Abstract
Background
Malaria is a major public health problem in India and accounts for about 88% of malaria burden in South-East Asia. India alone accounted for 2% of total malaria cases globally. Anti-malarial drug resistance is one of the major problems for malaria control and elimination programme. Artemether-lumefantrine (AL) is the first-line treatment of uncomplicated Plasmodium falciparum in north eastern states of India since 2013 after confirming the resistance against sulfadoxine-pyrimethamine. In the present study, therapeutic efficacy of artemether-lumefantrine and k13 polymorphism was assessed in uncomplicated P. falciparum malaria.
Methods
This study was conducted at four community health centres located in Koraput district of Odisha, Bastar district of Chhattisgarh, Balaghat district of Madhya Pradesh and Gondia district of Maharashtra state. Patients with uncomplicated P. falciparum malaria were administered with fixed dose combination (6 doses) of artemether-lumefantrine for 3days and clinical and parasitological response was recorded up to 28days as per World Health Organization protocol. Nucleotide sequencing of msp1 and msp2 gene was performed to differentiate between recrudescence and reinfection. Amplification and sequencing of k13 propeller gene region covering codon 450680 was also carried out to identify the polymorphism.
Results
A total 376 malaria patients who fulfilled the enrolment criteria as well as consented for the study were enrolled. Total 356 patients were followed up successfully up to 28days. Overall, the adequate clinical and parasitological response was 98.9% and 99.4% with and without PCR correction respectively. No case of early treatment failure was observed. However, four cases (1.1%) of late parasitological failure were found from the Bastar district of Chhattisgarh. Genotyping of msp1 and msp2 confirmed 2 cases each of recrudescence and reinfection, respectively. Mutation analysis of k13 propeller gene showed one non-synonymous mutation Q613H in one isolate from Bastar.
Conclusions
The study results showed that artemether-lumefantrine is highly effective in the treatment of uncomplicated P. falciparum malaria among all age groups. No functional mutation in k13 was found in the study area. The data from this study will be helpful in implementation of artemether-lumefantrine in case of treatment failure by artesunate plus sulfadoxine-pyrimethamine.
Keywords: Therapeutic efficacy, Artemether-lumefantrine, Plasmodium falciparum, Malaria
Background
Malaria is a major public health problem in India and accounts for about 88% of malaria burden in South-East Asia. India alone accounted for 2% of total malaria cases globally [1]. In India, a total of 3,38,494 malaria cases was reported in year 2019 and about 46.4% of malaria cases were caused by Plasmodium falciparum monoinfections [2]. Anti-malarial drug resistance is one of the major problems for malaria control and elimination programme [3].
First information of chloroquine resistant P. falciparum was reported from Assam in 1973 within India [4], and resistance gradually spread to other states covering almost entire country [5]. Emergence of sulfadoxine-pyrimethamine (SP) resistance was also reported in Karbi-Anglong, district of Assam in 1979 [6]. SP resistance was again reported in 1992 from Changlang district of Arunachal Pradesh, a state of north-east India [7]. Later artemisinin-based combination therapy (ACT), using artesunate plus sulfadoxine-pyrimethamine (AS+SP), was introduced as the second-line drug in 2005, in case of chloroquine treatment failures. In 2007, AS+SP was selected as the first-line treatment in areas with identified resistance [8]. In 2010, this treatment became the first-line treatment throughout India [9]. Later, due to reports of resistance to partner drug SP in North Eastern States, Co-formulated tablet of artemether-lumefantrine (AL) was introduced by programme for the treatment of P. falciparum cases [10].
Fixed dose combination of artemether (20mg) and lumefantrine (120mg) is commercially available. Artemether has a half-life of 13h, as it absorbs and metabolizes quickly leading to fast clearance of parasite from blood. Due to longer half-life of lumefantrine (36days), it further eliminates the residual parasite from the malaria patient and helps to prevent the recrudescence [11]. Therefore, combination of artemether and lumefantrine reduces the chance of parasite to develop resistance.
Mutation in k13 propeller gene has found to be associated with artemisinin resistance in Southeast Asia [12]. Many mutations Y493H, R539T, I543T, and C580Y are known which are responsible for in vivoandin vitroartemisinin resistance, while P553L is known for delayed parasite clearance in Southeast Asia [13]. India is a major contributor to the malaria load in Southeast Asia. Therefore, it is also important to keep track of emerging alleles of k13 in India.
Periodic monitoring of therapeutic efficacy is recommended by World Health Organization (WHO) to control the malaria and avoid the spread of resistant parasite in the population. Therefore, therapeutic efficacy of AL for the treatment of uncomplicated P. falciparum malaria was assessed in four states, Madhya Pradesh, Chhattisgarh, Maharashtra and Odisha and mutation in k13 propeller gene was analysed to confirm the artemisinin resistance.
