Abstract
Background
It has been shown that a subgroup of patients with differentiated thyroid cancer (DTC) and medullary thyroid carcinoma (MTC) would progress to advanced stages of thyroid cancer. Therefore, the present study was done to systematically review available evidence in order to investigate efficacy and safety of peptide receptor radionuclide therapy (PRRT) in the patients with advanced radioiodine refractory differentiated thyroid cancer (RR-DTC) and metastatic MTC.
Methods
For this purpose, relevant studies investigated safety and efficacy of PRRT in the patients with advanced RR-DTC and metastatic MTC were identified by searching Medline (Pubmed, Ovid, and Ebsco), Scopus, Embase, Web of Science, and Cochrane Library databases (from database inception to March 24, 2021). The review was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. Searching was done independently by two investigators. Two researchers independently extracted the data and any disagreement was adjudicated by consensus. Quality of the studies was assessed using the tool of case reports/series in systematic reviews.
Results
Among 2284 related papers, 41 papers met the inclusion criteria. A total of 157 patients with RR-DTC were treated with PPRT. Biochemical and objective responses (partial and complete) were observed in 25.3 and 10.5% of patients, respectively. Among 220 patients with metastatic MTC, biochemical and objective responses were observed in 37.2 and 10.6% of the patients, respectively.
Forty-six deaths were reported in 95 patients with advanced RR-DTC. In addition, 63 deaths were observed in 144 patients with metastatic MTC. Major side effects were reported in 124 patients treated with 90Y -based agent. In the patients treated with 177Lu-DOTA-TATE and 111In-Octreotide, mild and transient hematologic or renal complications were reported.
Conclusion
Findings of the study revealed that in the absence of the established treatment for the patients with RR-DTC and metastatic MTC, PRRT could be effective with few adverse events.
Trial registration
PROSPERO registration number: CRD42019125245.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08257-x.
Keywords: Peptide receptor radionuclide therapy, Radioiodine refractory-differentiated thyroid Cancer, Medullary thyroid carcinoma, Papillary thyroid carcinoma, Yttrium-90, 177Lu-DOTATATE, Indium-111, Systematic review
Background
Thyroid cancer is the most common endocrine malignancy and its incidence has increased by 4.4% per year during 20072011 [1, 2]. Differentiated thyroid cancer (DTC), is the most frequent subtype of thyroid cancer accounting for 8595% of the cases [3, 4]. Medullary thyroid cancer (MTC) originating from parafollicular or C cells of the thyroid gland accounts for approximately 5% of all thyroid cancer cases [5].
The standard of treatment for most patients with DTC includes thyroidectomy followed by radioiodine treatment. A 10-year overall survival rate of 8099% has been reported among these patients [6]. However, in spite of highly effective treatment strategies, there is a chance of recurrence in 20% of the subjects. Radioactive iodine plays a major role in diagnosis and treatment of recurrent disease [7]. However, some thyroid cancers are resistant to radioiodine despite the elevated level of thyroglobulin [8]. Radioiodine refractory-DTC (RR-DTC) has shown aggressive clinical behavior and a 10-year survival rate of 10% [9, 10]. Surgery and external beam radiation therapy can be used to manage local disease but not in case of widespread metastases. Moreover, chemotherapeutic agents have shown limited efficacy with considerable side effects [11, 12].
MTC is inherently non-sensitive to radioactive iodine. Hence, its management is more difficult and its prognosis is worse than DTC [7]. The overall survival rate is between 75 and 85% during 10years for individuals with MTC [13]. In spite of aggressive surgical treatment, there is almost a 50% of chance for persistent or recurrent disease, with deleterious effects on quality of life and the reduced 10-year survival rate by 40% [7, 13]. Reoperation, embolization, and perhaps radiotherapy could improve outcomes [14]. Meanwhile, response to conventional chemotherapy is limited with life-threatening toxicity [7]. Currently, other therapeutic options are scarce and not widely available.
There are few alternative treatments in the patients with advanced RR-DTC. Somatostatin receptor (SSTR) expression on cell surface of neuroendocrine and thyroid tumors regulates cell proliferation [15]. Targeting SSTR with radiotracer in peptide receptor radionuclide therapy (PRRT) can induce tumor cell death. Overexpression of somatostatin receptor subtypes on surface of cells is required for PRRT and therefore, tumor remission can be predicted based on the results of scintigraphy on somatostatin receptor. Thus, PRRT could be a therapeutic option based on scintigraphy results of somatostatin receptor. It has been used previously for treatment of metastatic neuroendocrine tumor and advanced pheochromocytomas and paragangliomas with high efficacy, tolerability, and low toxicity [16, 17].
Accordingly, the present study was conducted to systematically review available evidence in order to investigate efficacy and safety of PRRT in the patients with advanced RR-DTC and metastatic MTC.
Methods
Search strategy and selection criteria
A systematic review was performed on the published works to investigate safety and efficacy of PRRT in the patients with advanced RR-DTC and metastatic MTC, according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [18]. The study was registered before completing formal screening of search results (PROSPERO registration number: CRD42019125245).
Eligibility criteria
All the original studies containing data related to PRRT were considered eligible to be included in the review study. Exclusion criteria were irrelevant papers (based on screening of titles and abstracts), papers with insufficient data available, duplications, and review papers. All the eligible studies were included to assess efficacy, and/or safety of PRRT.
Study identification
For this systematic review, the Cochrane Central Register of Controlled Trials (Central), Medline (PubMed, Ovid, and Ebsco), Scopus, and Embase databases were searched (from database inception to March 24, 2021). Search terms for English-language publications included: peptide receptor radionuclide therapy, PRRT, radionuclide therapy, radiolabeled somatostatin analogues, thyroid cancer, thyroid carcinoma, thyroid neoplasm, differentiated thyroid cancer, differentiated thyroid carcinoma, differentiated thyroid neoplasm, medullary thyroid cancer, medullary thyroid carcinoma, and medullary thyroid neoplasm. Details regarding the search strategy are provided in the Supplementary Table1.
The first search was done independently by two investigators (ZE and ZM). Also, a complete updated search was performed on all databases available and new studies (if any exist) were identified to assess the details and incorporate findings in this review. The snowballing techniques were used to complete the search by screening reference lists of the included papers for relevant studies. Also, registry of prospective studies with accessible results was searched. Two authors (RM, ZM) independently determined studies that should be evaluated further by scanning the title, abstract, or both based on the inclusion/exclusion criteria, the reviewers were blinded to names of the journals and authors. All the potentially relevant papers as full texts were assessed and any disagreements were resolved by consensus or by arbitration of two experts (MK and MM). In case of duplicates or multiple publications of a primary study, yield of information was enhanced by collating all available data and using the most complete data set aggregated across all the known publications.
Data collection and management
Two reviewers (RM and ZM) independently extracted the data from the included trials and any disagreement was adjudicated by consensus or by arbitration of other reviewers (MK and MM). Published reports were obtained for every study, and standard information was extracted in a spreadsheet. The following data were extracted: authors name; year of publication; country where the study was performed; number of participants, sex and age of the participants; tumor classification, site of metastases; prior treatments (cumulative radioiodine in RR-DTC); cumulative activity (GBq) of PRRT; response to treatment criteria; time to progression (TTP); follow-up duration; response to treatment; complications (major/minor); mortality rate; and time to death.
Biochemical response was defined in the patients with DTC based on serum thyroglobulin (Tg) level and in the patients with MTC, it was defined based on serum calcitonin and carcino- embryonic antigen (CEA) levels. Different criteria were used to evaluate radiological responses to treatment, namely world health organization (WHO) criteria, response evaluation criteria in solid tumors (RECIST) criteria, and southwest oncology group (SWOG) criteria [19]. Moreover, the European organization for research and treatment of cancer (EORTC) has classified metabolic response to treatment based on the maximum standardized uptake value (SUVmax) [20].
For further analysis, proportions of complete and partial radiologic response were integrated as objective response.
Occurrence of adverse events was evaluated using common terminology criteria for adverse events (CTCAE) [21]. Two reviewers (RM and ZM) independently assessed methodological quality of the included studies using the tool of systematic reviews [22], and any disagreement was resolved by consensus.
