Abstract
Aims
Gastroenteritis is a digestive disorder among children with symptoms of abdominal cramps, diarrhoea, and vomiting. This study aimed to determine the prevalence of gastroenteritis in children in the Bannu district in 2019, and also contributed for adopting preventive measures to reduce mortality in children.
Subject and methods
A retrospective study was conducted to determine the prevalence of gastroenteritis in children in Bannu. The data were collected from official registers of admission maintained in the children wards in the Women and Children Hospital, Bannu. Patients with symptoms of gastroenteritis at the outpatient department were admitted to one of the children wards.
Results
Overall, 1456 children—897 (61.4%) males and 559 (38.6%) females—suffered from gastroenteritis during the study period. The age group ≤6 months demonstrated the highest share (37.8%), followed by >6 m ≤ 1y (35.5%), >1y ≤ 2y (15.8%), >2y ≤ 5y (7.3%), >5 ≤ 10y (3.2%), and > 10y ≤ 15y (0.4%). Overall, 89.1% of cases were from children ≤2 years old and 96.4% of cases were attributed to children ≤ 5 years of age. April demonstrated the highest percentage of prevalence of 17.7, followed by May (13.5%), November (13%), June (11.7%), September (10.4%), October (9.8%), July (9.5%), August (8.4%), March (3.4%), and February (2.6%). The age group ≤6 m was the dominant group during February through May and was replaced by the age group >6 m ≤ 1y during August through November.
Conclusion
Gastroenteritis showed a reduced prevalence when shifted from lower to higher age groups. Being male and age group ≤6 m showed the highest prevalence of gastroenteritis with the peak of disease in April. Further research is needed to determine the cause-based prevalence of different gastroenteritis cases in the study area.
Keywords: Prevalence, Gastroenteritis, Children, Bannu, Age group
Introduction
Gastroenteritis is an infectious disease and inflammation of the mucus membrane of the gastrointestinal tract, including the stomach and intestines (Jones et al. 2007; Chow et al. 2010; Schlossberg 2015). Gastroenteritis in children has three main causes: bacterial, viral, and parasitic agents (Johargy et al. 2010). The viruses include rotavirus, norovirus and coronavirus; bacteria include E. coli, Salmonella, Shigella, and Vibrio; parasites include Giardia lamblia and Entamoeba histolytica; and chemicals such as toxins (Elliott 2007; Chow et al. 2010). However, Shigella is the most common cause of bloody diarrhea in young children in developing countries (WHO 1994; Ozmert et al. 2011). Poor sanitation and inadequate personal hygiene are the main sources for spreading the intestinal parasites responsible for gastroenteritis, which are widely prevalent in developing countries (Ahmed et al. 2012).
The disease is characterized by vomiting and diarrhea (Chow et al. 2010; Eckardt and Baumgart 2011), nausea, stomach pain, fever, headache, loss of appetite and weight, and sweating (Jones 2003; Johargy et al. 2010). It is a water- and food-borne infection. The disease is spread through eating contaminated food, including improperly prepared food, or drinking contaminated water and other liquids, sharing personal objects/close contact with a person who is infected, and handling pets and other animals (Ciccarelli et al. 2013). Gastroenteritis is one of the most common illnesses in humans worldwide (Johargy et al. 2010; Okitsu-Negishi et al. 2004). The disease is the major/leading cause of mortality globally, particularly in children under 5 years of age (King et al. 2003; Okitsu-Negishi et al. 2004; Zamir et al. 2006; Onyon and Dawson 2018).
Approximately 70% of episodes of acute gastroenteritis in children are due to a virus (Webb and Starr 2005; Chow et al. 2010), and rotavirus is the most common virus causing the disease (Chow et al. 2010; Afzal et al. 2010). Both rotavirus and norovirus are dominant among viral causes of acute gastroenteritis. Bacterial infection accounts for 10 to 20% of all the acute gastroenteritis (Elliott 2007; Chow et al. 2010). Acute gastroenteritis is more prevalent in children than adults (Jeffs et al. 2019). The disease is responsible for millions of deaths each year in pediatric patients, particularly in children under 5 years old worldwide (Elliott 2007; Black et al. 2010; Pieścik-Lech et al. 2013; Sultan and Hassan 2018), because of severe dehydration and loss of minerals from the body as a result of rapid-onset diarrhea (Zaidi and Smith-Morris 2015). Rotavirus gastroenteritis is the leading cause of severe acute gastroenteritis in children worldwide and is associated with high hospitalization and mortality rates in children younger than 5 years of age (Choi et al. 2013). In 2015, there were two billion cases of gastroenteritis, resulting in 1.3 million deaths globally (GBD 2015).
