Abstract
Direct determination of muscle fiber composition is invasive and expensive, with indirect methods also requiring specialist resources and expertise. Performing resistance exercises at 80% 1RM is suggested as a means of indirectly estimating muscle fiber composition, though this hypothesis has never been validated against a direct method. The aim of the study was to investigate the relationship between the number of completed repetitions at 80% 1RM of back squat exercise and muscle fiber composition. Thirty recreationally active participants’ (10 females, 20 males) 1RM back squat load was determined, before the number of consecutive repetitions at 80% 1RM was recorded. The relationship between the number of repetitions and the percentage of fast-twitch fibers from vastus lateralis was investigated. The number of completed repetitions ranged from 5 to 15 and was independent of sex, age, 1RM, training frequency, training type, training experience, BMI or muscle fiber cross-sectional area. The percentage of fast-twitch muscle fibers was inversely correlated with the number of repetitions completed (r = –0.38, P = 0.039). Participants achieving 5 to 8 repetitions (n = 10) had significantly more fast-twitch muscle fibers (57.5 ± 9.5 vs 44.4 ± 11.9%, P = 0.013) than those achieving 11–15 repetitions (n = 11). The remaining participants achieved 9 or 10 repetitions (n = 9) and on average had equal proportion of fast- and slow-twitch muscle fibers. In conclusion, the number of completed repetitions at 80% of 1RM is moderately correlated with muscle fiber composition.
Keywords: Fiber type, Strength training, Vastus lateralis, Exercise prescription, Endurance
INTRODUCTION
The variable individual response to exercise training [1] is likely to be influenced by the heterogeneity of muscle fiber composition [2, 3]. Oxidative type I (slow-twitch) muscle fibers are predominantly suited to aerobic activities and repetitive submaximal contractions, whilst type II (fast-twitch, subdivided into oxidative type IIa and glycolytic type IIx) fibers have greater cross-sectional area and faster shortening velocity than type I fibers [4], and are preferentially recruited during anaerobic activities necessitating strength and power [5]. Whilst appropriate programme design is important in attaining the desired response to sustained training, variable inter-individual responses exist and are likely to be influenced by the muscle fiber composition of each individual. Accordingly, information concerning the individual variability in muscle fiber composition could assist coaches and practitioners when designing individualised training programmes tailored toward specific physiological adaptations [6].
The vastus lateralis (VL) muscle is key contributor to athletic movements such as running and jumping [7]. However, the proportion of fiber types in the VL varies considerably, ranging between 15–85% type I, 5–77% type IIa, and 0–44% type IIx [8–10]. The gold-standard to directly determine muscle fiber composition is the muscle biopsy, with the VL most commonly sampled due to its mixed fiber composition and accessibility [11]. Subsequently, the fiber composition of biopsies is determined using methods such as immunohistochemical analysis, histochemical staining for myosin ATPase, gel electrophoresis and mass-spectrometry [12–14], with transcriptomic analysis of the MYH1, MYH2 and MYH7 genes recently used to determine type IIx, type IIa and type I muscle fibers, respectively [15]. Nevertheless, collecting muscle biopsies is invasive, and subsequent analyses are expensive to perform, limiting accessibility to direct analytical methods.
Indirect methods to estimate muscle fiber composition offer alternative approaches that can be validated against direct methods. Tensiomyography (TMG) evaluates the morphofunctional potential of a muscle according to the radial enlargement of the muscle abdomen in response to electrical stimulation [16], with time to muscle contraction well correlated with slow-twitch muscle fiber proportion [17]. With fast-twitch fibers containing twice the carnosine content of slow-twitch fibers, magnetic resonance spectroscopy (MRS) of carnosine content is also indicative of fiber type [18]. This method was recently used to predict recovery from Wingate cycling, with considerable differences in knee extensor strength recovery between participants with predominantly slow-twitch fibers and participants with predominantly fast-twitch fibers [19]. Muscle fiber composition can also be estimated according to the vibrational properties of contracting skeletal muscle by mechanomyography (MMG), which demonstrates 80% accuracy in predicting VL fiber composition [20]. In addition to morphological measures, skeletal muscle phenotypes are associated with common genetic variants, suggesting that testing individuals for known genetic variants may aid the estimation of muscle fiber composition. Specific genes associated with muscle fiber type include ACE, ACTN3, AGTR2, CBLN2, CPNE5, HIF1A, FTO, PPARA, SPEG, TGFA, and VEGFR2 [21–33], with additional variants likely to exist. However, genomic research requires specialist knowledge and resources, further demonstrating the potential value of simple and cost-effective methods to indirectly estimate muscle fiber composition.