Methods
Study site details
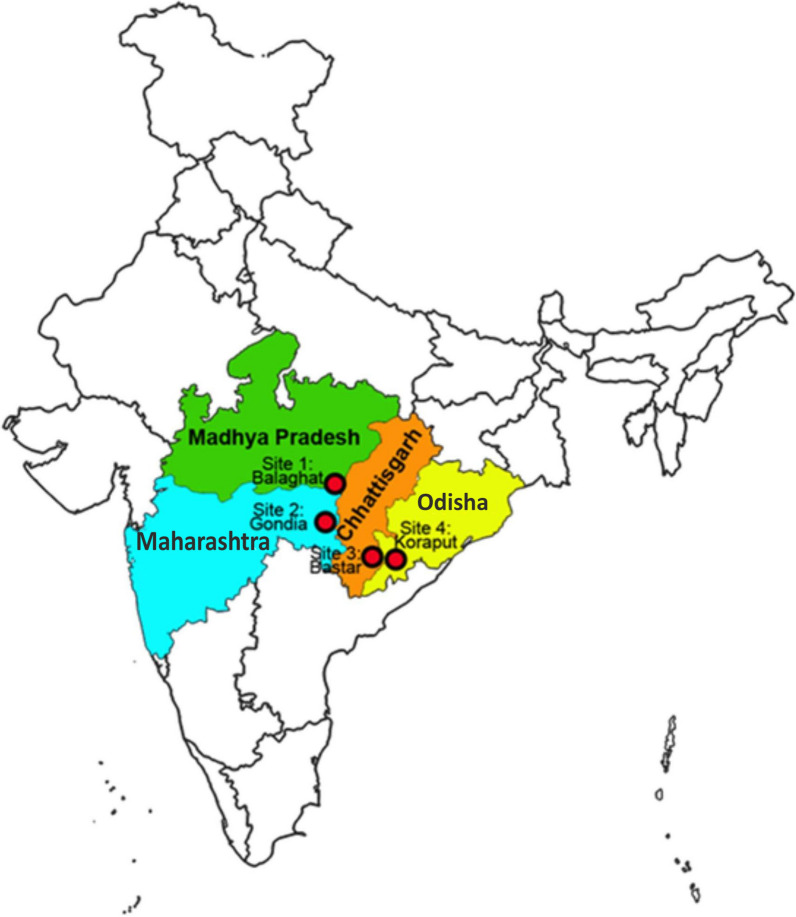
This study was a one-arm prospective study conducted at four sites during June 2017 to Dec 2017. These four community health centres (CHCs) located in Koraput district of Odisha, Baster district of Chhattisgarh, Balaghat district of Madhya Pradesh and Gondia district of Maharashtra state (Fig.1). Selection of these sites was based on malaria endemicity and geographic profile. These sites are tribal dominating located in remote forest and malaria is a major health problem in these areas. Both P. falciparum and Plasmodium vivax were reported from these sites. The selected CHCs were having sufficient facility for management of uncomplicated malaria. All these sites were at distance of 40 to 70km from their district headquarter for the management of severe malaria cases at district hospitals.
Fig. 1.
Map showing the study sites i.e. district Koraput (Odisha), district Bastar (Chhattisgarh), district Balaghat (Madhya Pradesh) and district Gondia (Maharashtra)
Screening of malaria patients
Febrile patients aged between 6months and 60years of age attending the CHC hospitals were screened for malaria parasite by microscopy and patients with uncomplicated P. falciparum infection were asked to participate in the study. The unmarried female (1218years) were excluded from the study as the request for pregnancy test and initiation of contraceptive was not acceptable in local areas.
Inclusion criteria
Symptomatic patients aged 6months (>5kg body weight) and 60years of age with uncomplicated malaria due to monoinfection of P. falciparum (parasitaemia of 1000 to 100,000/L asexual forms microscopically), fever or history of fever within 24 previous hours, ability to swallow oral medication and willing to comply with the study protocol for the duration of the study were included [14].
Exclusion criteria
Patients with general danger signs or signs of severe falciparum malaria; severe malnutrition; mixed or monoinfections other than P. falciparum species as detected by microscopy; febrile conditions caused by a disease other than malaria or another known underlying chronic or severe disease; female patients with positive pregnancy test or breastfeeding, who were unable to drink, had severe vomiting, presence of lethargy or decreased consciousness, inability to sit or stand, were all excluded [14].
Sample size
As the treatment failure rate to (artemether-lumefantrine) in the area is unknown, 5% was assumed. At a confidence level of 95% and a precision around the estimate of 5%, a minimum of 73 patients must be included. With a 20% increase to allow loss to follow-up and withdrawals during the 28day follow-up period, 88 patients were required in the study per site.
Enrolment and sample collection
The patient screening and enrolment started from June 2017 till December 2017. Patients presenting with fever or history of fever and symptoms of malaria were screened for malaria parasites by microscopy. Patients positive for P. falciparum malaria and fulfilling the enrolment criteria were enrolled in the study. The demographic information (age, gender, body temperature) were recorded. Two to three drops of finger prick blood was also blotted on to 3 MM filter paper (Whatman International Ltd., Maidstone, UK) at the time of enrolment and during the follow-up for molecular study.
