBPK, physiologically-based pharmacokinetic; t.i.d., 3 times daily.
Simulated values are reported as the arithmetic mean, and observed values are reported as the geometric mean.
Healthy men receiving 50 mg i.v. over 50 minutes and 50 mg p.o. capsule single dose plus 25, 50, 100, and 200 mg oral tablets single dose.47
Healthy men receiving 20 mg t.i.d. for 3 days followed by 80 mg p.o. tablet t.i.d. for 3 days.48
Adults with pulmonary arterial hypertension receiving 20 mg p.o. tablet t.i.d. for 30 days followed by 10 mg i.v. bolus.49
Healthy adult men receiving 25 mg i.v. over 25 minutes single dose or 50 mg p.o. solution single dose.13 AUC0–∞ was reported for 25–200 mg single oral dose, the 25 mg/25-minute infusion i.v., and the 50 mg/50-minute infusion i.v. AUC0–τ was reported for the 80 mg p.o. t.i.d. dose and 10 mg i.v. bolus (8 hours dosing interval).
Table 3.
Comparison of sildenafil CL and Vss between simulated and observed values
| Sildenafil CL, liters/houra | Sildenafil Vss, litersa | ||||||
|---|---|---|---|---|---|---|---|
| Population | Simulated | Observed | Ratio | Simulated | Observed | Ratio | References |
| Patients with PAH | 22.3 (38.6%) | 32.2 (N/A)c | 0.69 | 107 (44.1%) | 137 (N/A)c | 0.78 | 49 |
| Healthy men | 26.5 (37.4%) | 29.5 (31.2%)d | 0.90 | 87 (51.1%) | 107 (N/A)d | 0.81 | 50 |
| Preterm infants | 34.2 (19.6%) | 27.8 (33.3%)b,e | 1.23 | 157 (6.5%) | 144 (N/A)b,e | 1.09 | 21 |
| Preterm infants with 50% CV on fu | 34.7 (42.3%) | 27.8 (33.3%)b,e | 1.25 | 161 (36.7%) | 144 (N/A)b,e | 1.12 | 21 |
| Term infants | 37.5 (20.7%) | 24.7 (54.7%)b,f | 1.52 | 159 (8.0%) | 456 (N/A)b,f | 0.35 | 19 |
| Term infants with 50% CV on fu | 34.1 (46%) | 24.7 (54.7%)b,f | 1.38 | 147 (43%) | 456 (N/A)b,f | 0.32 | 19 |
CL, clearance; CV, coefficient of variation; fu, unbound fraction in plasma; N/A, not applicable; PAH, pulmonary arterial hypertension; Vss, volume of distribution at steady-state.
Simulated and observed values are reported as the mean (coefficient of variation, %). For observed values derived from population pharmacokinetic (PopPK) analyses, the coefficient of variation represents the inter-individual variability in the parameter.
Scaled to a 70 kg weight using typical values based on population pharmacokinetic (PopPK) models.
Adults with PAH receiving 20 mg oral tablet 3 times daily for 30 days followed by 10 mg i.v. bolus. The volume of distribution reported in this study is the volume of distribution during the terminal phase (Vz).49
Based on a PopPK analysis combining oral and i.v. data in healthy adult patients from three different studies.50
Based on a PopPK model developed in preterm infants receiving enteral and i.v. sildenafil.21
Based on a PopPK model developed in term neonates receiving i.v. sildenafil for persistent pulmonary hypertension of the newborn or hypoxemia.19
Fluconazole inhibition studies
The 6β-hydroxytestosterone production was linear upward 5 minutes for CYP3A4 and CYP3A5 and 30 minutes for CYP3A7 (Figures S7 and S8). Fluconazole was a mixed competitive inhibitor for CYP3A4, CYP3A5, and CYP3A7 (Figure S9). Alpha values > 1 indicate that fluconazole preferentially binds to the free enzyme (competitive inhibition). Fluconazole inhibition was higher for CYP3A4 relative to CYP3A5 and CYP3A7 (Table 4).
Table 4.
Fluconazole mixed inhibition parameters
| Enzyme | Inhibition type | KI, μM globala,c | Alphaa,b | KI, μM competitivea,c | KI, μM uncompetitivea,c |
|---|---|---|---|---|---|
| CYP3A4 | Mixed | 29.4 (20.3–43.8) | 16.6 (6.1–178) | 20.9 (16.8–25.9) | 83.1 (67.4–102.9) |
| CYP3A5 | Mixed | 182.5 (86.7–556.4) | 2.6 (0.5–13.9) | 70.8 (48.5–104.3) | 238.7 (183.2–318.9) |
| CYP3A7 | Mixed | 84.8 (30.5–296.8) | 13.5 (1.8–∞) | 45.9 (21.7–88.9) | 389.0 (266.7–610.3) |
CYP, cytochrome P450; KI, inhibition constants; KM, Michaelis–Menten constant, which describes the interaction of substrate and enzyme in the absence of inhibitor; Vmax, maximum enzyme velocity without inhibitor.
Value and the 90% confidence interval based on triplicate samples using recombinant enzyme expressing either cytochrome P450 3A4 (CYP3A4), CYP3A5, or CYP3A7.
Alpha determines the degree to which the binding of inhibitor changes the affinity of the enzyme for substrate. When alpha = 1, the mixed-model is identical to noncompetitive inhibition. When alpha is very large, the mixed-model becomes identical to competitive inhibition. When Alpha is very small (but greater than zero), the mixed model becomes nearly identical to an uncompetitive model.