Results
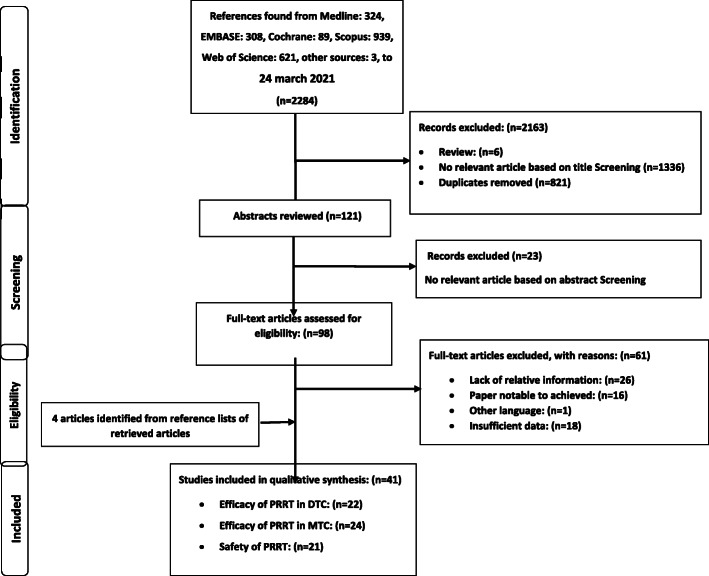
Search on the literature led to identification of 2284 publications, of which 98 papers were reviewed in full text (Fig.1. shows flow chart of literature search and paper selection). The risk of bias of the included studies was low (Supplementary Table2). Inter-reviewers agreement was excellent for the selected papers (Cohens test =0.96). Among 41 publications met the inclusion criteria, 12 papers were retrospective in terms of design; 19 papers were prospective studies and remaining 10 papers were case reports. Tables1 and 2 summarize characteristics of the included studies assessing efficacy of PRRT in the patients with advanced RR-DTC, and metastatic MTC, respectively. Data regarding safety of PRRT are presented in Table3. Cumulative activity of PRRT ranged between 0.92583.2GBq. For 90Y -based agent, most of the studies had used this agent with an administered activity ranging from 0.925 to 5.9GBq per cycle usually up to 4cycles. For 177Lu-DOTA-TATE, the administered activity rate was between 5.57.7GBq per cycle usually up to 4cycles. In terms of follow-up duration, in the patients with advanced RR-DTC, it was between 1 and 99months after commencement of PRRT (median: 12months). It was between 1 and 144months (median: 17months) in the patients with metastatic MTC. Death was recorded in 109 patients. Time to death varied from 1 to 63months (median: 11months). It should be noted that more than one criterion was used to evaluate efficacy of PRRT, and some patients did not complete their full course of treatment.
Fig. 1.
Flow chart of literature search and article selection
Table 1.
Efficacy of peptide receptor radionuclide therapy (PRRT) in patients with advanced RR-DTCa
| Reference (Publish Year) | Country | N | Sex | Age (year) | Tumor Classification | Site of metastasis | Prior treatments (Iodine cumulative activity GBq (med)) | Ligand (Radionuclide Chelator Peptide) | Cumulative activity (GBq) | Response criteria | TTP in SD (month) | Follow-Up duration median: (months) | Response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Czepczynski R et al. (2014) [15] | Poland | 6 | F/M: (9/2) | Median: 65 (4781) | 3FTC, 3HCTC | B/Lu/M | TT/ND/EBR/RIT (3.1) | 90Y-DOTATOC | 3.7_14.8 | Biochemical | NA | 21 (2_68) | 4PD, 1PR, 1SD |
| RECIST | 2PD, 2SD. 1PR | ||||||||||||
| Versari A et al. (2014) [23] | Italy | 11 | F/M | Median: 59 (1978) | 5PTC, 1Oxiphilic, 3FTC, 2Insular | Li/B/Lu | TT/ND/RIT (5.5533.3 (12.95)) | 90Y-DOTATOC | 4.329_17.95 | Biochemical | NA | 7.75 (3.5_11.5) | 11SD |
| RECIST | 2PR, 4SD, 4PD, 1NA | ||||||||||||
| EORTC | 2PR, 5SD, 4PD | ||||||||||||
| Iten F et al. (2009) [24] | Switzerland | 24 | F/M: (12/12) | Median: 58.8 (40.580.6) | 17FTC, 5PTC, 2No specified | NA | TT/ND/RIT | 90Y-DOTATOC | 5.630.3 | Biochemical | NA | 16.8 (1.8_99.1) | 7PR, 17PD |
| Gabriel M et al. (2004) [25] | Austria | 5 | F/M: (2/3) | Median:59 (51_72) | 3FTC, 2PTC | B/Lu/M | TT/ND/RIT (9.25_29.91 (18.87)) | 90Y-DOTATOC | 5.55_7.4 | NA | 5 | NA | 5SD |
| Gorges R et al. (2001) [26] | Germany | 3 | F/M: (2/1) | Median: 68 (51_72) | 1papillary-oxyphilic, 1follicular-oxyphilic, 1Hrthle cell carcinoma | Li/B/Lu/M | TT/ND/EBR/RIT | 90Y-DOTATOC | 1.7_9.62 | Biochemical | NA | 20 (16_31) | 2SD,1PD |
| RECIST | NA | 1SD, 2PD | |||||||||||
| Waldherr C et al. (2001) [27] | Switzerland | 7 | F/M: (4/3) | Median:60 (44_74) | 4PTC, 3FTC | NA | TT/ND/EBR/RIT/C/EN | 90Y-DOTATOC | 1.7_14.8 | WHO | 8 | 15 (1_31) | 2SD, 5PD |
| Virgolini I et al. (2002) [28] | UK | 25 | NA | NA | NA | NA | NA | 90Y-DOTA-Lanerotide | 0 .925_7.06 | WHO | NA | 36 | 3PR, 11SD, 11PD |
| Traub-Weidinger T et al. (2011) [29] | Austria | 4 | NA | Median:66 | 1FTC, 1HCTC | B/Lu | TT/ND/EBR/RIT | 90Y-DOTATOC | 7.2_7.4 | Imaging | NA | (22_27) | 4PD |
| 1FTC, 1PTC | Lu | 90Y-DOTA-Lanerotide | 1.85_3.7 | Imaging | NA | (4_12) | PD | ||||||
| Basu. S et al. (2020) [30] | India | 8 | M/F: (5/3) | 57_83 | 1FTC | B/Lu/M | TT/ND/RIT | 177Lu-DOTATATE | 5.5_25.4 | Biochemical | NA | 34(7_52) | 5PD, 3PR |
| RECIST | 6PD, 2SD | ||||||||||||
| Cinkir, H. Y et al. (2020) [31] | Turkey | 4 | M/F: (3/1) | Median: 64 (49,67) | 1FTC, 3PTC | Lu/B | TT/ND/RIT/C | 177Lu-DOTATATE | 14.8_30.8 | EORTC | 5.5 (1.7_9.4) | 13.8 (4.0_23.7) | 1PD, 2SD, 1PR |
| Roll. W et al. (2018) [32] | Germany | 5 | M/F: (4/1) | Median: 75 (62_89) | 3FTC, 1PTC, 1HCTC | NA | TT/ND/EBR/RIT | 177Lu-DOTATATE | Mean: 7.00.7 | Biochemical | NA | 6 (3_9) | 1FTC PR, 4PD |
| RECIST | 2PD, 2SD, 1PR | ||||||||||||
| EORTC | 3PD, 2SD | ||||||||||||
| Olivn-Sasot. P et al. (2017) [33] | Spain | 1 | F | 69 | FTC | B/Li | TT/ND/C/RIT (10.4) | 177Lu-DOTATATE | 2.6 | Biochemical | NA | 6 | PR |
| Elboa, U et al. (2016) [34] | Turkey | 1 | M | 64 | PTC (tall cell variant) | B/ Lu/M | TT/ND/RIT (27.75) | 177Lu-DOTATATE | 7.4 | Biochemical | NA | After second cycle | PR |
| Jois B et al. (2014) [35] | India | 1 | NA | NA | PTC | Lu | TT/ND/EBR/RIT (NA) | 177Lu-DOTATATE | 7.4 | Biochemical | NA | 3 | PR |
| Imaging | SD | ||||||||||||
| Teunissen JJ et al. (2005) [36] | Netherlands | 5 | F/M | Median: 52 (52_74) | 3HCTC, 1FTC, 1PTC | B/Lu | TT/ND/EBR/C/RIT (1.9_16.7 (12.9)) | 177Lu-DOTATATE | 22.430.1 | Biochemical | Median: 22(4_43) | (4_48) | 2PD, 3PR |
| WHO | 2SD, 1PD, 1PR, 1MRe | ||||||||||||
| Parihar AS et al. (2018) [37] | India | 1 | F | 54 | PTC | B/Lu | TT/ND/RIT (18.5) | 177Lu-DOTA-RGD2 | 5.5 | RECIST | NA | 4 | SD |
