Abstract
The immunogenicity of the novel mRNA COVID-19 vaccine in immunocompromised lung transplant recipients is still unknown. We compared the antibody response after the first and second doses of the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) with the response after natural SARS-CoV-2 infection in lung transplant recipients. None of the vaccinees tested after two doses of the mRNA BNT162b2 vaccine developed anti-SARS-CoV-2 IgG, while 85% patients presented an antibody response after SARS-CoV-2 infection. The absence of antibody response to vaccination led us to investigate the cellular response in a subset of patients. We detected SARS-CoV-2 specific T-cells in 4 out of 12 tested patients. Some patients therefore might have clinical benefit from the vaccine despite an absent antibody response. These results contrast with the excellent antibody response in immunocompetent individuals observed in mRNA BNT162b2 trials and indicate an urgent need to identify the best vaccine type and scheme for immunocompromised transplanted patients.
KEYWORDS: COVID-19, mRNA vaccine, antibody response, immunogenicity, lung transplantation
Abbreviations: LTRs, lung transplant recipients; SARS-CoV-2, severe acute respiratory syndrome coronavirus; ELISA, enzyme-linked immunoassay; CLIA, chemiluminiscent immunoassay; qRT-PCR, quantitative real-time polymerase chain reaction; S-RBD, spike receptor-binding domain; IFN-γ, interferon-γ; IL-2, interleukin-2; TNF-α, tumor necrosis factor-alpha; PBMCs, peripheral blood mononuclear cells
Lung Transplant Recipients (LTRs) are among the most vulnerable groups in the COVID-19 pandemic (mortality 14%-39%).1, 2, 3, 4 An early report provided evidence of a poor antibody response after the first mRNA vaccine dose in solid organ transplant recipients.5 However, the immunogenicity of the novel mRNA COVID-19 complete 2-dose vaccine in LTRs is unknown to date. We compared the antibody response after the first and second doses of the BNT162b2 mRNA COVID-19 vaccine with the response after natural SARS-CoV-2 infection in LTRs.
Methods
We included 48 LTRs without history of SARS-CoV-2 infection who received the first (n = 48) and second dose (n = 46) of the BNT162b2 vaccine (Pfizer-BioNTech) on December 29, 2020 and Jaunary 20, 2021, respectively. Seroconversion was assessed by anti-SARS-CoV-2 Spike S1 IgG ELISA (Euroimmun, Lubeck, Germany) and confirmed independently by Microblot-Array COVID-19 IgG against a mix of recombinant antigens (TestLine Clinical Diagnostics, Brno, Czech Republic) and chemiluminiscent immunoassay (CLIA) Liaison SARS-CoV-2 Trimeric S IgG against the trimeric spike S1 protein (Diasorin, Saluggia, Italy). The testing was conducted immediately before the first and second dose, 1 and 4-6 weeks after the second dose.
For comparison, 33 LTRs with a history of positive qRT-PCR were tested for SARS-CoV-2-specific IgG within 3 months after symptom onset or after the first qRT-PCR positivity in asymptomatic patients.
An additional group of 10 vaccinated healthy volunteers without history of COVID-19 was evaluated as a positive control to confirm the sensitivity of the IgG tests (Elisa, Microblot-Array, CLIA) and to evaluate seroconversion after the BNT162b2 vaccine. The volunteers' median age was 39.8 years (IQR, 33.3-47.8 years), the median time from the second dose to IgG testing was 31 days (IQR, 19-41 days).
In 12 vaccinated LTRs, SARS-CoV-2 specific T-cells were assessed by detecting intracellular cytokines interferon-γ (IFN-γ), interleukin-2 (IL-2) and tumor necrosis factor-alpha (TNF-α) after a 4h stimulation of 1 to 1.5 million of patients’ peripheral blood mononuclear cells (PBMCs) with 51 overlapping 11mer peptides of the S-RBD protein (JPT peptides, Berlin, Germany). A T-cell response study is laborious and expensive. It requires a fresh blood draw delivered to the laboratory on the same day, therefore we selected only patients living in Prague and Central Bohemia who were willing and able to visit our hospital for the blood draw. A positive response required an increase of > 1.5 fold above the non-stimulated controls and detection of more than 20 responding cells as previously described for CMV specific T-cell detection.6
The study was approved by the Motol University Hospital institutional review board and the participants provided written informed consent.
The Mann-Whitney test (2-tailed) was used for between-group comparison of numeric data, a chi-square test was used to test proportions. Statistical significance was set at p<0.05. The analyses were performed using Statistica version 13.5.0.17 (TIBCO Software).