The global KI reflects the net value for the mixed model. The KI is also reported separately for the individual contributions from competitive and uncompetitive inhibition.
Adult DDI evaluation
We evaluated DDI potential for sildenafil in combination with ritonavir and erythromycin in healthy adults. The simulated vs. observed geometric mean fold change in the AUC with and without ritonavir was 13 vs. 11-fold for sildenafil (AUC0–∞) and 2.0 vs. 1.7-fold for DMS (AUC0–24) on day 8 (Table S3; Figure S10).30 The simulated vs. observed mean fold change of AUC0–∞ with and without erythromycin was 2.24-fold vs. 2.82-fold for sildenafil and was 1.67-fold vs. 1.37-fold for DMS (Table S4).31
Pediatric PBPK model
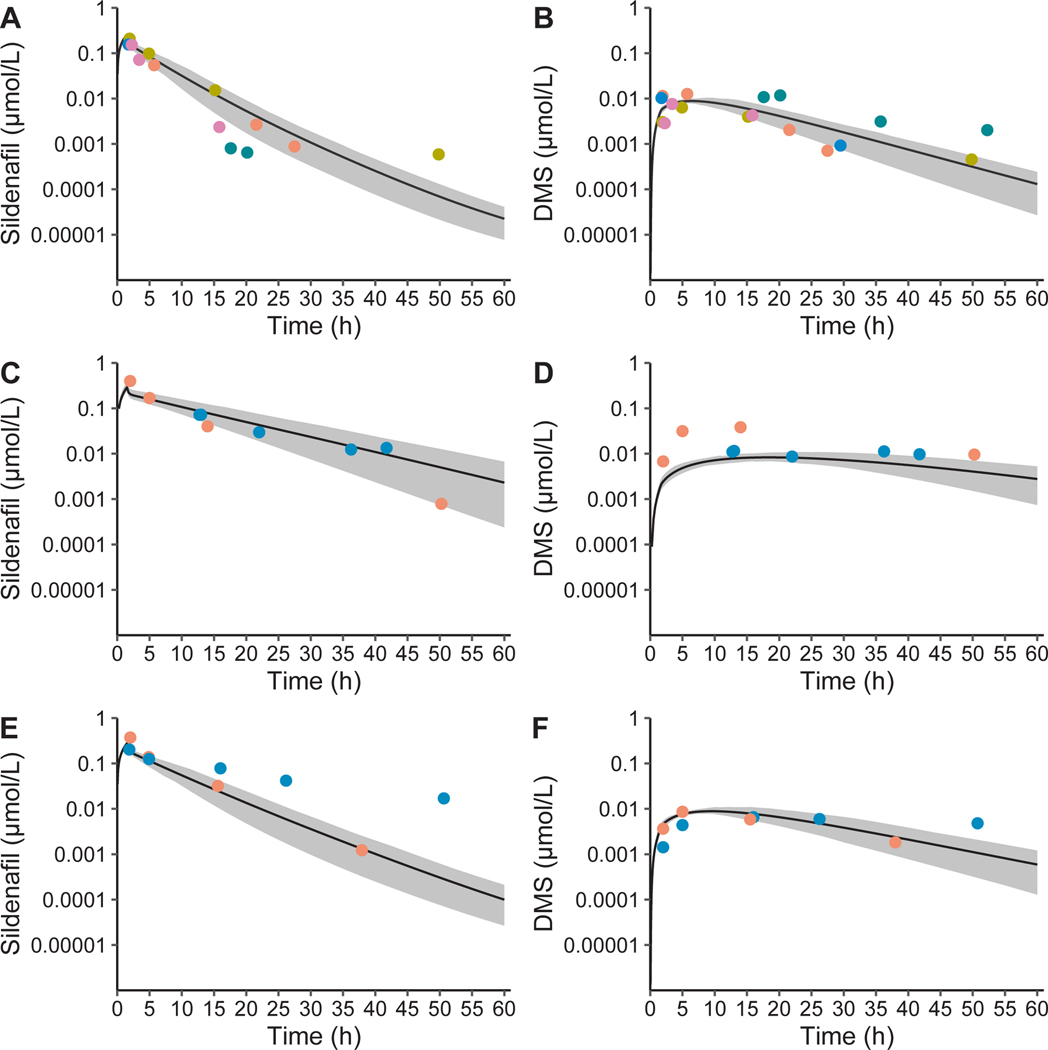
The adult PBPK model was scaled to infants using CYP3A ontogeny and anthropomorphic functions. Simulated concentrations were compared with 24 and 26 plasma samples for sildenafil and DMS, respectively, from 9 preterm infants (median [range] GA of 25 [23–27] weeks and PNA of 18 [7–40] days) receiving sildenafil, 4 of which also received fluconazole (Table S1). The simulated mean CL and Vss in the absence of fluconazole was similar to published values in preterm infants receiving enteral or i.v. doses, and to term infants receiving a continuous infusion of sildenafil (Table 3; Figure 1). However, the simulated variability in infants for CL and Vss was underpredicted, which could be overcome by an additional 50% coefficient of variability on protein binding (Table 3).
Figure 1.
