Abstract
Background
Acute bronchiolitis is a significant burden on children, their families and healthcare facilities. It mostly affects children younger than two years of age. Treatment involves adequate hydration, humidified oxygen supplementation, and nebulisation of medications, such as salbutamol, epinephrine, and hypertonic saline. The effectiveness of magnesium sulphate for acute bronchiolitis is unclear.
Objectives
To assess the effects of magnesium sulphate in acute bronchiolitis in children up to two years of age.
Search methods
We searched CENTRAL, MEDLINE, Embase, LILACS, CINAHL, and two trials registries to 30 April 2020. We contacted trial authors to identify additional studies. We searched conference proceedings and reference lists of retrieved articles. Unpublished and published studies were eligible for inclusion.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs, comparing magnesium sulphate, alone or with another treatment, with placebo or another treatment, in children up to two years old with acute bronchiolitis. Primary outcomes were time to recovery, mortality, and adverse events. Secondary outcomes were duration of hospital stay, clinical severity score at 0 to 24 hours and 25 to 48 hours after treatment, pulmonary function test, hospital readmission within 30 days, duration of mechanical ventilation, and duration of intensive care unit stay.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We used GRADE methods to assess the certainty of the evidence.
Main results
We included four RCTs (564 children). One study received funding from a hospital and one from a university; two studies did not report funding sources. Comparator interventions differed among all four trials. Studies were conducted in Qatar, Turkey, Iran, and India. We assessed two studies to be at an overall low risk of bias, and two to be at unclear risk of bias, overall. The certainty of the evidence for all outcomes and comparisons was very low except for one: hospital re‐admission rate within 30 days of discharge for magnesium sulphate versus placebo. None of the studies measured time to recovery, duration of mechanical ventilation, duration of intensive care unit stay, or pulmonary function.
There were no events of mortality or adverse effects for magnesium sulphate compared with placebo (1 RCT, 160 children). The effects of magnesium sulphate on clinical severity are uncertain (at 0 to 24 hours: mean difference (MD) on the Wang score 0.13, 95% confidence interval (CI) ‐0.28 to 0.54; and at 25 to 48 hours: MD on the Wang score ‐0.42, 95% CI ‐0.84 to ‐0.00). Magnesium sulphate may increase hospital re‐admission rate within 30 days of discharge (risk ratio (RR) 3.16, 95% CI 1.20 to 8.27; 158 children; low‐certainty evidence).
None of our primary outcomes were measured for magnesium sulphate compared with hypertonic saline (1 RCT, 220 children). Effects were uncertain on the duration of hospital stay in days (MD 0.00, 95% CI ‐0.28 to 0.28), and on clinical severity on the Respiratory Distress Assessment Instrument (RDAI) score at 25 to 48 hours (MD 0.10, 95% CI ‐0.39 to 0.59).
There were no events of mortality or adverse effects for magnesium sulphate, with or without salbutamol, compared with salbutamol (1 RCT, 57 children). Effects on the duration of hospital stay were uncertain (magnesium sulphate: 24 hours (95% CI 25.8 to 47.4), magnesium sulphate + salbutamol: 20 hours (95% CI 15.3 to 39.0), and salbutamol: 24 hours (95% CI 23.4 to 76.9)).
None of our primary outcomes were measured for magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine (1 RCT,120 children). Effects were uncertain for the duration of hospital stay in hours (MD ‐0.40, 95% CI ‐3.94 to 3.14), and for RDAI scores (0 to 24 hours: MD ‐0.20, 95% CI ‐1.06 to 0.66; and 25 to 48 hours: MD ‐0.90, 95% CI ‐1.75 to ‐0.05).
Authors' conclusions
There is insufficient evidence to establish the efficacy and safety of magnesium sulphate for treating children up to two years of age with acute bronchiolitis. No evidence was available for time to recovery, duration of mechanical ventilation and intensive care unit stay, or pulmonary function. There was no information about adverse events for some comparisons. Well‐designed RCTs to assess the effects of magnesium sulphate for children with acute bronchiolitis are needed. Important outcomes, such as time to recovery and adverse events should be measured.
Plain language summary
Magnesium sulphate for treating children up to two years old with bronchiolitis
Review question
We investigated the effectiveness and safety of magnesium sulphate for treating children up to two years old with acute bronchiolitis.
Background
Acute bronchiolitis is caused by a virus, and mostly affects children up to two years old. This condition blocks small airways in the lungs, causing coughing, wheezing, and breathing difficulties. Usual treatment involves supportive care, which includes providing adequate fluids and humidified oxygen. Children with bronchiolitis may need intensive care treatment.
Search date
Evidence is current to 30 April 2020.
Study characteristics
We included four studies (564 children) conducted in Qatar, Turkey, Iran, and India. Three studies included children with moderate to severe bronchiolitis. Studies compared magnesium sulphate, given via a tube into a vein (intravenous), or as a fine inhaled spray (nebulised), with a dummy treatment (known as a placebo – something that looks like magnesium sulphate treatment), medicines to open the airways (salbutamol or epinephrine), a salt water solution (hypertonic saline), or no treatment.
Study funding sources
One study received funding from a hospital and one from a university; two studies did not report funding sources.
Key results
There was not enough evidence to know if intravenous or nebulised magnesium sulphate, either alone or combined with other treatments, improved bronchiolitis outcomes in children up to two years old. We could not determine if magnesium sulphate reduced deaths, unexpected medical problems during treatment, time spent in hospital, or illness severity (based on doctors' clinical assessment scores). Time taken to recovery was not measured.
Certainty of the evidence
Overall, we are very uncertain of the results.
Summary of findings
Summary of findings 1. Magnesium sulphate compared with placebo for acute bronchiolitis in children.
| Magnesium sulphate versus placebo for acute bronchiolitis in children | ||||||
|
Patient or population: children up to 18 months old with acute bronchiolitis Settings: short stay unit, paediatric emergency department Intervention: magnesium sulphate Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Magnesium sulphate | |||||
| Time to recovery | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| Mortality | See comment | See comment | Not estimable | 160 (1 RCT) | See comment | Trial authors confirmed there were no deaths in either group (personal communication) |
| Adverse events | See comment | See comment | Not estimable | 160 (1 RCT) | See comment | Neither group reported adverse effects, such as apnoea, cyanosis, or haemodynamic instability |
| Duration of hospital stay | Geometric mean time to discharge was 25.3 hours in the placebo group. | Geometric mean time to discharge was 24.1 hours in the magnesium sulphate group. | Not estimable | 160 (1 RCT) | See comment | Results were taken directly from the published report. |
|
Clinical severity score (CSS) (Wang score; range 0 to 12; higher = poorer outcome) measured at 0 to 24 hours after treatment |
The mean (SD) CSS at 24 hours in the placebo group was 4.55 points (1.62) | The mean CSS at 24 hours in the magnesium sulphate group was 0.13 points higher (0.28 lower to 0.54 higher) | MD 0.13, (‐0.28 to 0.54) | 160 (1 RCT) | ⊕⊝⊝⊝ very lowa |
|
|
Clinical severity score (CSS) (Wang score; range 0 to 12; higher = poorer outcome) measured at 25 to 48 hours after treatment |
The mean (SD) CSS at 48 hours in the placebo group was 4.84 points (1.57) | The mean CSS at 48 hours in the magnesium sulphate group was 0.42 points lower (0.84 lower to 0) | MD ‐0.42, (‐0.84 to ‐0.00) |
160 (1 RCT) | ⊕⊝⊝⊝ very lowa |
|
|
Hospital re‐admission within 30 days of discharge (re‐admissions were reported at 14 days) |
62 per 1000 | 195 per 1000 | RR 3.16 (1.20 to 8.27) | 158 (1 RCT) | ⊕⊕⊝⊝ lowb | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; MD: mean difference; SD: standard deviation; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality. Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality. We are very uncertain about the estimate. | ||||||
aWe downgraded the quality of the evidence to very low for indirectness and very serious imprecision (1 study, small sample size, wide confidence intervals). bWe downgraded the quality of the evidence to low for indirectness and serious imprecision (1 study, small sample size).
Summary of findings 2. Magnesium sulphate compared with hypertonic saline for acute bronchiolitis in children.
| Magnesium sulphate versus hypertonic saline for acute bronchiolitis in children | ||||||
|
Patient or population: children up to 12 months old with acute bronchiolitis Settings: single‐centre, emergency department, paediatric intensive care unit and wards Intervention: magnesium sulphate Comparison: hypertonic saline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hypertonic saline | Magnesium sulphate | |||||
| Time to recovery | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| Mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| Duration of hospital stay | The mean (SD) duration of hospital stay in the hypertonic saline group was 3.2 days (1.0) | The mean duration of hospital stay in the magnesium sulphate group was 3.2 days (0.28 lower to 0.28 higher) | MD 0.00 (‐0.28 to 0.28) |
220 (1 RCT) |
⊕⊝⊝⊝ very lowa | |
|
Clinical severity score (CSS) (RDAI score; range 0 to 17; higher = poorer outcomes) measured at 0 to 24 hours after treatment |
‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
|
Clinical severity score (CSS) (RDAI score; range 0 to 17; higher = poorer outcomes) measured at 25 to 48 hours after treatment |
The mean (SD) CSS at 48 hours in the hypertonic saline group was 3.7 points (1.8) | The mean CSS at 48 hours in the magnesium sulphate group was 0.10 points higher (0.39 lower to 0.59 higher) | MD 0.10 (‐0.39 to 0.59) | 220 (1 RCT) | ⊕⊝⊝⊝ very lowa | |
| Hospital re‐admission within 30 days of discharge | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; SD: standard deviation; RCT: randomised controlled trial; RADI: respiratory distress assessment instrument | ||||||
| GRADE Working Group grades of evidence High quality. Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality. We are very uncertain about the estimate. | ||||||
aWe downgraded the quality of the evidence to very low for indirectness and very serious imprecision (1 study, small sample size).
Summary of findings 3. Magnesium sulphate or magnesium sulphate + salbutamol compared with salbutamol for acute bronchiolitis in children.
| Magnesium sulphate or magnesium sulphate + salbutamol versus salbutamol for acute bronchiolitis in children | ||||||
|
Patient or population: children from 1 month to 24 months old with acute bronchiolitis Settings: single‐centre, short‐stay unit, paediatric emergency department Intervention: magnesium sulphate or magnesium sulphate + salbutamol Comparison: salbutamol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Salbutamol | Magnesium sulphate or magnesium sulphate + salbutamol | |||||
| Time to recovery | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| Mortality | See comment | See comment | Not estimable | 56 (1 RCT)a | ‐ | No deaths occurred in any of the groups (personal communication) |
| Adverse events | See comment | See comment | Not estimable | 56 (1 RCT)a | ‐ | There were no instances of adverse effects, such as hypotension, arrhythmias, or loss of deep tendon reflexes reported in any of the groups. |
| Duration of hospital stay | The mean duration of hospital stay in the salbutamol group was 24 hours (95% CI 23.4 to 76.9). | The mean duration of hospital stay in the magnesium sulphate group was 24 hours (95% CI 25.8 to 47.4). The mean duration of hospital stay for the magnesium sulphate + salbutamol group was 20 hours (95% CI 15.3 to 39.0). |
Not estimable | 56 (1 RCT)a | ‐ | Results were taken directly from the published report. |
|
Clinical severity score (CSS) (Wang score; range 0 to 12; higher = poorer outcome) measured at 0 to 24 hours after treatment |
‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
|
Clinical severity score (CSS) (Wang score; range 0 to 12; higher = poorer outcome) measured 25 to 48 hours after treatment |
‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| Hospital re‐admission within 30 days of discharge | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; SD: standard deviation; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality. Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality. We are very uncertain about the estimate. | ||||||
a3‐arm trial: magnesium sulphate group (19 children), magnesium sulphate plus salbutamol (19 children), salbutamol (18 children)
Summary of findings 4. Magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine.
| Magnesium sulphate + epinephrine versus no treatment or normal saline + epinephrine | ||||||
|
Patient or population: children up to 12 months old with acute bronchiolitis Settings: 3 paediatric departments of 3 hospitals Intervention: magnesium sulphate + epinephrine Comparison: no treatment or normal saline + epinephrine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| no treatment or normal saline + epinephrine | Magnesium sulphate + epinephrine | |||||
| Time to recovery | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| Mortality | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| Duration of hospital stay | The mean (SD) duration of hospital stay in the epinephrine group was 84.7 hours (10.1) | The mean (SD) duration of hospital stay for the magnesium sulphate + epinephrine group was 0.40 hours less (3.94 lower to 3.14 higher) | MD ‐0.40 (‐3.94 to 3.14) |
120 (1 RCT) | ⊕⊝⊝⊝ very lowa | |
|
Clinical severity score (CSS) (RDAI score; range 0 to 17; higher = poorer outcomes) measured at 0 to 24 hours after treatment |
The mean (SD) CSS at 24 hours in the epinephrine group was 6.6 points (2.2) | The mean CSS at 24 hours in the magnesium sulphate + epinephrine group was 0.20 points lower (1.06 lower to 0.66 higher) | MD ‐0.20 (‐1.06 to 0.66) | 120 (1 RCT) | ⊕⊝⊝⊝ very lowa | |
|
Clinical severity score (CSS) (RDAI score; range 0 to 17; higher = poorer outcomes) measured 25 to 48 hours after treatment |
The mean (SD) CSS at 48 hours in the epinephrine group was 3.7 points (2.7) | The mean CSS at 48 hours in the magnesium sulphate + epinephrine group was 0.90 points lower (1.75 lower to 0.05 lower) | MD ‐0.90 (‐1.75 to ‐0.05) | 120 (1 RCT) | ⊕⊝⊝⊝ very lowa | Higher value indicates poor outcome |
| Hospital re‐admission within 30 days of discharge | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference; SD: standard deviation; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality. Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality. We are very uncertain about the estimate. | ||||||
aWe downgraded the evidence to very low for indirectness and very serious imprecision (1 study, small sample size).
