Abstract
The coronavirus disease 2019 (COVID-19) has spread rapidly as a pandemic around the world. In addition to severe acute respiratory syndrome, more and more studies have focused on the complication of COVID-19, especially ischemic stroke. Here, we propose several pathophysiological processes and possible mechanisms underlying ischemic stroke after COVID-19 for early prevention and better treatment of COVID-19-related stroke.
Keywords: COVID-19, ischemic stroke, SARS-CoV-2, coagulopathy, ACE2
The coronavirus disease 2019 (COVID-19) pandemic represents a challenging issue worldwide. The new coronavirus in this outbreak called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a lineage of β-CoVs and identified as the seventh human-infected corona viruse. Coronaviruses (CoVs) are a subfamily of single-stranded positive-sense RNA viruses which belong to the coronavirinae family [1]. Accroding to phylogenetic clustering, CoVs are classified into α, β, γ, and δ. Among them, α-CoVs (HCoV-NL63, HCoV-229E, HCoV-HKU2, HCoV-OC43) and β-CoVs (SARS-CoV, MERS-CoV) possess the ability to infect mammal and human cells and cause respiratory diseases [2]. α-CoVs infection is mild and self-limiting, accounting for 15% to 30% common cold, whlie β-CoVs infection is responsible for severe respiratory diseases, such as SARS and MERS [3].
This outbreak of COVID-19 has the characteristics of low pathogenicity and high transmissibility compared to the SARS in 2003 and MERS in 2012 [4]. Similar to MERS and SARS, the typical symptoms of COVID-19 are various including fever, breathing difficulties, shortness of breath, cough, and fatigue. Despite the characteristic symptoms of respiratory distress, patients with COVID-19 also have neurological manifestations, especially ischemic stroke (Table 1), which has attracted considerable public attention. However, the potential mechanisms underlying COVID-19-related stroke remain unknown.
Table 1.
Articles on Stroke in COVID-19.
| No. | Title | Type | Main points | Date | City, Country |
|---|---|---|---|---|---|
| 1 | Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Corona virus-infected Pneumonia in Wuhan | case series (n=138) | 5.1% (7/138) of COVID19 patients have a comorbidity with cerebrovascular disease | 2020.01.01-2020.02.03, | Wuhan, China |
| 2 | Neurologic Manifestation of Hospitalized Patients with Coronavirus Disease 2019 In Wuhan, china | systemic study (n=214) |
78/214 patients (36.4%) had neurologic manifestations. Acute cerebrovascular disease (mainly ischemic stroke) was more common among 88 patients with severe COVID-19 than those with no severe disease (5.7% vs 0.8%). | 2020.01.16-2020.02.19 | Wuhan, China |
| 3 | Stroke in Patients with Sars-cov-2 Infection: Case Series | case series (n=6) |
Six patients developed acute stroke during COVID-19 infection | 2020.03.16-2020.04.15 | Brescia or Udine, Italy |
| 4 | Acute Ischemic Stroke Complicating Common Carotid Artery Thrombosis During A Severe COVID -19 Infection | case report (n=1) |
the first case of acute brain infarction due to common carotid artery thrombus in the course of a severe COVID-19 infection | 2020.03.25 | Toulouse, France |
| 5 | Neurologic Features in Severe Sars-cov-2 Infection | systemic study(n=64) | 58 patients with severe COVID-19 infection had neurologic manifestations | 2020.03.03-2020.04.03 | Strasbourg, France |
| 6 | Large-vessel Stroke as A Presenting Feature Of COVID-19 In the Young | case series (n=5) |
Five cases of large-vessel stroke in patients younger than 50 with COVID-19 | 2020.03.23-2020.04.07 | New York, USA |
| 7 | Venous and Arterial Thromboembolic Complications In COVID19 Patients Admitted To An Academic Hospital In Milan, Italy | systemic study (n=388) | 2.5% (9/388) COVID-19 infected patients were diagnosed ischemic stroke | 2020.02.13-2020.04.10 | Milan, Italy |
| 8 | Characteristics of Ischemic Stroke Associated With COVID-19 | case series (n=6) | COVID-19 associated ischemic stroke is usually delayed | 2020.04.01-2020.04.16 | London, UK |
| 9 | COVID-19 Presenting as Stroke. | case series (n=4) | A series of 4 COVID-19 patients presented cerebrovascular accident in early stages of illness | Not mentioned | NY, USA |
| 10 | Cerebrovascular Disease In COVID-19 | case report (n=1) | The first reported case of COVID-19-related cerebral infarcts including brain imaging at multiple time points and CT angiographic imaging | Not mentioned | Pennsylvania, USA |
| 11 | Stroke in A Young COVID-19 Patient | case report (n=1) | a case of large-vessel stroke after COVID-19 infection | Not mentioned | Bridgeport, USA |
| 12 | Hemorrhagic Stroke And COVID-19 Infection: Coincidence or Causality? | case report (n=1) | a case of first-time intracerebral hemorrhage in a patient with APP gene duplication | 2020.09.22 | Petropolis, Brazil |
| 13 | Pediatric Stroke Associated with A Sedentary Lifestyle During the Sars-cov-2 (COVID-19) Pandemic: A Case Report on A 17-year-old | case report (n=1) | a case associated with a sedentary lifestyle | 2020.10.28 | Los Angeles, USA |
| 14 | The Impact Of COVID-19 On Ischemic Stroke | case report (n=1) | a case of right limb weakness with COVID-19 | 2020.06.29 | Wuhan, China |
| 15 | Hemorrhagic stroke in setting of severe COVID-19 Infection Requiring Extracorporeal Membrane Oxygenation (ECMO) | case report (n=1) | a case required ECMO support | 2020.08.19 | Florida, USA |
| 16 | Acute ischemic and hemorrhagic stroke in two Covid-19 Patients | case series (n=2) | two cases with coincident presentation of COVID-19 and cerebrovascular accident | 2020.10.30 | Italy |
| 17 | COVID-19 and stroke: casual or causal role? | case report (n=1) | a case of stroke in a 62-year-old COVID-19-positive patient, with multiple vascular risk factors. | 2020.07.08 | Frosinone, Italy |
| 18 | Ischemic-hemorrhagic stroke in patients with Covid-19 | case report (n=2) | 2 cases of patients infected with severe Covid-19 that were hospitalized in the Reanimation Unit that presented cerebrovascular symptoms and died afterwards | 2020.10.06 | Bizkaia, Espada |
| 19 | Complicacion Trombotica De Neumonia Grave Por COVID-19: Ictus Por Embolismo Paradojico Atipico. | case report (n=1) | a case with a central venous catheter, with a large vessel ischemic stroke, treated with mechanical thrombectomy for an atypical paradoxical embolism while in intensive care for bilateral COVID-19 pneumonia | 2020.07.31 | Barcelona, Espana |
