Abstract
Mindfulness-based relapse prevention (MBRP) is a therapy for addictive behaviors that incorporates cognitive-behavioral relapse prevention (RP) skills with mindfulness training to increase awareness and skillful action in high-risk situations. Stress is a common reason reported for substance use relapse, and using physiological measures to measure stress engagement may help us identify mechanisms of clinical improvement. Specifically, salutatory changes in HF-HRV post-treatment may serve as a marker of treatment efficacy. We investigated tonic and phasic heart rate variability (HRV) to a cognitive stressor (i.e., arithmetic challenge) following 8 weeks of RP, MBRP, or post-detox treatment known as treatment as usual (TAU; n = 34). MBRP was related to higher levels of tonic and phasic HF-HRV, lower levels of anxiety, and lower heart rate reactivity (than TAU only) compared to RP and TAU. This suggests that those who completed MBRP are engaging with stress, but perhaps in a more adaptive, flexible manner. MBRP is associated with higher cardiac vagal control and lower stress/anxious reactivity. Given that negative emotions are an important component of relapse, these results lend further support to say that mindfulness may be helpful for those with substance use disorders.
Keywords: Mindfulness, Substance use, Cardiac vagal control, Heart rate variability, Stress
Introduction
In the USA, upwards of 11% of the population abuses or is dependent upon alcohol and other drugs (Merikangas and McClair 2012). According to the 2010 National Survey on Drug Use and Health (2010), illicit drug use in the USA reached an 8-year peak with an estimated 22 million people qualifying as substance users. The impact of these rates is compounded by the fact that the majority of addicts who attempt abstinence relapse or return to problematic substance use (McLellan et al. 2000).
Stress (psychological distress) and stressors (stressful life events) are among the most frequently endorsed reasons for relapse in those with substance use disorders (Brownwell et al. 1986; Sinha 2001). Moreover, the frequency and severity of stressful life events predict use in substance abusers (Dawson et al. 2005). Activation of the brain stress system appears to be a key factor in the transition from drug use to abuse, in part, by playing a role in the generation of negative emotions which abusers are driven to abate (Koob 2008). In those with addiction, activation of the stress system (both central and peripheral) has profound effects on drug craving, which can enhance relapse susceptibility among those trying to remain abstinent (Sinha 2007). Stress-related psychopathology and substance use disorders have been theorized to share underlying etiological determinants (Brady and Sinha 2005). Thus, identifying markers of the stress process that may characterize addiction, recovery, or serve to predict relapse are of paramount importance.
Newer scientific models outlining adaptive stress processes modulated by the Xth cranial nerve have led to a burgeoning area of research that utilizes high-frequency heart rate variability as an index of inhibitory cardiac vagal control (Porges 2005, 2007; Thayer and Lane 2000, 2009). Heart rate variability (HRV) refers to time variations in the beat-to-beat interval (i.e., the interval between successive R waves on the electrocardiogram) produced by parasympathetic and sympathetic innervation of the sinoatrial (SA) node of the heart. Various quantifications of HRV exist, which are broadly classified into time and frequency domain measures of variability (Bernston et al. 1997). These variability measures can be further partitioned into high-and low-frequency components. At the level of the SA node, high-frequency heart rate variability (HF-HRV; 0.15–0.40 Hz) captures inhibitory parasympathetic (i.e., vagal) input. Conversely, low-frequency heart rate variability (LF-HRV; 0.04–0.15 Hz) effectively captures sympathetic and parasympathetic activity at the SA node. Thus, investigations of cardiac vagal control (or what some call vagal tone) report changes in HF-HRV or its analogous measure, respiratory sinus arrhythmia (RSA). As a result, the terms cardiac vagal control, vagal tone, HF-HRV, and RSA are used interchangeably in the literature to index vagal-mediated processes, such as those associated with stress. Some theories (Porges 2005, 2007) suggest that these cardiac vagal control processes allow for self-regulation when faced with stressors. This self-regulation may require attention, emotional attenuation, or communication, whereby activation of the parasympathetic nervous system would be more adaptive than the “classic” fight or flight response.
Research demonstrates that both tonic levels (i.e., baseline) and phasic changes (Beauchaine 2001; Castaldo et al. 2015; Ditto et al. 2006; Minami et al. 1999; Sarang and Telles 2006; Tang et al. 2009; Thayer et al. 1996) in HF-HRV are associated with various clinical conditions and treatment outcomes. In keeping with prior research of this type, tonic or resting vagal activity is measured in the current study during baseline, whereas phasic cardiac vagal activity is measured in response to the laboratory challenge. The theory of neurovisceral integration (Thayer and Lane 2000) argues that cardiac vagal control can be used as an index of the connection between physiological, affective, and cognitive processes. Specifically, neurovisceral integration argues that cardiac vagal control can potentially be used as an index of stress adaptability or even an endophenotype of clinical conditions. Under this model, tonic HF-HRV is a measure of adaptive capacity including inhibitory processes, whereas phasic changes in response to a challenge (e.g., a laboratory stressor) provide an index of self-regulation or adaptability. Thus, low tonic HF-HRV may indicate less capacity to regulate or adapt and the absence of a phasic response may demonstrate poor regulation or adaptability.
Indeed, reduced tonic cardiac vagal control has been observed in various clinical conditions including alcohol use disorder (Ingaldsson et al. 2003) and alcohol craving (Quintana et al. 2013), nicotine dependence (Conrad et al. 2015), post-traumatic stress disorder (Cohen et al. 1997, 1998), anxiety disorders (Chalmers et al. 2014; Thayer et al. 1996), panic disorder (Friedman and Thayer 1998), and depression (Chambers and Allen 2002; Friedman and Thayer 1998). Moreover, tonic cardiac vagal control has been shown to increase following treatments for some of the conditions listed above including depression (Bylsma et al. 2014), anxiety (Middleton and Ashby 1995), and smoking (Minami et al. 1999). These findings suggest that improved tonic cardiac vagal control may serve as marker of improvements following treatment.
Challenges such as stressors provoke changes in phasic HF-HRV, which is an index of self-regulation or adaptability. In the face of a threatening challenge, one adaptive response is to engage activation of the sympathetic nervous system in order to mobilize resources to deal with the threat. Activation of the sympathetic “fight or flight” response is partly dependent upon withdrawal of the parasympathetic nervous system, and investigators of the sympathetic response have adopted the phrase “task appropriate reduction in HF-HRV” in describing responses to threatening stimuli (Thayer and Lane 2009). Several studies demonstrate this reduction in healthy individuals used as control subjects (Castaldo et al. 2015). More exaggerated vagal withdrawal responses are associated with emotional liability and found to be associated with aggression, panic, and marital dysfunction (Beauchaine 2001), leading several researchers to investigate behaviors and/or practices that increase HF-HRV. Given that withdrawal of HF-HRV is associated with stress, behaviors that are associated with stress reduction or awareness of stress have been of particular interest. Among those studied to date, both relaxation (Sarang and Telles 2006) and mindfulness meditation (Ditto et al. 2006; Tang et al. 2009) increase HF-HRV.
Given that HF-HRV increases during mindfulness practice in healthy participants (Tang et al. 2009), it is reasonable to hypothesize that mindfulness training may prove to be one method of engaging the parasympathetic nervous system in response to stressors in substance abusers. Yet, few published studies test this latter hypothesis.
In one study, Brewer et al. (2009) investigated the effects of stress provocation on autonomic nervous system responses in participants meeting criteria for alcohol and/or cocaine abuse or dependence who completed a mindfulness training form of therapy vs standard cognitive-behavioral therapy (CBT). Results revealed greater vagal recruitment during stress provocation among those that received mindfulness training compared to those who received CBT. Parasympathetic nervous system activation may contribute to treatment success for smokers as Libby et al. (2012) demonstrated that smokers who increased HF-HRV during meditation practice smoked fewer cigarettes 17 weeks post-treatment than smokers who failed to engage the parasympathetic nervous system during meditation.
In another study, Garland (2011) investigated the effects of alcohol cue exposure on a time domain estimate of HF-HRV (i.e., square root of the mean-squared difference (RMSSD) between successive beat-to-beat intervals). Participants were members of a therapeutic community who met criteria for alcohol dependence including those with psychotic symptoms and comorbid substance use disorders. Utilizing a randomized controlled design, participants were assigned to either a mindfulness training form of therapy or a support group. Results of cue exposure effects on HF-HRV following 10 weeks of intervention revealed a task suppression effect in the support group while those that received mindfulness training showed an increase in HF-HRV, albeit not statistically significant. However, mindfulness was associated with improved physiological recovery (defined as RMSSD rate of return to baseline) from craving cue exposure.