Results
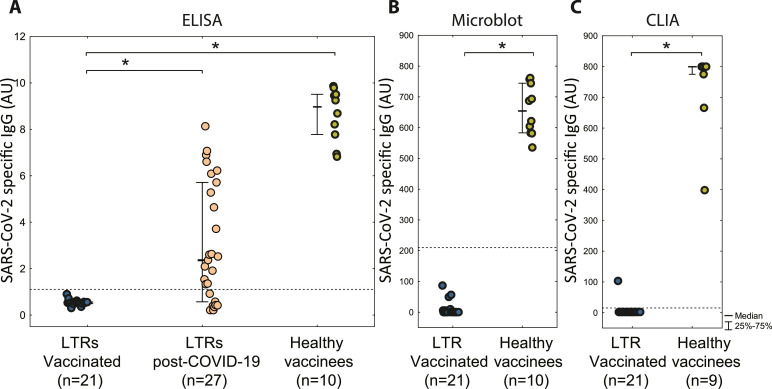
SARS-CoV-2-specific IgG were not detected in any of the vaccinated LTRs after the first or second vaccine dose. Serum samples were available before the first dose (n = 48; 100%), before the second dose (n = 46; 95.8%), 7 days after the second dose (n = 30; 62.5%) and 4-6 weeks after the second dose (n = 21; 43.8%) (Table. 1 ). Two patients did not receive the second dose: one of them had an episode of acute cellular rejection and the other one was infected with SARS-CoV-2 at day 9 after the first dose. Three patients were diagnosed with COVID-19 at days 15, 33 and 44 after the second dose, all of them with a mild course of the disease and with detectable SARS-CoV-2 spike IgG after the disease.
Table. 1.
Demographic and Clinical Characteristics of Study Participants
| No. (%) | |||
|---|---|---|---|
| LTRs Vaccinated | LTRs post-COVID-19 | ||
| n = 48 | n = 33 | p valuea | |
| Age, mean (SD), y | 52.1 (14.3) | 51.6 (15.5) | ns |
| Female | 19 (39.6) | 14 (42.4) | ns |
| Primary disease | |||
| Chronic obstructive pulmonary disease | 16 (33.3) | 10 (30.3) | ns |
| Interstitial lung disease | 17 (35.4) | 15 (45.5) | ns |
| Cystic fibrosis | 12 (25) | 6 (18.2) | ns |
| Other | 3 (6.3) | 2 (6.1) | ns |
| Transplant type | |||
| Double lung transplant | 45 (94) | 30 (91) | ns |
| Single lung transplant | 3 (6) | 3 (9) | ns |
| Time since transplant, median (range), d | 1552 (123-7349)b | 1287 (6-7745)c | ns |
| Maintenance immunosuppression | |||
| Calcineurin inhibitor | 48 (100) | 33 (100) | |
| Tacrolimus | 47 (97.9) | 33 (100) | ns |
| Cyclosporin | 1 (2.1) | 0 (0) | ns |
| Mycophenolate | 44 (91.7) | 8 (24.2)d | <0.0001 |
| Dose, mean (SD), mg | 1247 (703) | 197 (431)e | <0.0001 |
| Prednisone | 47 (97.9) | 33 (100) | ns |
| Dose, mean (SD), mg | 9.5 (4.4) | 10.9 (5.9)f | ns |
| Number of vaccine doses | |||
| 1st dose | 48 (100) | 0 | |
| 2nd dose | 46 (95.8) | 0 | |
| SARS-CoV-2 spike IgG detected after vaccinationg (patients positive/total tested) | |||
| Before 1st dose | 0/48 | ||
| Before 2nd dose | 0/46 | ||
| 1 week after 2nd dose | 0/30 | ||
| 4-6 weeks after 2nd dose | 0/21 | ||
| SARS-CoV-2 spike IgG detected after COVID-19h symptom onseti (patients positive/total tested) |
|||
| Total | 28/33 (84.8) | ||
| 0-2 weeks | 0/13 (0) | ||
| 3-6 weeks | 19/27 (70.4) | ||
| 7-12 weeks | 17/21 (81) | ||
| SARS-CoV-2 spike specific T cell response detected after vaccination (patients positive/total tested) | |||
| 9 weeks after 2nd dose | 4/12 (33.3) |
Comparison of LTRs Vaccinated and LTRs post-COVID-19 groups, nonsignificant as “ns”
Time since transplant to 1st vaccine dose
Time since transplant to onset of SARS-CoV-2 infection
Mycophenolate was temporarily discontinued in 25 patients upon the detection of SARS-CoV-2 infection
Mycophenolate was temporarily reduced in 5 patients upon the detection of SARS-CoV-2 infection
Prednisone dose before COVID-19 onset, 7 days after onset the dose was usually increased
Vaccinated LTRs were tested by ELISA, Microblot and CLIA
LTRs post-COVID-19 were tested by ELISA
Weeks after qRT-PCR SARS-CoV-2 positivity in case of asymptomatic infection
In contrast, in the post-COVID-19 group of LTRs on the same immunosuppressive regimen, anti-SARS-CoV-2 spike IgG were detected in 28 (85%) patients within 90 days following the diagnosis (Figure 1 ). Serum samples were collected at multiple time points for most patients, with 48% (n = 16) having ≥ 3 samples. SARS-CoV-2 IgG were detectable in 19 out of 27 (70%) patients between the third and sixth week post COVID-19, with further increase to 17 out of 21 (81%) between seven and 12th week, while no response occurred within 2 weeks after onset (Table). No antibodies were detected in 5 patients (15%), two of whom had previously received rituximab.
