Abstract
Introduction:
Small, dense low-density lipoprotein (sdLDL) is strongly associated with symptomatic carotid artery stenosis, but is not routinely evaluated in ischemic stroke patients. A method using the logarithmic transformation of the ratio of the plasma concentration of triglycerides (TGY) to HDL-cholesterol (HDL-C)[(Log [TGY/HDL-C])] has been described as a surrogate marker for sdLDL termed the atherogenic index of plasma (AIP).
Objective:
To determine if the AIP is independently associated with symptomatic carotid artery stenosis.
Methods:
We conducted a single center case-controlled study using a sample of ischemic stroke patients and compared risk factors of patients with and without symptomatic carotid artery stenosis. A multivariate logistic regression model was used to determine if the AIP divided into four quartiles was independently associated with symptomatic carotid artery stenosis. This model was compared to three other lipid models. Associations between non-lipid variables and the AIP were also identified.
Results:
31 cases of ischemic stroke due to symptomatic carotid artery stenosis and 236 controls of ischemic stroke not due to carotid artery stenosis were identified. Of the four lipid models assessed, only the model including the AIP (model 4) was found to be significantly associated with symptomatic carotid artery stenosis. The odd’s ratio (OR) for quartile 3 was 3.82 (95% CI 1.03–14.17) and the OR for quartile 4 was 4.13 (95% CI 1.09–15.54) using quartile 1 as a reference. Metabolic syndrome was the only variable associated with the AIP (OR 5.06 95% CI 2.6–9.7).
Conclusion:
At our single center, the AIP was the only lipid parameter independently associated with symptomatic carotid artery stenosis; and metabolic syndrome was independently associated with the AIP. The AIP may serve as a useful surrogate of sdLDL in patients with symptomatic carotid artery stenosis.
Keywords: Symptomatic carotid artery stenosis, Dyslipidemia, Atherogenic index of plasma, Small dense LDL
Introduction
A strong relationship exists between small dense LDL (sdLDL), carotid artery intima-media thickness (CIMT), and symptomatic carotid artery stenosis.1–4 Lipoprotein phenotyping, however, is not routinely done in hospitalized ischemic stroke patients. Dobiasova et al. have described a novel method to determine lipoprotein particle size using the logarithmic transformation of the ratio of the plasma concentration of triglycerides (TGY) to HDL-cholesterol (HDL-C)[(Log[TGY/HDL-C])].5 They have termed this parameter the “atherogenic index of plasma” (AIP) and other authors have confirmed their findings.6–10 Conventional assessment of lipoprotein particle size distribution requires gradient gel electrophoresis whereas the method described by Dobisaova can accurately estimate sdLDL by using routine serum lipid values. This is favorable for ischemic stroke research given that lipid profiles are routinely checked in ischemic stroke patients admitted to the hospital.
To our knowledge, the AIP has not been evaluated as a risk factor for symptomatic carotid artery stenosis. The purpose of this study is to determine if an independent relationship exists between the AIP and symptomatic carotid artery stenosis at our single center.
Materials and Methods
Study Design
We conducted a case-controlled study including all acute ischemic stroke patients from our electronic medical record (EMR) from January 2011 to December 2018. We utilized an analytical reporting software that abstracts data from a database within the EMR based on select criteria. The EMR database collects and stores all clinical information for all patients admitted to our hospital. Charts were identified by unique order sets that are only used for acute ischemic stroke patients. Three chart reviewers that were blinded to the study hypothesis (N.K., S. P., S. Z.) reviewed each chart manually to ensure accuracy; and also added information that could not be electronically abstracted. The chart reviewers underwent institutional training regarding ethical research practice; given a 30 min training session; and given written instructions on how to systematically review charts. A sample of charts (10%) from each abstractor was reviewed independently by the primary author to ensure accuracy. Institutional review board approval was obtained for this review.