Africa and South Asia had most of the diarrheal infections that led to a total 10% mortality in children (800,000 mortalities) worldwide (Liu et al. 2012; Alam et al. 2015). South Asia demonstrated 32% annual mortality out of an annual 7.6 million deaths globally because of diarrhea in children (You et al. 2010; Carvajal-Velez et al. 2016; Sohail and Neupane 2019). It is the second leading killer disease in low-income countries, including Pakistan, among infants and children <5 years (Black et al. 2010; Zaidi and Smith-Morris 2015). Pakistan demonstrated the highest rates of under-five children deaths among the 18 countries of the Eastern Mediterranean region, accounting for 464,886 annual deaths (Black et al. 2010; Alam et al. 2015). Rotavirus is the major cause of high disease burden of gastroenteric infections in Pakistan (Alam et al. 2013), and the country ranks fifth among the 15 developing countries that account for 73% of all diarrheal mortality in children less than 5 years of age globally (Boschi-Pinto et al. 2008). Treatment of the disease involves taking oral rehydration solution (oral rehydration therapy) and/or injecting intravenous fluids (intravenous fluid therapy). This is the first study undertaken in Khyber Pakhtunkhwa particularly in its southern part comprising Kohat, Karak, Bannu, Lakki Marwat, Dera Ismail Khan, and Tank districts. The aim of this study was to determine the prevalence of gastroenteritis/acute gastroenteritis in children in Bannu during February 2019 through November 2019 and to contribute to the policy makers for their future planning of healthcare policies, creating awareness about the disease in the general population, and in adopting preventive measures to reduce child mortality because of gastroenteritis in Bannu.
Materials and methods
Patient diagnosis and hospitalization
The patients visiting the Women and Children Hospital, Bannu undergo clinical diagnosis of gastroenteritis/acute gastroenteritis based on a person’s signs and symptoms (Eckardt and Baumgart 2011), including diarrhea, nausea, vomiting, and abdominal cramps. Those found with the symptoms are admitted to either child ward A or B. Patients admitted into the ward had their personal information recorded, including name, sex, age, and home address in the official registers maintained by the ward in-charge.
Data collection and data management
The data were collected from the official admission registers of both children A and B wards maintained by the ward in-charges. The register contains the patient name, gender, age, and home address of all 1456 children admitted, comprising 897 males and 559 females. The register data was in unconsolidated form, as monthly consolidated reports were not prepared. I prepared the consolidated report for each month for the study period. The data were classified into six age groups of children: ≤6 m, >6 m ≤ 1y, >1y ≤ 2y, >2y ≤ 5y, >5 ≤ 10y, and > 10y ≤ 15y.
Statistical analysis
The data were analyzed using Pearson’s chi-squared tests and Fisher’s exact tests (p = 0.05), followed by the post hoc test for pairwise comparisons. The chi-squared tests and Fisher’s exact tests were conducted to know significance of gender by months/age by months.
Results
The chi-squared tests (X2 = 9.268, df = 9, p value = 0.4129) and Fisher’s exact tests (p value = 0.4118) showed gender by months were not significant. The chi-squared tests for age by months demonstrated (X2 = 59.352, df = 45, p value = 0.0742) not significant. Nevertheless, the Fisher’s exact tests for age by months was (borderline) significant: p = 0.04798. However, none of the post hoc pairwise comparisons were significant.
Gender wise distribution of gastroenteritis
The male children showed higher prevalence of overall gastroenteritis than female children in all months of the study period (Table 1); males accounted for 897 (61.6%) cases out of 1456 cases of overall gastroenteritis. While acute gastroenteritis showed seven out of nine months with males as the dominant group, and overall, males accounted for 112 (60.2%) cases out of the 186 cases of acute gastroenteritis (Table 2).
Table 1.
Prevalence of gastroenteritis (including acute gastroenteritis) in children in Bannu district in 2019
| Mon | M | F | Age groups (months or years) | T | % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤6 m | >6 m ≤ 1y | >1y ≤ 2y | >2y ≤ 5y | >5 ≤ 10y | >10y ≤ 15y | |||||
| Febru | 25 | 13 | 19 | 9 | 6 | 4 | 0 | 0 | 38 | 2.6 |
| March | 32 | 18 | 22 | 15 | 8 | 4 | 1 | 0 | 50 | 3.4 |
| April | 166 | 92 | 119 | 77 | 39 | 16 | 6 | 1 | 258 | 17.7 |
| May | 127 | 70 | 83 | 68 | 24 | 14 | 7 | 1 | 197 | 13.5 |
| June | 106 | 65 | 67 | 69 | 26 | 6 | 2 | 1 | 171 | 11.7 |
| July | 88 | 50 | 53 | 48 | 17 | 15 | 4 | 1 | 138 | 9.5 |
| Augus | 79 | 43 | 38 | 43 | 22 | 12 | 7 | 0 | 122 | 8.4 |
| Septe | 85 | 66 | 43 | 60 | 29 | 11 | 8 | 0 | 151 | 10.4 |
| Octobe | 75 | 67 | 42 | 52 | 29 | 8 | 9 | 2 | 142 | 9.8 |
| Novem | 114 | 75 | 64 | 76 | 30 | 16 | 3 | 0 | 189 | 13 |
| Total | 897 | 559 | 550 | 517 | 230 | 106 | 47 | 6 | 1456 | 100 |
| % | 61.6 | 38.4 | 37.8 | 35.5 | 15.8 | 7.3 | 3.2 | 0.4 | 100 | – |
M, male, F, female, T, total children, m, month, y, year. Both Pearson’s chi-squared tests and Fisher’s exact tests (p = 0.05) did not showed significant difference in age by month. Nevertheless, the Fisher’s exact tests for age by months was (borderline) significant (p = 0.04798)
Table 2.