Anti-malarial treatment with artemether-lumefantrine
Enrolled patients were clinically examined by on duty medical officer and artemether-lumefantrine tablets were administered orally according to body weight, twice a day over 3days (Make-Ipca, Batch No.-DYI576027, Expiry date-01/2018) as per Indian National Malaria Drug Policy 2013 [15]. Female patients were asked for pregnancy test as the drug is contraindicated in the first trimester of pregnancy. Clinical and parasitological parameters were monitored over a 28-day follow-up period to evaluate drug efficacy. The day patient was enrolled and administered with first dose of artemether-lumefantrine was mentioned as 0day. Patients were observed for a minimum 30min after intake of drug to ensure that there was no vomiting after treatment. After three days of completed treatment (0, 1, 2day), clinical and microscopic follow up examination of patients on 3, 7, 14, 21 and 28day was also performed and outcomes were recorded into the following categories according to the WHO protocol [14].
Study definition
Early treatment failure (ETF)
Patient develops danger signs or severe malaria on Day 1, Day 2 or Day 3 and there are parasites in the blood, or the number of parasites in the blood on Day 2 is greater than that on the day of enrolment (Day 0), or axillary temperature on Day 3 is37.5C and there are parasites in the blood on Day 3, and Day 3 parasitaemia25% of those counted at the day of enrolment (Day 0).
Late clinical failure (LCF)
Danger signs or severe malaria in the presence of parasitaemia on any day between day 4 and day 28 in patients who did not previously meet any of the criteria of early treatment failure; and
Presence of parasitaemia on any day between day 4 and day 28 with axillary temperature37.5C in patients who did not previously meet any of the criteria of early treatment failure.
Late parasitological failure (LPF)
Presence of parasitaemia on any day between day 7 and day 28 with axillary temperature<37.5C in patients who did not previously meet any of the criteria of early treatment failure or late clinical failure.
Adequate clinical and parasitological response (ACPR)
Absence of parasitaemia on day 28, irrespective of axillary temperature, in patients who did not previously meet any of the criteria of early treatment failure, late clinical failure or late parasitological failure.
Microscopic examination of blood
Counting of parasite was done on Giemsa-stained thick blood films until 200 white blood cells (WBCs) counted by light microscopy. Parasite density (asexual parasites per L of blood), was calculated as number of asexual parasites divided by the number of WBCs counted, and then multiplying it by an assumed WBC number (6000 per L). When the number of asexual parasites was<100 per 200 WBCs in follow-up smears, counting was done against at least 500 WBCs. A blood slide sample was considered negative when no asexual parasite seen after examination of 1000 WBCs or 100 fields of thick smear. The presence of gametocytes on the day the patient was enrolled or on the day of follow-up was also recorded.
Quality assurance of microscopy
Blood smears of enrolled patients, including follow-up smears, were examined by two independent WHO level 1 qualified microscopists. If any discordance was found, a third reading was performed by another senior microscopist. Counting of parasite was also performed by two independent microscopists. If the difference between two readings varied by>25%, counting was again performed by a third microscopist. Each reader was unaware to the result of other reader.
Data entry and statistical analysis
Data from both clinical and parasitological assessments for each participant were entered and therapeutic efficacy was analysed using WHO standardized Microsoft Excel data collection sheet. Furthermore, survival analysis among total subjects from all study sites was performed using STATA software version 14.
Molecular analysis
If any patient was found positive for malaria parasite during follow up period, two to three drops of finger prick blood was also collected on 3 MM Whatman filter paper for identification of mixed infection using molecular tools. Genomic DNA was isolated and species-specific nested PCR was carried out using primers targeting the 18s rRNA gene for the identification of Plasmodium species [16]. PCR and sequencing of msp1, msp2 and k13 gene was performed as per previously published protocol [17, 18]. Recrudescence was defined as at least one identical allele for each of the two markers (msp1 & msp2) in the pre-treatment (Day 0) and post-treatment samples (day of parasite observed). Reinfection was diagnosed when all alleles for at least one of the markers differed between the two samples.
Sequence analysis
Sequencing result were analysed with analysis software v5.2 (Applied Biosystems) and sequence alignment was performed using GeneDoc software [19].
