Abstract
The inferior frontal gyrus and inferior parietal lobe have been characterized as human homologues of the monkey “mirror neuron” system, critical for both action production (AP) and action recognition (AR). However, data from brain lesion patients with selective impairment on only one of these tasks provide evidence of neural and cognitive dissociations. We sought to clarify the relationship between AP and AR, and their critical neural substrates, by directly comparing performance of 131 chronic left-hemisphere stroke patients on both tasks—to our knowledge, the largest lesion-based experimental investigation of action cognition to date. Using voxel-based lesion-symptom mapping, we found that lesions to primary motor and somatosensory cortices and inferior parietal lobule were associated with disproportionately impaired performance on AP, whereas lesions to lateral temporo-occipital cortex were associated with a relatively rare pattern of disproportionately impaired performance on AR. In contrast, damage to posterior middle temporal gyrus was associated with impairment on both AP and AR. The distinction between lateral temporo-occipital cortex, critical for recognition, and posterior middle temporal gyrus, important for both tasks, suggests a rough gradient from modality-specific to abstract representations in posterior temporal cortex, the first lesion-based evidence for this phenomenon. Overall, the results of this large patient study help to bring closure to a long-standing debate by showing that tool-related AP and AR critically depend on both common and distinct left hemisphere neural substrates, most of which are external to putative human mirror regions.
INTRODUCTION
The relationship between the neurocognitive substrates of action production (AP) and action recognition (AR) is a matter of controversy. On one hand, there is evidence that some aspects of action perception may be accomplished by accessing the same cognitive representations and neural regions that guide AP. Numerous studies, for example, have demonstrated that the ability to recognize actions to predict how they will unfold is conditioned by performance expertise (e.g., Makris & Urgesi, 2015; Balser et al., 2014; Moore & Müller, 2014). For example, elite basketball players can predict the outcome of free throw shots earlier and more accurately than individuals with comparable visual (but not motoric) experience (coaches or sports journalists) or novice basketball players (Aglioti, Cesari, Romani, & Urgesi, 2008). Such data have been hypothesized to reflect simulation (also called “resonance” or “direct matching”) mechanisms that operate automatically when actions are both viewed and planned, computed in a specialized brain network in ventral premotor and inferior parietal cortex known as “the mirror system” (e.g., Cross & Iacoboni, 2014; Enticott et al., 2012; Molenberghs, Cunnington, & Mattingley, 2012; Van Overwalle & Baetens, 2009; see also Caspers, Zilles, Laird, & Eickhoff, 2010). Some researchers argue for a strong interpretation of this hypothesis, suggesting that action simulation on its own is sufficient for action understanding. For example, Rizzolatti and colleagues have claimed that “action is understood when its observation causes the motor system of the observer to ‘resonate’ [with the observed action]” (Rizzolatti, Fogassi, & Gallese, 2001, p. 661). As pointed out by Hickok (2009), however, many such claims are based on paradigms in which there is no assessment of whether viewed actions are actually recognized and understood (e.g., Urgesi, Calvo-Merino, Haggard, & Agliotti, 2007; Urgesi, Candidi, Ionta, & Aglioti, 2007).
Functional neuroimaging studies of AP typically show widespread activation in frontoparietal cortex (e.g., Macuga & Frey, 2012; Grézes & Decety, 2001), including regions such as inferior frontal gyrus (IFG) and posterior inferior parietal lobe (IPL) that have been described as human homologues of the monkey mirror system (e.g., Cebolla, Palmero-Soler, Dan, & Cheron, 2014; Liepelt, Von Cramon, & Brass, 2008; Gallese, Fadiga, Fogassi, & Rizzolatti, 1996). In contrast, regions activated during action understanding consistently include the left posterior middle temporal gyrus (pMTG), a region not typically invoked as part of the mirror system, in addition to frontoparietal regions (e.g., Hoeren et al., 2014; Lingnau & Petris, 2013; Spunt & Lieberman, 2012; see Watson, Cardillo, Ianni, & Chatterjee, 2013, for a meta-analysis). On the basis of such data, it has been proposed that the mirror system is not sufficient for action understanding, but rather that an additional pathway linking pMTG and IFG is required (Kilner, 2011). Furthermore, some researchers have proposed that the posterior temporal cortex (pTC)1 implements a gradient of action knowledge in which concrete, modality-specific to increasingly abstract information is represented along a posterior-to-anterior axis that begins in visual motion area hMT+ and continues along the middle temporal gyrus (MTG; Watson & Chatterjee, 2011; Chatterjee, 2008; Kable, Kan, Wilson, Thompson-Schill, & Chatterjee, 2005).
Given that functional neuroimaging studies in healthy participants do not speak to the necessity of activated regions for a given task (cf. Fellows et al., 2005), research with brain lesion patients offers a more stringent test of the relationship between regions critical for AP and AR. On one hand, the co-occurrence of deficits in production and recognition in patients with relatively focal brain lesions might indicate that they both depend on the same neurocognitive substrates. On the other hand, double dissociations between the two tasks (i.e., sparing of one task in the context of deficits in the other, and vice versa) might be viewed as evidence supporting distinct mechanisms and neural bases (but see Plaut, 1995). Both of these positions have been taken in previous patient studies.
In a prior study, we assessed the ability of 44 left-hemisphere stroke patients to perform tool-related (i.e., transitive) pantomimed AP and AR tasks and found a highly significant relationship between the two (Buxbaum, Kyle, & Menon, 2005).2 Moreover, based on lesion overlap analyses, we identified the posterior IPL as critical to both tasks. We concluded, based on those data, that tool-related AP and AR are subserved by the same mechanisms. Similarly, in a study of 37 left-hemisphere stroke patients, Negri et al. (2007) observed a reliable correlation between tool use and AR. Importantly, however, when they examined single cases, they observed six patients who were impaired in tool use but within the normal range for pantomimed AR. They noted that the data permitted them to reject the strongest version of resonance theories in which the ability to perform actions is required for AR (see also Hickok, 2014).
However, as the authors acknowledge, production deficits in apraxia—a deficit in skilled action that cannot be attributed to weakness or sensory loss—may occur further “downstream” (closer to motor output) than representations subserving action knowledge (Buxbaum, 2001). Thus, findings of deficits in AP without accompanying deficits in recognition do not discount the possibility that action knowledge representations are necessary for AP. In addition, Negri et al. (2007) reported several patients who performed normally in tool use despite impairments in pantomime recognition. One of the challenges in interpreting this pattern is that tool use may be nearly normal in apraxic patients (despite deficits in pantomime production) because of the multimodal feedback provided by viewing and handling real tools (cf. Goldenberg et al., 2004), which in turn may plausibly facilitate activation of deficient tool use knowledge. Consequently, we employ pantomime production tasks in this study.
Other studies have assessed the relationship between brain lesions and action using voxel-based lesion-symptom mapping (VLSM). In an earlier study (Kalénine, Buxbaum, & Coslett, 2010), we demonstrated with data from 43 patients that the conceptual aspects of tool-related AR depend critically on left pMTG, whereas a more recent study from our laboratory with 71 patients showed that left frontal, parietal, and posterior temporal regions are critical for pantomimed tool-related AP (Buxbaum, Shapiro, & Coslett, 2014). Moreover, we demonstrated that a common region of the pTC was critical for both production of actions to the sight of tools as well as imitation of pantomimed tool actions. This novel finding indicated that the pTC has a greater role in tool AP than previously appreciated (see also Gallivan, McLean, Valyear, & Culham, 2013). However, a limitation ofthese separate studies for addressing theories of action simulation is that they do not permit us to directly assess the relationship between the tasks or the brain regions that are common and unique to each task, within the same patients.