Prevalence of acute gastroenteritis (age) in children in Bannu district in 2019
| Mon | M | F | Age groups (months or years) | T | % | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤6 m | >6 m ≤ 1y | >1y ≤ 2y | >2y ≤ 5y | >5 ≤ 10y | >10y ≤ 15y | |||||
| March | 8 | 7 | 4 | 5 | 3 | 1 | 2 | 0 | 15 | 8.1 |
| April | 9 | 3 | 9 | 1 | 1 | 0 | 1 | 0 | 12 | 6.5 |
| May | 2 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 3 | 1.6 |
| June | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 1.1 |
| July | 11 | 8 | 5 | 5 | 1 | 2 | 1 | 0 | 19 | 10.2 |
| Augus | 16 | 6 | 8 | 7 | 3 | 2 | 2 | 0 | 22 | 11.8 |
| Septe | 3 | 6 | 1 | 3 | 4 | 0 | 1 | 0 | 9 | 4.8 |
| Octobe | 6 | 9 | 6 | 4 | 2 | 3 | 0 | 0 | 15 | 8.1 |
| Novem | 55 | 34 | 29 | 28 | 20 | 10 | 2 | 0 | 89 | 47.8 |
| Total | 112 | 74 | 65 | 58 | 35 | 19 | 9 | 0 | 186 | – |
| % | 60.2 | 39.8 | 34.9 | 31.2 | 18.8 | 10.2 | 4.8 | 0 | – | – |
M, male, F, female, T, total children, m, month, y, year
Age wise distribution of gastroenteritis
Age groups play an important role in the overall prevalence of gastroenteritis (both acute and nonacute gastroenteritis) as well as only acute gastroenteritis because the prevalence of the disease steadily decreased (Tables 1 and 2) as the children aged, i.e., when age shifted from ≤6 months toward >10y ≤ 15y. The children of age group ≤6 months are the dominant group with 37.8% overall prevalence of gastroenteritis, while age group >10y ≤ 15y showed the lowest prevalence of the disease at 0.4% (Table 1). Similarly, acute gastroenteritis also showed the same pattern of age wise distribution (Table 2) with the highest in age group ≤6 months (34.9%) and the lowest in age group >10y ≤ 15y (0%).
Month wise/seasonal distribution of gastroenteritis
Monthly/seasonal variation in prevalence exists in overall gastroenteritis as well as acute gastroenteritis (Tables 1 and 2). April (late spring) demonstrated the highest number of cases of overall gastroenteritis (17.7%), and the lowest number of cases 38 (2.6%) were reported in February (late winter) in children (Table 1). Thus, the prevalence of the disease steadily increased from late winter though late spring, followed by steadily decreased from early through late summer, i.e., during May–August. The prevalence of the disease increased once again in November (late autumn/early winter) with 189 cases (13%). Nevertheless, the study reported acute gastroenteritis with a peak in November (89 cases: 47.8%) and demonstrated steadily reduced prevalence from spring through summer, i.e., during March–June (Table 2), followed by a sudden increased prevalence during summer, July–August. March, April, September, and October showed more or less similar distribution of acute gastroenteritis.
Age groups-based month-wise/seasonal comparison of the prevalence of gastroenteritis
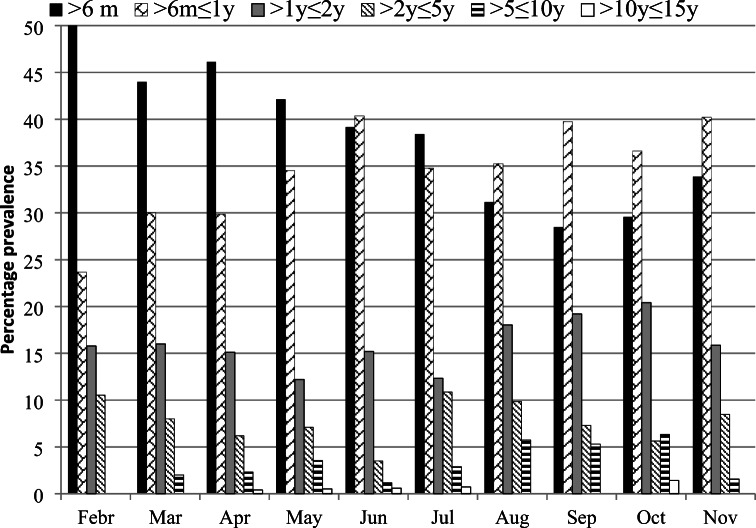
The age group ≤6 m was the dominant group among all the age groups during February through May and in July (Table 1, Fig. 1). While age group >6 m ≤ 1y was the dominant group in June and during August through September. Thus, the disease was more prevalent in the late winter and early spring in children ≤ 6 m old compared to other age groups and similarly age group of >6 m ≤ 1y revealed higher prevalence of the disease during late summer through autumn compared to the remaining age groups. The age group of >1y ≤ 2y showed higher prevalence of gastroenteritis in all months compared to the children of age groups >2y ≤ 15y.
