Abstract
Because of the COVID-19 pandemic, used face masks have increasingly littered the environment and are causes for concern since they are commonly made of plastics such as polypropylene. Understanding production of microplastics from face masks is essential for predicting the post COVID-19 pandemic impact on the soil ecosystem. We investigated the generation of nanofibers from meltblown face mask filters (MB filters) and their adverse effects on soil species, particularly the earthworm and springtail. Results of MB filter soil bioassays at a high concentration (1000 mg/kg dry soil) suggest inhibited reproduction and stunted growth in springtails, decreased intracellular esterase activity in earthworm coelomocytes, and inhibited spermatogenesis in male earthworm reproductive tissues. Moreover, it was estimated that generation of nanofibers from microfibers and fragments of MB filters might occur in the soil ecosystem post COVID-19. This study does not oppose the use of face masks but aims to encourage appropriate disposal of the masks. Preservation of human health and the ecosystem should be prioritized even amidst the COVID-19 pandemic.
Keywords: Face mask, Ecotoxicity, Meltblown filter
Graphical Abstract

1. Introduction
As of May 8, 2021, 156,496,592 confirmed COVID-19 cases had been reported globally by the World Health Organization (WHO) (WHO, 2021); confirmed cases and casualties have been increasing ever since WHO announced the COVID-19 outbreak on March 12, 2020 (WHO, 2020a).
It was established early on that the virus causing the COVID-19 disease (i.e., SARS-CoV-2—severe acute respiratory syndrome coronavirus 2) spreads through close contact with infected people (WHO, 2020b), thereby making personal protective equipment (PPE) essential for health care workers, patients, and the general public (WHO, 2020b). “PPE includes gloves, medical masks, goggles, face shields, and gowns; respirators and aprons are also required for specific procedures (i.e., N95 or FFP2 standard or equivalent)” (WHO, 2020b). Currently, to prevent transmission through close contact, wearing face masks in public transport and spaces has been made obligatory in several Asian countries including South Korea (KBS, 2020), China (NHC, 2020), and Taiwan (Taipei-City-Police-Department, 2020); in European countries including Germany (BMI, 2020), Switzerland (Swiss-government, 2020), and the UK (UK, 2020); and in North America, including Canada (Toronto, 2020) and the USA (CNN, 2020). A recent report from the World Wide Fund for Nature (WWF) stated that inappropriately disposing of masks—even in numbers as small as 1% of the total used face masks—would result in about 10 million face masks per month, equaling 30,000–40,000 kg of plastic dispersed in the environment (Patrício Silva et al., 2020, WWF, 2020). The types of masks can be classified as face masks, surgical masks, N95 masks, cotton masks, etc.
Masks and PPE are emerging pollutants in the post COVID-19 pandemic situation as large quantities of these materials are released into the environment (Fadare and Okoffo, 2020, Nghiem et al., 2020, Patrício Silva et al., 2020, Saadat et al., 2020). Masks that are incorrectly disposed of are evident in streets, gardens, and parks, and even on mountains ( Fig. 1). Because PPE is made of plastic (e.g., polypropylene or polyethylene), it is not expected to biodegrade over a short period (Aragaw, 2020, Fadare and Okoffo, 2020, Saliu et al., 2021). Moreover, there is a concern that masks release chemicals (Sullivan et al., 2021).
Fig. 1.
Improperly disposed of face masks in various terrestrial environments including gardens, parks, and mountains during the COVID-19 pandemic in South Korea. (Photo credit: J.I. Kwak).
To estimate the soil ecotoxicological effects of face masks that have been inappropriately disposed of beyond the COVID-19 pandemic, we investigated the effects of fibers and fragments derived from meltblown face mask filters (MB filters) on the soil ecosystem using earthworms and springtails, which are representative soil invertebrates. Earthworms and springtails are the recommended test species for evaluation of soil pollution (OECD, 1984, OECD, 2009) because of their important role as decomposers and consumers (ECB, 2003, CCME, 2006). Our study discusses potential soil ecotoxicity caused by microplastics derived from face masks, thereby elucidating their adverse effects beyond the COVID-19 pandemic.