In this study, we endeavored to gain a more nuanced understanding of the relationship between pantomimed, tool-related AP and AR—and their neuroanatomic substrates—by assessing a large group of stroke patients on both tasks and performing VLSM analyses to determine the regions that, when lesioned, predict deficits on each task. Our sample of 131 stroke patients makes this the largest lesion-based experimental investigation of action cognition to date. Importantly, we adopted a regression-based data analysis approach that assessed the neural substrates of disproportionate impairments in one task given performance on the other, and vice versa. In addition to the considerably larger sample size than has been tested in the past, this method goes beyond those previously used (e.g., Buxbaum, Kyle, et al., 2005) by controlling for overall behavioral severity as well as overall lesion volume. Furthermore, given the continuous nature of the distribution of scores (see Table 1), the approach represents an advance over those that have dichotomized patients’ performance by classifying them as intact or impaired (e.g., Negri et al., 2007).
Table 1.
Demographics and Behavioral Performance of the 131 Patients Participating in the Study
| Participant No. | Sex | Hand | Age at Testing | Education (years) | Months Post-stroke | Lesion Volume (cm3) | AP Avga | AR Avg |
|---|---|---|---|---|---|---|---|---|
| 1 | F | R | 50 | 18 | 5 | 60.2 | 87.5 | 100.0 |
| 2 | M | R | 59 | 16 | 29 | 8.6 | 85.0 | 87.5 |
| 3 | M | R | 60 | 12 | 79 | 71.8 | 85.0 | 100.0 |
| 4 | F | R | 49 | 16 | 71 | 89.1 | 95.0 | 95.5 |
| 5 | M | R | 37 | 12 | 76 | 17.0 | 97.2 | 100.0 |
| 6 | M | R | 53 | 13 | 65 | 172.2 | 92.5 | 100.0 |
| 7 | F | R | 73 | 12 | 57 | 21.5 | 62.5 | 85.7 |
| 8 | M | R | 67 | 19 | 41 | 84.9 | 92.5 | 87.5 |
| 9 | F | R | 61 | 14 | 44 | 5.6 | 95.0 | 95.8 |
| 10 | M | R | 53 | 19 | 71 | 219.9 | 75.0 | 79.2 |
| 11 | M | R | 76 | 21 | 52 | 8.0 | 94.4 | 91.3 |
| 12 | F | R | 64 | 12 | 32 | 3.6 | 85.0 | 91.7 |
| 13 | F | R | 56 | 11 | 11 | 20.6 | 87.5 | 95.8 |
| 14 | F | R | 56 | 17 | 397 | 227.6 | 27.5 | 75.0 |
| 15 | M | R | 73 | 20 | 33 | 35.7 | 91.7 | 95.8 |
| 16 | M | R | 67 | 12 | 49 | 102.4 | 60.0 | 87.5 |
| 17 | M | R | 53 | 14 | 44 | 52.3 | 80.0 | 95.7 |
| 18 | M | R | 42 | 15 | 184 | 41.8 | 95.0 | 79.2 |
| 19 | F | R | 61 | 14 | 30 | 37.0 | 82.5 | 83.3 |
| 20 | F | R | 52 | 14 | 41 | 31.4 | 97.2 | 100.0 |
| 21 | M | R | 59 | 11 | 7 | 4.5 | 100.0 | 95.8 |
| 22 | F | R | 72 | 12 | 9 | 17.9 | 95.0 | 83.3 |
| 23 | M | R | 49 | 14 | 21 | 5.4 | 83.3 | 82.6 |
| 24 | F | R | 48 | 13 | 83 | 85.2 | 90.0 | 100.0 |
| 25 | M | Both | 65 | 16 | 28 | 272.0 | 71.4 | 76.5 |
| 26 | M | R | 56 | 12 | 31 | 25.3 | 77.8 | 95.7 |
| 27 | F | R | 59 | 16 | 35 | 198.6 | 95.0 | 95.0 |
| 28 | M | R | 54 | 12 | 37 | 55.8 | 92.5 | 100.0 |
| 29 | F | R | 61 | 16 | 24 | 46.2 | 87.5 | 95.8 |
| 30 | M | R | 57 | 12 | 22 | 23.7 | 92.5 | 95.7 |
| 31 | F | R | 61 | 16 | 15 | 73.1 | 72.5 | 95.7 |
| 32 | M | R | 68 | 14 | 20 | 67.2 | 80.6 | 71.4 |
| 33 | F | R | 62 | 21 | 30 | 92.0 | 65.0 | 85.7 |
| 34 | F | R | 33 | 19 | 22 | 63.9 | 72.2 | 81.0 |
| 35 | F | R | 61 | 12 | 29 | 8.8 | 97.5 | 100.0 |
| 36 | F | R | 48 | 12 | 97 | 154.0 | 96.9 | 84.6 |
| 37 | F | R | 64 | 12 | 53 | 110.3 | 65.0 | 85.7 |
| 38 | F | R | 66 | 12 | 20 | 303.3 | 36.1 | 89.5 |
| 39 | M | R | 66 | 19 | 12 | 34.8 | 90.0 | 87.0 |
| 40 | F | R | 73 | 12 | 22 | 54.4 | 86.1 | 73.9 |
| 41 | F | R | 70 | 12 | 23 | 8.3 | 95.0 | 95.8 |
| 42 | F | R | 46 | 12 | 40 | 140.6 | 80.0 | 90.5 |
| 43 | F | R | 68 | 12 | 7 | 12.4 | 85.0 | 95.8 |
| 44 | M | R | 48 | 14 | 29 | 55.7 | 72.2 | 95.7 |
| 45 | F | R | 52 | 19 | 13 | 7.0 | 91.7 | 91.7 |
| 46 | M | R | 54 | 12 | 45 | 91.2 | 87.5 | 85.0 |
| 47 | M | R | 55 | 16 | 10 | 88.8 | 77.5 | 91.3 |
| 48 | F | R | 55 | 12 | 17 | 91.1 | 87.5 | 91.3 |
| 49 | F | R | 31 | 12 | 11 | 147.2 | 60.7 | 58.8 |
| 50 | M | R | 62 | 20 | 7 | 61.0 | 97.2 | 95.2 |
| 51 | M | R | 67 | 19 | 9 | 81.6 | 65.0 | 77.3 |
| 52 | F | R | 51 | 12 | 11 | 22.2 | 97.5 | 91.7 |
| 53 | F | R | 65 | 12 | 14 | 51.9 | 35.0 | 71.4 |
| 54 | M | R | 32 | 13 | 8 | 108.9 | 80.0 | 62.5 |