| Campenni A et al. (2015) [38] | Italy | 1 | M | 70 | PTC | Lu | TT/ND/RIT (3.7) | 177Lu-DOTATOC | 7.77 | Biochemical | 5 | 5 | PR |
| RECIST | SD | ||||||||||||
| Valkema R et al. (2002) [39] | Netherlands | 5 | NA | Median:70.8 (57.3_76.1) | 4PTC, 1FTC | Lu | TT/ND/BT/RIT | 1111n-Octerotide | 29.51_83.2 | Biochemical | NA | 15.8 (15_16.6) | 1SD, 1PD, 1PR, 2NA |
| SWOG | 15.8 (13.2_28.2) | 1SD, 4PD | |||||||||||
| Krenning E et al. (1999) [40] | Netherlands | 1 | NA | NA | PTC | NA | NA | 1111n-Octerotide | 20_75 | imaging | NA | 24 | 1SD |
| Stokkel MP et al. (2004) [41] | Netherlands | 11 | F/M: (7/4) | Median:67 (4469) | 6PTC, 5FTC | Li/B/Lu/M | TT/ND/EBR/C/Emb/RIT | 111In-DTPA-Octreotide | 14.3_33.1 | Biochemical | NA | 12 (1_12) | 7SD, 3PD, 1NA |
| Imaging | 4SD, 5PD, 2NA | ||||||||||||
| Budiawan H et al. (2013) [6] | Germany | 7 | F/M: (5/2) | Median: 64.5 (26_77) | 4FTC,3HCTC | A/Li/Lu/B | TT/ND/EBR/C/RIT/LITT/REDIFF | 90Y-DOTATATE and 177Lu-DOTATATE | NA | EORTC | NA | 50.4 (34.866) | 1SD, 5PD, 1PR |
| Scalorbi F et al. (2017) [42] | Italy | 21 | F/M: (13/8) | NA | NA | NA | TT/ND/RIT | NA | NA | EORTC | NA | NA | 2PR, 9PD, 10SD |
aAbbreviations: NA Not Available, FTC Follicular Thyroid Carcinoma, RR-DTC Radioiodine-Refractory Differentiated Thyroid Cancer, PTC Papillary Thyroid Carcinoma, HCTC Hurtle Cell Thyroid Carcinoma, TTP Time To Progression
Metastatic Site: A Adrenal, Li Liver, Lu Lung, B Bone, M Mediastinum
Prior treatments: TT Total Thyroidectomy, ND Node Dissection, EBR External Beam Radiation, C Chemotherapy, I Radioactive Iodine therapy, BT Biotherapy with octreotide, LITT Laser- induced thermotherapy, REDIFF Redifferentiation Using Roaccutane
Response: CR Complete Response, PR Partial Remission, SD Stable Disease, PD Progressive Disease, MR Minor Remission
Table 2.
Efficacy of peptide receptor radionuclide therapy in patients with metastatic MTCa
| Reference (Publish Year) | Country | N | Sex | Age | Site of metastasis | Prior treatment | Ligand (Radionuclide Chelator Peptide) | Cumulative activity (GBq) | Response criteria | TTP in SD (month) | Follow-Up duration (months) | Response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ksz M et al. (2014) [43] | Switzerland | 1 | NA | NA | NA | TT/ND/C | 90Y-DOTA-TOC | 5.65 | Biochemical | NA | 3 | PD |
| RECIST | PD | |||||||||||
| EORTC | PD | |||||||||||
| Bertagna F et al. (2009) [44] | USA | 1 | M | 74 | B/M/ H | TT/ND/RF | 90Y-DOTA-TOC | 9.01 | Biochemical | NA | 7 | SD |
| RECIST | NA | SD | ||||||||||
| WHO | NA | SD | ||||||||||
| EORTC | NA | PR | ||||||||||
| Iten F et al. (2007) [45] | Switzerland | 31 | F/M: 10/21 | Mean: 56.7 (24.076.9) | NA | TT/ND/C/EBR | 90Y-DOTA-TOC | 1.729.6 | Biochemical | NA | 15.7 (1.4_107) | 9R, 22NR |
| Bodei L et al. (2004) [46] | Italy | 21 | F/M: 8/13 | Median: 53 (3178) | Lu/Li/B/M | TT/ND/C/EBR/BT | 90Y-DOTA-TOC | 7.519.2 | Biochemical | NA | 40 | 3SD, 12PD, 5PR, 1CR |
| SWOG | NA | 12SD, 7PD, 2CR | ||||||||||
| Gao ZR et al. (2004) [47] | China | 1 | M | 58 | Lu/ M | NA | 90Y-DOTA-TOC | 3.33 | Biochemical | 6 | 10.5 | PR |
| WHO | SD | |||||||||||
| Waldherr C et al. (2001) [27] | Switzerland | 12 | F/M: 5/7 | Median: 60 (24_72) | NA | TT/ND/C/EBR/BT/EN | 90Y-DOTA-TOC | 1.7_14.8 | WHO | 10 (3_14) | 15 (1_31) | 5SD, 7PD |
| Otte A et al. (1999) [48] | Switzerland | 2 | F | 65 | NA | NA | 90Y-DOTA-TOC | 9.25_9.62 | WHO | NA | 24 | 2SD |
| Bilgic, S et al. (2020) [49] | Turkey | 19 | F/M: 6/13 | 32_87 | Lu/Li/B/M | TT/ND/C | 177Lu-DOTATATE | 6.5_52.3 | Biochemical | NA | NA | 7SD, 8PR, 4PD |
| Imaging | 15SD, 2PR, 2PD | |||||||||||
| Cinkir, H. Y et al. (2020) [31] | Turkey | 3 | M | Median: 53 (38,59) | Lu/B/M | TT/ND/C/EBR | 177Lu-DOTATATE | 14.8_44.4 | EORTC | 37.3 (17.6_56.9) | 24.2 (0_48.8) | 3SD |
| Parghane, R. V et al. (2020) [50] | India | 43 | F/M: 8/35 | Median: 48 (25,80) | Lu/Li/B/M | TT/ND/C/EBR | 177Lu-DOTATATE | 5.55_33.3 | Biochemical | 24 (15.1_32.9) | 26 (16.6_35.3) | 5CR, 4SD, 13PR, 21PD |
| RECIST | 22SD, 4PR, 17PD | |||||||||||
| Makis W et al. (2015) [51] | Canada | 2 | NA | Median: 56.5 (38,75) | B/M | TT/ND | 177Lu-DOTATATE | 22.2 | Biochemical | NA | 9.5 (9,10) | 1PR, 1PD |
| WHO | 2SD | |||||||||||
| Vaisman F et al. (2015) [52] | Brazil | 7 | NA | Median: 35.8 (20_54) | NA | NA | 177Lu-DOTATATE | 29.6 | RECIST | NA | 12 | 3PR, 3SD, 1PD |
| Soydal et al. (2014) [53] | Turkey | 2 | F/M | Median: 41 | Lu/Li | TT/ND/EBR | 177Lu-DOTATATE | 29.6 | RECIST | NA | 6weeks After fourth cycle | 2SD |
| Beukhof, C et al. (2019) [54] | Netherlands | 10 | F/M: 6/4 | Median: 62 (1975) | NA | NA | 177Lu-octreotide | 27.8_29.6 | Biochemical | 8.4 (3.6_144) | 16.88 (4.8_144) | 3SD, 4PR, 3PD |
| RECIST | 4SD, 6PD | |||||||||||
| Mathew, D et al. (2018) [55] | India | 2 | NA | NA | NA | NA | 177Lu-octreotide | NA | Imaging | NA | 7weeks After last cycle | 2SD |
| Pasieka JL et al. (2004) [56] | Canada | 1 | M | 46 | M | TT/ND/C | 1111n-Octerotide | 11.954 | Biochemical | NA | 9 | PD |
| SWOG | ||||||||||||
| Valkema R et al. (2002) [39] | Netherlands | 5 | NA | Median: 57.4 (27.7_77.4) | B/Lu | TT/ND/C/EBR/BT | 1111n-Octerotide | 25.14_87.28 | Biochemical | NA | 7.8 (2.76_26.8) | 2SD, 3PD |
| SWOG | 7.8 (2.76_26.8) | 3SD, 2PD | ||||||||||
| Caplin M et al. (2000) [57] | Poland | 1 | F | 46 | NA | NA | 1111n-Octerotide | 11.4 | Biochemical | NA | NA | CR |
