Abstract
Liver failure is one of the leading causes of death worldwide, and mortality from chronic liver disease is rising sharply in the United States. Healthy livers are capable of regenerating from toxic damage, but in advanced liver disease, the natural ability of the liver to regenerate is impaired. Zebrafish have emerged as a powerful experimental system for studying regeneration. They are an ideal model for studying liver regeneration from partial hepatectomy, a procedure with direct clinical relevance in which part of the liver is surgically removed, leaving the rest intact. There is no standard protocol for partial hepatectomy; previous studies using this model have used slightly different protocols and reported disparate results. Described here is an efficient, reproducible protocol for performing a partial hepatectomy in adult zebrafish. We use this technique to demonstrate that zebrafish are capable of epimorphic regeneration of the resected lobe. This protocol can be used to further interrogate the mechanisms required for liver regeneration in zebrafish.
SUMMARY:
This protocol describes the procedure for removing the ventral lobe of the liver in adult zebrafish to enable the study of liver regeneration.
INTRODUCTION:
Among the solid organs in humans, the liver is the only organ capable of regeneration1. This is critical, as the liver is an essential organ, responsible for key metabolic functions, energy storage, blood detoxification, secretion of plasma proteins, and bile production2. Hepatocytes lost due to toxic or inflammatory damage are replaced primarily via division of the remaining hepatocytes1. One classical experimental model for studying liver regeneration is partial hepatectomy, where individual lobes of the liver are removed, leaving the remaining lobes intact3. This procedure was initially developed in rats, in which approximately two-thirds of the liver mass is removed. After partial hepatectomy in mammals, compensatory regeneration occurs in the remaining lobes until the liver recovers its initial mass. Notably, the mammalian liver does not replace the missing lobes.
Zebrafish (Danio rerio) represent a tractable model for studying adult organ regeneration4. The zebrafish liver, while structurally different from the mammalian liver, is made up of the same cell types and serves the same function as in mammals2. It is composed of three lobes, with two dorsal lobes and a single ventral lobe that are flattened along the intestine. Partial hepatectomy has previously been performed in zebrafish, with conflicting accounts as to the precise mode of regeneration. Typically, a one-third partial hepatectomy is performed by removal of the entire ventral lobe. Initial reports indicated that after removal of the ventral lobe, the ventral lobe was fully regenerated within a week5-7, suggesting that in contrast to the mammalian liver, the zebrafish liver is capable of epimorphic regeneration. Subsequent studies demonstrated that removal of the ventral lobe resulted in compensatory regeneration in the dorsal lobes, rather than the regeneration of the missing ventral lobe, and ultimately the recovery of liver mass within a week8,9. Transcriptomic profiling of the dorsal lobes following resection of the ventral lobe revealed significant changes associated with compensatory regeneration10. Given that the mode of liver regeneration can vary with the extent of the injury8, we speculated that the discrepancies in results may be due to technical variation in the partial hepatectomy protocol between research groups.
This protocol describes a procedure for performing a one-third partial hepatectomy on adult zebrafish by removing the ventral lobe. This technique will be valuable for assessing mechanisms of liver regeneration.
PROTOCOL:
Zebrafish were raised and bred according to standard procedures. Experiments were approved by the Brigham and Women’s Hospital’s Institutional Animal Care and Use Committee (2016N000405). Adult zebrafish were fasted for 24 hours prior to the start of the protocol. System water refers to the water in zebrafish housing tanks in the aquatic facility.
1. Anesthetization
1.1. Prepare 0.016% Tricaine solution in system water. CAUTION: Tricaine is an irritant if it comes into contact with the eyes, skin or respiratory tract.
1.2. Prepare a sponge to hold anesthetized zebrafish during the dissection protocol. Cut a full sponge into quarters. Using a razor blade, remove a thin wedge of sponge that runs parallel to the long axis of the sponge quarter. The slit should be long enough to accommodate an adult fish (this will vary between different sizes of fish). For example, for an adult fish 35 mm in length, the length of the slit should be 20 mm. The head and body are snugly held in the sponge, but the tail runs past the edge of the sponge (Figure 1B).