| 20 | Clinical course of a 66-year-old man with an acute ischemic stroke in the setting of a COVID-19 Infection | case report (n=1) | A case with COVID-19 suffered from a right frontal cerebral infarct producing left-sided weakness and a deterioration in his speech pattern. | 2020.08.28 | Sutton-in-Ashfield, UK |
| 21 | COVID-19 is an independent risk factor for acute ischemic stroke | systemic study(n=123) | After adjusting for age, sex, and risk factors, COVID-19 infection had a significant independent association with acute ischemic stroke compared with control subjects (OR, 3.9; 95% CI, 1.7-8.9; P = .001) | 2020.03.16-2020.04.05 | New York, USA |
| 22 | Multiple embolic stroke on magnetic resonance imaging of the brain in a covid-19 case with persistent encephalopathy | case report | a case of multifocal ischemic stroke in a patient with COVID-19 | 2020.10.11 | WV, USA |
| 23 | Characteristics and Outcomes of COVID-19 Associated Stroke: A UK Multicenter Case-control Study | systemic study(n=1470) | Cases with ischemic stroke patients (admitted during the period of COVID-19 between 9 March and 5 July 2020) were more likely than ischemic controls (patients admitted during the same time period who never had evidence of COVID-19) to occur in Asians (18.8% vs 6.7%, p<0.0002) | 2020.03.07-2020.07.05 | London, UK |
| 24 | Unusual Pattern of Arterial Macrothrombosis Causing Stroke in A Young Adult Recovered From COVID-19 | case report (n=1) | a case with no significant past medical history who recently recovered from a mild COVID-19 infection and presented with unusual pattern of arterial macrothrombosis causing AIS | 2020.10.12 | Los Angeles, USA |
| 25 | Middle Cerebral Artery Ischemic Stroke and COVID-19: A Case Report | case report (n=1) | a case of a patient with SARS-CoV-2 infection and respiratory symptoms, complicated with a pro-thrombotic state involving multiple vascular territories and concomitant interleukin-6 increase | 2020.09.10 | Modena, Italy |
| 26 | Massive Bilateral Stroke in A COVID-19 Patient | case report (n=1) | a case of a woman with COVID-19 who suffered massive and bilateral middle cerebral artery strokes | 2020.08.21 | Birmingham, UK |
| 27 | Acute Ischemic and Hemorrhagic Stroke and COVID-19: Case Series | case series (n=5) | 5 cases of adults with COVID-19 indicate that COVID-19 may damage blood vessels in the brain and lead to stroke | 2020.10.08 | Sanandaj, Iran |
The characteristics of COVID-19 patients with ischemic stroke
Ischemic stroke is more likely to happen in severe cases
Studies have gradually uncovered the link between COVID-19 and ischemic stroke. Mao et al. first described that 36.4% of patients hospitalized with COVID-19 diagnosis exhibited neurological manifestations, and 4 in 88 cases (4.5%) with severe COVID-19 were affected by ischemic stroke, which was a higher frequency than that in mild cases (1 in 126 cases, 0.8%) [5]. Similarly, a higher proportion(67%) of cases with COVID-19 suffered from neurological feature in France, and this group revealed the association between disease severity and ischemic stroke incidence: 3 of 13 patients (23%) with severe COVID-19 had ischemic strokes [6].
Younger patients are also victims
Five cases of large-vessel stroke in patients younger than 50 years of age following SARS-CoV-2 infection have been reported in Mount Sinai Health System in New York City [7]. Increasing evidence suggests that ischemic stroke may occur after SARS-CoV-2 infection, even in young patients [8-11].
Acute ischemic stroke is the most common type of cerebrovascular diseases after COVID-19
Recently, Nannoni et al. reviewed published articles on acute cerebrovascular diseases (CVD) after COVID-19 (December 2019-September 2020) and found that among 108,571 patients with COVID-19, 1.4% of patients suffered from acute CVD. The most common manifestation in CVD was acute ischemic stroke (87.4%) [9,12]. Ischemic stroke could be the first manifestation after SARS-CoV-2 infection [8]. The incidence of COVID-19-related hemorrhagic stroke did not differ from that of non-COVID-19-hemorrhagic stroke [13].
COVID-19-related stroke has low incidence but poor outcome
As mentioned above, although the incidence of acute ischemic stroke was relatively low during hospitalization due to COVID-19, ranging from 1% to 6%, the mortality associated with it is substantially high, reaching as high as 38% [14,15]. Multivariate analysis demonstrated that patients with COVID-19 have more severe strokes and poorer outcomes compared with non-COVID-19 stroke [16]. Without other risk factors, ischemic stroke was an uncommon complication, exclusive of patients with a severe pulmonary injury. The presence of COVID-19 in patients who underwent EVT was an independent predictor of in-hospital mortality [14]. The presence of COVID-19 has been proved as an independent predictor of in-hospital mortality in ischemic stroke patients who underwent endovascular treatment [14].
These cases confirmed the connection between COVID-19 and ischemic stroke. Therefore, we conducted a commentary to investigate the possible pathophysiology of ischemic stroke after SARS-CoV-2 infection.
Pathogenic factors of SARS-CoV-2
Pathways of SARS-CoV-2 to invade the brain
The SARS-CoV-2 nucleotide sequence is 82% identical with that of human SARS-CoV and 50% identical with that of MERS-CoV [17-19]. SARS-CoV-2 has been identified in cerebrospinal fluid by polymerase chain reaction [20]. Several studies have suggested that SARS-CoV-2, like most coronaviruses, is neurotropic [21]. SARS-CoV-2 can plausibly invade the brain via several routes. First, SARS-CoV-2 binds to the angiotensin converting enzyme 2 (ACE2) receptor on the endothelial cells, which comprise the main component of the blood-brain barrier (BBB). SARS-CoV-2 transport across the vascular endothelium impairs the BBB and further enables virus invasion into the brain, where SARS-CoV-2 interacts with ACE2 on the surface of neurons, causing damage to the nervous system [22]. Second, SARS-CoV-2 passes across the BBB through adhesion to and subsequent infection of ACE2-expressing leukocytes, termed the Trojan horse mechanism [23]. Third, SARS-CoV-2 binds to the ACE2 receptor on the olfactory epithelium in the nasal cavity and invades the brain through the sieve plate near the olfactory bulb. This route is supported by observations of isolated anosmia and ageusia with or without respiratory symptoms [24,25].