2. Materials and methods
2.1. Preparation of fibers and fragments of masks
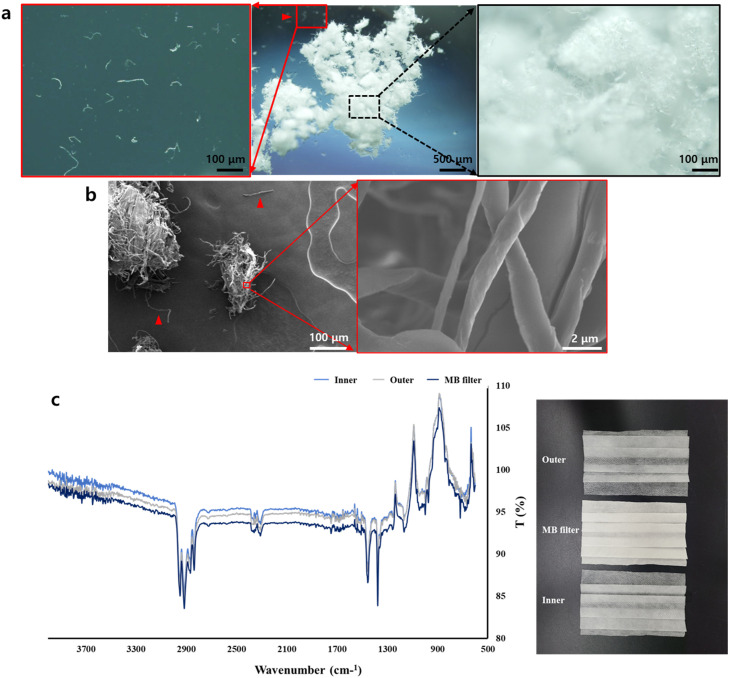
Triple-layered disposable white face masks, 145×95 mm in size, (Fig. S1) were purchased. This study focused on the MB filter (non-woven fabric), which is a common and key material for masks. The weight of the triple-layer fabric was 1976 mg and the weight of the MB filter was 448 mg without ear straps. To assess the soil ecotoxicity of the MB filter, white pristine MB filters were removed from masks, cut using micro-scissors, and then sieved using a stainless sieve (300 µm, Chunggye sieve, Gyeonggi-do, South Korea) and a stainless spatula. To characterize the tested face masks, Fourier transform infrared spectroscopy (FTIR, 4100 type A, resolution 0.96 cm−1, JASCO, Japan) was used with attenuated total reflection (ATR) mode. High-resolution field emission scanning electron microscopy (FE-SEM, 1.0–5.0 kV, SU8010, Hitachi High-Technologies Corporation, Japan) was also used for observing the shape of the MB filter fibers and fragments. Fig. 2a and b show microscopic images of tested fibers and fragments of the MB filter after sieving (300 µm) and Fig. 2c shows the polypropylene FTIR spectrum.
Fig. 2.
(a) Stereomicroscopic and (b) FE-SEM images of pristine MB filter fibers and fragments (sieve size: 300 µm). (c) FTIR spectrum of a pristine MB filter, and inner and outer layers of a face mask. Red arrow heads and red squares indicate microfibers detached from pristine MB filter fragments. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Preparation of contaminated soil with fibers and fragments of masks
LUFA 2.2 (Landwirtschaftliche Untersuchungs-und Forschungsanstalt) standard soil (loamy sand) with a soil pH of 5.6 (twin pH, HORIBA, Kyoto, Japan) and with a 0.5 mL/g water holding capacity was used in this study. Control groups were not treated with any chemicals or MB filters (<300 µm). Exposure groups were hand-mixed with soil and fragments of MB filters at 1000 mg/kg dry soil. To simulate the worst exposure scenario to the soil ecosystem, a high exposure concentration was assessed in the present study.
2.3. Springtails soil assay
The experiment was conducted on 12-day-old juvenile springtails Folsomia candida. OECD guidelines were adhered to when subjecting springtails to chemical testing (OECD, 2009). Ten juveniles were exposed to each replicate, which contained 40 g of wet test soil in a glass vial, with four replicates run in both the control and exposure groups in the dark 20 °C incubator (VISION SCIENTIFIC, Daejoen, South Korea). Survival, reproduction, and size of juveniles were examined 28 days after exposure (OECD, 2009). Additionally, adult survivors were collected, and esterase activity, oxidative stress, and light avoidance of adults were investigated. Adult survivors were stained with calcein acetoxymethyl ester (calcein-AM; Sigma-Aldrich, St. Louis, MO, USA) and 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 5 μM concentration in a phosphate buffer (pH 7) under dark conditions to evaluate esterase activity and oxidative stress by considering Sillapawattana et al. (2016). Eight to nine replicates for esterase activity and oxidative stress were evaluated. Green fluorescence was visualized at 510–550 nm emission and then green intensity was analyzed by the ImageJ software.
Methods of observing the light avoidance behavior of springtails followed modified Oliveira et al. (2018). Adult survivors from each replicate were moved to a Petri dish (90×15 mm) containing mixed plaster of Paris and powdered activated charcoal (DUCKSAN, Seoul, South Korea). The number of springtails in the light and dark sections was counted after 20 min in the dark 20 °C incubator (VISION SCIENTIFIC, Daejoen, South Korea). Four Petri dishes (6–10 springtails) were investigated.
2.4. Uptake test for springtails
To confirm the uptake of MB filter fibers and fragments by F. candida, the sieved fragments (<300 µm) were stained with Nile red (i.e., phenoxazone dye; Sigma-Aldrich, St. Louis, MO, USA). Nile red stock solution at 1000 mg/L in acetone (DUCKSAN, Seoul, South Korea) was prepared and then diluted to 10 mg/L with deionized water. MB filter fibers and fragments were stained for 30 min, dried in a 60 °C oven overnight, washed five times with 30 mL deionized water, and dried in a 60 °C oven again (Maes et al., 2017).
Juveniles (i.e., 12 days old) and one-month-old adults were tested on the Petri dish (90×15 mm) containing mixed plaster of Paris and powdered activated charcoal (DUCKSAN, Seoul, South Korea). Control groups were supplied with 4 mg of yeast for consumption and exposed groups were supplied with 3 mg of yeast and 1 mg of MB filter fibers and fragments. Immediately prior to exposure to the yeast, the MB filter fibers and fragments were sieved again (<300 µm) to prevent aggregation owing to electricity. Subsequently, 10 μL of deionized water was added and 10 springtails were exposed to the food mixed with MB filter fragments.
