Abstract
Neonatal calf survival and health is predominantly dependent on sufficient consumption of immunoglobulin G (IgG) and the resulting transfer of passive immunity (TPI). In this study, we investigate the potential for continued IgG secretion and temporal kinetics of mammary IgG output in sequential milkings performed at 0, 4, 16, 28, 40, and 52 hr postcalving in Holstein dairy cows. For colostrum (0 hr), we also scrutinize the relationships between IgG concentration, volume, refractometer readings (Bx values, Brix) and concentration of sugars (lactose and glucose). Mammary transcripts postpartum (0 hr) indicated that active IgG secretion continues beyond the first milking (colostrum; n = 4 to 5). IgG measurements at the different timepoints indicated that colostrum represents only 25.1% of the total IgG produced across the 6 sequential milking timepoints, with a substantial 48.9% being secreted into transition milk over the next 3 timepoints (4-, 6-, and 28-hr) combined. The differences on the basis of IgG concentrations across 0-, 4-, and 16-hr milking timepoints were not statistically significant (P = 0.1522; n = 9). For colostrum, volume remained highly variable, even with induced let-down prior to milking (n = 27). Nonetheless, colostrum IgG secretion was significantly co-regulated with volume (R2 = 0.915; P < 0.001; n = 18), an association that was stronger than that measured for lactose (R2 = 0.803; P < 0.001; n = 18) and glucose (R2 = 0.467; P = 0.002; n = 17). Comparing colostrum Bx values to absolute IgG concentrations showed no correlation (R2 = 0.127; P = 0.07; n = 27); biochemical separation of colostrum components indicated that both proteins and nonprotein solutes could affect Bx values (P < 0.0001 for both; n = 5). This suggests that Bx values do not reasonably indicate IgG concentration to serve as a measure of colostrum quality. Additionally, our finding that early transition milk (4-, 6-, and 28-hr) can contribute substantially more IgG than colostrum forces a rethink of existing feeding paradigms and means to maximize TPI in calves. Collectively, our results reveal the remarkable value of early transition milk and caveats to colostrum assessments that could advance application in enhancing neonatal calf health.
Keywords: calf, dairy, immunoglobulin, mammary, nutrition
Introduction
In 1922, it was first reported that neonatal calves fed only mature milk could not survive infections (>90% mortality within 27 d after birth) while calves fed colostrum or mature milk with added adult blood serum survived (Smith and Little, 1922a; Smith and Little, 1922b). Ensuing research uncovered that survival was due to the immediate antipathogenic protection provided by maternal immunoglobulin G (IgG) (transfer of passive immunity, TPI), which could not be transmitted in utero across the epitheliochorial placenta (Smith, 1930; Smith and Little, 1930; Johnson and Pierce, 1959; McEwan et al., 1970; Fey, 1971; Boyd, 1972). Subsequent use of radial immunodiffusion (RID) to quantify IgG in colostrum/serum (Mancini et al., 1965; Michalek et al., 1975) enabled investigation into how colostrum feeding modulations (timing and quantity) affect the extent/efficiency of TPI (Kruse, 1970a; McCoy et al., 1970; Stott et al., 1979a; Stott et al., 1979b; Stott and Fellah, 1983; Besser et al., 1991; Morin et al., 1997) and resulted in the projection that neonatal calves that acquired 10 mg/mL of IgG concentration in serum by 48 hr had successful TPI (McGuire et al., 1976; Chigerwe et al., 2008a, b). Ultimately, it was recommended that this could be achieved by feeding 200 g of IgG within 6 hr after birth (Besser et al., 1991), corresponding to a single 4 L feeding of colostrum with IgG concentration 50 g/L (reviewed by Godden, 2008).
Today, this target is still the mainstream recommendation for colostrum management on modern dairy farms (Kehoe et al., 2007; Fulwider et al., 2008; USDA-APHIS, 2008; Westhoff et al., 2020) and is instructed to be achieved by indirect IgG quantification with a Brix refractometer (Bx) to classify colostrum as good quality (22 Bx 50 g/L IgG) to be fed, or poor quality (<22 Bx <50 g/L IgG) to be discarded/provided to culls (Chigerwe et al., 2008b; Buczinski and Vandeweerd, 2016; Sutter et al., 2019). Yet as recently noted by others, the colostrum quality classification and TPI threshold are inadequate and outdated (Buczinski and Vandeweerd, 2016; Mcgee and Earley, 2019; Hare et al., 2020; Lombard et al., 2020), as evidenced by persistently high rates of TPI failures (12.1% to 37.1%) on North American dairies (Tyler et al., 1998; Trotz-Williams et al., 2008; Beam et al., 2009; Shivley et al., 2018). Cases of TPI failures are costly (Zwald et al., 2007; Raboisson et al., 2016; Hawkins et al., 2019) and invariably trigger the use of antibiotics (Braidwood and Henry, 1990; Weaver et al., 2000) which not only harms gut health/microbiota (Pereira et al., 2018) but also threatens public health with antibiotic resistance (Thames et al., 2012).
While several papers have reported IgG levels at several relatively distant timepoints after the first milking (Parrish et al., 1948, 1953; Bush et al., 1971; Oyeniyi and Hunter, 1978; Guidry et al., 1980; Chang et al., 1981; Stott et al., 1981; Hammon and Blum, 1998; Abdel-Salam et al., 2014; Silva-del-Ro et al., 2017; Dunn et al., 2018; Raimondo et al., 2019), IgG secretion in early transition milk has not been studied. Accordingly, our primary objective was to define the temporal kinetics of mammary IgG secretion by first testing whether IgG secretion occurs postpartum (0 hr; FCGRT and B2M gene expression in cows) and then measuring levels in colostrum and transition milk. In parallel, we investigated the accuracy of interpreting colostrum IgG concentrations using associated Bx values, and analyzed the relationship between colostrum volume and IgG, total protein, lactose, and glucose concentrations.
Materials and Methods
Animals
Holstein cows used for this study were from the Cornell University Ruminant Center (CURC; Harford, NY) and Beck Farms, LLC. (Freeville, NY). All animal procedures were approved by the Institutional Animal Care and Use Committee of Cornell University and only clinically healthy animals were included in this study. For mammary biopsies, 9 cows were randomly selected and sampled at CURC (postpartum day 0, n = 4; postpartum days 40 to 50, n = 5). To study the relationship between postpartum IgG secretion kinetics and colostrum/transition milk volume, 9 cows at CURC were randomly selected at parturition for sequential, complete milkings to obtain colostrum (<1) and transition milk (4, 16, 28, 40, and 52 hr postpartum). First milking colostrum volume data were obtained from CURC routine milking data (<2 hr postpartum) during the study (43 primiparous and 87 multiparous cows). To investigate if animal stress caused variability in colostrum volume, 27 cows immediately after parturition were administered oxytocin (40 units IM; n = 9 CURC, n = 18 Beck Farms) and the volume produced (<1 hr postpartum) was compared with volume data collected from CURC. To determine the relationship between Brix and IgG for colostrum samples, individual colostrum samples from the same 27 animals above were analyzed. The 18 colostrum samples from Beck Farms were used to compare volume with IgG, Brix, glucose, lactose and protein content. All cows were milked using a portable milking unit. From each collection, at least 6 aliquots (1.5 mL each) of colostrum/transition milk were immediately snap frozen in liquid nitrogen, and transported to the lab and stored in a 80 C freezer until processing.
Mammary gland biopsy
Multiparous Holstein cows were randomly selected after parturition (day 0, n = 4) and at 40 to 50 d after parturition (n = 5) for sampling (each cow was only biopsied once). For sampling, animals were prepared in a surgical suite at CURC with sedation using intravenous xylazine hydrochloride (35 g/kg BW). An ~10 cm2 area of skin on the posterior aspect of the mammary gland, at the midpoint in the right hind quarter was clipped, washed with soap, and prepared for sterile surgery (70% alcohol and an iodine surgical scrub). The area was locally anesthetized using subcutaneous distribution of lidocaine (3 to 5 mL of 1%, v/v). A scalpel blade was used to make an ~2.5 cm incision through the skin and gland capsule. The biopsy was taken at a depth of 2 cm using a core biopsy device (Bard Magnum Instrument), affixed with a 11G Bard TruGuide Coaxial Biopsy Needle. The full biopsy procedure was completed within 1 to 2 hr after parturition. Tissue samples collected were immediately snap frozen in liquid nitrogen, transported to the lab, and stored at 80 C before RNA extraction.
Gene expression analysis
Total RNA was extracted from mammary gland tissue using the RNeasy lipid tissue mini kit (Qiagen). Reverse transcription for cDNA synthesis was carried out for 1 g of total RNA using oligo-dT primers with the Multiscribe reverse transcriptase (ThermoFisher Scientific). Expression of specific genes was analyzed by quantitative PCR (qPCR) using SYBR-green detection method with intron-spanning primers: FcGRT5 GACAGTGGCTGCGGGAGGAG 3 and 5 ATCCTTGGACAGGCCGGGAGT 3; B2M5 TGCTG TCCCACGCTGAGTTCAC 3 and 5 GCTGCTTACAGGTCTCGAT CCCAC 3; PIGR5 GCCTGGAGGTCAGCCAAGATCC 3 and 5 GCACGCGTGAAAGGGCAGTT 3; HMBS5 GGTGGGTGTG TTGCACGATCC 3 and 5 CCAGTCAGGTACAGTTGCCCATCC 3. Results were analyzed after normalization to the expression of HMBS as internal control. Relative quantification of fold-change was performed comparing Ct values by applying the 2Ct method (Livak and Schmittgen, 2001).