| 55 | M | R | 51 | 12 | 6 | 89.6 | 97.2 | 100.0 |
| 56 | M | R | 62 | 12 | 43 | 214.4 | 85.0 | 87.0 |
| 57 | F | R | 71 | 19 | 23 | 133.3 | 77.5 | 100.0 |
| 58 | F | R | 42 | 19 | 11 | 26.7 | 82.5 | 88.2 |
| 59 | F | R | 43 | 16 | 18 | 71.1 | 83.3 | 90.0 |
| 60 | M | R | 64 | 14 | 14 | 53.1 | 66.7 | 77.3 |
| 61 | M | R | 67 | 21 | 10 | 68.4 | 92.5 | 72.7 |
| 62 | M | L | 51 | 10 | 13 | 7.5 | 95.0 | 95.8 |
| 63 | F | R | 48 | 17 | 23 | 181.8 | 75.0 | 79.2 |
| 64 | F | R | 79 | 12 | 69 | 6.6 | 45.0 | 66.7 |
| 65 | F | R | 51 | 12 | 16 | 60.3 | 85.0 | 100.0 |
| 66 | M | R | 64 | 12 | 14 | 155.5 | 62.5 | 91.7 |
| 67 | M | R | 60 | 13 | 142 | 135.9 | 65.0 | 95.8 |
| 68 | M | R | 58 | 16 | 135 | 138.7 | 55.0 | 70.8 |
| 69 | F | R | 72 | 16 | 170 | 189.8 | 82.5 | 91.7 |
| 70 | F | R | 41 | 12 | 12 | 27.1 | 50.0 | 95.8 |
| 71 | M | R | 68 | 12 | 319 | 89.0 | 87.5 | 91.7 |
| 72 | M | R | 54 | 12 | 89 | 90.5 | 92.5 | 95.8 |
| 73 | M | R | 42 | 11 | 22 | 40.8 | 92.5 | 87.5 |
| 74 | M | R | 56 | 16 | 9 | 41.4 | 62.5 | 70.8 |
| 75 | M | R | 40 | 12 | 26 | 83.1 | 57.5 | 66.7 |
| 76 | F | R | 63 | 12 | 143 | 188.6 | 37.5 | 70.8 |
| 77 | F | R | 54 | 18 | 165 | 196.0 | 63.9 | 83.3 |
| 78 | F | R | 41 | 10 | 127 | 202.6 | 50.0 | 87.5 |
| 79 | M | R | 62 | 11 | 45 | 40.1 | 97.5 | 95.8 |
| 80 | M | R | 55 | 16 | 22 | 165.8 | 97.2 | 91.7 |
| 81 | M | R | 51 | 11 | 82 | 44.5 | 92.5 | 83.3 |
| 82 | M | R | 64 | 13 | 42 | 185.3 | 75.0 | 79.2 |
| 83 | M | R | 49 | 16 | 35 | 68.5 | 70.0 | 100.0 |
| 84 | M | R | 57 | 21 | 114 | 105.1 | 85.0 | 100.0 |
| 85 | F | R | 77 | 13 | 8 | 10.6 | 90.0 | 91.7 |
| 86 | F | R | 40 | 16 | 3 | 124.9 | 55.0 | 95.8 |
| 87 | M | L | 71 | 8 | 152 | 204.9 | 80.0 | 91.7 |
| 88 | F | R | 44 | 12 | 10 | 16.0 | 87.5 | 95.8 |
| 89 | F | R | 39 | 14 | 50 | 118.9 | 85.0 | 95.8 |
| 90 | F | R | 80 | 16 | 10 | 56.1 | 60.0 | 50.0 |
| 91 | F | R | 35 | 12 | 7 | 46.6 | 90.0 | 100.0 |
| 92 | M | R | 78 | 12 | 41 | 70.4 | 85.0 | 95.8 |
| 93 | M | R | 61 | 11 | 27 | 76.0 | 87.5 | 62.5 |
| 94 | F | R | 56 | 16 | 59 | 6.3 | 75.0 | 70.8 |
| 95 | M | R | 78 | 8 | 39 | 34.2 | 85.0 | 95.8 |
| 96 | F | R | 69 | 12 | 21 | 191.7 | 28.1 | 70.8 |
| 97 | M | R | 57 | 11 | 62 | 20.4 | 87.5 | 91.7 |
| 98 | M | R | 74 | 8 | 20 | 16.7 | 63.9 | 66.7 |
| 99 | M | R | 59 | 19 | 55 | 264.7 | 77.5 | 79.2 |
| 100 | F | L | 52 | 12 | 9 | 1.9 | 87.5 | 91.7 |
| 101 | F | R | 50 | 17 | 23 | 114.0 | 87.5 | 95.8 |
| 102 | M | R | 63 | 16 | 7 | 12.3 | 82.5 | 91.7 |
| 103 | M | R | 64 | 16 | 20 | 43.8 | 85.0 | 83.3 |
| 104 | F | L | 49 | 12 | 14 | 54.8 | 50.0 | 66.7 |
| 105 | F | R | 74 | 14 | 21 | 57.3 | 80.6 | 95.8 |
| 106 | F | R | 60 | 12 | 8 | 19.8 | 82.5 | 95.8 |
| 107 | F | R | 66 | 12 | 77 | 3.9 | 92.5 | 100.0 |
| 108 | F | R | 55 | 16 | 79 | 266.7 | 56.3 | 62.5 |
| 109 | M | R | 55 | 18 | 14 | 45.2 | 97.5 | 95.8 |
| 110 | M | R | 42 | 14 | 9 | 68.9 | 97.2 | 95.8 |
| 111 | M | R | 60 | 12 | 10 | 10.1 | 97.5 | 70.8 |
| 112 | F | R | 56 | 12 | 22 | 41.7 | 80.6 | 87.5 |
| 113 | M | R | 66 | 16 | 14 | 114.4 | 97.5 | 95.8 |
| 114 | F | R | 50 | 12 | 145 | 23.4 | 80.0 | 100.0 |
| 115 | M | R | 61 | 12 | 36 | 58.0 | 82.5 | 87.5 |
| 116 | F | R | 50 | 12 | 30 | 173.6 | 47.5 | 79.2 |
| 117 | M | R | 71 | 12 | 14 | 71.1 | 100.0 | 87.5 |
| 118 | F | R | 54 | 12 | 20 | 27.4 | 90.0 | 75.0 |
| 119 | M | R | 68 | 14 | 9 | 50.2 | 90.0 | 91.7 |
| 120 | M | R | 55 | 20 | 74 | 72.7 | 77.5 | 100.0 |
| 121 | F | R | 54 | 14 | 8 | 41.0 | 92.5 | 100.0 |
| 122 | F | R | 58 | 12 | 6 | 36.7 | 65.0 | 95.8 |
| 123 | F | R | 66 | 11 | 61 | 33.9 | 87.5 | 100.0 |
| 124 | F | R | 44 | 15 | 9 | 14.7 | 90.0 | 100.0 |
| 125 | M | R | 79 | 11 | 27 | 31.4 | 60.0 | 100.0 |
| 126 | M | R | 50 | 12 | 17 | 49.4 | 75.0 | 83.3 |
| 127 | M | R | 64 | 19 | 65 | 48.3 | 90.0 | 100.0 |
| 128 | F | R | 47 | 13 | 82 | 109.6 | 97.5 | 100.0 |
| 129 | F | R | 55 | 16 | 12 | 14.0 | 87.5 | 91.7 |
| 130 | F | R | 67 | 12 | 91 | 27.2 | 67.5 | 95.8 |
| 131 | M | R | 64 | 12 | 82 | 20.1 | 87.5 | 100.0 |
Bolded scores are significantly below control mean (p < .05) by the Revised Standardized Difference Test (Crawford & Garthwaite, 2005).