| Krenning E et al. (1999) [40] | Netherlands | 3 | NA | NA | NA | NA | 1111n-Octerotide | NA | Imaging | NA | 24 | 1SD, 2PD |
| Buscombe JR et al. (2003) [58] | UK | 2 | NA | Median: 52 (4658) | NA | NA | 1111n-pentetreotide | 25_75 | RECIST | NA | 27.5 (22,33) | 2CR |
| Hayes AR et al. (2019) [59] | UK | 9 | NA | NA | NA | NA | 90Y-DOTATATE and/or 177Lu-DOTATATE | NA | Biochemical | 14 (820) | NA | 6PR, 3PD |
| Puranik A et al. (2019) [60] | India | 28 | F/M: 14/14 | Mean: 47.9 (26_72) | NA | TT/ND/C/EBR | 90Y-DOTATATE and 177Lu-DOTATATE | NA | EORTC | NA | 36 | 17SD |
| 72 | 5PR | |||||||||||
| 24 | 6PD | |||||||||||
| Budiawan H et al. (2013) [6] | Germany | 7 | F/M: 3/4 | Median: 66.5 (21_68) | Lu/Li/B | TT/ND/EBR/ | 90Y-DOTATATE and 177Lu-DOTA-TATE | NA | EORTC | NA | 50.4 (34.866) | 4SD, 1PD, 1PR |
| Scalorbi F et al. (2017) [42] | Italy | 7 | F/M: (4/3) | NA | NA | NA | NA | NA | NA | NA | NA | 5SD, 2PD |
aAbbreviations: NA Not Available, MTC Medullary Thyroid Carcinoma, TTP Time To Progression
Metastatic Site: A Adrenal, Li Liver, Lu Lung, B Bone, M Mediastinum
Prior treatments: TT Total Thyroidectomy, ND Node Dissection, EBR External Beam Radiation, C Chemotherapy, BT Biotherapy with Octreotide, LITT Laser Induced Thermotherapy, REDIFF Redifferentiation Using Roaccutane
Response: CR Complete Response, PR Partial Remission, SD Stable Disease, PD Progressive Disease, MR Minor Remission
Table 3.
Safety of Peptide Receptor Radionuclide Therapy in patients with Advanced RR-DTC & Metastatic MTCa
| Reference | Kind of PRRT | Number & Kind of Tumor | Cumulative activity (GBq) | Hematologic Toxicity | Gastrointestinal& Hepatobiliary Toxicity | Genitourinary Toxicity | Others | Mortality (Median time to death since the first course of PRRT (months)) |
|---|---|---|---|---|---|---|---|---|
| Bertagna F et al. [44] | 90Y-DOTATOC | 1MTC | 9.01 | None | None | None | None | 1 (NA) |
| Bodei L et al. [46] | 90Y-DOTATOC | 21MTC | 7.519.2 | 15 | NA | None | NA | 4 (NA) |
| Bodei L et al. [61] | 90Y-DOTATOC | 4MTC | 3.8_19.2 | None | None | None | None | None |
| Czepczynski R et al. [15] | 90Y-DOTATOC | 3FTC, 3HCTC | 3.7_14.8 | 6 | None | 2 | None | 1 (63) |
| Gorges R et al. [26] | 90Y-DOTATOC | 1papillary-oxyphilic, 1follicular-oxyphilic, 1Hrthle cell carcinoma | 1.7_9.62 | 3mild Lymphocytopenia | None | None | None | 1 (16) |
| Iten F et al. [24] | 90Y-DOTATOC | 17FTC,5PTC, 2No specified | 5.630.3 | 3Anemia, 3Transient Thrombocytopenia, 1Transient Leukopenia | 4nausea | 4permanent renal toxicity | None | 11FTC, 4PTC, 2No (13.7) |
| Iten F et al. [45] | 90Y-DOTATOC | 31MTC | 74.5 | 3Transient Leukopenia, 1Transient Thrombocytopenia | 5Nausea | 6 | NA | 22 (25.8) |
| Versari A et al. [23] | 90Y-DOTATOC | 5PTC, 1Oxiphilic, 3FTC, 2Insular | 4.329_17.95 | 2Transient Anemia, 2Transient Leukopenia | 4nausea, 1transient increase of transaminase | 1permanent renal toxicity | 2Asthenia | None |
| Waldherr C et al. [27] | 90Y-DOTATOC | 12MTC, 4PTC, 3FTC | 1.7_14 | 6Anemia, 10Transient Lymphocytopenia | NA | NA | NA | 1 (1) |
| Traub-Weidinger T et al. [29] | 90Y-DOTATOC | 1FTC, 1PTC | 7.2_7.4 | None | None | 1renal toxicity | None | 1 (22) |
| 90Y-DOTA-Lanerotide | 1FTC, 1HCTC | 1.85_3.7 | 2Transient Thrombocytopenia | None | 2 (4_12) | |||
| Basu. S et al. [30] | 177Lu-DOTATATE | 8 DTC | 5.5_25.4 | None | None | 1 transient | None | 2 (7_12) |
| Beukhof, C et al. [54] | 177Lu-Octreotate | 10MTC | None | 1Diarrhea | None | 1Hemoptysis | 7MTC, 1another cause | |
| Cinkir, H. Y et al. [31] | 177Lu-DOTATATE | 3MTC, 3PTC, 1FTC | 14.8_44.4 | 2Transient Anemia, 3Transient Leukopenia | None | None | None | 1MTC,2PTC |
| Parghane, R. V et al. [50] | 177Lu-DOTATATE | 43MTC | 5.55_33.3 | 1Transient | 1Nausea | None | None | 20 |
| Teunissen Jj et al. [12] | 177Lu-DOTATATE | 3HCTC, 1FTC, 1PTC | 22.430.1 | NA | NA | NA | NA | 1 (48), 1 (4) |
| Vaisman F et al. [52] | 177Lu-DOTATATE | 7MTC | NA | NA | NA | NA | 1transient sexual dysfunction, 2mild hair Loss, 1hypersensitivity dermatologic lesions | 2 (1/7 before the end of the protocol) |
| Valkema R et al. [39] | 1111n-Octerotide | 5MTC, 5DTC | NA | None | None | None | None | 4MTC (11.22 (2.76_26.8)), 5DTC (15.8 (13.2_28.2)) |
| Stokkel Mp et al. [41] | 111In-DTPA-Octreotide | 6PTC, 5FTC | 14.3_33.1 | 1thrombocytopenia | None | None | None | 1 (5), 2non related (1, 3) |
| Budiawan H et al. [6] | 90Y-DOTATATE and 177Lu-DOTATATE | 7MTC, 4FTC, 3HCTC | NA | 8minor hematology, 5Anemia, 1Leukopenia | 6transient increase of transaminase | 5mild renal toxicity | None | 1MTC (12), 2FTC (12), 1HCTC (24) |
Abbreviations: Na Not Available, RR-DTC Radioiodine-Refractory Differentiated Thyroid Cancer, FTC Follicular Thyroid Carcinoma, PTC Papillary Thyroid Carcinoma, HCTC Hurtle Cell Thyroid Carcinoma, MTC Medullary Thyroid Carcinoma
Efficacy of PRRT in RR-DTC
Overall, 157 patients with advanced RR-DTC were treated with PRRT. Based on biochemical response criteria, from 79 treated patients, 20 cases of partial response (PR), 22 cases of stable disease (SD), and 37 cases of persistent disease (PD) were determined. Out of 91 patients whose radiological response was assessed, 9 cases of PR, 39 cases of SD, and 43 cases of PD were recorded. Metabolic response was evaluated in 48 patients. Six cases of PR, 20 cases of SD, and 22 cases of PD were identified.