Simultaneously, it was necessary to carry out the Nile red leachate controls because Nile red is known to leach from stained microplastics (Catarino et al., 2019). By considering a balance of the provided yeast mass and water volume in this uptake test, 20 mg of MB filter fragments and 0.2 mL of deionized water in the 1.75 mL microtube was incubated in the dark condition at 20 °C for 24 h to make leachates from stained MB filters. Then, 10 μL of leachates filtered through a 0.45 µm syringe filter (cellulose acetate, ADVANTEC, Tokyo, Japan) was added to 4 mg of yeast on the Petri dish (90×15 mm) containing mixed plaster of Paris and powdered activated charcoal. In the Nile red leachate control groups, juveniles (i.e., 12 days old) and one-month-old adults were also introduced to compare the control and exposed groups. Three Petri dishes (10 springtails per Petri dish) were investigated. Seven days after exposure in the dark 20 °C incubator (VISION SCIENTIFIC, Daejoen, South Korea), the springtails were washed in an ultrasonic bath with deionized water for 30 s and examined using a fluorescent microscope (BX-51; Olympus, Tokyo, Japan) with a green filter (510–550 nm emission). The fluorescent images are shown in Fig. S3.
2.5. Earthworm soil assay
Adult earthworms Eisenia andrei (weight: 461±39 mg) were subject to soil exposure tests as mentioned in earlier research (Kwak and An, 2021), which had a relatively smaller scale than the standard test guideline. Each earthworm was exposed to a 20 mL flat-bottomed glass vial (one replicate) containing 10 g of dry soil. Five replicates for the control and exposure groups were run, and then an exposure test was conducted three times in the earthworm soil assay in the dark 20 °C incubator (VISION SCIENTIFIC, Daejoen, South Korea).
Survival, in vivo cytotoxicity (oxidative stress, lysosomal stability, and intracellular esterase activity in coelomocytes) using flow cytometry, and histopathological effect (seminal vesicle and ovary) using hematoxylin and eosin (H&E) assay, were evaluated 21 days after exposure. Oxidative stress and intracellular esterase activity in coelomocytes using a flow cytometer (FACScalibur; 10,000 events, FL1 500–560 nm band pass filter, BD Biosciences, San Jose, CA, USA) (Kwak et al., 2017), and histopathological evaluation were conducted according to earlier research (Kwak and An, 2021). For evaluation of the lysosomal stability in coelomocytes, collected coelomocytes from survivor earthworms were stained with 40 μg/mL of neutral red (eurhodin dye; Sigma-Aldrich, St. Louis, MO, USA) for 10 min and analyzed using a flow cytometer (Plytycz et al., 2007). Based on the H&E assay, matured oocytes in ovaries were counted and seminal vesicles were scored according to Table S1 (Gao et al., 2015, Li et al., 2017, Kwak and An, 2021),
2.6. Biofragmentation analyses
Biofragmentation of MB filter fibers and fragments were analyzed by combining high-resolution FE-SEM and Energy Dispersive X-ray (EDX) (X-MAXN, 20 kV, HORIBA, Kyoto, Japan) spot analysis. The FE-SEM/EDX analysis was the indirect tool for distinguishing between microplastic fragmentations and soil particles depending on the presence of Si or N (Kwak and An, 2021), since polypropylene is (C3H6)n while the Earth’s crust consist predominantly of Si (Gascho, 2001). Fibers and fragments of MB filters were collected from earthworm casts (21 days) and soil from the springtail soil assay (28 days) was tested under a microscope and washed with deionized water several times. The washed fibers and fragments of MB filters were placed on aluminum foil (thickness 16 µm), dried in the desiccator (Dry keeper, SANPLATEC, Osaka, Japan) for 7 days, and then analyzed by HR FE-SEM and EDX. Pristine fibers and fragments of MB filters were also analyzed by EDX to determine their composition. A minimum of four spots for the control and exposure groups were analyzed.
2.7. Statistical analysis
Statistical analysis was conducted using the Tukey test for a post-hoc test after one-way ANOVA (OriginPro, ver. 8, OriginLab Corporation, MA, USA) with p<0.05. The homogeneity of the variance was analyzed based on Levene’s test (OriginPro, ver. 8, OriginLab Corporation, MA, USA).