Background
Description of the condition
Acute bronchiolitis is a lower respiratory tract disease, which mostly affects children up to two years of age. Many viral infections can result in bronchiolitis; respiratory syncytial virus is the most common aetiological agent, and affects more than 60% of children with bronchiolitis (Calvo 2010; Hasegawa 2014b; Mansbach 2012b). Infection induces inflammation of the lower airways causing bronchiolar wall oedema, resulting in narrowing of the distal airways. Further tapering of the lumen of these airways occurs due to increased secretions and cellular debris. Bronchiolitis may cause significant airway obstruction via the bronchioles, leading to the need for mechanical ventilation in some circumstances. Irrespective of the causative virus, clinical presentation is typical; pathological investigation is not required for diagnosis. The diagnosis and management of bronchiolitis vary in different regions of the world. The American Academy of Pediatrics, together with the European Respiratory Society defined bronchiolitis as the first episode of wheezing preceded by "a constellation of clinical symptoms and signs suggestive of a viral upper respiratory prodrome, followed by increased respiratory effort in children less than two years of age" (AAP Subcommittee 2006). In the UK, a Delphi consensus exercise defined bronchiolitis as "a seasonal viral illness characterised by fever, nasal discharge, and dry, wheezy cough. On examination, there are fine inspiratory crackles, a high‐pitched expiratory wheeze, or both" (Lakhanpaul 2002). In the UK, the presence of a wheeze is not mandatory for diagnosis. Most European countries restrict the diagnosis of bronchiolitis to children up to the age of 12 months. We applied the AAP Subcommittee 2006 definition for this review. Some risk factors associated with bronchiolitis severity are low socioeconomic status, male gender, prematurity, prenatal exposure to steroids, and maternal smoking (Lanari 2015; Murray 2014).
Acute bronchiolitis imposes a significant burden on healthcare facilities. Increased rates of bronchiolitis‐related emergency department visits (Carroll 2008; Hasegawa 2014a), and hospitalisations have been reported (Green 2016; Rivera‐Sepulveda 2017). Reports of bronchiolitis range from 26.5% of infants younger than 12 months to 16.4% of children younger than two years (Carroll 2008; Muñoz‐Quiles 2016). It has been estimated that 17% of children with bronchiolitis who are admitted to hospital are treated in intensive care units (Mansbach 2012a). Between 42% and 75% of these children are treated using both invasive and non‐invasive mechanical ventilation techniques (Flores‐González 2017; Mansbach 2012a). Previously, the percentage of children reported to die from bronchiolitis was 4.2% (Shay 2001). Recent studies show a mortality rate of 0.24% in hospitalised children (Muñoz‐Quiles 2016). However, the number of children with advanced disease has increased and consequently, healthcare costs have also increased (Hasegawa 2013).
Description of the intervention
No specific treatment is currently available for acute bronchiolitis. Treatment involves supportive care, including adequate hydration and humidified oxygen supplementation. Various treatment options have been proposed, but only nebulised epinephrine and hypertonic saline have been shown to be useful (Hartling 2011; Zhang 2017).
Magnesium is the active component of its sulphate salt of magnesium, sulphur, and oxygen, and has many different pharmacological actions. Magnesium is an important cofactor of many enzymes involved in biological reactions, and is present in tissues, including the brain, smooth muscles of the uterus, bronchioles, and the gastrointestinal tract (Swaminathan 2003). It has many therapeutic uses, such as controlling convulsions, preventing preterm labour, treating constipation, controlling tachyarrhythmias, and reversing bronchoconstriction in status asthmaticus (Schwalfenberg 2017). The action of magnesium on the bronchioles results in dilation of the airways by several mechanisms. Use of magnesium sulphate for children with bronchiolitis is based on its treatment effectiveness for adults (Kew 2014), and children with asthma (Griffiths 2016).
Treatment with magnesium sulphate may reduce hospital admissions of children with acute asthma presenting to the emergency department; evidence on the efficacy of magnesium sulphate is required for children with bronchiolitis. Magnesium sulphate can be administered intravenously or nebulised. The dose of intravenous magnesium sulphate for a bronchodilation effect used in trials ranges from 25 mg/kg/dose to 100 mg/kg/dose (maximum 2 g; (Alansari 2017; Ciarallo 1996; Scarfone 2000)). Nebulisation doses range from 40 mg/kg (Modaresi 2015), to 150 mg per dose (Kose 2014). Common adverse effects of magnesium sulphate therapy include hypotension (Ciarallo 1996; Goodacre 2013; Scarfone 2000), cardiac arrhythmias (Goodacre 2013; Lu 2000), and respiratory depression (Lu 2000; Mahajan 2004). Respiratory depression can be monitored by examining deep tendon reflex; loss of this reflex is the first sign of impending toxicity (Lu 2000).
How the intervention might work
Acute bronchiolitis results in the narrowing of small distal airways, causing airflow obstruction. Treatment to dilate airways may prove useful for children with acute bronchiolitis. Several ways that magnesium sulphate may act on the bronchiolar wall, resulting in bronchodilation, have been proposed. Magnesium sulphate has been shown to dilate bronchial muscle in animal studies (Hirota 1999; Kumasaka 1996; Spivey 1990), and may result in bronchial smooth muscle relaxation by blocking the voltage‐dependent calcium channels, preventing calcium influx (Gourgoulianis 2004). Magnesium sulphate decreases acetylcholine accumulation at nerve endings, further preventing bronchoconstriction (Castillo 1954). Magnesium sulphate may offer a safe, widely available, and low‐cost therapy to relieve airway obstruction in children with bronchiolitis.
Why it is important to do this review
Several Cochrane Reviews have investigated treatment options for children with acute bronchiolitis, including nebulised deoxyribonuclease (Enriquez 2012), heliox inhalation (Liet 2015), bronchodilators other than magnesium sulphate (Gadomski 2014), nebulised epinephrine (Hartling 2011), nebulised hypertonic saline (Zhang 2017), and steroids (Fernandes 2013). However, none of these interventions have been adopted as treatment. The role of magnesium sulphate therapy for bronchiolitis has not been reviewed previously. This review complements other Cochrane Reviews, to achieve consensus on treatment for children with acute bronchiolitis.
Objectives
To assess the effects of magnesium sulphate in acute bronchiolitis in children up to two years of age.
Methods
Criteria for considering studies for this review
Types of studies
In the protocol we planned to include randomised controlled trials (RCTs) and non‐RCTs, as we were expecting to identify few RCTs. We included RCTs only, as we did not identify any non‐RCTs.
Types of participants
We included children up to two years of age, diagnosed with acute bronchiolitis on clinical assessment, irrespective of confirmation of viral aetiology, presenting to emergency departments, outpatient departments, admitted to hospital or intensive care units. We did not exclude studies enrolling children with comorbidities, high risk factors, or both. Comorbidities and high risk factors for the population of interest for this review included children younger than 12 weeks, preterm birth at 34 weeks' gestation or less, histories of diagnosed chronic lung or congenital heart disease, or immunodeficiency.
Types of interventions
We compared magnesium sulphate (any dose, route of administration, or timing) alone, or as an adjunct to conventional treatments including:
nebulised hypertonic saline;
nebulised epinephrine; or
nebulised bronchodilators.
The possible comparisons were:
magnesium sulphate compared with placebo;
magnesium sulphate compared with hypertonic saline;
magnesium sulphate compared with epinephrine;
magnesium sulphate compared with conventional bronchodilator;
magnesium sulphate + bronchodilator compared with no treatment or normal saline + the same bronchodilator;
magnesium sulphate + hypertonic saline compared with no treatment or normal saline + hypertonic saline;
magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine
Types of outcome measures
We added the primary outcome time to recovery, as it is a person‐centred, clinically important outcome, likely to be modified by the treatment. In the protocol, the primary outcome, clinical severity score, had four components: clinical severity score measured using any validated scoring system, duration of mechanical ventilation, duration of hospital stay, or duration of intensive care unit stay. We moved clinical severity score to the secondary outcomes, and separated the four components into four secondary outcomes as these are different independent outcomes with different meaning. We specified time points of assessment of the clinical severity score.
Primary outcomes
Time to recovery
Mortality
Adverse effects of magnesium sulphate treatment
Secondary outcomes
Duration of hospital stay
Clinical severity score (at 0 to 24 hours and 25 to 48 hours after treatment) measured using any validated scoring system
Pulmonary function test
Hospital readmission rate within 30 days of discharge
Duration of mechanical ventilation
Duration of intensive care unit stay
Search methods for identification of studies
Electronic searches
We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 3), in the Cochrane Library, which includes the Cochrane Acute Respiratory Group's Specialised Register (searched 30 April 2020); MEDLINE via PubMed (1966 to 30 April 2020); Embase (1980 to 30 April 2020); LILACS (Latin American and Caribbean Health Science Information database; 1982 to 30 April 2020), and CINAHL (1992 to 30 April 2020; Appendix 1). We did not apply any language or publication restrictions. We also searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP; searched 30 April 2020).
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We contacted experts in the field to identify additional, unpublished material.
Data collection and analysis
Selection of studies
Two review authors (SC, DK) independently screened titles and abstracts of studies identified as a result of the search, for potential inclusion in the review. We retrieved full‐text study reports of studies deemed potentially eligible, and two review authors (SC, DK) independently screened the full text to identify studies for inclusion, and record reasons for exclusion of ineligible studies. We resolved any disagreements through discussion, or by consulting a third review author (NC), if necessary. We identified and excluded duplicates, and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in a PRISMA flow diagram (Figure 1), and 'Characteristics of excluded studies' table (Moher 2009).
1.
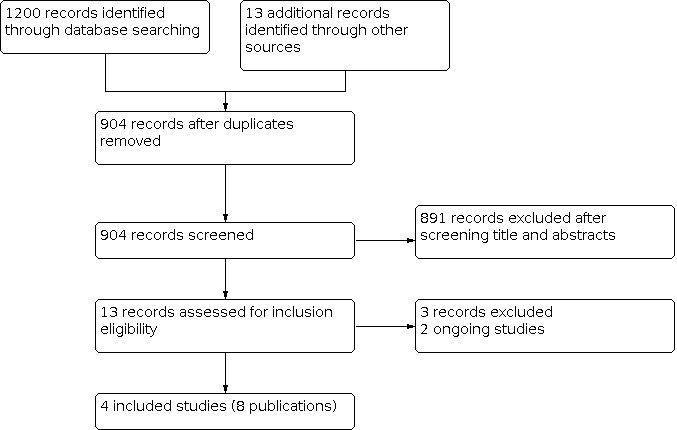
Study flow diagram
Data extraction and management
We used a data collection form for study characteristics and outcome data, which was piloted on at least one study in the review. One review author (SC) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any run‐in period, number of study centres and location, study setting, withdrawals, and date of study
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, inclusion criteria and exclusion criteria
Interventions: intervention, comparison, concomitant medications, and excluded medications
Outcomes: primary and secondary outcomes specified and collected, and time points reported
Notes: funding for trial, and notable conflicts of interest for trial authors.