The science of delineating HF-HRV tonic and phasic response patterns as unique markers of clinical symptoms and/or recovery or treatment effects is in its infancy. While Bowen and colleagues (Bowen et al. 2006, 2009) have shown that mindfulness-based relapse prevention (MBRP)is an effective aftercare treatment for substance use disorders, HRV profiles have yet to be explored. Those treated with MBRP evince less post-treatment substance use and substance-related problems than participants who receive standard treatments without a mindfulness component. MBRP also exerts salutary effects on negative affect and craving which include both a reduction in self-reported craving and negative affect (Bowen et al. 2009) and a buffering of the relationship between negative affect (depression symptoms) and craving (Witkiewitz and Bowen2010).Considering research explicating adaptive stress processes, we speculate that if the mindfulness strategies taught in MBRP allow one to pause in the face of a stressor (e.g., craving), accept such negative feelings without trying to alter, or behaviorally act on them, we should see a measurable increase in HF-HRV during a laboratory stressor among those who have practiced mindfulness.
Thus, the purpose of this study was to delineate psychophysiological responses, including tonic HF-HRV differences post-treatment and phasic HF-HRV differences to a laboratory challenge, among those treated with MBRP, relapse prevention (RP), and standard post-detox aftercare among clients from a private, non-profit, community recovery center. We expected that MBRP would produce more favorable responses than the other two treatment groups. Specifically, we hypothesized that MBRP would be related to less heart rate and blood pressure reactivity to the stress- or than the other two treatment groups. Also, compared to RP and treatment as usual (TAU), we expected MBRP to have higher tonic HF-HRV and to respond to the stressor with increased HF-HRV (i.e., greater phasic HF-HRV). Using psychological self-report measures for state anxiety and craving, we hypothesized that those who received MBRP would report less state anxiety and craving in response to the stressor than the other two treatment groups and that HF-HRV would be inversely related to self-reported measures.
Method
Participants
Participants (n = 34) were recruited from a phase III clinical trial (NIH/NIDA 1 R01 DA025764-01A1), hereafter referred to as the parent study, assessing the therapeutic efficacy of mindfulness-based relapse prevention among clients at a private, non-profit substance abuse care facility in the Pacific Northwest. The treatment agency provides addiction services to a broad population. More information about the agency can be found in Bowen et al. (2014). Participants were eligible for the parent study if they were (1) fluent in English, (2) between the ages of 18 to 70, (3) medically cleared to participate in treatment, (4) had completed intensive outpatient treatment at least within the last 2 weeks with a continuing care plan set up within the target treatment center, and (5) willing to be randomly assigned to either the MBRP, RP, or TAU treatment group. Participants with psychosis and/or endorsed suicidal thoughts were excluded. In the present study, eligible participants had completed the MBRP, RP, or TAU groups within the last 2 months, and had no pre-existing health condition (e.g., hypertension) or taking any medications (e.g., antipsychotics) known to affect the stress response.
Procedure
Participants in the parent study were randomly assigned to participate in 8 weeks of MBRP, RP, or TAU. All groups were held once a week. If participants were required by the treatment agency (e.g., mandated treatment) to attend more than one group a week, the second group was a TAU supplement. All treatment contact including urine analyses, weekly checkins, and sober support groups (e.g., alcoholics anonymous) were tracked.
MBRP (Bowen et al. 2010) was performed in accordance with the published manual. MBRP is an 8-week therapy program based off of RP and mindfulness-based stress reduction (MBSR; Kabat-Zinn 1990). MBRP begins by establishing a foundation of mindfulness in formal and informal practices, and then builds in cognitive-based relapse prevention strategies aimed at experiencing substance use-related cues with a new awareness.
RP (Marlatt and Gordon 1985) was modified to be used in a group format. RP is considered the gold standard for alcohol and other drug use. RP utilizes cognitive behavioral strategies, such as urge surfing, to target substance use-related cues.
TAU included process-oriented groups, such as 12-step, as facilitated on a regular basis at the community treatment agency.
Following approval of the University of Washington and Seattle Pacific University Institutional Review Boards, participants who had completed the MBRP, RP, or TAU groups within the last 2 months were contacted over the phone. A researcher described the study and provided interested participants with a number to call for screening. Potential participants were screened over the phone (n = 77). Individuals who were not interested in participation (n = 4) or with health conditions known to affect the stress response such as a respiratory or cardiovascular disorder (n = 25) were excluded. Out of the eligible participants (n = 48; MBRP n = 16; RP n = 16; TAU n = 16), approximately 25% of participants (n = 13) did not come to their stress testing appointment, resulting in n = 35 participants who completed the stress testing protocol. One additional TAU participant was subsequently excluded from analyses for protocol violation (i.e., completed the parent study as an RP participant and re-enrolled as TAU participant), resulting in a final sample of 34 participants (MBRP n = 12; RP n = 12; TAU n = 10). Because the luteal phase of the menstrual cycle is associated with increased hemodynamic responses to laboratory stressors (Lustyk et al. 2010), craving (Carpenter et al. 2006; Lustyk et al. 2011), anxiety in women with substance use disorders (Fox and Sinha 2009), and reduced HF-HRV (Bai et al. 2009; Sato et al. 1995), all female participants were tested during the follicular menstrual cycle phase (cycle days 5–9, n = 4). Female participants who were not menstruating (e.g., menopausal, n = 3) were scheduled for the next convenient stress-testing time block. Analyses were run to confirm that there was no systematic time bias between finishing the treatment group and the stress testing session by gender or group. A one-way ANOVA revealed no significant differences between the groups on time between the treatment end date and the stress testing session (F(2) = 0.01, p = 0.99), and an independent sample t test revealed no significant gender differences in time between the treatment end date and the stress testing session (t(32) = −0.76, p = 0.46).
Eligible participants were scheduled for one 1-h stress testing session (Fig. 1) that was conducted at the Recovery Center in a quiet and isolated room conducive to physiological monitoring without interference. The stress testing session was scheduled mid-day, between the hours of 11:00 and 17:00, to control for cortisol diurnal rhythm. Participants were asked to refrain from exercise the hour prior to testing, and maintain abstinence from alcohol and other drug use before the stress test. We observed 100% compliance with alcohol and other drug abstinence as indicated from salivary drug screening. Two salivary drug screens were used to assess for cocaine, opiates, amphetamine, methamphetamine, phencyclidine, THC (3 days of sensitivity; Oral Lab +6 Panel, Varian Medical Systems, Palo Alto, CA), and alcohol (24 h of sensitivity; Alcohol Panel, Varian Medical Systems, Palo Alto, CA). Following consent, participants completed the Oral Lab +6 Panel which took 3 min to collect and 10 min to read. Next, participants completed the Alcohol Panel which took less than 30 s to collect and 2 min to read. Following the salivary screen, participants completed the demographic, Non-Exercise Inventory (Non-Ex), and State/Trait Anxiety Inventory–Trait (STAI-T) measures. After completing these measures, the 15-min baseline began. During the baseline, participants completed the verbal analogue scales and the State/Trait Anxiety Inventory–State (STAI-S). Following the baseline, participants were instructed in how to engage in the Paced Auditory Serial Addition Task (PASAT) with standardized instruction protocol. Immediately following the PASAT, participants completed the verbal analogue scales and the STAI-S for the second time and transitioned into a 15-min recovery. After the 15-min recovery, participants completed the verbal analogue scales and the STAI-S for a final time. Following the stress testing session, participants were compensated with a $20 gift card to a major grocery store chain and continued enrollment in the phase iii clinical trial assessing the therapeutic efficacy of mindfulness-based relapse prevention.
Fig. 1.