Fig. 1.
Monthly percentage age-wise prevalence of gastroenteritis in children in Bannu from February through November 2019
Discussion
In Pakistan, very little research has been conducted regarding gastroenteritis. Very few studies have explored the viral etiology for gastroenteritis and a majority have focused only on the rotavirus in the country (Iftikhar et al. 2012; Tamim et al. 2013; Alam et al. 2015). Most of the studies conducted in Pakistan, including Khyber Pakhtunkhwa, were based on molecular epidemiology, i.e., prevalence of viruses such as rotavirus causing gastroenteritis.
Thus, literature is not available on the general epidemiology of gastroenteritis/acute gastroenteritis in children in the country, particularly in Khyber Pakhtunkhwa (KP). Therefore, the present study is the first undertaken in the KP to fill the gap in knowledge. Nevertheless, we can compare our results with the prevalence of gastroenteritis in other countries.
The present study found males showed higher prevalence of the disease compared to females. Kazi et al. (2014) collected 6679 stool samples from hospitalized children under five years of age with severe acute watery diarrhea in the cities of Karachi, Lahore, Rawalpindi, and Peshawar and found 58.7% (1196) of the positive results among children for rotavirus were males.
The highest number of cases of acute gastroenteritis were reported in November and the lowest during May–June in the present study. While Saluja et al. (2014) recorded overall 4711 cases of acute severe gastroenteritis with the highest prevalence in May 2012 and the lowest number of cases in April 2011 in India. They also recorded the northern, southern, eastern, and western parts of India reported the highest numbers of cases in June, July, May, and June, respectively, and recorded the lowest number of cases in March, August, April, and November, respectively. The prevalence of gastroenteritis continuing from February to November was supported by Kawai et al. (2012) and Alam et al. (2013), who found rotavirus year-round. Similarly, Bishop (1996) also concluded that rotavirus infections exist throughout the year in tropical areas. The present study demonstrated 89.1% of the overall gastroenteritis prevailed in the children of age ≤ 2 years and is supported by Alam et al. (2013), who recorded higher prevalence of rotavirus among the children hospitalized between 12 and 17 months of age. Furthermore, Qazi et al. (2009) found most of the rotavirus infections among children less than 12 months of age in low income communities of Karachi. This variation might reflect a different study design and target population.
The rate of prevalence of gastroenteritis age-wise showed a steady decrease when age shifted from lower to higher ages. The present study reported 73.3% overall gastroenteritis in the children aged ≤ 12 months, followed by 15.8% in age > 1y ≤ 2y, and 7.3% in age >2y ≤ 5 and was supported by Kazi et al. (2014), who found 60.9% of children with rotavirus were ≤ 12 months old in Pakistan, followed by 26% children between 12 to 23 months, and 13.1% of children between 24 to 59 months. The present study also reported 96.4% prevalence of the disease in children aged ≤5 y and was supported by Wildi-Runge et al. (2009), who also recorded 94% of RV hospitalizations of children <3 years of age.
The current study investigated and found April through June and November to be the peak season of the disease with the highest prevalence in April and was supported by Van Lierde et al. (1989) who found that adenovirus causing gastroenteritis occurred mostly in children less than 3 years old with the peak incidence of virus in April. However, in contrast the peak prevalence of severe rotavirus in Iran was reported in September through January; in China during November through January (Eesteghamati et al. 2009); and in India during December to February (Mullick et al. 2014; Satter et al. 2017). Alam et al. 2015 found that diarrhea in Pakistan is associated with several virus strains: rotavirus contributes the highest (66%) among the enrolled children, followed by parechovirus (21%), norovirus (19.5%), and astrovirus (8.5%). Previous studies demonstrated astrovirus and norovirus prevalence in Pakistan was 11% and 10%, respectively, based on stool samples collected during 1990–1994 (Phan et al. 2004). Similarly, a study conducted by Phan et al. (2004) found norovirus prevalence in children admitted to Civil Hospital, Karachi. In addition, Phan et al. (2004) and Alam et al. (2013) found astrovirus, during the 1990s and in 2013, respectively, focusing on gastroenteritis patients from Karachi and Rawalpindi, respectively. Rotavirus infections accounted for one third of children hospitalized with severe gastroenteritis in urban centers in Pakistan (Nisa and Ahmad 2018). Pakistan’s Extended Program of Immunization (EPI) decided to incorporate rotavirus vaccine as a part of EPI in Pakistan from Nov 2016 (Nisa and Ahmad 2018). Peak age of rotavirus diarrhea in children is 3 months to 2 years (Bass 2004; Afzal et al. 2010).