Two review authors (SC, DK) independently extracted outcome data from the included studies. We noted if outcome data were not reported in a usable way in the 'Characteristics of included studies' tables. We resolved any disagreements by consensus. One review author (SC) entered data into the Review Manager 5 file (Review Manager 2014). We double‐checked that data were entered correctly by having a second review author (DK) spot‐check study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (SC, DK) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion, if necessary. We assessed the risk of bias according to the following domains.
Allocation (selection bias)
Blinding (performance bias and detection bias)
Incomplete outcome data (attrition bias)
Selective outcome reporting (reporting bias)
Other bias
We graded each potential source of bias as high, low, or unclear, and provided a quote from the study report, together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary. When information on risk of bias related to unpublished data, or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
We entered outcome data for each study into the Data and analyses tables in Review Manager 5 to calculate the treatment effects (Review Manager 2014). We used risk ratio (RR) for dichotomous outcome, such as hospital readmission rate. We did not analyse adverse effects and mortality, as no events were reported.
For continuous outcomes, we calculated mean difference (MD), as we only identified one study for analysis. We planned to use standardised mean difference (SMD) if different scales were used, but we did not find such data. We confirmed that higher scores for continuous outcomes had the same meaning for the particular outcome, explained the direction, and reported if the directions were reversed, when required. We reported all pooled effect sizes with 95% confidence intervals (CIs).
We did not conduct meta‐analyses, as the treatments were not similar enough to pool.
Unit of analysis issues
The unit of analysis was the child. For studies with more than two arms, we included the relevant arms for analysis, and excluded other arms. We planned to combine intervention groups to make a single pair comparison, but we did not find such data.
Dealing with missing data
We contacted investigators to verify key study characteristics, and to obtain missing numerical outcome data, when needed (e.g. when a study was identified as abstract only or a different format of reporting was used by the authors). We did not conduct sensitivity analyses, as either missing data were not likely to introduce bias, or data were insufficient.
Assessment of heterogeneity
We did not measure heterogeneity, as only one study was available for each comparison.
Assessment of reporting biases
We did not create a funnel plot to explore possible small‐study and publication biases, as we did not include more than 10 trials for any comparison.
Data synthesis
We planned to pool data from studies that we judged to be clinically homogeneous, using Review Manager 5 (Review Manager 2014). If more than one eligible study provided usable data in any single comparison, we planned to perform a meta‐analysis. We did not find sufficient data for pooling or meta‐analysing.
We planned to use the random‐effects model to analyse data, as we expected the included studies would differ in terms of intervention dose, route of delivery, and study location. We used the fixed‐effect model to calculate effect size and 95% CIs, as we identified and included only one study for each comparison.
Subgroup analysis and investigation of heterogeneity
We were unable to conduct analyses for many outcomes, due to the lack of data. Those outcomes and comparisons are described in Table 5. We did not perform any of the planned subgroup analyses due to insufficient data. Only one study was available for each comparison.
1. Comparisons and outcomes for which analyses could not be conducted.
| Outcome | Comparison | Reason for not conducting analysis |
| Time to recovery | All comparisons | No data available |
| Mortality | Magnesium sulphate compared with placebo | No deaths reported |
| magnesium sulphate compared with conventional bronchodilator | ||
| Magnesium sulphate + bronchodilator compared with no treatment or normal saline + the same bronchodilator | ||
| Magnesium sulphate compared with hypertonic saline | No data available | |
| Magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine | ||
| Adverse effects of magnesium sulphate treatment | Magnesium sulphate compared with placebo | No adverse effects reported |
| Magnesium sulphate compared with conventional bronchodilator | ||
| Magnesium sulphate + bronchodilator compared with no treatment or normal saline + the same bronchodilator | ||
| Magnesium sulphate compared with hypertonic saline | No data available | |
| Magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine | ||
| Duration of hospital stay | Magnesium sulphate compared with placebo | Reporting format was different. Study authors used geometric mean timea. We did not transform data for analysis, as only single study was available for this comparison. |
| Magnesium sulphate compared with conventional bronchodilator | Reporting format was different. Study authors used 95% confidence interval. We did not transform data for analysis, as only single study was available for this comparison. | |
| Magnesium sulphate + bronchodilator compared with no treatment or normal saline + the same bronchodilator | ||
| Clinical severity score at 0 to 24 hours after treatment | Magnesium sulphate compared with hypertonic saline | No data available |
| Magnesium sulphate compared with conventional bronchodilator | ||
| Magnesium sulphate + bronchodilator compared with no treatment or normal saline + the same bronchodilator | ||
| Clinical severity score at 25 to 48 hours after treatment | Magnesium sulphate compared with conventional bronchodilator | No data available |
| Magnesium sulphate + bronchodilator compared with no treatment or normal saline + the same bronchodilator | ||
| Pulmonary function test | All comparisons | No data available |
| Hospital readmission rate within 30 days of discharge | All comparisons except magnesium sulphate compared with placebo | No data available |
| Duration of mechanical ventilation | All comparisons | No data available |
| Duration of intensive care unit stay | All comparisons | No data available |
Note: these analyses were planned in the protocol, but were not carried out due to limited or no available data.
aGeometric mean is equal to the exponential of the mean of the log‐transformed values. The value of geometric mean is usually lesser than the arithmetic mean. Geometric mean is used as it is not much affected by the skewed distribution of data.
Sensitivity analysis
We did not conduct any sensitivity analyses due to insufficient data.
Summary of findings and assessment of the certainty of the evidence
We created 'Summary of findings' tables using the following outcomes: time to recovery, mortality, adverse events, duration of hospital stay, clinical severity score at 0 to 24 hours and at 25 to 48 hours after treatment, and hospital readmission rate within 30 days of discharge. We created a single 'Summary of findings' table for the comparisons, Magnesium sulphate compared with conventional bronchodilator, and Magnesium sulphate + bronchodilator compared with no treatment or normal saline + same bronchodilator. The intervention groups in both of these comparisons come from a single trial (Kose 2014). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence, as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade the quality of the evidence in footnotes, and provided comments to aid readers' understanding of the review, when necessary.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables for details.
Results of the search
We searched five databases, and retrieved 1200 records to 30 April 2020 (Electronic searches). Searches of other resources, internet, and expert contacts identified 13 additional records that appeared to meet the inclusion criteria, bringing our total yield to 1213 records. After removal of duplicates, we assessed 904 records, based on information presented in the title and abstract, and removed 891 records. We obtained the full text for 13 records. We excluded three studies, and identified two ongoing studies. We included four studies, reported in eight publications (Figure 1). We screened the reference lists of the included publications, but did not identify any additional randomised controlled trials (RCTs).
We sought additional information from study authors when data were not reported in a usable way, or were not reported. Two study authors responded and provided additional data (Alansari 2017; Kose 2014), one did not respond (Modaresi 2015), and another could not be contacted (Priya 2018). The authors of two excluded studies could not provide additional data (Gupta 2015; Rady 2018).
Included studies
Study design
We did not identify any non‐RCTs comparing magnesium sulphate alone, or in addition to conventional treatments, with any interventions. We included four RCTs, reported in eight publications. Three studies were published as full‐text papers in peer reviewed journals (Alansari 2017; Kose 2014; Modaresi 2015); one was a Master's thesis, posted on a university website (Priya 2018). Three RCTs were parallel‐arm designs; one included three parallel arms (Kose 2014).
Setting
The included studies were conducted in Qatar (Alansari 2017), Turkey (Kose 2014), Iran (Modaresi 2015), and India (Priya 2018). Three were single‐centre studies, and one was a three‐centre study (Modaresi 2015). Three studies enrolled children from emergency rooms; one study did not specify the setting (Modaresi 2015).
Population
Two studies included children up to 12 months old (Modaresi 2015; Priya 2018), one study included children up to 18 months (Alansari 2017), and one study included children from 1 month to 24 months old (Kose 2014). Three studies excluded children with a history of premature birth (Alansari 2017; Kose 2014; Priya 2018); two studies excluded children with atopy (allergy) history or family history of asthma (Kose 2014; Modaresi 2015). Two studies used the Wang score (range 0 to 12; higher scores indicate increased illness severity) for clinical severity scoring, and included children whose scores were ≥ 4 (moderate to severe bronchiolitis; (Alansari 2017; Kose 2014; Wang 1992)). Two studies used the Respiratory Distress Assessment Instrument (RDAI; range 0 to 17; higher scores mean increased illness severity; (Lowell 1987)). One included children whose scores were at least 5 (moderate to severe bronchiolitis; (Modaresi 2015)). Severity of illness was unclear in Priya 2018.
Intervention
Comparator interventions differed amongst all four trials: Alansari 2017 compared magnesium sulphate with placebo; Kose 2014 compared it with a bronchodilator (salbutamol); Modaresi 2015 compared magnesium sulphate in combination with epinephrine with epinephrine alone ; and Priya 2018 compared magnesium sulphate with hypertonic saline. Three studies administered the nebulised form of magnesium sulphate to children, and one administered intravenous magnesium sulphate (Alansari 2017). Magnesium sulphate was given as a single intravenous dose in Alansari 2017, as two nebulised doses in Kose 2014, and as multiple doses until discharge of the child in two studies (Modaresi 2015; Priya 2018).
Study funding sources
Alansari 2017 was hospital‐sponsored, and Modaresi 2015 was funded by the Child Growth and Development Research Center, Isfahan University of Medical Sciences. Two studies did not mention sources of funding (Kose 2014; Priya 2018).
Excluded studies
We excluded three studies (Gupta 2015; Pruikkonen 2018; Rady 2018).
All three studies included different populations; study authors were unable to provide specific outcome data for children up to two years old, with the first episode of wheezing.
Gupta 2015 was available as a conference abstract. The trial enrolled children, of unspecified ages, with first or second episodes of moderate to severe acute bronchiolitis. We contacted the trial authors to request data on children with their first wheezing episode, but additional data were not available. Two studies examined a different population. Pruikkonen 2018 included children aged six months to four years, with more than one episode of wheezing. Rady 2018 included children with wheezy chest of all causes, between the ages of 2 months and 12 years; the trial authors were unable to provide details of outcome on children up to two years old.
Studies awaiting classification
We did not identify any studies awaiting classification.
Ongoing studies
We identified two ongoing trials being conducted in India. Both are placebo controlled, two parallel‐arm trials. CTRI/2017/02/007919 plans to enrol 104 children from 2 to 12 months old. CTRI/2018/06/014400 plans to enrol 50 children from 1 to 24 months old. The intervention drug in both studies is the nebulised form of magnesium sulphate, but doses and frequency of administration differ. CTRI/2017/02/007919 prescribes three doses of magnesium sulphate at one hour intervals; CTRI/2018/06/014400) prescribes magnesium sulphate every 4 hours over 24 hours (6 doses). The primary outcome, bronchiolitis severity, is measured with the RDAI score in CTRI/2017/02/007919, and the Wang bronchiolitis severity score in CTRI/2018/06/014400. See Characteristics of ongoing studies.
Risk of bias in included studies
Figure 2 provides a summary of risk of bias judgements. Figure 3 depicts the risk of bias for each domain, presented as percentages across all included studies. We assessed two studies at low risk of bias overall, and two as unclear risk of bias overall.
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
3.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item, presented as percentages across all included studies
Allocation
We judged two studies at low risk of bias for selection bias; both provided adequate reporting of sequence generation and allocation (Alansari 2017; Modaresi 2015). Two studies did not provide adequate information on the method of sequence generation, and allocation and we rated them as unclear risk of bias (Kose 2014; Priya 2018).