In 85 patients treated with 90Y -based agent; 44 patients were assessed based on biochemical response among whom 8 cases of PR, 14 cases of SD, and 22 cases of PD were observed. Seven cases of PR, 23 cases of SD, and 25 cases of PD were identified in 55 patients assessed based on radiological response. Moreover, 2 cases of PR, 5 cases of SD, and 4 cases of PD were reported in 11 patients assessed based on metabolic response.
In 26 patients treated with Lutetium-177 -based agent, 10 cases of PR, and 11 cases of PD showed biochemical response. Considering 20 patients assessed for radiological response, 2 cases of PR, 9 cases of SD, and 9 cases of PD were reported. Out of 9 patients assessed for metabolic response, 1 case of PR, 4 cases of SD, and 4 cases of PD were identified.
Moreover, in 18 patients treated with Indium-111, biochemical response was assessed in 14 patients. Two patients with PR, 8 cases with SD, and 4 cases with PD were reported. Seven SD cases and 9 PD cases were recorded based on radiological response in 16 patients.
Among 157 patients with RR-DTC, biochemical and objective responses (partial and complete) were observed in 25.3 and 10.5% of the patients, respectively.
Efficacy of PRRT in metastatic MTC
In total, 220 patients with metastatic MTC were treated with PRRT. Based on biochemical response to the treatment in 145 patients, 7 cases of complete response (CR), 47 cases of PR, 20 cases of SD, and 71 cases of PD were recognized.
Radiologic response was evaluated among 134 patients. Four cases of CR, 9 cases of PR, 75 cases of SD, and 46 cases of PD were observed. Considering metabolic response among 46 patients, 7 cases of PR, 29 cases of SD, and 10 cases of PD were identified.
Sixty-nine patients were treated by 90Y-DOTATOC, 88 patients were treated with 177Lu-DOTA-TATE, and 12 patients were treated with 111_Indium -based agent. Type of treatment was unknown in other patients.
In 69 patients treated with 90Y-DOTATOC, 1 case of CR, 15 cases of PR, 4 cases of SD, and 35 cases of PD (based on biochemical response criteria in 55 patients) as well as 2 cases of CR, 21 cases of SD and 15 cases of PD (based on radiological response criteria in 38 patients) and 1 case of PR and 1 case of PD (based on metabolic response criteria in 2 patients) were reported. Out of 74 patients treated with 177Lu-DOTA-TATE, 5 cases of CR, 26 cases of PR, 14 cases of SD, and 29 cases of PD were observed based on biochemical response criteria. Moreover, 9 cases of PR, 50 cases of SD, and 26 cases of PD were achieved in 85 patients based on radiological response criteria. Furthermore, SD was found in 3 patients based on metabolic response criteria. In the patients treated with 111_Indium -based agent; 1 case of CR, 2 cases of SD, and 4 cases of PD (in 7 patients assessed based on biochemical response) and also, 2 cases of CR, 4 cases of SD, and 5 cases of PD (in 11 patients assessed based on radiological criteria) were reported.
Overall, in the patients with metastatic MTC, biochemical and objective responses were observed in 37.2 and 10.6% of the patients, respectively.
Safety of PRRT
Safety of PRRT was assessed in 19 studies (totally, 239 patients). Death was observed in 109 patients. In addition, time to death varied from 1 to 63months.
In 95 patients with advanced RR-DTC, 46 patients died. Time to death ranged from 1 to 63months from commencement of PRRT. Based on type of PRRT, death occurred in 29/55 patients treated with 90Y -based agent, 6/17 patients treated with 177Lu-DOTA-TATE, and 8/16 patients treated with 111In-Octreotide. Among 44 patients with metastatic MTC, 63 patients died. Time to death ranged from 1 to 26.8months since initiating the first course of PRRT. Based on the type of PRRT, death occurred in 27/69 patients treated with 90Y-DOTATOC, 31/63 patients treated with 177Lu-DOTA-TATE, and 4/5 patients treated with 111In- Octreotide. Major side effects were reported in 124 patients treated with 90Y -based agent. Fourteen patients developed renal toxicity (2 cases of grade 4, 2 cases of grade 3, 2 cases of grade 2, and 8 cases of grade 1). Furthermore, hematologic toxicity was observed in 64 patients (3 cases developed grade 4 of thrombocytopenia, and 1 patient reported to suffer from grade 4 of anemia). Moreover, in 80 patients treated with 177Lu-DOTA-TATE, mild and transient hematologic and renal complications were reported (4 patients with grade 1 and one case with grade 2 of hematologic toxicity and one patient with grade 2 of renal toxicity). Among 21 patients treated with 111In- Octreotide, one patient developed transient thrombocytopenia (grade1).
Discussion
Herein, a comprehensive systematic review was done to investigate efficacy and safety of PRRT in management of advanced RR-DTC and metastatic MTC. The results suggested that PRRT could maintain disease stability with few adverse events. In short-term, toxicity is mild and transient. In addition, long-term toxicity is rare and with low grade. To the best of our knowledge, no similar systematic review or meta-analysis has been done previously to investigate efficacy and safety of PRRT in RR-DTC and metastatic MTC.
There are few recommended treatments for the patients with RR-DTC and therapeutic options are associated with certain limitations in case of the patients with metastatic DTC. The choice of treatment depends on bulk of the tumor. Simple observation, multi-targeted, or mutation-selected kinase inhibitors (MKI), and traditional cytotoxic chemotherapy are the available options [12, 62]. Despite approval of doxorubicin by the food and drug administration (FDA), treatment with cytotoxic agents has shown disappointing results [63]. Therefore, benefit-risk ratio must be carefully evaluated before starting treatment [62].
For majority of the patients with MTC, primary surgery is curative at early stages. However, local and distant metastases after surgery are the major causes of mortality [14]. Resurgery, chemotherapy, external beam radiation therapy, and biological agents, such as RET and MEK inhibitors have yielded disappointing and limited results. Although, treatment with tyrosine kinase inhibitors (TKIs) (Vandetanib and Cabozantinib) improves progression-free survival (PFS), severe adverse events could limit the use of them. There is no curative treatment for these patients, and all the available treatment modalities have been shown to have certain limitations and complications [6].
In the 1990s, the role of SSTR in regulation and proliferation of normal thyroid cells and tumoral tissues was reported that led to introduction of peptide receptor imaging and PRRT in management of metastatic MTC and advanced RR-DTC [15]. Type of SSTRs expression could have an effect on survival rate of these patients [64]. From 5 subtypes of SSTR described in human cells, SSRT2 is expressed in MTC [7]. However, SSRT2 expression has not been identified in papillary or follicular thyroid cancer, and it is irregularly expressed in Hurthle cell adenoma and Hurthle cell carcinoma [65].
Generally, PRRT is able to deliver a high dose of radiation to intracellular components of cancer cells, and induce tumor shrinkage [7]. Currently, PRRT is considered as a safe and effective treatment modality for metastatic inoperable well-differentiated neuroendocrine tumors and advanced pheochromocytomas and paragangliomas [16, 17].