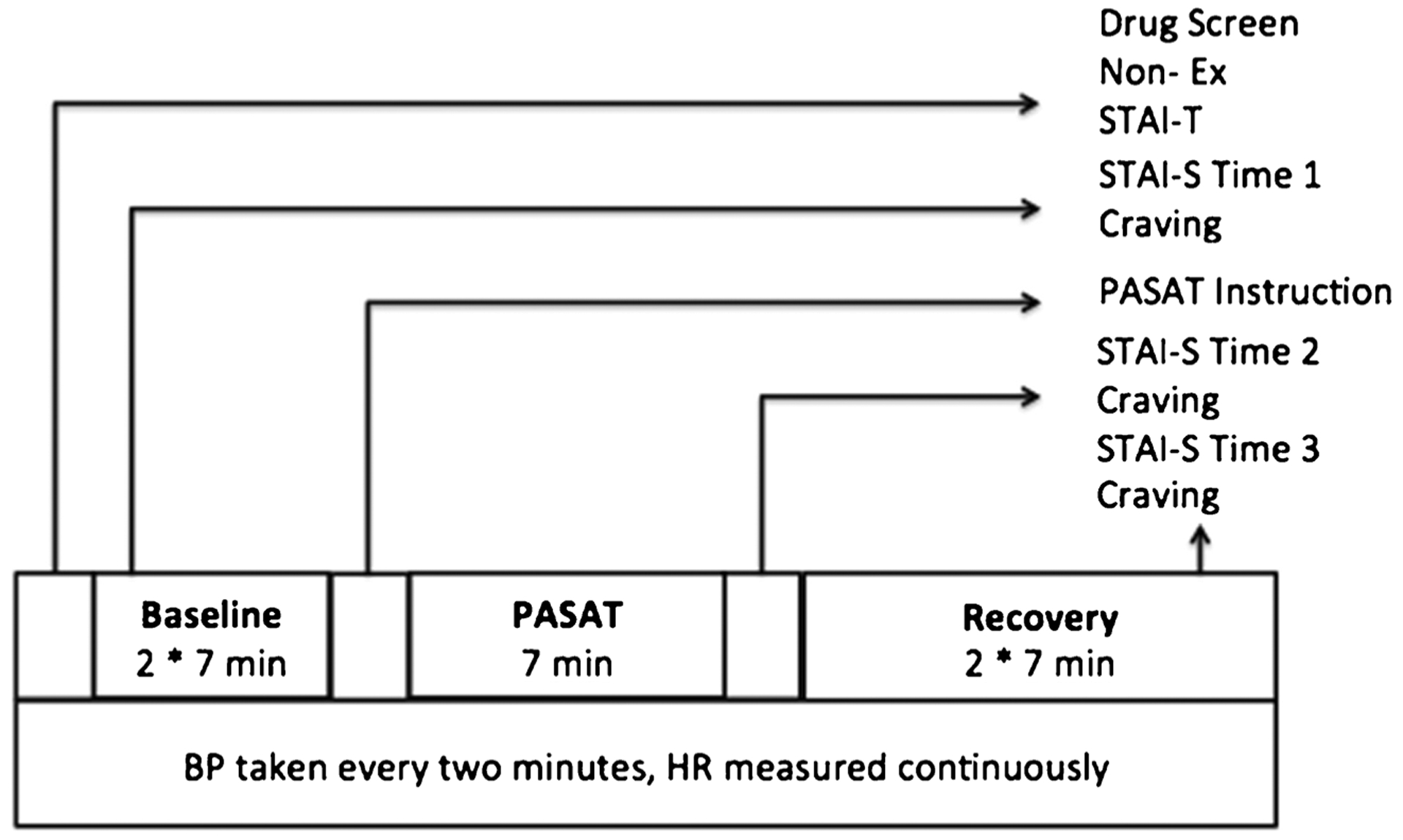
Visual representation of laboratory protocol. Non-Ex Non-Exercise Inventory, STAI-T State/Trait Anxiety Inventory–Trait, STAI-S State/Trait Anxiety Inventory–State, Craving Visual Analogue Craving Scale, PASAT Paced Auditory Serial Addition Task, HR heart rate, BP blood pressure
Measures
Questionnaires included in the present analyses were collected via two mechanisms. The demographic information was collected during computer-administered assessments at the community health clinic where treatment took place as a part of the parent study. The measures collected during the physiological protocol as part of the present study include a self-report measure of maximum oxygen volume (V02 max), state and trait anxiety, and visual analogue scales.
Demographic information was assessed as a part of the parent phase III clinical trial. Variables such as participant gender, age, and ethnicity were all assessed at baseline via a computer assessment administered at the community health clinic where treatment took place.
University of Houston Non-Exercise Test
The Non-E (Rossy and Thayer 1998) is a self-report measure that assesses basic health and fitness levels of participants to estimate V02 max. The Non-E uses an equation that considers participant gender, body mass index (BMI), and frequency and intensity of activity in a typical week to calculate an approximate V02 max.
State-Trait Anxiety Inventory
STAI-S and STAI-T were assessed using the Spielberger State/Trait Anxiety Inventory (Spielberger et al. 1982). The STAI asks participants to evaluate their feelings of restlessness, failure, calm, contentment, and other on a scale from 1 to 4, where “1” indicated not at all, “2” indicated somewhat, “3” indicated moderately so, and “4” indicated very much so. STAI-T measures such feelings based on the participant’s typical experience, whereas the STAI-S measures the participants’ present moment emotive experience. The STAI-S was measured during the physiological protocol (see “Procedure” section) and participants were unable to write, so this portion was administered verbally.
Craving
A Visual Analogue Scale (VAS) assessed present moment state craving with the verbal prompt “how much craving or desire to drink or use drugs do you feel now” rated from 1 to 10, where 1 indicated no feeling at all and 10 indicated very extreme feeling, the most extreme you have ever experienced. The verbal prompt was concurrently represented with a visual/written representation of these questions and the rating scale on a flip-board.
Cognitive Stressor
The stressor employed was the cognitive PASAT (Gronwall 1977). The PASAT involved four trials of 50 single digits presented audibly with increasing pace. Participants added each newly presented digit to the one immediately preceding it while answers were recorded.
Data Analyses
Electrocardiography (ECG) was continuously measured throughout the experiment via PowerLab 8/35 high-performance data acquisition system at a sampling rate of 1000 Hz (ADInstruments, Colorado Springs, CO). After gently abrading the skin, three leads equipped with disposable Ag/AgCl electrodes were placed just below the right clavicle at five to six intercostal and grounded below the left clavicle. A Piezo respiratory belt transducer (ADInstruments) placed at diaphragmatic level continuously monitored respiration. An auto-inflating sphygmomanometer placed on the non-dominant arm took blood pressure (BP; Dinamap 1846, Critikon Inc., Tampa, FL) at 5-min intervals during the 15-min baseline and recovery periods. To acquire more than one BP measurement during the 7-min stressor (the PASAT discussed later), BP was taken at 2-min intervals during the stressor (i.e., at 2, 4, and 6 min during the stressor).
ECG and respiratory data pre-processing was performed using LabChart 7 Pro (ADInstruments). All HRV processing conformed to the guidelines put forth by the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (Bernston et al. 1997). The Lab Chart R wave marker was used to identify beats in four 7-min periods: one during baseline, the entire PASAT period, and two during recovery. The 7-min duration was chosen because of the length of the PASAT, which was 7 min. We needed to be able to score the PASAT to determine if participants were actively invested in the stressor task. This requires using the entire 7-min period. To follow Task Force guidelines of comparing across time periods of equal duration, we used the last 7 min of baseline and the first two 7-min blocks of recovery to assess changes in rate of recovery post-stressor. In further keeping with Task Force guidelines, R-R intervals were visually inspected and corrected for artifacts, ectopic beats, and missed beats using internal driven algorithms tools in Lab Chart. A fast Fourier transformation (FFT size of 1024) with a Hann window was applied R-R interval variability to yield power density as a function of frequency with a high-frequency range of 0.15–0.4 Hz. Finally, the skewed HF-HRV data were logarithmically corrected prior to repeated measure analyses.
Whether it is necessary to control for respiration factors when quantifying HF-HRV is a point of contention in psychophysiology research. While the Task Force (Bernston et al. 1997) suggests utilizing respiratory frequency as a covariate in analysis, they also acknowledge that using such covariates can remove experimental effects in analyses. Additionally, including respiration as a covariate does not fix the underlying respiration problem (Allen et al. 2007). Thus, if systematic differences in respiration between experimental conditions/phases do not exist, it is perfectly reasonable to use spectrally generated power data without making adjustments due to respiration (Grossman and Taylor 2007). Test phase comparative analysis between baseline and stressor revealed no significant differences in respiration frequency, t(25) = −1.34, p = 0.19. As such, no respiratory adjustments to our HF-HRV data were made.
Per Task Force guidelines (Bernston et al. 1997), we report both time and frequency measures of HRV. As a time domain estimate of total HRV, we report SDNN (i.e., the standard deviation of the beat-to-beat intervals or the square root of the variance). Since total variance within the recording period is included in the equation, SDNN is a measure of total heart rate variability. Among the various time domain estimates of high-frequency variations in heart rate, the Task Force recommends RMSSD because it is more statistically robust and appropriate for short-duration testing, and as such, we report RMSSD as well. Inclusion here will further facilitate comparison between our findings and other studies (Garland 2011) that opted to use this time domain estimate in their analyses.