Based on previous patient (e.g., Buxbaum et al., 2014; Kalénine et al., 2010; Buxbaum, Kyle, et al., 2005) and neuroimaging studies (e.g., Johnson-Frey, 2004; Rumiati et al., 2004), we predicted that a broad swath of frontal, temporal, and parietal cortex would be critical for tool-related AP, whereas a relatively posterior subset of these same regions would be critical for AR. Accordingly, disproportionate impairments on AP should result from lesions more anterior than those that cause disproportionate impairments on recognition. Prior functional neuroimaging data (e.g., Kable et al., 2005; see Watson & Chatterjee, 2011; Chatterjee, 2008, for reviews) suggest that we may find a posterior-to-anterior gradient of abstraction within the temporal lobe, such that recognition will disproportionately rely on modality-specific areas specialized for processing visual motion, whereas both production and recognition will rely on more anterior regions less strongly tied to any single modality (and therefore critical for both).
METHODS
Participants
One hundred thirty-one chronic left-hemisphere stroke survivors recruited from Moss Rehabilitation Research Institute’s patient research database participated in this study (65 men; average age = 57.8 years, SD = 10.8; average education = 14 years, SD = 3). All participants were at least 3 months post-stroke (average = 45.1 months, SD = 55.4), and all participants’ lesions affected both cortical and subcortical regions of the brain. Ninety of the 131 stroke participants had ischemic strokes, 26 had hemorrhagic strokes, and 15 had strokes of unknown etiology. Patients with a history of psychosis, brain injury, drug or alcohol abuse, severely impaired verbal comprehension (a score of 4 or less on the auditory verbal comprehension subtest of the Western Aphasia Battery; Kertesz, 1982), or who were over 80 years old were excluded from the study. Data from 49 of the 131 patients have not previously been reported. AR (but not production) data from 13 of the 131 patients were included in a previous study (Kalénine et al., 2010), AP (but not recognition) data from 39 patients were included in a second study (Buxbaum et al., 2014), and 30 patients provided data to both studies.
Additionally, control participants completed both tasks. All controls achieved a minimum score of 27/30 on the Mini-Mental State Examination (Folstein, Folstein, & McHugh, 1975). Ten right-handed control participants (five women, mean age = 64.7 years, range = 43–77 years; mean education = 14 years, range = 10–18 years) completed the AR task. Their average score was 97.5 (SD = 3.8, range = 91.7–100). Twenty-two right-handed control participants (13 women, mean age = 57.7 years, range = 38–80 years; mean education = 15.5 years, range = 12–22 years) completed the AP task. Their average score was 94.9 (SD = 2.7, range = 90–100). There were no significant differences between the patients and the control group tested on AP for age, t(151) = .04, p = .97, or between the patients and the control group tested on AR for education, t(139) = .01, p = .99. However, compared to the patients, the controls tested on AP were significantly more educated (an average of 15.5 years vs. 14 for the patients, t(151) = 2.16, p = .03) and the controls tested on AR were significantly older (an average of 65 years vs. 57.8 for the patients, t(139) = 2.08, p = .04). However, age did not significantly correlate with performance among controls who performed AR, r(9) = −.79, p = .82; similarly, education did not significantly correlate with performance among controls who performed AP, r(21) = .20, p = .36.
For 109 of the stroke participants, we obtained a research quality structural MRI or CT scan at the Hospital of the University of Pennsylvania. Table 1 provides demographic information as well as raw scores on the experimental tasks. All participants were compensated for participation time and travel expenses and gave informed consent according to guidelines laid out by the Institutional Review Board of Einstein Healthcare Network and the Declaration of Helsinki.
Behavioral Tasks
Action Production
Participants were presented, one at a time, with 10 familiar tools (scissors, watch, toothbrush, comb, fork, bottle opener, cigarette lighter, razor, eraser, and nail clippers) and asked to pantomime the correct use of each tool, without touching it, using their left (less affected) hand only. Participants had unlimited time to respond on each trial. Before the experimental trials, they completed one practice trial (keys), during which they received corrective feedback from the experimenter as needed. Performance was videotaped and later coded according to guidelines outlined in Buxbaum, Kyle, et al. (2005; see Buxbaum et al., 2014 for a detailed appendix of scoring methods). Any trial for which the participant failed to respond was excluded from analysis. Coding was performed by trained research team members whose interrater reliability exceeded a Cohen’s Kappa of 0.85 on a subset of six action videos scored by all coders.
As in our past work (e.g., Buxbaum et al., 2014; Buxbaum, Kyle, et al., 2005), each pantomime received an independent score of 0 (error) or 1 (correct) in each of five categories (Content, Hand Posture, Arm Posture, Amplitude, and Timing), based on control performance on the same task. A Content score of 0 was given when the participant produced a recognizable semantic substitution (e.g., smoking a cigarette instead of using a lighter). Only actions receiving content scores of 1 were scored on the remaining components. In actions meeting that criterion, Hand Posture was scored as 0 if the manner of movement of the hand and wrist or the positioning of the hand and fingers relative to one another were flagrantly incorrect. So-called “body-part-as-object” errors that substituted the hand or fingers for a tool (e.g., gesturing to scissors by moving the fingers like scissor blades) also earned a 0 in this category. The first time a participant committed such an error, the experimenter reminded them of the task’s instructions and gave them a second chance to attempt the action. A score of 0 for Arm Posture indicated that the arm moved in a flagrantly incorrect plane or direction. A score of 0 for Amplitude indicated that an action was flagrantly too large or too small. A score of 0 for Timing indicated a movement that was too quick or too slow or performed with too many or too few iterations. A total score for each pantomime was computed by averaging the Hand Posture, Arm Posture, Amplitude, and Timing scores.3
Action Recognition
Participants performed a forced-choice task consisting of 24 transitive action names, which they matched to a video of the correct action. The task was described previously in Kalénine et al. (2010) and designed to tax semantic understanding of action. On each trial, participants saw a phrase describing an everyday action (e.g., “combing hair”) written on a computer screen while the experimenter read the phrase aloud. After a 2-sec delay, two videos of an experimenter performing pantomimed actions played in succession. The participant was told to select the video (labeled “A” or “B”) that matched the action phrase. The incorrect choice was an action semantically related to the correct choice—for example, the correct video for “combing hair” showed a pantomime of using a comb, whereas the incorrect video (semantic foil) showed a pantomime of brushing teeth (see Figure 1). Patients also completed a verb comprehension pretest, in which they chose a tool picture from an array of three tools to match with an action name (e.g., matching a hammer to the verb “hammering”). Actions that patients failed to match to the relevant tool were excluded from their final AR score, and average AR performance was calculated based on an adjusted total number of trials. This pretest ensured that patients understood the verb phrases used in the AR task.
Figure 1.

Sample trial from the AR task. Participants see and hear an action name (“combing hair”), then see videos of (A) a correct action and (B) a semantic foil (here, “brushing teeth”), and must indicate which video matches the action name.