Brix refractometry, protein quantitation, protein precipitation, dialysis, and osmolality
The Bx value for all fresh colostrum/milk samples was determined within 1 hr of collection using 2 calibrated optical Brix Refractometers (range 0% to 32% or 28% to 64%; Fisher Scientific) at the farm site. Frozen-thawed colostrum/milk was used for all laboratory analyses. For protein quantification a defined volume of colostrum was used and coarse lipids were removed first in a 2-step centrifugation (4,000 g for 30 min, followed by 20,000 g for 17 min), with the coalesced fat layer removed at each step. Then the samples were treated with SDS (2.5%, w/v, final concentration) and additionally diluted in phosphate-buffered saline (1:100), and the bicinchoninic protein assay (Pierce, ThermoFisher Scientific), was used to measure protein concentration in the samples as previously described (Tu et al., 2016; Schalich et al., 2020). To remove proteins from coarse lipid removed colostrum samples, trichloroacetic acid (TCA) protein precipitation was performed. TCA was added (10% final concentration) and mixed well and samples were incubated on ice for 30 min. Precipitated proteins were pelleted by centrifugation at 3,200 g at 4 C for 40 min and the supernatant was collected. To remove dissolved solutes including sugars, dialysis was performed on coarse lipid-removed colostrum samples. In brief, samples were loaded into a 6-8kDa molecular weight cut off (MWCO) dialysis tubing, and dialyzed overnight (12 hr) at 4 C against 0.9% saline. Osmolality of colostrum, de-fatted colostrum, and defatted and dialyzed colostrum was measured using a calibrated micro-osmometer (Fiske, Advanced Instruments).
Quantitative IgG Western blots
Quantitation of IgG content in colostrum was performed by precisely estimating the 50 kDa heavy chain using a specific antibody with near-infrared fluorescence detected in Western blots. A standard curve was constructed using known concentrations of bovine IgG (Millipore-Sigma) in 3 independent replicates. Optimal dilutions were predetermined for the colostrum samples as 1:1000 for the first milking and 1:300 to 1:500 for the subsequent milking samples before running the quantitation for all samples. Procedural steps in Western blot detection are as previously described (Morohaku et al., 2013). In brief, coarse-lipid-removed colostrum samples were prepared in Laemmli sample buffer containing dithiothreitol, incubated at 100 C for 15 min, and separated by SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes, nonspecific binding was blocked using rabbit serum (5%, v/v) in tris-buffered saline containing 0.2% Tween 20 (TBST). Membranes were then incubated in TBST containing DyLight649-conjugated polyclonal anti-bovine IgG that recognizes both heavy and light chains produced in rabbits and affinity purified (1:10,000 dilution; Jackson ImmunoResearch), for 12 hr on a rocking platform at 4 C. Membranes were then washed in TBST and checked using a fluorescence imaging system (Azure Biosystems), and imaged for quantification using a laser fluorescence scanner (Odyssey, Li-Cor). An IgG standard sample (4 g) was included as an internal control for all test gels to validate quantification accuracy. Intensity of the IgG heavy chain band was measured using ImageJ (Schneider et al., 2012), as previously described (Morohaku et al., 2014). Known concentrations were used to construct the standard curves without saturation, and concentrations per unit volume of colostrum in test samples were analyzed. Standard curves were reproduced four times, and all samples were independently quantified 2 to 3 times with dilutions made in the appropriate range.
Quantifying lactose and glucose
Free d-glucose and lactose levels in colostrum were measured using a sequential lactose assay kit (K-LOLAC, Megazyme), following manufacturer instructions. In brief, coarse-lipid-removed colostrum samples were diluted in ultrapure water and incubated at 60C for 15 min in an orbital platform. Solutions of potassium hexacyanoferrate (II) trihydrate and zinc sulfate heptahydrate were used to precipitate nonsolute components; precipitates were filtered through a Whatman No. 1 filter paper and the filtrate was collected. A 3-step hydrolysis of d-glucose (hexokinase, glucose-6-phosphate dehydrogenase, 6-phosphoguconated dehydrogenase) and measurement of the NADPH produced (absorbance at = 340 nm) was used to estimate free glucose. Subsequently, addition of the lactose specific -galactosidase enzyme to hydrolyze lactose and repeatition of the 3-step hydrolysis above to detect the d-glucose released was used to estimate the lactose content of the samples. Blanks were used as controls and each sample was analyzed in duplicates.
Statistics
For quantitative comparisons, differences between groups (qPCR, osmolarity, protein concentration, and total IgG) were compared using Students t-test; comparisons of more than 2 groups (colostrum volume and biochemistry) were performed using ANOVA and post hoc Tukeys test (P < 0.05 was considered significant). Simple or multivariable linear regression was used to examine scalar responses and model the relationship between two or more explanatory variables (IgG standard curve). Orthogonal regression was used to examine linear relationships between two continuous variables (Bx vs. IgG; volume vs. IgG concentration, Bx, total IgG, total protein, total glucose, and total lactose). Time course data were subjected to repeated measures analysis with post hoc Tukeys test correction for multiple comparisons (IgG concentration and volume over time). For statistical analyses Prism 5 (GraphPad) was used. In the results, data are represented as mean SEM. In all figures independent data points are displayed when possible.
Results and Discussion
Mammary gland IgG transport system is sustained postpartum
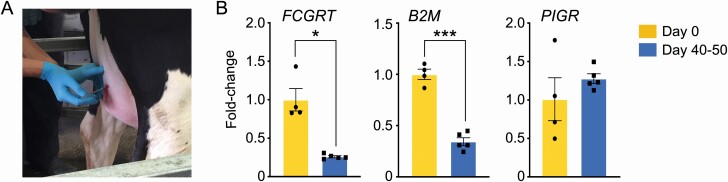
Bovine neonatal Fc receptor (FCRN; a heterodimer of FCGRT and B2M gene products) is considered the pivotal system responsible for transfer of maternal IgG from the serum to milk (Mayer et al., 2005; Lu et al., 2007). It is known that colostrum IgG secretion begins 2 to 3 wk preceding parturition (Brandon et al., 1971); however, it remains to be documented when this process ends. In examining mRNA levels for FCGRT and B2M in mammary gland tissue after the first milking (day 0), we found that expression was high and significantly greater than that observed in cows producing mature milk (40 to 50 d in milk/DIM; Figure 1). This reflects elevated IgG transport from systemic circulation into colostrum and suggests that IgG transport remains active postpartum. We do not find a similar pattern for the IgA and IgM transporter PIGR expression between the days 0 and 40 to 50 DIM cows (P = 0.32). This is in agreement with what has been reported for the sheep mammary gland in which PIGR levels, regulated by prolactin, were maintained during established lactation (Rincheval-Arnold et al., 2002). IgA and IgM form minor immune components in colostrum, but they continue to play a critical role in immunosurveillance and pathogen recognition in the mammary gland during production of mature milk (Mach and Pahud, 1971).
Figure 1.
Transport system that incorporates IgG into colostrum is actively transcribed in the mammary gland postpartum. (A) Site of mammary gland biopsy at the midpoint of the right hind quarter of cows. Biopsies were collected after the first/colostrum milking, within 1 to 2 hr after parturition. Step of passing the needle guide, prior to the core biopsy procedure is shown. (B) Physiological expression of the FcRN heterodimer components, FcGRT and B2M, is significantly higher at day 0 compared with baseline levels at days 40 to 50 of lactation (*P < 0.05; ***P < 0.001). This is indicative of an active process extending to the period of transition milk secretion. As a control, expression of the IgA and IgM transporter, PIGR, was not different between day 0 and days 40 to 50 of lactation.
Mean colostrum volume variability is related to mammary secretion
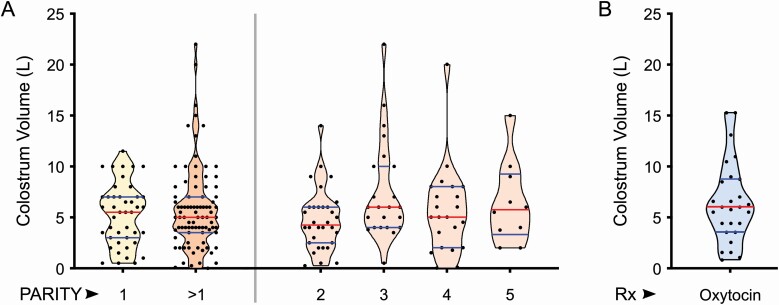
In routine colostrum milking (<2 hr after parturition), we found no significant difference in the mean volume of colostrum produced by primiparous vs. multiparous Holstein cows (P = 0.44), and across lactations (P = 0.09) (Figure 2A). We did observe a high range of volumes recorded among cows within each parity (between 0.1 and 22 L). These results are in accordance with 2 previous reports (Oyeniyi and Hunter, 1978; Kehoe et al., 2011) but in contrast to others (Kruse, 1970b; Devery-Pocius and Larson, 1983; Pritchett et al., 1991; Kessler et al., 2014). One possibility for first milking volume variability among cows could be factors surrounding parturition and postpartum stress, which could have idiosyncratic effects on colostrum let-down. In order to test whether colostrum volume variability is related to mammary secretion or idiosyncratic effects, we induced let-down in cows using oxytocin for complete milking (<1 hr after parturition), and made accurate measurements of colostrum volume. In the group tested (n = 27), our results showed that colostrum volume variability persisted (Figure 2B), indicating that volume variability is directly related to the extent of mammary secretion.