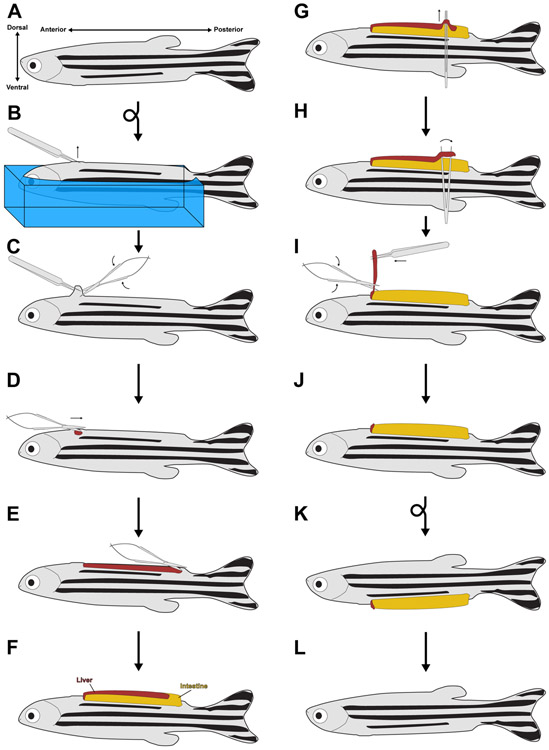
Figure 1. The partial hepatectomy protocol.
(A) Diagram of an adult zebrafish, with the anterior-posterior and dorsal-ventral axes noted. (B) After anesthetization, an animal is embedded in a sponge (shown in blue) so that it is ventral side up. Fine forceps are used to pinch the skin just posterior to the heart. Sponge is not shown in subsequent panels for clarity. (C) Scissors are used to break the skin just posterior to the heart. (D-E) The scissors are used at the now open wound to make a 3-4 mm incision running from anterior to posterior, terminating just before the pelvic fins. (F) Squeezing the sides of the sponge causes the visceral organs to pop out of the larger wound. (G) Fine forceps with both tines touching are slid between the ventral lobe of the liver and the intestine. (H) The forceps are relaxed, causing them to break the portal vein attachments between the live and the intestine. This process is repeated along the length of the ventral lobe. (I-J) The ventral lobe is pulled up from its posterior end, while the scissors are used to sever the ventral lobe from the rest of the liver. (K) The animal is placed into a recovery chamber with system water, where it regains consciousness and rights itself. (L) With time, the viscera are pulled back into the body cavity and the wound heals.
1.3. Soak the sponge in 0.016% Tricaine solution.
1.4. Place adult zebrafish (either male or female) in 625 mL of 0.016% Tricaine solution.
1.5. Incubate for 6 minutes or until the fish is unresponsive to touch.
1.6. Using forceps, carefully remove fish from the Tricaine tank and place fish ventral side up in the groove of the sponge (Figure 1A-B).
1.7. Place the sponge under a dissecting microscope with top-down illumination.
2. Surgery
2.1. Using fine forceps, pinch the skin and scales medially, just posterior to the heart (Figure 1B).
2.2. Using spring-loaded scissors, make a cut under the forceps to create a hole in the body cavity (Figure 1C-D). Take care not to injure the heart or a major blood vessel, as this will result in increased mortality.
2.3. Using spring-loaded scissors, make a 3-4 mm incision along the abdomen, processing posteriorly until the incision arrives at the pelvic fins (Figure 1D-E). By this point, the ventral lobe of the liver may be visible through the incision.
2.4. Squeeze the sides of the sponge with one hand to force the visceral organs out of the body cavity. The ventral lobe of the liver will be visible on top of the intestine (Figures 1F,2A-B). The liver will appear as a pink or orange structure spread out over the golden-brown intestine. Animals designated as sham controls are recovered at this point.