ACE2, the door for SARS-CoV-2 to enter the body
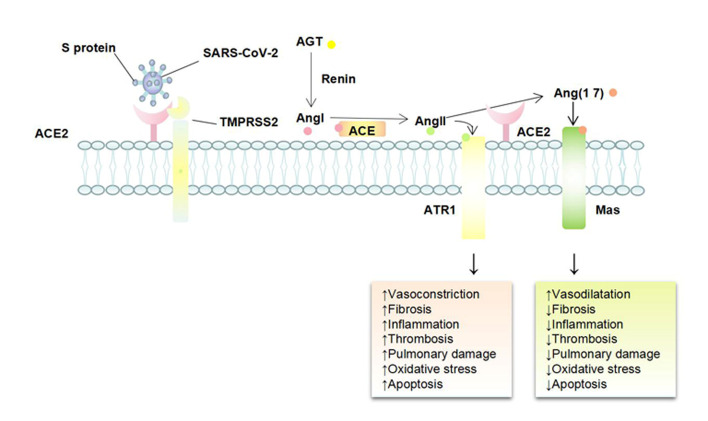
Recently, ACE2 was identified as the receptor for SARS-CoV-2 that caused the COVID-19 pandemic. SARS-CoV and SARS-CoV-2, which both can bind to ACE2 receptors, have 76% homology in amino acid sequence [26], but the affinity of ACE2 for SARS-CoV-2 is 10-20 times higher than that of SARS-CoV, which explains why SARS-CoV-2 is more infectious. In the brain, ACE2 is expressed in several cell types, especially in cerebral vascular endothelial cells [27]. As shown in Figure 1, Following SARS-CoV-2 binds to ACE2, the membrane receptor ACE2 is functionally removed from the outer membrane, resulting in the downregulation of ACE2 surface expression [28]. Therefore, the ACE2-Ang-(1-7)-Mas receptor axis is substantially weakened, whereas the ACE-angiotensin II (Ang II)-Ang II receptor 1 (AT1R) axis mediating vasoconstriction, neuroinflammation, oxidative stress, apoptosis, and cell proliferation functions is relatively strengthened. In addition to protecting the brain from inflammation, apoptosis, and oxidative stress, Ang-(1-7) and the Mas receptor can also reduce platelet proliferation and glycoprotein VI activation by increasing nitric oxide (NO) and prostacyclin, thereby inhibiting thrombosis [29].
Figure 1.
Mechanism of SARS-CoV-2 invasion and its effects on the vascular endothelium. Angiotensin converting enzyme 2 (ACE2), a dipeptidyl carboxypeptidase and a homolog of ACE, is an essential negative regulator of the renin-angiotensin system. Renin cleaves angiotensinogen (AGT) into angiotensin I (Ang I), which is hydrolyzed by ACE to angiotensin II (Ang II). Ang II has a high affinity to Ang II receptor 1 (AT1R) and plays a major physiological role in mediating vasoconstriction, neuroinflammation, oxidative stress, apoptosis, and cell proliferation, known as the classic ACE-Ang II-AT1R axis. ACE2 cleaves Ang II into Ang-(1-7), which effectively binds to the Mas receptor and counteracts adverse effects of the ACE-Ang II-AT1R axis, known as the ACE2-Ang-(1-7)-Mas receptor axis.
Taken together, SARS-CoV-2-mediated loss of ACE2 impairs endothelial cell function, leading to the occurrence or worsening of acute ischemic stroke [30].
COVID-19 and thrombosis
Thrombotic events include both venous thromboembolism (VTE) (deep vein thrombosis [DVT] and pulmonary embolism [PE]) and arterial thrombosis (myocardial infarction [MI], ischemic stroke, and others systemic thromboembolism). Thrombosis is commonly happened in acute infections, such as SARS [31,32] and H1N1 influenza [33,34]. The thrombotic incidence appears higher in COVID-19, even with the utilization of thromboprophylaxis [35]. A study assessed hospitalized patients with COVID-19 in a New York city health system demonstrated that thrombotic events occurred in 16.0% patients [36].
The incidence of venous thrombotic events is higher than arterial. A pooled analysis including thirty-five observational studies, showed that the pooled incidence of VTE was up to 41.9% and the pooled incidence of arterial thrombosis was 11.3% [37]. Klok et al. studied 184 ICU patients with COVID-19 and showed that PE was the most frequent thrombotic complication (81%) [38]. As to arterial thrombotic events, Modin et al found that 44 and 17 in 5119 COVID-19 patients suffered from ischemic stroke and AMI, respectively [39].
Higher incidence of thrombotic events was observed in COVID-19 patients with a severe condition [36,40]. Bilaloglu et al reported 244 in 829 ICU patients (29.4%) and 289 in 2505 non-ICU patients (11.5%) underwent a thrombotic event [36]. COVID-19 patients diagnosed with thrombotic complications were at higher risk of all-cause death (HR 5.4; 95% CI 2.4-12) [41]. Some researchers pointed out that PE was the direct cause of death behind COVID-19 [42]. In severe COVID-19 patients, the significant increase of D-dimer is a good indicator for identifying high-risk group of thrombotic complications [43], especially for PE [44]. Increased D-dimer concentrations of greater than 1.0 μg/ml effectively predict the risk of VTE in COVID-19 patients [45]. D-dimer level-guided aggressive thromboprophylaxis treatments in patients with COVID-19 has essential clinical value [46].
COVID-19 is an endothelial disease, in the end. Small fibrinous thrombi were observed in small pulmonary vessels in areas of both damaged and more preserved lung parenchyma from severe COVID-19 patients [47-49]. Two studies involved 10 and 11 decedents both found thrombosis and microangiopathy in the small vessels and capillaries of the lungs, which may lead to death [50,51]. The high incidence of thrombotic events suggests an important role of SARS-CoV-2-induced endothelial injury.
There are three triggers for thrombosis: hyper-coagulability, endothelial damage, and abnormal hemodynamics. SARS-CoV-2 infection and inflammatory reactions lead to vascular damage, hyper-coagulability, thrombin activation, platelet aggregation, as well as plaque shedding due to hemodynamic changes, thereby promoting the occurrence of ischemic stroke.
The potential thrombosis pathogenesis behind COVID-19-related stroke
As described above, many papers have reported that patients with COVID-19 are at increased risk of thrombosis, which results in COVID-19-related stroke. We summarize several pathogeneses and propose the potential mechanisms.
Coagulation dysfunction
Most patients with COVID-19, especially severe cases and critically ill patients, have varying degrees of coagulation dysfunction. Abnormal coagulation parameters are predictors of poor prognosis in COVID-19 patients [52]. Patients with COVID-19-related stroke have significantly higher D-dimer levels and blood viscosity than those with stroke alone [53]. A retrospective study of 191 COVID-19 patients in Wuhan demonstrated that 42% of patients with COVID-19 and 81% of deceased patients with COVID-19 had D-dimer levels higher than 1.0 μg/mL [54]. Chen et al. reported that 36 of 99 (36%) non-severe COVID-19 patients exhibited elevated D-dimer levels [55]. Tang et al. reported that non-survivors had significantly higher D-dimer and fibrin degradation products (FDP) than survivors. In the late stage of the disease, 71.4% of non-survivors and 0.6% of survivors had different degrees of disseminated intravascular coagulation, due to the excessive consumption of coagulation factors [56]. These findings suggest that coagulation disorders in COVID-19 patients are closely related to the severity of the disease.