Repeated measure analysis of covariance (RM-ANCOVA) assessed treatment group differences in psychophysiological measures across the testing period. Variables assessed that are known to affect HRV were covaried in all analyses included: age, gender, severity of substance use at intake (i.e., phase III clinical trial baseline), VO2 max, and trait anxiety. In addition, baseline craving at intake was covaried in our craving analyses. To assess whether tonic HF-HRV differentially predicted psychophysiological reactivity (from baseline to stressor), a series of moderation analyses were performed in accordance with the methods of Aiken and West (1991).
RM ANCOVAs were initially performed on all four time points: the last 7 min of baseline, the 7-min PASAT, and the two 7-min recovery points. However, none of our analyses comparing recovery rates across treatment groups revealed significant differences in rates of recovery, so for simplicity, recovery was collapsed to one 7-min block. Thus, the analyses reported here are on three time points.
Results
Participant characteristics and demographic information are summarized in Table 1. Our sample was mainly male, fairly ethnically diverse, and the primary drug of choice was alcohol. There were no statistically significant treatment group differences in any of these variables except gender; eight of the nine women in the study were in the RP group. Gender was covaried in all analyses.
Table 1.
Characteristics of study participants (n = 34)
| Characteristic | Number | Percenta | |
|---|---|---|---|
| Gender | |||
| Male | 25 | 73 | |
| Female | 9 | 27 | |
| Race/ethnicityb | |||
| Asian | 1 | 3 | |
| Black | 7 | 21 | |
| Hispanic | 4 | 12 | |
| Native American | 4 | 12 | |
| White | 16 | 47 | |
| Educationb | |||
| No high school | 2 | 6 | |
| Through high school | 17 | 32 | |
| Some college | 9 | 27 | |
| Community college | 2 | 6 | |
| Bachelor’s degree | 3 | 9 | |
| Some graduate school | 1 | 3 | |
| Employment status | |||
| Employed | 15 | 44 | |
| Unemployed | 19 | 56 | |
| Household incomeb | |||
| 0–$4,999 | 17 | 590 | |
| $5,000–$9,999 | 1 | 3 | |
| $10,000–$14,999 | 2 | 6 | |
| $15,000–$19,999 | 4 | 12 | |
| Unknown | 8 | 24 | |
| Partnered status | |||
| Single (never married) | 22 | 65 | |
| Divorced | 8 | 24 | |
| Separated | 4 | 12 | |
| Living with partner | 0 | 0 | |
| Primary drug of choice | |||
| Alcohol | 20 | 59 | |
| Crack cocaine | 8 | 24 | |
| Marijuana | 1 | 3 | |
| Methamphetamine | 3 | 9 | |
| Heroin | 2 | 6 | |
| Secondary drug of choicec | |||
| None | 8 | 24 | |
| Alcohol | 3 | 9 | |
| Crack cocaine | 5 | 15 | |
| Powder cocaine | 4 | 12 | |
| Marijuana | 8 | 24 | |
| Methamphetamine | 2 | 6 | |
| Heroin (and other opiates) | 2 | 6 | |
| Other (whatever available) | 1 | 3 | |
| Mean | SD | Range | |
| Age | 43.4 | 9.7 | 23–60 years |
Percentages rounded to nearest whole number
Two people did not provide an answer
One person did not provide an answer
Heart Rate
Heart rate increased during the stressor and quickly subsided during recovery in all three treatment groups. RM-ANCOVA revealed a statistically significant two-way interaction of time × treatment condition F(4, 54) = 9.12, p < 0.01, partial η2 = 0.4 that was accounted for by the increase from baseline to stressor (i.e., reactivity or delta (Δ) = stressor maximum − baseline average). All treatment group comparisons were statistically significant at the p < 0.05 level with TAU demonstrating the greatest reactivity with a 10 beat per minute (BPM) increase followed by MBRP with a 6-BPM increase and RP with a 3-BPM increase. There were no significant group differences in baseline or recovery values, nor were there differences in rates of recovery (i.e., return to baseline values) across treatment groups, and as such, Fig. 2 depicts only the reactivity score results.
Fig. 2.
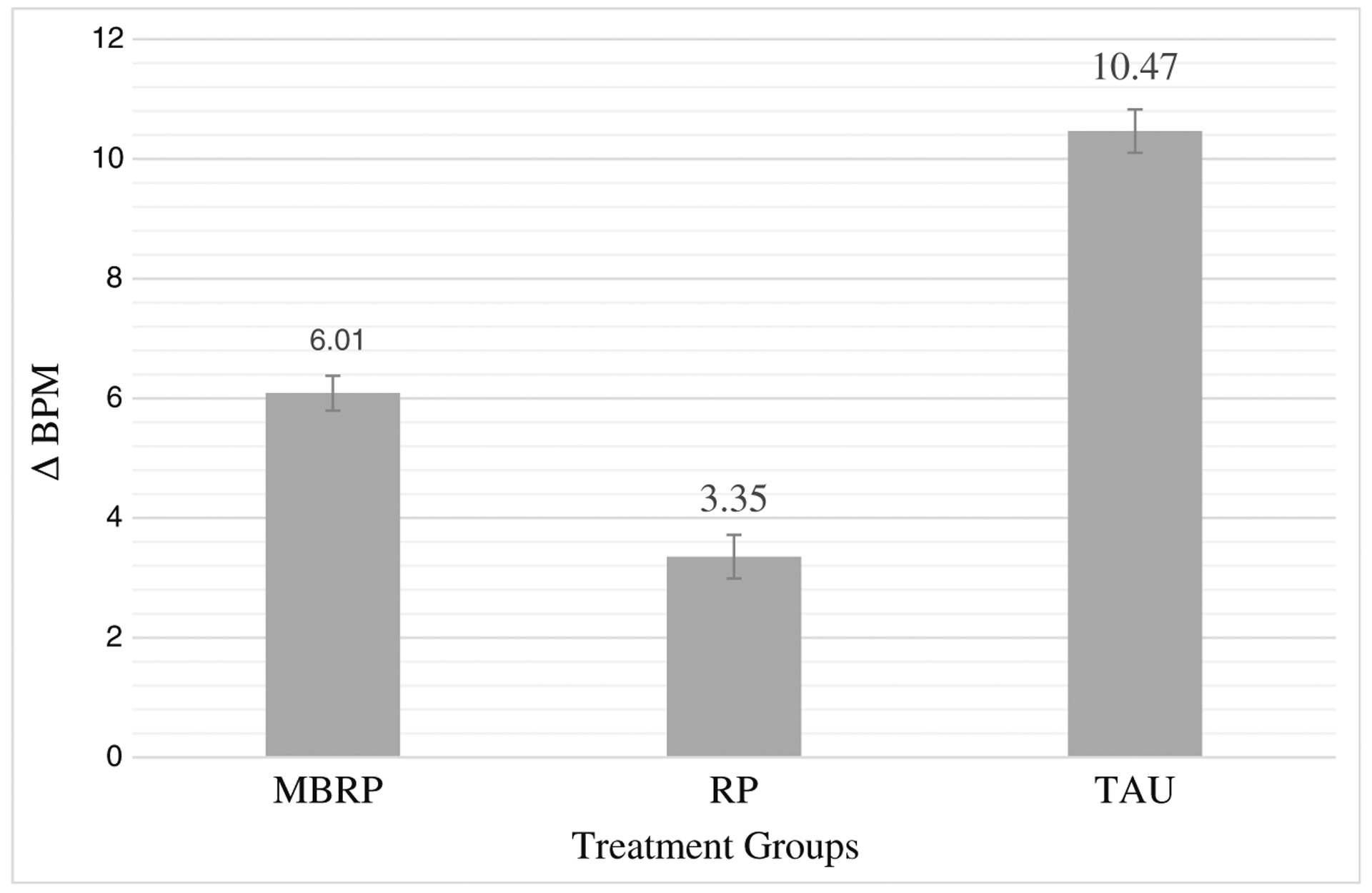
Change in heart rate from baseline to stressor. Significant differences were observed among all three treatment groups. Standard errors are represented in the figure by the error bars attached to each column. ΔBPM delta or change in heart rate (beats per minute) from baseline to stressor, MBRP mindfulness-based relapse prevention, RP relapse prevention, TAU treatment as usual
Blood Pressure
RM-ANCOVA did not reveal any significant effects of treatment on blood pressure across baseline, PASAT, and recovery. All groups showed a typical increase in blood pressure during the laboratory stressor. However, group differences in neither systolic blood pressure reactivity (MBRPΔ = 7.5-mmHg increase, RPΔ = 6.6-mmHg increase, and TAUΔ = 8.4 mmHg) nor diastolic blood pressure reactivity were observed (MBRP Δ = 4.1 mmHg, RPΔ = 4.7 mmHg, TAUΔ = 6.6 mmHg).