Figure 2.
Volume of first milking/colostrum within 2 hr postpartum is highly variable. (A) First milking colostrum volume data from Holstein dairy cows (n = 130) was collected and sorted based on cow parity. When primiparous and multiparous groups were compared, there was no difference in colostrum volume produced. Within the multiparous group, comparing colostrum volume produced across the 5 different lactations, also did not show a significant difference in volume produced. The full range of colostrum volumes was between 0.1 and 22 L as recorded across all animals/groups. (B) First milking after oxytocin for inducing complete let-down also showed significant variability in volume between individual animals (range = 0.75 to 15.36 L), indicating differences to mammary secretion eliminating environmental factors that might have affected milking yield.
Colostrum Bx values are not correlated to IgG concentrations
Methods used previously for IgG quantification in colostrum have mostly relied on simplicity rather than precision. For example, RID first used on colostrum samples in 1975 depends on determining the relative distance of insoluble IgG+anti-IgG precipitate formation in agarose gels (Michalek et al., 1975). This provides only a rough estimate as attaining equivalence in this approach is not linear (Berne, 1974). Techniques such as the enzyme-linked immunosorbent assay/ELISA based on enzyme kinetics have shown variability among commercial kits in estimating colostrum IgG (Gelsinger et al., 2015; Dunn et al., 2018). Common to these two methods, any non-specific binding by antibodies is incorporated into the estimated results. As a clear deficiency, studies directly comparing ELISA and single RID measurements on the same colostrum samples reported an almost 2-fold lower absolute IgG values with ELISA (Vetter et al., 2013; Gelsinger et al., 2015; Marie Lkke et al., 2016; Dunn et al., 2018). This not only indicates a weak relationship between the 2 IgG estimation methods but also highlights a void in knowledge of absolute values for colostrum IgG concentrations in dairy cows.
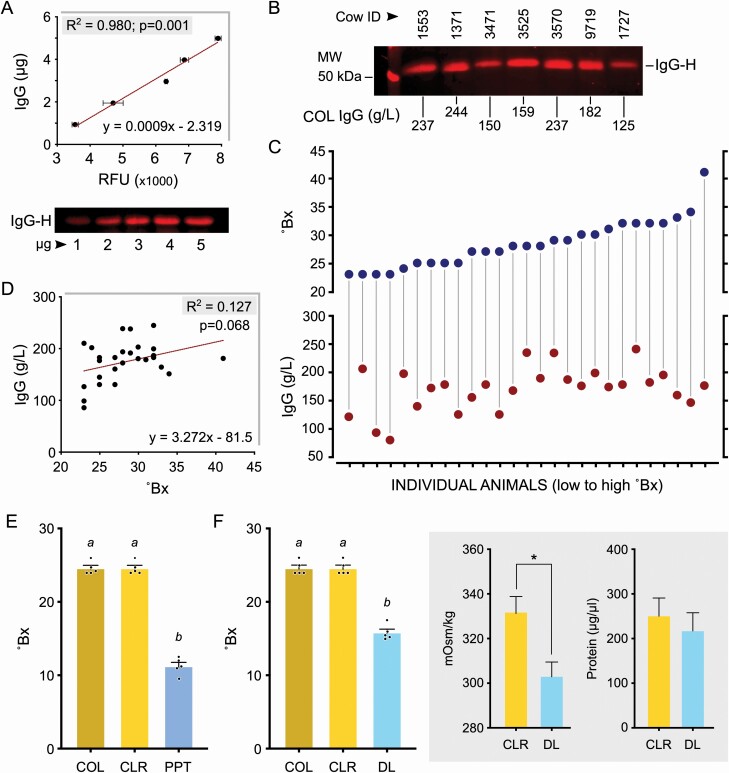
To precisely measure colostrum IgG content, we optimized a fluorescent Western blot assay using quantitative detection of the IgG heavy chain (IgG-H). By distinct visualization of individual bands even in complex biological fluids, this method provided highly selective sensitivity and stability across a quantifiable linear range (Gingrich et al., 2000; Zellner et al., 2008). For IgG standards, it was possible to measure a corresponding standard range of IgG-H relative fluorescence units (RFU) indicative of concentrations consistently across independent runs (R2 = 0.98; P < 0.001; Figure 3A). Reproducible IgG concentration in appropriately diluted colostrum samples could be calculated comparing this reference range, specifically visualizing the IgG-H band (Figure 3B). Investigating Bx values and corresponding colostrum IgG concentration indicated an extremely poor association between these variables; some low Bx values were associated with high IgG concentrations and vice versa (Figure 3C). Statistically, the scatter plot did not look linear and there was no significant linear relationship between Bx and IgG concentrations (R2 = 0.127; P = 0.068; Figure 3D). Moreover, comparing R to the table of critical values indicated that a relationship did not exist between Bx and IgG concentrations, and that the regression line cannot be used for prediction.
Figure 3.
Refractive index measurements (Bx values) does not directly correlate to IgG concentrations and is affected by both protein and nonprotein solute levels. (A) Standard curve generated using purified IgG detecting the heavy chain (IgG-H) in fluorescent Western blots for quantitating IgG concentration in colostrum (n = 3). Quantitative measurements of band intensity profiles are presented as RFU/relative fluorescence units. Representative standard blot with defined concentrations of purified bovine IgG is shown. (B) Representative blot with sample-specific variable dilutions as used to quantify colostrum (COL) IgG concentrations is shown with calculated values. (C) Concurrent measurements of Bx values and IgG concentration in 27 colostrum samples indicating the lack of a strong relationship between these 2 dimensions. (D) Absence of a significant correlation between IgG concentration and Bx values in the range of the 27 colostrum samples tested. (E) Removal of lipids/fat globules (CLR/coarse lipid removed), from colostrum had no effect on Bx values. Protein precipitated (PPT) CLR colostrum showed a significant reduction in Bx values (different letters indicate P < 0.0001). (F) Removal of nonprotein solutes (<6 kDa) from CLR colostrum by dialysis (DL) also caused substantial reduction in Bx values (different letters indicate P < 0.0001). Dialysis was validated by the significant decrease to osmolality (*P < 0.05), and confirming the absence of protein loss during this process.
These findings do not support previous RID studies that concluded Bx values provide a reliable estimate to IgG concentrations (Morrill et al., 2012; Quigley et al., 2013; Silva-del-Ro et al., 2017; Gamsjger et al., 2020). To explain this disparity, we would like to point out that there are three aspects of analytical difference. First, high lipid content, as is characteristic of whole colostrum, is known to interfere with immunoassays and can thus inhibit antigen-antibody binding and produce false low IgG concentrations (Steen et al., 2011; Wild, 2013). Removal of lipids prior to our assay in frozen-thawed samples likely enhanced precision. Second, isolation of a denatured IgG-H band provided specificity, eliminating any concerns regarding nonspecific binding/adherence of antibodies. Third, quantification using fluorophores through consistent laser excitation scanning provides high resolution and uniformity for sensitive estimations. On this basis, we detected higher absolute values for colostrum IgG concentrations (average: 150 14.738 g/L) compared with some key RID-based studies (Oyeniyi and Hunter, 1978; Stott et al., 1981). However, detection at this range is not entirely novel as other studies have reported overlapping high ranges (Kehoe et al., 2011; Conneely et al., 2013; Gross et al., 2014), underscoring the unexplained variation in previously reported measurements.