Sildenafil and N-desmethyl sildenafil (DMS) with and without fluconazole physiologically-based pharmacokinetic model population simulations in preterm infants. Population simulations in 100 preterm infants (33% girls, 7–40 days postnatal age, 24–27 weeks gestational age, and 590–1,242 g) for sildenafil (a) and DMS (b) in infants receiving sildenafil alone, and for sildenafil (c) and DMS (d) in infants receiving sildenafil with steady-state administration of fluconazole for treatment (12 mg/kg i.v. daily) and for sildenafil (e) and DMS (f) in infants receiving sildenafil with fluconazole for prophylaxis (6 mg/kg i.v. every 72 hours). A single dose of 0.25 mg/kg i.v. sildenafil with 6 mg/kg fluconazole i.v. in preterm infants resulted in a simulated mean fold-change of 1.08 for maximal concentration (Cmax) and 1.40 for the area under the curve extrapolated to infinity (AUC0–∞) for sildenafil plus DMS accounting for different phosphodiestesterase type 5 inhibitory activity and protein binding (sildenafil + 0.5*1.25*DMS). A single dose of 0.25 mg/kg i.v. sildenafil with 6 days of fluconazole dosing of 12 mg/kg fluconazole i.v. in preterm infants resulted in a simulated mean fold-change of 1.13 for Cmax and 2.59 for AUC0–∞ for sildenafil plus DMS. The solid grey area is the 95% prediction interval and the dots are concentrations colored by individuals. Results were obtained using the default PK-Sim® ontogeny functions for alpha-1-acid glycoprotein without additional variability introduced on the fraction unbound. Observed concentrations were dose normalized to 0.25 mg/kg.
Sensitivity analysis
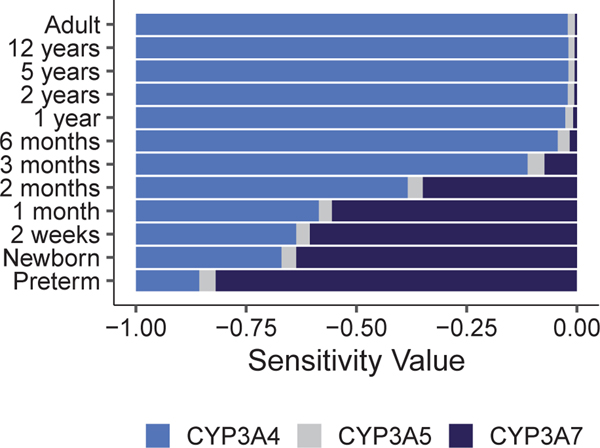
In order to evaluate the critical parameters influencing sildenafil Cmax and AUC0–∞, a sensitivity analysis was performed from preterm infants to adults (Tables S5 and S6). The most sensitive parameters for sildenafil AUC0–∞ included CYP3A4 KM/Vmax, CYP3A4 ontogeny factor and reference concentration, fraction unbound, lipophilicity, and the plasma protein scale factor (Table S5). Sildenafil AUC0–∞ was more sensitive to the reference concentration of CYP3A7 compared with CYP3A4 in infants ≤ 2 months of age (Figure 2; Table S5).
Figure 2.
Results of a sensitivity analysis comparing the influence of cytochrome P450 3A4 (CYP3A4), cytochrome P450 3A5 (CYP3A5), and cytochrome P450 3A7 (CYP3A7) reference concentration on sildenafil AUC0−∞ after a single oral dose for all ages, except that an i.v. dose was simulated for preterm infants, as a function of age. Comparison of sensitivity values for the impact of reference concentration of CYP3A4 (blue), CYP3A5 (grey), and CYP3A7 (navy) on sildenafil AUC0–∞ in typical subjects of various ages. A sensitivity analysis was performed for a typical healthy adult, a preterm infant (22 days PNA, 25 weeks GA, and 849 g weight), a term infant at birth (neonate), a term infant at 2 weeks of age, as well as infants, children, and adolescents 1, 2, 3, and 6 months, and 1, 2, 5, and 12 years of age. A sensitivity of −1.0 implies that a 10% increase of CYP3A reference concentration leads to a 10% decrease of AUC0–∞, and a sensitivity of + 1 implies that a 10% increase of CYP3A reference concentration leads to a 10% increase of AUC0–∞. AUC0–∞, area under the plasma concentration vs. time curve from zero to infinity; GA, gestational age; PNA, postnatal age.
Pediatric DDI evaluation and dosing recommendations
Based on simulations in virtual adults, fluconazole (800 mg i.v. loading dose, then 400 mg i.v. daily) administered with sildenafil (10 mg i.v. 3 times daily) resulted in an increase in sildenafil plus DMS (accounting for differences in activity and protein binding) AUC0–24 at steady-state (AUCss,0–24) of 2.11-fold. The adult PBPK model was scaled to infants, and was compared with observations in preterm infants receiving i.v. sildenafil with prophylaxis and steady-state treatment doses of fluconazole including the minor role of CYP2C9 (Figure 1). When sildenafil (0.25 mg/kg i.v. 3 times daily) was administered with and without fluconazole (12 mg/kg i.v. daily), the simulated AUCss,0–24 fold change of sildenafil plus DMS was 2.82-fold in virtual infants (24–40 weeks GA and 0–14 days PNA). Stratifying by postmenstrual age < 36 and ≥ 36 weeks, the simulated AUCss,0–24 fold change of sildenafil plus DMS was 2.87 and 2.55 in virtual preterm and term infants, respectively (Table S7).