3. Results
3.1. Microplastic fragments from face masks and their distribution in the soil environment
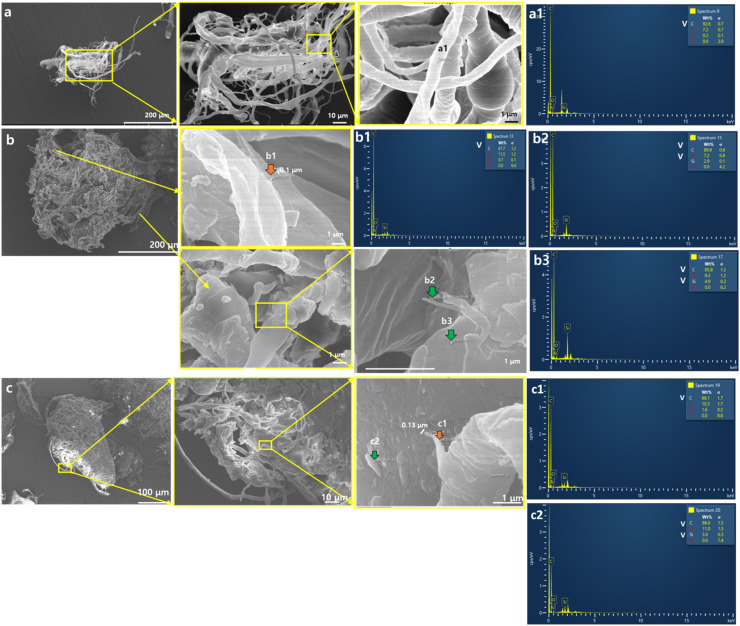
We confirmed the occurrence and distribution via ingestion by soil invertebrates of MB filter microplastic fibers and fragments in the soil environment. As reported by earlier studies (Aragaw, 2020, Fadare and Okoffo, 2020), materials of pristine face masks such as the MB filter and the inner and outer layers of face masks are characterized by polypropylene; we confirmed this finding using Fourier-transform infrared (FTIR) spectroscopy (Fig. 2c) in this study. Stereomicroscopy and FE-TEM verified that the MB filter and the inner and outer layers were made of microfibers (Fig. S1). The morphology of the cut pristine MB filter fragments (<300 µm sieved) and fibers which were detached from the fragments are shown in Fig. 2b,c (see also Fig. S2). It was indicated that mixtures of MB filter fibers and fragments can be introduced in the soil environment in tandem. Subsequently, ingestion and egestion of these pristine MB filter fibers and fragments by earthworms Eisenia andrei and springtails Folsomia candida were observed through bioassays (Fig. S3,S4). As shown in Fig. S2, single small microfibers were detached from fragments of the MB filters and these fibers or smaller fragments of the MB filters might be ingested by sectional F. candida. Excreted earthworm casts containing MB filter fragments were evident on the soil surface since Day 1 of the experiment. In the case of F. candida, ingested MB filter fragments were observed in the F. candida compared to controls and nile red leaching controls as well. Total 13% of juvenile and 27% of adult individuals ingested mask MB filter fragments after 7 days exposure to the uptake test (Fig. S3). These results suggested that MB filter fibers and fragments are ingestible by soil invertebrates and hence, they can be distributed via feeding activity. Moreover, we observed roughness increase, surface deterioration, and fragmentation of thinner MB filter fibers of <1 µm size on the surface of MB filter fibers, after exposure to earthworms and springtails ( Fig. 3; see also Fig. S5,S6). As shown in Fig. 3 a1, it was determined that pristine microfibers of MB filters showed dominant carbon signals based on the EDX spot analysis. In addition, it was also separated between fragmented nanofibers and soil particles based on the presence of a Si signal (Fig. 3 b1–b2, c1–c2). Fig. 3 b1 and c1 suggested fragmented nanofibers (~0.1 µm thickness) with predominant carbon signals without meaningful Si signals. These results indicated that if soil invertebrates ingest and egest MB filter fibers, nanofibers would probably be produced due to biofragmentation. However, there are some limitations due to the characterization of the fragmented nanofibers in the present study, and these limitations are discussed in the Discussion Section.
Fig. 3.
FE-SEM images (a–c) and EDX spot spectrum (a1–c2) of (a) pristine MB filter fibers and fragments, (b) collected MB filter fibers and fragments from springtail test soil 28 days after exposure and (c) collected MB filter fibers and fragments from earthworm casts 21 days after exposure. Orange arrows (b1 and c1) indicate fragmented nano-sized fibers on the surface of MB filter microfibers. White symbol of element in the inner box of a1–c2 indicates meaningfully detected elements. Green arrows (b2–b3 and c2) indicate what we interpret as soil particles exhibiting Si signals. White chemical element symbols in (a1–c2) show significant signals detected by EDX. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Adverse effects of MB filters on soil invertebrates
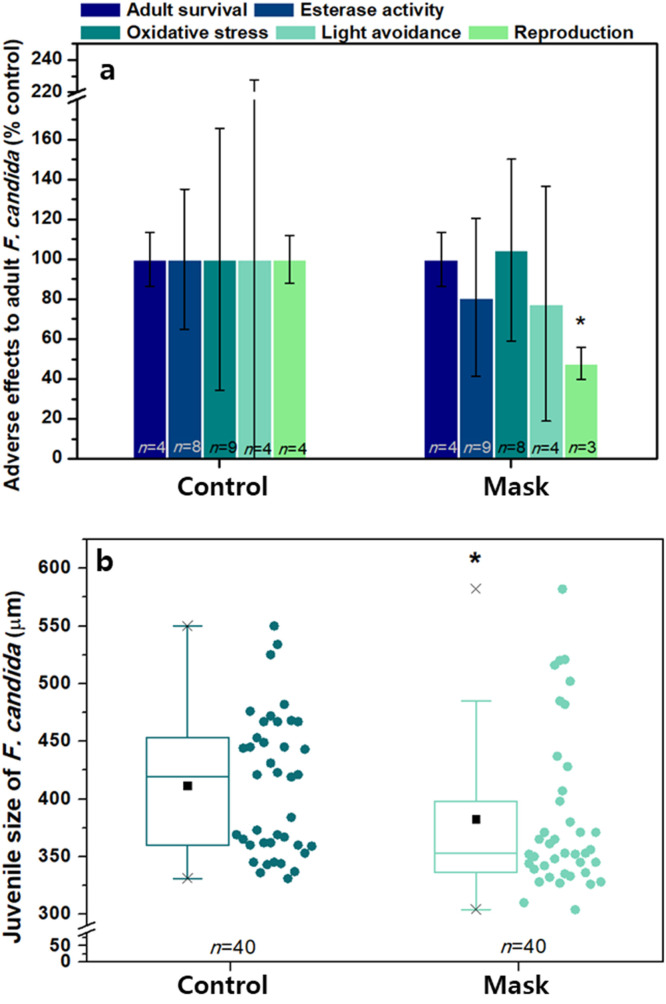
To estimate the soil ecotoxicological effects of MB filter fibers and fragments beyond the COVID-19 pandemic, we performed earthworm subchronic assay and springtail chronic assay. In the springtail assay, reproduction and growth of juveniles were suppressed after chronic exposure (on Day 28) to MB filter fibers and fragments in the soil. Compared to the control groups (i.e., 100%), the reproduction and growth rates dropped to 48.2% (p<0.05, F-value=14, DF=2) and 92.9% (p<0.05, F-value=44, DF=2), respectively, both of which were significantly different from those of the control groups. Conversely, no adverse effects on survival, esterase activity, oxidative stress, and light avoidance behavior of adult springtails were caused by MB filter fibers and fragments ( Fig. 4; see also Table 1). These springtail assay results indicated that MB filter fibers and fragments did not affect adult springtails but affected juvenile springtails adversely.
