Abstract
BACKGROUND
Diurnal variation of natriuretic peptide (NP) levels and its relationship with 24-h blood pressure (BP) rhythm has not been established. Obese individuals have a relative NP deficiency and disturbed BP rhythmicity.
OBJECTIVES
This clinical trial evaluated the diurnal rhythmicity of NPs (B-type natriuretic peptide [BNP], mid-regional pro-atrial natriuretic peptide [MR-proANP], N-terminal pro–B-type natriuretic peptide [NT-proBNP]) and the relationship of NP rhythm with 24-h BP rhythm in healthy lean and obese individuals.
METHODS
On the background of a standardized diet, healthy, normotensive, lean (body mass index 18.5 to 25 kg/m2) and obese (body mass index 30 to 45 kg/m2) individuals, age 18 to 40 years, underwent 24-h inpatient protocol involving ambulatory BP monitoring starting 24 h prior to the visit, controlled light intensity, and repeated blood draws for assessment of analytes. Cosinor analysis of normalized NP levels (normalized to 24-h mean value) was conducted to assess the diurnal NP rhythm and its relationship with systolic BP.
RESULTS
Among 52 participants screened, 40 participants (18 lean, 22 obese; 50% women; 65% Black) completed the study. The median range spread (percentage difference between the minimum and maximum values) over 24 h for MR-proANP, BNP, and NT-proBNP levels was 72.0% (interquartile range [IQR]: 50.9% to 119.6%), 75.5% (IQR: 50.7% to 106.8%), and 135.0% (IQR: 66.3% to 270.4%), respectively. A cosine wave-shaped 24-h oscillation of normalized NP levels (BNP, MR-proANP, and NT-proBNP) was noted both in lean and obese individuals (prhythmicity < 0.05 for all). A larger phase difference between MR-proANP BP rhythm (−4.9 h vs. −0.7 h) and BNP BP rhythm (−3.3 h vs. −0.9 h) was seen in obese compared with lean individuals.
CONCLUSIONS
This human physiological trial elucidates evidence of diurnal NP rhythmicity and the presence of an NP-BP rhythm axis. There exists a misalignment of the NP-BP diurnal rhythm in the obese, which may contribute to the disturbed diurnal BP pattern observed among obese individuals. (The Diurnal Rhythm in Natriuretic Peptide Levels; NCT03834168)
Keywords: blood pressure, diurnal rhythm, hypertension, natriuretic peptides, obesity
Natriuretic peptides are cardiac-derived hormones that regulate the cardiovascular system by causing natriuresis (1,2), vasodilation (3), and direct inhibition of the renin-angiotensin-aldosterone system (RAAS) (4). Data from small human studies and animal models indicate that there may exist a diurnal rhythm of NP levels (5–9). An inverse association of body mass index (BMI) with NP levels has been well described (10,11), which is consistent across sex (10,11) and peptide subgroups of NPs (11,12). The relative NP deficiency seen among obese individuals has been postulated to contribute to the development of cardiovascular diseases (13–16).
Obesity and hypertension (HTN) are increasing among American adults nationwide, and these findings are evidenced even in young adults across all racial groups (17–20). Obese individuals have a high prevalence of vascular dysfunction, abnormal salt handling, nondipping blood pressure (BP), and nocturnal HTN (21). The reasons for this are poorly understood. We and others have shown that nocturnal HTN and nondipping systolic BP are associated with an increased risk of adverse cardiovascular events and mortality, independent of other BP parameters (22–27). The postulated rhythmicity of NPs and its relationship with 24-h systolic BP patterns in healthy, lean, and obese populations in the setting of a standardized salt intake have not been previously evaluated.
We conducted a mechanistic, single-center, physiological, clinical trial in the setting of a standardized diet to evaluate: 1) the diurnal rhythm of NPs and the differences in NP rhythm in lean and obese individuals; and 2) the relationship of diurnal variation of NPs with systolic BP in the overall cohort and in lean and obese individuals. We also assessed the differences in timed urinary sodium excretion, 24-h renin, and aldosterone levels between young, healthy, lean, and obese individuals.
METHODS
STUDY DESIGN, SETTING, AND LOCATION.
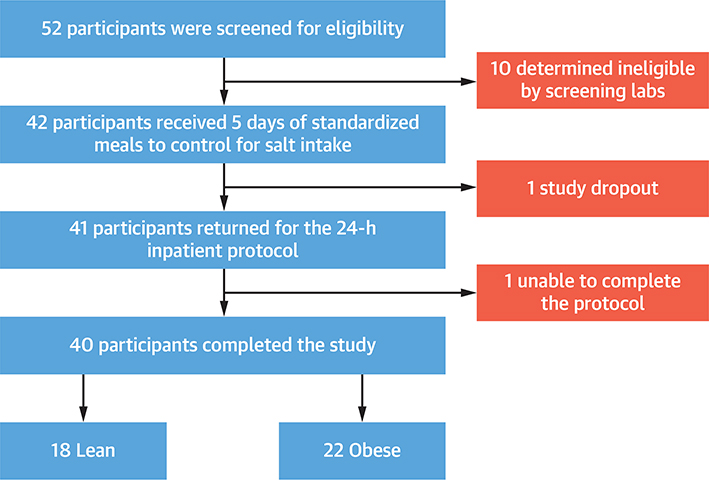
The prospective, single-center, mechanistic clinical trial was conducted from February 2019 to January 2021 at the Clinical Research Unit at the University of Alabama at Birmingham). The trial was approved by the Institutional Review Board (IRB No: 300004115), and participants provided written and informed consent. Healthy participants were recruited from the Birmingham metropolitan area and the University of Alabama at Birmingham campus (Figure 1).
FIGURE 1. Selection of Study Participants.
Flowchart of participant selection for this study.
SAMPLE SELECTION.
We included self-identified White and Black individuals with BMI between 18.5 and 25 kg/m2 (lean) or 30 and 45 kg/m2 (obese) who had a seated BP ≤140/90 mm Hg, were willing to comply with the standardized study diet, and provided written, informed consent. The inclusion and exclusion criteria, study protocol, and laboratory assessment details are detailed in Supplemental Table 1 and the Supplemental Methods (28–35).
MEASURES AND OUTCOMES.
The primary study measures included systolic ambulatory BP measures (in mm Hg) and plasma levels of NPs that included mid-regional pro-atrial natriuretic peptide (MR-proANP), B-type natriuretic peptide (BNP), and N-terminal pro–B-type natriuretic peptide (NT-proBNP). Other study measures included daytime and nighttime urinary sodium, urinary potassium, and 24-h renin and aldosterone levels. The BNP, MR-proANP, NT-proBNP, renin, aldosterone, and BP at each time point was normalized against their respective mean value of the entire day in each individual. The maximum number of analyzable ambulatory BP measurements were 48 daytime and 16 nighttime readings (Total = 64). The NP and RAAS analytes were measured at 10 time-points over the 24-h period, with 5 recordings during wakefulness (7 AM to 10 PM) and 5 recordings during the sleep period (10 PM to 7 AM) (Supplemental Figure 1).