Results
Prevalence of malaria and enrolment of study subject
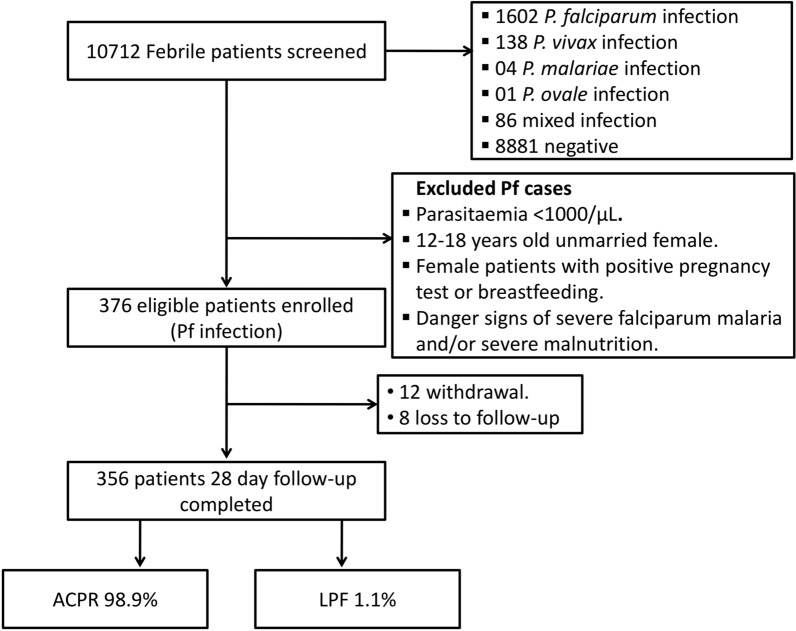
Overall, 10,712 patients from 1 to 60years were screened for malaria parasite during the study period. Malaria positivity rate was 17.9% (1915/10712) with 84% (1602/1915) P. falciparum monoinfection. A total 12% (224/1915) case of P. vivax and 4% (86/1915) mixed infection of P. falciparum and P. vivax was recorded. Out of 1602 mono P. falciparum cases, a total 376 malaria patients who fulfilled the enrolment criteria as well as consented for the study were enrolled. Therapeutic efficacy was determined in 356 (94.7%) patients who had completed their 28days follow-up while 20 patients were either withdrawn from the study or loss to follow up due to various reasons (Fig.2).
Fig. 2.
Flowchart showing screening, enrolment and follow-up of malaria patients at all four study sites. ACPR: adequate clinical and parasitological response; LPF: late parasitological failure
Demographic information of study participant
Background characteristics of the enrolled patients are summarized in Table 1. The median (range) age of the patients was 11years (1year60years). Most of the patients (49%) were 515years old followed by adults (35%) and rest 16% were under 5years and this difference was statistically significant (P<0.001). A similar trend of patients age was observed at each site and no significant difference between the sites was recorded. Overall 62% patients were male and 38% were female which is significant statistically (P<0.0001) and similarly at each site male were significantly more than female. Geometric Mean (95% CI) parasite density at baseline was recorded lowest at Madhya Pradesh (5262.6; 4218.36565.3) followed by Maharashtra (6770.8; 5065.49050.4), Odisha (7380.8; 5480.19940.8) and highest in Chhattisgarh (14,044; 11,617.616,977.2). The difference of mean parasite density between the sites was found significant statistically (P<0.0001).
Table 1.
Summary statistics of the study participants at four sites
| MP (n=105) | CG (n=117) | MH (n=82) | OD (n=72) | Total (n=376) | |
|---|---|---|---|---|---|
| Age (years) | |||||
| Median | 11 | 10 | 18 | 8 | 11 |
| Mean | 15.5254 | 14.27778 | 23.37805 | 11.95833 | 16.16667 |
| SD | 13.27809 | 10.95415 | 17.77544 | 10.49673 | 13.82476 |
| 25th percentile | 7 | 7 | 8 | 6 | 7 |
| 75th percentile | 19 | 20 | 38 | 14 | 21 |
| Min. | 1 | 2 | 1.5 | 2 | 1 |
| Max. | 59 | 52 | 60 | 50 | 60 |
| Age group (years) | |||||
| Under 5 (%) | 12 (11.4) | 19 (16.2) | 13 (15.9) | 17 (23.6) | 61 (16.22) |
| 515 (%) | 65 (61.9) | 58 (49.6) | 21 (25.6) | 40 (55.6) | 184 (48.94) |
| Adult (%) | 28 (26.7) | 40 (34.2) | 48 (58.5) | 15 (20.8) | 131 (34.84) |
| Sex | |||||
| Male (%) | 49 (46.7) | 85 (72.6) | 48 (58.5) | 50 (69.4) | 232 (61.70) |
| Female (%) | 56 (53.3) | 32 (27.4) | 34 (41.5) | 22 (30.6) | 144 (38.30) |
| Weight (kg) | |||||
| Median | 20 | 29 | 40 | 22 | 26 |
| Mean | 26 | 31 | 36 | 26 | 29.77287 |
| SD | 14.4 | 15.9 | 15.7 | 14.7 | 15.65803 |
| 25th percentile | 15 | 18 | 22 | 14.5 | 16 |
| 75th percentile | 35 | 46 | 47 | 38 | 44 |
| Min. | 3 | 6 | 8 | 9 | 3 |
| Max. | 65 | 65 | 76 | 69 | 76 |
| Height (cm) | |||||
| Median | 125 | 140 | 148 | 120.5 | 133 |
| Mean | 129 | 136 | 141 | 122 | 132.3378 |
| SD | 22.8 | 28.2 | 24.5 | 25.2 | 26.22478 |
| 25th percentile | 113 | 116 | 124 | 99 | 112 |
| 75th percentile | 148 | 161 | 160 | 146.5 | 155 |
| Min. | 75 | 48 | 80 | 73 | 48 |