Selection of Participants and Variables
Our hospital is a 547-bed academic center and regional comprehensive stroke center. Ischemic stroke patients ≥18 years of age who had radiographic evidence of ischemic infarction were included. Patients treated via our telestroke network and outside hospital transfers were excluded. Our institutional stroke protocols are consistent with the American Heart Association guidelines for the management of acute ischemic stroke.11 A fasting lipid profile is checked in all patients within 24 h of admission. The following baseline variables were collected: age, gender, glucose on presentation, National Institute of Health Stroke Scale (NIHSS) on presentation, mean arterial pressure (MAP) on presentation, body mass index (BMI), and hemoglobin A1c (HgbA1c). The patient’s past medical history and lipid profiles were also reviewed. Symptomatic carotid artery stenosis was defined as stenosis ≥ 50% and the most likely etiology of the ischemic stroke. The designation of symptomatic carotid artery stenosis was determined by review of non-invasive or angiographic imaging reports; and attending stroke physician documentation. The AIP was calculated by taking the logarithm to the base 10 of the ratio of the fasting plasma TGY to HDL-C measured in mmol/l. TGY measured in mg/dl was converted to mmol/l by dividing by 88.57 and HDL-C was converted by dividing by 38.67.12
Analyses and Outcome
Baseline characteristics comparing symptomatic and asymptomatic carotid stenosis were described as means with standard deviations or medians with interquartile ranges (IQR); and percentages. Continuous parametric variables were compared using a t-test. Continuous nonparametric data were analyzed utilizing the Mann-Whitney U test. A Shapiro—Wilk test was used to determine normality. Chi-square test or Fisher’s exact test were used to compare categorical variables.
Lipid variables (TGY, HDL-C, LDL) were included using four different logistic regression models: TGY, HDL-C, and LDL variables included in model 1; only the LDL/HDL-C ratio in model 2; only the TGY/HDL-C ratio in model 3; and the AIP separated into four quartiles in model 4 with quartile 1 as the reference. A logistic regression model was also used to determine associations between non-lipid variables and the AIP. A backward stepwise regression technique was used for all logistic regression models to determine the model with best fit. All models were hierarchically well-formulated. A pvalue ≤ .05 was considered significant in univariate and multivariate analysis.
Results
Our analytical software identified 267 charts based on our criteria. Table 1 compares the variables between the groups. 236 patients had ischemic infarctions not due to carotid artery stenosis while 31 patients had symptomatic carotid artery stenosis. Univariate analysis for symptomatic carotid artery stenosis identified male sex (p = .024) as the only non-lipid variable associated with symptomatic carotid artery stenosis. Results for the four logistic regression models for the dependent variable symptomatic carotid stenosis are noted in Table 2. Only model 4, the model that included the AIP as an independent variable, found that quartile 3 and 4 were independently associated with symptomatic carotid artery stenosis (AIP Quartile 3 odd’s ratio 3.82 95% CI 1.03–14.15; AIP Quartile 4 odd’s ratio 4.13 95% CI 1.09–15.54). We did not identify any non-lipid variables that were independently associated with symptomatic carotid stenosis within any of the models. In the regression model that assessed AIP as a dependent variable, metabolic syndrome (odd’s ratio 5.06 95% CI 2.6–9.7) was the only variable independently associated with AIP.
Table 1.
Comparison of Ischemic Stroke Risk Factors Variables expressed as medians (IQR) or N (%).