Fig. 4.
Effects of toxicity of MB filter fibers and fragments on springtail Folsomia candida 28 days after exposure. (a) Adult survival rate, esterase activity, oxidative stress, light avoidance, and reproduction. (b) Juvenile size (growth). The asterisk indicates significant decreases in the exposed groups (p<0.05).
Table 1.
Average adult survival, reproduction (number of juveniles), adult light avoidance behavior (28 days), juvenile growth in the springtail chronic soil assay (28 days), and adult earthworm survival, in vivo cytotoxicities in coelomocytes, and histopathological alterations in earthworm reproductive tissues in the earthworm subchronic soil assay (21 days). The asterisk indicates significant decreases in the exposed groups compared to the control groups (p<0.05).
| Control group | Exposed group | ||
|---|---|---|---|
| Springtail F. candida | Adult survival (%) | 93±10 | 93±10 |
| Adult light avoidance behavior (%) | 30±38 | 23±18 | |
| Adult esterase activity (% control) | 100±35 | 81±40 | |
| Adult oxidative stress (% control) | 100±65 | 105±45 | |
| Reproduction (% control) | 100±12 | 48±8* | |
| Juvenile growth (μm) | 412±60 | 383±68* | |
| Earthworm E. andrei | Adult survival (%) | 87±12 | 87±12 |
| Oxidative stress in coelomocytes (% control) | 100±19 | 94±14 | |
| Lysosomal stability in coelomocytes (% control) | 100±13 | 88±8 | |
| Esterase activity in coelomocytes (% control) | 100±22 | 62±20* | |
| Seminal vesicle (H&E scoring) | 2.1±0.9 | 0.8±0.9* | |
| Number of mature oocytes | 3.0±2.1 | 2.0±2.0 |
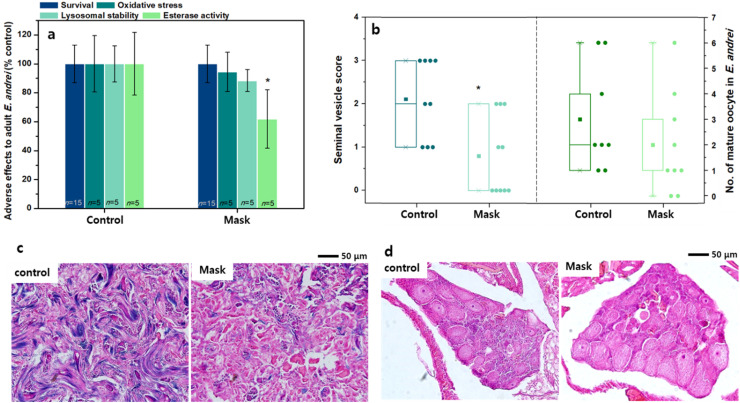
Intracellular esterase activity and spermatogenesis in seminal vesicles of earthworms were significantly affected by MB filter fibers and fragments (p<0.05; Fig. 5; see also Fig. S7,S8 and Table S1). Intracellular esterase activity in exposed earthworm coelomocytes dropped considerably to 62% (p<0.05, F-value=5, DF=2) as compared to that of the control groups (i.e., 100%). In the case of seminal vesicle tissues, less mature sperms and spermids (blue-stained nuclei) were observed in the exposed groups (Fig. S7) and the seminal vesicle score declined to 0.8 as compared to that of the control groups (Fig. 5) (p<0.05, F-value=5, DF=2). On the contrary, MB filter fibers and fragments did not adversely affect earthworm survival or result in pathological symptoms such as bleeding, swelling, thinning, and severance. The lysosomal stability and oxidative stress in coelomocytes were also not significantly impaired (p>0.05) (Fig. 5; see also Fig. S8). The number of matured oocytes in the exposed groups (3.0±2.1) decreased slightly without statistical difference compared to that of the control groups (2.0±2.0; see Fig. S9). These results of the earthworm assay suggested that MB filter fibers and fragments do not affect earthworms at the individual level but affect them at the tissue and cellular levels.
Fig. 5.
Effects of toxicity of MB filter fibers and fragments on earthworm Eisenia andrei 21 days after exposure. (a) Adult earthworm survival rate and in vivo cytotoxicities in coelomocytes (esterase activity, oxidative stress, and lysosomal stability). (b) Seminal vesicle score for normalcy evaluation and the number of mature oocytes in ovary. Representative images of earthworm (c) seminal vesicles and (d) ovaries tissues after H&E staining. The asterisk indicates significant decreases in the exposed groups (p<0.05).
Overall, the inhibited reproduction rate and juvenile growth of springtails, and the damaged spermatogenesis of earthworms indicated that the presence of MB filter fibers and fragments in the soil possibly affects the next generation of soil species.