| Max. | 170 | 182 | 170 | 168 | 182 |
| Parasite density/L | |||||
| Median | 4520 | 16,320 | 5920 | 6958.5 | 7764.5 |
| Mean | 10,912.3 | 21,995.6 | 15,757.1 | 15,901.4 | 16,373.02 |
| SD | 16,912.8 | 20,083.3 | 21,535.4 | 20,995.7 | 20,151.2 |
| 25th percentile | 2160 | 6392 | 1800 | 2399.5 | 2667 |
| 75th percentile | 11,640 | 29,980 | 21,800 | 20,027.5 | 22,573.5 |
| Min. | 1000 | 1080 | 1200 | 1053 | 1000 |
| Max. | 99,240 | 98,200 | 92,280 | 98,746 | 99,240 |
| Geometric mean | 5262.6 | 14,044.0 | 6770.8 | 7380.8 | 8051.0 |
| 95% CI | (4218.36565.3) | (11,617.616,977.2) | (5065.49050.4) | (5480.19940.8) | (7101.99127.0) |
n number, L micro liter, MP Madhya Pradesh, CG Chhattisgarh, MH Maharashtra, OD Odisha
Efficacy of artemether-lumefantrine
Among the total 376 enrolled cases, 8 patients were loss to follow-up because of remote area and migration of population for their earning and livelihood. Therefore, these patients could not be traced. A total of 12 patients were withdrawn from the study as they were unable to complete the treatment due to persistent vomiting or failure to attend the scheduled visits during the first three days. However, 356 patients were followed up successfully up to 28days. The adequate clinical and parasitological response (ACPR) without PCR correction was 98.9%; 95% CI (97.199.7) with four cases (1.1%; 95% CI 0.32.8) of late parasitological failure (LPF) (Table 2). The ACPR with PCR correction was 99.4%; 95% CI (97.899.9). All four cases of LPF were recorded from study site Chhattisgarh. KaplanMeier survival curve of cumulative incidence of success with and without PCR correction at study site Chhattisgarh is shown in Fig.3. Furthermore, survival analysis curve with and without PCR correction from all the study sites is shown in Fig.4. Neither early treatment failure (ETF) nor late clinical failure (LCF) was observed in this study. Site wise treatment outcomes and parasite clearance time with AL in presented in Table 3. Also, in most of the patients (65%) parasitaemia was cleared within24h. The parasite clearance time was more in the Chhattisgarh and Odisha in comparison to Madhya Pradesh and Maharashtra.
Table 2.
Site wise summary of patient enrolment and follow up status and treatment result with and without PCR correction
| Variables | Study Sites | ||||
|---|---|---|---|---|---|
| MP | CG | MH | OD | TOTAL | |
| No. of malaria positive cases Screened | 251 | 987 | 87 | 506 | 1831 |
| No. enrolled | 105 | 117 | 82 | 72 | 376 |
| Withdrawal | 2 | 5 | 0 | 5 | 12 |
| Loss to follow-up | 0 | 4 | 0 | 4 | 8 |
| Without PCR correction | |||||
| Early treatment failure | 0 | 0 | 0 | 0 | 0 |
| Late clinical failure | 0 | 0 | 0 | 0 | 0 |
| Late parasitological failure | 0 | 4 | 0 | 0 | 4 |
| Adequate clinical and parasitological response (%) | 103 (100) | 104 (96.3) | 82 (100) | 63 (100) | 352 (98.9) |
| With PCR correction | |||||
| Early treatment Failure | 0 | 0 | 0 | 0 | 0 |
| Late clinical failure | 0 | 0 | 0 | 0 | 0 |
| Late parasitological failure | 0 | 2 | 0 | 0 | 2 |
| Adequate clinical and parasitological response (%) | 103 (100) | 104(98.1) | 82 (100) | 63 (100) | 352 (99.4) |
| Pf recrudescence | 0 | 2 | 0 | 0 | 0 |
| Pf re-infection | 0 | 2 | 0 | 0 | 0 |
| Mixed with Pf recrudescence | 0 | 0 | 0 | 0 | 0 |
| PCR negative (unknown) | 0 | 0 | 0 | 0 | 0 |
MP Madhya Pradesh, CG Chhattisgarh, MH Maharashtra, OD Odisha
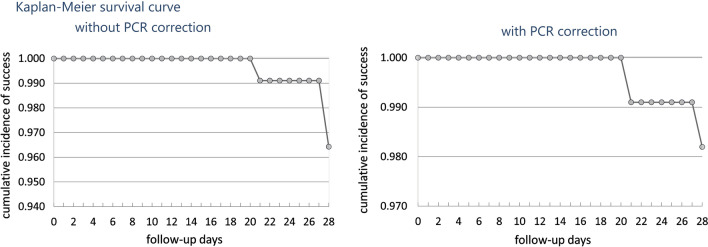
Fig. 3.