The most frequently used radionuclides in PRRT are 90Y and Lutetium-177. They have different physical characteristics, namely different emission ranges. This results in various maximum tissue penetrations ranging from 3mm for Lutetium-177 to 12mm for 90Y. Since, 90Y has the highest energy and maximum tissue penetration; it is a preferable radionuclide for tumors with large size and poor vascularization. On the other hand, Lutetium-177 emits intermediate-energy suitable for small-sized tumors. Few studies had used 111In-Octreotide, with tissue penetration ranging from 0.2 to 10mm (Table 1) [7]. Krenning et al., for the first time reported treatment of the patients with advanced DTC with 111In-Octreotide analogs. One patient, who received total cumulative activity of at least 20GBq showed disease stabilization [40]. In a pilot study conducted in Netherlands, 9 patients with advanced RR-DTC were treated with high, fixed doses of 111In -Octreotide. Six months after the last therapy, 4 patients had SD, and 5 patients showed PD. Mean Tg value was higher in PD cases than patients with SD. They concluded low Tg value could have a positive effect on the outcome [41].
Grges et al., in a study regarding the first cases of treatment with 90Y-DOTATOC in 3 patients with advanced RR-DTC and pulmonary metastasis showed deceleration in short-term disease progression [26]. In the last report on treatment with 90Y-DOTATOC in RR-DTC, median survival was found to be 21months from initiating the first course of PRRT with only minor and transient hematological toxicity in some patients [15]. Recently, 177Lu-DOTA-TATE has been used more than 90Y but, number of patients treated with this somatostatin analog was limited.
In the patients with metastatic MTC, limited experience with PRRT treatment has been reported. Results of a study on the patients with metastatic MTC suggested that treatment with 90Y-DOTATOC is associated with a long-term survival benefit. However, treatment response was independent of pre-treatment scintigraphy results [45]. Recently, Beukhof et al., reported 17years of experiences with 177Lu-octreotate treatment. They concluded that this treatment could be considered as a treatment in the patients with high uptake on 111In-DTPA-Octreotide scan (uptake grade 3) and positive SSTR2a expression in tumor histology [54]. Budiawan et al., found that the patients with RR-DTC having good response had less undergone other treatment modalities prior to PRRT than non-responders. In addition, they introduced lung metastasis as a poor prognostic factor for survival after PRRT [6].
However, PRRT is not free from adverse effects and minor complications,such as nausea, asthenia, and elevation in liver enzyme level are observed in up to 16.7% of patients, while major complications,such as nephrotoxicity and hematologic adverse events are rare and transient [23, 24]. Proximal tubular reabsorption of radio peptide and its interstitial retention lead to glomerular fibrosis [40], which is markedly observed after treatment with 90Y-DOTATOC. Hence, kidney protection is mandatory along with co-administration of positively-charged amino acids,such as L-lysine and/or L-arginine competitively inhibiting proximal tubular reabsorption of the radio peptide, or prolonged infusion over 10h to 2days after administration of radio peptide. Despite kidney protection, loss of renal function may become clinically evident years after PRRT, especially after administration of 90Y- DOTATOC. Sporadic reported cases of delayed renal failure have received activities greater than 7.4GBq /m2 in very few cycles, without kidney protection [61]. Cumulative and per-cycle renal uptake dose, age, hypertension, diabetes and previous chemotherapy with nephrotoxic agents could accelerate the decrease in renal function after PRRT [66]. Considering these risk factors, one can modify treatment plan or change choice of radio peptide based on burden of tumors. Hematologic side effects generally are mild and temporary,such as reduction in count of lymphocytes and platelets [57].
Our systematic review demonstrated that treatment with PRRT not only could lead to minor complications in approximately 10% of cases but also it can cause very rare and transient major complications.
This systematic review benefited from a comprehensive search conducted by two independent investigators, no time limits, independent reviews by two reviewers, and no publication bias. However, the main limitation of the present study was low quality of the available evidence. However, other underlying problems and limitations included retrospective nature of the studies, a selection bias, the amount of radioactivity administered (183GBq), non-uniform response criteria, huge difference in follow-up periods (199months),and the limited number of patients per report. Also, our search was restricted to English -language papers.
This systematic review investigated efficacy and safety of PRRT in treatment of RR-DTC and metastatic MTC. Given paucity of evidence, it is recommended to perform further multi-center randomized controlled clinical trials.
Conclusions
According to findings of our study, due to lack of various treatment modalities, PRRT could be an option for treatment of advanced RR-DTC, as well as metastatic MTC, with few adverse events.
Supplementary Information
Additional file 1: Supplemental Table1. Medline (Pubmed, Ovid and Ebsco), Scopus, Embase, Web of Science and the Cochrane Library database (Last Updated March 24, 2021).
Additional file 2: Supplemental Table2. Risk of bias assessment.
Acknowledgements
None.
Abbreviations
- RR-DTC
Radioiodine-refractory differentiated thyroid cancer
- MTC
Medullary thyroid cancer
- PFS
Progression-free survival
- SSTR
Somatostatin receptor
- PRRT
Peptide receptor radionuclide therapy
- TTP
Time to progression
- Tg
Thyroglobulin
- CEA
Carcino embryogenic antigen
- WHO
World health organization
- RECIST
Response evaluation criteria in solid tumors
- SWOG
Southwest oncology group
- EORTC
European organization for research and treatment of cancer
- RAI
Radioactive iodine
- CR
Complete response
- SD
Stable disease
- PR
Partial response
- PD
Persistent disease
- TKI
Tyrosine kinase inhibitors
- MKI
Mutation-selected kinase inhibitors
- FDA
Food and drug administration
Authors contributions
MK had the original idea of this work. MK, MM, RM and ZM designed and conceived the protocol. ZE and ZM designed the search strategies. MK, ZM and RM performed the data extraction and wrote the manuscript. All authors critically revised the draft of the manuscript and approved its final version.
Authors information
Affiliations
Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences (IUMS), Tehran, Iran
Zohreh Maghsoomi, Zahra Emami, Ramin Malboosbaf & Mohammad E. Khamseh
Research Center for Prevention of Cardiovascular Disease, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences (IUMS), Tehran, Iran
Mojtaba Malek
Funding
For this study no funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publishers Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al (eds). SEER Cancer Statistics Review, 1975-2011. Bethesda: National Cancer Institute; 2014. https://seer.cancer.gov/archive/csr/1975_2011/.