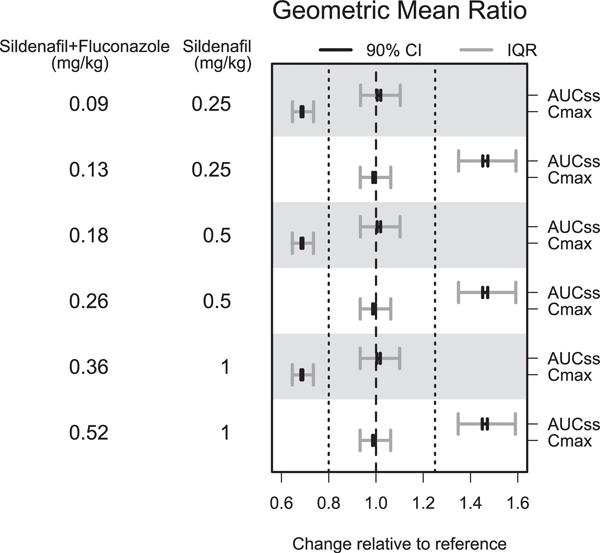
Optimal dosing simulations were performed for sildenafil with fluconazole in infants with and without CYP2C9 DMS formation and CYP2C9 inhibition and the results were nearly identical. When given in combination with treatment doses of fluconazole (12 mg/kg i.v. daily), reducing the sildenafil dose by 64% (administered i.v. over a 90-minute infusion) resulted in a geometric mean ratio of 1.01 for simulated AUCss,0–24 relative to virtual infants (24–42 weeks (postmenstrual age)) receiving sildenafil alone (Figure 3; Table S8). Simulated unbound sildenafil Cmax values, targeted to achieve 53%, 77%, and 90% phosphodiesterase type 5 inhibition based on a dose-ranging study of oral sildenafil in children aged 1–17 years with pulmonary arterial hypertension, were slightly lower for virtual infants receiving sildenafil with fluconazole compared with sildenafil alone (Figure S11).37 To achieve similar Cmax values, we reduced the sildenafil dose by 48% (administered i.v. over a 90-minute infusion) with 12 mg/kg i.v. daily fluconazole, which resulted in a geometric mean ratio for simulated Cmax of 0.99 relative to infants receiving sildenafil alone, but overestimated simulated AUCss,0–24 (Figure 3; Table S9). Additionally, reducing the sildenafil dose by 64%, but shortening the i.v. infusion time to 30 minutes, resulted in a geometric mean ratio for simulated Cmax of 0.90 (Table S10).
Figure 3.
Changes in daily AUCss and Cmax in preterm and term infants receiving modified doses of i.v. sildenafil given t.i.d. in combination with fluconazole compared to preterm and term infants receiving sildenafil alone. Data presented as the geometric mean and associated 90% prediction interval of the change in sildenafil plus 0.5*1.25*DMS (accounting for differences in potency and protein binding) AUCss and Cmax in infants receiving sildenafil with fluconazole relative to infants receiving sildenafil without fluconazole. The reference sildenafil doses were 0.25 mg/kg i.v., 0.5 mg/kg i.v., or 1 mg/kg i.v., each dose administered over a 90-minute infusion every 8 hours. The fluconazole dose was 12 mg/ kg daily, administered i.v. over a 60-minute infusion. When given in combination with fluconazole, reducing the sildenafil dose by 64% resulted in a geometric mean ratio of 1.01 for AUCss, relative to infants receiving sildenafil alone but Cmax was underpredicted. To achieve similar Cmax values, reducing the sildenafil dose by 48% with fluconazole resulted in a geometric mean ratio for Cmax of 0.99 relative to infants receiving sildenafil alone, however, but AUCss was overpredicted. AUCss, area under the plasma concentration vs. time curve at steady-state; Cmax, maximal concentration; CI, confidence interval; DMS, N-desmethylsildenafil; IQR, inter-quartile range.
DISCUSSION
We developed an adult and pediatric PBPK model for sildenafil with fluconazole incorporating CYP3A and CYP2C9 activity and ontogeny to characterize age-related differences in CYP3A-mediated DDI potential. We first developed and evaluated a sildenafil PBPK model in adults to gain confidence in the structural model before scaling to pediatrics (Figure S1). We assumed that DMS CL was mediated through CYP3A based on a proteomics study suggesting that DMS undergoes modification by CYP3A to form two additional metabolites.38 The adult sildenafil PBPK model captured the observed DDI in healthy adults receiving sildenafil in combination with ritonavir or erythromycin.30,31 CYP3A7 CLint was optimized using preterm infant PK data because CYP3A7 expression is minimal in adults and highest in preterm infants.
The simulated sildenafil CL and Vss for adults and infants were comparable and all within twofold of observed values, except for one study in infants, which suggests reasonable agreement between our model predictions and observations (Table 3). This one study reported a higher Vss in full-term neonates (456 L/70 kg (22.4 L)) than reported in preterm infants and adults, which the authors suggested may be due to lower protein binding of sildenafil in infants relative to adults (93.9% vs. 96%).20 It seems unlikely that term infants would have a significantly higher volume of distribution than preterm infants. In addition, the CL and Vss reported in infants was much higher than the variability generated using the infant virtual population in PK-Sim® (Table 3). Critically ill infants receiving sildenafil with fluconazole may have higher or more variable protein binding associated with illness, stress, or inflammation. For example, AAG is an acute phase protein that has been shown to increase and fluctuate in infants and children with illness and inflammation.39,40 To test this hypothesis, we increased the coefficient of variation on protein binding through AAG in the simulated infant population, a reasonable assumption based on the high variability of plasma AAG levels observed in infants, resulting in similar observed and simulated CL and Vss variability (Table 3).41 Infants with pulmonary arterial hypertension may have differences in organ function and/or blood flow relative to the virtual infant population in PK-Sim® possibly leading to higher variability.