Inter-individual differences are evident in the number of completed repetitions at different percentages of 1 repetition maximum (1RM), with heterogeneity in muscle fiber composition suggested as an underlying mechanism [34]. Accordingly, the number of repetitions achieved at specific percentages of 1RM has been proposed as a practical approach to estimate muscle fibre composition [35]. However, despite the association of exertion at specific percentages of 1RM with the variability in repetitions performed [36], there are no published studies reporting significant correlations between direct estimates of muscle fiber composition and multiple repetition testing. In one study by Terzis et al. [37], leg press machine testing was used to determine the association of 70% and 85% 1RM repetition tests with muscle fibre composition, with participants possessing greater proportions of slow-twitch muscle fibres completing more repetitions. However, this result did not reach statistical significance, potentially due to a limited sample size (n = 12). With access to a leg press machine not always possible, back squat exercise using an Olympic barbell offers a viable alternative for exercise testing. Furthermore, there is also a need to study participants of both genders and to do so with a sample size greater than previous investigations. Therefore, the aim of this study was to determine the interrelationship between muscle fiber composition of the vastus lateralis of 30 participants (10 females and 20 males) and the total number of consecutive repetitions achieved at 80% of 1RM using back squat exercise. It was hypothesised that the number of repetitions completed would be inversely correlated with the proportion of fast-twitch muscle fibers and would be independent of gender.
MATERIALS AND METHODS
Subjects
Thirty recreationally active participants (10 females, age 26.0 ± 7.3; 20 males; age 32.2 ± 7.6) participated in the study. Participant characteristics are shown in Table 1. Participants’ training parameters were assessed using a standardised questionnaire, with participants classified according to training frequency as moderately active (3–4 training sessions per week; n = 11), highly active (5–7 training sessions per week; n = 11) and extremely active (1–2 training sessions per day; n = 8). Participants’ training backgrounds were classified as either resistance type (3 females, 10 males) or mixed type (aerobic + resistance; 7 females, 10 males), with training experience expressed as the total number training years. The procedures adopted in this study were conducted ethically according to the Declaration of Helsinki for research involving human subjects. Ethical approval was obtained from the Ethics Committee of the Physiological Section of the Russian National Committee for Biological Ethics in Russia and the Ethics Committee of the Federal Research and Clinical Center of Physical-chemical Medicine of the Federal Medical and Biological Agency of Russia. Written informed consent was obtained from all participants prior to participation.
TABLE 1.
Characteristics and exercise testing results of study participants
| Characteristics | Female (n = 10) |
Male (n = 20) |
P |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age, years | 26.0 ± 7.3 | 32.2 ± 7.6 | 0.024 |
| Training experience, years | 10.4 ± 7.0 | 11.8 ± 7.8 | 0.725 |
| Height, cm | 169.0 ± 5.2 | 179.4 ± 5.8 | 0.0003 |
| Weight, kg | 57.3 ± 6.2 | 80.1 ± 13.8 | < 0.0001 |
| BMI, kg/m2 | 20.0 ± 1.2 | 24.9 ± 4.3 | 0.0005 |
| Proportion of fast-twitch MF, % | 48.4 ± 10.9 | 52.2 ± 15.3 | 0.619 |
| Proportion of slow-twitch MF, % | 56.3 ± 9.8 | 51.1 ± 15.4 | 0.397 |
| CSA of fast-twitch MF, μm2 | 4173 ± 1488 | 6625 ± 2530 | 0.004 |
| CSA of slow-twitch MF, μm2 | 4662 ± 1013 | 5333 ± 1743 | 0.176 |
| 1RM squat (kg) | 70.5 ± 31.3 | 155.2 ± 62.7 | 0.0005 |
| Completed repetitions at 80% 1RM | 9.8 ± 2.7 | 10.3 ± 2.8 | 0.659 |
Procedures
Back squat testing
All participants were familiar with back squat exercise with a minimum of two years experience. Participants performed a 1RM protocol to determine the maximal load they could lift for the back squat with correct technique using a standard 20 kg Olympic barbell. Briefly, participants performed a warm-up with a self-selected load that allowed them to complete a minimum of 8 repetitions. For each 1RM attempt, participants squatted (descending and ascending for 2 s and 1 s, respectively) with the bar placed across the posterior deltoids and until the tops of their thighs were parallel to the ground [38]. One repetition maximum was determined according to guidelines published by the National Strength and Conditioning Association [38]. After successfully achieving their 1RM load, participants rested passively for 15 minutes. After resting, participants were instructed to perform as many consecutive repetitions at 80% of their individualised 1RM load in one continuous attempt, adhering to the same criteria for descent, ascent and bar positioning as during 1RM attempts. All attempts were vocally encouraged, directed and supervised by the test administrator who was qualified in exercise prescription. A load of 80% 1RM was selected because this load is recommended for increasing muscle strength and achieving muscle hypertrophy [39], meaning this load is commonly prescribed by practitioners working with the athletes and general population, and because 80% 1RM was originally proposed as the most appropriate load to indirectly estimate muscle fiber composition [35].