KaplanMeier survival curve showing cumulative incidence of success with (98.2%; 95% CI 9399.5) and without PCR correction (96.4%; 95% CI 90.898.6) at study site Bastar (CG)
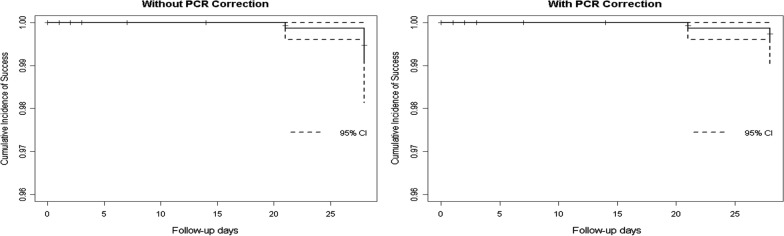
Fig. 4.
KaplanMeier survival curve showing cumulative incidence of success with (99.4%; 95% CI 97.899.9) and without PCR correction (98.9%; 95% CI 97.199.7) at all study sites
Table 3.
Site wise treatment outcome and parasite clearance time with artemether-lumefantrine
| S. no. | Outcome of treatment (28days) | MP | CG | MH | OD | Total |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| 1 | Primary classification | |||||
| ACPR. | 103 (100) | 104 (96.3) | 82 (100) | 63(100) | 352 (98.9) | |
| ETF | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| LCF | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| LPF | 0 (0.0) | 4 (3.7) | 0 (0.0) | 0 (0.0) | 4 (1.1) | |
| Loss to follow-up | 0 (0.0) | 4 (3.4) | 0 (0.0) | 4 (5.6) | 8 (2.1) | |
| Withdrawal | 2 (1.9) | 5 (4.3) | 0 (0.0) | 5 (6.9) | 12 (3.2) | |
| Total | 105 (100) | 117 (100) | 82 (100) | 72 (100) | 376 (100) | |
| 2 | Parasite clearance time (hours) | |||||
| 24 | 86 (83.5) | 39 (36.1) | 79 (96.3) | 24(38.1) | 228 (64.0) | |
| >2448 | 17 (16.5) | 68(63.0) | 3 (3.7) | 37 (58.7) | 125 (35.1) | |
| >4872 | 0 (0.0) | 1 (0.9) | 0 (0.0) | 2 (3.2) | 3 (0.8) | |
| >72 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
ACPR adequate clinical and parasitological response, ETF early treatment failure, LCF late clinical failure, LPF late parasitological failure, MP Madhya Pradesh, CG Chhattisgarh, MH Maharashtra, OD Odisha
Molecular analysis of samples with late parasitological failure
Samples from 0day and day of late parasitological failure (LPF) were analysed for Plasmodium species by species-specific nested PCR. All the four cases were found positive for P. falciparum on both days (0day and day of failure). To understand whether treatment failures were recrudescence or reinfection, DNA samples were sequenced for P. falciparum merozoite surface protein 1 and 2. Out of four cases, two contain the same allele for both the genes msp1 and msp2 among both time (0day and day of failure), whereas other two patients had re-infection in which msp1 and msp2 allele were different at the time of treatment failure.
Mutations in k13 propeller gene
Total 352 samples were taken for amplification of k13 gene. Out of these, successful sequencing was done from 308 samples. Alignment of these sequences from reference sequence (PF3D7 1,343,700) showed one Non-synonymous mutation Q613H in one sample from Madhya Pradesh only, while rest of the samples were wild type.
Discussion
In the present study, efficacy of AL was tested in four states (Madhya Pradesh, Chhattisgarh, Maharashtra and Odisha). These states are major contributor of malaria in India. Among these, Chhattisgarh (18%) and Odisha (11.7%) is the second and third highest contributor of malaria in the country [2]. AL is recommended by National Policy for the treatment of malaria in North Eastern states due to reports of resistance in SP, a partner drug of combination therapy, AS+SP. Although AS+SP is recommended in all other states of the country. Efficacy of AL was assessed in these states, so that AL can be used in case of treatment failure by AS+SP and can be applied by the National Programme in other states as and when required. In the present study, no case of early treatment failure (ETF or ECF) was found, and the ACPR was 98.9%. Previous study carried out three states Madhya Pradesh, Chhattisgarh and Jharkhand in the 2015 also showed that AL is effective in treatment of uncomplicated P. falciparum malaria [18]. Moreover, PCR uncorrected efficacy was 100% at all study sites except Chhattisgarh as the enrolled patients were advised to use bed nets (provided by the government) regularly. AL was found equally effective in both the study in 2015 and 2017 among all age groups and areas with high transmission intensity. Cases of Plasmodium species mixed infection also have been reported from these study area [20, 21]. Present study results were consistent with other studies from India [22,25], Brazil and Papua New Guinea [26, 27].