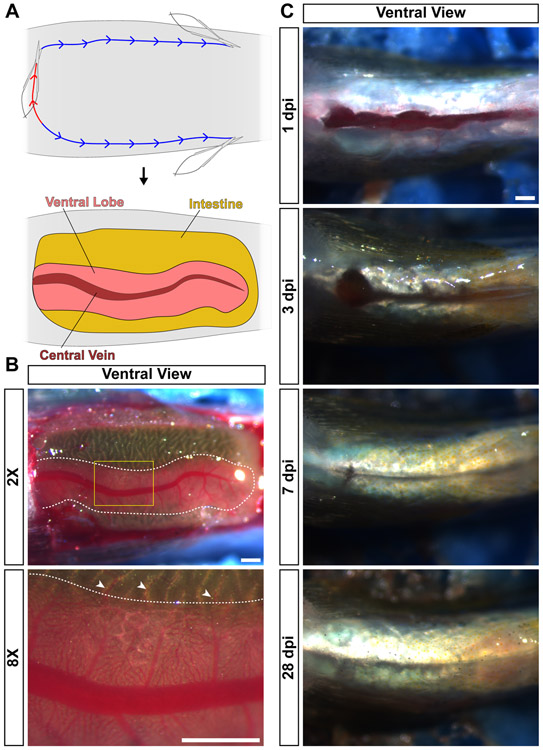
Figure 2. Zebrafish anatomy and recovery from partial hepatectomy.
(A) For analysis of the visceral organs, animals are euthanized and placed ventral side up in a sponge. First, an incision is performed at the anterior-posterior position of the heart (red line). Then, two incisions are generated that run along the anterior-posterior axis to the pelvic fins (blue line). Then, the skin and muscle are peeled back, revealing the visceral organs. Labeled are the intestine, ventral lobe of the liver, and the central vein in that lobe. (B) Live image of a ventral view of a zebrafish prepped for analysis of the visceral organs. The ventral lobe of the liver is outlined with a dotted white line. Yellow box in the 2X image indicates the location of the 8X image. White arrowheads indicate portal vein connections between the intestine and the liver. (C) Ventral view of the wound generated by the partial hepatectomy procedure at the indicated timepoints. Over time, the incision heals completely. Scale bars, 500 μm.
2.5. Squeeze the fine forceps so that the two tines are touching. While keeping pressure on the sponge, slide the tines of the fine forceps in between the liver and the intestine (Figure 1G). Take care to not puncture the intestine, as this will result in increased mortality.
2.6. Slowly relax the pressure on the forceps so that the tines move away from one another (Figure 1H). This sliding action severs the numerous portal vein attachments between the liver and the intestine (Figure 2B), and is necessary to cleanly remove the ventral lobe. Repeat this process until all of the portal connections between the liver and intestine have been severed.
2.7. Peel back the ventral lobe from the intestine using fine forceps, and cut the ventral lobe free from the rest of the liver (Figure 1I).
2.8. This procedure results in a one-third partial hepatectomy (Figure 1J)
3. Recovery
3.1. Carefully remove the fish from the sponge and place it in a tank of system water.
3.2. Pipette system water over the gills for a few minutes until the fish is swimming on its own (Figure 1K).
3.3. Monitor fish for 2-4 hours before placing them back onto the system. Fish should not be fed for a full 24 hours after the surgery.
3.4. Monitor fish daily for the duration of the experiment.
3.5. With time, the incision in the body wall will heal naturally without the need for sutures (Figures 1L,2C).
4. Ventral Lobe to Intestine Length Analysis
4.1. Euthanize all animals destined for analysis in ice water for 10 minutes until all opercular movements cease.
4.2. Remove the fish from ice water and place it ventral side up in the groove of a sponge.
4.3. Using spring-loaded scissors, make an incision in the ventral body wall at the anterior-posterior position of the heart. Then make two more incisions that run along the anterior-posterior axis from the first incision all the way to the pelvic fins. (Figure 2A).
4.4. Peel back the skin and muscle to reveal the visceral organs (Figure 2A).
4.5. Acquire bright field and fluorescent images of the visceral organs using an epifluorescence microscope. This field of view will include the area where the ventral lobe was resected. Because animals are euthanized prior to analysis, this kind of analysis precludes long term imaging of the same fish.
5. Liver to Body Weight Ratio Analysis
5.1. Euthanize all animals destined for analysis in ice water for 10 minutes until all opercular movements cease.
5.2. Place fish in a 50 mL conical tube.
5.3. Add 25 mL of 4% paraformaldehyde in 1XPBS+0.3% Tween to the tube. CAUTION: Formaldehyde is toxic, and solutions containing formaldehyde should always be processed in a chemical hood.