Evaluation of muscle fiber composition and cross-sectional area
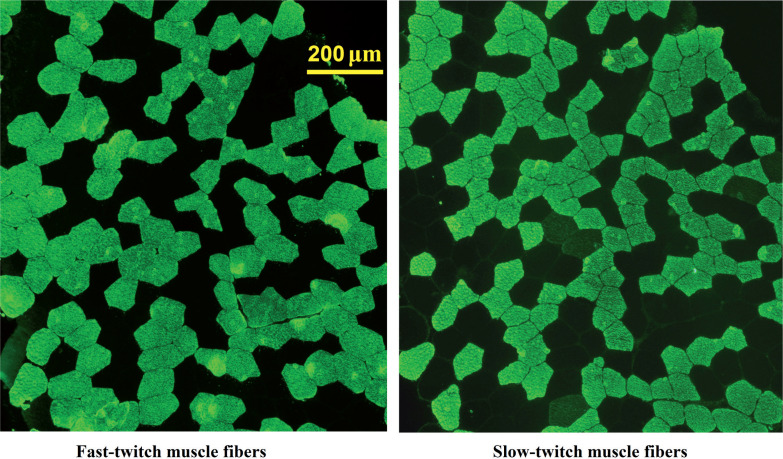
Percutaneous muscle biopsies were collected from the vastus lateralis of the left leg using the modified Bergström needle procedure with aspiration under local anesthesia with 2% lidocaine solution. Samples were immedately frozen in liquid nitrogen and stored at -80°C. For analysis, serial cross-sections (7 μm) were obtained from frozen samples using an ultratom (Leica Microsystems, Germany). Sections were thaw-mounted on polysine glass slides, maintained at room temperature (RT) for 15 min and incubated in PBS (3 5 min). The sections were then incubated at RT in primary antibodies against slow or fast isoforms of the myosin heavy chains (M8421, 1:5000; M4276; 1:600, respectively; Sigma-Aldrich, USA) for 1 h and incubated in PBS (3 × 5 min). Next, the sections were incubated at RT in secondary antibodies conjugated with FITC (F0257; 1:100; Sigma-Aldrich) for 1 h. The antibodies were removed and the sections washed in PBS (3 × 5 min), placed in mounting media and covered with a cover slip. Images were captured by fluorescent microscope (Eclipse Ti-U, Nikon, Japan). All analyzed images contained 318 23 fibers (Fig. 1). The ratio of the number of stained fibers to the total fiber number was calculated. Fibers stained in serial sections with antibodies against slow and fast isoforms were considered hybrid fibers. The cross-sectional area (CSA) of fast- and slow-twitch muscle fibers was evaluated using ImageJ software (NIH, USA).
FIG. 1.
Microphotographs of the labelled muscle sections.
Statistical analyses
Statistical analyses were conducted using GraphPad InStat (GraphPad Software, Inc., USA) software. Differences in traits between different groups were analysed using Mann-Whitney test. Relationships between participant characteristics, exercise testing performance and muscle fiber measurements was tested using Spearman’s (non-parametric) correlation. Multiple regression analysis was used to detect independent associations between the number of repetitions achieved at 80% of 1RM and different characteristics. All data are presented as mean (SD). P values < 0.05 were considered statistically significant.