The main study outcomes included: 1) the diurnal rhythm of normalized NP levels (overall and in lean and obese); and 2) the relationship between diurnal rhythms of normalized NPs with normalized BP (overall and in lean and obese). Additional study outcomes included: 1) range and range spread of 24-h MR-proANP, NT-proBNP, renin, and aldosterone levels in the overall population and among lean and obese individuals; 2) differences in the 24-h, daytime, and nighttime mean BNP, MR-proANP, NT-proBNP, renin, and aldosterone levels between lean and obese individuals; 3) timed urinary sodium and potassium excretion between lean and obese; 4) diurnal rhythm of normalized renin and aldosterone levels (overall and in lean and obese); and 5) diurnal rhythm of NPPA gene expression in the whole blood mRNA of the trial participants. In the absence of diurnal cardiac NPPA gene expression, we assessed the diurnal variation in the whole blood NPPA mRNA levels as a surrogate for cardiac expression.
STATISTICAL ANALYSIS.
All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina) and R version 4.0.1 (R Core Team, Vienna, Austria). The baseline characteristics were compared using descriptive statistics. Continuous variables were summarized as the median and interquartile range (IQR). Continuous data were compared using the Wilcoxon rank-sum test. Pearson chi-square test was used to compare the categorical variables. The range was computed as the difference between the minimum and maximum value of the analyte over the 24-h period. The range spread (%) was computed as (range/minimum value) × 100. The cosinor analysis of the normalized analyte values (normalized to the 24-h mean value) was conducted using the cosinor2 package in R Statistical Software version 4.0.1 (R Core Team) (8,36–38). This package uses linear regression to identify the cosine curve that best fits the data and tests the overall significance of the cosinor model using an F-ratio (8,36–38). The 24-h data was considered rhythmic if it fit the cosinor function (i.e., f(t) = M + AMP × Cos [(2πt)/24 + ϕ]+ εt, where M refers to the Mesor [rhythm-adjusted mean], AMP refers to the amplitude [difference between peak and M], and ϕ refers to the phase [time of peak]). Data was considered rhythmic if the p value of the R2 of the cosine function was <0.05. The rhythm parameters are based on the grouped analysis of the lean and obese population groups. The cosinor parameters of interest while comparing the normalized NP rhythms between lean and obese individuals were the amplitude and acrophase. The reported cosinor parameter of interest while comparing the normalized NP and BP rhythms was acrophase. The amplitude and acrophase are defined based on the fitted cosinor curve. Linear regression models were used to assess the relative percentage difference with a 95% confidence interval (adjusted for age, sex, race) for the 24-h, daytime, and nighttime mean MR-proANP, BNP, NT-proBNP, renin, and aldosterone levels between lean and obese individuals. This was calculated using the following formula: (eβ – 1) × 100 (β coefficient from linear regression). A 2-sided type-I error of <0.05 was considered statistically significant for all analyses.
RESULTS
Of 52 patients screened, there were 40 participants (18 lean and 22 obese) that were enrolled and completed the study. The study recruited a relatively young (31 years [IQR: 26 to 35 years]) population, which included 50% women and 65% Black individuals. The baseline characteristics of the study population are described in Table 1.
TABLE 1.
Baseline Characteristics of the Study Population
| Overall (N = 40) | Lean (n = 18) | Obese (n = 22) | p Value | |
|---|---|---|---|---|
| Age, yrs | 31.0 (26.0–35.0) | 30.5 (24.0–32.0) | 33.0 (26.0–37.0) | 0.15 |
| Women | 20 (50.0) | 8 (45.0) | 12 (54.6) | 0.53 |
| Black | 26 (65.0) | 10 (55.6) | 16 (72.7) | 0.26 |
| BMI, kg/m2 | 29.6 (24.4–32.9) | 24.3 (21.9–25.0) | 32.2 (30.7–36.0) | <0.001 |
| Hip circumference, cm | 106.1 (96.1–114.8) | 97.3 (93.8–101.0) | 111.4 (106.1–118.7) | <0.001 |
| Waist circumference, cm | 97.6 (78.3–105.4) | 76.9 (73.5–85.7) | 103.6 (99.8–111.8) | <0.001 |
| Systolic blood pressure, mm Hg | 116.2 (106.0–123.0) | 110.7 (102.0–122.7) | 116.5 (108.3–126.0) | 0.24 |
| Diastolic blood pressure, mm Hg | 71.0 (65.3–78.2) | 69.5 (64.3–76.0) | 73.7 (67.7–80.0) | 0.12 |
| Heart rate, beats/min | 77.0 (69.0–80.0) | 76.5 (66.0–80.0) | 77.0 (71.0–80.0) | 0.49 |
| Fasting glucose, mg/dl | 87.5 (81.0–97.0) | 85.0 (79.0–96.0) | 91.5 (82.0–100.0) | 0.32 |
| Renal function | ||||
| BUN, mg/dl | 12.0 (10.5–14.0) | 12.0 (11.0–13.0) | 12.0 (10.0–14.0) | 0.89 |
| Serum creatinine, mg/dl | 0.9 (0.8–0.9) | 0.9 (0.8–0.9) | 0.9 (0.8–1.0) | 0.16 |
| Total bilirubin, mg/dl | ||||
| Serum electrolytes | 0.5 (0.4–0.8) | 0.5 (0.4–0.8) | 0.5 (0.4–0.7) | 0.86 |
| Sodium, mEq/l | 138.0 (136.5–138.5) | 137.5 (137.0–139.0) | 138.0 (136.0–138.0) | 0.85 |
| Potassium, mEq/l | 4.2 (3.9–4.3) | 4.2 (3.9–4.3) | 4.2 (4.0–4.3) | 0.98 |
| Calcium, mg/dl | 9.6 (9.3–9.7) | 9.6 (9.3–9.7) | 9.6 (9.2–9.8) | 0.72 |
| Chloride, mEq/l | 103.0 (102.0–104.0) | 102.5 (102.0–104.0) | 103.5 (102.0–105.0) | 0.15 |
| Bicarbonate, mEq/l | ||||
| Liver function | 26.0 (25.0–27.0) | 26.0 (25.0–28.0) | 26.0 (25.0, 27.0) | 0.78 |
| ALT | 14.0 (12.0–20.0) | 13.5 (11.0–16.0) | 17.0 (12.0–27.0) | 0.15 |
| AST | 17.0 (14.0–20.0) | 17.0 (14.0–20.0) | 17.0 (14.0–23.0) | 0.79 |
| GGT | 20.0 (12.5–25.5) | 16.0 (12.0–24.0) | 22.5 (15.0–27.0) | 0.17 |
| Hematological parameters | ||||
| WBC, × 10,000/mm3 | 5.4 (4.4–6.3) | 5.3 (4.3–6.7) | 5.5 (4.6–6.1) | 0.86 |
| RBC, × 1,000,000/mm3 | 4.7 (4.1–5.0) | 4.7 (4.1–5.0) | 4.7 (4.1–5.0) | 0.82 |
| Platelet, × 100,000/mm3 | 243.4 (202.2–271.6) | 217.7 (192.8–274.9) | 248.7 (223.6–268.2) | 0.33 |
| Hemoglobin, gm/dl | 14.2 (13.2–14.8) | 14.0 (13.1–14.9) | 14.2 (13.2–14.8) | 0.99 |
| Hematocrit | 41.0 (37.5–43.0) | 40.5 (37.0–43.0) | 41.0 (38.0–43.0) | 0.93 |
| MCV | 88.0 (86.0–91.0) | 87.5 (84.0–90.0) | 89.0 (86.0–92.0) | 0.17 |
| MCH | 30.0 (29.0–31.0) | 30.0 (29.0–31.0) | 31.0 (29.0–32.0) | 0.22 |
| MCHC | 34.0 (33.0–34.5) | 34.0 (33.0–34.0) | 34.0 (33.0–35.0) | 0.7 |
| RDW | 13.5 (12.8–14.0) | 13.5 (13.1–13.9) | 13.3 (12.8–14.5) | 0.73 |
| MPV | 9.0 (8.0–9.0) | 9.0 (8.0–9.0) | 8.0 (8.0–9.0) | 0.38 |
| Analytes | ||||
| 24-h mean BNP, pg/ml | 21.0 (15.9–30.5) | 24.0 (19.3–34.6) | 18.7 (13.4–25.5) | 0.01 |
| 24-h mean MR-proANP, pmol/l | 29.7 (25.4–38.5) | 33.1 (29.4–44.3) | 26.8 (20.2–37.3) | 0.006 |
| 24-h mean NT-proBNP, pg/ml | 16.9 (6.2–32.4) | 24.5 (12.5–43.5) | 9.7 (6.0–20.5) | 0.02 |
| 24-h mean renin, pg/ml | 8.7 (4.1–15.7) | 13.3 (5.6–22.9) | 6.9 (3.4–11.7) | 0.02 |
| 24-h mean aldosterone, ng/l | 6.5 (4.3–8.3) | 5.0 (3.7–7.7) | 7.1 (6.3–8.6) | 0.99 |
Values are mean (interquartile range) or n %. p values are for lean versus obese comparisons.