Anandan et al. (2014) investigated and found that of 626 stool samples from older children and adults (>12 years of age) screened, 8.4% were initially positive for rotavirus with gastroenteritis by antigen detection in southern India. Mullick et al. (2014) found 47.5% cases were positive for rotaviruses. The peak in incidence (70–80%) was reported during the winter season (December–February). The age group of 6–12 months followed by 12–24 months of children revealed the highest rotavirus positivity both for severe or mild diarrhea cases. In New Zealand, Jeffs et al. (2019) found from 1997 to 2015, 56.4% of non-viral gastroenteritis hospitalized children were male aged <15 years.
Johargy et al. (2010) found that of 270 stool samples tested for gastroenteritis from different hospitals of Makkah and Jeddah in Saudi Arabia, 90 (33%) had viral etiology, of which rotavirus type A (serotype G) accounted for 60 (22%) cases, adenovirus in 20 (7%), followed by astrovirus in the remaining 10 (4%) patients. Thirteen (5%) were of bacterial origins of which nine (3%) were Salmonella species, four (2%) were Shigella species, and only three (1%) of the samples were positive for Giardia lamblia. Nevertheless, the present study does not deal with the causes of gastroenteritis as viral or nonviral.
Ahmed et al. (2012) reported that out of 237 examined fecal samples of gastroenteritis patients at District Headquarter Hospital Gilgit laboratory, 60% of patients were males with higher prevalence in the age group (1 ≥ 5 y) as compared to others infected with different protozoan and helminth parasites, including four types of helminth viz., the highest frequency 22.8% (Ascaris lumbricoides), followed by 4.6% (Hymenolepis nana), 2.5% (Trichurus trichiura), 1.7% (Taenia saginata), and two types of protozoan parasites: the highest frequency 19.8% of Giardia lamblia, followed by 2.5% (Entamoeba histolytica). There were 1.7% of mixed infestations of A. lumbricoides and T. trichura, while 0.8% had A. lumbricoides and G. lamblia.
Sadiq et al. (2019) described 500 stool samples of patients with symptoms of acute diarrhea with C. jejuni accounting for 48.2% and campylobacter for 52% of all studied cases. The median age of children with both rotaviruses A (RVA) and C. jejuni infection was 6–11 months. The prevalence of RVA was high in winter months, while C. jejuni was high in summer over a 1-year study period. The overall results have demonstrated the high prevalence of C. jejuni in Rawalpindi and Islamabad (Pakistan) in 2014.
Children particularly in the developing countries are at high risk of the disease (Chow et al. 2010). This may be because parasites and Escherichia coli infections are more prevalent in less developed nations relative to in the industrialized countries, where a virus is the major cause of the disease (Kapikian 1993). Lack of immunity contributes to higher risk of infection in children (Singh 2010), particularly those ≤5 years, and thus they are more vulnerable to the disease. While, the development of immunity contributed to the lower rate of gastroenteritis among the older children or the adults (Eckardt and Baumgart 2011). Gastroenteritis also has non-infectious causes (Singh 2010), and therefore one of the reason of the highest prevalence of the disease in children age ≤ 6 m and the higher concurrence of the disease in males is because of the fact that mothers usually feed their babies, particularly males, with lactose containing food. Thus, males are exposed to the disease more compared to female children. The highest prevalence of the disease in April (Table 1) is because of the pre-monsoon rainfall in Bannu in March. Water becomes contaminated during the rainy season, resulting in the common outbreaks at this time (Webber 2009). Furthermore, hot and humid weather also contributes to the rise of gastroenteritis infection, and hence after March the steady increase in temperature helps bacteria to grow faster and leads to the high prevalence during April through June.
The unawareness/ignorance among parents in Pakistan is the key factor contributing to the high children mortality of those aged less than 5 years. Unsanitary conditions result in fecal to oral transmission such as failure to wash hand before using food and failure to properly dispose of feces (Elliott et al. 1996; Zaidi and Smith-Morris 2015), feeding young ones with improperly boiled milk instead of using mother’s milk, not cleaning/sterilizing bottle before using milk, no proper control of flies (vector), and so on. Acute gastroenteritis is spread via fecal-to-oral transmission (Jorge et al. 2010). Thus, improvement of sanitary conditions, health education, and nutrition contribute to preventing the disease (Bajolet and Chippaux-Hyppolite 1998). The treatment involves oral rehydration therapy (major treatment), use of antiemetic and probiotics; nevertheless, the severe dehydration is treated with intravenous (IV) therapy (Onyon and Dawson 2018).
Conclusion and recommendations
The present study analyzed epidemiological changes in the prevalence of gastroenteritis according to age, sex, and month/season wise. Male children were the dominant group in all months, with 61.4% cases of gastroenteritis compared to female children. Both age and season determined the prevalence of disease in children in the Bannu district, as the rate of prevalence of the disease decreased with the increase in age of the children as we shift from ≤6 m toward >10y ≤ 15y, with the highest in the former (37.8%) and lowest in the latter (0.4%). While late spring (April) and early and mid-summer (May–June) and November showed peak prevalence of the disease. Children ≤5 years of age had the largest share of cases (96.4%), and 89.1% of the cases were reported in children ≤ 2 years of age.