RESULTS
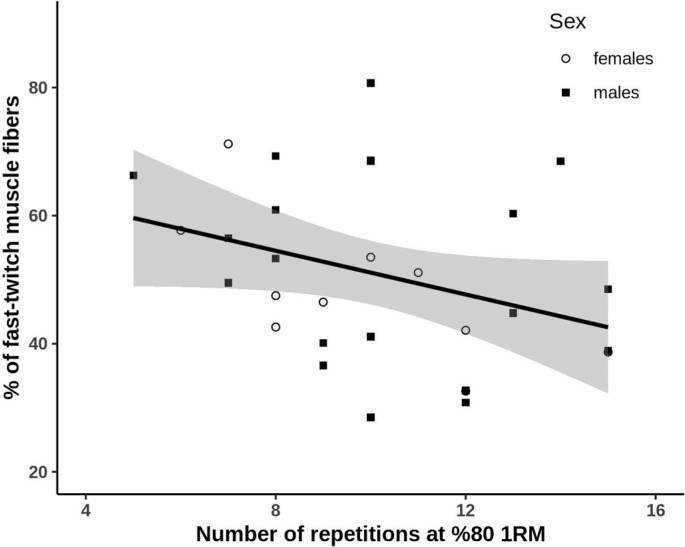
Participants exercise performance during 1RM and 80% 1RM testing, as well as muscle fiber characteristics are presented separately for females and males in Table 1. The number of repetitions achieved at 80% of 1RM ranged from 5 to 15 and was independent of sex, age, 1RM, training frequency, type and experience, BMI and CSA of muscle fibers (all P > 0.05). The percentage of fast-twitch muscle fibers was inversely correlated with the number of repetitions completed (r = –0.38, P = 0.039, Fig. 2). Participants who completed 5 to 8 repetitions (n = 10; four females and six males) had significantly more fast-twitch muscle fibers (57.5 ± 9.5 vs 44.4 ± 11.9%, P = 0.013) than participants who completed 11 to 15 repetitions (n = 11; four females and seven males). The remaining participants completed 9 or 10 repetitions (n = 9; two females and seven males) and on average had equal proportion of fast (51.6 ± 17.5%) and slow-twitch (50.8 ± 16.9%) muscle fibers.
FIG. 2.
Relationship between muscle fiber composition and the number of completed repetitions at 80% 1RM. Shaded areas represent 95% confidence intervals.
DISCUSSION
The aim of the present study was to determine whether muscle fiber composition of the VL is associated with the number of consecutive back squat repetitions completed at 80% 1RM. The main finding was a moderate inverse correlation between the percentage of fasttwitch muscle fibers and the number of repetitions completed, which was unrelated to sex, age, 1RM, training frequency, training type, training age, BMI or fast-twitch muscle fiber CSA. This study is the first to demonstrate concordance between the use of an 80% 1RM load during back squat exercise and the muscle fiber composition of VL biopsies, signifying the potential of this approach to indirectly estimate VL muscle fiber composition.
In the present study, participants achieving 5 to 8 repetitions had a higher percentage of fast-twitch fibers than those achieving 11 to 15 repetitions (57.5 vs 44.3%). Those who completed 9 to 10 repetitions exhibited an equal proportion of each fiber type. Our hypothesis was centred on the theory that loads of 80% 1RM load could be used to estimate the muscle fiber composition of untrained individuals, which had not been previously tested using back squat exercise. It was suggested that participants completing more than 12 repetitions would possess more than 50% slow-twitch fibers, individuals completing fewer than 7 repetitions would possess more than 50% fast-twitch fibers, and that those completing between 7 and 12 repetitions would exhibit an equal proportion of each fiber type [35]. To our knowledge, the present study is the first to find a significant interrelationship between muscle fiber composition of the VL and the variability in the number of back squat repetitions completed at 80% 1RM.
Our results are supported by previous literature where participants estimated to posses more fast-twitch muscle fibers completed fewer repetitions at an individualised load than those estimated to possess more slow-twitch fibers [36]. Comparison with that study is limited by the recruitment of only female participants, the investigation of a 70% 1RM load, and the fact that testing was performed on an isokinetic dynamometer. However, participants in the present study completed a similar range of repetitions (5 to 15) to the previous study (7 to 15), with a slightly lower mean number of repetitions, which may reflect the current participants lifting a relatively greater load. Whilst the present study investigated this relationship using conventional resistance exercise and muscle biopsies, the previous study associated isokinetic dynamometry measures with an indirect estimate of muscle fiber composition derived from a regression equation [40]. The equation described incorporates relative torque after 55 contractions of a fatigue test with power output at an angular velocity of 280°s-1 normalised to fat-free mass of the thigh. Together, these variables explained 51.8% of the variance in the percentage of VL fast-twitch fibers. Whilst this association of skeletal muscle phenotypes with muscle fiber composition is of interest, the practical use of this equation is limited by the requirement to access an isokinetic dynamometer and to accurately quantify fat-free-mass. In contrast, the present study demonstrates the relationship between directly measured VL fiber composition and a universally recognised resistance exercise, with individualised loads derived using a reliable assessment of 1RM [41]. Whilst further studies in larger cohorts are required to support our findings, the present study highlights the potential of this approach as an accessible and cost-effective alternative for estimating muscle fiber composition.