ALT = alanine transaminase; AST = aspartate transaminase; BMI = body mass index; BNP = B-type natriuretic peptide; BUN = blood urea nitrogen; GGT = gamma- glutamyl transferase; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; MPV = mean platelet volume; MR-proANP = mid-regional pro-atrial natriuretic peptide; NT-proBNP = N-terminal pro-B-type natriuretic peptide; RBC = red blood cells; RDW = red-cell distribution width; WBC = white blood cells.
DIURNAL RHYTHM OF NPs.
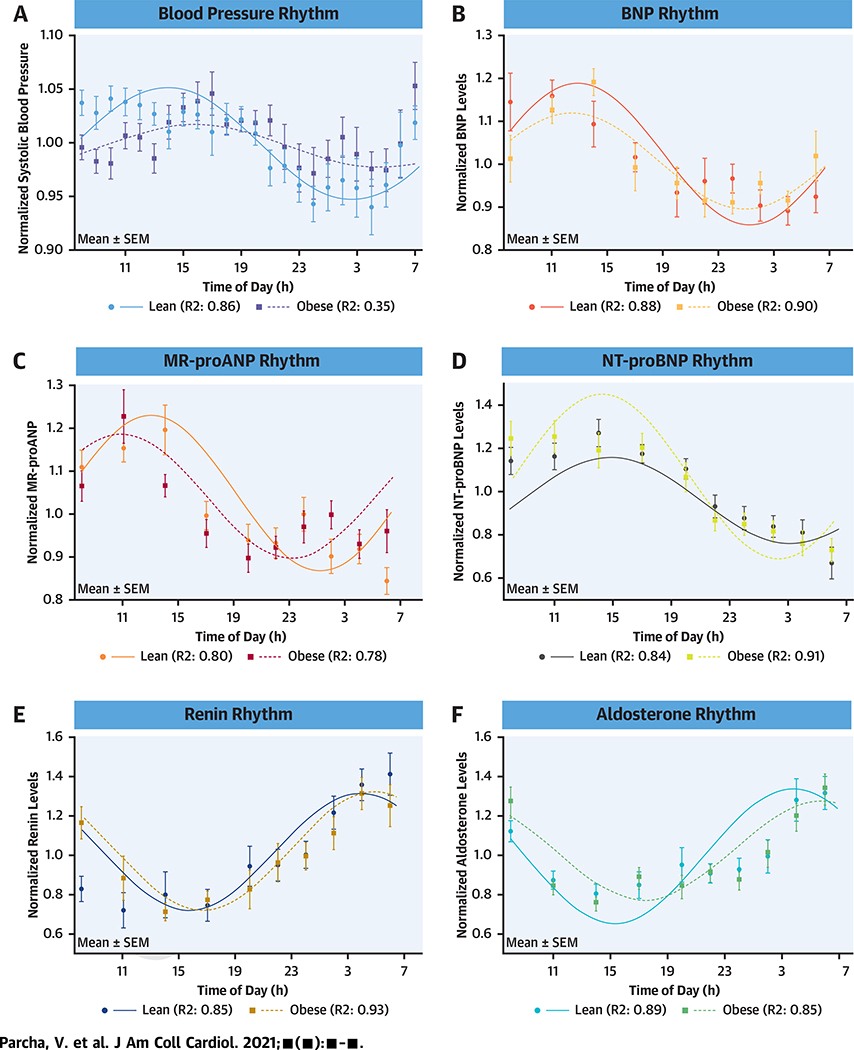
The median range and range spread of MR-proANP levels over the 24-h period was 16.73 pmol/l (IQR: 11.89 to 24.25 pmol/l) and 75.5% (IQR: 50.7% to 106.8%), respectively (Table 2). The median range for BNP and NT-proBNP levels over 24 h was 12.0 pg/ml (IQR: 8.0 to 17.0 pg/ml) and 16.46 pg/ml (IQR: 3.95 to 30.58 pg/ml), respectively. The range spread of BNP and NT-proBNP over the 24-h period was 72.0% (IQR: 50.9% to 119.6%) and 135.0% (IQR: 66.3% to 270.4%), respectively. Among obese individuals, the MR-proANP levels were 25.9% (95% confidence interval [CI]: −40.5% to −7.7%) lower over 24 h, with daytime and nighttime levels 28.1% (95% CI: −42.3% to −10.4%) and 22.9% lower (95% CI: −39.3% to −3.0%), respectively. Obese individuals had 31.6% (95% CI: −47.8% to −10.4%), 31.6% (95% CI: −48.8% to −8.6%), and 30.9% (95% CI: −47.3% to −9.5%) lower 24-h, daytime, and nighttime BNP levels compared with lean individuals, respectively (Table 3). Similarly, compared with lean individuals, obese participants had 48.8% (95% CI: −69.3% to −15.6%), 48.8% (95% CI: −69.3% to −14.8%), and 49.8% (95% CI: −69.3% to −18.1%) lower 24-h, daytime, and nighttime NT-proBNP levels, respectively. There was a significant cosine-shaped diurnal rhythm of normalized MR-proANP (p < 0.001), BNP (p < 0.001), and NT-proBNP (p < 0.001) levels among the study participants (Central Illustration). The acrophase of MR-proANP, BNP, and NT-proBNP rhythm was noted at 12:56 PM (95% CI: 11:24 AM to 2:06 PM), 1:30 PM (95% CI: 12:12 PM to 2:36 PM), and 3:18 PM (95% CI: 2:36 PM to 4:00 PM), respectively (Supplemental Table 2, Supplemental Figure 2). A cosine-shaped diurnal rhythm in the normalized NPPA gene expression was seen in the overall population (p < 0.001) and in both lean (p = 0.002) and obese participants (p = 0.01) (Supplemental Figures 3 and 4).