5.4. Nutate for 48 hours at 4C.
5.5. Perform four 10 minute washes in 1XPBS+0.3% Tween.
5.6. Retrieve fish with forceps, and blot dry on a paper towel.
5.7. Record the weight of the entire fish.
5.8. Using spring-loaded scissors, make an incision in the ventral body wall at the anterior-posterior position of the heart. Then make two more incisions that run along the anterior-posterior axis from the first incision all the way to the pelvic fins. (Figure 2A).
5.9. Peel back the skin and muscle to reveal the visceral organs (Figure 2A).
5.10. Acquire bright field images of the liver using an epifluorescence microscope.
5.11. Dissect out the liver, placing the pieces of liver on a weighing boat.
5.12. Record the weight of the liver.
REPRESENTATIVE RESULTS:
In order to examine the regenerative potential of the adult zebrafish liver, we performed partial hepatectomy (PHX) in adult zebrafish. In general, large adults (30-40 mm in length) were selected, ranging from 1.5-2.5 years old. Within individual experiments, animals were selected from the same tank, and were age- and size-matched. As an appropriate control, we utilized sham surgeries in which the animal was both aestheticized and received a large incision in the ventral body wall, but was recovered without removing any tissue. The survival rates for sham controls ranged from 90-100% for both male and female zebrafish. The survival rates for PHX animals ranged from 60-75% for male zebrafish and 60-90% for female zebrafish, with all deaths occurring in the first 24 hours following the surgery.
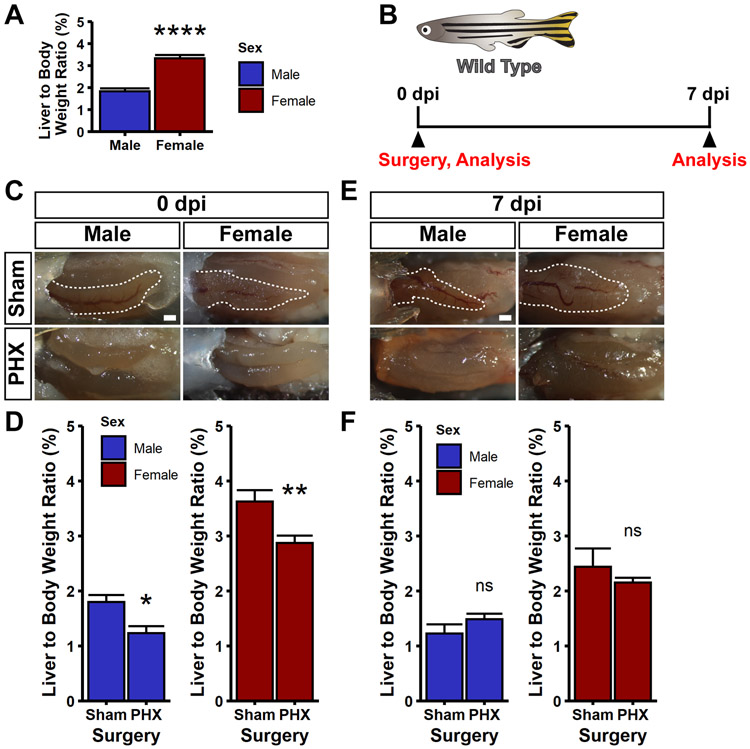
The gold standard for quantifying the recovery of the liver following PHX is the liver to body weight ratio, or LBR. This assay has been used for both measurement of liver mass recovery in both mammalian11 and zebrafish models8. To reliably quantify the weight of the liver, we made weight measurements on fixed animals, as previously described8. We performed LBR measurements on wild type, uninjured zebrafish, and found that as previously reported8, female zebrafish had almost double the LBR as compared to male zebrafish (3.3% for female zebrafish and 1.8% for male zebrafish) (Figure 3A). We performed both sham and PHX on adult zebrafish of both sexes and analyzed the LBR at 0 and 7 days post injury (dpi) (Figure 3B). At 0 dpi, sham animals had a clearly visible ventral lobe, whereas in PHX animals the ventral lobe was completely absent (Figure 3C). Notably, this resulted in a significant reduction in LBR (30% reduction in male fish, 20% reduction in female fish) (Figure 3D). In the cohort of fish analyzed at 7 dpi, we found that the ventral lobe had not regenerated (Figure 3E), and yet the LBR of PHX and sham controls were comparable (Figure 3F). These measurements indicate that by 7 dpi, the liver has regained mass relative to sham controls, presumably by compensatory regeneration in the dorsal lobes, in agreement with prior reports8,9.