Figure 1.
SARS-CoV-2-specific IgG response after BNT162b2 mRNA vaccine and SARS-CoV-2 Infection (A) SARS-CoV-2 spike S1 protein specific IgG detected by ELISA (B) SARS-CoV-2 IgG against a mix of recombinant antigens detected by Microblot-Array (C) SARS-CoV-2 specific IgG against trimeric spike S1 detected by CLIA. Vaccinated LTRs are evaluated at 4-6 weeks post 2nd dose (doses 3 weeks apart). LTRs post-COVID-19 were evaluated 3-6 weeks post onset. Healthy non-transplant vaccines were evaluated at median of 4 weeks (1-8 weeks). Significant difference (p < 0.001) is highlighted by a star. The dotted line shows the positivity threshold (the only value above the threshold in vaccinated LTR is considered false positive since it was not confirmed by the other two assays).
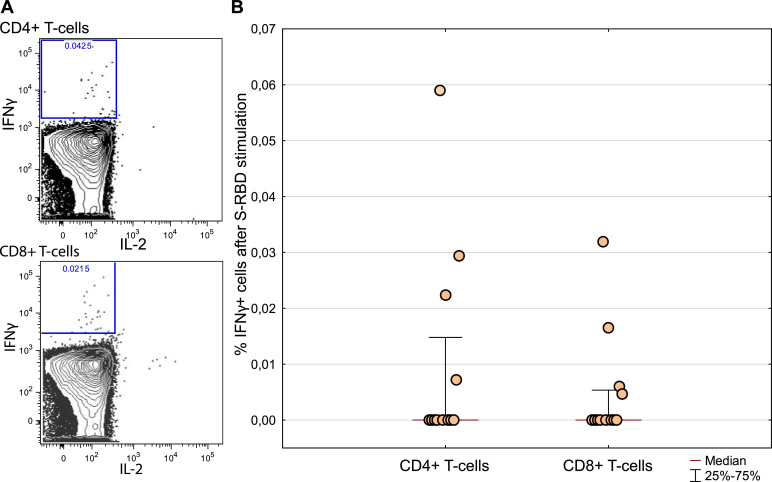
The absence of specific IgG in vaccinated LTRs prompted us to investigate the T-cell response to vaccination. T cells of four of twelve tested patients responded to the S-RBD antigen. Among the responders, we detected 0.03% of CD4+ T cells (range 0.007%-0.06%) and 0.015% of CD8+ T cells (range 0.005%-0.03%) 9 weeks after the second dose (Figure 2 ).
Figure 2.
SARS-CoV-2 S-RBD specific response of CD4+ and CD8+ T cells. (A) A representative dot plot of flow cytometry measurement of IFN-γ producing CD4+ and CD8+ T cells (B) Twelve LTRs were assessed for SARS-CoV-2 S-RBD specific response of CD4+ and CD8+ T cells. Magnitude of the response is calculated as % of IFN-γ responding T-cells after S-RBD stimulation less % of IFN-γ without any stimulation.
The maintenance immunosuppression regimen in both groups included calcineurin inhibitors, mycophenolate and corticosteroids. However, in the post-COVID-19 group, mycophenolate was temporarily discontinued in 25 (76%) patients upon detection of SARS-CoV-2 infection. 22 out of 25 (88%) patients without mycophenolate and 6/8 (75%) patients with reduced/maintained mycophenolate dose had detectable SARS-CoV-2 IgG. We found no difference in the frequency of antibody response between the post-COVID-19 subgroups divided according to the presence of mycophenolate (p = 0.74). The mycophenolate dosage in vaccinated patients remained unchanged.