TABLE 2.
Diurnal Variation in Natriuretic Peptide Levels Among Lean and Obese Individuals
| Overall | Lean | Obese | |
|---|---|---|---|
| Rhythmicity | |||
| Normalized MR-proANP | <0.001 | 0.003 | 0.006 |
| Normalized BNP | <0.001 | 0.02 | <0.001 |
| Normalized NT-proBNP | <0.001 | 0.001 | 0.01 |
| 24-h range | |||
| MR-proANP, pmol/l | 16.73 (11.89–24.25) | 17.92 (12.94–25.21) | 15.51 (10.61–22.02) |
| BNP, pg/ml | 12.0 (8.0–17.0) | 14.0 (10.0–19.0) | 10.5 (8.0–14.0) |
| NT-proBNP, pg/ml | 16.46 (3.95–30.58) | 19.43 (7.16–32.92) | 13.52 (3.23–22.92) |
| 24-h range spread | |||
| MR-proANP, % | 75.5 (50.7–106.8) | 64.4 (46.6–120.2) | 79.4 (51.5–105.9) |
| BNP, % | 72.0 (50.9 –119.6) | 54.7 (46.2–119.2) | 76.6 (66.7–120.0) |
| NT-proBNP, % | 135.0 (66.3–270.4) | 121.3 (68.0–309.7) | 190.0 (64.4–269.0) |
Values are p value or median (interquartile range).
TABLE 3.
Differences in Natriuretic Peptide Levels Between Lean and Obese Individuals
| 24-h Natriuretic Peptide Level Differences |
||||
|---|---|---|---|---|
| Lean | Obese, β (95% CI) | % Difference (95% CI)* | p Value | |
| Log MR-proANP | ||||
| 24-h MR-proANP | Ref. | −0.30 (−0.52 to −0.08) | −25.9 (−40.5 to −7.7) | 0.008 |
| Daytime MR-proANP | Ref. | −0.33 (−0.55 to −0.11) | −28.1 (−42.3 to −10.4) | 0.004 |
| Nighttime MR-proANP | Ref. | −0.26 (−0.50 to −0.03) | −22.9 (−39.3 to −3.0) | 0.03 |
| Log BNP | ||||
| 24-h BNP | Ref. | −0.38 (−0.65 to −0.11) | −31.6 (−47.8 to −10.4) | 0.01 |
| Daytime BNP | Ref. | −0.38 (−0.67 to −0.09) | −31.6 (−48.8 to −8.6) | 0.02 |
| Nighttime BNP | Ref. | −0.37 (−0.64 to −0.10) | −30.9 (−47.3 to −9.5) | 0.01 |
| Log NT-proBNP | ||||
| 24-h NT-proBNP | Ref. | −0.67 (−1.18 to −0.17) | −48.8 (−69.3 to −15.6) | 0.01 |
| Daytime NT-proBNP | Ref. | −0.67 (−1.18 to −0.16) | −48.8 (−69.3 to −14.8) | 0.01 |
| Nighttime NT-proBNP | Ref. | −0.69 (−1.18 to −0.20) | −49.8 (−69.3 to −18.1) | 0.01 |
Adjusted for age, sex, and race. Multivariable linear regression models using natural log-transformed NP as the dependent variable and race as the independent variable was used to assess statistical difference by obesity. Lean individuals were chosen as reference. Values shown were β coefficient (95% confidence interval [CI]), which were on the log NP scale. Percentage lower than lean was the estimated % difference in NP levels in lean vs. obese, which was calculated by using the formula (eβ − 1) × 100, assuming all other variables in the model remained constant.
Abbreviations as in Table 1.
CENTRAL ILLUSTRATION. Diurnal Rhythm of Natriuretic Peptides, Renin, Aldosterone, and Systolic Blood Pressure in Lean and Obese Individuals.
The solid line depicts the fitted cosinor curve for the data in lean individuals, and the dashed line represents the fitted cosinor curve for the data in obese individuals. The curves depict the diurnal rhythm of systolic blood pressure (A), B-type natriuretic peptides (BNP) (B), mid-regional pro-atrial natriuretic peptide (MR-proANP) (C), N-terminal pro–B-type natriuretic peptides (NT-proBNP) (D), renin (E), and aldosterone (F) in lean and obese individuals. The circles represent the mean value at respective time points in lean individuals and boxes represent the mean value at respective time points in obese individuals. The error bars represent the standard error of mean (SEM). R2 indicates the proportion of variance explained by the 24 h variation.
A cosine-shaped diurnal rhythm of normalized MR-proANP, BNP, and NT-proBNP levels was evident among both lean and obese individuals (p < 0.05 for all). There was a significant phase difference between the MR-proANP (134 min; pacrophase < 0.001) and BNP rhythm (24 min; pacrophase = 0.08) between lean and obese individuals. The differences in the rhythm parameters between lean and obese individuals for the study participants are depicted in Supplemental Table 3.
24-H SYSTOLIC BP AND RELATIONSHIP WITH NP LEVELS.
The 24-h, daytime, and nighttime average systolic BPs in the study population were 114 mm Hg (IQR: 109 to 127 mm Hg), 118 mm Hg (IQR: 109 to 127 mm Hg), and 111 mm Hg (IQR: 103 to 119 mm Hg), respectively. The 24-h average systolic BP was relatively lower in lean (110 mm Hg [IQR: 99 to 120 mm Hg]) compared with obese (118 mm Hg [IQR: 110 to 122 mm Hg]) individuals (p = 0.09). The nighttime systolic BP was higher among obese individuals (114 mm Hg [IQR: 107 to 125 mm Hg] vs. 112 mm Hg [IQR: 101 to 123 mm Hg]; p = 0.03). The daytime systolic BP was similar among lean and obese individuals (115 mm Hg [IQR: 105 to 125 mm Hg] vs. 120 mm Hg [IQR: 112 to 128 mm Hg]; p = 0.41).