Self-Reported Anxiety
State anxiety increased during the stressor and quickly subsided during recovery in all three treatment groups. RM-ANCOVA revealed a statistically significant two-way interaction of time × condition that was accounted for by the increase from baseline to stressor (i.e., reactivity): F(3, 47) = 5.04, p < 0.01, partial η2 = 0.3. There were no significant group differences in baseline or recovery values, nor were there differences in rates of recovery (i.e., return to baseline values) across treatment groups. Figure 3 depicts these reactivity scores. Pairwise comparisons revealed statistically significant group differences in reactivity between MBRP (Δ = 2.2 rating increase) and TAU (Δ = 16.9 rating increase) only, p < 0.01.
Fig. 3.
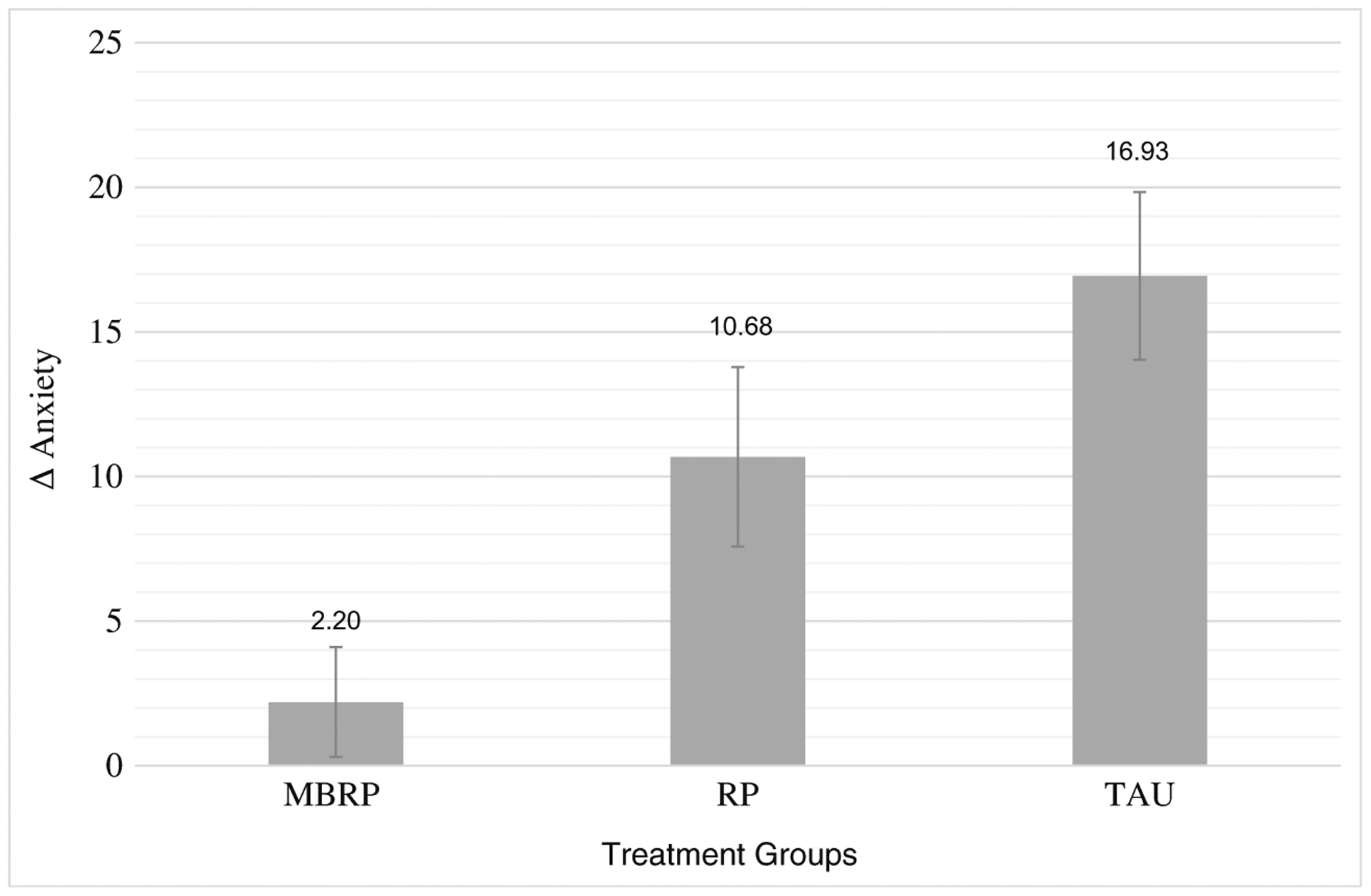
Change in anxiety from baseline to stressor. Significant differences were observed between MBRP and TAU. Standard errors are represented in the figure by the error bars attached to each column. Δ anxiety delta or change in anxiety reports from baseline to stressor, MBRP mindfulness-based relapse prevention, RP relapse prevention, TAU treatment as usual
Self-Reported Craving
RM-ANCOVA revealed a statistically significant two-way interaction of time × condition that was accounted for by reactivity: F(3, 47) = 6.04, p < 0.05, partial η2 = 0.5. Group differences in baseline, recovery, or rates of recovery were not observed, and as such, reactivity scores are again used in Fig. 4. Pairwise comparisons revealed statistically significant group differences in reactivity between MBRP (Δ = 0 rating change) and TAU (Δ = 2-point rating change) only, p < 0.05.
Fig. 4.
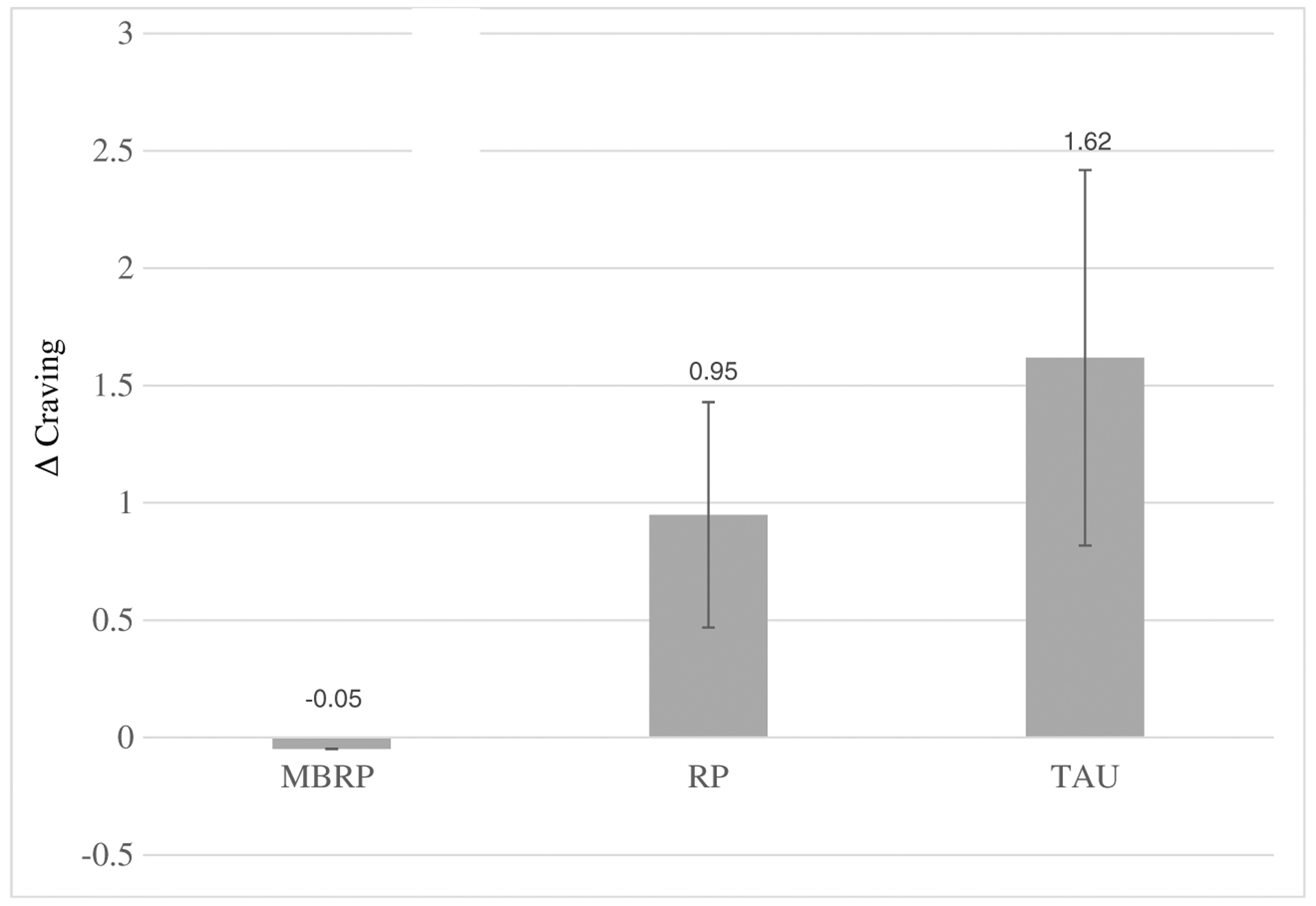
Change in craving from baseline to stressor. Significant differences were observed between MBRP and TAU. Standard errors are represented in the figure by the error bars attached to each column. Δ craving delta or change in craving reports from baseline to stressor, MBRP mindfulness-based relapse prevention, RP relapse prevention, TAU treatment as usual
Figure 5a depicts HF-HRV findings across the testing session in untransformed values to facilitate comparison to the few published norms in the literature. Spectral analysis of stationary supine 5-min recordings reveals high-frequency values in the range of 772–1178 ms2 (975 ± 203 ms2; Bernston et al. 1997). As we would expect in a clinical population such as the one tested in this study, all tonic HF-HRV values were lower than the norms. RM-ANCOVA, run on log-transformed data, revealed a significant two-way interaction of time by condition that was accounted for by differences at baseline (i.e., tonic HF-HRV) and in response to the stressor (i.e., phasic HF-HRV): F(4, 52) = 4.44, p < 0.01, partial η2 = 0.3. Log-transformed data across testing periods are depicted in Fig. 5b, whereas tonic and phasic changes, described below, are depicted in Fig. 6.