Sensitivity analysis for sildenafil across age highlights that CYP3A7 should be included for CYP3A substrates in infants ≤ 2 months of age (Figure 2). However, we often do not have CYP3A7 in vitro parameters and instead only scale using CYP3A4 activity. This assumption is reasonable for children because CYP3A4 expression reaches full capacity by ~ 1.3 years of age, but may lead to model misspecification in neonates primarily expressing CYP3A7.42 CYP3A7 expression decreases from 142.2 pmol/mg in neonates to 4 pmol/mg in adults.2,3,7 CYP3A7 changes with age with mean values of 201 pmol/mg in fetal liver samples (estimated GA of 31–41 weeks) and 158 pmol/mg in premature birth liver samples (estimated GA < 40 weeks).2 In contrast, CYP3A4 increases from 5 pmol/mg in neonates to 98 pmol/mg in adults.2,3,7
Fluconazole CYP3A inhibition data were experimentally generated to characterize DDI potential between sildenafil with fluconazole in infants. Fluconazole was a mixed inhibitor of CYP3A using testosterone as the probe substrate, and CYP3A4 was a more potent inhibitor relative to CYP3A5 and CYP3A7 (Table 4). When comparing the metabolic capacity for CYP3A using 10 different CYP3A substrates, one study reported an equal or reduced metabolic capability for CYP3A5 compared with CYP3A4 and a significantly lower catalytic activity for CYP3A7 compared with CYP3A4.4 The KI for CYP3A4 and CYP3A5 from this study differed from another study using midazolam as the CYP3A probe substrate (29.4 μM vs. 9.21 μM for CYP3A4 and 182.5 μM vs. 84.6 μM for CYP3A5).5 Results can differ based on the CYP3A probe substrate used, and two distinct substrate groups have been postulated, including testosterone in one group and midazolam in the other group.43 The magnitude of the simulated DDI between sildenafil with fluconazole was slightly greater in neonates than adults (a fold change in AUCss,0–24 of 2.82 vs. 2.11). This may be attributed to a lower catalytic activity for CYP3A7 relative to CYP3A4 particularly because the difference was greater for preterm than term infants. Additionally, neonates receive a higher fluconazole treatment dose (12 mg/kg i.v. daily) relative to adults (6 mg/kg i.v. daily) and fluconazole inhibition is dose-dependent. Given the lack of adult DDI data available for sildenafil with fluconazole model evaluation, it is difficult to determine if this difference is significant and clinically relevant.
The pediatric sildenafil with fluconazole PBPK model was applied to provide dosing recommendations in infants and compared against pharmacodynamic and efficacy end points. Based on the FDA clinical drug interaction studies guidance, we targeted geometric mean ratios for Cmax and AUCss,0–24 with and without fluconazole within the 0.8 to 1.25 equivalence range.1 This approach was applied because there is no optimal dosing or exposure-response relationship established for sildenafil in infants. However, in adults with pulmonary arterial hypertension, the concentration-response relationship for pulmonary vascular resistance has a concentration of half-maximal effect of 17 ng/mL and the concentration that produces the maximal effect is around 100 ng/mL.44 There was a dose-ranging, placebo-controlled study performed in children with pulmonary arterial hypertension from 1 to 17 years old receiving 3 sildenafil doses targeted to achieve steady-state Cmax values of 47, 140, and 373 ng/mL corresponding with 53%, 77%, and 90% unbound inhibition of in vitro phosphodiesterase type 5 activity, respectively. Interestingly, in this study, the low dose (10 mg in children > 20 kg) was ineffective whereas the high dose (20, 40, and 80 mg for children among ≥ 8–20, > 20–45, and > 45 kg, respectively) was associated with an increased risk of mortality after 2 years of treatment relative to children receiving lower doses of sildenafil.24 Another study reported that newborns with persistent pulmonary hypertension who achieved an initial sildenafil concentration of 58.4 ± 44.8 ng/mL, experienced significant improvements in oxygenation after 4 hours of a continuous sildenafil infusion, whereas those with levels of 3.7 ± 4.6 ng/mL did not experience improvements in oxygenation.45 These model simulations suggest that sildenafil dosing of 0.5 mg/kg i.v. every 8 hours alone and 0.18 mg/kg sildenafil i.v. every 8 hours when given in combination with 12 mg/kg i.v. daily fluconazole, results in simulated Cmax values exceeding the 77% unbound inhibition target. Additional studies are needed to further evaluate the relationship among sildenafil exposure, efficacy, and safety in premature infants.
We present a novel approach for characterizing DDIs in pediatric patients; yet, there are limitations that warrant further discussion. All simulations were performed in infants receiving i.v. sildenafil with fluconazole because the preterm infants who received sildenafil with fluconazole received sildenafil i.v. Furthermore, oral absorption in preterm infants is not enabled within the PK-Sim®/MoBi® software. Therefore, these dose recommendations may differ for infants receiving oral sildenafil with fluconazole. Another limitation is that the sildenafil with fluconazole DDI data used for model evaluation were available for preterm infant data only. However, the percentage reduction in CL in this study following i.v. administration was similar to another study in neonates with a median (range) PNA of 20 days (2–121 days; GA not reported) who received sildenafil via nasogastric tube in combination with fluconazole (65 vs. 47%).20 The interaction with fluconazole is also dose-dependent and our simulations focused on treatment doses of fluconazole and infants in the published studies may have received prophylaxis doses.20 Additionally, there was no available clinical DDI data in adults receiving sildenafil with fluconazole to confirm the adult DDI model predictions. Nonetheless, we were able to model the DDI between sildenafil with ritonavir and erythromycin in healthy adults (Tables S3 and S4).