Figure 3. Compensatory regeneration after partial hepatectomy.
(A) Liver to body weight (LBR) ratios were measured for wild type, uninjured zebrafish. Female zebrafish have almost double the LBR compared to male zebrafish. Female animals were compared to male animals using a Wilcoxon rank sum test, ****p<0.0001. (B) Schematic indicating that wild type zebrafish were used for this experiment. Animals were subjected to sham or partial hepatectomy (PHX), and then either fixed at 0 or 7 days post injury (dpi). Fixed animals were imaged and subjected to LBR measurements. (C,E) Shown are representative images of animals subjected to sham or PHX at 0 dpi (C) and 7 dpi (E). Note the complete absence of the ventral lobes in PHX animals. For all images, shown is a ventral view of the visceral organs. Images are bright field images. The ventral lobe of the liver is outlined in white. Scale bars, 500 μm. (D,F) Bar graphs of the liver to body weight ratios for both male and female zebrafish after sham and PHX surgeries at 0 dpi (D) and 7 dpi (F). The height of the bar is the mean value, and the error bars represent SEM. Whereas there is a reduction in LBR following partial hepatectomy at 0 dpi, there is no significant difference between PHX and sham animals at 7 dpi, indicating restoration of LBR. Partial hepatectomy samples were compared to sham controls using a Wilcoxon rank sum test, ns = not significant, *p<0.05, **p<0.01.
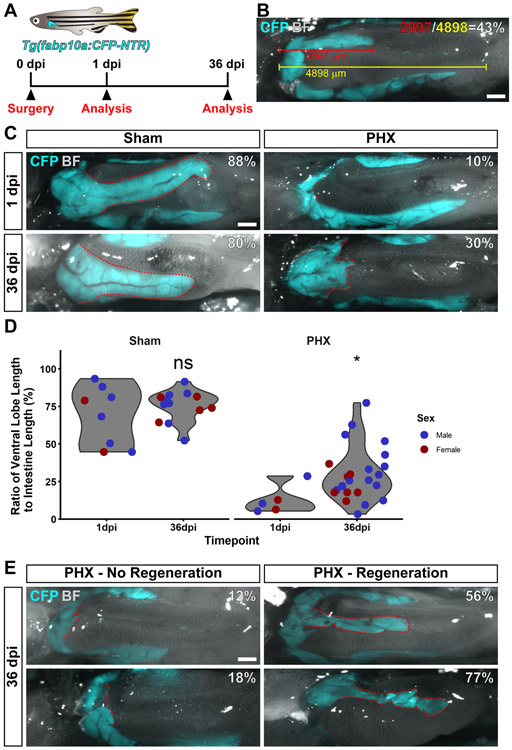
We decided to investigate whether, given enough time, the ventral lobe of the liver was capable of regeneration. Adult Tg(fabp10a:CFP-NTR) fish were selected for experimentation, as these fish express CFP in hepatocytes, allowing for visualization of the liver using fluorescence microscopy. A cohort of animals was subjected to the full one-third partial hepatectomy protocol. Animals were analyzed at 1 or 36 dpi (Figure 4A). For each animal, the ratio of ventral lobe length to the intestine was measured (Figure 4B).
Figure 4. Zebrafish can regenerate their ventral lobes after partial hepatectomy.