Fig. 5.
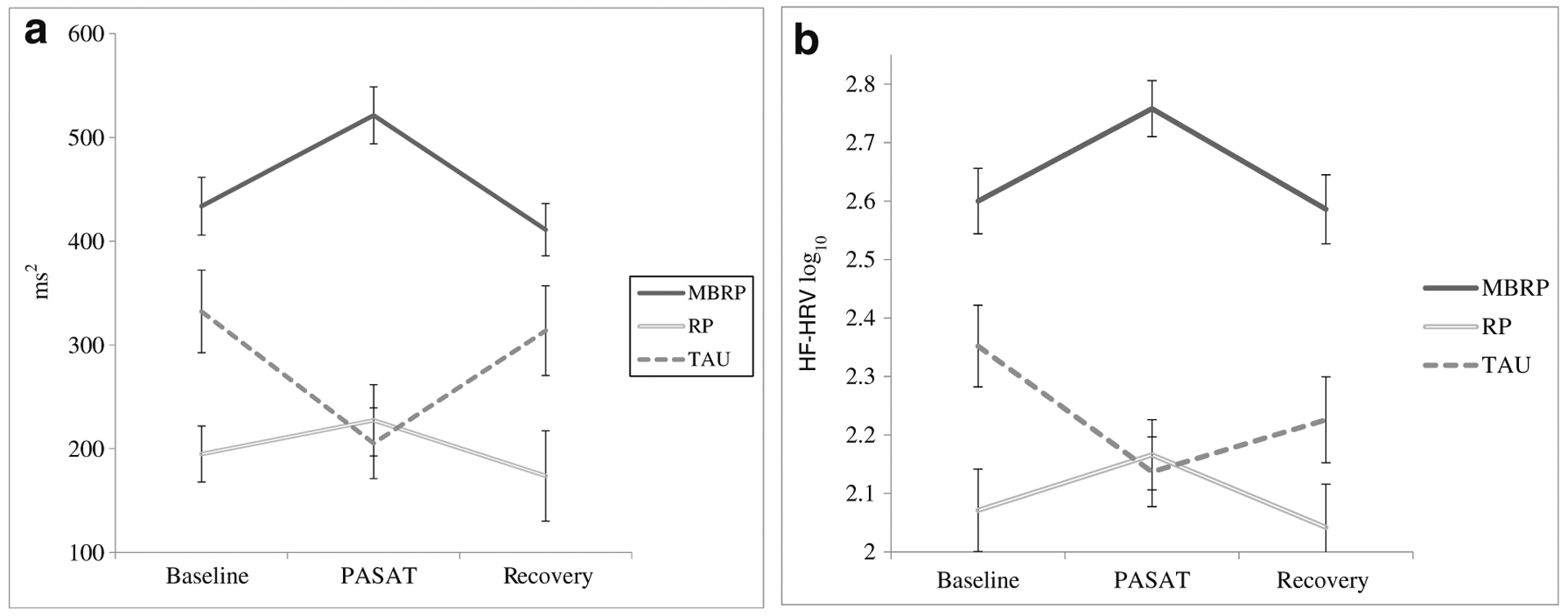
a High-frequency heart rate variability as a function of time across testing shown in actual values to facilitate comparisons with reported norms. Standard errors are represented in the figure by the error bars attached to each data point in the line. ms2 milliseconds squared, PASAT Paced Auditory Serial Addition Task, MBRP mindfulness-based relapse prevention, RP relapse prevention, TAU treatment as usual. b High-frequency heart rate variability as a function of time across testing shown in log-transformed values as used in analyses. Standard errors are represented in the figure by the error bars attached to each data point in the line. HF-HRV high-frequency heart rate variability, PASAT Paced Auditory Serial Addition Task, MBRP mindfulness-based relapse prevention, RP relapse prevention, TAU treatment as usual
Fig. 6.
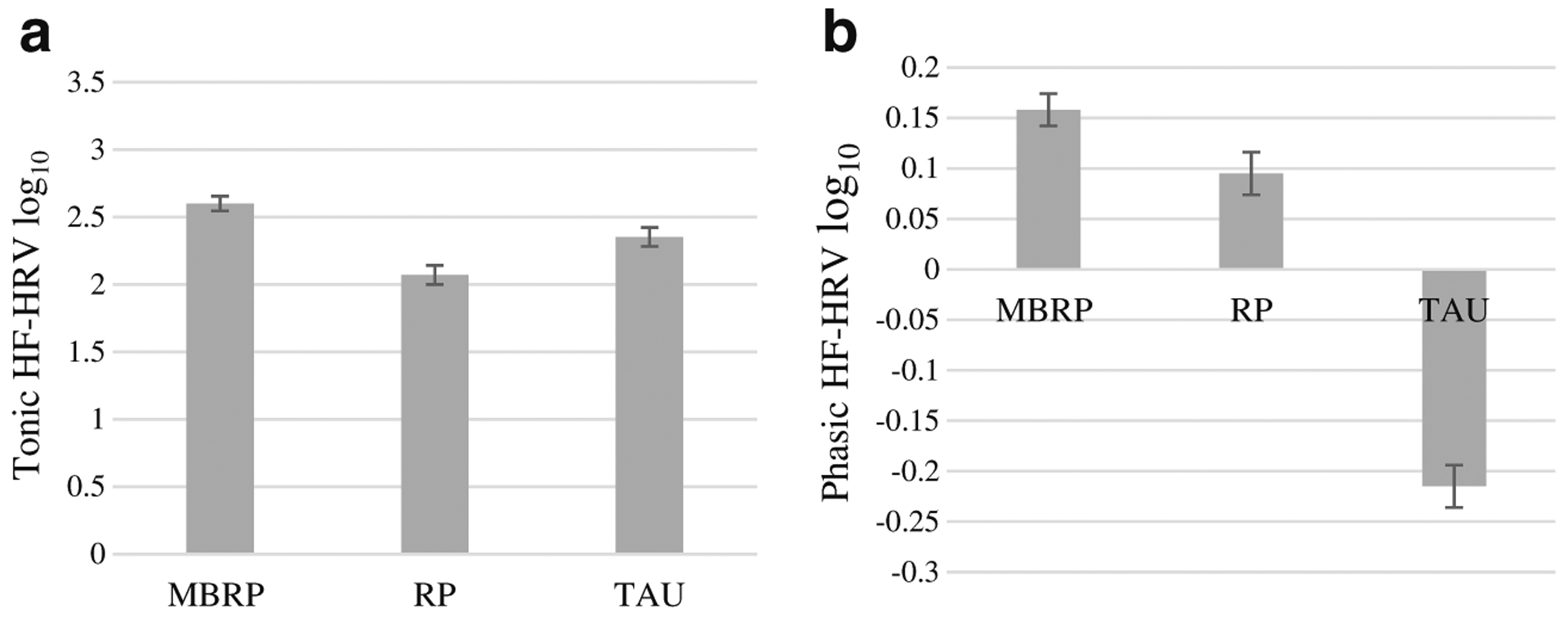
Tonic (a) and phasic (b) high-frequency heart rate variability (HF-HRV) by treatment condition. a Average tonic HF-HRV at baseline. Significant differences were observed between MBRP and RP. b Delta or change in HF-HRV (i.e., phasic) from baseline to stressor. Significant differences were observed among all three treatment groups. Values are log transformed as used in analyses. Standard errors are represented in the figure by the error bars attached to each column. HF-HRV high-frequency heart rate variability, MBRP mindfulness-based relapse prevention, RP relapse prevention, TAU treatment as usual
Tonic HF-HRV
Treatment group differences were observed at baseline. Prior to performing the stressor task, MBRP evinced greater tonic HF-HRV than RP, p < 0.01. Also, a nearly statistically significant difference in tonic HF-HRV was observed between MBRP and TAU (p = 0.056). HF-HRV did not significantly differ between RP and TAU. These findings are depicted in Fig. 6a.