Blinding
We judged one study, which maintained blinding of investigators and parents, at low risk of bias (Modaresi 2015). We judged three studies as unclear risk of bias, because they did not give details on how they maintained blinding (Alansari 2017; Kose 2014; Priya 2018).
Incomplete outcome data
We considered all four studies to be at low risk of attrition bias; fewer than two to three children in each intervention group were lost to follow‐up or withdrew from three studies (Alansari 2017; Kose 2014; Modaresi 2015); no participant data were missing from Priya 2018.
Selective reporting
We considered all included studies to be at a low risk of bias for selective reporting. Alansari 2017 reported all outcomes as specified in the protocol. The protocols for the other three studies were not available, but they reported all outcomes planned in the methods section of the published report. We did not detect any selective omission of outcomes.
Other potential sources of bias
We judged two studies at low risk of other bias (Alansari 2017; Modaresi 2015). Authors of these trials did not disclose the receipt of any grants, funds, or shares. We considered two trials as unclear risk of bias (Kose 2014; Priya 2018). These trials did not mention sources of funding, or conflicts of interest. There was no imbalance in baseline clinical severity scores, or other baseline characteristics of the children in the included studies. We found nothing to suggest inappropriate administration of study drugs, pre‐randomisation administration of interventions, interim analysis affecting conduct of research, or selective reporting of subgroups.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Comparison 1: magnesium sulphate compared with placebo
One RCT, which enrolled 162 children, compared intravenous (IV) magnesium sulphate with placebo (Table 1; Alansari 2017). They excluded the data for two enrolled children in the analysis, because one child was immunodeficient, and the other was discharged against medical advice. A total of 160 children were analysed for all outcomes except hospital re‐admission rate (a secondary outcome), for which data from 158 children were analysed.
Primary outcomes
1. Time to recovery
This outcome was not measured.
2. Mortality
We contacted the trial authors, who confirmed there were no deaths in either group (Alansari 2019).
3. Adverse effects of magnesium sulphate treatment
There were no reported incidences of adverse effects, such as apnoea, cyanosis, or haemodynamic instability in either group.
Secondary outcomes
1. Duration of hospital stay
Alansari 2017 did not measure simple (arithmetic) mean and standard deviation (SD), but instead, measured geometric means. Geometric mean is equal to the exponential of the mean of the log‐transformed values. The value of geometric mean is lesser than the arithmetic mean. Geometric mean is not much affected by the skewed distribution of data. We contacted the trial authors to request simple mean and SD. Although we received a response, the trial authors did not provide the requested data (Alansari 2019). We did not calculate arithmetic means from the geometric mean for this analysis, because there was only one study available for this comparison. Geometric mean time until discharge was 24.1 hours in the magnesium sulphate group and 25.3 hours in the placebo group.
2. Clinical severity score (at 0 to 24 hours and 25 to 48 hours after treatment) measured using any validated scoring system
Alansari 2017 reported clinical severity scores for bronchiolitis at a number of time points up to 72 hours post‐treatment, using the Wang score. The magnesium sulphate group included 78 children, and the placebo group included 82 children at all measurement time points. Mean bronchiolitis severity scores at baseline were 6.85 points (SD 1.30) in the magnesium sulphate group, and 6.97 points (SD 1.17) in the placebo group. The study found no statistical differences in clinical severity score at any time point.
Clinical severity score at 0 to 24 hours after treatment
The mean clinical severity score at 24 hours was 4.68 points (SD 0.94) in the magnesium sulphate group, and 4.55 points (SD 1.62) in the placebo group. It is uncertain whether magnesium sulphate improves clinical severity score at 24 hours (MD 0.13, 95% confidence interval (CI) ‐0.28 to 0.54; 1 RCT; 160 children; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Magnesium sulphate compared with placebo for acute bronchiolitis in children, Outcome 1: Clinical severity score at 0 to 24 hours after treatment
Clinical severity score at 25 to 48 hours after treatment
The mean clinical severity score at 48 hours was 4.42 points (SD 1.08) in the magnesium sulphate group, and 4.84 points (SD 1.57) in the placebo group. It is uncertain whether magnesium sulphate reduces clinical severity score at 48 hours (MD ‐0.42, 95% CI ‐0.84 to ‐0.00; 1 RCT; 160 children; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Magnesium sulphate compared with placebo for acute bronchiolitis in children, Outcome 2: Clinical severity score at 25 to 48 hours after treatment
3. Pulmonary function test
This outcome was not measured.
4. Hospital readmission rate within 30 days of discharge
Hospital readmission rates within 14 days of discharge were available. Fifteen children in the magnesium sulphate group and five children in the placebo group were readmitted to an infirmary or hospital. Magnesium sulphate may increase hospital readmissions amongst children up to two years old with bronchiolitis, compared with placebo (risk ratio (RR) 3.16, 95% CI 1.20 to 8.27; 1 RCT; 158 children; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Magnesium sulphate compared with placebo for acute bronchiolitis in children, Outcome 3: Hospital readmission rate within 30 days of discharge
5. Duration of mechanical ventilation
None of the children were mechanically ventilated.
6. Duration of intensive care unit stay
The mean duration of intensive care unit stay was 101.00 hours in the magnesium sulphate group (SD not available; there was only one child in the magnesium sulphate group), and 154.37 hours (SD 62.62) in the placebo group. Although the mean duration was shorter in the magnesium sulphate group, it is uncertain whether magnesium sulphate reduced duration of intensive care unit stay compared with placebo, due to the small number of participants (1 RCT; 160 children; very low‐certainty evidence).
Comparison 2: magnesium sulphate compared with hypertonic saline
One RCT, which enrolled 220 children, compared nebulised magnesium sulphate with hypertonic saline (Table 2; Priya 2018).
Primary outcomes
1. Time to recovery
This outcome was not measured.
2. Mortality
This outcome was not measured. The thesis did not describe mortality. We were unsuccessful in our attempts to contact the study author or thesis supervisor.
3. Adverse effects of magnesium sulphate treatment
This outcome was not measured.
Secondary outcomes
1. Duration of hospital stay
The mean duration of hospital stay was 3.2 days (SD 1.1) in the magnesium sulphate group, and 3.2 days (SD 1.0) in the hypertonic saline group. It is uncertain whether there is a difference between magnesium sulphate and hypertonic saline on duration of hospital stay (MD 0.00, 95% CI ‐0.28 to 0.28; 1 RCT; 220 children; very low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Magnesium sulphate compared with hypertonic saline for acute bronchiolitis in children, Outcome 1: Duration of hospital stay
2. Clinical severity score (at 0 to 24 hours and 25 to 48 hours after treatment) measured using any validated scoring system
The study used the RDAI score for clinical severity for bronchiolitis, measured at a number of time points until discharge. The magnesium sulphate and hypertonic saline groups each included 110 children at all time points. Mean bronchiolitis severity scores at baseline were 8.0 points (SD 1.8) in the magnesium sulphate group, and 7.9 points (SD 1.7) in the hypertonic saline group.
Clinical severity score at 0 to 24 hours after treatment
This outcome was not measured.
Clinical severity score at 25 to 48 hours after treatment
The mean clinical severity score at 48 hours was 3.8 points (SD 1.9) in the magnesium sulphate group, and 3.7 points (SD 1.8) in the hypertonic saline group. It is uncertain whether magnesium sulphate improves clinical severity score at 48 hours (MD 0.10, 95% CI ‐0.39 to 0.59; 1 RCT; 220 children; very low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Magnesium sulphate compared with hypertonic saline for acute bronchiolitis in children, Outcome 2: Clinical severity score at 25 to 48 hours after treatment
3. Pulmonary function test
This outcome was not measured.
4. Hospital readmission rate within 30 days of discharge
This outcome was not measured.
5. Duration of mechanical ventilation
This outcome was not measured. The study only included children with mild to moderate bronchiolitis; they excluded children with severe bronchiolitis.
6. Duration of intensive care unit stay
This outcome was not measured.
Comparison 3: magnesium sulphate compared with epinephrine
None of our included trials compared magnesium sulphate with epinephrine.
Comparison 4: magnesium sulphate compared with conventional bronchodilator
We included one RCT, enrolling 57 children, which compared nebulised magnesium sulphate with salbutamol, a conventional bronchodilator (Table 3; Kose 2014). One child in the magnesium sulphate group was withdrawn due to deteriorating clinical status. Children were allocated to one of three interventional arms: magnesium sulphate (19 children); salbutamol (18 children); and salbutamol + magnesium sulphate (19 children). We used data from two study arms (the magnesium sulphate group and the salbutamol group, N = 37 children) for analysis.
Primary outcomes
1. Time to recovery
This outcome was not measured.
2. Mortality
The trial authors confirmed that no deaths occurred in either group (Kose 2019).
3. Adverse effects of magnesium sulphate treatment
There were no reported incidences of adverse effects, such as hypotension, arrhythmias, or loss of deep tendon reflexes in either group (Kose 2019).
Secondary outcomes
1. Duration of hospital stay
The trial authors did not provide the SD for duration of hospital stay, but reported the 95% CI (Kose 2019). We did not calculate the SD from the 95% CI for this analysis because: 1) there was only a single study available for this comparison; 2) there were very few children in either intervention arm; and 3) the 95% CIs were not symmetrical. The mean duration of hospital stay was 24 hours (95% CI 25.8 to 47.4) in the magnesium sulphate group, and 24 hours (95% CI 23.4 to 76.9) in the salbutamol group. It is uncertain whether magnesium sulphate has any effect on duration of hospital stay compared with salbutamol, a conventional bronchodilator (1 RCT; 37 children; very low‐certainty evidence).
2. Clinical severity score (at 0 to 24 hours and 25 to 48 hours after treatment) measured using any validated scoring system
The study used the Wang score for clinical severity for bronchiolitis, and documented scores at one and four hours after treatment, but not at 24 or 48 hours after treatment.
Clinical severity score at 0 to 24 hours after treatment
This outcome was not measured.
Clinical severity score at 25 to 48 hours after treatment
This outcome was not measured.
3. Pulmonary function test
This outcome was not measured (Kose 2019).
4. Hospital readmission rate within 30 days of discharge
This outcome was not measured (Kose 2019).
5. Duration of mechanical ventilation
No children were mechanically ventilated (Kose 2019).
6. Duration of intensive care unit stay
No children required admission for intensive care (Kose 2019).
Comparison 5: magnesium sulphate + bronchodilator compared with no treatment or normal saline + the same bronchodilator
We included one RCT, enrolling 57 children, which compared nebulised magnesium sulphate with a conventional bronchodilator (salbutamol; Table 3; Kose 2014). Children were allocated to one of three interventional arms: magnesium sulphate (19 children); salbutamol (18 children); and salbutamol + magnesium sulphate (19 children). We used data from two study arms (magnesium sulphate + salbutamol group and the no treatment or normal saline + salbutamol group; N = 37 children) for the analyses.
Primary outcomes
1. Time to recovery
This outcome was not measured.
2. Mortality
The trial authors confirmed there were no deaths in either group (Kose 2019).
3. Adverse effects of magnesium sulphate treatment
There were no reported incidences of adverse effects, such as hypotension, arrhythmias, or loss of deep tendon reflexes in either group (Kose 2019).
Secondary outcomes
1. Duration of hospital stay
The trial authors did not provide the SD for duration of hospital stay, but reported the 95% CI. We did not calculate the SD from the 95% CI for this analysis because: 1) there was only a single study available for this comparison; 2) there were very few children in either intervention arm; and 3) the 95% CIs were not symmetrical. The mean duration of hospital stay was 20 hours (95% CI 15.3 to 39.0) in the magnesium sulphate + salbutamol group, and 24 hours (95% CI 23.4 to 76.9) in the salbutamol group. Due to very low‐certainty evidence, the effect of magnesium sulphate + salbutamol on duration of hospital stay is uncertain.
2. Clinical severity score (at 0 to 24 hours and 25 to 48 hours after treatment) measured using any validated scoring system
The study used the Wang score for clinical severity for bronchiolitis, and documented it at one and four hours after treatment, but not at 24 or 48 hours after treatment.
Clinical severity score at 0 to 24 hours after treatment
This outcome was not measured.
Clinical severity score at 25 to 48 hours after treatment
This outcome was not measured.