Low immunity in the children and an unhygienic way of life are the primary factors contributing to the high prevalence of the disease in the study area. It is recommended that breast-fed infants continue to be nursed in the usual fashion. Moreover, to improve efficacy, mass vaccination of children through a national immunization program is required. Furthermore, educating people to create mass awareness in them of the causes and sources of the high rate of the disease and use of preventive measures and adopting hygienic practices such as by encouraging children and their caretakers to wash their hands and teaching them to avoid improperly stored foods and contaminated water should be carried out to aid in effective disease management and prevention of large scale outbreaks.
Limitations
The current study only has a general description of the gastroenteritis in children in Bannu during the study period and does not encompass the cause (etiology) based prevalence of the disease. Therefore, future studies are needed to determine the etiology of the disease to determine the prevalence of the origin of the disease: viral-, bacterial-, and parasitic-based gastroenteritis, for a more exact picture of the disease and to more effectively control gastroenteritis in the study area.
Acknowledgments
The additional director of the Women and Children Hospital, Bannu is highly acknowledged for allowing me to obtain the research data and for granting me ethical approval of the study. I am also grateful to Mr. Shafiq-ur-Rehman, children ward in-charge for providing the admission register to obtain the data. Thanks also to Jos Feys, senior research fellow at the KU Leuven University (Catholic University of Leuven, Belgium) for statistically analyzing the data.
Availability of data and material
Data is provided in the tables in the drafted manuscript.
Authors’ contributions
I am the sole author of this manuscript and contributed to the study conception and design, material preparation, data collection, while analysis of the data was performed by Jos Feys, senior research fellow at the KU Leuven University (Catholic University of Leuven, Belgium). I read and approved the final manuscript.
Declarations
Conflict of interest
The author declares no competing interest.
Ethics approval
The study was approved by the additional director of the Women and Children Hospital, Bannu with reference No. 1221, dated: November 07, 2020.
Informed consent
This study does not include human participants.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afzal A, Tariq PA, Rota SC, Choudhry S. Virus gastroenteritis in children upto five years of age. J Rawalp Med Colle. 2010;14(1):33–35. [Google Scholar]
- Ahmed K, Shezana JM, Imran R, Shuja N, Shah G. Prevalence of intestinal parasitic pathogens among gastroenteritis patients in district Gilgit, Gilgit-Baltistan, Pakistan. Pak J Zool. 2012;44(4):1059–1063. [Google Scholar]
- Alam MM, Khurshid A, Shaukat S, Rana MS, Sharif S, Angez M, Nisar N, Aamir UB, Naeem M, Zaidi SSZ. Viral etiologies of acute dehydrating gastroenteritis in Pakistani children: confounding role of Parechoviruses. Viruses. 2015;7:378–393. doi: 10.3390/v7010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MM, Khurshid A, Shaukat S, Suleman RM, Sharif S, et al. Epidemiology and genetic diversity of rotavirus strains in children with acute gastroenteritis in Lahore, Pakistan. PLoS One. 2013;8(12):e67998. doi: 10.1371/journal.pone.0067998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandan S, Peter R, Aramugam R, Ismail N, Veeraraghavan B, Kang G. Group a rotavirus gastroenteritis in older children and adults at a hospital in southern India. Vacci. 2014;32S:A33–A35. doi: 10.1016/j.vaccine.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Bajolet O, Chippaux-Hyppolite C. Rotavirus and other viruses of diarrhea. Bull Soc Pathol Exot. 1998;91(5):432–437. [PubMed] [Google Scholar]
- Bass M (2004) Rotavirus & other agents of viral gastroenteritis. In: Behrman RE, Kliegman RM, Jenson HB (eds) Nelson textbook of paediatrics, 17th edn. Fletcher, Philadelphia
- Bishop RF. Natural history of human rotavirus infection. Arch Virol Suppl. 1996;12:119–128. doi: 10.1007/978-3-7091-6553-9_14. [DOI] [PubMed] [Google Scholar]