Colostrum protein and nonprotein solutes affect Bx values
In other industries such as viticulture, the Bx values are used to determine the sugar content of must and wine (Son et al., 2009). As we did not find a strong correlation between Bx values and IgG concentrations, we decided to examine other components in colostrum that might contribute to the variability in Bx values. Given that the mean refractive indices of proteins and disaccharides differ distinctly in scale [e.g., refractive index is 1.336 for a 15% albumin solution (Barer and Tkaczyk, 1954) vs. only 0.348 for a 10% glucose solution (Lide, 2006)], we sought to determine the contribution of fat/lipids, proteins, and nonprotein solutes (sugars) to the Bx value of colostrum. Removal of coarse lipids made no difference in Bx between samples (P = 0.736) (Figure 3E, F). However, subsequent acid precipitation of proteins (Figure 3E) and removal of low molecular weight solutes by dialysis (Figure 3F) resulted in significant reduction in Bx values (P < 0.0001 for both). Dialysis of coarse-lipid-removed colostrum samples resulted in a significant decrease in osmolality, without changes to protein concentration (Figure 3F), indicating that the major components lost are non-protein solutes such as sugars. Interestingly, coarse-lipid-removed colostrum samples were hypertonic (332 mOsm/kg), and although dialysis reduced this value (to 303 mOsm/kg) (Figure 3F), they remained hypertonic suggesting that soluble proteins significantly contribute to the osmotic pressure, similar to the oncotic pressure (colloid osmotic pressure) regulated by albumin and immunoglobulins in serum (Hall and Guyton, 1976; Kaysen and Al Bander, 1990; Kiyosawa, 2003). The fact that lipids do not affect Bx values can be explained by their insoluble presence as mobile droplets which can scatter visible light; dissolved solutes (proteins and sugars) are apparently exclusive in their involvement in refractive index measurements. Given these possible variables that affect Bx values and the known individual distinctions to colostrum volume, Bx values are not a specific indicator of IgG levels, and its use for colostrum quality assessment is unsubstantiated.
Seventy-five percent of the mammary IgG secreted postpartum is part of transition milk
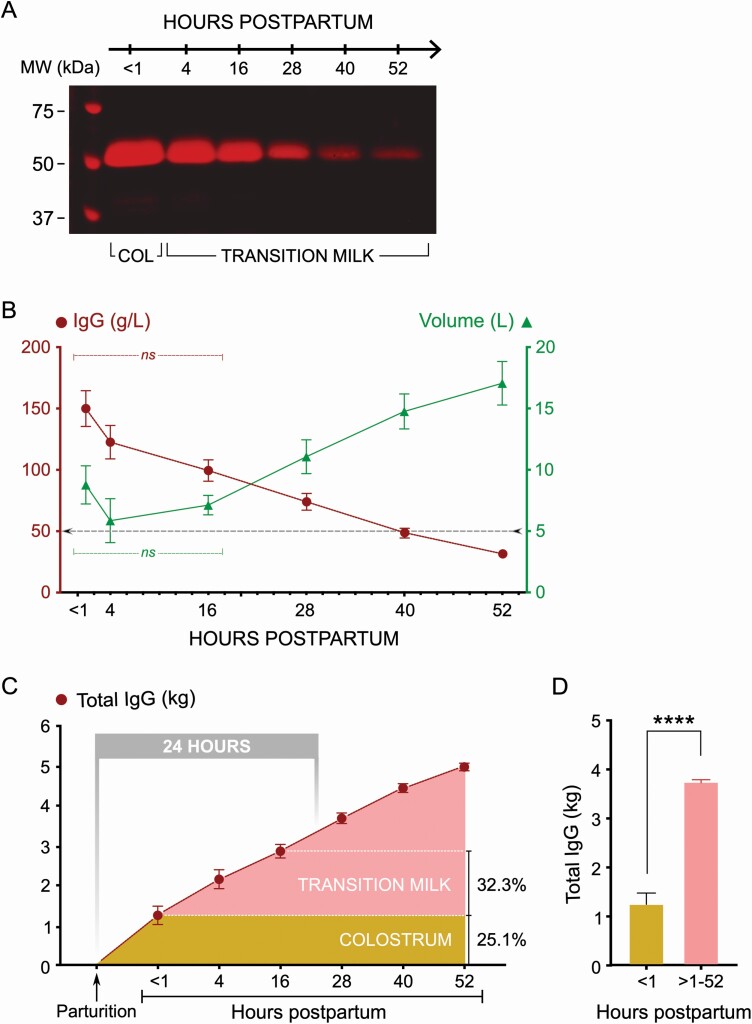
In order to evaluate the temporal kinetics of IgG secretion by the postpartum mammary gland, we collected colostrum (<1 hr) and transition milk (4, 16,28, 40, and 52 hr) from cows and measured both volume and IgG concentration across these timepoints. Visual levels of IgG in colostrum and transition milk clearly indicated its robust secretion after the first milking (Figure 4A). Estimation of sequential samples for IgG concentration indicated that decline in IgG levels was quite gradual with significant amounts being released in transition milk over the first 28 hr (Figure 4B). Mean IgG concentrations were 150 g/L at <1-hr, 123 g/L at 4-hr, 100 g/L at 16-hr, 74 g/L at 28-hr, 49 g/L at 40-hr and 32 g/L at 52-hr milking. Volume of postpartum mammary secretions with this milking frequency was not different between <1-, 4-, and 16-hr milking, but increased with an inverse correlation to IgG concentrations at 28-, 40-, and 52-hr milking. Total IgG contents calculated for the volume produced were 1.25 kg at <1-hr, 0.91 kg at 4-hr, 0.70 kg at 16-hr, 0.83 kg at 28-hr, 0.76 kg at 40-hr, and 0.53 kg at 52-hr milking (Figure 4C). These results indicated that 3.73 kg (74.9%) of the postpartum mammary IgG is released in transition milk; the first milking, with the characteristic appearance of colostrum, contained only 1.25 kg (25.1%) of the postpartum mammary IgG production (Figure 4D). Postpartum mammary IgG production was not significantly different for both volume and IgG concentration at the 4-hr milking compared with <1-hr milking. The combined transition milk at 4-, 16-, and 28-hr milking contained ~49% of the postpartum mammary IgG production.
Figure 4.
Time course of IgG secretion by the mammary gland reveals substantially greater amounts secreted in transition milk. (A) Representative Western blot showing colostrum (COL) and transition milk IgG levels in periodic postpartum milking (1, 4, 16, 28, 40, and 52 hr). (B) Graph showing changes to IgG concentration and colostrum/transition milk volume during the periodic post-partum milking (n = 9). The threshold line (at 50 g/L) indicates minimum concentration recommended for feeding by industry standards. Data show that colostrum/milk IgG secretion in the mammary gland continues beyond the first milking (colostrum), with a gradual decline over the sampling period in transition milk. IgG concentrations were not significantly different (ns) between <1-, 4-, and 16-hr milking. Increases to volume was not significantly different between <1-, 4-, and 16-hr milking, but progressively increased at the 28-, 40-, and 52-hr milkings. (C) Graph showing total postpartum mammary IgG secretion (cumulative values) across the different timepoints indicating the overall mammary output over time. Notable are the increased combined IgG output over 4- and 16-hr milking that are timepoints without significant changes to volume. Overall, there remains a linear increase in total mammary IgG released over time (R2 = 0.7076; P = 0.0004). Of the total mammary IgG released, 25% is contained in colostrum and 32.3% is contained in the 4- and 16-hr transition milk. (D) Comparing total IgG produced in the first milking compared with transition milk combined (4 to 52 hr) indicated that an ~3-fold significantly higher levels of total IgG is secreted as part of transition milk (****P < 0.00001).
In previous studies, partial documentation of mammary IgG secretion kinetics, albeit at different timepoints, is consistent with our findings. It was demonstrated that mammary IgG release remains elevated at 0 and 12 hr postpartum (Sasaki et al., 1976; Oyeniyi and Hunter, 1978; Stott et al., 1981; Elfstrand et al., 2002; Moore et al., 2005; Abdel-Salam et al., 2014). Although maternal-offspring coevolution with regard to the precise physiological kinetics of IgG absorption remains to be fully elucidated (Stott et al., 1979c), estimates of termination or closure of intestinal permeability to IgG indicate that it occurs near 24 hr of age in calves (McCoy et al., 1970; Stott et al., 1979a). Interpreting our results in this light specifies that including transition milk, just extending to the second (4-hr) or third (16-hr) milking in feeding calves, could easily more than double the total IgG levels provided to calves compared to colostrum feeding alone.