3. Pulmonary function test
This outcome was not measured (Kose 2019).
4. Hospital readmission rate within 30 days of discharge
This outcome was not measured (Kose 2019).
5. Duration of mechanical ventilation
No children were mechanically ventilated (Kose 2019).
6. Duration of intensive care unit stay
No children required intensive care admission (Kose 2019).
Comparison 6: magnesium sulphate + hypertonic saline compared with no treatment or normal saline + hypertonic saline
No trials compared magnesium sulphate + hypertonic saline versus no treatment or normal saline + hypertonic saline.
Comparison 7: magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine
We included one RCT, with 125 enrolled children, which compared these interventions (Table 4; Modaresi 2015). Data from 120 children (60 in each group) were available for analysis; three children from the magnesium sulphate group and two children from the epinephrine group were lost during follow‐up. The trial authors did not respond to our request for additional data.
Primary outcomes
1.Time to recovery
This outcome was not measured.
2. Mortality
This outcome was not measured.
3. Adverse effects of magnesium sulphate treatment
This outcome was not measured.
Secondary outcomes
1. Duration of hospital stay
The mean duration of hospital stay was 84.3 hours (SD 9.7) in the magnesium sulphate + epinephrine group, and 84.7 hours (SD 10.1) in the epinephrine group (MD ‐0.40, 95% CI ‐3.94 to 3.14; 1 RCT; 120 children; very low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine for acute bronchiolitis in children, Outcome 1: Duration of hospital stay
2. Clinical severity score (at 0 to 24 hours and 25 to 48 hours after treatment) measured using any validated scoring system
The study used the RDAI score for clinical severity for bronchiolitis, and documented it at a number of time points until discharge. The mean bronchiolitis severity scores at baseline were 11.4 points (SD 2.7) in the magnesium sulphate + epinephrine group, and 11.0 points (SD 2.7) in the epinephrine group.
Clinical severity score at 0 to 24 hours after treatment
The mean clinical severity score at 24 hours was 6.4 points (SD 2.6) in the magnesium sulphate + epinephrine group, and 6.6 points (SD 2.2) in the epinephrine group. It is uncertain whether magnesium sulphate + epinephrine reduces clinical severity score at 24 hours compared with epinephrine alone (MD ‐0.20, 95% CI ‐1.06 to 0.66; 1 RCT; 120 children; very low‐certainty evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: Magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine for acute bronchiolitis in children, Outcome 2: Clinical severity score at 0 to 24 hour after treatment
Clinical severity score at 25 to 48 hours after treatment
The mean clinical severity score at 48 hours was 2.8 points (SD 2.0) in the magnesium sulphate + epinephrine group, and 3.7 points (SD 2.7) in the epinephrine group. It is uncertain whether magnesium sulphate + epinephrine reduces clinical severity score at 48 hours compared with epinephrine alone (MD ‐0.90, 95% CI ‐1.75 to ‐0.05; 1 RCT; 120 children; very low‐certainty evidence; Analysis 3.3).
3.3. Analysis.

Comparison 3: Magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine for acute bronchiolitis in children, Outcome 3: Clinical severity score at 25 to 48 hour after treatment
3. Pulmonary function test
This outcome was not measured.
4. Hospital readmission rate within 30 days of discharge
This outcome was not measured.
5. Duration of mechanical ventilation
This outcome was not measured.
6. Duration of intensive care unit stay
This outcome was not measured.
Discussion
Summary of main results
We included four randomised controlled trials (RCT; 564 children). Each trial compared magnesium sulphate, either alone or in combination, with different interventions. Of seven proposed comparisons, data were available for five. Studies compared magnesium sulphate with placebo or no treatment, a conventional bronchodilator, or hypertonic saline. No treatment was standard care, epinephrine, or salbutamol for both groups. If measured, the outcomes were reported by a single trial only, resulting in low‐quality or very low‐quality evidence for all outcomes.
Therefore, we are uncertain about the effects of magnesium sulphate on mortality, adverse events, the duration of hospital stay, clinical severity at 24 or 48 hours, or the risk of readmission within 30 days.
None of the studies reported on two of our primary outcomes, time to recovery and mortality. However, correspondence with authors of two trials confirmed there were no deaths in any of the groups (Alansari 2017; Kose 2014). It may be that mortality rates associated with acute bronchiolitis are low, and only one study included children with severe bronchiolitis, while three studies included children with moderate disease severity. The studies either reported no adverse events, or did not measure them as an outcome.
Duration of hospital stay was reported in different formats in each study, but there was little or no difference found between groups. Effects on clinical severity at 24 or 48 hours were uncertain for magnesium sulphate alone compared to placebo or hypertonic saline, or when combined with epinephrine and compared to normal saline + epinephrine.
Pulmonary function tests were not measured in any of the included trials. Hospital re‐admission rate within 30 days of discharge was measured by one trial. Magnesium sulphate may increase hospital readmission rate within 14 days of discharge compared with placebo. Duration of mechanical ventilation and intensive care unit stay were not available for any comparison.
We found that the available evidence was insufficient to establish the efficacy and safety of magnesium sulphate due to single studies analysing small numbers of children for each comparison.
Overall completeness and applicability of evidence
The available evidence is insufficient to determine the superiority or inferiority of magnesium sulphate over other treatments for children with acute bronchiolitis. Our searches identified relevant studies, abstracts, and ongoing studies. Of the four included studies, only Alansari 2017 had a published protocol available, and reported all predefined outcomes. The overall applicability of the evidence was low, due to imprecision and risk of bias. Studies differed regarding intervention groups, and could not be pooled for analysis. No studies were available for two comparisons: magnesium sulphate versus epinephrine, and magnesium sulphate + hypertonic saline versus no treatment or normal saline + hypertonic saline. The evidence determining the role of magnesium sulphate on the primary outcome, time to recovery, was not available, as this outcome was not measured in any of the trials. There was a lack of data for the remaining primary outcomes, mortality and adverse events. Evidence from single studies only were available for the remainder of the outcomes.
Quality of the evidence
We used GRADE methods to assess the overall certainty of evidence. We assessed it as very low for most outcomes for which data were available, due to very serious imprecision (i.e. single trial analysing few participants for each comparison). We assessed the domains regarding allocation and blinding as unclear, which suggests incomplete reporting. We assessed one trial at low risk of bias for all domains; and one trial at unclear risk of bias in most domains.
Potential biases in the review process
The search strategy was designed to detect both RCTs and non‐RCTs, and it is unlikely that relevant studies were not identified and assessed. We attempted to obtain additional information from the trial authors as required. Two review authors independently screened the search results, determined studies for inclusion, assessed risk of bias, extracted relevant data, and conducted GRADE assessments. We resolved discrepancies with an independent review author three times, regarding risk of bias and GRADE assessment. There were some deviations from the protocol, but these were unlikely to introduce bias. We could not assess publication bias due to the small number of studies available. It is possible that some studies with negative results were missed, if they were not available in the public domain.
Agreements and disagreements with other studies or reviews
We did not find any published reviews that addressed the same clinical question considered in our review.
Authors' conclusions
Implications for practice.
There are insufficient data and evidence to establish the efficacy and safety of magnesium sulphate for children under two years of age with acute bronchiolitis. We are uncertain, if compared with placebo, magnesium sulphate may be associated with an increased readmission rate within 14 days of discharge from hospital (low‐certainty evidence).
There is no evidence available for our primary outcome, time to recovery, for any comparison; and no evidence for secondary outcomes, duration of mechanical ventilation, intensive care unit stay, and pulmonary function.
Implications for research.
Well‐designed randomised controlled trials to assess the effects of magnesium sulphate for children with acute bronchiolitis are needed. Future trials enrolling children from different settings, and including very ill children with bronchiolitis are also needed. Outcomes, such as time to recovery, adverse effects, need for mechanical ventilation, duration of mechanical ventilation, need for intensive care unit admission, and duration of intensive care unit stay must be included.
History
Protocol first published: Issue 2, 2018 Review first published: Issue 12, 2020
Acknowledgements
We thank Khalid Ansari and Mehmat Kose for their gracious assistance in providing additional data and responding to our questions (Alansari 2019; Kose 2019). We thank Arun K Yadav for his support and help in this review. We thank Dr Kalpana Kumari and Dr Rahul Teotia for their help with analyses.
The Methods section of the protocol for this review was based on a standard template developed by Cochrane Airways, and adapted by the Cochrane Acute Respiratory Infections Group.
We would also like to acknowledge the reviewers, Professor David Isaacs, University of Sydney, Australia and Linjie Zhang, Faculty of Medicine, Federal University of Rio Grande, Brazil for giving us valuable suggestions and comments. We acknowledge Anne Lyddiatt and Dee Schneiderman, the consumer reviewers, for providing valuable suggestions during the course of peer review. We also acknowledge the contact editor, Lubna Al‐Ansary, and statistical editor, Conor Teljeur, for giving their valuable time to this review.