In conclusion, it is critical to incorporate CYP3A7 parameters into PBPK models to accurately predict CYP3A mediated drug distribution and DDI potential in infants ≤ 2 months of age. Additional PBPK models developed for other CYP3A inhibitors or inducers can be comodeled using this sildenafil PBPK model to guide model-informed precision dosing in infants receiving sildenafil with other interacting drugs, such as erythromycin and protease inhibitors.
Supplementary Material
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
 Cytochrome P450 (CYP) 3A mediated drug-drug interactions (DDIs) may differ between adults and infants because CYP3A7 has higher expression in infants and typically lower catalytic activity relative to CYP3A4, the predominant isoform expressed in adults.
Cytochrome P450 (CYP) 3A mediated drug-drug interactions (DDIs) may differ between adults and infants because CYP3A7 has higher expression in infants and typically lower catalytic activity relative to CYP3A4, the predominant isoform expressed in adults.
WHAT QUESTION DID THIS STUDY ADDRESS?
 Using sildenafil with fluconazole as an example, this study leverages physiologically-based pharmacokinetic modeling and sparse DDI data collected in preterm infants to characterize age-related differences in CYP3A-mediated DDIs.
Using sildenafil with fluconazole as an example, this study leverages physiologically-based pharmacokinetic modeling and sparse DDI data collected in preterm infants to characterize age-related differences in CYP3A-mediated DDIs.
WHAT DOES THIS STUDY ADD TO OUR KNOW-LEDGE?
 This study highlights that it is critical to incorporate CYP3A7 parameters for CYP3A substrates in infants ≤ 2 months of age. Additionally, reducing the sildenafil dose by 64% in combination with 12 mg/kg i.v. daily fluconazole resulted in comparable simulated daily area under the plasma concentration vs. time curve at steady-state (AUCss,0–24) values as virtual infants receiving sildenafil alone.
This study highlights that it is critical to incorporate CYP3A7 parameters for CYP3A substrates in infants ≤ 2 months of age. Additionally, reducing the sildenafil dose by 64% in combination with 12 mg/kg i.v. daily fluconazole resulted in comparable simulated daily area under the plasma concentration vs. time curve at steady-state (AUCss,0–24) values as virtual infants receiving sildenafil alone.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
 This novel approach can be applied to other metabolic DDIs in pediatrics where clinical DDI data is available in adults, but lacking or limited in the pediatric population.
This novel approach can be applied to other metabolic DDIs in pediatrics where clinical DDI data is available in adults, but lacking or limited in the pediatric population.
ACKNOWLEDGMENTS
A special thanks to Leonard Collins for developing the HPLC-MS/MS assay for 6β-hydroxytestosterone. The assay measuring sildenafil and DMS concentrations in the clinical PK samples was developed by Robert M. Wurm, BS, at OpAns, LLC (Durham, NC).
FUNDING
This Pediatric Trials Network (PTN) study was funded under Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) contract HHSN275201000003I (Principal Investigator [PI]: Daniel K. Benjamin, Jr.). The Best Pharmaceuticals for Children Act (BPCA) Data Coordinating Center was funded under HHSN275201700002C (PI: Ravinder Anand). S.N.S. was supported by the National Institute of General Medical Sciences (NIGMS) and the NICHD of the National Institutes of Health under award T32GM086330. J.G.G. received research support from an NIGMS-funded T32 program under award T32GM122741. D.G. received research support from the NICHD (K23HD083465 and R01HD096435). M.M.L. received research support from the FDA (R01FD006099) and the National Heart, Lung, and Blood Institute (NHBLI) (K24HL143283). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix.
APPENDIX 1
Pediatric Trials Network Steering Committee Members: Daniel K. Benjamin Jr, Christoph Hornik, Kanecia Zimmerman, Phyllis Kennel, and Rose Beci, Duke Clinical Research Institute, Durham, NC; Chi Dang Hornik, Duke University Medical Center, Durham, NC; Gregory L. Kearns, Texas Christian University and UNTHSC School of Medicine, Fort Worth, TX; Matthew Laughon, The University of North Carolina at Chapel Hill, Chapel Hill, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Janice Sullivan, University of Louisville, Louisville, KY; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; and Paula Delmore, Wichita Medical Research and Education Foundation, Wichita, KS.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): Perdita Taylor-Zapata and June Lee.
The Emmes Company, LLC (Data Coordinating Center): Ravinder Anand, Gaurav Sharma, Gina Simone, Kim Kaneshige, and Lawrence Taylor.
Pediatric Trials Network Publications Committee: Chaired by Thomas Green, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL.