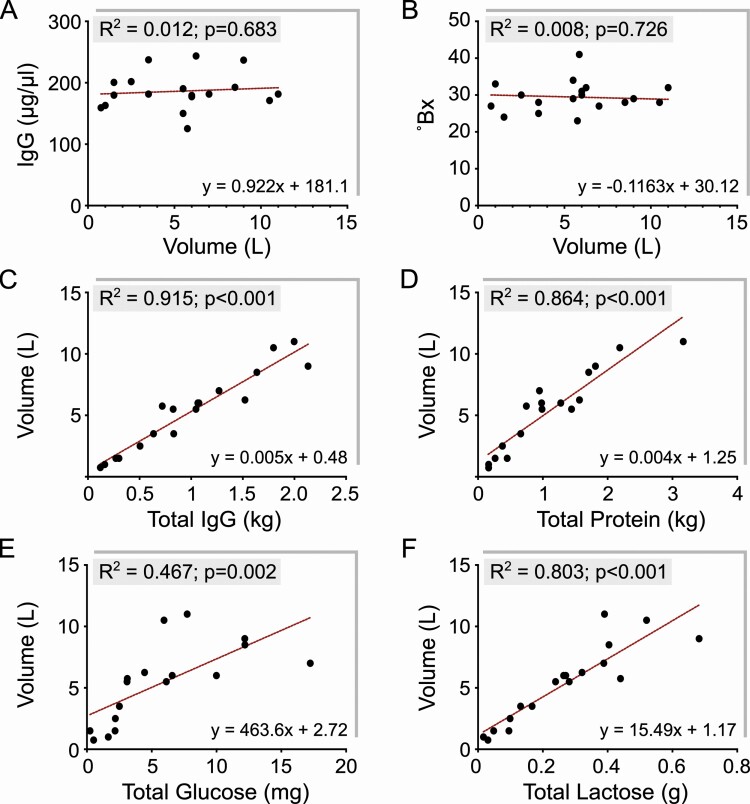
IgG and colostrum volume are coregulated
To better understand the basis of variability in colostrum volume (Figure 2B), and its relationship to result that Bx was not correlated to IgG concentration (Figure 3D), we examined the associated levels of IgG, glucose, and lactose. Our results indicated that colostrum volume had no significant correlation to IgG concentration or Bx values. IgG concentration more or less remained unaltered and uncoupled to volume changes (Figure 5A). Similarly, Bx values were not affected by volume changes (Figure 5B). However, total IgG and total protein in colostrum were strongly associated with colostrum volume and statistically significant (Figure 5C, D). Similarly, total glucose and total lactose in colostrum, solutes that can also affect Bx values, were also associated with colostrum volume and statistically significant, albeit with a weaker effect than that evident for total IgG. These results indicated that IgG secretion in the postpartum mammary gland is coregulated with volume of colostrum. Examining the impact of dialysis on osmolality of coarse lipid removed colostrum (Figure 3F), and the fact that IgG is the major fraction of soluble protein in colostrum, it is conceivable that IgG secretion might be a postpartum driver for colostrum volume. This would be in synergy with disaccharides (lactose and glucose), that are considered established regulators of mammary secretion volume for mature milk (Taylor and Husband, 1922).
Figure 5.
IgG secretion and colostrum volume are coregulated, with a stronger association than other secreted components such as glucose and lactose. (A,B) IgG concentration and Bx values were not correlated to colostrum volume. Measurements for IgG concentration and Bx values remained within a constant range even with the highly variable range in colostrum volume (0.75 to 11 L; n = 18). (C, D) Total IgG secreted (and total protein secreted) was in strong correlation to colostrum volume (P < 0.001), indicating that IgG secretion and acquisition of colostrum fluid volume are significantly associated. (D, E) Total glucose and total lactose secreted also showed a significant association with colostrum volume (P = 0.002 and P < 0.001, respectively), but the relationships appeared weaker than that observed for total IgG towards higher volumes.
The finding that colostrum IgG concentration was independent of volume is indeed supported by previous observations (Baumrucker et al., 2010). However, subsequent studies have claimed the opposite (Kehoe et al., 2011; Silva-del-Ro et al., 2017). By using oxytocin for complete let-down during milking, our results control for factors that may have negatively impacted colostrum volume variability in these above studies. Early investigations have speculated that with IgG secretion beginning 2 to 3 wk prior to parturition (Brandon et al., 1971), colostrum in the teat and gland cisterns might have higher IgG concentrations compared with the alveoli (Stott et al., 1979c). Therefore, it is plausible that incomplete milking could have led to an erroneous assumption that IgG concentrations have an inverse relationship to colostrum volume in prior studies.
Translation of physiology to applied management
Based on our results, the current classification of good and poor-quality colostrum as interpreted by Bx values is unfounded; IgG concentration is not reflected in Bx values, and any extrapolation for on-farm management is unsubstantiated. Moreover, variability in absolute IgG concentrations in prior work weakens the basis of existing feeding recommendations for neonatal management. This is significant as studies across U.S. dairy farms have suggested that approximately 30% of colostrum is poor quality and failing to meet industry threshold standards for IgG concentration based on Bx values (Morrill et al., 2012; Shivley et al., 2018). Moreover, our finding that transition milk examined at 4- and 16-hr contained 18.2% and 14.1%, respectively, of the total postpartum mammary IgG produced is quite significant. These values parallel losses to circulating IgG levels (Butler et al., 1972; Guidry et al., 1980), indicating a significant investment of resources by the cow to provide IgG in postpartum mammary secretions to ensure survival and health of the calf. Therefore, a single feeding restricted to colostrum, which contains only 1.25 kg (25.1%) of the postpartum mammary IgG output, is not based on the maternal-offspring coevolution of physiology and needs reconsideration for improving calf management. Lack of physiological biomimicry could be the underlying reason for high TPI failures recorded in farms (Tyler et al., 1998; Trotz-Williams et al., 2008; Beam et al., 2009; Shivley et al., 2018). Feeding transition milk from 4- and 6-hr milkings would provide an additional 1.61 kg (32.3%) IgG to calves, this is more than that available via colostrum. Such an adjustment of management practice to align with cowcalf physiology is bound to improve TPI and be of immense improvement to the long-term health of calves.
Our recording of IgG secretion kinetics in the postpartum mammary gland suggests that the absorptive kinetics in the calf intestine before full cessation of IgG uptake/gut closure also requires precise reevaluation. There are already fragments of evidence in the literature that multiple feedings of colostrum and feedings beyond 24 hr could result in acquisition of significantly higher serum IgG levels in neonatal calves (Hammon and Blum, 1998; Jaster, 2005; Conneely et al., 2014; Wojtas et al., 2019; Hare et al., 2020) and other metabolic benefits (Hammon and Blum, 1998; Steinhoff-Wagner et al., 2011). There are currently no studies on the benefits of transition milk to TPI and calf health.
Therefore, our results show that the concept of colostrum quality for calf management is arbitrary. Biomimicry to time and feed at least 50% to 75% of the IgG secreted in the postpartum mammary gland via colostrum and transition milk is a physiologically relevant feeding scheme to maximize TPI. Developing this practice would be aligned to core benefits in farm economics via reducing antibiotic use and enhancing calf health and long-term productivity. In future studies, direct parallel investigations on the calf intestinal IgG absorption mechanism and sustenance during the neonatal period are necessary to provide the precise physiological capacity for uptake to further refine management.
Acknowledgments
This project was funded by the Northeast Sustainable Agriculture Research and Education (NE-SARE) grant GNE19-220-33243 to K.S., the Cornell CALS Charitable Trust Research fund to O.R., and startup funds from the College of Agriculture and Life Sciences, Cornell University to V.S. The authors would like to thank the staff at CURC for their assistance in this project, especially Ms. Lisa Furman and Mr. Josh VanDeWeert for their assistance in collecting mammary gland biopsies and postbiopsy cow health management. The authors would also like to thank Mr. Tyler Beck and the Beck Farms LLC, Freeville, NY for their participation and help in this research.