Appendices
Appendix 1. MEDLINE PubMed search
#1 Bronchiolitis[Mesh] #2 bronchiolitis[tiab] #3 bronchioli tides[tiab] #4 bronchiole*[tiab] #5 bronchial[tiab] #6 bronchiolar[tiab] #7 bronchodilat*[tiab] #8 bronchoconstrict*[tiab] #9 Respiratory Syncytial Viruses[MeSH:NoExp] #10 Respiratory Syncytial Virus, Human[MeSH] #11 Respiratory Syncytial Virus Infections[MeSH] #12 Respiratory Syncytial Virus[tiab] #13 Respiratory Syncytial Viruses[tiab] #14 rsv[tiab] #15 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 #16 "Magnesium Sulfate"[Mesh] #17 "Magnesium Compounds"[Mesh:NoExp] #18 "Magnesium"[Mesh] #19 MgSO4*[tiab] #20 Mg S04*[tiab] #21 magnesium[tiab] #22 magnesium[nm] #23 #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 #24 #15 AND #23 #25 animals [mh] NOT humans [mh] #26 #24 NOT #25
Appendix 2. Embase Elsevier search
#1 'bronchiolitis'/exp #2 bronchiolitis:ti,ab #3 bronchiolitides:ti,ab #4 bronchiole*:ti,ab #5 bronchial:ti,ab #6 bronchiolar:ti,ab #7 bronchodilat*:ti,ab #8 bronchoconstrict*:ti,ab #9 'pneumovirus'/de #10 'human respiratory syncytial virus'/exp #11 'respiratory syncytial virus infection'/exp #12 "respiratory syncytial virus":ti,ab #13 "respiratory syncytial viruses":ti,ab #14 rsv:ti,ab #15 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 #16 'magnesium sulfate'/de #17 'magnesium derivative'/de #18 'magnesium'/de #19 MgSO4*:ti,ab #20 "Mg S04*":ti,ab #21 magnesium:ti,ab #22 magnesium:tn)) #23 #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 #24 #15 AND #23 #25 'animal '/exp NOT 'human '/exp #26 #24 NOT #25
Appendix 3. CENTRAL search (via Wiley)
#1 MeSH descriptor: [Bronchiolitis] explode all trees #2 "bronchiolitis":ti,ab,kw (Word variations have been searched) #3 "bronchiolitides":ti,ab,kw (Word variations have been searched) #4 bronchiole*:ti,ab,kw (Word variations have been searched) #5 bronchial:ti,ab,kw (Word variations have been searched) #6 "bronchiolar":ti,ab,kw (Word variations have been searched) #7 bronchodilat*:ti,ab,kw (Word variations have been searched) #8 bronchoconstrict*:ti,ab,kw (Word variations have been searched) #9 MeSH descriptor: [Respiratory Syncytial Viruses] this term only #10 MeSH descriptor: [Respiratory Syncytial Virus, Human] explode all trees #11 MeSH descriptor: [Respiratory Syncytial Virus Infections] explode all trees #12 "respiratory syncytial virus":ti,ab,kw (Word variations have been searched) #13 "respiratory syncytial viruses":ti,ab,kw (Word variations have been searched) #14 "RSV":ti,ab,kw (Word variations have been searched) #15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 #16 MeSH descriptor: [Magnesium Sulfate] explode all trees #17 MeSH descriptor: [Magnesium Compounds] this term only #18 MeSH descriptor: [Magnesium] explode all trees #19 MgSO4*:ti,ab,kw (Word variations have been searched) #20 Mg S04*:ti,ab,kw (Word variations have been searched) #21 "magnesium":ti,ab,kw (Word variations have been searched) #22 #16 or #17 or #18 or #19 or #20 or #21 #23 #15 and #22 #24 MeSH descriptor: [Animals] explode all trees #25 MeSH descriptor: [Humans] explode all trees #26 #24 not #25 #27 #23 not #26
Appendix 4. LILACS Virtual health library search
mh:C08.127.446.135$ OR Bronquiolitis OR Bronquiolite OR bronchiolitis OR bronchiolitides OR bronchiole* OR bronchial OR bronchiolar OR bronchodilat* OR bronchoconstrict* OR mh:"Respiratory Syncytial Viruses" OR mh:"Respiratory Syncytial Virus, Human" OR mh:"Respiratory Syncytial Virus Infections" OR (Virus AND Sincitial AND Respiratorio) OR (Vírus AND Respiratório AND Sincicial) OR respiratory syncytial virus OR respiratory syncytial viruses OR rsv) AND (mh:"Magnesium Sulfate" OR mh:"Magnesium Compounds" OR mh:"Magnesium" OR Magnesio OR Magnésio OR mgso4* OR "mg s04*" OR magnesium
Appendix 5. CINAHL EBSCO search
S27 S23 NOT S26
S26 S24 NOT S25
S25 (MH "Human")
S24 (MH "Animals+")
S23 S15 AND S22
S22 S16 OR S17 OR S18 OR S19 OR S20 OR S21
S21 TI Magnesium OR AB Magnesium
S20 TI Mg S04* OR AB Mg S04*
S19 TI MgSO4* OR AB MgSO4*
S18 (MH "Magnesium")
S17 (MH "Magnesium Compounds+")
S16 (MH "Magnesium Sulfate")
S15 S1 OR S2 OR S3 OR S4 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S13 OR S14
S14 TI rsv OR AB rsv
S13 (MH "Respiratory Syncytial Virus Infections")
S12 TI "Respiratory syncytial virus" OR AB "Respiratory syncytial virus"
S11 TI "Respiratory Syncytial Viruses" OR AB "Respiratory Syncytial Viruses"
S10 (MH "Respiratory Syncytial Viruses")
S9 TI pneumovirus OR AB pneumovirus
S8 TI bronchoconstrict* OR AB bronchoconstrict*
S7 TI bronchodilat* OR AB bronchodilat*
S6 TI bronchiolar OR AB bronchiolar
S5 TI bronchial OR AB bronchial
S4 TI bronchiole* OR AB bronchiole*
S3 TI bronchiolitides OR AB bronchiolitides
S2 TI Bronchiolitis OR AB Bronchiolitis
S1 (MH "Bronchiolitis+")
Data and analyses
Comparison 1. Magnesium sulphate compared with placebo for acute bronchiolitis in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Clinical severity score at 0 to 24 hours after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Clinical severity score at 25 to 48 hours after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Hospital readmission rate within 30 days of discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Magnesium sulphate compared with hypertonic saline for acute bronchiolitis in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Duration of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Clinical severity score at 25 to 48 hours after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Magnesium sulphate + epinephrine compared with no treatment or normal saline + epinephrine for acute bronchiolitis in children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Duration of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 Clinical severity score at 0 to 24 hour after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3 Clinical severity score at 25 to 48 hour after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alansari 2017.
| Study characteristics | ||
| Methods |
Trial registration: NCT02145520 Study design: randomised, double‐blind placebo‐controlled study Setting and centres: single‐centre, short stay unit, Paediatric Emergency Center, Hamad General Hospital, Qatar Dates of study: October 2012 to May 2015 Duration of study: until child discharged Inclusion criteria: children aged ≤ 18 months, with moderate to severe viral bronchiolitis, with prodromal history consistent with viral upper respiratory tract infection, followed by wheezing or crackles on auscultation, or both, and a Wang bronchiolitis severity score ≥ 4 on presentation Exclusion criteria: children were excluded if they had 1 or more of the following characteristics: preterm birth ≤ 34 weeks' gestation, previous history of wheezing, steroid use within 48 hours of presentation, obtundation and progressive respiratory failure requiring intensive care unit admission, history of apnoea within 24 hours before presentation, oxygen saturation ≤ 85% on room air, history of a diagnosis of chronic lung disease, congenital heart disease, immunodeficiency, exposure to varicella within 21 days before enrolment, known magnesium or calcium metabolism disturbance, or known adverse reaction to magnesium sulphate |
|
| Participants |
Randomised: 162 infants; 80 in the magnesium group and 82 in the placebo group. Analysed: 160 infants; 78 in the magnesium group and 82 in the placebo group; 2 enrolled infants could not be included in the analysis: 1 was immunodeficient and 1 was discharged against medical advice Analysis for follow‐up: at 2 weeks: 158 infants; magnesium 77; placebo 81 (2 children were lost to follow‐up, 1 from each group) Age: (mean (SD)) was 4 (3) months in the magnesium group and 5 (4) months in the placebo group Gender distribution: (male: female) was 58:22 in the magnesium group and 48:34 in the placebo group Baseline Wang bronchiolitis severity score: mean (SD) was 6.85 (1.30) in the magnesium group and 6.97 (1.17) in the placebo group |
|
| Interventions |
Intervention: 100 mg/kg magnesium sulphate intravenously by a syringe pump over 60 minutes; single dose Comparator: placebo; 0.9% saline Concomitant treatment in both study arms: 5 mL nebulised 5% hypertonic saline in 1 mL 1:1000 epinephrine given at 4 hour intervals throughout the infirmary stay |
|
| Outcomes |
Planned outcomes: the study protocol was available, so we could determine the planned outcomes. Primary outcome was improvement percentage of discharge after a dose of intravenous magnesium sulphate (time frame: time to medical readiness for discharge). Secondary outcomes were
Reported outcomes: all outcomes planned in study protocol were reported in the paper. Outcome data not provided in a usable way for improvement percentage of discharge after a dose of intravenous magnesium sulphate. Study authors used geometric mean (95% CI) time until discharge and expressed results as ratio of geometric mean time in 2 interventions. Mean (SD) was not provided by the authors. |
|
| Notes |
Funding source: this study was hospital sponsored by Hamad Medical Corporation Conflicts of interest: none Comment: the study did not provide mean and standard deviations for clinical severity score at various time points. They included a bar chart from which it was difficult to obtain the exact scores. The trial author provided this information in the form of a table. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate methods of sequence generation and allocation concealment used. "Randomisation was stratified into two groups, patients with a history of eczema and/or known to have a parent or full sibling with prior physician diagnosis of asthma, and patients without. Block randomisation was used for allocation". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Details of blinding were not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 child was lost to follow‐up in either group, and excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes planned in the protocol were reported in the published report |
| Other bias | Low risk | None of the trial authors disclosed receipt of any grants, funds, or shares from any pharmaceutical company. Authors reported no conflicts of interest |
Kose 2014.
| Study characteristics | ||
| Methods |
Trial registration: not traceable Study design: double‐blind, prospective clinical trial Setting and centres: single‐centre, short‐stay unit, Pediatric Emergency Department of Erciyes University Hospital, Central Anatolia, Kayseri, Turkey Dates of study: October 2012 to March 2013 Duration of study: until child was discharged Inclusion criteria: children aged 1 to 24 months, history of preceding viral upper respiratory infection followed by wheezing and crackles on auscultation, first wheezing episode, and clinical severity score 4 to 8 on admission. Viral respiratory infection was diagnosed on clinical grounds. Exclusion criteria: infants with clinical severity score < 4 or > 8, oxygen saturation (SaO₂) < 86 % in room air, chronic cardiopulmonary or neurological disease, premature birth, birthweight < 2500 g, history of recurrent wheezing episodes, history of atopy, proven immune deficiency, age < 1 month or > 2 years, proven or suspected acute bacterial infection, previous treatment with bronchodilators or corticosteroids, the presence of symptoms > 7 days, proven asthma in parents and siblings, and consolidation or atelectasis on a chest roentgenogram |
|
| Participants |
Randomised: 57 infants Analysed: 56 infants; magnesium 19; salbutamol 18; salbutamol plus magnesium sulphate 19 (1 child in the magnesium sulphate group subsequently withdrew because of deteriorated clinical status Analysis for follow‐up: there was no follow‐up of participants Age: median age (min to max) was 7.5 (3 to 19) months in the salbutamol group; 8 (4 to 22) months in the magnesium sulphate group; and 8 (3 to 21) months in the salbutamol + magnesium sulphate group Gender distribution (male:female) was 12:6 in the salbutamol group; 12:7 in the magnesium sulphate group; and 13:6 in the magnesium sulphate + salbutamol group Baseline clinical severity score: mean (95% CI) clinical severity score was 5.9 (5.4 to 6.4) in the salbutamol group; 6.1 (5.6 to 6.5) in the magnesium sulphate group; and 6.3 (5.7 to 6.8) in the salbutamol + magnesium sulphate group Baseline heart rate: mean heart rate was 149.4 (95% CI 136.9 to 161.8) in the salbutamol group; 147.8 (95% CI 139.4 to 156.1) in the magnesium sulphate group; and 156.7 (95% CI 149.2 to 165.2) in the salbutamol + magnesium sulphate group |
|
| Interventions |
Intervention: inhaled magnesium sulphate 150 mg, diluted to 4 mL with 0.9 % saline solution Comparator:
All groups received medications twice, at 30‐minute intervals |
|
| Outcomes |
Planned outcomes: no protocol available Reported outcomes: main outcomes were:
Outcome data not provided in a usable way for duration of hospital stay. Authors used mean (95% CI) time and expressed result as P value for significance comparing two interventions. Mean (SD) was not provided by the trial authors |
|
| Notes | Funding source: not mentioned Conflicts of interest: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | "All eligible patients were randomly assigned to one of the three groups". Details of randomisation method, or how randomisation was done were not available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial report mentioned double‐blinded study, but did not report for whom, or how double‐blinding was conducted |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 child in the magnesium sulphate group subsequently withdrew because of deteriorating clinical status. This was unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | The planned outcomes in the methods section were reported in the results section |
| Other bias | Unclear risk | Source of funding not mentioned |
Modaresi 2015.
| Study characteristics | ||
| Methods |
Trial registration: not traceable Study design: double‐blind randomised clinical trial Setting and centres: 3 hospitals paediatric departments, Isfahan University of Medical Sciences, Isfahan, Iran Dates of study: January 2010 to December 2011 Duration of study: until child was discharged Inclusion criteria: acute onset of respiratory distress, positive wheezing on physical examination, chest radiograph compatible with bronchiolitis, aged < 12 months, and RDAI score of at least 5 Exclusion criteria: family history of asthma; chronic pulmonary, cardiac, neurologic and oncologic disease; previous use of glucocorticoids, bronchodilators or monoamine oxidase inhibitors (MAOI); tachycardia exceeding 200 beats per minute; tachypnoea exceeding 100 breaths per minute |
|
| Participants |
Randomised: 125 infants. 2 study arms: magnesium sulphate + epinephrine 63; epinephrine 62 Analysed: magnesium sulphate + epinephrine 60; epinephrine 62. Three children in the magnesium sulphate + epinephrine group, and two children in the epinephrine group were subsequently withdrawn because of deteriorating clinical status Analysis for follow‐up: there was no follow‐up of participants Age: mean (SD) age was 5.2 (2.1) months in the magnesium sulphate + epinephrine group; 4.8 (3.2) months in the epinephrine group Gender distribution: (male:female) was 34:26 in the magnesium sulphate + epinephrine group; 38:22 in the epinephrine group Baseline clinical severity score: mean (SD) 11.4 (2.7) in the magnesium sulphate plus epinephrine group; 11 (2.7) in the epinephrine group Baseline heart rate: mean (SD) 104 (5) in the magnesium sulphate + epinephrine group; 104 (4) in the epinephrine group Baseline O₂ saturation: mean (SD) 88 (1) in the magnesium sulphate + epinephrine group; 87 (1) in the epinephrine group |
|
| Interventions |
Intervention: 40 mg/kg magnesium sulphate (3.25%) and 0.1 mL/kg epinephrine (1/1000) mixed with normal saline nebulisation Comparator: 0.1 mL/kg epinephrine (1/1000) mixed with normal saline nebulisation Concomitant treatment in both groups: 0.1 mL/kg epinephrine (1/1000) mixed with normal saline nebulisation All groups received 3 doses of each medication at 20‐minute intervals, and then every 4 hours |
|
| Outcomes |
Planned outcomes: no protocol available Reported outcomes: primary outcome was length of hospital stay (defined as the time from the first study inhalation until discharge from the hospital, as recorded in the medical record for each child). Other outcomes were
All outcomes recorded at admission, first 20 minutes, second 20 minutes, third 20 minutes, after 4 hours, and then daily during hospitalisation |
|
| Notes |
Funding source: supported financially by Child Growth and Development Research Center, Isfahan University of Medical Sciences, Isfahan, Iran Conflicts of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate methods of sequence generation and allocation concealment used. Random number tables and third party randomisation were used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Knowledge of allocations was never broken throughout the study, as stated by the trial authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 children in the magnesium sulphate + epinephrine group and 2 children in the epinephrine group were subsequently withdrawn because of deteriorating clinical status |
| Selective reporting (reporting bias) | Low risk | The planned outcomes in the methods section were reported in the results section |
| Other bias | Low risk | None of the trial authors disclosed receipt of any grants, or funds, or shares from any pharmaceutical company. Authors reported no conflicts of interest |
Priya 2018.