Pediatric Trials Network Sildenafil Study Team, Principal Investigators, and Study Coordinators
Namasivayam Ambalavanan, MD (Principal Investigator [PI]), Tara E. McNair, RN, BSN (Study Coordinator [SC]), and Vivien Phillips, RN, BSN (SC), University of Alabama at Birmingham, Birmingham, AL, USA; Andrew Atz, MD (PI) and Kalyan Janakiram Choudhary, MSCR (SC), Medical University of South Carolina Children’s Hospital, Charleston, SC, USA; Gregory M. Sokol, MD (PI), Susan Gunn, NNP-BC, CCRC (SC), Dianne Herron, RN, CCRC (SC), Lucy Smiley, BS, CCRC (SC), Riley Hospital for Children at Indiana University Health, Indiana University, Indianapolis, IN, USA; Chi D. Hornik, PharmD, BCPS, CPP (PI), C. Michael Cotten, MD (Investigator), Lacey Andrews, BS (SC), and Duke University Medical Center, Durham, NC, USA; Dan Stewart, MD (PI), Janice Sullivan, MD (Investigator), and Andrew Michael, RN, BSN (SC), University of Louisville Norton Children’s Hospital, Louisville, KY, USA; Gratias Mundakel, MD (PI) and Subhatra Limbu, MBBS, MPH (SC), Kings County Hospital Center/SUNY Downstate Medical Center, Brooklyn, NY, USA; Brenda Poindexter, MD, MS (PI), and Sandra Wuertz, RN-BSN, CCRP, CLC (SC), Cincinnati Children’s Hospital Medical Center and the University of Cincinnati School of Medicine.
Footnotes
CONFLICTS OF INTEREST
M.M.L. has received support for work on data safety and monitoring boards and drug development from Medipost, United Therapeutics, and Aguettant. All other authors declared no competing interests for this work.
SUPPORTING INFORMATION
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
References
- 1.US Food and Drug Administration Center for Drug Evaluation and Research. Clinical drug interaction studies-cytochrome P450 enzyme- and transporter-mediated drug interactions. Guidance for industry <https://www.fda.gov/media/134581/download> (2020). Accessed August 5, 2020. [Google Scholar]
- 2.Stevens JC et al. Developmental expression of the major human hepatic CYP3A enzymes. J. Pharmacol. Exp. Ther 307, 573–582 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Sim SC, Edwards RJ, Boobis AR & Ingelman-Sundberg M. CYP3A7 protein expression is high in a fraction of adult human livers and partially associated with the CYP3A7*1C allele. Pharmacogenet. Genomics 15, 625–631 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Williams JA et al. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab. Dispos 30, 883–891 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Gibbs MA, Thummel KE, Shen DD & Kunze KL Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: comparison of Ki values and impact of CYP3A5 expression. Drug Metab. Dispos 27, 180–187 (1999). [PubMed] [Google Scholar]
- 6.Soars MG, Grime K & Riley RJ Comparative analysis of substrate and inhibitor interactions with CYP3A4 and CYP3A5. Xenobiotica 36, 287–299 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Hines RN Ontogeny of human hepatic cytochromes P450. J. Biochem. Mol. Toxicol 21, 169–175 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Li A, Yeo K, Welty D & Rong H. Development of guanfacine extended-release dosing strategies in children and adolescents with ADHD using a physiologically based pharmacokinetic model to predict drug–drug interactions with moderate CYP3A4 inhibitors or inducers. Pediatr. Drugs 20, 181–194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olafuyi O, Coleman M & Badhan RKS Development of a paediatric physiologically based pharmacokinetic model to assess the impact of drug-drug interactions in tuberculosis co-infected malaria subjects: a case study with artemetherlumefantrine and the CYP3A4-inducer rifampicin. Eur. J. Pharm. Sci 106, 20–33 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Perez KM & Laughon M. Sildenafil in term and premature infants: a systematic review. Clin. Ther 37, 2598–2607.e1 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Greenberg RG & Benjamin DK Neonatal candidiasis: diagnosis, prevention, and treatment. J. Infect 69, S19–S22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyland R, Roe EG, Jones BC & Smith DA Identification of the cytochrome P450 enzymes involved in the N-demethylation of sildenafil. Br. J. Clin. Pharmacol 51, 239–248 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muirhead GJ, Rance DJ, Walker DK & Wastall P. Comparative human pharmacokinetics and metabolism of single-dose oral and intravenous sildenafil. Br. J. Clin. Pharmacol 53 (suppl. 1), 13S–20S (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warrington JS, Shader RI, von Moltke LL & Greenblatt DJ In vitro biotransformation of sildenafil (Viagra): identification of human cytochromes and potential drug interactions. Drug Metab. Dispos 28, 392–397 (2000). [PubMed] [Google Scholar]
- 15.Walker DK Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica 29, 297–310 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Haspel HC et al. Binding of phosphodiesterase type 5 inhibitors sildenafil, tadalafil, and vardenafil to total human plasma proteins and human serum albumin (HSA) and 1-acid glycoprotein (AGP). In 14th North American International Society for the Study of Xenobiotics Meeting. Rio Grande, Puerto Rico <https://www.researchgate.net/publication/268138068_Binding_of_Phosphodiesterase_Type_5_Inhibitors_Sildenafil_Tadalafil_and_Vardenafil_to_Total_Human_Plasma_Proteins_and_Human_Serum_Album_in_HSA_and_1-Acid_Glycoprotein_AGP> (2006). Accessed March 20, 2020. [Google Scholar]
- 17.Pfizer, Inc. Revatio® (sildenafil) Product Insert <https://www.pfizermedicalinformation.com/en-us/revatio/clinical-pharmacology> (2020). Accessed March 20, 2020.