| Ischemic Stroke not due to Carotid Stenosis (N = 236) | Ischemic Stroke due to Symptomatic Carotid Stenosis (N = 31) | |
|---|---|---|
| Age | 66 (19) | 66 (16) |
| Male Sex* | 109 (46%) | 21 (68%) |
| LOS | 4 (5) | 4 (3) |
| Glucose on Presentation | 121.5 (63.5) | 130 (41) |
| NIHSS on Admission | 5 (7) | 7 (12) |
| MAP on Presentation | 107.3 (24.2) | 105.7 (38) |
| BMI | 28.3 (9.5) | 27.4 (11.7) |
| HgbA1C | 5.8 (1.4) | 6.1 (1.8) |
| History of Hypertension | 186 (78%) | 23 (74%) |
| History of Diabetes | 78 (33%) | 13 (42%) |
| History Atrial Fibrillation | 59 (25%) | 3 (10%) |
| History of Obstructive Sleep Apnea | 16 (7%) | 1 (3%) |
| History of Ischemic Stroke | 50 (21%) | 4(13%) |
| History of Coronary Artery Disease | 92 (39%) | 12 (39%) |
| History of Venous Thromboembolism | 18 (8%) | 0 (0%) |
| Chronic Kidney Disease | 30 (13%) | 3 (10%) |
| Tobacco Abuse | 120 (53%) | 22 (71%) |
| TGY ≥ 150 mg/dl | 60 (25%) | 7 (22.5%) |
| HDL-C ≤ 49 mg/dl | 156 (66.6%) | 26 (84%) |
| LDL ≥ 100 mg/l | 101 (44%) | 11 (35%) |
| LDL/HDL-C | 2.0 (1.3) | 2.3 (1.3) |
| TGY/HDL-C | 2.3 (2.7) | 2.8 (2.4) |
| AIP | .004 (.480) | .101 (.329) |
| Metabolic Syndrome | 64 (31%) | 11 (37%) |
p ≤ .05.
Abbreviations: NIHSS, National Institute of Health Stroke Scale; MAP, mean arterial pressure; HgbA1C, Hemoglobin A1C; TGY, Triglycerides; HDL-C, High-density Lipoprotein; LDL, Low-density Lipoprotein; AIP, Atherogenic Index of Plasma.
Table 2.
Lipid Parameters Independently Associated with Symptomatic Carotid Stenosis.
| Model* | Lipid Parameters Assessed | OR (95% CI) |
|---|---|---|
| 1 | LDL ≥ 100 mg/dl | 1.30 (.57–2.95) |
| TGY ≥ 150 mg/dl | 1.26 (.98–7.46) | |
| HDL ≤ 49 mg/dl | 2.70 (.98–7.46) | |
| 2 | LDL/HDL ratio | 1.10 (.82–1.48) |
| 3 | TGY/HDL ratio | .98 (.89–1.11) |
| 4 | AIP Quartile 2 | 2.14 (.49–9.41) |
| AIP Quartile 3 | *3.82 (1.03–14.17) | |
| AIP Quartile 4 | *4.13 (1.09–15.54) | |
p < .05.
Abbreviations: OR, Odd’s Ratio; CI, Confidence Interval; TGY, Triglycerides; HDL-C, High-density Lipoprotein; LDL, Low-density Lipoprotein; AIP, Atherogenic Index of Plasma.
Discussion
The major finding from our single center case-controlled study is the AIP is independently associated with symptomatic carotid artery stenosis. We did not identify any other lipid variables associated with symptomatic carotid artery stenosis.
The primary benefit from using the AIP over other lipid parameters is its strong association with sdLDL5. Many different lipoprotein abnormalities have been described in patients with carotid artery stenosis including low HDL-C;13–15 elevated LDL;16 elevated TGY;17 the LDL/HDL-C ratio;18–20 and the TGY/HDL-C ratio.21,22 These lipid parameters were not associated with symptomatic carotid artery stenosis in our cohort in regression models 1–3. This discrepancy may be because most of these lipid parameters were studied in cohorts with asymptomatic carotid artery stenosis patients16,18–22 while our cohort only included recently symptomatic patients. Analogous to stable versus unstable coronary heart disease (CHD), there are important difference between stable and unstable carotid atherosclerotic plaques including plaque vascularity, intraplaque hemorrhage, and fibrous cap thinning; and these differences may be independent of the degree of stenosis.23,24 The point estimates for the odd’s ratios (OR) of the AIP for quartile 3 and 4 were high, albeit with low precision; and, an exposure-response relationship in which the point estimates increase as the AIP quartile increases is supportive of causality.