Glossary
Abbreviations
- Bx
Brix
- COL
colostrum
- ELISA
enzyme-linked immunosorbent assay
- IgG
immunoglobulin G
- IgG-H
IgG heavy chain
- mOsm
milliosmole
- MWCO
molecular weight cut off
- NADPH
nicotinamide adenine dinucleotide phosphate
- PIGR
polymeric immunoglobulin receptor
- RFU
relative fluorescence units
- RID
radial immunodiffusion
- SDS
sodium dodecyl sulfate
- TBST
tris-buffered saline with 0.1% Tween 20
- TCA
trichloroacetic acid
- TPI
transfer of passive immunity
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abdel-Salam, Z., Abdel Ghany S. h., and Harith M. A.. . 2014. Evaluation of immunoglobulins in bovine colostrum using laser induced fluorescence. Talanta 129:15–19.. doi: 10.1016/j.talanta.2014.04.033 [DOI] [PubMed] [Google Scholar]
- Barer, R., and Tkaczyk S.. . 1954. Refractive index of concentrated protein solutions. Nature 173:821–822.. doi: 10.1038/173821b0 [DOI] [PubMed] [Google Scholar]
- Baumrucker, C. R., Burkett A. M., Magliaro-Macrina A. L., and Dechow C. D.. . 2010. Colostrogenesis: mass transfer of immunoglobulin G1 into colostrum. J. Dairy Sci. 93:3031–3038.. doi: 10.3168/jds.2009-2963 [DOI] [PubMed] [Google Scholar]
- Beam, A. L., Lombard J. E., Kopral C. A., Garber L. P., Winter A. L., Hicks J. A., and Schlater J. L.. . 2009. Prevalence of failure of passive transfer of immunity in newborn heifer calves and associated management practices on US dairy operations. J. Dairy Sci. 92:3973–3980.. doi: 10.3168/jds.2009-2225 [DOI] [PubMed] [Google Scholar]
- Berne, B. H. 1974. Differing methodology and equations used in quantitating immunoglobulins by radial immunodiffusion-a comparative evaluation of reported and commercial techniques. Clin. Chem. 20:61–69.. doi: 10.1093/clinchem/20.1.61 [DOI] [PubMed] [Google Scholar]
- Besser, T. E., Gay C. C., and Pritchett L.. . 1991. Comparison of three methods of feeding colostrum to dairy calves. J. Am. Vet. Med. Assoc. 198:419–422.. [PubMed] [Google Scholar]
- Boyd, J. W. 1972. The relationship between serum immune globulin deficiency and disease in calves: a farm survey. Vet. Rec. 90:645–649.. doi: 10.1136/vr.90.23.645 [DOI] [PubMed] [Google Scholar]
- Braidwood, J. C., and Henry N. W.. . 1990. Clinical efficacy of chlortetracycline hydrochloride administered in milk replacer to calves. Vet. Rec. 127:297–301.. [PubMed] [Google Scholar]
- Brandon, M. R., Watson D. L., and Lascelles A. K.. . 1971. The mechanism of transfer of immunoglobulin into mammary secretion of cows. Aust. J. Exp. Biol. Med. Sci. 49:613–623.. doi: 10.1038/icb.1971.67 [DOI] [PubMed] [Google Scholar]
- Buczinski, S., and Vandeweerd J. M.. . 2016. Diagnostic accuracy of refractometry for assessing bovine colostrum quality: a systematic review and meta-analysis. J. Dairy Sci. 99:7381–7394.. doi: 10.3168/jds.2016-10955 [DOI] [PubMed] [Google Scholar]
- Bush, L. J., Aguilera M. A., Adams G. D., and Jones E. W.. . 1971. Absorption of colostral immnunoglobulins by newborn dairy calves. J. Dairy Sci. 54:1547–1549.. doi: 10.3168/jds.S0022-0302(71)86063-0 [DOI] [PubMed] [Google Scholar]
- Butler, J. E., Kiddy C. A., Pierce C. S., and Rock C. A.. . 1972. Quantitative changes associated with calving in the levels of bovine immunoglobulins in selected body fluids. I. Changes in the levels of IgA, IgGl and total protein. Can. J. Comp. Med. 36:234–242.. [PMC free article] [PubMed] [Google Scholar]
- Chang, C. C., Winter A. J., and Norcross N. L.. . 1981. Immune response in the bovine mammary gland after intestinal, local, and systemic immunization. Infect. Immunol. 31:650–659.. doi: 10.1128/IAI.31.2.650-659.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigerwe, M., Tyler J. W., Middleton J. R., Spain J. N., Dill J. S., and Steevens B. J.. . 2008a. Comparison of four methods to assess colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 233:761–766.. doi: 10.2460/javma.233.5.761 [DOI] [PubMed] [Google Scholar]
- Chigerwe, M., Tyler J. W., Schultz L. G., Middleton J. R., Steevens B. J., and Spain J. N.. . 2008b. Effect of colostrum administration by use of oroesophageal intubation on serum IgG concentrations in Holstein bull calves. Am. J. Vet. Res. 69:1158–1163.. doi: 10.2460/ajvr.69.9.1158 [DOI] [PubMed] [Google Scholar]
- Conneely, M., Berry D. P., Murphy J. P., Lorenz I., Doherty M. L., and Kennedy E.. . 2014. Effect of feeding colostrum at different volumes and subsequent number of transition milk feeds on the serum immunoglobulin G concentration and health status of dairy calves. J. Dairy Sci. 97:6991–7000.. doi: 10.3168/jds.2013-7494 [DOI] [PubMed] [Google Scholar]
- Conneely, M., Berry D. P., Sayers R., Murphy J. P., Lorenz I., Doherty M. L., and Kennedy E.. . 2013. Factors associated with the concentration of immunoglobulin G in the colostrum of dairy cows. Animal 7:1824–1832.. doi: 10.1017/S1751731113001444 [DOI] [PubMed] [Google Scholar]
- Devery-Pocius, J. E., and Larson B. L.. . 1983. Age and previous lactations as factors in the amount of bovine colostral immunoglobulins. J. Dairy Sci. 66:221–226.. doi: 10.3168/jds.S0022-0302(83)81780-9 [DOI] [PubMed] [Google Scholar]
- Dunn, A., Duffy C., Gordon A., Morrison S., Argello A., Welsh M., and Earley B.. . 2018. Comparison of single radial immunodiffusion and ELISA for the quantification of immunoglobulin G in bovine colostrum, milk and calf sera. J. Appl. Anim. Res. 46:758–765.. doi: 10.1080/09712119.2017.1394860 [DOI] [Google Scholar]
- Elfstrand, L., Lindmark-Mnsson H., Paulsson M., Nyberg L., and kesson B.. . 2002. Immunoglobulins, growth factors and growth hormone in bovine colostrum and the effects of processing. Int. Dairy J. 12:879–887.. doi: 10.1016/S0958-6946(02)00089-4 [DOI] [Google Scholar]
- Fey, H. 1971. Immunology of the newborn calf: its relationship to colisepticemia. Ann. NY Acad. Sci. 176:49–63.. doi: 10.1111/j.17496632.1971.tb34992.x [DOI] [Google Scholar]
- Fulwider, W. K., Grandin T., Rollin B. E., Engle T. E., Dalsted N. L., and Lamm W. D.. . 2008. Survey of dairy management practices on one hundred thirteen north central and northeastern United States dairies. J. Dairy Sci. 91:1686–1692.. doi: 10.3168/jds.2007-0631 [DOI] [PubMed] [Google Scholar]
- Gamsjger, L., Elsohaby I., Pearson J. M., Levy M., Pajor E. A., Haines D. M., and Windeyer M. C.. . 2020. Assessment of Brix refractometry to estimate immunoglobulin G concentration in beef cow colostrum. J. Vet. Intern. Med. 34:1662–1673.. doi: 10.1111/jvim.15805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelsinger, S. L., Smith A. M., Jones C. M., and Heinrichs A. J.. . 2015. Technical note: comparison of radial immunodiffusion and ELISA for quantification of bovine immunoglobulin G in colostrum and plasma. J. Dairy Sci. 98:4084–4089.. doi: 10.3168/jds.2014-8491 [DOI] [PubMed] [Google Scholar]
- Gingrich, J. C., Davis D. R., and Nguyen Q.. . 2000. Multiplex detection and quantitation of proteins on western blots using fluorescent probes. Biotechniques 29:636–642.. doi: 10.2144/00293pf02 [DOI] [PubMed] [Google Scholar]
- Godden, S. 2008. Colostrum management for dairy calves. Vet. Clin. North Am. Food Anim. Pract. 24:19–39.. doi: 10.1016/j.cvfa.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. J., Kessler E. C., and Bruckmaier R. M.. . 2014. Colour measurement of colostrum for estimation of colostral IgG and colostrum composition in dairy cows. J. Dairy Res. 81:440–444.. doi: 10.1017/S0022029914000466 [DOI] [PubMed] [Google Scholar]
- Guidry, J., Butler J. E., Pearson R. E., and Weinland B. T.. . 1980. IgA, igG1, IgG2, IgM, and BSA in serum and mammary secretion throughout lactation. Vet. Immunol. Immunopathol. 1:329–341.. doi: 10.1016/0165-2427(80)90012-4 [DOI] [PubMed] [Google Scholar]
- Hall, J. E., and Guyton A. C.. . 1976. Changes in renal hemodynamics and renin release caused by increased plasma oncotic pressure. Am. J. Physiol. 231(5 Pt. 1):1550–1556.. doi: 10.1152/ajplegacy.1976.231.5.1550 [DOI] [PubMed] [Google Scholar]
- Hammon, H. M., and Blum J. W.. . 1998. Metabolic and endocrine traits of neonatal calves are influenced by feeding colostrum for different durations or only milk replacer. J. Nutr. 128:624–632.. doi: 10.1093/jn/128.3.624 [DOI] [PubMed] [Google Scholar]
- Hare, K. S., Pletts S., Pyo J., Haines D., Guan L. L., and Steele M.. . 2020. Feeding colostrum or a 1:1 colostrum:whole milk mixture for 3 days after birth increases serum immunoglobulin G and apparent immunoglobulin G persistency in Holstein bulls. J. Dairy Sci. 103:11833–11843.. doi: 10.3168/jds.2020-18558 [DOI] [PubMed] [Google Scholar]