(A) Schematic indicating that Tg(fabp10a:CFP-NTR) were used for this experiment. Surgery and analysis by imaging were performed at the indicated timepoints. (B) An example image to demonstrate how animals were analyzed. Animals were quantified by measuring the length of the ventral lobe (in red), the length of the intestine (in yellow) and computing the percentage of the intestine that the ventral lobe occupies (in white). (C) Shown is a representative image of animals subjected to sham or partial hepatectomy (PHX) at 1 and 36 days post injury (dpi). (D) Violin plots of the ratio of ventral lobe length to intestine length for animals subjected to sham or partial hepatectomy at 1 and 36 dpi. Each dot represents the value from a single fish. Values for male fish are in blue, female fish in red. Partial hepatectomy samples were compared to sham controls using a Wilcoxon rank sum test, ns = not significant, *p<0.05. (E) Shown are two examples of partial hepatectomy animals at 36 dpi that did not regenerate, and two examples that did regenerate. For all images, shown is a ventral view of the visceral organs. Images are a merge of a CFP fluorescence image and a bright field image. CFP fluorescence is only present in the liver. The ventral lobe of the liver is outlined in red. The percentage of the intestine that the ventral lobe occupies is in white. Scale bars, 500 μm.
Animals subjected to sham surgeries had a prominent ventral lobe that occupied 50-100% of the intestine length, whereas animals subjected to one-third partial hepatectomy had a severely reduced ventral lobe. Strikingly, many partial hepatectomy animals at 36 dpi had an increased ventral lobe to intestine ratio as compared to partial hepatectomy animals at 1 dpi (Figure 4C). There was no increase in the size of the ventral lobe in sham controls; however, a statistically significant increase in the size of the ventral lobe was observed after recovery from partial hepatectomy (Figure 4D). We noted that there was a wide variation in the response to the surgery, with some animals displaying no regeneration, and others clearly regenerating a well-defined ventral lobe (Figure 4E). These results demonstrate that the ventral lobe is capable of regenerating from surgical liver resection in adult zebrafish. Taken together, our work indicates that the zebrafish liver is capable of both epimorphic and compensatory regeneration.
DISCUSSION:
The anatomical differences between zebrafish and mammalian models for liver regeneration present unique challenges to liver resection. The liver in zebrafish is in close proximity to the heart and the intestine; inadvertently damaging either organ results in increased mortality. The zebrafish liver is not encapsulated, making it more difficult to separate from the intestine. The liver receives nutrient-rich blood from the intestine through portal veins. In mammals, veins leaving the intestine converge on a primary portal vein that then splits as it enters the liver12. In contrast, the zebrafish liver receives portal circulation from a series of tiny vessels that move directly from the intestine into the liver (Figure 2B). Thus, each lobe of the liver, flattened out over the intestine, is securely attached to the intestine by these vessels. Attempting to remove the ventral lobe without first severing the portal veins can often result in incomplete resection (data not shown). Some of the initial protocols describe pulling the ventral lobe out through a relatively minor incision, which does not allow for severing these portal attachments5,6.
The protocol described here is designed to address these challenges to enable consistent ventral lobe removal. Anesthetized zebrafish are placed snugly in the groove of sponge to keep their gills wet and their bodies immobile for the duration of the procedure. Pinching the skin just posterior to the heart makes it possible to place an incision in the skin without damaging any of the internal organs. Opening a large (3-4 mm) incision makes removal of the liver and assessing the degree of removal straightforward (Figure 2C). Importantly, zebrafish can recover from and ultimately heal a wound of this size without any primary wound closure. By squeezing the sponge and compressing the body wall, the visceral organs become more accessible and it is possible to slide the tines of a forceps in between the liver and the intestine. The forceps can then be used to sever the portal connections between the liver and the intestine. Once this has been achieved, fine forceps can be used to peel back the ventral lobe so that it can be separated from the rest of the liver.