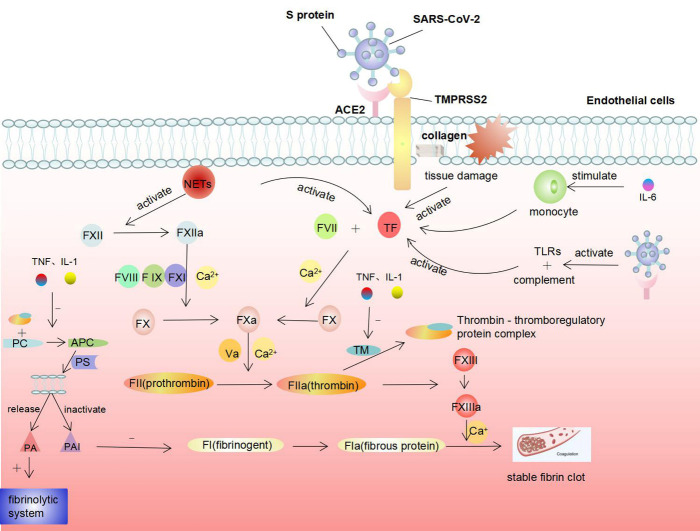
Coagulation and anticoagulation systems include platelet thrombus formation, fibrin formation, and fibrinolysis. Under physiological conditions, anti-coagulation is the main factor that ensures normal blood flow. Upon vascular endothelium damage, the subendothelial collagen is exposed to blood, thereby activating the coagulation system [57], resulting in a large amount of thrombin generation and fibrin deposition, which is the key factor of hypercoagulability. We suspect five mechanisms behind SARS-CoV-2-mediated coagulopathy (Fig. 2).
Figure 2.
Mechanisms of coagulation dysfunction triggered by SARS-CoV-2.
(1)SARS-CoV-2 directly damages the endothelium and activates coagulation.
SARS-CoV-2 directly damages endothelial cells in various organs [58]. Autopsies have shown that SARS-CoV-2 virus particles are present in capillary endothelial cells, resulting in the disruption of their tight junctions, cell swelling, and loss of contact with the basement membrane, and endotheliitis [58,59]. After a vascular endothelial injury, the subendothelial collagens are exposed and release tissue factor (TF), triggering an exogenous coagulation cascade that leads to thrombin production and fibrin deposition [60].
(2)Excessive cytokines activate coagulation.
Excessive production and release of cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6 are characteristic of COVID-19. TNF and IL-1 promote blood coagulation by inhibiting the expression of thromboregulatory protein (TM) and reducing the production of activated protein C (APC), thus promoting the coagulation reaction [61,62]. TM is expressed on the surface of endothelial cells and forms a thrombin-thrombomodulin complex, inhibiting the activity of thrombin. Moreover, the complex can also activate APC. APC is a strong anticoagulant that promotes the release of tissue-type plasminogen activator, thereby activating the fibrin dissolution system and inactivating tissue plasminogen activator inhibitor [62].
IL-6 stimulates fibrinogen synthesis in the liver and induces megakaryocytes to produce a large number of platelets, both of which can promote the coagulation reaction. IL-6 can also stimulate monocytes to release TF, thereby initiating the exogenous coagulation cascade [63].
(3)SARS-CoV-2 activates the body's immune response, leading to activation of coagulation.
Thrombosis is one of the ways in which innate immunity strives to reduce the spread of infection [64]. After infecting the human body, SARS-CoV-2 is recognized by pathogen-associated molecular patterns (PAMP) receptors like Toll-like receptors (TLRs), initiating innate immune system. In addition, the complement system is activated after SARS-CoV-2 infection. C3a and C5 cleavage products (C5a and C5b) collectively influence many aspects of coagulation, including the activation of TF, the release of von Willebrand factor (vWF) from endothelial cells, and enhancement of P-selectin exposure [65].
Peripheral lymphocyte counts (mainly CD4+ and CD8+ T cells) were consistently and significantly reduced in COVID-19 patients, especially in severe cases, suggesting that the adaptive immunity is also weakened [54,66]. The degree of lymphopenia is correlated with the severity of COVID-19 [67]. SARS-CoV-2 can directly invade lymphocytes, particularly T cells, resulting in a compromised antiviral response due to a reduction in the number of lymphocytes [66]. However, it was demonstrated that although the counts of peripheral T cells were substantially reduced, their status was highly pro-inflammatory, manifested by increased numbers of Th17 cells and high cytotoxicity of CD8 T cells [48]. Dysregulation of T cell subsets can also increase the secretion of negative hematopoietic regulators (IL-2, interferon [IFN]-γ, and TNF), leading to coagulation dysfunction.
(4)Neutrophils are involved in the activation of the coagulation cascade.
Stimulated by inflammatory cytokines or infectious pathogens, neutrophil extracellular traps (NETs) are released from neutrophils to capture pathogens [68]. This fibrous network provides a scaffold for platelets to adhere to and capture erythrocytes and leukocytes, leading to platelet aggregation and thrombosis. Autopsies of deceased COVID-19 patients revealed neutrophil infiltration in pulmonary capillaries [68] and the level of NETs in the blood of COVID-19 patients is increased [69]. NETs can directly activate factor XII and TF and initiate an exogenous coagulation cascade. Moreover, histones in NETs also function as ligands for platelet TLRs to promote platelet activation and thrombosis [70].
(5)Extramedullary megakaryocytes induce microvascular thrombosis.
Extramedullary megakaryocytes are present in the microvessels of most organs. The number of pulmonary megakaryocytes is increased by infection, impairing the respiratory system and the circulatory system [71]. Approximately 90% of lung-derived megakaryocytes are present in the pulmonary microcirculation. However, in cases of severe infection, a large proportion of megakaryocytes leave the lungs and enter the arterial circulation [72]. Megakaryocytes in the peripheral circulation can produce platelets, resulting in microvascular thrombosis in COVID-19 patients.
In summary, coagulation dysfunction increases the risk of thrombosis in COVID-19 patient.
Role of cytokine storm in COVID-19-related stroke
The cytokine storm is characteristic in patients with the most severe forms of COVID-19. Huang et al. first noted elevated levels of pro-in?ammatory cytokines, such as IL-1, TNF-α, IFN-γ, IP-10, and MCP-1, in the serum of patients with COVID-19 [54]. Subsequently, Mehta et al. confirmed the increase of inflammatory cytokines (IL-6, IL-10, IL-2, and IFN-γ) in severe cases of COVID-19 [73]. Compared to non-ICU patients, ICU patients have higher concentrations of G-CSF, IP-10, MCP-1, TNF-α, and IL-6 [74].
The cytokine storm, also called hypercytokinemia, is the phenomenon of the aggressive release of pro-inflammatory cytokines and insufficient control of anti-inflammatory responses due to immune dysfunction [75]. Under normal circumstances, the immune system can respond to external stimuli by secreting cytokines, helping the body to overcome the attack by pathogens. Following SARS-CoV-2 invasion, the virus is first recognized by innate immune system and activates immune cells, such as macrophages and dendritic cells (DCs), which can phagocytose and hydrolyse the virus [76]. Macrophages can release TNF-α in an endocrine manner, and dendritic cells secrete IL-12 and IL-6 in a paracrine manner [77]. Once the virus escapes recognition by the innate immune system, it is recognized by cell surface RNA pattern recognition receptors and form a protein complex, which promotes the translocation of transcription factors and upregulates the expression of pro-inflammatory factors. The release of cytokines facilitates the recruitment of more immune cells and secretion of more cytokines, thereby forming a positive feedback loop to continuously amplify the inflammatory response. This positive feedback results in two outcomes: 1) Pro-inflammatory cytokines are massively produced, disrupting the balance between pro- and anti-inflammatory cytokines. 2) Due to excessive response by the immune system, the cytokines start to attack healthy tissues rather than the virus, causing a systemic inflammatory response.