- Hawkins, A., Burdine K., Amaral-Phillips D., and Costa J. H. C.. . 2019. An economic analysis of the costs associated with pre-weaning management strategies for dairy heifers. Animals. 9:471. doi: 10.3390/ani9070471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaster, E. H. 2005. Evaluation of quality, quantity, and timing of colostrum feeding on immunoglobulin G1 absorption in Jersey calves. J. Dairy Sci. 88:296–302.. doi: 10.3168/jds.S0022-0302(05)72687-4 [DOI] [PubMed] [Google Scholar]
- Johnson, P., and Pierce A. E.. . 1959. Ultracentrifugal and electrophoretic studies on neonatal calf sera and maternal colostrum. J. Hyg. (Lond.). 57:309–320.. doi: 10.1017/s0022172400020179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen, G. A., and al Bander H.. . 1990. Metabolism of albumin and immunoglobulins in the nephrotic syndrome. Am. J. Nephrol. 10 (Suppl. 1:36–42.. doi: 10.1159/000168192 [DOI] [PubMed] [Google Scholar]
- Kehoe, S. I., Heinrichs A. J., Moody M. L., Jones C. M., and Long M. R.. . 2011. Comparison of immunoglobulin G concentrations in primiparous and multiparous bovine colostrum. Prof. Anim. Sci. 27:176–180.. doi: 10.15232/S1080-7446(15)30471-X [DOI] [Google Scholar]
- Kehoe, S. I., Jayarao B. M., and Heinrichs A. J.. . 2007. A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. J. Dairy Sci. 90:4108–4116.. doi: 10.3168/jds.2007-0040 [DOI] [PubMed] [Google Scholar]
- Kessler, E. C., Bruckmaier R. M., and Gross J. J.. . 2014. Milk production during the colostral period is not related to the later lactational performance in dairy cows. J. Dairy Sci. 97:2186–2192.. doi: 10.3168/jds.2013-7573 [DOI] [PubMed] [Google Scholar]
- Kiyosawa, K. 2003. Theoretical and experimental studies on freezing point depression and vapor pressure deficit as methods to measure osmotic pressure of aqueous polyethylene glycol and bovine serum albumin solutions. Biophys. Chem. 104:171–188.. doi: 10.1016/s0301-4622(02)00365-4 [DOI] [PubMed] [Google Scholar]
- Kruse, V. 1970a. Absorption of immunoglobulin from colostrum in newborn calves. Anim. Prod. 12:627–638.. doi: 10.1017/S0003356100029275 [DOI] [Google Scholar]
- Kruse, V. 1970b. Yield of colostrum and immunoglobulin in cattle at the first milking after parturition. Anim. Prod. 12:619–626.. doi: 10.1017/S0003356100029263 [DOI] [Google Scholar]
- Lide, D. R., ed. 2006. CRC handbook of chemistry and physics.Boca Raton, FL:Taylor and Francis. [Google Scholar]
- Livak, K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408.. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lombard, J., Urie N., Garry F., Godden S., Quigley J., Earleywine T., McGuirk S., Moore D., Branan M., Chamorro M., . et al. 2020. Consensus recommendations on calf- and herd-level passive immunity in dairy calves in the United States. J. Dairy Sci. 103:7611–7624.. doi: 10.3168/jds.2019-17955 [DOI] [PubMed] [Google Scholar]
- Lu, W., Zhao Z., Zhao Y., Yu S., Zhao Y., Fan B., Kacskovics I., Hammarstrm L., and Li N.. . 2007. Over-expression of the bovine FcRn in the mammary gland results in increased IgG levels in both milk and serum of transgenic mice. Immunology 122:401–408.. doi: 10.1111/j.1365-2567.2007.02654.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach, J. P., and Pahud J. J.. . 1971. Secretory IgA, a major immunoglobulin in most bovine external secretions. J. Immunol. 106:552–563.. [PubMed] [Google Scholar]
- Mancini, G., Carbonara A. O., and Heremans J. F.. . 1965. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry 2:235–254.. doi: 10.1016/0019-2791(65)90004-2 [DOI] [PubMed] [Google Scholar]
- Marie Lkke, M., Engelbrecht R., and Wiking L.. . 2016. Covariance structures of fat and protein influence the estimation of IgG in bovine colostrum. J. Dairy Res. 83:58–66.. doi: 10.1017/S0022029915000734 [DOI] [PubMed] [Google Scholar]
- Mayer, B., Doleschall M., Bender B., Bartyik J., Bosze Z., Freny L. V., and Kacskovics I.. . 2005. Expression of the neonatal Fc receptor (FcRn) in the bovine mammary gland. J. Dairy Res. 72 Spec No:107–112.. doi: 10.1017/s0022029905001135 [DOI] [PubMed] [Google Scholar]
- McCoy, G. C., Reneau J. K., Hunter A. G., and Williams J. B.. . 1970. Effects of diet and time on blood serum proteins in the newborn calf. J. Dairy Sci. 53:358–362.. doi: 10.3168/jds.S0022-0302(70)86209-9 [DOI] [PubMed] [Google Scholar]
- McEwan, A. D., Fisher E. W., and Selman I. E.. . 1970. Observations on the immune globulin levels of neonatal calves and their relationship to disease. J. Comp. Pathol. 80:259–265.. doi: 10.1016/0021-9975(70)90093-9 [DOI] [PubMed] [Google Scholar]
- McGee, M., and Earley B.. . 2019. Review: passive immunity in beef-suckler calves. Animal 13:810–825.. doi: 10.1017/S1751731118003026 [DOI] [PubMed] [Google Scholar]
- McGuire, T. C., Pfeiffer N. E., Weikel J. M., and Bartsch R. C.. . 1976. Failure of colostral immunoglobulin transfer in calves dying from infectious disease. J. Am. Vet. Med. Assoc. 169:713–718.. [PubMed] [Google Scholar]
- Michalek, S. M., Rahman A. F., and McGhee J. R.. . 1975. Rat immunoglobulins in serum and secretions: comparison of IgM, IgA and IgG in serum, colostrum, milk and saliva of protein malnourished and normal rats. Proc. Soc. Exp. Biol. Med. 148:1114–1118.. doi: 10.3181/00379727-148-38699 [DOI] [PubMed] [Google Scholar]
- Moore, M., Tyler J. W., Chigerwe M., Dawes M. E., and Middleton J. R.. . 2005. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 226:1375–1377.. doi: 10.2460/javma.2005.226.1375 [DOI] [PubMed] [Google Scholar]
- Morin, D. E., McCoy G. C., and Hurley W. L.. . 1997. Effects of quality, quantity, and timing of colostrum feeding and addition of a dried colostrum supplement on immunoglobulin G1 absorption in Holstein bull calves. J. Dairy Sci. 80:747–753.. doi: 10.3168/jds.S0022-0302(97)75994-0 [DOI] [PubMed] [Google Scholar]
- Morohaku, K., Pelton S. H., Daugherty D. J., Butler W. R., Deng W., and Selvaraj V.. . 2014. Translocator protein/peripheral benzodiazepine receptor is not required for steroid hormone biosynthesis. Endocrinology 155:89–97.. doi: 10.1210/en.2013-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohaku, K., Phuong N. S., and Selvaraj V.. . 2013. Developmental expression of translocator protein/peripheral benzodiazepine receptor in reproductive tissues. PLoS One. 8:e74509. doi: 10.1371/journal.pone.0074509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill, K. M., Conrad E., Lago A., Campbell J., Quigley J., and Tyler H.. . 2012. Nationwide evaluation of quality and composition of colostrum on dairy farms in the United States. J. Dairy Sci. 95:3997–4005.. doi: 10.3168/jds.2011-5174 [DOI] [PubMed] [Google Scholar]
- Oyeniyi, O. O., and Hunter A. G.. . 1978. Colostral constituents including immunoglobulins in the first three milkings postpartum. J. Dairy Sci. 61:44–48.. doi: 10.3168/jds.S0022-0302(78)83549-8 [DOI] [PubMed] [Google Scholar]
- Parrish, D. B., Bartley E. E., Burris D. U., and McIntyre R. T.. . 1953. Properties of the colostrum of the dairy cow. VIII. digestibility of colostrum and milk by calves during the early postnatal days of life. J. Dairy Sci. 36:489–494.. doi: 10.3168/jds.S0022-0302(53)91525-2 [DOI] [Google Scholar]
- Parrish, D. B., Wise G. H., Hughes J. S., and Atkeson F. W.. . 1948. Properties of the colostrum of the dairy cow. II. effect of prepartal rations upon the nitrogenous constituents. J. Dairy Sci. 31:889–895.. doi: 10.3168/jds.S0022-0302(48)92273-5 [DOI] [Google Scholar]
- Pereira, R. V. V, Carroll L. M., Lima S., Foditsch C., Siler J. D., Bicalho R. C., and Warnick L. D.. . 2018. Impacts of feeding preweaned calves milk containing drug residues on the functional profile of the fecal microbiota. Sci. Rep. 8:1–12.. doi: 10.1038/s41598-017-19021-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett, L. C., Gay C. C., Besser T. E., and Hancock D. D.. . 1991. Management and production factors influencing immunoglobulin G1 concentration in colostrum from Holstein cows. J. Dairy Sci. 74:2336–2341.. doi: 10.3168/jds.S0022-0302(91)78406-3 [DOI] [PubMed] [Google Scholar]
- Quigley, J. D., Lago A., Chapman C., Erickson P., and Polo J.. . 2013. Evaluation of the Brix refractometer to estimate immunoglobulin G concentration in bovine colostrum. J. Dairy Sci. 96:1148–1155.. doi: 10.3168/jds.2012-5823 [DOI] [PubMed] [Google Scholar]