We measured the liver to body weight ratios (LBR) of animals subjected to sham and PHX surgeries. We found that by 7 dpi, the LBR of PHX animals was comparable to controls (Figure 3F). Given that the ventral lobe had not regenerated at this point (Figure 3E), we inferred that the regeneration occurred through compensation in the dorsal lobes. To address the question of whether the ventral lobe can ultimately regenerate in zebrafish, we performed PHX and examined liver re-growth. On average, the ratio of the length of the ventral lobe to the intestine was higher at 36 dpi than at 1 dpi, indicating regeneration of the ventral lobe (Figure 4C-D). There is a wide variation in animal response to the injuries, with some animals experiencing very little growth and others experiencing substantial recovery of the ventral lobe (Figure 4E). We conclude that the liver can regenerate with a mixture of epimorphic regeneration of the ventral lobe and compensatory regeneration in the dorsal lobes, albeit at different timescales. As prior studies have used partial hepatectomies to assay to role of individual genes5,7,9 and signaling pathways6,8,10 after resection, we anticipate that this protocol will advance our understanding of the molecular and cellular mechanisms of liver regeneration in an adult vertebrate.
Table of Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| Dumont #55 Forceps | Fine Science Tools | 11295-51 | |
| Epifluorescence microscope | Zeiss | Discovery.V8 | |
| Mastertop Cellulose Cleaning Scrub Sponge | Amazon | B07CBSM53Z | |
| Super Fine Micro Scissors, 3 1/4" straight | Biomedical Research Instruments | 11-1020 | |
| Tricaine methanesulfonate | Syndel | TRIC-M-GR-0010 | |
| 16% Paraformaldehyde Aqueous Solution, EM Grade | Electron Microscopy Sciences | 15700 | |
| PBS10X Liquid Conc 4L | EMD Millipore | 6505-4L | |
| Tween™ 20, Fisher BioReagents | Fischer Scientific | BP337-500 | |
| EMS Kuehne Coverglass/Specimen Forceps | Electron Microscopy Sciences | 72997-07 | |
| 50 mL Falcon® Centrifuge Tubes, Polypropylene, Sterile | Corning | 352098 | |
| AS 82/220.R2 PLUS Analytical Balance | Bay State Scale & Systems, INC. | WL-104-1051 |
ACKNOWLEDGMENTS:
I.M.O. is supported by the NIAAA (F32AA027135). W.G. is supported by R01DK090311, R01DK105198, R24OD017870, and the Claudia Adams Barr Program for Excellence in Cancer Research. W.G. is a Pew Scholar in Biomedical Sciences.
Footnotes
DISCLOSURES:
The authors declare that they have no competing financial interests.
REFERENCES:
- 1.Michalopoulos GK Principles of liver regeneration and growth homeostasis. Comprehensive Physiology 3, 485–513 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Wang S, Miller SR, Ober EA and Sadler KC Making It New Again: Insight Into Liver Development, Regeneration, and Disease From Zebrafish Research. Current Topics in Developmental Biology vol. 124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalopoulos GK and Bhushan B Liver regeneration: biological and pathological mechanisms and implications. Nature Reviews Gastroenterology and Hepatology (2020) doi: 10.1038/s41575-020-0342-4. [DOI] [PubMed] [Google Scholar]
- 4.Gemberling M, Bailey TJ, Hyde DR and Poss KD The zebrafish as a model for complex tissue regeneration. Trends in Genetics 29, 611–620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadler KC, Krahn KN, Gaur NA and Ukomadu C Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator, uhrf1. Proceedings of the National Academy of Sciences 104, 1570–1575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goessling W et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Developmental Biology 320, 161–174 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Dovey M et al. Topoisomerase II Is Required for Embryonic Development and Liver Regeneration in Zebrafish. Molecular and Cellular Biology 29, 3746–3753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kan NG, Junghans D and Belmonte JCI Compensatory growth mechanisms regulated by BMP and FGF signaling mediate liver regeneration in zebrafish after partial hepatectomy. The FASEB Journal 23, 3516–3525 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z, Chen J, Xiong JW and Peng J Haploinsufficiency of Def activates p53-dependent TGFβ signalling and causes scar formation after partial hepatectomy. PLoS ONE 9, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng G, Long Y, Peng J, Li Q and Cui Z Transcriptomic characterization of the dorsal lobes after hepatectomy of the ventral lobe in zebrafish. BMC Genomics 16, 979 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalopoulos GK Liver regeneration. Journal of cellular physiology 213, 286–300 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grisham JW Organizational Principles of the Liver. The Liver: Biology and Pathobiology: Fifth Edition 1–15 (2009) doi: 10.1002/9780470747919.ch1. [DOI] [Google Scholar]