Phasic HF-HRV
Figure 6b also shows the treatment group differences in phasic HF-HRV or the change from baseline to stressor values. ANCOVA revealed a significant effect of treatment: F(1, 27) = 2.86, p < 0.001, partial η2 = 0.79. All of the pairwise comparisons were significant (TAU vs RP, p = 0.013; TAU vs MBRP, p < 0.001; RP vs MBRP, p = 0.011).
Discussion
We hypothesized that MBRP would be related to the lowest levels of heart rate, blood pressure, anxiety, and craving reactivity, and the highest levels of tonic and phasic HF-HRV when compared to MBRP and TAU. In general, MBRP was associated with favorable hemodynamic and psychological outcomes as our hypotheses were mostly supported. As we hypothesized, we found that MBRP evinced the highest levels of tonic and phasic HF-HRV and the lowest level of anxiety reactivity. Our hypothesis was partially supported with MBRP demonstrating lower heart rate reactivity than TAU, but higher reactivity than RP. For blood pressure and craving, we saw overall patterns of responses and reactivity as we would expect, but no measureable group differences between active treatment groups in the patterns of reactivity.
While the heart rate reactivity in MBRP was higher than RP, both active treatment groups show substantial improvements in heart rate reactivity over TAU. Affecting cardiac responses to stress is of paramount concern in the treatment of substance use disorders given the comorbidity of cardiac disease in this population (Rehm et al. 2009). Additionally, the pattern of increased heart rate reactivity for all treatment groups in response to the stressor confirms that our stress protocol performed as expected and that all groups engaged in the task. This was further supported by increased systolic blood pressure reactivity for all groups; however, we did not see any group differences in blood pressure reactivity. This suggests that MBRP performed as well as the other treatment groups in regards to blood pressure reactivity.
Our observed increases in tonic and phasic HF-HRV following treatment with MBRP align with other research. Beyond findings from other substance use research on MBRP presented earlier (Brewer et al. 2009), these findings align with findings of increases of HF-HRV post-treatment for depression (Balogh et al. 1993), anxiety (Middleton and Ashby 1995), and smoking (Minami et al. 1999). Higher tonic HF-HRV, long viewed as a marker of superior self-regulation and adaptability to stress, is increasingly seen as a possible peripheral physiological marker of effective and flexible cortical control associated with adaptive emotion regulation (Thayer et al. 2012). To the degree that increased HF-HRV is a mark of PFC activity, our findings may indicate top-down modulation over vagal input, or what could be thought of as vagal braking, being associated with self-regulation (Thayer and Lane 2009). Conversely, we could be observing bottom-up reductions in stress reactivity (Westbrook et al. 2013). Ultimately, understanding whether we are observing top-down modulation or bottom-up reductions in stress needs further investigation.
The findings that anxiety reactivity was lower for those who completed MBRP than other treatment groups is favorable for long-term substance use outcomes. Stress and negative life events are among the most commonly reported reasons for problematic alcohol and other drug consumption (Marlatt and Donovan 2005). In addition, negative emotions are a common precipitant in problematic drinking (Otto et al. 2005). Many individuals with substance use disorders are drawn to alcohol as a means of emotional coping given its temporary, stress-dampening effects (Sinha and O’Malley 1999). Risk for substance use increases when individuals consistently resort to alcohol consumption instead of more adaptive coping strategies.
In recent years, with the debate and release of the newest version of the Diagnostic and Statistical Manual 5 (Diagnostic and Statistical Manual of Mental Disorders (DSM-5) 2013), craving has become a central point of interest in substance use research. While we only found differences between MBRP and TAU in craving reactivity due to our stressor, we did observe increases in craving in all groups. Other research published from the parent study suggests that mindfulness moderates the relationship between treatment with MBRP and craving outcomes (Witkiewitz et al. 2013). However, craving reactivity due to stress was not different for MBRP than the other treatment groups. This suggests that MBRP performs as well as RP and TAU for craving reactivity, but given the connections between stress, craving, and relapse, further research should target these relationships.
Limitations
A few limitations of this study exist. This laboratory assessment was conducted as an extension of a large-scale clinical trial (Bowen et al. 2014), and in consideration of participant burden, we only have one assessment time point with a relatively small sample. This study would be strengthened by including pre-treatment levels of HF-HRV, and even more post-treatment follow-ups. Thus, these results should be considered exploratory. Future studies should continue to assess these concepts before and after treatment with larger sample sizes. This study also has strengths, of the few similar studies in HF-HRV and mindfulness; few studies are covarying age, gender, smoking, severity of use, and fitness level. These variables are important predictors of HF-HRV, and we considered them in the present analyses. Moreover, the present study contributes to an emerging literature investigating the effects of mindfulness therapies on HF-HRV in substance users.
Thus, MBRP demonstrated favorable hemodynamic and psychological outcomes performing as good or better than the gold standard treatments for substance use disorders. Most noteworthy, MBRP evinced measureable increases in HF-HRV and was related to lower levels of state anxiety. These findings may be interpreted as “pausing” or applying the vagal brake in the face of stress among those who received MBRP. So in the face of stress, as social beings, we may want to throw a fit, or if a substance user, get a “fix” (i.e., consume drug), but we could engage our flexible vagus to self-regulate and self-sooth. Those that practice mindfulness produce psychophysiological responses to stress that suggest that they are engaging the latter. These results are an important step to identifying the mechanisms of action by which MBRP, as one in a set of many emerging new therapies involving mindfulness, functions. The relationships of these results to therapeutic outcomes are currently being explored. Of particular interest is understanding how HF-HRV relates in the moment to drug-taking behavior. Future research will continue to identify the physiological, neurobiological, and psychological mechanisms of action in MBRP. Future research should also continue to examine the other mechanisms by which mindfulness-based interventions exert salutary effects, which include many different components of treatment besides meditation.