Our data is consistent with the literature that the AIP may be used as a surrogate for sdLDL and its cardiovascular consequences. Multiple studies have shown an association between the AIP and CHD.8,9,25 Additionally, Yildiz et al. have reported a positive correlation between the AIP and increased CIMT in end-stage renal disease (ESRD) patients undergoing hemodialysis.6 sdLDLs have certain properties that make them prone to be atherogenic. These include reduced blood stream clearance; enhanced oxidation compared to other lipoproteins; enhanced binding to endothelial proteoglycans; enhanced penetration through the endothelial barrier; and increased uptake by macrophage scavenger receptors. These properties increase foam cell formation and are the initial stages of atherogenesis.26–30
When compared to typical lipid parameters, Shoji et al. found that sdLDL is the best marker of carotid atherosclerosis.4 The authors used a multivariate linear regression model adjusted for multiple non-lipid covariates in a cohort of 326 patients to determine the relationship between lipid parameters and CIMT. sdLDL had the highest regression coefficient suggestive of a more positive linear relationship compared to other lipid parameters. Additionally, sdLDL was also positively correlated with plasma TGY and inversely associated with HDL-C consistent with the relationship identified using the AIP. Our data is consistent with their findings when the AIP is used as a surrogate marker for sdLDL.
In addition to extracranial carotid artery stenosis, sdLDL has been associated with short term mortality and functional outcome after ischemic stroke.31 Despite the strong relationship between carotid artery atherosclerotic disease and sdLDL, evaluation of lipoprotein particle size is not routinely done in ischemic stroke patients. Given that serum lipids are routinely checked in ischemic stroke patients, the AIP is a promising marker for the assessment of lipoprotein particle size.
We additionally found that metabolic syndrome is positively and independently associated with the AIP. Metabolic syndrome has previously been associated with the AIP,9,32 carotid artery atherosclerotic disease,33–35 and ischemic stroke. A recent analysis of the Atherosclerosis Risk in Communities (ARIC) and Jackson Heart Study (JHS) cohorts found that the risk of ischemic stroke increased with metabolic syndrome severity when stratified into quartiles.36 Given that the major dyslipidemic consequence of metabolic syndrome is the development of sdLDL,37,38 we suspect that sdLDL is a mediator for the causal pathway between metabolic syndrome and symptomatic carotid artery stenosis (Fig. 1, pathway 1). Given that metabolic syndrome is made up of components that are also independently associated with carotid artery stenosis,35 there likely are additional mechanisms independent of sdLDL (Fig. 1, pathway 2).