4. Discussion
Owing to the increasing use of face masks, their improper disposal (Patrício Silva et al., 2020, WWF, 2020), and the consequent littering during the COVID-19 pandemic (Patrício Silva et al., 2021), plastic pollution is inevitably going to be a pressing issue globally. In order to predict the environmental impact of microplastics derived from face masks beyond the COVID-19 pandemic, we focused on the ecotoxicological effects of microplastic fibers and fragments and investigated the possibility of plastic nanofiber generation due to biofragmentation. Earthworms and springtails are good ecological indicators; they are ideal species for assessing the biofragmentation of nanofibers from MB filter microfibers and the adverse effects, as they are representative consumers and invertebrates in the soil ecosystem (ECB, 2003). We observed ingestion of MB filter fragments and fibers by earthworms and springtails on Day 1 and Day 7 of the experiment, respectively. We observed rapid uptake of microplastic particles by these organisms, thereby confirming earlier studies (Dawson et al., 2018a, Kwak and An, 2021). After confirming ingestion or egestion of MB filter fragments by earthworms or springtails (see Fig. S3, S4), fragments were collected from earthworm casts (Day 21) and springtail test soil (Day 28); these were analyzed using high-resolution FE-SEM and EDX spectroscopy to obtain images of plastic nanofiber fragmentations. As shown in Fig. 3 b1 and c1, we observed nanofibers at the surface of the MB filter fibers after exposure to earthworms and springtails. This observation indicated that face masks can be the source of polypropylene nanofibers by biofragmentation, which can subsequently be distributed by soil species and dispersed to soil ecosystems. Such biofragmented nanoplastics from microplastics have been recently detected by observing the Antarctic krill (Dawson et al., 2018b), earthworm gut bacteria (Huerta Lwanga et al., 2018), and earthworm (Kwak and An, 2021). Even without biofragmentation in the environment, face masks may become weathered and fragmented, thereby releasing cracked polypropylene microplastics when exposed to visible and UV light (Tang et al., 2019, Uheida et al., 2021, Wu et al., 2021). In addition, polypropylene microplastics derived from face masks can absorb existing pollutants in the ecosystem such as heavy metals (Zhou et al., 2020), organic pollutants (Wang et al., 2020, Wu et al., 2020, Xu et al., 2021), and cosmetic additives (Zhang et al., 2018); hence, soil species ingesting polypropylene microplastics combined with other pollutants essentially act as vectors of negative health impacts. For example, synergistic toxicity of cadmium and polypropylene microplastic were observed in earthworms species (Zhou et al., 2020). Recently, it was also reported that face masks themselves release heavy metals (Pb, Cd, Sb, and Cu) or organic chemicals (polyethylene glycol) (Sullivan et al., 2021). By considering the fiber shape, we expect a possibility of a similar toxicity between fibers and fragments of MB filters (PP) and polyester fiber cushions (Prendergast-Miller et al., 2019) because polyester fibers did not cause mortality and genes related oxidative stress but affected the mt and hsp70 gene expression in earthworms Lumbricus terrestris (Prendergast-Miller et al., 2019).
Based on earthworm and springtail toxicity tests, we established that MB filter fibers and fragments significantly impacted the number and size of juvenile springtails, intracellular esterase activity in earthworm coelomocytes, and spermatogenesis in earthworm seminal vesicles (Fig. 4, Fig. 5) at the tested concentration (1000 mg/kg dry soil), which is relatively high. By considering the worst exposure scenario with high concentration, these soil ecotoxicities were related to the long-term toxicity of polypropylene, based on previous research (Zhou et al., 2020). Zhou et al. (2020) that observed time- and concentration-dependent toxicities of polypropylene microplastics (<150 µm) and their effect on earthworm growth rate and mortality over 14, 28, and 42 days; the longer the duration of the exposure, the higher the mortality and growth inhibition of earthworms. MB filter leachates can be toxic for organisms because MB filters might contain additives such as anti-oxidants, flame retardants, and stabilizers (Dutton, 2008). Polypropylene microplastics may also release volatile organic compounds such as benzene and propanal (Lomonaco et al., 2020). For instance, it was reported that leachates from polypropylene microplastics impaired the larval development of pearl oysters (Gardon et al., 2020) and brown mussels (Gandara e Silva et al., 2016), and reduced the survival of copepods (Bejgarn et al., 2015).
Despite these findings, the toxic impacts of face mask fibers and fragments on the soil ecosystem and the effect of face mask polypropylene plastic nanofibers on soil species are still largely unexplored. The limitations of the present study can be described as follows. (1) The modes of toxic action and bio transportation of face mask fibers and fragments need to be elucidated. (2) This study simulated the worst-case exposure scenario of a relatively high concentration; therefore, the impacts of lower exposure concentrations of mask fragments should be investigated. (3) This study focused only on the MB filter. An MB filter would rarely be discarded from a mask in a realistic environment and some masks have no MB filters; therefore, mixture toxicity of all the mask components should be considered, and results of this study were limited within effects of MB filters not outer nor inner layer of masks. (4) How nanofibers were generated by soil invertebrates, particularly by springtails, remains unclear because the present study did not use defaunated or sterilized soil. Therefore, multiple factors such as soil microorganisms, soil exoenzymes, gut microbiomes, digestive enzymes, and burrowing behaviors might impact the generation of nanofibers from microfibers. (5) Lastly, quantified results such as the measurement of the decreased size of the egested MB filters was not possible in the present study because the size of the MB filter fragments was not fixed and the fragments tended to clump together.