Funding
This study was funded by the National Center for Research Resources (grant number UL1RR025014).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Aiken L, & West S (1991). Multiple regression: testing and interpreting interactions. Newbury Park: Sage Publications. [Google Scholar]
- Allen JJB, Chambers AS, & Towers DN (2007). The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biological Psychology, 74(2), 243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Bai X, Li J, Zhou L, & Li X (2009). Influence of the menstrual cycle on nonlinear properties of heart rate variability in young women. AJP: Heart and Circulatory Physiology, 297(2), H765–H774. doi: 10.1152/ajpheart.01283.2008. [DOI] [PubMed] [Google Scholar]
- Balogh S, Fitzpatrick DF, Hendricks SE, & Paige SR (1993). Increases in heart rate variability with successful treatment in patients with major depressive disorder. Psychopharmacology Bulletin, 29(2), 201–206. [PubMed] [Google Scholar]
- Beauchaine T (2001). Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13, 183–214. [DOI] [PubMed] [Google Scholar]
- Bernston GG, Bigger T, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … Van de Molen MW (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophsyiology, 34, 623–648. [DOI] [PubMed] [Google Scholar]
- Bowen S, Witkiewitz K, Dillworth TM, Chawla N, Simpson TL, Ostafin BD, … Marlatt GA (2006). Mindfulness meditation and substance use in an incarcerated population. Psychology of Addictive Behaviors, 20(3), 343–347. doi: 10.1037/0893-164X.20.3.343. [DOI] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, … Marlatt A (2009). Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Substance Abuse, 30(4), 295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Marlatt GA, & Parks GA (2010). Mindfulness-based relapse prevention. New York: Guilford. [Google Scholar]
- Bowen S, Witkiewitz K, Clifasefi SL, Grow J, Chawla N, Hsu SH, et al. (2014).Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: a randomized clinical trial. JAMA Psychiatry, 71(5), 547–556. doi: 10.1001/jamapsychiatry.2013.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady K, & Sinha R (2005). Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. American Journal of Psychiatry, 162, 1483–1493. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Sinha R, Chen JA, Michalsen RN, Babuscio TA, Nich C, … Rounsaville BJ (2009). Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Substance Abuse, 30(4), 306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownwell K, Marlatt GA, Lichtenstein E, & Wilson GT (1986). Understanding and preventing relapse. American Psychologist, 41, 765–782. [DOI] [PubMed] [Google Scholar]
- Bylsma L, Salomon K, Taylor-Clift A, Morris BH, & Rottenberg J (2014). Respiratory sinus arrhythmia reactivity in current and remitted major depressive disorder. Psychosomatic Medicine, 76, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, & Brady K (2006). Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine & Tobacco Research, 8, 627–638. [DOI] [PubMed] [Google Scholar]
- Castaldo R, Melillo P, Casterta M, Triassi M, & Pecchia L (2015). Acute mental stress assessment via short term HRV analysis in healthy adults: a systematic review with meta-analysis. Biomedical Signal Processing and Control, 18, 370–377. [Google Scholar]
- Chalmers JA, Quintana DS, Maree J, Abbott A, & Kemp AH (2014). Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Frontiers in Psychiatry, 5(80), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AS, & Allen J (2002). Vagal tone as an indicator of treatment response in major depression. Psychophysiology, 39(6), 861–864. doi: 10.1017/S0048577202010442. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, & Cassuto Y (1997). Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biological Psychiatry, 41, 627–629. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Matar MA, Kaplan Z, Lowenthal U, Miodownik H, & Cassuto Y (1998). Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biological Psychiatry, 44, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Conrad M, Gorka SM, & Kassel J (2015). Smoking’s effects on respiratory sinus arrhythmia in adolescent smokers. International Journal of Psychophysiology, 97, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, & Ruan W (2005). Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction, 100(3), 281–292. [DOI] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders: DSM-5. (2013). Washington DC: American Psychiatric Association. [Google Scholar]
- Ditto B, Eclache M, & Goldman N (2006). Short-term autonomic and cardiovascular effects of mindfulness body scan meditation. Annals of Behavioral Medicine, 32(3), 227–234. [DOI] [PubMed] [Google Scholar]
- Fox HC, & Sinha R (2009). Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harvard Review of Psychiatry, 17, 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B, & Thayer JF (1998). Autonomic balance revisited: panic anxiety and heart rate variability. Journal of Psychiatric Research, 44, 133–151. [DOI] [PubMed] [Google Scholar]
- Garland EL (2011). Trait mindfulness predicts attentional and autonomic regulation of alcohol cue-reactivity. Journal of Psychophysiology, 25(4), 180–189. doi: 10.1027/0269-8803/a000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall DMA (1977). Paced auditory serial-addition task: a measure of recovery from concussion. Perceptual and Motor Skills, 44, 367–373. [DOI] [PubMed] [Google Scholar]
- Grossman P, & Taylor EW (2007). Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and bio-behavioral functions. Biological Psychology, 74, 263–285. [DOI] [PubMed] [Google Scholar]
- Ingaldsson JT, Laberg JC, & Thayer JF (2003). Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry, 54, 1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J (1990). Full catastrophe living: using the wisdom of your mind and body to face stress, pain, and illness. New York: Delacorte. [Google Scholar]
- Koob GF (2008). A role for brain stress systems in addiction. Psychology, 83, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby DJ, Worhunsky PD, Pilver CE, & Brewer JA (2012). Meditation-induced changes in high-frequency heart rate variability predict smoking outcomes. Frontiers in Human Neuroscience, 6(54), 1–8. doi: 10.3389/fnhum.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustyk MKB, Olson KC, Gerrish WG, Holder A, & Widman L (2010). Psychophysiological and neuroendocrine responses to laboratory stressors in women: implications of menstrual cycle phase and stressor type. Biological Psychology, 83(2), 84–92. doi: 10.1016/j.biopsycho.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Lustyk MKB, Gerrish WG, Douglas H, Bowen S, & Marlatt GA (2011). Relationships among premenstrual symptom reports, menstrual attitudes, and mindfulness. Mindfulness, 2(1), 37–48. doi: 10.1007/s12671-011-0041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, & Donovan DM (2005). Relapse prevention: maintenance strategies in the treatment of addictive behaviors. New York: Guilford. [Google Scholar]
- Marlatt GA, & Gordon JR (1985). Relapse prevention: a self-control strategy for the maintenance of behavior change. New York: Guilford. [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, & Kleber HD (2000). Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA, 284, 1689–1695. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, & McClair VL (2012). Epidemiology of substance use disorders. Human Genetics, 131(6), 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton HC, & Ashby M (1995). Clinical recovery from panic disorder is associated with evidence of changes in cardiovascular regulation. Acta Psychiatrica Scandinavica, 91, 108–113. [DOI] [PubMed] [Google Scholar]
- Minami J, Ishimitsu T, & Matsuoka H (1999). Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension, 33(1), 586–590. [DOI] [PubMed] [Google Scholar]
- Otto M, Powers M, & Fischmann D (2005). Emotional exposure in the treatment of substance use disorders: conceptual model, evidence, and future directions. Clinical Psychology Review, 25, 824–839. [DOI] [PubMed] [Google Scholar]
- Porges SW (2005). A phylogenetic perspective. In Carter CS, Ahnert L, Grossmann KE, & Hrdy SB (Eds.), Attachment and bonding: A new synthesis (pp. 33–54). Cambridge: MIT Press. [Google Scholar]
- Porges SW (2007). A phylogenetic journey through the vague and ambiguous Xth cranial nerve: a commentary on contemporary heart rate variability research. Biological Psychology, 74(2), 301–307. doi: 10.1016/j.biopsycho.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, Guastella AJ, McGregor IS, Hickie IB, & Kemp AH (2013). Heart rate variability predicts alcohol craving in alcohol dependent outpatients: further evidence for HRV as a psychophysiological marker of self-regulation. Drug and Alcohol Dependence, 132, 395–398. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, & Patra J (2009). Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet, 373, 2223–2233. [DOI] [PubMed] [Google Scholar]
- Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of national findings. Office of Applied Studies, NSDUH Series H-38A, HHS. (2010) (Vol. Publication, No. SMA 10–4586). Rockville, MD: SAMSHA. [Google Scholar]
- Rossy LA, & Thayer JF (1998). Fitness and gender-related differences in heart period variability. Psychosomatic Medicine, 60(6), 773–781. [DOI] [PubMed] [Google Scholar]
- Sarang P, & Telles S (2006). Effects of two yoga based relaxation techniques on heart rate variability (HRV). International Journal of Stress Management, 13, 460–475. [Google Scholar]
- Sato N, Miyake S, Akatsu JL, & Kumashiro M (1995). Power spectral analysis of heart rate variability in healthy young women during the normal menstrual cycle. Psychosomatic Medicine, 57, 331–335. [DOI] [PubMed] [Google Scholar]
- Sinha R (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology, 158, 343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R (2007). The role of stress in addiction relapse. Current Psychiatry Reports, 9, 388–395. [DOI] [PubMed] [Google Scholar]
- Sinha R, & O’Malley S (1999). Craving for alcohol: findings from the clinic and the laboratory. Alcohol and Alcoholism, 34, 223–230. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, & Lushene RE (1982). Manual for the STAI.
- Tang Y-Y, Ma Y, Fan Y, Feng H, Wang J, Feng S, … … & Zhang Y (2009). Central and autonomic nervous system interaction is altered by short-term meditation. Proceedings of the National Academy of Sciences, 106(22), 8865–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61, 201–216. [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart–brain connection: further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33(2), 81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman B, & Borkovec T (1996). Autonomic characteristics of genderalized anxiety disorder and worry. Biological Psychiatry, 39, 255–266. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers J, & Wagner T (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36, 747–756. [DOI] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, & Tindle HA (2013). Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social Cognitive and Affective Neuroscience, 8(1), 73–84. doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, & Bowen S (2010). Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. Journal of Consulting and Clinical Psychology, 78(3), 362–374. doi: 10.1037/a0019172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, Douglas H, & Hsu SH (2013). Mindfulness-based relapse prevention for substance craving. Addictive Behaviors, 38, 1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]