Figure 1.
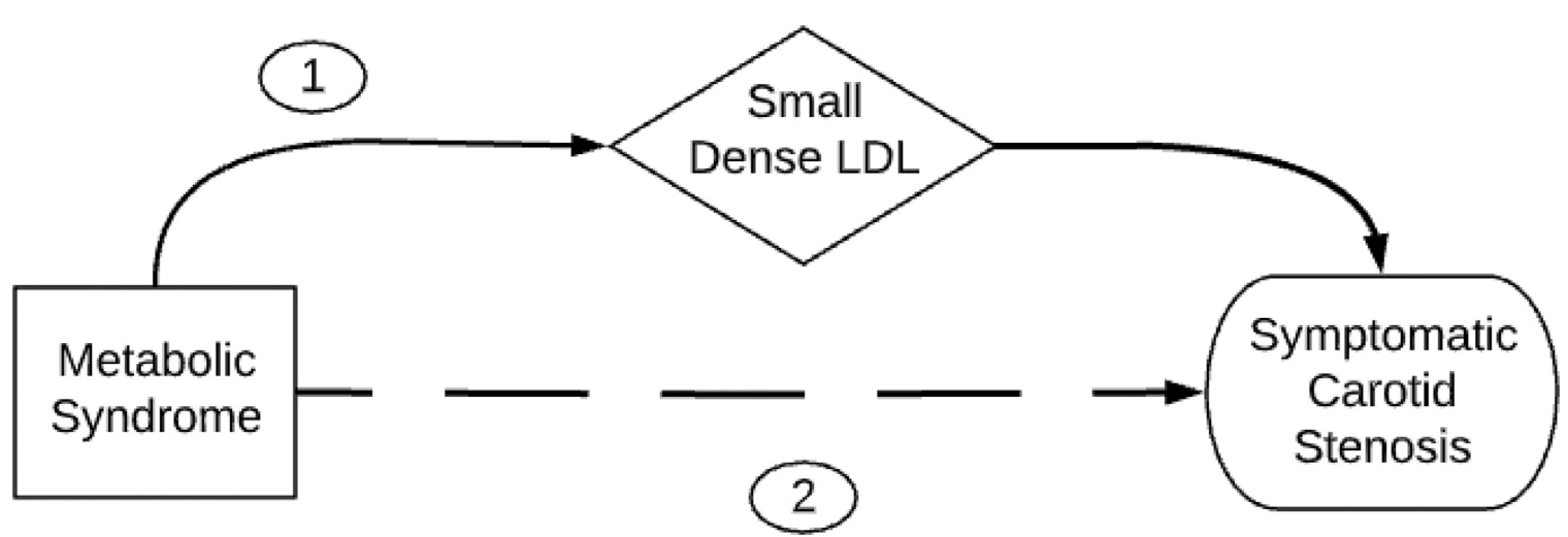
Directed acyclic graph depicting two direct causal pathways to symptomatic carotid stenosis. Pathway 1 depicts small dense LDL (sdLDL) as a mediator between metabolic syndrome and symptomatic carotid stenosis. Pathway 2 depicts a causal pathway way between metabolic syndrome and symptomatic carotid stenosis independent of sdLDL.
Prior studies have also suggested that the AIP is inversely associated with measures of insulin sensitivity; and the clustering of sdLDL, metabolic syndrome, and insulin resistance may have important therapeutic implications.39,40 For example, pioglitazone is a thiazolidinedione that reduces insulin resistance.39,41 Kernan et al. studied the effect of pioglitazone in patients with recent ischemic stroke and transient ischemic attack (TIA) in patients with insulin resistance; and without diabetes in the Insulin Resistance Intervention after Stroke (IRIS) study. The primary outcome that was a composite of ischemic stroke or myocardial infarction occurred in 9.0% of patients in the pioglitazone group and 11.8% of patients in the control arm (hazard ratio .76; 95% confidence interval [CI] .62 to .93; p = .007). In addition to improving insulin sensitivity, pioglitazone also reduces sdLDL.42 Given the biological relationship between sdLDL and insulin resistance, we suspect that the reduction in cerebrovascular events due to pioglitazone may also be due to improvements in lipoprotein particle size. To our knowledge, this has not been studied and may be an important area of future research.
The AIP would be well suited as a biomarker for research in carotid atherosclerosis lipidology. For example, currently statin agents are the only lipid directed pharmacotherapy for patients with carotid atherosclerosis43. Conversely, fibrates are known to target atherogenic dyslipidemia, lower sdLDL, and reduce major coronary events, yet have not shared similar success to statin agents in reducing cerebrovascular events.44,45 Future studies would benefit from testing fibrates, and other agents, in patients with cerebrovascular disease stratified by sdLDL status. As the AIP can be calculated from a plasma lipid profile, this marker would be well suited for multicenter studies from a cost and external validity standpoint.