Lesion Analyses
One hundred nine patients consented to a research quality CT or MRI scan at the Hospital of the University of Pennsylvania. Research MRI scans (n = 65) included whole-brain T1-weighted MR images collected on a 3T (Siemens Trio, Erlangen, Germany; repetition time = 1620 msec, echo time = 3.87 msec, field of view = 192 × 256 mm, 1 × 1 × 1 mm voxels) or 1.5T (Siemens Sonata, repetition time = 3,000 msec, echo time = 3.54 msec, field of view = 24 cm, 1.25 × 1.25 × 1.25 mm voxels) scanner, using a Siemens eight-channel head coil. Patients who were contraindicated for MRI (n = 44) underwent whole-brain research CT scans without contrast (60 axial slices, 3–5 mm slice thickness) on a 64-slice Siemens SOMATOM Sensation scanner. The remaining 22 declined to participate in a research scan but provided clinical scans which a team neurologist determined to be of sufficiently high quality to reliably draw the outlines of the lesion.
For research MRI scans, a research team member manually segmented lesions to produce a 3-D lesion mask of 0s and 1s, with 1 indicating a lesioned voxel. Segmentation included both gray and white matter voxels. Furthermore, intact gray matter voxels surrounded on all sides by lesioned gray and/or white matter were also drawn as “lesioned.” As a result, the analysis potentially reveals both gray and white matter damage associated with behavioral impairments (see, e.g., Watson & Buxbaum, 2015; Schwartz, Faseyitan, Kim, & Coslett, 2012). Thresholded, binarized lesion drawings were then warped to a 1 mm × 1 mm × 1 mm common template brain (Montreal Neurological Institute “Colin27”) using a symmetric diffeomorphic registration algorithm (Avants, Epstein, Grossman, & Gee, 2008, www.picsl.upenn.edu/ANTS) to translate manual lesion segmentations to standardized space via a two-step process: First, they were registered to an intermediate template comprising healthy brain images acquired from the same scanner at the Hospital of the University of Pennsylvania that was used to collect MRI scans from the patients; then, volumes were mapped from the intermediate template to the “Colin27” template. A team neurologist naive to the behavioral data (Dr. H. Branch Coslett) inspected all warped lesions to ensure that no errors had occurred. Lesions from research CT or clinical scans were drawn by the same neurologist directly onto the template brain, which had been rotated to match the pitch of the patient’s scan. This method has achieved high intra- and interrater reliability in a previous study (Schnur et al., 2009).
It is possible for regions irrigated by the same blood vessels to incorrectly appear to be important for the same behavioral deficit. To account for these possible effects of vascular association between voxels, we controlled for total lesion volume. For each patient’s lesion drawing, we divided the value in each voxel (1 if lesioned, 0 if not lesioned) by the square root of the patient’s total lesion volume (see Mirman, Zhang, Wang, Coslett, & Schwartz, 2015; Zhang, Kimberg, Coslett, Schwartz, & Wang, 2014, for more details). Thus, lesioned voxels that belong to a patient with a large lesion carried less weight in the analyses than those belonging to a patient with a small lesion.
Voxel-based Lesion-symptom Mapping
VLSM analyses utilized the VoxBo brain imaging package (Kimberg & Aguirre, 2001) to perform a regression in which the transformed lesion values (i.e., controlled for total lesion volume) were used to predict behavioral scores across all patients. Only voxels lesioned in at least 10 patients were included in the analysis. A total of 368,499 voxels (49.9% of the 738,535 voxels in the left hemisphere, according to the Automated Anatomical Labeling (AAL) atlas; Tzourio-Mazoyer et al., 2002) survived this criterion. We corrected for multiple comparisons by calculating a false discovery rate (FDR) threshold (Benjamini & Hochberg, 1995). Only voxels whose p values survived the FDR threshold at q = 0.05 were considered significant, that is, a false positive rate of 5% of suprathreshold voxels. Results were subjected to a minimum cluster size criterion, such that only clusters containing >50 voxels were included.
In addition to patients’ raw scores on AP and AR, VLSM analyses were also conducted on residualized scores for both tasks. A residualized score results from regressing the raw score from one task onto the raw score from the other to remove any shared variance. The residual thus represents the degree to which a patient is more impaired on one task (e.g., AP) than would be expected given performance on the other (e.g., AR). VLSM analyses on residualized scores thus aim to find brain regions differentially involved in one task versus another.
Lesion Subtraction Analyses
Although it is usually the case, a significant VLSM result at a given voxel does not mathematically require that patients with the worst performance have lesions at that voxel. Therefore, we also conducted lesion subtraction analyses for patients with the greatest disproportionate impairments on either AP or AR. In doing so, we determined whether patients with clinically significant deficits had lesions to the same areas that emerge as significant in VLSM analyses, and vice versa. Patients with residualized scores more than two standard deviations below the group mean on AP given their performance on AR (n = 9) and on AR given their performance on AP (n = 6) were included. Lesion subtraction analyses calculate the difference between the percentages of lesions at each voxel in these two patient groups. We used percentages rather than raw numbers to account for the different number of patients in each group. Thus, the result of a lesion subtraction reveals voxels with greater involvement for one performance group than another. Results of lesion subtraction analyses were assigned a threshold based on the difference size that yielded a chi-square test significant at p < .05 at each voxel (see Kemmerer, Rudrauf, Manzel, & Tranel, 2012; Mirman & Graziano, 2012, for details). For the above group sizes, this difference size was determined to be 55.6%.
RESULTS
Behavioral Results
Table 1 shows the broad distribution of raw scores on AP and AR. The mean score was 79.90% (SD = 16.19) for AP and 88.34% (SD = 11.06) for AR. On the basis of the Revised Standardized Difference Test (Crawford & Garthwaite, 2005), which determines the difference between each patient’s score and the control mean using a modified t test, 48 patients (36.6%) scored abnormally (p < .05) on AP only, 9 patients (6.9%) scored abnormally on AR only, and 47 patients (35.9%) scored abnormally on both tasks. When we compared the magnitude of t values calculated for AR and AP, we found that the nine patients who scored abnormally solely on AR showed numerically smaller differences from normal values (average = 3.9, maximum = 7.6) than the 47 patients who scored abnormally solely on AP (average = 4.8, maximum = 15.8). AR and AP scores were moderately correlated in a partial correlation controlling for total lesion volume, r(128) = .44, p < .005 (Figure 2). To ensure that an outlier (AR residual score = −39.18, AP residual score = −21.97) was not driving this correlation, we conducted the analysis a second time without this data point. The partial correlation value remained essentially unchanged [r(127) = .43, p < .005].
Figure 2.