Four cases of LPF were observed from Bastar district of Chhattisgarh in the present study. After genotyping, two cases were reinfection and two cases of recrudescence were revealed. Previous study showed 1 case of LCF and 2 cases of LPF from Bastar region while another study from India also reported 1 case of ECF [18, 22]. Parasite resistance to drugs, poor drug absorption and altered pharmacokinetics may result in the treatment failure.
Polymorphism in k13 propeller gene is associated with artemisinin resistance. In this study, only one non-synonymous mutation (Q613H) was found in one isolate (0.28%) which is not linked to resistance. Previous study by our group from Madhya Pradesh, India showed M579T (1.6%) which is also not linked to artemisinin resistance [18]. Other study showed presence of mutation G533A, S549Y, R561H and A578S in isolate from Tripura, West Bengal, Arunachal Pradesh and Mizoram respectively at low frequency (0.26%) [28]. Among these R561H was reported to associate with delayed parasite clearance [29] and none of these mutations was observed in the present study.
Conclusion
AL was highly effective for treatment of uncomplicated P. falciparum malaria in all age groups and in the areas where more than one Plasmodium species are prevalent. Study revealed no functional mutation in k13 gene. This indicates no immediate threat of artemisinin resistance in study area. However regular monitoring of antimalarial drug efficacy and genotyping is important to tract the emerging resistance and alleles circulating in the malaria endemic areas.
Acknowledgements
We are thankful to the study participant and parents/guardian for their support during enrolment and follow-up. We are also thankful to the medical officers and staff of all study sites for their efforts and hard work under the remote areas during the study period.
Disclaimer
MGB, EC, PR and RK are staff member of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
HJ, KS, MSD and MMP are staff members of the health department of corresponding states. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the states of India.
SSK, AK and ND are the staff members of National Vector Borne Disease Control Programme (NVBDCP), New Delhi, India. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the NVBDCP.
Authors contributions
NS conceived the study; NS, MMS, PKB, Anil K. Verma, SS, AD and DB designed the study protocol; Sri Krishna, SM, Prakash Tiwari, Anup K. Vishwakarma, Sushrikanta Khandai, ST, HB, SJ and Pradeep Tiwari carried out the sample and data collection; Sri Krishna, SM, Prakash Tiwari and Anup K. Vishwakarma analysed the data; BMV, CRT, SST, KPB, HJ, KS, MSD, MMP provided the logistic and technical support; SSK, AK, ND, MGB, EC, PR and RK provided the technical support; MGB, EC, PR, RK and PKB carried out the interpretation of data; AST, SS, NY, PLM, MM, SS and MMS provided clinical supervision at study sites; Sri Krishna, SM, Prakash Tiwari, Anup K. Vishwakarma and PKB did molecular analysis; Sri Krishna, SM, Prakash Tiwari, Anup K. Vishwakarma, Anil K. Verma, SS and PKB drafted the manuscript; SSK, AK, ND, MGB, EC, PR, RK, AD and PKB critically revised the manuscript. All authors have read and approved the final manuscript.
Funding
This study was funded by WHO country office, India. The funder has no role in study design, sample collection, analysis and writing of the manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
This study was approved by institutional ethics committee (IEC) of ICMR-National Institute of Research in Tribal health, Jabalpur and Indian Council of Medical research (ICMR), New Delhi.
Consent for publication
All authors have given their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Neeru Singh: deceased on 19th August 2017.
References
- 1.WHO. World Malaria Report 2018. Geneva, World Health Organization, 2018. http://www.who.int/malaria/publications/world-malaria-report-2018/report/en/ Accessed 18 Oct 2019
- 2.National vector borne disease control programme (NVBDCP) http://nvbdcp.gov.in/Doc/malaria-situation.pdf. Accessed 18 Oct 2019
- 3.Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2007;77(Suppl 6):69–78. doi: 10.4269/ajtmh.2007.77.69. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal PN, Sharma MI, Sharma SL, Gogai S. Resistance to chloroquine in falciparum malaria in Assam State. India J Commun Dis. 1973;5:175–180. [Google Scholar]
- 5.Farooq U, Mahajan RC. Drug resistance in malaria. J Vector Borne Dis. 2004;41:45–53. [PubMed] [Google Scholar]
- 6.Das S, Barkakaty BN, Roy JR, Guha AK, Rastogi AC. Pyrimethamine in combination with sulfadoxine or sulfalene in P. falciparum infected cases in India. Indian J Malariol. 1981;18:109–116. [Google Scholar]
- 7.NMEP. Drug resistance and chemotherapy of malaria in India, an update. In: National Malaria Eradication ProgrammeEnhanced Malaria Control Project. GPS Dhillon, GS Sonal, U Arora, J Nandi, Eds. Ministry of Health & Family Welfare, Government of India, New Delhi. 1997
- 8.Shah NK, Dhillon GP, Dash AP, Arora U, Meshnick SR, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011;11:57–64. doi: 10.1016/S1473-3099(10)70214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Directorate of National Vector Borne Disease Control Programme. National drug policy on malaria, New Delhi, 2010.http://nvbdcp.gov.in/Doc/drug-policy-2010.pdf. Accessed 20 Oct 2019
- 10.Directorate of National Vector Borne Disease Control Programme. National drug policy on malaria, New Delhi, 2013. http://nvbdcp.gov.in/Doc/National-Drug-Policy-2013.pdf. Accessed. 20 Oct 2019
- 11.Byakika-Kibwika P, Lamorde M, Mayanja-Kizza H, Khoo S, Merry C. Artemether-lumefantrine combination therapy for treatment of uncomplicated malaria: the potential for complex interactions with antiretroviral drugs in HIV-infected individuals. Malar Res Treat. 2011;2011:703730. doi: 10.4061/2011/703730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boussaroque A, Fall B, Madamet M, Camara C, Benoit N, Fall M, et al. Emergence of mutations in the K13 propeller gene of Plasmodium falciparum isolates from Dakar, Senegal, in 20132014. Antimicrob Agents Chemother. 2016;60:624–627. doi: 10.1128/AAC.01346-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, 2009
- 15.NVBDCP. National drug policy 2013. National Vector Borne Disease Control Programme; New Delhi, 2013. http://www.nvbdcp.gov.in/Doc/National-Drug-Policy-2013.pdf. Accessed 20 Oct 2019.