Our study has several limitations that are worth noting. First, we did not account for ethnic differences between our groups due to the frequency of this missing information from the medical records. Second, our statistical analysis was limited by the number of cases identified. Given the modest number of cases compared to controls, a type I error is possible. The limited number of cases is also the most likely explanation for low precision with wide confidence intervals. However, in a large CHD cohort where multiple lipid parameters were correlated with CHD risk, the OR of CHD risk was highest in the model including AIP compared to models using other lipid parameters9. Therefore, we suspect we were able to find a positive association using the AIP despite a modest number of cases because of a similarly strong association. Furthermore, biological plausibility and an exposure-response relationship favor a true association over a type I error. We also did not account for lipid lowering therapies administered that may confound lipid profiles. We are doubtful that this meaningfully biases the internal validity of this study as we do not routinely use treatments directed at the variable of interest. This may, however, be the cause of type II errors in models 1–3. Finally, our study is retrospective and we cannot be certain of unmeasured confounders between the groups. Therefore, the conclusions from our study should be considered hypothesis generating until a prospective cohort study is done.
In conclusion, we found an independent relationship between symptomatic carotid artery stenosis and atherogenic dyslipidemia as measured by the AIP; and metabolic syndrome was an independent predictor of the AIP at our single center.
Acknowledgement
The authors acknowledge the Stritch School of Medicine at Loyola University for their support in the publication of this manuscript.
Funding Sources
The authors had no sources of funding for this study.
Footnotes
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Statement of Ethics
Our study was performed in concordance with ethical research practice guidelines at our institution, including approval from our institutional IRB.
References
- 1.Landray MJ, Sagar G, Muskin J, Murray S, Holder RL, Lip GY. Association of atherogenic low-density lipoprotein subfractions with carotid atherosclerosis. QJM 1998;91(5):345–351. [DOI] [PubMed] [Google Scholar]
- 2.Shen H, Zhou J, Shen G, Yang H, Lu Z, Wang H. Correlation between serum levels of small, dense low-density lipoprotein cholesterol and carotid stenosis in cerebral infarction patients>65 years of age. Ann Vasc Surg 2014;28(2):375–380. [DOI] [PubMed] [Google Scholar]
- 3.Hulthe J, Bokemark L, Wikstrand J, Fagerberg B. The metabolic syndrome, LDL particle size, and atherosclerosis: the atherosclerosis and insulin resistance (AIR) study. Arterioscler Thromb Vasc Biol 2000;20(9):2140–2147. [DOI] [PubMed] [Google Scholar]
- 4.Shoji T, Hatsuda S, Tsuchikura S, et al. Small dense low-density lipoprotein cholesterol concentration and carotid atherosclerosis. Atherosclerosis 2009;202(2):582–588. [DOI] [PubMed] [Google Scholar]
- 5.Dobiášová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate inapob-lipoprotein-depleted plasma (FER HDL). Clin Biochem 2001;7(34):583–588. [DOI] [PubMed] [Google Scholar]
- 6.Yildiz G, Duman A, Aydin H, et al. Evaluation of association between atherogenic index of plasma and intima media thickness of the carotid artery for subclinic atherosclerosis in patients on maintenance hemodialysis. Hemodial Int 2013;17(3):397–405. [DOI] [PubMed] [Google Scholar]
- 7.Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, et al. Atherogenic index of plasma (AIP): a marker of cardiovascular disease. Med J Islam Repub Iran 2015;29:240. [PMC free article] [PubMed] [Google Scholar]