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of mortality in 2008: a systematic analysis. Lance. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- Boschi-Pinto C, Velebit L, Shibuya K. Estimating child mortality due to diarrhoea in developing countries. Bull W Heal Organ. 2008;86:710–717. doi: 10.2471/BLT.07.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Velez L, Amouzou A, Perin J, et al. Diarrhoea management in children under 5 in sub-Saharan Africa: does the source of care matter? A countdown analysis. BMC Publ Heal. 2016;16:830. doi: 10.1186/s12889-016-3475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi UY, Lee SY, Ma SH, et al. Epidemiological changes in rotavirus gastroenteritis in children under 5 years of age after the introduction of rotavirus vaccines in Korea. Eur J Pediatr. 2013;172:947–952. doi: 10.1007/s00431-013-1974-y. [DOI] [PubMed] [Google Scholar]
- Chow CM, Leung AKC, Hon KL. Acute gastroenteritis: from guidelines to real life. Clin Exp Gastroenter. 2010;3:97–112. doi: 10.2147/ceg.s6554.Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli S, Stolfi I, Caramia G. Management strategies in the treatment of neonatal and pediatric gastroenteritis. Infec Drug Resis. 2013;6:133–161. doi: 10.2147/IDR.S12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt AJ, Baumgart DC. Viral gastroenteritis in adults. Recent Patents on Anti-Infect Drug Discov. 2011;6(1):54–63. doi: 10.2174/157489111794407877. [DOI] [PubMed] [Google Scholar]
- Eesteghamati A, Gouya M, Keshtkar A, Najafi L, Zali MR, Sanaei M, et al. Sentinel hospital-based surveillance of rotavirus diarrhea in Iran. J Infect Dis. 2009;200(Suppl 1):S244–S247. doi: 10.1086/605050. [DOI] [PubMed] [Google Scholar]
- Elliott EJ. Acute gastroenteritis in children. BMJ. 2007;334(7583):35–40. doi: 10.1136/bmj.39036.406169.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott EJ, Backhouse JA, Leach JW. Pre-admission management of acute gastroenteritis. J Paedia Child Heal. 1996;32(1):18–21. doi: 10.1111/j.1440-1754.1996.tb01534.x. [DOI] [PubMed] [Google Scholar]
- GBD Disease and injury incidence and prevalence, collaborators. Lanc. 2015;388:1545–1602. [Google Scholar]
- Iftikhar T, Butt A, Nawaz K, Sarwar Y, Ali A, Mustafa T, Haque A. Genotyping of rotaviruses detected in children admitted to hospital from Faisalabad region, Pakistan. J Med Virol. 2012;84:2003–2007. doi: 10.1002/jmv.23402. [DOI] [PubMed] [Google Scholar]
- Jeffs E, Williman J, Martin N, Brunton C, Walls T (2019) The epidemiology of non-viral gastroenteritis in New Zealand children from 1997 to 2015: an observational stud. BMC Pub Heal 19:18. 10.1186/s12889-018-6229-4 [DOI] [PMC free article] [PubMed]
- Johargy A, Ghazi H, Mumenah A. Frequency of viral, bacterial and parasitic enteropathogens among young children with acute diarrhea in Saudi Arabia. J Pak Med Assoc. 2010;60(6):456–459. [PubMed] [Google Scholar]
- Jones M, Harrach B, Ganac R, Gozum M, Dela Cruz W, Riedel B. New adenovirus species found in a patient presenting with gastroenteritis. J Virol. 2007;81(11):5978–5984. doi: 10.1128/JVI.02650-06.Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. A clinical pathway for pediatric gastroenteritis. Gastroenterol Nurs. 2003;26(1):7–18. doi: 10.1097/00001610-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Jorge W, Domingo S, Nicholas J, Avanish KA, Panikkar (2010) Fecal pollution of water. In: Cleveland CJ (ed) Encyclopedia of Earth. National Council for Science and the Environment, Washington DC
- Kapikian AZ. Viral gastroenteritis. JAMA. 1993;269(5):627–630. doi: 10.1001/jama.1993.03500050105035. [DOI] [PubMed] [Google Scholar]
- Kawai K, O’Brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vacci. 2012;30(7):1244–1254. doi: 10.1016/j.vaccine.2011.12.092. [DOI] [PubMed] [Google Scholar]
- Kazi AM, Warraich GJ, Qureshi S, Qureshi H, Khan MMA, et al. Sentinel hospital-based surveillance for assessment of burden of rotavirus gastroenteritis in children in Pakistan. PLoS One. 2014;9(10):e108221. doi: 10.1371/journal.pone.0108221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CK, Glass R, Bresee JS, Duggan C (2003) Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. Centers for Disease Control and Prevention. MMWR Recomm Rep 21;52(RR-16):1–16 [PubMed]
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lanc. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- Mullick S, Mandal P, Nayak MK, Ghosh S, De P, Rajendran K, et al. Hospital based surveillance and genetic characterization of rotavirus strains in children (<5 years) with acute gastroenteritis in Kolkata, India, revealed resurgence of G9 and G2 genotypes during 2011–2013. Vacci. 2014;32(Suppl 1):A20–A28. doi: 10.1016/j.vaccine.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Nisa T-U, Ahmad A. Surveillance studies for Rota virus vaccine implication in Pakistan. Pak j Med Denti. 2018;7(04):66–70. [Google Scholar]
- Okitsu-Negishi S, Nguyen TA, Phan TG, Ushijima H. Molecular epidemiology of viral gastroenteritis in Asia. Pediatr Int. 2004;46(2):245–252. doi: 10.1046/j.1442-200x.2004.01896.x. [DOI] [PubMed] [Google Scholar]