- 16.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 17.Bharti PK, Alam MT, Boxer R, Shukla MM, Gautam SP, Sharma YD, et al. Therapeutic efficacy of chloroquine and sequence variation in pfcrt gene among patients with falciparum malaria in central India. Trop Med Int Health. 2010;15:33–40. doi: 10.1111/j.1365-3156.2009.02425.x. [DOI] [PubMed] [Google Scholar]
- 18.Bharti PK, Shukla MM, Ringwald P, Krishna S, Singh PP, Yadav A, et al. Therapeutic efficacy of artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria from three highly malarious states in India. Malar J. 2016;15:498. doi: 10.1186/s12936-016-1555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholas K, Nicholas HB., Jr . GeneDoc: a tool for editing and annotating multiple sequence alignment, ver. 2.7. 000. Pittsburgh Supercomputing Centers National Resource for Biomedical Supercomputing; 1997. [Google Scholar]
- 20.Krishna S, Bharti PK, Chandel HS, Ahmad A, Kumar R, Singh PP, et al. Detection of mixed infections with Plasmodium spp. by PCR, India, 2014. Emerg Infect Dis. 2015;21:1853–1857. doi: 10.3201/eid2110.150678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishna S, Yadav A, Bhandari S, Vishwakarma AK, Bharti PK, Mandavi PL, et al. Prevalence of malaria in two highly endemic Community Health Centers in the Bastar district, Chhattisgarh showing mixed infections with Plasmodium species. Sci Rep. 2017;7:16860. doi: 10.1038/s41598-017-16974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valecha N, Srivastava P, Mohanty SS, Mittra P, Sharma SK, Tyagi PK, et al. Therapeutic efficacy of artemether-lumefantrine in uncomplicated falciparum malaria in India. Malar J. 2009;8:107. doi: 10.1186/1475-2875-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pareek A, Chandurkar N, Srivastav V, Lakhani J, Karmakar PS, Basu S, et al. Comparative evaluation of efficacy and safety of artesunatelumefantrine vs. artemetherlumefantrine fixed-dose combination in the treatment of uncomplicated Plasmodiumfalciparum malaria. Trop Med Int Health. 2013;18:578–587. doi: 10.1111/tmi.12088. [DOI] [PubMed] [Google Scholar]
- 24.Anvikar AR, Kuepfer I, Mishra V, Bruce J, Arya T, Mishra DR, et al. Efficacy of two artemisinin-based combinations for the treatment of malaria in pregnancy in India: a randomized controlled trial. Malar J. 2018;17:246. doi: 10.1186/s12936-018-2393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Pluijm RW, Tripura R, Hoglund RM, Phyo AP, Lek D, Ul Islam A, et al. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial. Lancet. 2020;395:1345–1360. doi: 10.1016/S0140-6736(20)30552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh M, do Valle SN, Farias S, de Souza TM, Viana GM, Lucchi N, et al. Efficacy of artemetherlumefantrine for uncomplicated Plasmodium falciparum malaria in Cruzeiro do Sul, Brazil, 2016. Am J Trop Med Hyg. 2018;98:88–94. doi: 10.4269/ajtmh.17-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavul L, Hetzel MW, Teliki A, Walsh D, Kiniboro B, Rare L, et al. Efficacy of artemetherlumefantrine and dihydroartemisininpiperaquine for the treatment of uncomplicated malaria in Papua New Guinea. Malar J. 2018;17:350. doi: 10.1186/s12936-018-2494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra N, Prajapati SK, Kaitholia K, Bharti RS, Srivastava B, Phookan S, et al. Surveillance of artemisinin resistance in Plasmodium falciparum in India using the kelch13 molecular marker. Antimicrob Agents Chemother. 2015;59:2548–2553. doi: 10.1128/AAC.04632-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.