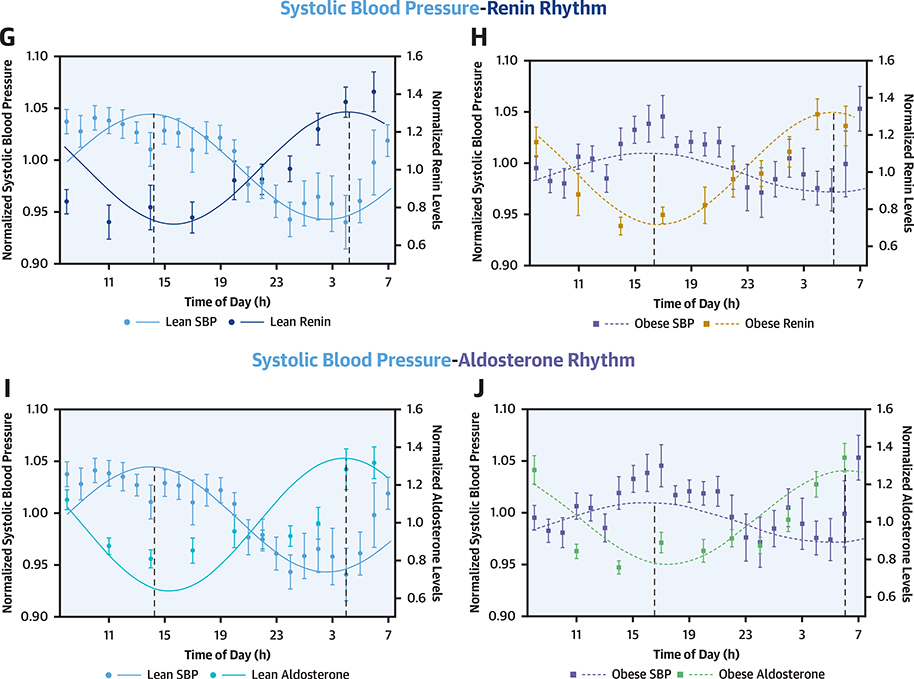
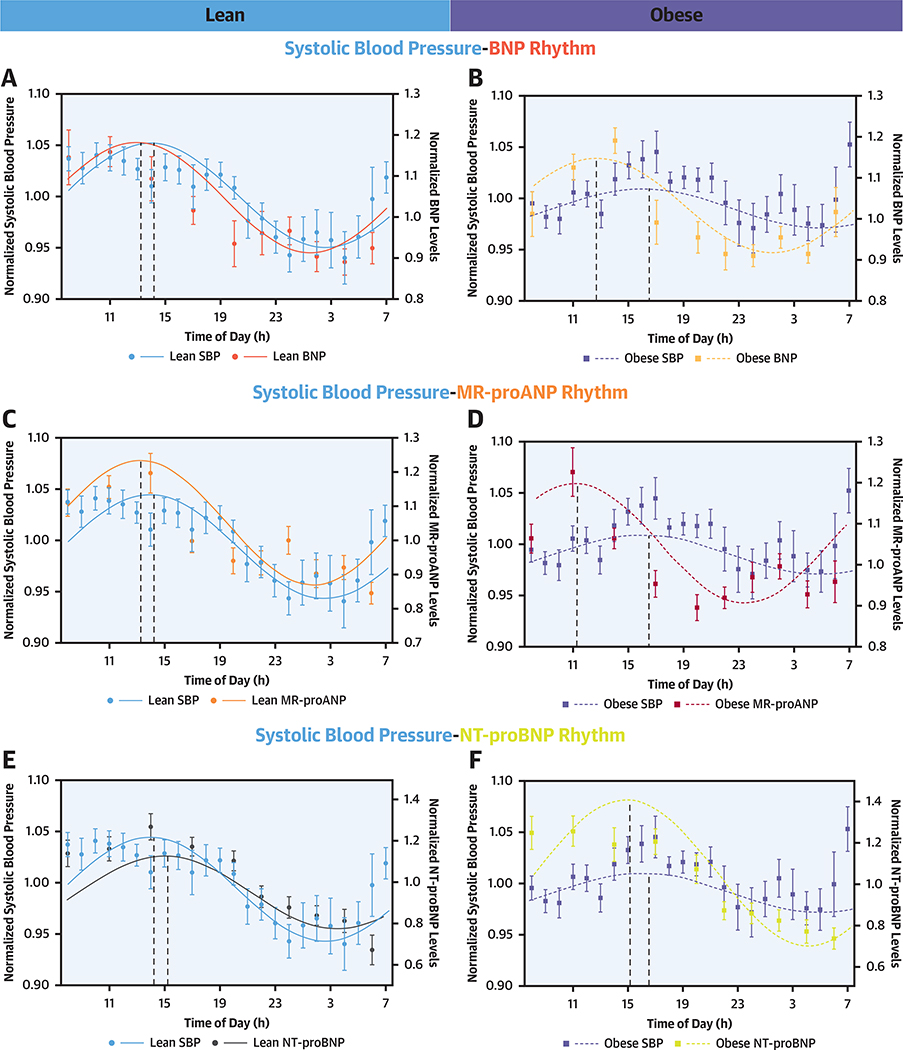
There was significant cosine-shaped variation in the normalized systolic BP over 24 h in the overall study population (p = 0.002) and lean individuals (p = 0.008) but not in obese individuals (p = 0.29) (Supplemental Table 3). The acrophase of the systolic BP rhythm was noted at 3:10 PM (95% CI: 1:30 PM to 5:18 PM), 2:38 PM (95% CI: 1:06 PM to 4:54 PM), and 4:36 PM (95% CI: 2:06 PM to 7:00 PM) in the overall, lean, and obese study populations, respectively (Supplemental Table 2). There was an acrophase delay of ~2 h between lean and obese individuals (pacrophase <0.001). The acrophase difference between the normalized MR-proANP and systolic BP rhythm was −4.9 h in obese and −0.7 h in lean individuals (Figure 2). There was a significant phase difference between the overall systolic BP and the BNP and MR-proANP rhythms (pacrophase <0.001 for all), with the peak of NP rhythm preceding the BP rhythm by ~2 h. The acrophase difference between the normalized BNP and systolic BP rhythm was −3.3 h in obese and −0.9 h in lean individuals. The comparison of the BP and NP phases is depicted in Table 4.
FIGURE 2. Relationship of Diurnal Rhythm of Natriuretic Peptides, Renin, and Aldosterone With Systolic Blood Pressure in Lean and Obese.
The solid line depicts the fitted cosinor curve for the data in lean individuals, and the dashed line represents the fitted cosinor curve for the data in obese individuals. The Y1 axis depicts the spread of the normalized systolic blood pressure (SBP) over the 24-h period. The Y2 axis depicts the spread of the B-type natriuretic peptides (BNPs) (A and B), mid-regional pro-atrial natriuretic peptide (MR-proANP) (C and D), N-terminal-pro B-type natriuretic peptide (NT-proBNP) (E and F), renin (G and H), and aldosterone (I and J) in lean and obese individuals. The circles represent the mean value of the parameter at respective time points in lean individuals, and boxes represent the mean value of the parameter at respective time points in obese individuals. The error bars represent the standard error of mean (SEM).
TABLE 4.
Relationship of Natriuretic Peptide, Renin, and Aldosterone Rhythm With Systolic Blood Pressure Rhythm
| Acrophase Relationship With Systolic Blood Pressure |
|||
|---|---|---|---|
| Overall | Lean | Obese | |
| MR-proANP | |||
| Phase difference, radians | −0.55 | −0.18 | −1.27 |
| Phase difference, h | −2.1 | −0.7 | −4.9 |
| p value | <0.001 | 0.02 | <0.001 |
| BNP | |||
| Phase difference, radians | −0.39 | −0.23 | −0.86 |
| Phase difference, h | −1.5 | −0.9 | −3.3 |
| p value | <0.001 | 0.02 | <0.001 |
| NT-proBNP | |||
| Phase difference, radians | −0.08 | −0.30 | −0.40 |
| Phase difference, h | −0.3 | −1.1 | −1.5 |
| p value | 0.23 | 0.06 | 0.09 |
| Renin | |||
| Phase difference, radians | 3.63 | 3.62 | 3.32 |
| Phase difference, h | 13.9 | 13.8 | 12.7 |
| p value | 0.03 | 0.04 | 0.18 |
| Aldosterone | |||
| Phase difference, radians | 4.04 | 3.5 | 3.51 |
| Phase difference, h | 15.4 | 13.4 | 13.4 |
| p value | <0.001 | 0.02 | 0.01 |
Phase difference calculated as: acrophase of SBP – acrophase of analyte.