- Raboisson, D., Trillat P., and Cahuzac C.. . 2016. Failure of passive immune transfer in calves: a meta-analysis on the consequences and assessment of the economic impact. PLoS One. 11. doi: 10.1371/journal.pone.0150452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo, R. F. S., Ferro J. S. P., Miyashiro S. I., Ferreira P. T., Saut J. P. E., Birgel D. B., and Birgel Junior E. H.. . 2019. The dynamics of individual whey protein concentrations in cows mammary secretions during the colostral and early lactation periods. J. Dairy Res. 86:88–93.. doi: 10.1017/S0022029918000808 [DOI] [PubMed] [Google Scholar]
- Rincheval-Arnold, A., Belair L., and Djiane J.. . 2002. Developmental expression of pIgR gene in sheep mammary gland and hormonal regulation. J. Dairy Res. 69:13–26.. [PubMed] [Google Scholar]
- Sasaki, M., Davis C. L., and Larson B. L.. . 1976. Production and turnover of IgG1 and IgG2 immunoglobulins in the bovine around parturition. J. Dairy Sci. 59:2046–2055.. doi: 10.3168/jds.S0022-0302(76)84486-4 [DOI] [PubMed] [Google Scholar]
- Schalich, K. M., Herren A. W., and Selvaraj V.. . 2020. Analysis of differential strategies to enhance detection of low-abundance proteins in the bovine serum proteome. Anim. Sci. J. 91:e13388. doi: 10.1111/asj.13388 [DOI] [PubMed] [Google Scholar]
- Schneider, C. A., Rasband W. S., and Eliceiri K. W.. . 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675.. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivley, C. B., Lombard J. E., Urie N. J., Haines D. M., Sargent R., Kopral C. A., Earleywine T. J., Olson J. D., and Garry F. B.. . 2018. Preweaned heifer management on US dairy operations: Part II. Factors associated with colostrum quality and passive transfer status of dairy heifer calves. J. Dairy Sci. 101: 9185–9198.. doi: 10.3168/jds.2017-14008 [DOI] [PubMed] [Google Scholar]
- Silva-Del-Ro, N., Rolle D., Garca-Muoz A., Rodrguez-Jimnez S., Valldecabres A., Lago A., and Pandey P.. . 2017. Colostrum immunoglobulin G concentration of multiparous Jersey cows at first and second milking is associated with parity, colostrum yield, and time of first milking, and can be estimated with Brix refractometry. J. Dairy Sci. 100:5774–5781.. doi: 10.3168/jds.2016-12394 [DOI] [PubMed] [Google Scholar]
- Smith, T. 1930. The immunological significance of colostrum: I. The relation between colostrum, serum, and the milk of cows normal and immunized towards B. coli. J. Exp. Med. 51:473–481.. doi: 10.1084/jem.51.3.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T., and Little R. B.. . 1922a. The significance of colostrum to the new-born calf. J. Exp. Med. 36:181–198.. doi: 10.1084/jem.36.2.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T., and Little R. B.. . 1922b. Cow serum as a substitute for colostrum in new-born calves. J. Exp. Med. 36:453. doi: 10.1084/jem.36.4.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T., and Little R. B.. . 1930. The immunological significance of colostrum ii. the initial feeding of serum from normal cows and cows immunized towards b. coli in place of colostrum. J. Exp. Med. 51:483–492.. doi: 10.1084/jem.51.3.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son, H. S., Hong Y. S., Park W. M., Yu M. A., and Lee C. H.. . 2009. A novel approach for estimating sugar and alcohol concentrations in wines using refractometer and hydrometer. J. Food Sci. 74:C106–C111.. doi: 10.1111/j.1750-3841.2008.01036.x [DOI] [PubMed] [Google Scholar]
- Steen, G., Klerk A., Laan K. v., and Eppens E. F.. . 2011. Evaluation of the interference due to haemoglobin, bilirubin and lipids on Immulite 2500 assays: a practical approach. Ann. Clin. Biochem. 48(Pt 2):170–175.. doi: 10.1258/acb.2010.010187 [DOI] [PubMed] [Google Scholar]
- Steinhoff-Wagner, J., Grs S., Junghans P., Bruckmaier R. M., Kanitz E., Metges C. C., and Hammon H. M.. . 2011. Maturation of endogenous glucose production in preterm and term calves 1. J. Dairy Sci. 94:5111–5123.. doi: 10.3168/jds.20114355 [DOI] [PubMed] [Google Scholar]
- Stott, G. H., and Fellah A.. . 1983. Colostral immunoglobulin absorption linearly related to concentration for calves. J. Dairy Sci. 66:1319–1328.. doi: 10.3168/jds.S0022-0302(83)81941-9 [DOI] [PubMed] [Google Scholar]
- Stott, G. H., Fleenor W. A., and Kleese W. C.. . 1981. Colostral immunoglobulin concentration in two fractions of first milking postpartum and five additional milkings. J. Dairy Sci. 64:459–465.. doi: 10.3168/jds.S0022-0302(81)82594-5 [DOI] [PubMed] [Google Scholar]
- Stott, G. H., Marx D. B., Menefee B. E., and Nightengale G. T.. . 1979a. Colostral immunoglobulin transfer in calves I. Period of absorption. J. Dairy Sci. 62:1632–1638.. doi: 10.3168/jds.S0022-0302(79)83472-4 [DOI] [PubMed] [Google Scholar]
- Stott, G. H., Marx D. B., Menefee B. E., and Nightengale G. T.. . 1979b. Colostral immunoglobulin transfer in calves. III. Amount of absorption. J. Dairy Sci. 62:1902–1907.. doi: 10.3168/jds.S0022-0302(79)835213 [DOI] [PubMed] [Google Scholar]
- Stott, G. H., Marx D. B., Menefee B. E., and Nightengale G. T.. . 1979c. Colostral immunoglobulin transfer in calves. IV. Effect of suckling. J. Dairy Sci. 62:1908–1913.. doi: 10.3168/jds.S0022-0302(79)835225 [DOI] [PubMed] [Google Scholar]
- Sutter, F., Borchardt S., Schuenemann G. M., Rauch E., Erhard M., and Heuwieser W.. . 2019. Evaluation of 2 different treatment procedures after calving to improve harvesting of high-quantity and high-quality colostrum. J. Dairy Sci. 102: 9370–9381.. doi: 10.3168/jds.2019-16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, W., and Husband A. D.. . 1922. The effect on the percentage composition of the milk of (a) Variations in the daily volume and (b) Variations in the nature of the diet. J. Agric. Sci. 12:111–124.. doi: 10.1017/S002185960000486X [DOI] [Google Scholar]
- Thames, C. H., Pruden A., James R. E., Ray P. P., and Knowlton K. F.. . 2012. Excretion of antibiotic resistance genes by dairy calves fed milk replacers with varying doses of antibiotics. Front. Microbiol. 3:139. doi: 10.3389/fmicb.2012.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotz-Williams, L. A., Leslie K. E., and Peregrine A. S.. . 2008. Passive immunity in Ontario dairy calves and investigation of its association with calf management practices. J. Dairy Sci. 91:3840–3849.. doi: 10.3168/jds.2007-0898 [DOI] [PubMed] [Google Scholar]
- Tu, L. N., Zhao A. H., Hussein M., Stocco D. M., and Selvaraj V.. . 2016. Translocator Protein (TSPO) affects mitochondrial fatty acid oxidation in steroidogenic cells. Endocrinology 157:1110–1121.. doi: 10.1210/en.2015-1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, J. W., Hancock D. D., Wiksie S. E., Holler S. L., Gay J. M., and Gay C. C.. . 1998. Use of serum protein concentration to predict mortality in mixed-source dairy replacement heifers. J. Vet. Intern. Med. 12:79–83.. doi: 10.1111/j.1939-1676.1998.tb02099.x [DOI] [PubMed] [Google Scholar]
- USDA-APHIS. 2008. Colostrum feeding and management on U.S. dairy operations, 19912007. USDA, editor. 1–4.. https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy07/Dairy07_is_Colostrum.pdf. [Google Scholar]
- Vetter, A., Argello A., Baumrucker C., and Bruckmaier R. M.. . 2013. Short communication: fractional milking distribution of immunoglobulin G and other constituents in colostrum. J. Dairy Sci. 96:5919–5922.. doi: 10.3168/jds.2013-6745 [DOI] [PubMed] [Google Scholar]
- Weaver, D. M., Tyler J. W., VanMetre D. C., Hostetler D. E., and Barrington G. M.. . 2000. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 14:569–577.. doi: [DOI] [PubMed] [Google Scholar]
- Westhoff, T., Overton T., and Mann S.. . 2020. Survey on colostrum management practices in New York State. Progress. Dairy. [Google Scholar]
- Wild, D. 2013. The immunoassay handbook, 4th ed. Oxford, UK: Elsevier Science. [Google Scholar]
- Wojtas, E., Iwaszkiewicz M., Zachwieja A., Pecka-Kieb E., Paczyska K., and Tumanowicz J.. . 2019. Effects of additional colostrum feeding on the levels of protein fractions in calves serum. Mljekarstvo. 69:206–210.. doi: 10.15567/mljekarstvo.2019.0306 [DOI] [Google Scholar]
- Zellner, M., Babeluk R., Diestinger M., Pirchegger P., Skeledzic S., and Oehler R.. . 2008. Fluorescence-based Western blotting for quantitation of protein biomarkers in clinical samples. Electrophoresis 29:3621–3627.. doi: 10.1002/elps.200700935 [DOI] [PubMed] [Google Scholar]
- Zwald, A., Kohlman T. L., Gunderson S. L., Hoffman P. C., and Kriegl T.. . 2007. Economic costs and labor efficiencies associated with raising dairy herd replacements on Wisconsin dairy farms and custom heifer raising operations. Madison, WI: University of Wisconsin-Extension. [Google Scholar]