No relationship was observed between participants’ training background and muscle fiber composition in the present study. Previously, endurance athletes completed more repetitions of leg press exercise at 70% and 80% 1RM compared to strength athletes, with no difference at 90% [42], whilst non-athletes accustomed to endurance training achieved more repetitions at 80% 1RM of half-squat exercise than those accustomed to strength training [43]. These results are of importance because endurance trained individuals typically possess a greater percentage of slow-twitch fibers [3] demonstrating increased lactate buffering capacity [44], fiber capillarisation [45] and mitochondrial content [46], i.e. the muscles of these individuals have greater oxidative capacity and fatigue resistance than their strength trained counterparts [11]. Slow-twitch muscle fibers are suited to repetitive and submaximal contractile activity, which might explain why participants with a greater proportion of slow-twitch fibers completed a greater number of repetitions at 80% 1RM in the present study. It is possible that discrepancies between this and previous studies reflect the exercises studied, or the different athletic backgrounds of participants. Given that the number of repetitions completed at 80% of 1RM was related to fiber composition but was independent of age, 1RM, training frequency, training type, training experience, BMI and fast-twitch muscle fiber CSA, it is plausible to suggest that this trait is partly heritable, with further investigation required to test this hypothesis.
Direct determination of muscle fiber composition is invasive and expensive, and therefore unfavourable to field settings and the general population. Many indirect methods require specialist resources and have their own limitations. For example, TMG requires a highly trained operator to minimise measurement error [47], the detection of carnosine content by MRS is heavily influenced by dietary factors [48], and crosstalk from neighbouring muscles during MMG affects the myographic signal from the investigated region [49]. Accordingly, there is a need for an indirect and accessible method to estimate fiber composition that can be executed by exercise professionals using standard equipment. The present study is the first to experimentally validate a previous hypothesis [35] using back squat exercise and to demonstrate that the number of repetitions achieved at 80% 1RM is associated with muscle fiber composition. Our findings highlight the potential to develop a non-invasive and cost-effective approach for estimating muscle fiber composition which, following further validation, could aid the design of training programmes for improving the strength or endurance capacity of skeletal muscle. With fast-twitch fibers preferentially damaged following fast and forceful contractions [50], knowledge of fiber composition may also assist recovery from strenuous training. Accordingly, this field test could help exercise professionals identify individuals who may be more susceptible to exercise induced muscle damage, and who may require extended rest or additional recovery strategies following strenuous training. At present, our findings are restricted to males and females aged 18 to 40, with replication required before this test can be applicable to other ages, such as youths and the elderly. Furthermore, because the reliability of maximal back squat testing increases after 6–12 months of resistance training [51], the use of this test may be inappropriate for less experienced individuals.
The advantages of the present study include the direct determination of VL muscle fiber composition, a common and reliable method for 1RM testing [41], and the potential practical application of these findings for individuals without access to the facilities and expertise required to directly determine fiber composition. However, this study also has limitations. Firstly, we recognise that a greater sample size could improve the statistical power of the results. Secondly, our findings are limited to the muscle fiber composition of the VL, and due to the heterogeneity of fiber composition within skeletal muscles of the human body, the validity of this method is limited to the VL. We also recognise that information regarding participants’ endurance exercise capacity and genetic profile was not included in the present study, and that including such data may improve the prediction of muscle fiber composition in future studies, in addition to investigating a series of external loads. Finally, we recognise that validation of this approach in untrained populations and in elite athletes is required for this approach to be used with sedentary individuals and within highlevel sport, for example in weightlifting and powerlifting where the back squat is a fundamental exercise.
CONCLUSIONS
The present study is the first to demonstrate the interrelationship between back squat performance at 80% 1RM and muscle fiber composition. We suggest that completing 8 or fewer repetitions at 80% 1RM is indicative that participants possess more than 50% fast-twitch fibers in their VL, that completing 11 or more repetitions at 80% 1RM is indicative that participants possess more than 50% slow-twitch fibers in their VL, and that completing 9 or 10 repetitions is indicative of participants possessing an equal distribution of each fiber type. Though further validation is required, the present study demonstrates the association of muscle fiber type with back squat performance and highlights potential to develop a non-invasive and cost-effective approach to estimate muscle fiber composition.