- 8.Onat A, Can G, Kaya H, Hergenç G. Atherogenic index of plasma”(log10 triglyceride/high-density lipoprotein—cholesterol) predicts high blood pressure, diabetes, and vascular events. Journal of clinical lipidology 2010;4 (2):89–98. [DOI] [PubMed] [Google Scholar]
- 9.Cai G, Shi G, Xue S, Lu W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the chinese han population. Medicine (Baltimore) 2017;96(37):e8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nwagha U, Ikekpeazu E, Ejezie F, Neboh E, Maduka I. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr Health Sci 2010;10(3). [PMC free article] [PubMed] [Google Scholar]
- 11.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2019;50(12): e344–e418. [DOI] [PubMed] [Google Scholar]
- 12.Rugge B, Balshem H, Sehgal R, Relevo R, Gorman P, Helfand M. Screening and treatment of subclinical hypothyroidism or hyperthyroidism. 2011. [PubMed]
- 13.Exner M, Minar E, Mlekusch W, et al. Myeloperoxidase predicts progression of carotid stenosis in states of low high-density lipoprotein cholesterol. J Am Coll Cardiol 2006;47(11):2212–2218. [DOI] [PubMed] [Google Scholar]
- 14.Garvey L, Makaroun MS, Muluk VS, Webster MW, Muluk SC. Etiologic factors in progression of carotid stenosis: a 10-year study in 905 patients. J Vasc Surg 2000;31 (1):31–38. [DOI] [PubMed] [Google Scholar]
- 15.Mathiesen EB, Bønaa KH, Joakimsen O. Low levels of high-density lipoprotein cholesterol are associated with echolucent carotid artery plaques: the tromsø study. Stroke 2001;32(9):1960–1965. [DOI] [PubMed] [Google Scholar]
- 16.Grotta JC, Yatsu FM, Pettigrew LC, et al. Prediction of carotid stenosis progression by lipid and hematologic measurements. Neurology 1989;39(10):1325–1331. [DOI] [PubMed] [Google Scholar]
- 17.Labreuche J, Touboul P, Amarenco P. Plasma triglyceride levels and risk of stroke and carotid atherosclerosis: a systematic review of the epidemiological studies. Atherosclerosis 2009;203(2):331–345. [DOI] [PubMed] [Google Scholar]
- 18.Yang C, Sun Z, Li Y, Ai J, Sun Q, Tian Y. The correlation between serum lipid profile with carotid intima-media thickness and plaque. BMC Cardiovasc Disord 2014;14 (1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamada M, Makita S, Abiko A, Naganuma Y, Nagai M, Nakamura M. Low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a useful marker for early-stage carotid atherosclerosis. Metab Clin Exp 2010;59(5):653–657. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto M, Adachi H, Hirai Y, et al. LDL-C/HDL-C ratio predicts carotid intima-media thickness progression better than HDL-C or LDL-C alone. J Lipids 2011;2011:549137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacifico L, Bonci E, Andreoli G, et al. Association of serum triglyceride-to-HDL cholesterol ratio with carotid artery intima-media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr Metab Cardiovasc Dis 2014;24(7):737–743. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Deng Y, Yang M, Wu Y, Sun S, Sun J. Triglyceride to high—density lipoprotein cholesterol ratio and carotid intima—medial thickness in chinese adolescents with newly diagnosed type 2 diabetes mellitus. Pediatr Diabetes 2016;17(2):87–92. [DOI] [PubMed] [Google Scholar]
- 23.Mofidi R, Crotty T, McCarthy P, Sheehan S, Mehigan D, Keaveny T. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. Br J Surg 2001;88(7):945–950. [DOI] [PubMed] [Google Scholar]
- 24.Carr S, Farb A, Pearce WH, Virmani R, Yao JS. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg 1996;23(5):755–766. [DOI] [PubMed] [Google Scholar]
- 25.Frohlich J, Dobiasova M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin Chem 2003;49(11):1873–1880. [DOI] [PubMed] [Google Scholar]
- 26.Sigala F, Kotsinas A, Savari P, et al. Oxidized LDL in human carotid plaques is related to symptomatic carotid disease and lesion instability. J Vasc Surg 2010;52(3): 704–713. [DOI] [PubMed] [Google Scholar]