Abbreviations as in Table 1.
DIURNAL RHYTHM OF RENIN AND ALDOSTERONE.
The median range and range spread of renin over 24 h were 10.6 pg/ml (IQR: 5.4 to 23.7 pg/ml) and 253.4% (IQR: 130.2% to 435.3%), respectively. The median range and range spread of aldosterone over 24-hs were 6.4 ng/l (IQR: 3.2 to 8.3) ng/l and 270.5% (IQR: 146.2% to 415.2%), respectively (Supplemental Table 4). The 24-h mean renin levels were relatively lower among obese (13.3 pg/ml [IQR: 5.6 to 22.9 pg/ml]) compared with lean individuals (6.9 pg/ml [IQR: 3.4 to 11.7 pg/ml) (adjusted p = 0.06) (Supplemental Table 5). The 24-h mean aldosterone levels were similar between lean (5.0 ng/l [IQR: 3.7 to 7.7 ng/l]) and obese individuals (7.1 ng/l [IQR: 6.3 to 8.6 ng/l]) (adjusted p = 0.99). There was a significant cosine-shaped diurnal rhythm of normalized renin and aldosterone levels in the study population, and this was seen in both lean and obese individuals (p for rhythmicity <0.05) (Central Illustration). The acrophase of normalized renin and aldosterone rhythm were observed at 4:55 AM (95% CI: 4:06 AM to 05:54 AM) and 6:26 AM (95% CI: 5:30 AM to 07:36 AM), respectively. There was an acrophase delay of nearly 2 h (6:10 AM vs. 4:00 AM) in obese individuals compared with lean individuals (pacrophase = 0.02) (Supplemental Table 3). The diurnal rhythm of renin and aldosterone levels was in antiphase with the BP rhythm in the overall population and both lean and obese individuals (pacrophase < 0.001 for all) (Table 4).
DIURNAL VARIATION IN URINARY SODIUM AND POTASSIUM.
The total, daytime, and nighttime urinary volume was similar between lean and obese individuals (p > 0.05 for all) (Table 5). The total urinary sodium (392 mmol/24 h [IQR: 275 to 476 mmol/24 h] vs. 403 mmol/24 h [IQR: 290 to 506 mmol/24 h]) and total potassium excretion (79 mmol/24 h [IQR: 50 to 132 mmol/24 h] vs. 82 mmol/24 h [IQR: 64 to 122 mmol/24 h]) was similar between lean and obese individuals (p > 0.05 for both). The daytime excretion of urinary sodium and potassium were similar between lean and obese individuals (p > 0.05 for both). The nocturnal sodium excretion was 189 mmol (IQR: 152 to 214 mmol) among obese individuals and 144 mmol (IQR: 89 to 200 mmol) in lean individuals (adjusted p = 0.07). The nocturnal potassium excretion in the obese was 39 mmol (IQR: 27 to 53 mmol) and among lean individuals was 29 mmol (IQR: 21 to 42 mmol) (adjusted p = 0.06).
TABLE 5.
Differences in Timed Urinary Parameters Between Lean and Obese Individuals
| p Value |
|||||
|---|---|---|---|---|---|
| Overall | Lean | Obese | Unadjusted | Adjusted* | |
| Urinary volume | |||||
| 24-h total urinary volume, l | 2.70 (1.85–3.00) | 2.70 (2.20–2.88) | 2.64 (1.70–3.30) | 0.73 | 0.62 |
| Daytime urinary volume, l | 1.75 (1.20–2.30) | 1.75 (1.33–2.20) | 1.53 (1.05–2.30) | 0.77 | 0.84 |
| Nighttime urinary volume, l | 0.81 (0.55–1.18) | 0.69 (0.55–1.03) | 0.91 (0.60–1.19) | 0.28 | 0.16 |
| Urinary sodium | |||||
| 24-h total urinary sodium, mmol | 394 (283–502) | 392 (275–476) | 403 (290–506) | 0.90 | 0.92 |
| Daytime urinary sodium, mmol | 190 (134–343) | 190 (137–394) | 192 (131–279) | 0.34 | 0.33 |
| Nighttime urinary sodium, mmol | 179 (105–200) | 144 (89–200) | 189 (152–214) | 0.053 | 0.07 |
| Urinary potassium | |||||
| 24-h total urinary potassium, mmol | 79 (60–131) | 79 (50–132) | 82 (64–122) | 0.78 | 0.31 |
| Daytime urinary potassium, mmol | 43 (31–92) | 41 (31–102) | 45 (33–69) | 0.97 | 0.74 |
| Nighttime urinary potassium, mmol | 35 (24–48) | 29 (21–42) | 39 (27–53) | 0.10 | 0.06 |
Values are median (interquartile range).
Adjusted for age, sex, and race.
DISCUSSION
In this physiological clinical trial, we observed a diurnal rhythm of MR-proANP, BNP, and NT-proBNP levels. This rhythmic oscillation of NPs was present in the overall cohort and among lean and obese individuals, with NP levels being highest in the daytime and lowest at night. Healthy obese individuals have approximately 26% lower MR-proANP, 32% lower BNP, and 49% lower NT-proBNP levels over a 24-h period. These differences persist in the daytime and nighttime periods. The diurnal rhythm of NPs tracks closely with the diurnal rhythm of systolic BP and precedes by ~2 h. There was a larger time difference (~3 to 5 h) between NP and BP rhythm peaks among obese individuals indicating an NP-BP rhythm misalignment. We also observed a 24-h rhythm for normalized renin and aldosterone levels, with a median range spread of ~253% and ~271%, respectively. The renin and aldosterone rhythms were in antiphase with the NP rhythms with the highest levels in the early mornings. The renin levels were 99% lower among obese individuals during the 24-h period.
The diurnal rhythm in NPs has been seen previously in animal models, and inconclusive evidence of the diurnal NP rhythm exists from small human studies (5–9). Prior investigations evaluating the chronobiology of NPs were limited because they had a small population size, were not racially diverse, excluded women, and did not involve individuals with obesity, which is a well-recognized putative “NP deficiency” state (5–7). Additionally, earlier investigations of the NP rhythms have been on a random salt intake background and did not involve assessing the NP gene expression and estimation of timed-urinary sodium excretion in humans (5–7). This is the largest physiological study evaluating the chronobiology of NPs and the influence of obesity on this rhythm in the setting of standardized salt intake using a human model to understand the implication of this physiological system on the development of high-risk diurnal BP rhythm. We confirm the existence of a diurnal rhythm of NPs in both lean and obese individuals. The diurnal variation in NPPA expression from whole blood tracked well with the diurnal variation in MR-proANP levels in our study. However, whether the rhythm is centrally or peripherally regulated requires further investigation in larger studies incorporating measures of the central circadian rhythm. Furthermore, longer inpatient studies (7 days) with more frequent NP assessment and assessment of central and peripheral clock gene expressions may yield greater insights into the circadian rhythm of this cardiac-derived hormone system. The large intra-individual biological variations in the BNP and NT-proBNP levels of up to ~169% and 92%, respectively, over 24 h have been noted in heart failure patients previously (8,39). The large degree of biological variance in the NT-proBNP, BNP, and MR-proANP during the 24-h period identified in our study has important clinical implications. BNP and NT-proBNP are surrogate biomarkers for diagnosis and prognosis in heart failure and are often used to titrate guideline-directed medical therapy (40–42). Our study highlights the need for a formal investigation of the need to account for the “time” of the day in addition to the putative NP deficiency states (obesity or Black individuals) while making clinical assessments among heart failure patients using the NP levels (14,40–43). Analogous to cortisol, formal cut-off values of NPs inclusive of the timing of blood draw may need to be established while ruling out heart failure based on the currently accepted thresholds for heart failure (14,40–43).