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- 1.Hubal MJ, Gordish-Dressman H, Thomspon PD, Price TB, Hoffman EP, Angelopolous TJ, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37(6):964–972. [PubMed] [Google Scholar]
- 2.Ahmetov II, Vinogradova OL, Williams AG. Gene polymorphisms and fiber-type composition of human skeletal muscle. Int J Sport Nutr Exerc Metab. 2012;22(4):292–303. doi: 10.1123/ijsnem.22.4.292. [DOI] [PubMed] [Google Scholar]
- 3.Fuku N, Kumagai H, Ahmetov II. Sports, Exercise, and Nutritional Genomics. Academic Press; 2019. Genetics of muscle fiber composition; pp. 295–314. [Google Scholar]
- 4.Bottinelli R, Canepari M, Pellegrino M, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol. 1996;495(2):573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abernethy PJ, Jurimae J, Logan PA, Taylor AW, Thayer RE. Acute and chronic response of skeletal muscle to resistance exercise. Sports Med. 1994;17(1):22–38. doi: 10.2165/00007256-199417010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hughes DC, Ellefsen S, Baar K. Adaptations to Endurance and Strength Training. Cold Spring Harb Perspect Med. 2018;8(6):a029769. doi: 10.1101/cshperspect.a029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Brito Fontana H, Herzog W. Vastus lateralis maximum force-generating potential occurs at optimal fascicle length regardless of activation level. Eur J Appl Physiol. 2016;116(6):1267–1277. doi: 10.1007/s00421-016-3381-3. [DOI] [PubMed] [Google Scholar]
- 8.Komi PV, Karlsson J. Skeletal muscle fibre types, enzyme activities and physical performance in young males and females. Acta Physiol Scand. 1978;103(2):210–218. doi: 10.1111/j.1748-1716.1978.tb06208.x. [DOI] [PubMed] [Google Scholar]
- 9.Lexell J, Taylor CC. Variability in muscle fibre areas in whole human quadriceps muscle. How much and why? Acta Physiol Scand. 1989;136(4):561–568. doi: 10.1111/j.1748-1716.1989.tb08702.x. [DOI] [PubMed] [Google Scholar]
- 10.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol. 1989;257(4 Pt 1):E567–572. doi: 10.1152/ajpendo.1989.257.4.E567. [DOI] [PubMed] [Google Scholar]
- 11.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, et al. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48(5):623–629. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- 12.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 13.Murgia M, Toniolo L, Nagaraj N, Ciciliot S, Vindigni V, Schiaffino S, et al. Single muscle fiber proteomics reveals fiber-type-specific features of human muscle aging. Cell Rep. 2017;19(11):2396–2409. doi: 10.1016/j.celrep.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 14.Semenova E, Khabibova S, Borisov O, Generozov E, Ahmetov I. The variability of DNA structure and muscle-fiber composition. Hum Physiol. 2019;45(2):225–232. [Google Scholar]
- 15.Taylor DL, Jackson AU, Narisu N, Hemani G, Erdos MR, Chines PS, et al. Integrative analysis of gene expression, DNA methylation, physiological traits, and genetic variation in human skeletal muscle. Proc Natl Acad Sci U S A. 2019;116(22):10883–10888. doi: 10.1073/pnas.1814263116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rusu LD, Cosma GG, Cernaianu SM, Marin MN, Rusu PA, Ciocănescu DP, Neferu FN. Tensiomyography method used for neuromuscular assessment of muscle training. J Neuroeng Rehabil. 2013;10(1):67. doi: 10.1186/1743-0003-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahmane R, Djordjevič S, Šimunič B, Valenčič V. Spatial fiber type distribution in normal human muscle: histochemical and tensiomyographical evaluation. J Biomech. 2005;38(12):2451–2459. doi: 10.1016/j.jbiomech.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Baguet A, Everaert I, Hespel P, Petrovic M, Achten E, Derave W. A new method for non-invasive estimation of human muscle fiber type composition. PLoS One. 2011;6(7):e21956. doi: 10.1371/journal.pone.0021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lievens E, Klass M, Bex T, Derave W. Muscle fiber typology substantially influences time to recover from high-intensity exercise. J Appl Physiol. 2020;128(3):648–659. doi: 10.1152/japplphysiol.00636.2019. [DOI] [PubMed] [Google Scholar]