- 2.Atlanta G. Cancer facts and figures. 2015. [Google Scholar]
- 3.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338(5):297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande HA, Gettinger SN, Sosa JA. Novel chemotherapy options for advanced thyroid tumors: small molecules offer great hope. Curr Opin Oncol. 2008;20(1):19–24. doi: 10.1097/CCO.0b013e3282f28373. [DOI] [PubMed] [Google Scholar]
- 5.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns] Cancer. 1998;83(12):2638–2648. doi: 10.1002/(SICI)1097-0142(19981215)83:12<2638::AID-CNCR31>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Budiawan H, Salavati A, Kulkarni HR, Baum RP. Peptide receptor radionuclide therapy of treatment-refractory metastatic thyroid cancer using (90) yttrium and (177) lutetium labeled somatostatin analogs: toxicity, response and survival analysis. Am J Nucl Med Mol Imaging. 2013;4(1):39–52. [PMC free article] [PubMed] [Google Scholar]
- 7.Salavati A, Puranik A, Kulkarni HR, Budiawan H, Baum RP. Peptide receptor radionuclide therapy (PRRT) of medullary and nonmedullary thyroid cancer using radiolabeled somatostatin analogues. Semin Nuclear Med. 2016;46(3):21524. 10.1053/j.semnuclmed.2016.01.010. [DOI] [PubMed]
- 8.Silberstein EB. The problem of the patient with thyroglobulin elevation but negative iodine scintigraphy: the TENIS syndrome. Semin Nucl Med. 2011;41(2):113–120. doi: 10.1053/j.semnuclmed.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Sherman SI. Thyroid carcinoma. Lancet. 2003;361(9356):501–511. doi: 10.1016/S0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 10.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, de Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 11.Santini F, Bottici V, Elisei R, Montanelli L, Mazzeo S, Basolo F, Pinchera A, Pacini F. Cytotoxic effects of carboplatinum and epirubicin in the setting of an elevated serum thyrotropin for advanced poorly differentiated thyroid cancer. J Clin Endocrinol Metab. 2002;87(9):4160–4165. doi: 10.1210/jc.2001-011151. [DOI] [PubMed] [Google Scholar]
- 12.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13(5):539–547. doi: 10.1634/theoncologist.2007-0239. [DOI] [PubMed] [Google Scholar]
- 14.Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M. New therapeutic approaches to treat medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab. 2008;4(1):22–32. doi: 10.1038/ncpendmet0717. [DOI] [PubMed] [Google Scholar]
- 15.Czepczynski R, Matysiak-Grzes M, Gryczynska M, Baczyk M, Wyszomirska A, Stajgis M, et al. Peptide receptor radionuclide therapy of differentiated thyroid cancer: efficacy and toxicity. Arch Immunol Ther Exp. 2015;63(2):147–154. doi: 10.1007/s00005-014-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satapathy S, Mittal BR, Bhansali A. Peptide receptor radionuclide therapy in the management of advanced pheochromocytoma and paraganglioma: A systematic review and meta-analysis. Clin Endocrinol. 2019;91(6):71827. 10.1111/cen.14106. [DOI] [PubMed]
- 17.Gulenchyn K, Yao X, Asa S, Singh S, Law C. Radionuclide therapy in neuroendocrine tumours: a systematic review. Clin Oncol. 2012;24(4):294–308. doi: 10.1016/j.clon.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julka PK, Doval DC, Gupta S, Rath GK. Response assessment in solid tumours: a comparison of WHO, SWOG and RECIST guidelines. Br J Radiol. 2008;81(966):444–449. doi: 10.1259/bjr/32785946. [DOI] [PubMed] [Google Scholar]
- 20.Pinker K, Riedl C, Weber WA. Evaluating tumor response with FDG PET: updates on PERCIST, comparison with EORTC criteria and clues to future developments. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):55–66. doi: 10.1007/s00259-017-3687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Institute NC . Common terminology criteria for adverse events (CTCAE) version 4.0. 2010. [Google Scholar]
- 22.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Versari A, Sollini M, Frasoldati A, Fraternali A, Filice A, Froio A, Asti M, Fioroni F, Cremonini N, Putzer D, Erba PA. Differentiated thyroid cancer: a new perspective with radiolabeled somatostatin analogues for imaging and treatment of patients. Thyroid. 2014;24(4):715–726. doi: 10.1089/thy.2013.0225. [DOI] [PubMed] [Google Scholar]
- 24.Iten F, Muller B, Schindler C, Rasch H, Rochlitz C, Oertli D, Maecke HR, Muller-Brand J, Walter MA. (90) yttrium-DOTA -TOC response is associated with survival benefit in iodine-refractory thyroid Cancer long-term results of a phase 2 clinical trial. Cancer. 2009;115(10):2052–2062. doi: 10.1002/cncr.24272. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel M, Froehlich F, Decristoforo C, Ensinger C, Donnemiller E, von Guggenberg E, Heute D, Moncayo R. 99mTc-EDDA/HYNIC-TOC and (18) F-FDG in thyroid cancer patients with negative (131) I whole-body scans. Eur J Nucl Med Mol Imaging. 2004;31(3):330–341. doi: 10.1007/s00259-003-1376-x. [DOI] [PubMed] [Google Scholar]
- 26.Gorges R, Kahaly G, Muller-Brand J, Macke H, Roser HW, Bockisch A. Radionuclide-labeled somatostatin analogues for diagnostic and therapeutic purposes in nonmedullary thyroid cancer. Thyroid. 2001;11(7):647–659. doi: 10.1089/105072501750362718. [DOI] [PubMed] [Google Scholar]
- 27.Waldherr C, Schumacher T, Pless M, Crazzolara A, Maecke HR, Nitzsche EU, et al. Radiopeptide transmitted internal irradiation of non-iodophil thyroid cancer and conventionally untreatable medullary thyroid cancer using. Nucl Med Commun. 2001;22(6):673–678. doi: 10.1097/00006231-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Virgolini I, Britton K, Buscombe J, Moncayo R, Paganelli G, Riva P. In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin Nucl Med. 2002;32(2):148–155. doi: 10.1053/snuc.2002.31565. [DOI] [PubMed] [Google Scholar]
- 29.Traub-Weidinger T, Raderer M, Uffmann M, Angelberger P, Kurtaran A, Leimer M, et al. Improved quality of life in patients treated with peptide radionuclides. World J Nucl Med. 2011;10(2):115–121. doi: 10.4103/1450-1147.89779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu S, Parghane RV, Naik C. Clinical efficacy of (177) Lu-DOTATATE peptide receptor radionuclide therapy in thyroglobulin-elevated negative iodine scintigraphy: a "not-so-promising" result compared to GEP-NETs. World J Nucl Med. 2020;19(3):205–210. doi: 10.4103/wjnm.WJNM_21_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cinkir HY, Elboga U. An alternative therapy option in metastatic thyroid Cancer: peptide receptor radionuclide therapy. J Istanb Fac Med. 2020;83(4):339–344. [Google Scholar]
- 32.Roll W, Riemann B, Schfers M, Stegger L, Vrachimis A. 177Lu-DOTATATE therapy in radioiodine-refractory differentiated thyroid Cancer: a single center experience. Clin Nucl Med. 2018;43(10):e346–ee51. doi: 10.1097/RLU.0000000000002219. [DOI] [PubMed] [Google Scholar]
- 33.Olivan-Sasot P, Falgas-Lacueva M, Garcia-Sanchez J, Vera-Pinto V, Olivas-Arroyo C, Bello-Arques P. Use of (177) Lu-dotatate in the treatment of iodine refractory thyroid carcinomas. Rev Esp Med Nucl Imagen Mol. 2017;36(2):116–119. doi: 10.1016/j.remn.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Elboa U, zkaya M, Sayiner ZA, elen YZ. Lu-177 labelled peptide treatment for radioiodine refractory differentiated thyroid carcinoma. BMJ Case Rep. 2016;8;2016:bcr2015213627. 10.1136/bcr-2015-213627. [DOI] [PMC free article] [PubMed]
- 35.Jois B, Asopa R, Basu S. Somatostatin receptor imaging in non131I-avid metastatic differentiated thyroid carcinoma for determining the feasibility of peptide receptor radionuclide therapy with 177Lu-DOTATATE: low fraction of patients suitable for peptide receptor radionuclide therapy and evidence of Chromogranin a levelpositive neuroendocrine differentiation. Clin Nucl Med. 2014;39(6):505–510. doi: 10.1097/RLU.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 36.Teunissen JJ, Kwekkeboom DJ, Kooij PP, Bakker WH, Krenning EP. Peptide receptor radionuclide therapy for non-radioiodine-avid differentiated thyroid carcinoma. J Nucl Med. 2005;46(Suppl 1):107s–114s. [PubMed] [Google Scholar]