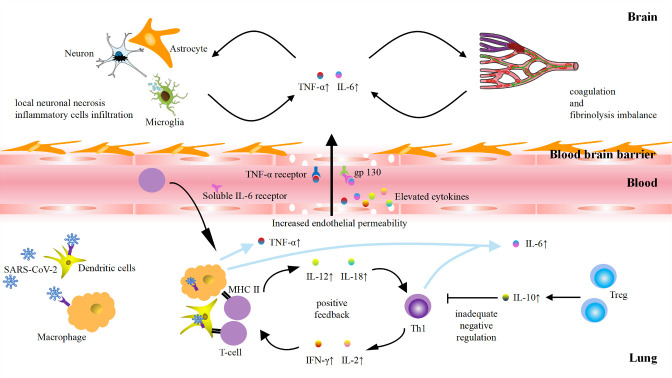
We hypothesize three possible mechanisms underlying cytokine storm-induced strokes after SARS-CoV-2 infection (Fig. 3). 1) Endothelial cell dysfunction leads to a disruption of the BBB. Brain endothelial cells express TNF-α and IL-6 receptors that may mediate local cell dysfunction [78], inducing the rupture of the BBB. Therefore, the integrity of the BBB is altered with increased permeability, leading to an elevated concentration of pro-inflammatory cytokines in the brain parenchyma [79]. 2) Excessive cytokines directly cause local neuronal necrosis. Enhanced permeability of the BBB enables excessive cytokine levels in the brain parenchyma, directly cause local neuronal necrosis. Necrosis may also recruit more infiltrating inflammatory cells around neurons to induce a stroke. 3) Injured endothelial cells induce coagulation and fibrinolysis imbalance. The injured endothelial cells induced by cytokines, in turn, release inflammatory factors in excess. This positive feedback promotes platelet activation and fibrinogen deposition, as well as inhibit fibrinolysis and thrombomodulin activity, leading to the imbalance of coagulation and fibrinolysis. The endothelial cells transform from an anti- to a procoagulant state, thereby facilitating thrombosis.
Figure 3.
Possible mechanisms of the cytokines storm in COVID-19-associated stroke.
Antiphospholipid antibodies
Antiphospholipid antibodies (aPLs) mainly include anticardiolipin antibodies (aCL), anti-β2GPi antibodies (anti-β2GPi), and lupus anticoagulant (LAC). Harzallah et al. reported that 25 of 56 (45%) of patients with COVID-19 were LAC-positive, and 5 (10%) of the patients were positive for aCL or anti-β2GPi antibodies [80]. In Wuhan, China, Zhang and colleagues first described that three COVID-19 patients with ischemic stroke were positive for aCL and anti-β2GPi antibodies [81]. Beyrouti et al. reported that five COVID-19 patients with ischemic stroke were LAC-positive [82]. These cases aroused our curiosity regarding the relationship between aPL and COVID-19-related stroke.
Among aPLs, LAC, the complex of aPL and plasma proteins (mainly β2-glycoproteins), is the greatest risk factor for arterial and venous thrombosis [83] and β2GPi is the main binding cofactor for these antibodies [84]. Patients positive for LAC, aCL, and anti-β2GPi antibodies, called triple-positive patients, have incidence rates for VTE of 9.8% (after 2 years) and 37.1% (after 10 years) during the follow-up period [85].
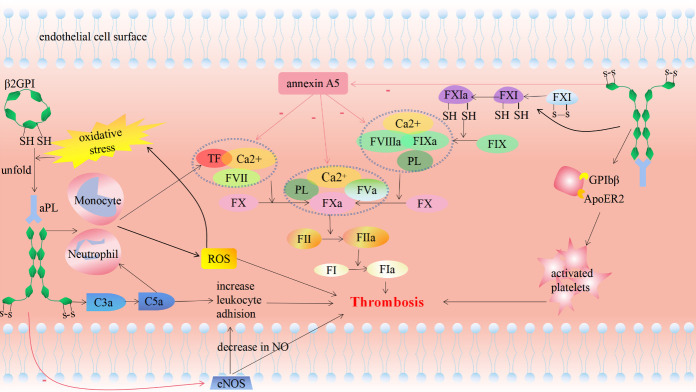
The pathogenesis of aPL behind COVID-19-related stroke is multifaceted (Fig. 4). 1) Increased oxidative stress: Autoantibodies can disrupt mitochondrial function of monocytes and neutrophils, resulting in excessive reactive oxygen species (ROS) release in patients [86,87]. Following ROS stimulation, free thiols of β2GPi form disulfide bonds, and the ring conformation of β2GPi unfolds, exposing the normally shielded epitopes, thereby inducing autoantibody formation [88]. Thus, a positive feedback is formed to further promote oxidative stress. Increased levels of oxidative stress mediate damage to cell structures and serve as a trigger for ischemic stroke [89]. 2) Changes in coagulation factors: aPLs upregulate the release of TF, which is the key promoters of the exogenous coagulation cascade, from monocytes [86], neutrophils [87] and endothelial cells [90]. Furthermore, aPLs increase the level of coagulation factor Ⅺ containing free thiols (reduced factor Ⅺ), which is more prone to be activated by thrombin, factor Ⅻa, or factor Ⅺa compared to its unreduced form [91], participating in the endogenous coagulation pathway. LAC activates thrombin by promoting the binding of prothrombin to surface phospholipids and affecting its affinity [92]. 3) Platelet activation: Anti-β2GPi cross-links vWF receptor glycoprotein ibα and ApoE receptor 2 to enhance platelet activation, promote thromboxane A2 (TXA2) release, and increase platelet adhesion [93]. Platelet activation initiates thrombus formation [94]. 4) Complement activation: Complement activation is involved in antiphospholipid antibody-induced thrombosis. Activation of complement by aPL generates C3a and C5a, which mediate leukocyte adhesion and thrombus formation,[95,96]. 5) Inhibition of endothelial nitric oxide synthase (eNOS): By inhibiting eNOS activity and reducing bioavailable NO, aPL promotes leukocyte-endothelial cell adhesion and thrombosis [97]. Autopsy reports of patients with COVID-19 revealed infiltration of leukocytes in small pulmonary vessels; therefore, we hypothesized that aPL also promotes leukocyte adhesion in cerebral vessels [58]. 6) Inhibition of annexin A5: AnnexinV is a calcium-dependent protein that binds to phosphatidylserine residues, which form a shield that inhibits the formation of procoagulant complexes, including the TF-Ⅶa complex, the Ⅸa-Ⅷa complex, and the Ⅹa-Ⅴa complex [98]. The complex of anti-β2GPi and β2GPi can disrupt this shield, exposing procoagulant phosphatidylserines and, hence, promote thrombosis [99].
Figure 4.