With this study, we have elucidated the ecotoxicity of face mask waste. Currently, the COVID-19 pandemic poses a serious global threat to human health and survival; therefore, the use of face masks is inevitable, and, unfortunately, so is face mask waste production. With this study, we advocate the proper use—as well as disposal—of face masks for the sake of human health and ecosystem stability during and beyond the COVID-19 pandemic.
CRediT authorship contribution statement
Jin Il Kwak: Conceptualization, Investigation, Writing - original draft. Youn-Joo An: Conceptualization, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2020R1A2B5B02001734). We are grateful to Y. Park for assistance with the experiments.
Editor: Dr. R Teresa
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jhazmat.2021.126169.
Appendix A. Supplementary material
Supplementary material
.
References
- Aragaw T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020;159 doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejgarn S., MacLeod M., Bogdal C., Breitholtz M. Toxicity of leachate from weathering plastics: an exploratory screening study with Nitocra spinipes. Chemosphere. 2015;132:114–119. doi: 10.1016/j.chemosphere.2015.03.010. [DOI] [PubMed] [Google Scholar]
- BMI, 2020. Coronavirus: Frequently Asked Questions.
- Catarino A.I., Frutos A., Henry T.B. Use of fluorescent-labelled nanoplastics (NPs) to demonstrate NP absorption is inconclusive without adequate controls. Sci. Total Environ. 2019;670:915–920. doi: 10.1016/j.scitotenv.2019.03.194. [DOI] [PubMed] [Google Scholar]
- CCME, 2006. A Protocol for the Derivation of Environmental and Human Health Soil Quality Guidelines.
- CNN, 2020. These are the States Requiring People to Wear Masks When Out in Public.
- Dawson A., Huston W., Kawaguchi S., King C., Cropp R., Wild S., Eisenmann P., Townsend K., Bengtson Nash S. Uptake and depuration kinetics influence microplastic bioaccumulation and toxicity in antarctic krill (Euphausia superba) Environ. Sci. Technol. 2018;52:3195–3201. doi: 10.1021/acs.est.7b05759. [DOI] [PubMed] [Google Scholar]
- Dawson A.L., Kawaguchi S., King C.K., Townsend K.A., King R., Huston W.M., Bengtson Nash S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018;9:1001. doi: 10.1038/s41467-018-03465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton K.C. Overview and analysis of the meltblown process and parameters. J. Text. Appar. Technol. Manag. 2008;6:1–25. [Google Scholar]
- ECB, 2003. Technical Guidance Document on Risk Assessment. TGD Part II.
- Fadare O.O., Okoffo E.D. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandara e Silva P.P., Nobre C.R., Resaffe P., Pereira C.D.S., Gusmão F. Leachate from microplastics impairs larval development in brown mussels. Water Res. 2016;106:364–370. doi: 10.1016/j.watres.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Gao Y., Li X., Guo J., Sun X., Sun Z. Reproductive responses of the earthworm (Eisenia fetida) to antiparasitic albendazole exposure. Chemosphere. 2015;120:1–7. doi: 10.1016/j.chemosphere.2014.05.030. [DOI] [PubMed] [Google Scholar]
- Gardon T., Huvet A., Paul-Pont I., Cassone A.-L., Sham Koua M., Soyez C., Jezequel R., Receveur J., Le Moullac G. Toxic effects of leachates from plastic pearl-farming gear on embryo-larval development in the pearl oyster Pinctada margaritifera. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115890. [DOI] [PubMed] [Google Scholar]
- Gascho G.J. In: Studies in Plant Science. Datnoff L.E., Snyder G.H., Korndörfer G.H., editors. Elsevier; 2001. Silicon sources for agriculture; pp. 197–207. [Google Scholar]
- Huerta Lwanga E., Thapa B., Yang X., Gertsen H., Salánki T., Geissen V., Garbeva P. Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: a potential for soil restoration. Sci. Total Environ. 2018;624:753–757. doi: 10.1016/j.scitotenv.2017.12.144. [DOI] [PubMed] [Google Scholar]
- KBS, 2020. Face Masks Mandatory on Public Transport.
- Kwak J.I., An Y.-J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard. Mater. 2021;402 doi: 10.1016/j.jhazmat.2020.124034. [DOI] [PubMed] [Google Scholar]
- Kwak J.I., Park J.-W., An Y.-J. Effects of silver nanowire length and exposure route on cytotoxicity to earthworms. Environ. Sci. Pollut. Res. 2017;24:14516–14524. doi: 10.1007/s11356-017-9054-x. [DOI] [PubMed] [Google Scholar]
- Li C., Chen M., Li X., Yang M., Wang Y., Yang X. Purification and function of two analgesic and anti-inflammatory peptides from coelomic fluid of the earthworm, Eisenia foetida. Peptides. 2017;89:71–81. doi: 10.1016/j.peptides.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Lomonaco T., Manco E., Corti A., La Nasa J., Ghimenti S., Biagini D., Di Francesco F., Modugno F., Ceccarini A., Fuoco R., Castelvetro V. Release of harmful volatile organic compounds (VOCs) from photo-degraded plastic debris: a neglected source of environmental pollution. J. Hazard. Mater. 2020;394 doi: 10.1016/j.jhazmat.2020.122596. [DOI] [PubMed] [Google Scholar]
- Maes T., Jessop R., Wellner N., Haupt K., Mayes A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017;7:44501. doi: 10.1038/srep44501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Stud. Chem. Environ. Eng. 2020;1 doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHC, 2020. Beijing Issues Guidance for the Public on the Proper Use of Face Masks Under Regular Containment measures.