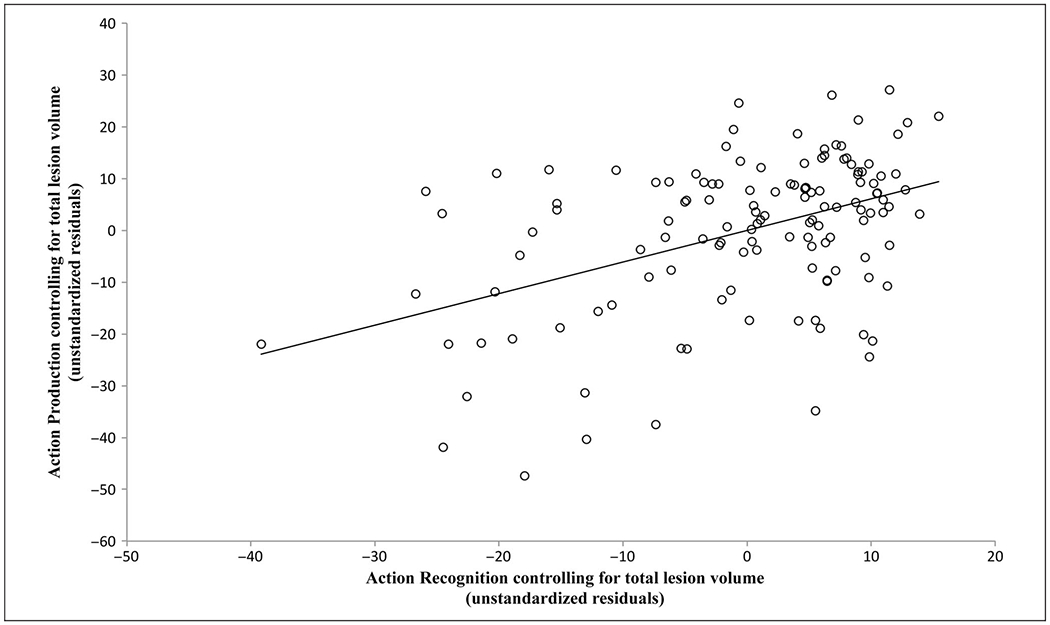
Partial correlation between performance on AP and AR tasks, controlling for total lesion volume [r(128) = .44, p < .005]. The x axis displays the unstandardized residual scores that resulted from regressing AR onto lesion volume, and the y axis displays the same residual scores for regressing AP onto lesion volume.
VLSM Results
Figure 3 shows the overlap of all 131 lesions included in the analysis. The lesion coverage is well distributed over the left hemisphere, especially in ROIs, including the IFG, IPL, and pTC.
Figure 3.
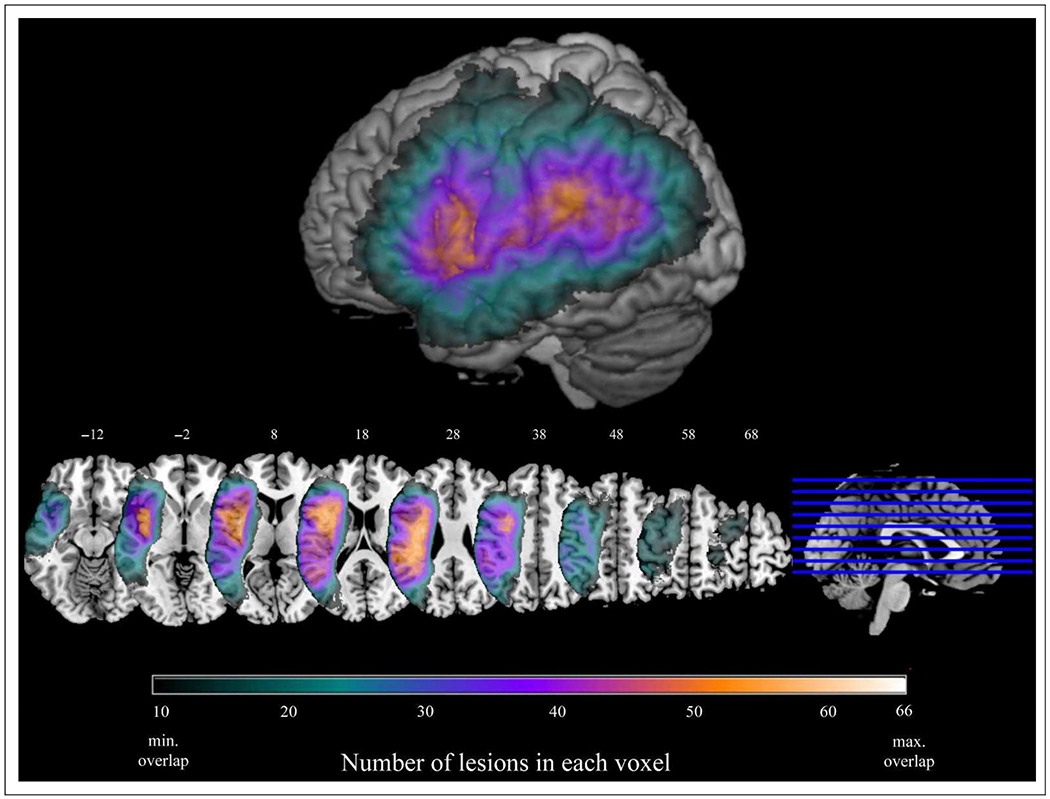
Overlap of all 131 lesions included in the analyses. Only voxels with a minimum of 10 lesions in each voxel are displayed. The maximum overlap was 66 lesions. Surface rendering displayed at a search depth of 8 mm. Z coordinates of axial slices are listed in MNI standardized space.
Analyses with Raw Scores
Figure 4 and Table 2 present the results of VLSM analyses conducted on the raw AP and AR scores. For AP, 7161 voxels survived an FDR-corrected threshold of q = 0.05, including superior temporal gyrus (STG) and MTG, IFG, middle frontal gyrus (MFG), primary somatosensory cortex (S1), primary motor cortex (M1), thalamus, and supramarginal gyrus (SMG). For AR, 18,588 voxels survived an FDR-corrected threshold of q = 0.05. Suprathreshold voxels clustered around the posterior part of the temporal lobe, including STG, MTG, inferior temporal gyrus, and lateral temporo-occipital cortex (LTO), with another smaller cluster in MFG and IFG. Next, we looked for regions where damage was significantly tied to poor performance on both tasks by calculating the intersection (conjunction) of significant voxels resulting from the separate VLSM analyses of raw AP scores and AR scores (Figure 4C and Table 3). The conjunction analysis revealed three clusters in the posterior temporal lobe (MTG and STG), LTO, and angular gyrus, as well as one in MFG and IFG.
Figure 4.
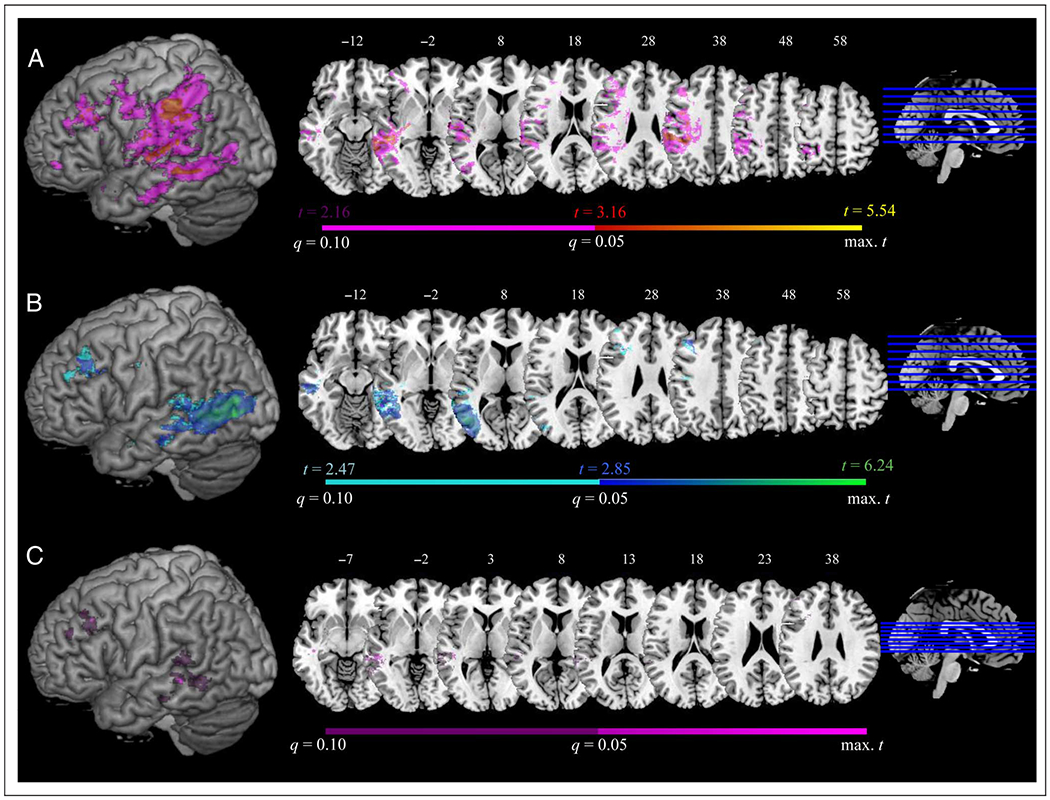
VLSM results showing areas critical for (A) AP, (B) AR, and (C) conjunction of regions significantly involved in AP and AR in individual VLSM analyses (FDR q = 0.05). Results at an FDR threshold of 0.05 < q ≤ 0.1 are displayed in violet for AP, cyan for AR, and dark purple for the conjunction. Surface rendering displayed at a search depth of 8 mm. Z coordinates of axial slices are listed in MNI standardized space.