Prior studies indicate that increased receptor-mediated clearance in adipose tissue may be responsible for the relative NP deficiency in obese noted in our study (44,45). Additionally, transcriptional regulators of NP expression in the peripheral blood may also contribute to the putative NP deficiency in peripheral NP levels (46). Future investigations incorporating these measures in humans may help elicit the underlying mechanisms responsible for NP deficiency in obese individuals. Consistent with prior published data from healthy individuals with different NP levels, the difference in endogenous NP levels between lean and obese did not result in a difference in the total urinary sodium excretion on a standardized salt background. This may be due to the tight regulation of the sodium homeostasis in healthy individuals, lack of established relationship of endogenous NP differences and urinary sodium excretion, and salt-standardized diet taken by study participants.
We observed an intimate relationship between NP and BP levels in the study population overall and stratified by obesity status. The larger phase difference of ~3 to 5 h between the MR-proANP, BNP, and BP rhythms, and the lower NP levels in obese contributes to the higher nocturnal BP and the nocturnal nondipping of BP seen commonly among obese individuals. The renin and aldosterone levels were in antiphase with the NP and BP rhythm. This indicates the existence of an NP-RAAS-BP rhythm axis. Our findings suggest that obese individuals have attenuated NP levels throughout the 24 h, which could impair their ability to vasodilate under normal physiological conditions. The NP rhythmicity was intact in healthy lean and obese individuals. However, there was a phase difference of nearly 2 h between the peak (acrophase) of the MR-proANP rhythms between lean and obese individuals. The phase differences in the NP rhythmand the disrupted NP-BP rhythm relationship in the obese indicate that there is a misalignment of the NP-BP chronobiology in obese individuals. The leftward shift of the NP rhythm in the obese may contribute to the pathophysiological basis of the 24-h systolic BP rhythm pattern observed among obese individuals. Further investigation is needed to understand the clinical implications of these phase differences.
There are several physiological and clinical implications of this investigation. The intricate NP-RAAS-BP rhythm axis identified in our study may help us understand the pathophysiology behind nocturnal nondipping and other high-risk nocturnal BP phenotypes that are associated with adverse cardiovascular events (27). Our study identifies a plausible mechanistic pathway that may be targeted using chronopharmacology to counter the disturbances in the diurnal BP rhythm. NP chronotherapy with nocturnal NP augmentation may prevent the nocturnal surge of BP. Safe and efficacious NP augmenting treatment options (through neprilysin inhibition) (47) have been approved for use in heart failure and have also been shown to cause a significant reduction in 24-h mean BP (48). Future investigation may build upon the evidence from our study to assess a physiologically driven precision medicine approach through chronopharmacological perturbation of the NP-RAAS-BP rhythm axis.
STUDY LIMITATIONS.
This is a single-center study with a relatively small sample size of healthy young adults. The protocol’s complexity, use of a standardized diet prior to inpatient visit, plausible physiological influences of sleep patterns, frequent interruptions, and stress from the repeated blood draw impacting the diurnal rhythm are other limitations worth noting. Although the protocol was complex, it was standardized and rigorously implemented, which allowed us to reduce inherent variations that occur when NP levels are measured on a random salt intake background. The stringent protocol also allowed us to provide a relatively accurate simultaneous assessment of the numerous physiological measures in the study participants. Although BMI is a validated tool to assess cardiometabolic disease risk (49) and mortality (50), magnetic resonance imaging has been recognized as an ideal means to characterize adiposity (51). Due to budgetary constraints and high subject-burden, we were not able to analyze the adiposity using imaging studies. Although the study excluded those who were shift-workers or had sleep or psychiatric disorders, the study did not account for circadian rhythm confounders such as sleep quality, sleep duration, and diurnal activity profile (28). In the absence of detailed sleep studies, we cannot rule out the possibility of some degree of sleep disorders such as sleep apnea in our study population. There was a poor expression of the NPPB gene in the PAXGene specimens that prevented us from assessing the diurnal variation in peripheral NPPB gene expression. Our study also excluded overweight individuals (BMI 25 to 30 kg/m2) as it may be considered as an intermediate phenotype. We were unable to measure mature ANP in the study participants due to the known analytical issues with the manual assay, and have hence used an automated assay-based surrogate analyte (MR-proANP) instead (52,53). The generalizability of our findings warrants replication of the observed results in a cohort of patients with hypertension or cardiovascular diseases such as heart failure, including individuals from across the BMI range, across the spectrum of age (by including children and adolescents), and those from different ethnicities such as Asians, Hispanics, and so on. The impact of sleep disturbances, meal timing, and activity on the chronobiology of the NP-RAAS-BP axis needs further evaluation. A larger diverse sample size with a racially balanced population would help in understanding the physiological and clinical implications of the disturbances in the NP-RAAS-BP diurnal rhythm axis.
CONCLUSIONS
There exists a diurnal rhythm of the NP hormones, which tracks with the BP rhythm, and this is evident in both lean and obese individuals. The misalignment of the NP-BP rhythm may contribute to the nondipping nocturnal BP pattern seen among the obese. This study elucidates the chronobiology of the NP- RAAS-BP axis in healthy lean and obese individuals.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
There is a wide variation in circulating NP hormone levels over 24 h. Diurnal variation in NP hormone levels tracks closely with variation in BP.
TRANSLATIONAL OUTLOOK:
The interactive variations in NP, renin-angiotensin-aldosterone system activity, and BP opens avenues for pharmacological interventions to improve clinical outcomes, particularly among obese patients.
Acknowledgments
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This research was partly supported by the National Center for Advancing Translational Research of the National Institutes of Health under award number UL1TR001417 to the University of Alabama at Birmingham Center for Clinical and Translational Science. Dr. Margulies has received research grant support from Sanofi-Aventis, Merck, Sharp, and Dohme, and GlaxoSmithKline; and has consulted for MyoKardia, Pfizer, and Luitpold Pharmaceuticals. Drs. Wang and P. Arora are named as coinventors on a patent application relating to the use of miRNAs for the treatment of hypertension and other disorders. Dr. P. Arora is supported by the National Institutes of Health Mentored Patient-Oriented Research Award 5K23HL146887–02. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- BNP
B-type natriuretic peptides
- HTN
hypertension
- MR-proANP
mid-regional pro-atrial natriuretic peptides
- NT-proBNP
N-terminal pro-B-type-natriuretic peptides
- RAAS
renin-angiotensin-aldosterone System
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For an expanded Methods section as well as supplemental tables and figures, please see the online version of this paper.