Pathophysiological mechanisms of antiphospholipid antibodies induced thrombogenesis.
SARS-CoV-2 triggers the production of aPL by molecular mimicry and increased levels of cytokines[100]. It is reasonable to speculate that aPL serves as a potential mediator of cerebrovascular events in patients with COVID-19.
Abnormal ferritin metabolism
Ferritin was proven to be a predictor of mortality among 150 COVID-19 patients in Wuhan, since its level is significantly higher in non-survivors than in survivors [101]. Zhou et al. found that COVID-19 patients with elevated serum ferritin levels (>300 µg/L) had a 9-fold increase in pre-discharge mortality [54]. Subsequently, a growing number of researchers have recognized serum ferritin as a powerful index for COVID-19 severity, helping to identify cases with dismal prognosis [102,103]. The first reported case of COVID-19-related stroke showed that the patient’s serum ferritin level was markedly elevated [104], which has attracted researchers’ attention to the role of serum ferritin in COVID-19-related stroke.
Ferritin is an iron-binding molecule that stores major intracellular iron in all organisms [104]. A higher ferritin level is associated with an increased risk of ischemic stroke [105,106]. Ruddell et al. proposed the role of serum ferritin (mainly composed of L-ferritin subunits) as a pro-inflammatory signaling molecule in hepatic stellate cells [107]. Ferritin is involved in inflammatory/fibrotic states associated with the infection of various organs, such as the heart, lung, brain, kidney, and pancreas, all of which have cell types similar to hepatic stellate cells to mediate the fibrotic response to injury [107,108]. Serum ferritin can initiate the production of thrombus-like fibers through a variety of pathways and induce inflammation, thereby damaging cerebral blood vessels and causing neurological symptoms.
Treatment of COVID-19-related stroke
Multidisciplinary treatment of COVID-19-related stroke, including antiviral drugs, supportive therapy, and stroke treatment, has shown cheerful clinical effects. Alharthy et al. have shown that in life-threatening COVID-19, especially with immune dysregulation features such as antiphospholipid antibodies, therapeutic plasma exchange could be an effective rescue therapy [109]. Similar to stroke alone, treatment for ischemic stroke in COVID-19 patients is individualized and complicated, including thrombolysis [110,111] and thrombectomy [112,113] for acute interventions and antiplatelets and anticoagulants for secondary prevention. It should be noted that many patients with acute ischemic stroke lose the opportunity for acute intervention because of isolation and reluctance to present to the hospital [114,115]. The treatment efficacy for ischemic stroke following COVID-19 needs further evaluation. Ischemic stroke therapy in COVID-19 patients should not only be based on traditional guidelines, but on the experience and new insights from healthcare workers who are combating COVID-19 worldwide [116].
Conclusions
Despite the characteristic symptoms of respiratory distress, less than 2% of hospitalized patients with COVID-19 have an ischemic stroke [9,14,15,117]. The exact stroke pathophysiology following SARS-CoV-2 infection remains to be established by autopsies and pathology reports. Based on current knowledge, several possible mechanisms exist, including hypercoagulability, activation of the cytokine storm, excessive levels of antiphospholipid antibodies, and abnormal ferritin levels. During the lockdown period, factors such as lifestyle changes and sedentary lifestyle must be taken into consideration when assessing stroke risks [118]. It is unclear whether the patients who recover from COVID-19-related stroke experience any transient or long-lasting cerebrovascular sequelae. Clinical physicians should be aware that severe COVID-19 can lead to ischemic stroke, and close monitoring of the neurological status of COVID-19 patients is imperative.
Footnotes
Conflicts of interests
The authors declare that they do not have any conflicts of interest.
References
- [1].Weiss SR, Leibowitz JL (2011). Coronavirus pathogenesis. Adv Virus Res, 81: 85-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cui J, Li F, Shi ZL (2019). Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol, 17:181-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Su S, Wong G, Shi W, Liu J, Lai A, Zhou J, et al. (2016). Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol, 24: 490-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jiang S, Shi Z, Shu Y, Song J, Gao GF, Tan W, et al. (2020). A distinct name is needed for the new coronavirus. Lancet, 395: 949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. (2020). Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. (2020). Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med, 382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cavallieri F, Marti A, Fasano A, Dalla Salda A, Ghirarduzzi A, Moratti C, et al. (2020). Prothrombotic state induced by COVID-19 infection as trigger for stroke in young patients: A dangerous association. eNeurologicalSci, 20: 100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nannoni S, de Groot R, Bell S, Markus HS (2020). Stroke in COVID-19: A systematic review and meta-analysis. Int J Stroke, 1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rameez F, McCarthy P, Cheng Y, Packard LM, Davis AT, Wees N, et al. (2020). Impact of a Stay-at-Home Order on Stroke Admission, Subtype, and Metrics during the COVID-19 Pandemic. Cerebrovasc Dis Extra, 10:159-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Majidi S, Fifi JT, Ladner TR, Lara-Reyna J, Yaeger KA, Yim B, et al. (2020). Emergent Large Vessel Occlusion Stroke During New York City's COVID-19 Outbreak: Clinical Characteristics and Paraclinical Findings. Stroke, 51:2656-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Szegedi I, Orbán-Kálmándi R, Csiba L, Bagoly Z (2020). Stroke as a Potential Complication of COVID-19-Associated Coagulopathy: A Narrative and Systematic Review of the Literature. J Clin Med, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].John S, Hussain SI, Piechowski-Jozwiak B, Dibu J, Kesav P, Bayrlee A, et al. (2020). Clinical characteristics and admission patterns of stroke patients during the COVID 19 pandemic: A single center retrospective, observational study from the Abu Dhabi, United Arab Emirates. Clin Neurol Neurosurg, 199:106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Requena M, Olivé-Gadea M, Muchada M, García-Tornel Á, Deck M, Juega J, et al. (2020). COVID-19 and Stroke: Incidence and Etiological Description in a High-Volume Center. J Stroke Cerebrovasc Dis, 29: 105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rajdev K, Lahan S, Klein K, Piquette CA, Thi M (2020). Acute Ischemic and Hemorrhagic Stroke in COVID-19: Mounting Evidence. Cureus, 12: e10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fuentes B, Alonso de Leciñana M, García-Madrona S, Díaz-Otero F, Aguirre C, Calleja P, et al. (2021). Stroke Acute Management and Outcomes During the COVID-19 Outbreak: A Cohort Study From the Madrid Stroke Network. Stroke, 52(2):552-562 [DOI] [PubMed] [Google Scholar]
- [17].Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. (2020). Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect, 9:221-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 395:565-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wan Y, Shang J, Graham R, Baric RS, Li F (2020). Receptor Recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS Coronavirus. J Virol, 94(7):e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. (2020). A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis, 94:55-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Steardo L, Steardo L Jr, Zorec R, Verkhratsky A (2020). Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol (Oxf), 229(3):e13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bohmwald K, Gálvez N, Ríos M, Kalergis AM (2018). Neurologic Alterations Due to Respiratory Virus Infections. Front Cell Neurosci, 12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ (2014). Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol, 807:75-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Baig AM, Khaleeq A, Ali U, Syeda H (2020). Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci, 11: 995-8. [DOI] [PubMed] [Google Scholar]