- 37.Parihar AS, Sood A, Kumar R, Bhusari P, Shukla J, Mittal BR. Novel use of (177) Lu-DOTA-RGD2 in treatment of (68) Ga-DOTA-RGD2-avid lesions in papillary thyroid cancer with TENIS. Eur J Nucl Med Mol Imaging. 2018;45(10):1836–1837. doi: 10.1007/s00259-018-4036-x. [DOI] [PubMed] [Google Scholar]
- 38.Campenn A, Pignata SA, Baldari S. Can peptide receptor radionuclide therapy (PRRT) be useful in radioiodine-refractory differentiated thyroid cancer? Endocrine. 2015;50(2):516–518. doi: 10.1007/s12020-014-0491-8. [DOI] [PubMed] [Google Scholar]
- 39.Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, et al. Phase I study of peptide receptor radionuclide therapy with [in-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32(2):110–122. doi: 10.1053/snuc/2002.31025. [DOI] [PubMed] [Google Scholar]
- 40.Krenning E, De Jong M, Kooij P, Breeman W, Bakker W, De Herder W, et al. Radiolabelled somatostatin analogue (s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol. 1999;10(suppl_2):S23–SS9. doi: 10.1093/annonc/10.suppl_2.S23. [DOI] [PubMed] [Google Scholar]
- 41.Stokkel MP, Verkooijen RB, Bouwsma H, Smit JW. Six month follow-up after 111In-DTPA-octreotide therapy in patients with progressive radioiodine non-responsive thyroid cancer: a pilot study. Nucl Med Commun. 2004;25(7):683–690. doi: 10.1097/01.mnm.0000130244.14444.5e. [DOI] [PubMed] [Google Scholar]
- 42.Scalorbi F, Filice F, Sollini M, Menga M. Peptide receptor radionuclide therapy (PRRT) in metastatic thyroid tumors: an opportunity after traditional treatment failure. Clin Transl Imaging. 2017;5(Suppl 1):S100. [Google Scholar]
- 43.ksz M, Winter L, Pfannenberg C, Reischl G, Mssig K, Bares R, et al. Peptide receptor radionuclide therapy of neuroendocrine tumors with 90Y-DOTATOC: is treatment response predictable by pre-therapeutic uptake of 68Ga-DOTATOC? 2014;95(3):289300. [DOI] [PubMed]
- 44.Bertagna F, Giubbini R, Savelli G, Pizzocaro C, Rodella C, Biasiotto G, Lucchini S, Maroldi R, Rosenbaum J, Alavi A. A patient with medullary thyroid carcinoma and right ventricular cardiac metastasis treated by (90) Y-Dotatoc. Hell J Nucl Med. 2009;12(2):161–164. [PubMed] [Google Scholar]
- 45.Iten F, Muller B, Schindler C, Rochlitz C, Oertli D, Macke HR, et al. Response to (90) yttrium-DOTA -TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clin Cancer Res. 2007;13(22):6696–6702. doi: 10.1158/1078-0432.CCR-07-0935. [DOI] [PubMed] [Google Scholar]
- 46.Bodei L, Handkiewicz-Junak D, Grana C, Mazzetta C, Rocca P, Bartolomei M, Lopera Sierra M, Cremonesi M, Chinol M, Mcke HR, Paganelli G. Receptor radionuclide therapy with 90Y-DOTATOC in patients with medullary thyroid carcinomas. Cancer Biother Radiopharm. 2004;19(1):65–71. doi: 10.1089/108497804773391694. [DOI] [PubMed] [Google Scholar]
- 47.Gao ZR, Biersack HJ, Ezziddin S, Logvinski T, An R. The role of combined imaging in metastatic medullary thyroid carcinoma: In-111-DTPA-octreotide and I-131/I-123-MIBG as predictors for radionuclide therapy. J Cancer Res Clin Oncol. 2004;130(11):649–656. doi: 10.1007/s00432-004-0588-1. [DOI] [PubMed] [Google Scholar]
- 48.Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, Maecke HR, Muller J. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med. 1999;26(11):1439–1447. doi: 10.1007/s002590050476. [DOI] [PubMed] [Google Scholar]
- 49.Bilgic S, Saer MS, Beytur MF, Nazari A, Uslu Beli RL, Asa S, et al. The effectiveness of 177Lu-DOTATATE in patients with metastatic medullary thyroid cancer. Eur J Nucl Med Mol Imaging. 2020;47(SUPPL 1):S27–SS8. [Google Scholar]
- 50.Parghane RV, Naik C, Talole S, Desmukh A, Chaukar D, Banerjee S, Basu S. Clinical utility of Lu-177-DOTATATE PRRT in somatostatin receptor-positive metastatic medullary carcinoma of thyroid patients with assessment of efficacy, survival analysis, prognostic variables, and toxicity. Head Neck-J Sci Spec Head Neck. 2020;42(3):401–416. doi: 10.1002/hed.26024. [DOI] [PubMed] [Google Scholar]
- 51.Makis W, McCann K, McEwan AJ. Medullary thyroid carcinoma (MTC) treated with 177Lu-DOTATATE PRRT: a report of two cases. Clin Nucl Med. 2015;40(5):408–412. doi: 10.1097/RLU.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 52.Vaisman F, Rosado de Castro PH, Lopes FP, Kendler DB, Pessoa CH, Bulzico DA, et al. Is there a role for peptide receptor radionuclide therapy in medullary thyroid cancer? Clin Nucl Med. 2015;40(2):123–127. doi: 10.1097/RLU.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 53.Soydal , Peker A, zkan E, Kk N, Kir MK. The role of baseline Ga-68 DOTATATE positron emission tomography/computed tomography in the prediction of response to fixed-dose peptide receptor radionuclide therapy with lu-177 DOTATATE. Turkish J Med Sci. 2016;46(2):409–413. doi: 10.3906/sag-1412-11. [DOI] [PubMed] [Google Scholar]
- 54.Beukhof CM, Brabander T, van Nederveen FH, van Velthuysen MF, de Rijke YB, Hofland LJ, et al. Peptide receptor radionuclide therapy in patients with medullary thyroid carcinoma: predictors and pitfalls. BMC Cancer. 2019;19(1):325. doi: 10.1186/s12885-019-5540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mathew D, Hephzibah J, Shanthly N, Oommen R. Overview of peptide receptor radionuclide therapy with 177Lutetium-DOTATATE in our institution: 4 years' experience. Indian J Nucl Med. 2018;33(5):S65. [Google Scholar]
- 56.Pasieka JL, McEwan AJB, Rorstad O. The palliative role of 131I-MIBG and 111In-octreotide therapy in patients with metastatic progressive neuroendocrine neoplasms. Surgery. 2004;136(6):1218–1226. doi: 10.1016/j.surg.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 57.Caplin M, Mielcarek W, Buscombe J, Jones A, Croasdale P, Cooper M, et al. Toxicity of high-activity 111In-Octreotide therapy in patients with disseminated neuroendocrine tumours. Nucl Med Commun. 2000;21(1):97–102. doi: 10.1097/00006231-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Buscombe JR, Caplin ME, Hilson AJW. Long-term efficacy of high-activity 111in-pentetreotide therapy in patients with disseminated neuroendocrine tumors. J Nucl Med. 2003;44(1):1–6. [PubMed] [Google Scholar]
- 59.Hayes AR, Crawford A, Al Riyami K, Tang C, Wild D, Khoo B, et al. Metastatic medullary thyroid cancer (MTC): is there a role for peptide receptor radionuclide therapy (PRRT)? Neuroendocrinology. 2019;108:273. [Google Scholar]
- 60.Puranik A, Baum RP, Kulkarni H, Singh A, Rangarajan V, Agrawal A, et al. Peptide receptor radionuclide therapy using 177Lu and 90Y-DOTATATE in metastatic treatment-refractory medullary thyroid cancer. Neuroendocrinology. 2019;108:228. [Google Scholar]
- 61.Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90 Y-DOTATOC and 177 Lu-DOTATATE: the role of associated risk factors. 2008;35(10):184756. [DOI] [PubMed]
- 62.Weitzman SP, Sherman SI. Novel drug treatments of progressive radioiodine-refractory differentiated thyroid Cancer. Endocrinol Metab Clin N Am. 2019;48(1):253–268. doi: 10.1016/j.ecl.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Oncol. 2010;22(6):464–468. doi: 10.1016/j.clon.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 64.de Vries LH, Lodewijk L, Willems SM, Dreijerink KMA, de Keizer B, van Diest PJ, Schepers A, Bonenkamp HJ, van Engen-van Grunsven IACH, Kruijff S, van Hemel BM, Links TP, Nieveen van Dijkum EJM, van Eeden S, Valk GD, Borel Rinkes IHM, Vriens MR. SSTR2A expression in medullary thyroid carcinoma is correlated with longer survival. Endocrine. 2018;62(3):639–647. doi: 10.1007/s12020-018-1706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forssell-Aronsson EB, Nilsson O, Bejegard SA, Kolby L, Bernhardt P, Molne J, et al. 111In-DTPA-D-Phe1-octreotide binding and somatostatin receptor subtypes in thyroid tumors. J Nucl Med. 2000;41(4):636–642. [PubMed] [Google Scholar]
- 66.Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90) Y-DOTA (0),Tyr (3)-octreotide and (177) Lu-DOTA (0), Tyr (3)-octreotate. J Nucl Med. 2005;46(Suppl 1):83s–91s. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table1. Medline (Pubmed, Ovid and Ebsco), Scopus, Embase, Web of Science and the Cochrane Library database (Last Updated March 24, 2021).
Additional file 2: Supplemental Table2. Risk of bias assessment.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].