Table 2.
Brodmann and AAL Regions for Clusters of Significanta Voxels in the AP and AR VLSM Analyses, Controlling for Total Lesion Volume
| Peak Voxel |
|||||||
|---|---|---|---|---|---|---|---|
| Task | AAL Regionsb | BAb | Total Voxels | t | Xc | Y | Z |
| AP | Parietal_Inf, SupraMarginal, Postcentral, Precentral | 40, 2, 3, 6d | 2,850 | 4.78 | −32 | −31 | 41 |
| Temporal_Mid, Temporal_Sup, Thalamus | 21, 41, 42, 20, 37, 22 | 1,581 | 5.54 | −32 | −23 | 0 | |
| Frontal_Mid, Frontal_Inf_Tri, Frontal_Inf_Oper | 44, 46, 45 | 392 | 4.24 | −38 | 25 | 35 | |
| Postcentral | 3, 43 | 385 | 4.41 | −41 | −20 | 31 | |
| Temporal_Sup | 22, 42 | 296 | 4.39 | −64 | −23 | 12 | |
| Temporal_Sup, Temporal_Mid | 20 | 266 | 5.26 | −40 | −22 | −7 | |
| Temporal_Mid | 20, 21 | 150 | 4.21 | −39 | −6 | −17 | |
| Precentral | 6d | 135 | 3.95 | −42 | 0 | 38 | |
| Parietal_Inf | 40 | 74 | 3.79 | −43 | −41 | 54 | |
| AR | Temporal_Mid, Occipital_Mid, Temporal_Inf, Temporal_Sup, Occipital_Inf | 37, 21, 19, 20, 22, 39, 18 | 17,018 | 6.24 | −60 | −64 | 2 |
| Frontal_Mid, Frontal_Inf_Tri, Frontal_Inf, Oper | 44, 46, 45 | 865 | 3.93 | −38 | 25 | 36 | |
| Temporal_Mid, Temporal_Sup | 22, 42 | 113 | 3.32 | −63 | −40 | 10 | |
| Temporal_Sup | 20 | 71 | 3.40 | −39 | −17 | −8 | |
q = 0.05.
Brodmann and AAL (Tzourio-Mazoyer et al., 2002) areas listed in descending order of voxels involved in the region. Areas containing fewer than 10 significant voxels were not reported.
Peak voxel coordinates listed in MNI space.
BA 6 is commonly identified as the premotor cortex (PMC); however, the “AAL Regions” column does not list this label because the AAL atlas does not include a region labeled as “PMC.” Instead, BA 6 in the Brodmann atlas corresponds to portions of the AAL regions labeled as Precentral, Frontal_Mid, and Frontal_Sup.
Table 3.
Brodmann and AAL Regions for Clusters of Voxels Significantlya Involved in AP Controlling for AR, in AR Controlling for AP, and in the Conjunction between Analyses of AP and AR Raw Accuracies
| Peak Voxel |
|||||||
|---|---|---|---|---|---|---|---|
| Task | AAL Regionsb | BAb | Total Voxels | t | Xc | Y | Z |
| AP controlling for AR | Parietal_Inf, SupraMarginal, Postcentral | 40, 2, 3 | 2,804 | 4.79 | −20 | −21 | 31 |
| Precentral | 6d | 277 | 4.06 | −35 | 0 | 37 | |
| Parietal_Sup, Parietal_Inf | 7, 2, 40 | 274 | 4.48 | −26 | −51 | 58 | |
| Postcentral | 3 | 220 | 4.07 | −47 | −16 | 31 | |
| Thalamus | – | 113 | 4.94 | −32 | −22 | 0 | |
| SupraMarginal | 40, 2 | 63 | 3.72 | −43 | −41 | 54 | |
| Postcentral_L, Temporal_Sup | 3, 22, 40 | 51 | 3.96 | −32 | −34 | 51 | |
| AR controlling for AP | Temporal_Mid, Occipital_Mid, Temporal_Inf | 37, 19, 21, 18, 39, 22 | 8,088 | 6.04 | −60 | −64 | 2 |
| Temporal_Mid | 20 | 117 | 3.7 | −53 | −30 | −12 | |
| Temporal_Mid, Temporal_Inf | 20 | 115 | 3.67 | −39 | −25 | −15 | |
| Conjunction between | Temporal_Mid, Temporal_Sup | 21 | 293 | 5.03 | −43 | −33 | 2 |
| AP and AR | Frontal_Mid, Frontal_Inf_Tri | 44, 45, 46 | 181 | 4.24 | −38 | 25 | 35 |
| Temporal_Mid | 20 | 93 | 4.94 | −42 | −23 | −8 | |
| Temporal_Mid | 21 | 66 | 4.01 | −62 | −37 | −2 | |
All analyses controlled for total lesion volume.
q = 0.05.
Brodmann and AAL (Tzourio-Mazoyer et al., 2002) areas listed in descending order of voxels involved in the region. Areas containing fewer than 10 significant voxels were not reported.
Peak voxel coordinates listed in MNI space.
BA 6 is commonly identified as the premotor cortex (PMC); however, the “AAL Regions” column does not list this label because the AAL atlas does not include a region labeled as “PMC.” Instead, BA 6 in the Brodmann atlas corresponds to portions of the AAL regions labeled as Precentral, Frontal_Mid, and Frontal_Sup.
Residual Analyses
We next assessed regions that, when lesioned, indicated disproportionate deficits on one or the other behavioral task. Figure 5 and Table 3 present the results of VLSM analyses conducted on residualized scores (AR controlling for shared variance with AP, and AP controlling for shared variance with AR; see Methods). Five thousand two hundred thirty voxels survived an FDR-corrected threshold of q = 0.05 for residualized AP scores; suprathreshold voxels included portions of IPL, S1, M1, and STG. For residualized AR scores, 8454 voxels survived an FDR-corrected threshold of q = 0.05. Suprathreshold voxels clustered around pMTG and LTO.
Figure 5.