| Study characteristics | ||
| Methods |
Trial registration: not traceable Study design: single‐blind randomised controlled trial Setting and centres: single‐centre, emergency department, paediatric intensive care unit and wards of Institute of Social Paediatrics, Stanley Medical College and Hospital, Tamilnadu, India. Dates of study: January 2017 to September 2017 Duration of study: until child was discharged Inclusion criteria: children less than 12 months old, with acute onset of respiratory symptoms associated with fever, cough, tachypnoea, or chest radiograph findings consistent with bronchiolitis (such as hyperinflation), and first episode of wheezing Exclusion criteria: previous history of wheezing
|
|
| Participants |
Randomised: 220 infants. 2 study arms: nebulised magnesium sulphate 110; nebulised hypertonic saline 110 Analysed: magnesium sulphate 110; hypertonic saline 110. No missing data. Analysis for follow‐up: none Age: the mean (SD) age was 7.1 (3.1) months in the magnesium sulphate group; 6.9 (3) months in the hypertonic saline group. Gender distribution: (male:female) 63:47 in the magnesium sulphate group; 63:47 in the hypertonic saline group. Baseline clinical severity score (RDAI): mean (SD) 8.0 (1.8) in the magnesium sulphate group; 7.9 (1.7) in the hypertonic saline group |
|
| Interventions |
Intervention: magnesium sulphate; 0.1 to 0.2 mL/kg/dose of 25% magnesium sulphate, mixed to 4 mL with 0.9% normal saline nebulisation Comparator: 4 mL hypertonic saline nebulisation All children received medications at 20‐minute intervals for the first 3 doses, and then every 4 hours Concomitant treatment in both groups: none mentioned |
|
| Outcomes |
Planned outcomes: no protocol available Reported outcomes:
|
|
| Notes |
Funding source: not mentioned Conflicts of interest: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Deatails of randomisation not available. Sealed envelopes used, but it is not known whether these were sequentially numbered and opaque |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Authors reported the study to be single‐blinded, but due to unclear allocations, blinding method was not robust |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts |
| Selective reporting (reporting bias) | Low risk | The planned outcomes in the methods section were reported in the results section |
| Other bias | Unclear risk | Details related to source of funding and the conflicts of interests were not shared. |
CI: confidence interval SD: standard deviation RDAI: respiratory distress assessment instrument min: minimum max: maximum
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gupta 2015 | The study was published as a conference abstract. It included children presenting with first or second episodes of moderate to severe acute bronchiolitis. Trial authors responded, but could not provide additional data related to first episodes of bronchiolitis (inclusion criteria for this review). |
| Pruikkonen 2018 | Study population did not match the review inclusion criteria. Trial authors included children with more than one episode of wheezing, while inclusion eligibility was limited to children with first episode of wheezing in this review, as per the definition by AAP Subcommittee 2006. |
| Rady 2018 | This study included children with a wheezy chest from all causes, which is not part of our inclusion criteria. The trial authors stated that they included infants and children between 2 months and 12 years. However, the trial authors could not provide details of outcomes on children up to 2 years of age, which was part of our inclusion criteria for this review. |
Characteristics of ongoing studies [ordered by study ID]
CTRI/2017/02/007919.
| Study name | CTRI/2017/02/007919 |
| Methods |
Trial registration: CTRI/2017/02/007919 Study design: randomised, parallel‐group, placebo‐controlled trial Setting and centres: single centre, emergency department, Department of Paediatrics, GMSH, Chandigarh, India Dates of study: first enrolment 12 January 2017 Duration of study: intervention duration: 3 hour; outcome assessment duration: until child was discharged Inclusion criteria: children aged 2 to 12 months with diagnosis of acute bronchiolitis, with short history of cough, with or without fever of less than 7 days duration, wheezing on examination, first attack of wheezing. Exclusion criteria: past history of wheezing, family history of bronchial asthma, presence of congenital heart disease or congenital malformation, history of prematurity or mechanical ventilation in newborn period, pneumonia or pleural effusion, SAM, very sick child with shock, seizures, tachycardia (HR > 200/minute), and respiratory failure, foreign body ingestion, history of nebulisation of any drug |
| Participants | Plan to randomise 104 infants |
| Interventions |
Intervention: magnesium sulphate nebulisation: enrolled cases in the intervention group shall be given 40 mg/kg magnesium sulphate nebulisation, diluted with 2 mL to 3 mL normal saline. 3 doses of medication will be given at 1‐hour intervals, and will not be repeated after 3 doses Comparator: oxygen therapy, IV fluids or oral feeds: this group will be treated with oxygen therapy, IV fluids, or oral feeds, symptomatic treatment for fever, and supportive care Concomitant treatment in both study arms: none written |
| Outcomes |
Primary outcomes: improvement in severity of bronchiolitis, measured with the Respiratory Distress Assessment Instrument (RDAI) score. Timepoint: RDAI score shall be assessed at admission, at 3, 6, 12, 18, 24, 36, 48, 60, 72, 84, 96 hours, and at discharge. Secondary outcomes:
|
| Starting date | 12 January 2017 |
| Contact information | Name: Dr Neeraj Dhawan Email: ndhawan_6144@yahoo.com; p.sadbhavna@yahoo.com Address: Department of Paediatrics, Government Multispeciality Hospital, Chandigarh, India |
| Notes |
CTRI/2018/06/014400.
| Study name | CTRI/2018/06/014400 |
| Methods |
Trial registration: CTRI/2018/06/014400 Study design: randomised, parallel‐group, placebo‐controlled trial Setting and centres: single centre in India Dates of study: first enrolment 2 July 2018 Duration of study: interventions: 24 hours; follow‐up: 2 weeks Inclusion criteria: infants aged 1 month to 24 months presenting to paediatric emergency or OPD, with diagnosis of bronchiolitis and BSS ≥ 4, and guardian of child willing to give informed consent Exclusion criteria:
|
| Participants |
Randomised: plan to randomise 50 Infants Analysed: not available Analysis for follow‐up at 2 weeks: not available Age: infants 1 month to 24 months of age Gender distribution: not available Baseline Wang bronchiolitis severity score: > 4 |
| Interventions |
Intervention: nebulisation with 3 mL 3.2% magnesium sulphate every 4 hours for 24 hours, in addition to standard care. Oxygen saturation will be noted before starting treatment, and oxygen will be supplemented if < 92%. If the saturation falls below 90% in either group, and persists despite oxygen supplementation, the child will be considered for ventilation. Comparator: standard care: maintenance of adequate hydration and oxygenation Concomitant treatment in both study arms: none written |
| Outcomes |
Primary outcomes: to compare improvement of bronchiolitis severity scores between treatment groups. Secondary outcomes:
|
| Starting date | 12 January 2017 |
| Contact information | Name: Dr Daisy Khera Email daisykhera78@gmail.com Department of Pediatrics AIIMS Jodhpur, Jodhpur, Rajasthan, India |
| Notes |
OPD: out patient department BSS: bronchiolitis severity score SAM: severe acute malnutrition SpO₂: Oxygen saturation
Differences between protocol and review
In the protocol, we planned to include non‐randomised controlled trials (non‐RCTs), as we were expecting to identify few studies. In the review, we only included RCTs, as we did not identify any non‐RCTs.
We added the primary outcome, time to recovery, as it is a person‐centred, and clinically important outcome, likely to be modified by the treatment.
In the protocol, the primary outcome, clinical severity score, had four components: clinical severity score measured using any validated scoring system, duration of mechanical ventilation, duration of hospital stay, or duration of intensive care unit stay. These could not be described under one outcome. Therefore, we separated these into four outcomes. We moved 'clinical severity score' to the secondary outcomes.
We did not specify time points of clinical severity score in the protocol. Clinical severity scoring was conducted at multiple time points amongst the studies. Therefore, we specified the time points of clinical severity score at 0 to 24 hours, and 25 to 48 hours after treatment for the review.
There was a change in authorship: Arun K Yadav, an author on the protocol, did not co‐author the review.
We planned to meta‐analyse using the random‐effects model, as we anticipated the studies would differ. We used the fixed‐effect model to calculate effect size and 95% confidence intervals (CIs) when we could not pool results, and included only one study for comparison.
We planned to conduct sensitivity and subgroup analyses. However, there were insufficient data.
Contributions of authors
Dr Sudha Chandelia (SC): conceptualised, designed the search strategy, performed the literature search, screened search results, screened and retrieved papers against eligibility criteria, collected and extracted data, obtained and screened data on unpublished studies, entered data into RevMan 5, conducted data analysis and interpretation, assessed certainty of evidence, co‐ordinated the review, wrote and drafted the review.
Dr Dinesh Kumar (DK): provided intellectual input for the background, screened search results, retrieved and screened papers against eligibility criteria, conducted data extraction, and assessed certainty of evidence.
Ms Neelima Chadha (NC): provided intellectual input into the development of the search strategy, literature search; and provided full‐text articles.
Nishant Jaiswal (NJ): provided intellectual input for 'Risk of bias' assessment, reported and wrote revised drafts of the review, provided intellectual input and guidance for GRADE application.
All authors reviewed and approved the review.
Sources of support
Internal sources
-
PGIMER and Dr RML Hospital, New Delhi and PGIMER, Chandigarh, India
Library and Internet facility
External sources
No sources of support supplied
Declarations of interest
SC: None known DK: None known NC: None known NJ: None known
New
References
References to studies included in this review
Alansari 2017 {published and unpublished data}
- Alansari K, Sayyed R, Davidson BL, Al Jawala S, Ghadier M. Intravenous magnesium sulfate for bronchiolitis: A randomized trial. European Journal of Pediatrics 2016;175(11):1511. [DOI: 10.1007/s00431-016-2785-8] [DOI] [Google Scholar]
- *.Alansari K, Sayyed R, Davidson BL, Al Jawala S, Ghadier M. IV magnesium sulfate for bronchiolitis: a randomized trial. Chest 2017;152(1):113-9. [DOI: 10.1016/j.chest.2017.03.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alansari K. Mean and standard deviations in our trial [personal communication]. Email to: S Chandelia 17 April 2019.
Kose 2014 {published and unpublished data}
- *.Kose M, Ozturk MA, Poyrazoğlu H, Elmas T, Ekinci D, Tubas F, et al. The efficacy of nebulized salbutamol, magnesium sulfate, and salbutamol/magnesium sulfate combination in moderate bronchiolitis. European Journal of Pediatrics 2014;173(9):1157-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Kose M. Mean and standard deviations in our trial [personal communication]. Email to: S Chandelia 29 April 2019.