- OECD, 1984. Earthworm acute toxicity tests. OECD Guideline for Testing of Chemicals, Paris.
- OECD, 2009. OECD guidelines for the testing of chemicals No. 232. Collembolan Reproduction Test in Soil.
- Oliveira M., Cardoso D.N., Soares A.M.V.M., Loureiro S. Toxic effects of human pharmaceuticals to Folsomia candida – a multigeneration approach. Sci. Total Environ. 2018;625:1225–1233. doi: 10.1016/j.scitotenv.2017.12.319. [DOI] [PubMed] [Google Scholar]
- Patrício Silva A.L., Prata J.C., Walker T.R., Campos D., Duarte A.C., Soares A.M.V.M., Barcelò D., Rocha-Santos T. Rethinking and optimising plastic waste management under COVID-19 pandemic: policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrício Silva A.L., Prata J.C., Walker T.R., Duarte A.C., Ouyang W., Barcelò D., Rocha-Santos T. Increased plastic pollution due to COVID-19 pandemic: challenges and recommendations. Chem. Eng. J. (Lausanne, Switz.: 1996) 2021;405 doi: 10.1016/j.cej.2020.126683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plytycz B., Klimek M., Homa J., Tylko G., Kolaczkowska E. Flow cytometric measurement of neutral red accumulation in earthworm coelomocytes: novel assay for studies on heavy metal exposure. Eur. J. Soil Biol. 2007;43:S116–S120. [Google Scholar]
- Prendergast-Miller M.T., Katsiamides A., Abbass M., Sturzenbaum S.R., Thorpe K.L., Hodson M.E. Polyester-derived microfibre impacts on the soil-dwelling earthworm Lumbricus terrestris. Environ. Pollut. 2019;251:453–459. doi: 10.1016/j.envpol.2019.05.037. [DOI] [PubMed] [Google Scholar]
- Saadat S., Rawtani D., Hussain C.M. Environmental perspective of COVID-19. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliu F., Veronelli M., Raguso C., Barana D., Galli P., Lasagni M. The release process of microfibers: from surgical face masks into the marine environment. Environ. Adv. 2021;4 [Google Scholar]
- Sillapawattana P., Gruhlke M.C.H., Schäffer A. Effect of silver nanoparticles on the standard soil arthropod Folsomia candida (Collembola) and the eukaryote model organism Saccharomyces cerevisiae. Environ. Sci. Eur. 2016;28:27. doi: 10.1186/s12302-016-0095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G.L., Delgado-Gallardo J., Watson T.M., Sarp S. An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks - linked to the COVID-19 pandemic. Water Res. 2021;196 doi: 10.1016/j.watres.2021.117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiss-government, 2020. Coronavirus: Masks Compulsory on Public Transport; Quarantine for Travellers from High-risk Regions; Lifting of Certain Entry Restrictions from 20 July.
- Taipei-City-Police-Department, 2020. Passengers Required to Wear Face Mask When Taking MRT Starting April 4.
- Tang C.-C., Chen H.-I., Brimblecombe P., Lee C.-L. Morphology and chemical properties of polypropylene pellets degraded in simulated terrestrial and marine environments. Mar. Pollut. Bull. 2019;149 doi: 10.1016/j.marpolbul.2018.05.062. [DOI] [PubMed] [Google Scholar]
- Toronto, 2020. COVID 19: Mandatory Mask or Face Covering Bylaw.
- Uheida, A., Mejía, H.G., Abdel-Rehim, M., Hamd, W., Dutta, J., 2021. Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system. J. Hazard. Mater. In press, 124299. [DOI] [PubMed]
- UK, 2020. Face Coverings to Become Mandatory on Public Transport.
- Wang J., Coffin S., Schlenk D., Gan J. Accumulation of HOCs via precontaminated microplastics by earthworm Eisenia fetida in soil. Environ. Sci. Technol. 2020;54:11220–11229. doi: 10.1021/acs.est.0c02922. [DOI] [PubMed] [Google Scholar]
- WHO, 2020a. Archived: WHO Timeline - COVID-19.
- WHO, 2020b. Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19). Interim Guidance.
- WHO, 2021. WHO Coronavirus Disease (COVID-19) Dashboard.
- Wu X., Liu P., Huang H., Gao S. Adsorption of triclosan onto different aged polypropylene microplastics: critical effect of cations. Sci. Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2020.137033. [DOI] [PubMed] [Google Scholar]
- Wu X., Liu P., Shi H., Wang H., Huang H., Shi Y., Gao S. Photo aging and fragmentation of polypropylene food packaging materials in artificial seawater. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116456. [DOI] [PubMed] [Google Scholar]
- WWF, 2020. In the Disposal of Masks and Gloves, Responsibility is Required.
- Xu B., Huang D., Liu F., Alfaro D., Lu Z., Tang C., Gan J., Xu J. Contrasting effects of microplastics on sorption of diazepam and phenanthrene in soil. J. Hazard. Mater. 2021;406 doi: 10.1016/j.jhazmat.2020.124312. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zheng M., Wang L., Lou Y., Shi L., Jiang S. Sorption of three synthetic musks by microplastics. Mar. Pollut. Bull. 2018;126:606–609. doi: 10.1016/j.marpolbul.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Liu X., Wang J. Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J. Hazard. Mater. 2020;392 doi: 10.1016/j.jhazmat.2020.122273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material