- 27.Nishi K, Itabe H, Uno M, et al. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol 2002;22(10):1649–1654. [DOI] [PubMed] [Google Scholar]
- 28.Chiesa R, Melissano G, Castellano R, et al. In search of biological markers of high-risk carotid artery atherosclerotic plaque: Enhanced LDL oxidation. Ann Vasc Surg 1998;12(1):1–9. [DOI] [PubMed] [Google Scholar]
- 29.Hulthe J, Bokemark L, Wikstrand J, Fagerberg B. The metabolic syndrome, LDL particle size, and atherosclerosis: The atherosclerosis and insulin resistance (AIR) study. Arterioscler Thromb Vasc Biol 2000;20(9):2140–2147. [DOI] [PubMed] [Google Scholar]
- 30.Zeljkovic A, Vekic J, Spasojevic-Kalimanovska V, et al. LDL and HDL subclasses in acute ischemic stroke: Prediction of risk and short-term mortality. Atherosclerosis 2010;210(2):548–554. [DOI] [PubMed] [Google Scholar]
- 31.Song T, Cho H, Chang Y, et al. Low-density-lipoprotein particle size predicts a poor outcome in patients with atherothrombotic stroke. J Clin Neurol 2015;11(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunes SOV, Melo de, Piccoli Luiz Gustavo, de Castro Marcia, Pizzo Regina, et al. Atherogenic index of plasma and atherogenic coefficient are increased in major depression and bipolar disorder, especially when comorbid with tobacco use disorder. J Affect Disord 2015;172:55–62. [DOI] [PubMed] [Google Scholar]
- 33.Ishizaka N, Ishizaka Y, Toda E, Hashimoto H, Nagai R, Yamakado M. Association between cigarette smoking, metabolic syndrome, and carotid arteriosclerosis in japanese individuals. Atherosclerosis 2005;181(2):381–388. [DOI] [PubMed] [Google Scholar]
- 34.Kawamoto R, Tomita H, Oka Y, Kodama A, Kamitani A. Metabolic syndrome amplifies the LDL-cholesterol associated increases in carotid atherosclerosis. Intern Med 2005;44(12):1232–1238. [DOI] [PubMed] [Google Scholar]
- 35.Kawamoto R, Tomita H, Ohtsuka N, Inoue A, Kamitani A. Metabolic syndrome, diabetes and subclinical atherosclerosis as assessed by carotid intima-media thickness. J Atheroscler Thromb 2007;14(2):78–85. [DOI] [PubMed] [Google Scholar]
- 36.DeBoer MD, Filipp SL, Sims M, Musani SK, Gurka MJ. Risk of ischemic stroke increases over the spectrum of metabolic syndrome severity. Stroke 2020;120:028944. STROKEAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakano S, Kuboki K, Matsumoto T, Nishimura C, Yoshino G. Small, dense LDL and high-sensitivity C-reactive protein (hs-CRP) in metabolic syndrome with type 2 diabetes mellitus. J Atheroscler Thromb. 2010:1003020189. [DOI] [PubMed] [Google Scholar]
- 38.Ruotolo G, Howard BV. Dyslipidemia of the metabolic syndrome. Curr Cardiol Rep 2002;4(6):494–500. [DOI] [PubMed] [Google Scholar]
- 39.Tan MH, Johns D, Glazer NB. Pioglitazone reduces atherogenic index of plasma in patients with type 2 diabetes. Clin Chem 2004;50(7):1184–1188. [DOI] [PubMed] [Google Scholar]
- 40.Adiels M, Olofsson S, Taskinen M, Borén J. Overproduction of very low density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28(7):1225–1236. [DOI] [PubMed] [Google Scholar]
- 41.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374(14):1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winkler K, Konrad T, Fullert S, et al. Pioglitazone reduces atherogenic dense LDL particles in nondiabetic patients with arterial hypertension: a double-blind, placebo-controlled study. Diabetes Care 2003;26(9):2588–2594. [DOI] [PubMed] [Google Scholar]
- 43.Ricotta JJ, AbuRahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated society for vascular surgery guidelines for management of extracranial carotid disease. J Vasc Surg 2011;54(3):e1–e31. [DOI] [PubMed] [Google Scholar]
- 44.Katsiki N, Nikolic D, Montalto G, Banach M, Mikhailidis DP, Rizzo M. The role of fibrate treatment in dyslipidemia: an overview. Curr Pharm Des 2013;19(17): 3124–3131. [DOI] [PubMed] [Google Scholar]
- 45.Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet North Am Ed 2010;375(9729):1875–1884. [DOI] [PubMed] [Google Scholar]