- Onyon C, Dawson T. Symposium: gastroenterology. Paediatri Child Health. 2018;28(11):527–532. doi: 10.1016/j.paed.2018.08.010. [DOI] [Google Scholar]
- Ozmert EN, Ince OT, Orun E, Yalcın S, Yurdakok K, Gur D. Clinical characteristics and antibiotic resistance of Shigella gastroenteritis in Ankara, Turkey between 2003 and 2009, and comparison with previous reports. Intern j infect diseas. 2011;15(12):e849–e853. doi: 10.1016/j.ijid.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Phan TG, Okame M, Nguyen TA, Maneekarn N, Nishio O, Okitsu S, Ushijima H. Human astrovirus, norovirus (GI, GII), and sapovirus infections in Pakistani children with diarrhea. J Med Virol. 2004;73(2):256–261. doi: 10.1002/jmv.20084. [DOI] [PubMed] [Google Scholar]
- Pieścik-Lech M, Shamir R, Guarino A, Szajewska H. Review article: the management of acute gastroenteritis in children. Aliment Pharmacol Ther. 2013;37(3):289–303. doi: 10.1111/apt.12163. [DOI] [PubMed] [Google Scholar]
- Qazi R, Sultana S, Sundar S, Warraich H, un-Nisa T et al (2009) Population based surveillance for severe rotavirus gastroenteritis in children in Karachi, Pakistan. Vaccine 27(Suppl 5):F25–F30. 10.1016/j.vaccine.2009.08.064 [DOI] [PubMed]
- Sadiq A, Bokhari B, Noreen Z, Asghar RM, Bostan N, Sadiq, et al. Magnitude of rotavirus a and Campylobacter jejuni infections in children with diarrhea in twin cities of Rawalpindi and Islamabad, Pakistan. BMC Infect Disea. 2019;19:978. doi: 10.1186/s12879-019-4575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja T, Sharma SD, Gupta M, Kundu R, Kar S, Dutta A, et al. A multicenter prospective hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children less than five years of age in India. Vacci. 2014;32S:A13–A19. doi: 10.1016/j.vaccine.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Satter SM, Gastanaduy PA, Islam K, Rahman M, Rahman M, Luby SP, et al. Hospital-based surveillance for rotavirus gastroenteritis among young children in Bangladesh: defining the potential impact of a rotavirus vaccine program. Pediatr Infect Dis J. 2017;36(2):168–172. doi: 10.1097/inf.0000000000001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossberg D (2015) Clinical infectious disease, 2nd edn.. Cambridge University Press, New York, p 334
- Singh A (2010) Pediatric emergency medicine practice acute gastroenteritis—an update. Pedia Emerg Medi Pract 7 (7)
- Sohail H, Neupane S. Prevalence of and factors associated with under-5 mortality in South Asia. Int Health. 2019;11(2):119–127. doi: 10.1093/inthealth/ihy065. [DOI] [PubMed] [Google Scholar]
- Sultan MA, Hassan Z. Assessment of severity of acute gastroenteritis in the paediatric Pakistani population by modified Vesikari score. J Pak Med Assoc. 2018;68(2):159–164. [PubMed] [Google Scholar]
- Tamim S, Hasan F, Matthijnssens J, Sharif S, Shaukat S, Alam MM, et al. Epidemiology and phylogenetic analysis of VP7 and VP4 genes of rotaviruses circulating in Rawalpindi, Pakistan during 2010. Infect Genet Evol: J Molec Epidem Evolut Gene in Infec Disea. 2013;14:161–168. doi: 10.1016/j.meegid.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Van Lierde S, Corbeel L, Eggermont E. Clinical and laboratory findings in children with adenovirus infections. Eur J Pediatr. 1989;148:423–425. doi: 10.1007/BF00595902. [DOI] [PubMed] [Google Scholar]
- Webb A, Starr M. Acute gastroenteritis in children. Aust Fam Physic. 2005;34(4):227–231. [PubMed] [Google Scholar]
- Webber R (2009) Communicable disease epidemiology and control: a global perspective (3rd edn.). CAB Int, Wallingford, p 79
- WHO (1994) Diarrhoeal disease control programme. The management of bloody diarrhoea in young children. WHO, Geneva. https://apps.who.int/iris/handle/10665/62462
- Wildi-Runge S, Allemann S, Schaad UB, et al. A 4-year study on clinical characteristics of children hospitalized with rotavirus gastroenteritis. Eur J Pediatr. 2009;168:1343–1348. doi: 10.1007/s00431-009-0934-z. [DOI] [PubMed] [Google Scholar]
- You D, Wardlaw T, Salama P, Jones G. Levels and trends in under-5 mortality, 1990–2008. Lanc. 2010;375(9709):100–103. doi: 10.1016/S0140-6736(09)61601-9. [DOI] [PubMed] [Google Scholar]
- Zaidi SH, Smith-Morris C (2015) Diapers in war zones: ethnomedical factors in acute childhood gastroenteritis in Peshawar, Pakistan. PLoS One 10(3): e0119069. 10.1371/journal.pone.0119069 [DOI] [PMC free article] [PubMed]
- Zamir D, Weiler Z, Kogan E, Ben-Valid E, Polishchuk I, Hay E, Reitblat T. Single-dose quinolone treatment in acute gastroenteritis. J Clinical Gastroenter. 2006;40(3):186–190. doi: 10.1097/00004836-200603000-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided in the tables in the drafted manuscript.