- 18.Takahiro R. et al. Contribution of CYP3A isoforms to dealkylation of PDE5 inhibitors: a comparison between sildenafil N-demethylation and tadalafil demethylenation. Biol. Pharm. Bull 38, 58–65 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee A, Dombi T, Wittke B & Lalonde R. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin. Pharmacol. Ther 85, 56–63 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Ahsman MJ et al. Sildenafil exposure in neonates with pulmonary hypertension after administration via a nasogastric tube. Arch. Dis. Child. Fetal Neonatal Ed 95, F109–F114 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez D. et al. Population pharmacokinetics of sildenafil in extremely premature infants. Br. J. Clin. Pharmacol 85, 2824–2837 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippert J. et al. Open systems pharmacology community-an open access, open source, open science approach to modeling and simulation in pharmaceutical sciences. CPT Pharmacometrics Syst. Pharmacol 8, 878–882 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer M, Schneckener S, Ludewig B, Kuepfer L & Lippert J. Using expression data for quantification of active processes in physiologically based pharmacokinetic modeling. Drug Metab. Dispos 40, 892–901 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues AD Integrated cytochrome P450 reaction phenotyping. Attempting to bridge the gap between cDNA-expressed cytochrome P450 and native human liver microsomes. Biochem. Pharmacol 57, 465–480 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Pelkonen O. Drug metabolism in the human fetal liver. Relationship to fetal age. Arch. Int. Pharmacodyn. Ther 202, 281–287 (1973). [PubMed] [Google Scholar]
- 26.Barter ZE et al. Covariation of human microsomal protein per gram of liver with age: absence of influence of operator and sample storage may justify interlaboratory data pooling. Drug Metab. Dispos 36, 2405–2409 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Ku H-Y et al. The contributions of cytochromes P450 3A4 and 3A5 to the metabolism of the phosphodiesterase type 5 inhibitors sildenafil, udenafil, and vardenafil. Drug Metab. Dispos 36, 986–990 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Hindmarsh AC et al. Open systems pharmacology suite manual, version 7.4 <http://www.open-systems-pharmacology.org> (2018). Accessed March 20, 2020.
- 29.Rodgers T & Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci 95, 1238–1257 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Muirhead GJ, Wulff MB, Fielding A, Kleinermans D & Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Clin. Pharmacol 50, 99–107 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muirhead GJ, Faulkner S, Harness JA & Taubel J. The effects of steady-state erythromycin and azithromycin on the pharmacokinetics of sildenafil citrate in healthy volunteers. Br. J. Clin. Pharmacol 53, 37S–43S (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watt KM et al. Physiologically based pharmacokinetic approach to determine dosing on extracorporeal life support: fluconazole in children on ECMO. CPT Pharmacometrics Syst. Pharmacol 7, 629–637 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claassen K. et al. Development of a physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr. Pharm. Des 21, 5688–5698 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Mayer H, Burghaus R, Coböken K, Frenchen S, Ince I & Schlender J. A novel approach to estimate ontogenies for PBPK applications – from literature data to simulations. Population Approach Group Europe (PAGE). 27th PAGE Annual Meeting <https://www.page-meeting.org/default.asp?abstract=8583> (2018). [Google Scholar]
- 35.Gerhart JG et al. Physiologically-based pharmacokinetic modeling of fluconazole using plasma and cerebrospinal fluid samples from preterm and term infants. CPT Pharmacometrics Syst. Pharmacol 8, 500–510 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pappas PG et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis 62, e1–e50 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barst RJ et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 125, 324–334 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Kim J-H et al. Non-targeted metabolomics-guided sildenafil metabolism study in human liver microsomes. J. Chromatogr. B 1072, 86–93 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Klein M. et al. Predictors of inflammation in a cohort of Bolivian infants and toddlers. Am. J. Trop. Med. Hyg 95, 954–963 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merrill R. et al. Factors associated with inflammation in preschool children and women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr 106, 348S–358S (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maharaj AR, Gonzalez D, Cohen-Wolkowiez M, Hornik CP & Edginton AN Improving pediatric protein binding estimates: an evaluation of α1-acid glycoprotein maturation in healthy and infected subjects. Clin. Pharmacokinet 57, 577–589 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salem F, Johnson TN, Abduljalil K, Tucker GT & Rostami-Hodjegan A. A re-evaluation and validation of ontogeny functions for cytochrome P450 1A2 and 3A4 based on in vivo data. Clin. Pharmacokinet 53, 625–636 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Kenworthy KE, Bloomer JC, Clarke SE & Houston JB CYP3A4 drug interactions: correlation of 10 in vitro probe substrates. Br. J. Clin. Pharmacol 48, 716–727 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.US Food and Drug Administration Center for Drug Evaluation and Research. Pharmacometrics Addendum to Clinical Pharmacology & Biopharmaceutics Review < https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021845s000_Revatio_biopharmr.pdf> (2004). Accessed March 2, 2020.
- 45.Steinhorn RH et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J. Pediatr 155, 841–847.e1 (2009). [DOI] [PubMed] [Google Scholar]
- 46.DrugBank. version 5.1.2 <https://www.drugbank.ca>. Accessed March 20, 2020.
- 47.Nichols DJ, Muirhead GJ & Harness JA Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br. J. Clin. Pharmacol 53 (suppl. 1), 5S–12S (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgess G, Hoogkamer H, Collings L & Dingemanse J. Mutual pharmacokinetic interactions between steady-state Bosentan and sildenafil. Eur. J. Clin. Pharmacol 64, 43–50 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Vachiery J-L et al. Safety, tolerability and pharmacokinetics of an intravenous bolus of sildenafil in patients with pulmonary arterial hypertension. Br. J. Clin. Pharmacol 71, 289–292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.US Food and Drug Administration Center for Drug Evaluation and Research. Clinical pharmacology review NDA 22–473, Intravenous sildenafil<https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022473s000_ClinPharmR.pdf> (2009). Accessed March 20, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.