REFERENCES
- 1.Melo LG, Steinhelper ME, Pang SC, Tse Y, Ackermann U. ANP in regulation of arterial pressure and fluid-electrolyte balance: lessons from genetic mouse models. Physiol Genomics 2000;3: 45–58. [DOI] [PubMed] [Google Scholar]
- 2.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sciences 1981;28: 89–94. [DOI] [PubMed] [Google Scholar]
- 3.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature 1988;332:78–81. [DOI] [PubMed] [Google Scholar]
- 4.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med 1998;339:321–8. [DOI] [PubMed] [Google Scholar]
- 5.Portaluppi F, Montanari L, Bagni B, degli Uberti E, Trasforini G, Margutti A. Circadian rhythms of atrial natriuretic peptide, blood pressure and heart rate in normal subjects. Cardiology 1989;76:428–32. [DOI] [PubMed] [Google Scholar]
- 6.Portaluppi F, Bagni B, degli Uberti E, et al. Circadian rhythms of atrial natriuretic peptide, renin, aldosterone, cortisol, blood pressure and heart rate in normal and hypertensive subjects. J Hypertens 1990;8:85–95. [DOI] [PubMed] [Google Scholar]
- 7.Goetze JP, Jorgensen HL, Sennels HP, Fahrenkrug J. Diurnal plasma concentrations of natriuretic propeptides in healthy young males. Clin Chem 2012;58:789–92. [DOI] [PubMed] [Google Scholar]
- 8.Crnko S, Printezi MI, Jansen TPJ, et al. Prognostic biomarker soluble ST2 exhibits diurnal variation in chronic heart failure patients. ESC Heart Fail 2020;7:1224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goetze JP, Georg B, Jorgensen HL, Fahrenkrug J. Chamber-dependent circadian expression of cardiac natriuretic peptides. Regul Pept 2010;160:140–5. [DOI] [PubMed] [Google Scholar]
- 10.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594–600. [DOI] [PubMed] [Google Scholar]
- 11.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation 2005;112:2163–8. [DOI] [PubMed] [Google Scholar]
- 12.Krauser DG, Lloyd-Jones DM, Chae CU, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J 2005;149:744–50. [DOI] [PubMed] [Google Scholar]
- 13.Arora P, Wu C, Khan AM, et al. Atrial natriuretic peptide is negatively regulated by microRNA-425. J Clin Invest 2013;123:3378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel N, Russell GK, Musunuru K, et al. Race, natriuretic peptides, and high-carbohydrate challenge: a clinical trial. Circ Res 2019;125:957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton-Cheh C, Larson MG, Vasan RS, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet 2009;41:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton-Cheh C, Johnson T, Gateva V, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009;41:666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalra R, Parcha V, Patel N, et al. Increased awareness, inadequate treatment, and poor control of cardiovascular risk factors in American young adults: 2005–2016. Eur J Prev Cardiol 20202047487320905190. [DOI] [PubMed] [Google Scholar]
- 18.Patel N, Kalra R, Bhargava A, Arora G, Arora P. Ideal cardiovascular health among american adults after the economic recession of 2008–2009: insights from NHANES. Am J Med 2019;132: 1182–90.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in us youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 2018;319: 1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parcha V, Patel N, Kalra R, Arora G, Arora P. Prevalence, awareness, treatment, and poor control of hypertension among young american adults: race-stratified analysis of the National Health and Nutrition Examination Survey. Mayo Clin Proc 2020;95:1390–403. [DOI] [PubMed] [Google Scholar]
- 21.Kotsis V, Stabouli S, Bouldin M, Low A, Toumanidis S, Zakopoulos N. Impact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension 2005;45:602–7. [DOI] [PubMed] [Google Scholar]
- 22.Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008;51:55–61. [DOI] [PubMed] [Google Scholar]
- 23.Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007;370:1219–29. [DOI] [PubMed] [Google Scholar]
- 24.Salles GF, Reboldi G, Fagard RH, et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC-H) meta-analysis. Hypertension 2016;67:693–700. [DOI] [PubMed] [Google Scholar]
- 25.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int 2010;27:1629–51. [DOI] [PubMed] [Google Scholar]
- 26.Kario K Nocturnal hypertension: new technology and evidence. Hypertension 2018;71: 997–1009. [DOI] [PubMed] [Google Scholar]
- 27.Parcha V, Kalra R, Li P, Oparil S, Arora G, Arora P. Nocturnal blood pressure dipping in treated hypertensives: insights from the SPRINT trial. Eur Jor Prev Cardiol 2020. November 19 [E-pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016;149:631–8. [DOI] [PubMed] [Google Scholar]
- 29.de Greeff A, Shennan AH. Validation of the Spacelabs 90227 OnTrak device according to the European and British Hypertension Societies as well as the American protocols. Blood Press Monit 2020;25:110–4. [DOI] [PubMed] [Google Scholar]
- 30.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005;45:142–61. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien E, Coats A, Owens P, et al. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British hypertension society. BMJ 2000;320:1128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hyperten 2003;21:821–48. [DOI] [PubMed] [Google Scholar]
- 33.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354:2368–74. [DOI] [PubMed] [Google Scholar]
- 34.Melander O, Newton-Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA 2009;302:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arora P, Wu C, Hamid T, et al. Acute metabolic influences on the natriuretic peptide system in humans. J Am Coll Cardiol 2016;67:804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornelissen G Cosinor-based rhythmometry. Theor Biol Med Model 2014;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 2007;38:275–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia 1982;9:397–439. [PubMed] [Google Scholar]
- 39.Wu AH, Smith A, Wieczorek S, et al. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol 2003;92:628–31. [DOI] [PubMed] [Google Scholar]
- 40.Chow SL, Maisel AS, Anand I, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation 2017;135:e1054–91. [DOI] [PubMed] [Google Scholar]
- 41.Parcha V, Patel N, Kalra R, et al. Racial differences in serial NT-proBNP levels in heart failure management: insights from the GUIDE-IT trial. Circulation 2020;142:1018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Januzzi JL Jr., Ahmad T, Mulder H, et al. Natriuretic peptide response and outcomes in chronic heart failure with reduced ejection fraction. J Am Coll Cardiol 2019;74:1205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel N, Gutierrez OM, Arora G, et al. Race-based demographic, anthropometric and clinical correlates of N-terminal-pro B-type natriuretic peptide. Int J Cardiol 2019;286:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovacova Z, Tharp WG, Liu D, et al. Adipose tissue natriuretic peptide receptor expression is related to insulin sensitivity in obesity and diabetes. Obesity (Silver Spring) 2016;24:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu W, Shi F, Liu D, et al. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci Signal 2017;10:eaam6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parcha V, Arora P. Glycosylation of natriuretic peptides in obese heart failure: mechanistic insights. Ann Transl Med 2019;7:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMurray JJ, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 48.Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010;375:1255–66. [DOI] [PubMed] [Google Scholar]
- 49.Lyall DM, Celis-Morales C, Ward J, et al. Association of body mass index with cardiometabolic disease in the UK Biobank: a Mendelian randomization study. JAMA Cardiol 2017;2:882–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373: 1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obesity 2008;32:959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semenov AG, Katrukha AG. Analytical issues with natriuretic peptides - has this been overly simplified? EJIFCC 2016;27:189–207. [PMC free article] [PubMed] [Google Scholar]
- 53.Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem 2004;50:234–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.