- [25].Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. (2020). Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis, 71(15):889-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Batlle D, Wysocki J, Satchell K (2020). Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy. Clin Sci (Lond), 134: 543-5. [DOI] [PubMed] [Google Scholar]
- [27].Chen J, Zhao Y, Chen S, Wang J, Xiao X, Ma X, et al. (2014). Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology, 79: 550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. (2005). A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med, 11: 875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fang C, Stavrou E, Schmaier AA, Grobe N, Morris M, Chen A, et al. (2013). Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood, 121: 3023-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hess DC, Eldahshan W, Rutkowski E (2020). COVID-19-Related Stroke. Transl Stroke Res, 11: 322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chong PY, Chui P, Ling AE, Franks TJ, Tai DY, Leo YS, et al. (2004). Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med, 128: 195-204. [DOI] [PubMed] [Google Scholar]
- [32].Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, et al. (2003). The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol, 200: 282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bunce PE, High SM, Nadjafi M, Stanley K, Liles WC, Christian MD (2011). Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis, 52: e14-7. [DOI] [PubMed] [Google Scholar]
- [34].Grimnes G, Isaksen T, Tichelaar Y, Brækkan SK, Hansen JB (2018). Acute infection as a trigger for incident venous thromboembolism: Results from a population-based case-crossover study. Res Pract Thromb Haemost, 2: 85-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB, et al. (2020). Registry of Arterial and Venous Thromboembolic Complications in Patients With COVID-19. J Am Coll Cardiol, 76: 2060-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS (2020). Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA, 324: 799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Patell R, Chiasakul T, Bauer E, Zwicker JI (2021). Pharmacologic Thromboprophylaxis and Thrombosis in Hospitalized Patients with COVID-19: A Pooled Analysis. Thromb Haemost, 121: 76-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Klok FA, Kruip M, van der Meer N, Arbous MS, Gommers D, Kant KM, et al. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res, 191:145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Modin D, Claggett B, Sindet-Pedersen C, Lassen M, Skaarup KG, Jensen J, et al. (2020). Acute COVID-19 and the Incidence of Ischemic Stroke and Acute Myocardial Infarction. Circulation, 142: 2080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller M, et al. (2020). Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost, 18: 1995-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Klok FA, Kruip M, van der Meer N, Arbous MS, Gommers D, Kant KM, et al. (2020). Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res, 191: 148-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. (2020). Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med, 173: 268-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cui S, Chen S, Li X, Liu S, Wang F (2020). Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost, 18: 1421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Garcia-Olivé I, Sintes H, Radua J, Abad Capa J, Rosell A (2020). D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir Med, 169: 106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Artifoni M, Danic G, Gautier G, Gicquel P, Boutoille D, Raffi F, et al. (2020). Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis, 50: 211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Price LC, McCabe C, Garfield B, Wort SJ (2020). Thrombosis and COVID-19 pneumonia: the clot thickens. Eur Respir J, 56(1): 2001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY (2020). Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in two patients with lung cancer. J Thorac Oncol, 15: 700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med, 8: 420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva L, de Oliveira EP, Saldiva P, et al. (2020). Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost, 18: 1517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS (2020). Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med, 8: 681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, et al. (2020). Pulmonary Arterial Thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann Intern Med, 173: 350-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Levi M, Toh CH, Thachil J, Watson HG (2009). Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol, 145:24-33. [DOI] [PubMed] [Google Scholar]
- [53].Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G (2020). Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta, 507: 167-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 395: 1054-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 395: 507-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tang N, Li D, Wang X, Sun Z (2020). Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost, 18: 844-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Verdecchia P, Cavallini C, Spanevello A, Angeli F (2020). The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med, 76: 14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. (2020). Endothelial cell infection and endotheliitis in COVID-19. Lancet, 395: 1417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, et al. (2020). Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J, 41: 1804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Morrissey JH (1995). Tissue factor interactions with factor VII: measurement and clinical significance of factor VIIa in plasma. Blood Coagul Fibrinolysis, 6 Suppl 1: S14-9. [PubMed] [Google Scholar]
- [61].Nawroth PP, Stern DM (1986). Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med, 163: 740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Joseph L, Fink LM, Hauer-Jensen M (2002). Cytokines in coagulation and thrombosis: a preclinical and clinical review. Blood Coagul Fibrinolysis, 13: 105-16. [DOI] [PubMed] [Google Scholar]
- [63].Tanaka T, Narazaki M, Kishimoto T (2016). Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy, 8: 959-70. [DOI] [PubMed] [Google Scholar]
- [64].Keragala CB, Draxler DF, McQuilten ZK, Medcalf RL (2018). Haemostasis and innate immunity - a complementary relationship: A review of the intricate relationship between coagulation and complement pathways. Br J Haematol, 180: 782-98. [DOI] [PubMed] [Google Scholar]
- [65].Foley JH (2016). Examining coagulation-complement crosstalk: complement activation and thrombosis. Thromb Res, 141 Suppl 2: S50-4. [DOI] [PubMed] [Google Scholar]
- [66].Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. (2020). SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet, 395: 1517-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. (2020). Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine, 55: 102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. (2020). Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. (2020). Neutrophil extracellular traps in COVID-19. JCI Insight, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. (2011). Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood, 118: 1952-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sharma GK, Talbot IC (1986). Pulmonary megakaryocytes: "missing link" between cardiovascular and respiratory disease. J Clin Pathol, 39: 969-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hansson GK, Libby P, Schönbeck U, Yan ZQ (2002). Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res, 91: 281-91. [DOI] [PubMed] [Google Scholar]
- [73].Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet, 395: 1033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395: 497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ferrara JL, Abhyankar S, Gilliland DG (1993). Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc, 25: 1216-7. [PubMed] [Google Scholar]
- [76].Channappanavar R, Perlman S (2017). Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol, 39: 529-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. (2020). Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol, 189: 428-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nadeau S, Rivest S (1999). Regulation of the gene encoding tumor necrosis factor alpha (TNF-alpha) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol, 58: 61-77. [DOI] [PubMed] [Google Scholar]