All three anti-SARS-CoV-2-specific IgG tests detected high levels of antibodies in all healthy volunteers after the second dose (Figure 1).
Discussion
The complete lack of antibody response in LTRs after the second mRNA BNT162b2 vaccine dose contrasts with a very good response in 85% of LTRs after COVID-19. Possible causes include a more complex and durable antigenic stimulation during natural infection or the significantly lower proportion of patients receiving mycophenolate in the post-COVID-19 group. However, none of the four vaccinated patients without mycophenolate developed antibodies either. While no antibody response was detectable by any of the three IgG tests, we have been able to detect a T-cell response to the vaccination antigen in 4 out of 12 patients by sensitive functional response. Thus, some patients might have a clinical benefit from the vaccine despite having no antibody response. Only a mild course of the COVID disease was observed in all three fully vaccinated patients infected post-vaccination. Furthermore, the finding of a poor but significantly higher anti-spike antibody response in organ transplant recipients (25% vs 10%, p = 0.006) after the first dose of the mRNA-1273 vaccine (Moderna) compared to the BNT162b2 vaccine (Pfizer-BioNTech) suggests that vaccine properties might be important in immunosuppressed patients.5 Immunocompromised LTRs without memory response might benefit from early revaccination with different vaccine types.
In conclusion, none of the LTRs developed anti-SARS-CoV-2 antibodies after two doses of the mRNA BNT162b2 vaccine (Pfizer-BioNTech), while 85% presented an antibody response after SARS-CoV-2 infection. The detection of specific CD4+ and CD8+ T cells might suggest that the inclusion of cellular response testing in the evaluation of post-vaccine immune responses might be beneficial in immunocompromised patients and additional boosting may be required for the development of post-vaccination antibody response in LTRs.
Author Contributions
Dr Havlin and Dr Kalina had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Hubacek and Lischke are co–senior authors.
Concept and design: Havlin, Kalina, Hubacek, Lischke
Acquisition, analysis and interpretation of data: Havlin, Kalina, Hubacek, Lastovicka
Drafting of the manuscript: Havlin, Kalina, Hubacek
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Havlin, Kalina, Hubacek
Obtained funding: None
Administrative, technical, or material support: Lastovicka, Svorcova, Dvorackova
Supervision: Kalina, Havlin, Hubacek, Lischke
Data visualization: Havlin, Kalina
Disclosure statement
The authors have declared no conflict of interest exists.
Acknowledgment
This work was supported by the grant of the Ministry of Health of the Czech Republic for conceptual development of research organization, No. 0064203 (Motol University Hospital, Prague, Czech Republic).
Role of the Funder/Sponsor: The support was used for covering the costs of the detection kits used in the study. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
The authors would like to thank Tereza Martinu, MD, Krystof Slaby, MD, Jan Simonek, MD, Andrea Zajacova, MD, Jiri Pozniak, MD, Jiri Vachtenheim, MD, Jan Kolarik, MD, Rene Novysedlak, MD, for contributing to this study.
References
- 1.Myers CN, Scott JH, Criner GJ. COVID-19 in lung transplant recipients. Transpl Infect Dis. 2020;22:1–5. doi: 10.1111/tid.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saez-Giménez B, Berastegui C, Barrecheguren M. COVID-19 in lung transplant recipients: a multicenter study. Am J Transplant. 2020 doi: 10.1111/ajt.16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aversa M, Benvenuto L, Anderson M. COVID-19 in lung transplant recipients: a single center case series from New York City. Am J Transplant. 2020;20:3072–3080. doi: 10.1111/ajt.16241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messika J, Eloy P, Roux A. COVID-19 in lung transplant recipients. Transplantation. 2021;105:177–186. doi: 10.1097/TP.0000000000003508. [DOI] [PubMed] [Google Scholar]
- 5.Boyarsky BJ, Werbel WA, Avery RK. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. Jama. 2021:2–4. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelak O, Stuchly J, Krol L. Appearance of cytomegalovirus-specific T-cells predicts fast resolution of viremia post hematopoietic stem cell transplantation. Cytometry B Clin Cytom. 2017;92:380–388. doi: 10.1002/cyto.b.21348. [DOI] [PubMed] [Google Scholar]