- 20.Fry AC, Housh TJ, Cramer JB, Weir JP, Beck TW, Schilling BK, et al. Noninvasive assessment of skeletal muscle myosin heavy chain expression in trained and untrained men. J Strength Cond Res. 2017;31(9):2355–2362. doi: 10.1519/JSC.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Tanaka H, Shono N, Miura S, Kiyonaga A, Shindo M, Saku K. The I allele of the angiotensin-converting enzyme gene is associated with an increased percentage of slow-twitch type I fibers in human skeletal muscle. Clin Genet. 2003;63(2):139–144. doi: 10.1034/j.1399-0004.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 22.Ahmetov II, Mozhayskaya IA, Flavell DM, Astratenkova IV, Komkova AI, Lyubaeva EV, et al. PPARalpha gene variation and physical performance in Russian athletes. Eur J Appl Physiol. 2006;97(1):103–108. doi: 10.1007/s00421-006-0154-4. [DOI] [PubMed] [Google Scholar]
- 23.Vincent B, De Bock K, Ramaekers M, Van den Eede E, Van Leemputte M, Hespel P, Thomis MA. ACTN3 (R577X) genotype is associated with fiber type distribution. Physiol Genomics. 2007;32(1):58–63. doi: 10.1152/physiolgenomics.00173.2007. [DOI] [PubMed] [Google Scholar]
- 24.Ahmetov II, Hakimullina AM, Lyubaeva EV, Vinogradova OL, Rogozkin VA. Effect of HIF1A gene polymorphism on human muscle performance. Bull Exp Biol Med. 2008;146(3):351–353. doi: 10.1007/s10517-008-0291-3. [DOI] [PubMed] [Google Scholar]
- 25.Ahmetov II, Hakimullina AM, Popov DV, Lyubaeva EV, Missina SS, Vinogradova OL, Williams AG, Rogozkin VA. Association of the VEGFR2 gene His472Gln polymorphism with endurance-related phenotypes. Eur J Appl Physiol. 2009;107(1):95–103. doi: 10.1007/s00421-009-1105-7. [DOI] [PubMed] [Google Scholar]
- 26.Ahmetov II, Druzhevskaya AM, Lyubaeva EV, Popov DV, Vinogradova OL, Williams AG. The dependence of preferred competitive racing distance on muscle fibre type composition and ACTN3 genotype in speed skaters. Exp Physiol. 2011;96(12):1302–1310. doi: 10.1113/expphysiol.2011.060293. [DOI] [PubMed] [Google Scholar]
- 27.Mustafina LJ, Naumov VA, Cieszczyk P, Popov DV, Lyubaeva EV, Kostryukova ES, et al. AGTR2 gene polymorphism is associated with muscle fibre composition, athletic status and aerobic performance. Exp Physiol. 2014;99(8):1042–1052. doi: 10.1113/expphysiol.2014.079335. [DOI] [PubMed] [Google Scholar]
- 28.Willems SM, Wright DJ, Day FR, Trajanoska K, Joshi PK, Morris JA, et al. Large-scale GWAS identifies multiple loci for hand grip strength providing biological insights into muscular fitness. Nat Commun. 2017;8:16015. doi: 10.1038/ncomms16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumagai H, Tobina T, Ichinoseki-Sekine N, Kakigi R, Tsuzuki T, Zempo H, et al. Role of selected polymorphisms in determining muscle fiber composition in Japanese men and women. J Appl Physiol. 2018;124(5):1377–1384. doi: 10.1152/japplphysiol.00953.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilherme J, Egorova ES, Semenova EA, Kostryukova ES, Kulemin NA, Borisov OV, et al. The A-allele of the FTO gene rs9939609 polymorphism is associated with decreased proportion of slow oxidative muscle fibers and over-represented in heavier athletes. J Strength Cond Res. 2019;33(3):691–700. doi: 10.1519/JSC.0000000000003032. [DOI] [PubMed] [Google Scholar]
- 31.Pickering C, Suraci B, Semenova EA, Boulygina EA, Kostryukova ES, Kulemin NA, et al. A genome-wide association study of sprint performance in elite youth football players. J Strength Cond Res. 2019;33(9):2344–2351. doi: 10.1519/JSC.0000000000003259. [DOI] [PubMed] [Google Scholar]
- 32.Guilherme JPLF, Semenova EA, Zempo H, Martins GL, Lancha Junior AH, Miyamoto-Mikami E, et al. Are GWAS-identified SNPs associated with sprint athletic status? A replication study with three different cohorts. Int J Sports Physiol Perform. 2020 doi: 10.1123/ijspp.2019-1032. [DOI] [PubMed] [Google Scholar]