- [79].Rochfort KD, Cummins PM (2015). The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans, 43: 702-6. [DOI] [PubMed] [Google Scholar]
- [80].Harzallah I, Debliquis A, Drénou B (2020). Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost, 18(8):2064-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. (2020). Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med, 382: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. (2020). Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry, 91(8):889-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Galli M, Luciani D, Bertolini G, Barbui T (2003). Lupus anticoagulants are stronger risk factors for thrombosis than anticardiolipin antibodies in the antiphospholipid syndrome: a systematic review of the literature. Blood, 101: 1827-32. [DOI] [PubMed] [Google Scholar]
- [84].McNeil HP, Simpson RJ, Chesterman CN, Krilis SA (1990). Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: beta 2-glycoprotein I (apolipoprotein H). Proc Natl Acad Sci U S A, 87: 4120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pengo V, Ruffatti A, Legnani C, Testa S, Fierro T, Marongiu F, et al. (2011). Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood, 118: 4714-8. [DOI] [PubMed] [Google Scholar]
- [86].Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, et al. (2007). Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum, 56: 2687-97. [DOI] [PubMed] [Google Scholar]
- [87].Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, et al. (2006). A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol, 177: 4794-802. [DOI] [PubMed] [Google Scholar]
- [88].de Laat B, van Berkel M, Urbanus RT, Siregar B, de Groot PG, Gebbink MF, et al. (2011). Immune responses against domain I of β(2)-glycoprotein I are driven by conformational changes: domain I of β(2)-glycoprotein I harbors a cryptic immunogenic epitope. Arthritis Rheum, 63: 3960-8. [DOI] [PubMed] [Google Scholar]
- [89].Sugamura K, Keaney JF Jr (2011). Reactive oxygen species in cardiovascular disease. Free Radic Biol Med, 51: 978-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Vega-Ostertag M, Casper K, Swerlick R, Ferrara D, Harris EN, Pierangeli SS (2005). Involvement of p38 MAPK in the up-regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum, 52: 1545-54. [DOI] [PubMed] [Google Scholar]
- [91].Giannakopoulos B, Gao L, Qi M, Wong JW, Yu DM, Vlachoyiannopoulos PG, et al. (2012). Factor XI is a substrate for oxidoreductases: enhanced activation of reduced FXI and its role in antiphospholipid syndrome thrombosis. J Autoimmun, 39: 121-9. [DOI] [PubMed] [Google Scholar]
- [92].Rao LV, Hoang AD, Rapaport SI (1996). Mechanism and effects of the binding of lupus anticoagulant IgG and prothrombin to surface phospholipid. Blood, 88: 4173-82. [PubMed] [Google Scholar]
- [93].Urbanus RT, Pennings MT, Derksen RH, de Groot PG (2008). Platelet activation by dimeric beta2-glycoprotein I requires signaling via both glycoprotein Ibalpha and apolipoprotein E receptor 2'. J Thromb Haemost, 6: 1405-12. [DOI] [PubMed] [Google Scholar]
- [94].Holinstat M (2017). Normal platelet function. Cancer Metastasis Rev, 36: 195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R, et al. (2005). Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood, 106: 2340-6. [DOI] [PubMed] [Google Scholar]
- [96].Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J (2005). Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum, 52: 2120-4. [DOI] [PubMed] [Google Scholar]
- [97].Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, et al. (2011). Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. J Clin Invest, 121: 120-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rand JH, Wu XX, Andree HA, Lockwood CJ, Guller S, Scher J, et al. (1997). Pregnancy loss in the antiphospholipid-antibody syndrome--a possible thrombogenic mechanism. N Engl J Med, 337: 154-60. [DOI] [PubMed] [Google Scholar]
- [99].de Laat B, Wu XX, van Lummel M, Derksen RH, de Groot PG, Rand JH (2007). Correlation between antiphospholipid antibodies that recognize domain I of beta2-glycoprotein I and a reduction in the anticoagulant activity of annexin A5. Blood, 109: 1490-4. [DOI] [PubMed] [Google Scholar]
- [100].Cervera R, Asherson RA (2005). Antiphospholipid syndrome associated with infections: clinical and microbiological characteristics. Immunobiology, 210: 735-41. [DOI] [PubMed] [Google Scholar]
- [101].Ruan Q, Yang K, Wang W, Jiang L, Song J (2020). Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med, 46: 846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gill D, Monori G, Tzoulaki I, Dehghan A (2018). Iron Status and Risk of Stroke. Stroke, 49: 2815-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Alunno A, Carubbi F, Rodríguez-Carrio J (2020). Storm, typhoon, cyclone or hurricane in patients with COVID-19? Beware of the same storm that has a different origin. RMD Open, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Goldberg MF, Goldberg MF, Cerejo R, Tayal AH (2020). Cerebrovascular Disease in COVID-19. AJNR Am J Neuroradiol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gillum RF, Sempos CT, Makuc DM, Looker AC, Chien CY, Ingram DD (1996). Serum transferrin saturation, stroke incidence, and mortality in women and men. The NHANES I Epidemiologic Followup Study. National Health and Nutrition Examination Survey. Am J Epidemiol, 144: 59-68. [DOI] [PubMed] [Google Scholar]
- [106].Chang YL, Hung SH, Ling W, Lin HC, Li HC, Chung SD (2013). Association between ischemic stroke and iron-deficiency anemia: a population-based study. PLoS One, 8: e82952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ, et al. (2009). Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology, 49: 887-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV (2010). Serum ferritin: Past, present and future. Biochim Biophys Acta, 1800: 760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Alharthy A, Faqihi F, Balhamar A, Memish ZA, Karakitsos D (2020). Life-threatening COVID-19 presenting as stroke with antiphospholipid antibodies and low ADAMTS-13 activity, and the role of therapeutic plasma exchange: A case series. SAGE Open Med Case Rep, 8: 2050313X20964089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhou Y, Hong C, Chang J, Xia Y, Jin H, Li Y, et al. (2020). Intravenous thrombolysis for acute ischaemic stroke during COVID-19 pandemic in Wuhan, China: a multicentre, retrospective cohort study. J Neurol Neurosurg Psychiatry, 2(2):226-228. [DOI] [PubMed] [Google Scholar]
- [111].Carneiro T, Dashkoff J, Leung LY, Nobleza C, Marulanda-Londono E, Hathidara M, et al. (2020). Intravenous tPA for Acute Ischemic Stroke in Patients with COVID-19. J Stroke Cerebrovasc Dis, 29: 105201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mansour OY, Malik AM, Linfante I (2020). Mechanical Thrombectomy of COVID-19 positive acute ischemic stroke patient: a case report and call for preparedness. BMC Neurol, 20: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yaeger KA, Fifi JT, Lara-Reyna J, Rossitto C, Ladner T, Yim B, et al. (2020). Initial Stroke Thrombectomy Experience in New York City during the COVID-19 Pandemic. AJNR Am J Neuroradiol, 41: 1357-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Candelaresi P, Manzo V, Servillo G, Muto M, Barone P, Napoletano R, et al. (2021). The Impact of Covid-19 Lockdown on Stroke Admissions and Treatments in Campania. J Stroke Cerebrovasc Dis, 30: 105448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Rose DZ, Burgin WS, Renati S (2020). Untreated Stroke as Collateral Damage of COVID-19: "Time Is Brain" Versus "Stay at Home". Neurohospitalist, 10: 291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wang Z, Yang Y, Liang X, Gao B, Liu M, Li W, et al. (2020). COVID-19 Associated Ischemic Stroke and Hemorrhagic Stroke: Incidence, Potential Pathological Mechanism, and Management. Front Neurol, 11: 571996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, et al. (2020). Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA Neurol, 77:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lam K, Lee JH, Cheng P, Ajani Z, Salem MM, Sangha N (2020). Pediatric stroke associated with a sedentary lifestyle during the SARS-CoV-2 (COVID-19) pandemic: a case report on a 17-year-old. Neurol Sci, 42:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]