Modaresi 2015 {published data only (unpublished sought but not used)}
- Modaresi MR, Faghihinia J, Kelishadi R, Reisi M, Mirlohi S, Pajhang F, et al. Erratum to: nebulized magnesium sulfate in acute bronchiolitis: a randomized controlled trial. Indian Journal of Pediatrics 2016;83(5):487. [DOI] [PubMed] [Google Scholar]
- *.Modaresi MR, Faghihinia J, Kelishadi R, Reisi M, Mirlohi S, Pajhang F, et al. Nebulized magnesium sulfate in acute bronchiolitis: a randomized controlled trial. Indian Journal of Pediatrics 2015;82(9):794-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Modaresi MR, Faghihinia J, Mirlohi H, Pajhang F. The effectiveness of magnesium sulfate nebulizer in treatment of acute bronchiolitis. Paediatric Respiratory Reviews 2012;13(suppl 1):S58. [DOI: 10.1016/S1526-0542(12)70078-7] [DOI] [Google Scholar]
- Modaresi MR, Faghihinia J, Mirlohi S, Pajhang F. Evaluating the effectiveness of magnesium sulphate nebulizer in treatment of acute bronchiolitis. Journal of Isfahan Medical School 2011;29(152):1142-7. [Google Scholar]
Priya 2018 {unpublished data only}
- Priya SS. Nebulized magnesium sulfate versus hypertonic saline in acute bronchiolitis: a randomized control trial [Masters thesis]. repository-tnmgrmu.ac.in/9235/2/200700218sathiya_priya.pdf (accessed 5 March 2019).
References to studies excluded from this review
Gupta 2015 {published data only}
- Gupta S. Additonal information in our trial [personal communication]. Email to: S Chandelia 1 May 2019.
- *.Gupta S. Add-on use of magnesium sulfate in children with bronchiolitis. Pediatric Pulmonology 2015;50(39):S58-9. [Google Scholar]
Pruikkonen 2018 {published data only}
- Pruikkonen H, Tapiainen T, Kallio M, Dunder T, Pokka T, Uhari M, et al. Intravenous magnesium sulfate for acute wheezing in young children: a randomised double-blind trial. European Respiratory Journal 2018;51(2):1701579. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rady 2018 {published data only (unpublished sought but not used)}
- *.Rady HI, Shaaban HH, Bazaraa HM, Abdelmonem SE, Aly YS. Magnesium sulphate as an adjuvant therapy in critically ill infants and children presenting with wheezy chest. Journal of Pediatrics & Neonatal Biology 2018;3(1):5. [Google Scholar]
- Rady HI. Additonal information in our trial [personal communication]. Email to: S Chandelia 25 November 2019.
- Saied Y, Hamdy H, Bazaraa H, Rady H, Elanwary S. Magnesium sulphate as an adjuvant therapy in critically ill infants and children presenting with wheezy chest in addition to standard treatment. European Respiratory Journal 2018;52(Suppl 62):PA2321. [DOI: 10.1183/13993003.congress-2018.PA2321] [DOI] [Google Scholar]
References to ongoing studies
CTRI/2017/02/007919 {published data only}
- CTRI/2017/02/007919. Magnesium sulphate nebulization in acute bronchiolitis: a randomized controlled trial [Effect of nebulized magnesium sulfate in acute bronchiolitis]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=17290 (first received 20 February 2017).
CTRI/2018/06/014400 {published data only}
- CTRI/2018/06/014400. Role of magnesium sulfate in bronchiolitis: a randomized control trial [Role of magnesium sulfate in children having bronchiolitis]. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=26334 (first received 4 June 2018).
Additional references
AAP Subcommittee 2006
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics 2006;118(4):1774-93. [DOI: 10.1542/peds.2006-2223] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calvo 2010
- Calvo C, Pozo F, Garcia-Garcia ML, Sanchez M, Lopez-Valero M, Perez-Brena P, et al. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatrica 2010;99(6):883-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2008
- Carroll KN, Gebretsadik T, Griffin MR, Wu P, Dupont WD, Mitchel EF, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics 2008;122(1):58-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castillo 1954
- Castillo JD, Engbaek L. The nature of the neuromuscular block produced by magnesium. Journal of Physiology 1954;124(2):370-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ciarallo 1996
- Ciarallo L, Sauer AH, Shannon MW. Intravenous magnesium therapy for moderate to severe pediatric asthma: results of a randomized, placebo-controlled trial. Journal of Pediatrics 1996;129(6):809-14. [DOI: 10.1016/S0022-3476(96)70023-9] [DOI] [PubMed] [Google Scholar]
Enriquez 2012
- Enriquez A, Chu IW, Mellis C, Lin WY. Nebulised deoxyribonuclease for viral bronchiolitis in children younger than 24 months. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD008395. [DOI: 10.1002/14651858.CD008395.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandes 2013
- Fernandes RM, Bialy LM, Vandermeer B, Tjosvold L, Plint AC, Patel H, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD004878. [DOI: 10.1002/14651858.CD004878.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flores‐González 2017
- Flores-González JC, Mayordomo-Colunga J, Jordan I, Miras-Veiga A, Montero-Valladares C, Olmedilla-Jodar M, et al. Prospective multicentre study on the epidemiology and current therapeutic management of severe bronchiolitis in Spain. BioMed Research International 2017 Mar 22 [Epub ahead of print];2017:2565397. [DOI: 10.1155/2017/2565397] [DOI] [PMC free article] [PubMed]
Gadomski 2014
- Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD001266. [DOI: 10.1002/14651858.CD001266.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodacre 2013
- Goodacre S, Cohen J, Bradburn M, Gray A, Benger J, Coats T. Intravenous or nebulised magnesium sulphate versus standard therapy for severe acute asthma (3Mg trial): a double-blind, randomised controlled trial. Lancet Respiratory Medicine 2013;1(4):293-300. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gourgoulianis 2004
- Gourgoulianis KI, Chatziparasidis G, Chatziefthimiou A, Molyvdas PA. Magnesium as a relaxing factor of airway smooth muscles. Journal of Aerosol Medicine 2004;14(3):301-7. [DOI: 10.1089/089426801316970259] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 23 November 2017. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Green 2016
- Green CA, Yeates D, Goldacre A, Sande C, Parslow RC, McShane P, et al. Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Archives of Disease in Childhood 2016;101(2):140-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffiths 2016
- Griffiths B, Kew KM. Intravenous magnesium sulfate for treating children with acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD011050. [DOI: 10.1002/14651858.CD011050.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartling 2011
- Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No: CD003123. [DOI: 10.1002/14651858.CD003123.pub3] [DOI] [PubMed] [Google Scholar]
Hasegawa 2013
- Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000–2009. Pediatrics 2013;132(1):28-36. [DOI: 10.1542/peds.2012-3877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasegawa 2014a
- Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Temporal trends in emergency department visits for bronchiolitis in the United States, 2006 to 2010. Pediatric Infectious Disease Journal 2014;33(1):11-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasegawa 2014b
- Hasegawa K, Mansbach JM, Teach SJ, Fisher ES, Hershey D, Koh JY, et al. Multicenter study of viral etiology and relapse in hospitalized children with bronchiolitis. Pediatric Infectious Disease Journal 2014;33(8):809-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirota 1999
- Hirota K, Sato T, Hashimoto Y, Yoshioka H, Ohtomo N, Ishihara H, et al. Relaxant effect of magnesium and zinc on histamine-induced bronchoconstriction in dogs. Critical Care Medicine 1999;27(6):1159-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kew 2014
- Kew KM, Kirtchuk L, Michell CI. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD010909. [DOI: 10.1002/14651858.CD010909.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumasaka 1996
- Kumasaka D, Lindeman KS, Clancy J, Lande B, Croxton TL, Hirshman CA. MgSO₄ relaxes porcine airway smooth muscle by reducing Ca2+ entry. American Journal of Physiology 1996;270(3):L469-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lakhanpaul 2002
Lanari 2015
- Lanari M, Prinelli F, Adorni F, Santo SD, Vandini S, Silvestri M, et al, Study Group of Italian Society of Neonatology on Risk Factors for RSV Hospitalization. Risk factors for bronchiolitis hospitalization during the first year of life in a multicenter Italian birth cohort. Italian Journal of Pediatrics 2015;41:40. [DOI: 10.1186/s13052-015-0149-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liet 2015
- Liet JM, Ducruet T, Gupta V, Cambonie G. Heliox inhalation therapy for bronchiolitis in infants. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD006915. [DOI: 10.1002/14651858.CD006915.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowell 1987
- Lowell DI, Lister G, Von Koss H, McCarthy P. Wheezing in infants: the response to epinephrine. Pediatrics 1987;79:939-45. [PMID: ] [PubMed] [Google Scholar]
Lu 2000
- Lu JF, Nightingale CH. Magnesium sulfate in eclampsia and pre-eclampsia: pharmacokinetic principles. Clinical Pharmacokinetics 2000;38(4):305-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mahajan 2004
- Mahajan P, Haritos D, Rosenberg N, Thomas R. Comparison of nebulized magnesium sulfate plus albuterol to nebulized albuterol plus saline in children with acute exacerbations of mild to moderate asthma. Journal of Emergency Medicine 2004;27(1):21-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Mansbach 2012a
- Mansbach JM, Piedra PA, Stevenson MD, Sullivan AF, Forgey TF, Clark S, et al. Prospective multicenter study of children with bronchiolitis requiring mechanical ventilation. Pediatrics 2012;130(3):e492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansbach 2012b
- Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Archives of Pediatrics and Adolescent Medicine 2012;166(8):700-6. [DOI: 10.1001/archpediatrics.2011.1669] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muñoz‐Quiles 2016
- Muñoz-Quiles C, López-Lacort M, Úbeda-Sansano I, Alemán-Sánchez S, Pérez-Vilar S, Puig-Barberà J, et al. Population-based analysis of bronchiolitis epidemiology in Valencia, Spain. Pediatric Infectious Disease Journal 2016;35(3):275-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Murray 2014
- Murray J, Bottle A, Sharland M, Modi N, Aylin P, Majeed A, et al. Risk factors for hospital admission with RSV bronchiolitis in England: a population-based birth cohort study. PLoS One 2014;9(2):e89186. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivera‐Sepulveda 2017
- Rivera-Sepulveda A, Garcia-Rivera EJ. Epidemiology of bronchiolitis: a description of emergency department visits and hospitalizations in Puerto Rico, 2010–2014. Tropical Medicine and Health 2017;45:24. [DOI: 10.1186/s41182-017-0064-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scarfone 2000
- Scarfone RJ, Loiselle JM, Joffe MD, Mull CC, Stiller S, Thompson K, et al. A randomized trial of magnesium in the emergency department treatment of children with asthma. Annals of Emergency Medicine 2000;36(6):572-8. [DOI: 10.1067/mem.2000.111060] [DOI] [PubMed] [Google Scholar]
Schwalfenberg 2017
- Schwalfenberg GK, Genuis SJ. The importance of magnesium in clinical healthcare. Scientifica (Cairo) 2017 Sep 28 [Epub ahead of print]:4179326. [DOI: 10.1155/2017/4179326] [DOI] [PMC free article] [PubMed]
Shay 2001
- Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979-1997. Journal of Infectious Diseases 2001;183(1):16-22. [DOI: 10.1086/317655] [DOI] [PubMed] [Google Scholar]
Spivey 1990
- Spivey WH, Skobeloff EM, Levin RM. Effect of magnesium chloride on rabbit bronchial smooth muscle. Annals of Emergency Medicine 1990;19(10):1107-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Swaminathan 2003
- Swaminathan R. Magnesium metabolism and its disorders. Clinical Biochemist. Reviews 2003;24(2):47-66. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Wang 1992
- Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. American Review of Respiratory Disease 1992;145:106-9. [DOI: 10.1164/ajrccm/145.1.106] [DOI] [PubMed] [Google Scholar]
Zhang 2017
- Zhang L, Mendoza-Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD006458. [DOI: 10.1002/14651858.CD006458.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Chandelia 2018
- Chandelia S, Yadav AK, Kumar D, Chadha N. Magnesium sulphate for acute bronchiolitis in children under two years of age. Cochrane Database of Systematic Reviews 2018, Issue 2. Art. No: CD012965. [DOI: 10.1002/14651858.CD012965] [DOI] [PMC free article] [PubMed] [Google Scholar]