- 33.Kusic D, Connolly J, Kainulainen H, Semenova EA, Borisov OV, Larin AK, et al. Striated muscle-specific serine/threonine-protein kinase beta segregates with high versus low responsiveness to endurance exercise training. Physiol Genomics. 2020;52(1):35–46. doi: 10.1152/physiolgenomics.00103.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoeger WWK, Barette SL, Hale DF, Hopkins DR. Relationship between repetitions and selected percentages of one repetition maximum. J Strength Cond Res. 1987;1(1):11–13. [Google Scholar]
- 35.Karp JR. Muscle fiber types and training. Strength Cond J. 2001;23(5):21. [Google Scholar]
- 36.Douris PC, White BP, Cullen RR, Keltz WE, Meli J, Mondiello DM, Wenger D. The relationship between maximal repetition performance and muscular fiber type as estimated by noninvasive technique in the quadriceps of untrained women. J Strength Cond Res. 2006;20(3):699–703. doi: 10.1519/17204.1. [DOI] [PubMed] [Google Scholar]
- 37.Terzis G, Spengos K, Manta P, Sarris N, Georgiadis G. Fiber type composition and capillary density in relation to submaximal number of repetitions in resistance exercise. J Strength Cond Res. 2008;22(3):845–850. doi: 10.1519/JSC.0b013e31816a5ee4. [DOI] [PubMed] [Google Scholar]
- 38.Haff G, Triplett N. Essentials of Strength & Conditioning. Champaign, IL: Human Kinetics; 2016. Exercise technique for free weight and machine training; p. 380. [Google Scholar]
- 39.American College of Sports Medicine American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 40.Suter E, Herzog W, Sokolosky J, Wiley JP, Macintosh BR. Muscle fiber type distribution as estimated by Cybex testing and by muscle biopsy. Med Sci Sports Exerc. 1993;25(3):363–370. [PubMed] [Google Scholar]
- 41.McMaster DT, Gill N, Cronin J, McGuigan M. A brief review of strength and ballistic assessment methodologies in sport. Sports Med. 2014;44(5):603–623. doi: 10.1007/s40279-014-0145-2. [DOI] [PubMed] [Google Scholar]
- 42.Richens B, Cleather DJ. The relationship between the number of repetitions performed at given intensities is different in endurance and strength trained athletes. Biol Sport. 2014;31(2):157–161. doi: 10.5604/20831862.1099047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panissa VL, Azevedo NR, Julio UF, Andreato LV, Pinto ESCM, Hardt F, Franchini E. Maximum number of repetitions, total weight lifted and neuromuscular fatigue in individuals with different training backgrounds. Biol Sport. 2013;30(2):131–136. doi: 10.5604/20831862.1044458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(4):831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 45.Andersen P, ad Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingjer F. Effects of endurance training on muscle fibre ATP-ase activity, capillary supply and mitochondrial content in man. J Physiol. 1979;294:419–432. doi: 10.1113/jphysiol.1979.sp012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin-Rodriguez S, Alentorn-Geli E, Tous-Fajardo J, Samuelsson K, Marin M, Alvarez-Diaz P, Cugat R. Is tensiomyography a useful assessment tool in sports medicine? Knee Surg Sports Traumatol Arthrosc. 2017;25(12):3980–3981. doi: 10.1007/s00167-017-4600-0. [DOI] [PubMed] [Google Scholar]
- 48.Everaert I, Mooyaart A, Baguet A, Zutinic A, Baelde H, Achten E, et al. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids. 2011;40(4):1221–1229. doi: 10.1007/s00726-010-0749-2. [DOI] [PubMed] [Google Scholar]
- 49.Islam MA, Sundaraj K, Ahmad RB, Sundaraj S, Ahamed NU, Ali MA. Cross-talk in mechanomyographic signals from the forearm muscles during sub-maximal to maximal isometric grip force. PLoS One. 2014;9(5):e96628. doi: 10.1371/journal.pone.0096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chapman DW, Newton M, McGuigan M, Nosaka K. Effect of lengthening contraction velocity on muscle damage of the elbow flexors. Med Sci Sports Exerc. 2008;40(5):926–933. doi: 10.1249/MSS.0b013e318168c82d. [DOI] [PubMed] [Google Scholar]
- 51.Comfort P, McMahon JJ. Reliability of maximal back squat and power clean performance in inexperienced athletes. J Strength Cond Res. 2015;29(11):3089–3096. doi: 10.1519/JSC.0000000000000815. [DOI] [PubMed] [Google Scholar]