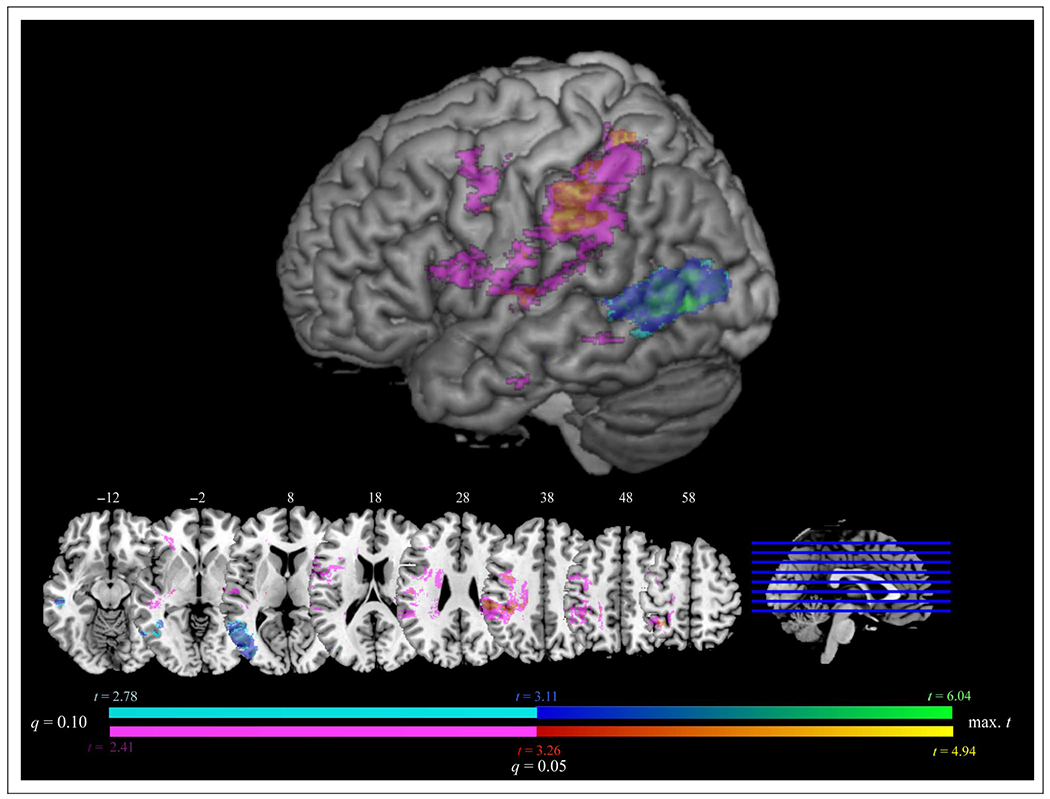
VLSM results displaying regions involved in AP after controlling for the shared variance with AR (red–yellow) and regions involved in AR after controlling for the shared variance with AP (blue–green; FDR q = 0.05). Results at an FDR threshold of 0.05 < q ≤ 0.1 are displayed in violet for AP controlling for AR and in cyan for AR controlling for AP. Surface rendering displayed at a search depth of 8 mm. Z coordinates of axial slices are listed in MNI standardized space.
To examine the relationship in pTC between residualized AR scores and the conjunction of AP and AR, we plotted these results together (Figure 6) and found that the majority of voxels associated with disproportionate AR impairments (peak voxel at y = −64) were posterior to those associated with the AP/AR conjunction (y = −22 to 11). In a post hoc analysis, we further investigated the overlap of our results with regions known to be important to visual perception. As shown in Figure 6, we found that voxels in LTO associated with residualized AR scores overlapped with hMT+, a region specialized for processing visual motion (e.g., Watson, Cardillo, Bromberger, & Chatterjee, 2014; Dumoulin et al., 2000).
Figure 6.
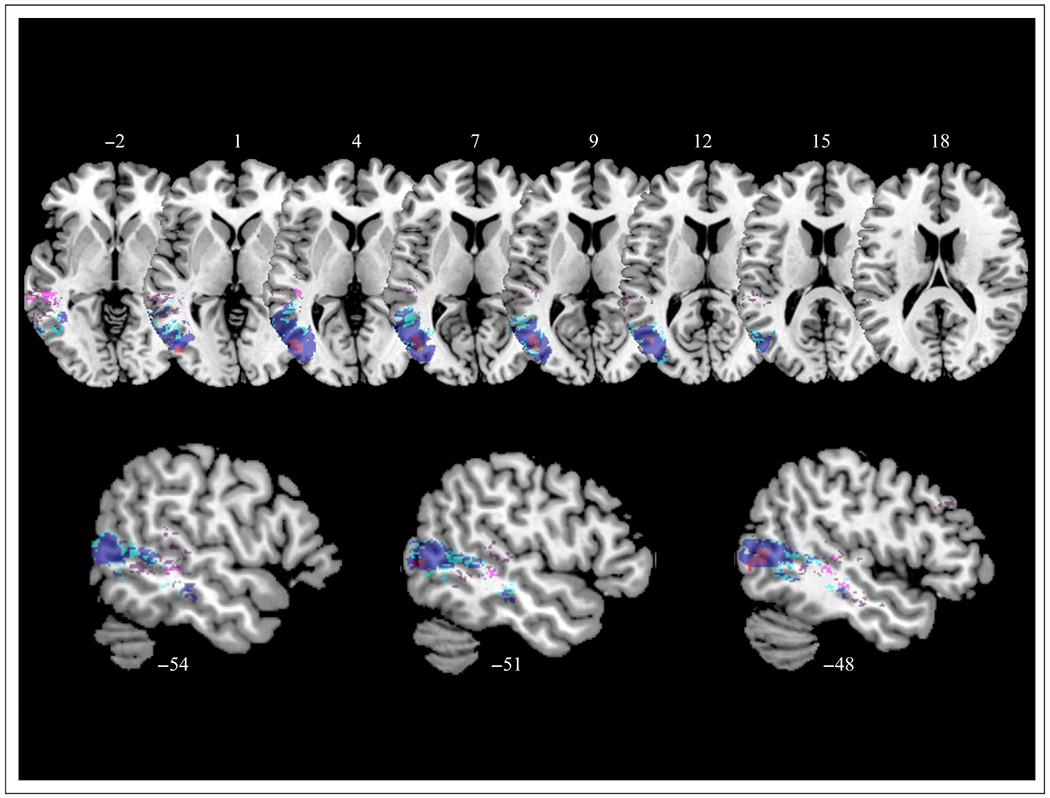
VLSM results displaying regions involved in AR after controlling for the shared variance with AP (blue at FDR q = 0.05, cyan at 0.05 < q ≤ 0.1), regions involved in both AR and AP (i.e., conjunction; violet at FDR q = 0.05, dark purple at 0.05 < q ≤ 0.1), and hMT+ (red; Watson et al., 2014). Z coordinates of axial slices and x coordinates of sagittal slices are listed in MNI standardized space.
Lesion Subtraction Analyses
We also sought to determine whether patients with clinically significant deficits have lesions to the same areas that emerged as significant in VLSM analyses. To do so, we used lesion subtractions to determine the regions in which patients with disproportionate AP impairments had greater lesion overlap than that observed among patients with disproportionate AR impairments, and vice versa. In other words, we subtracted the two groups’ lesions from each other (see also Kemmerer et al., 2012; Mirman & Graziano, 2012). When thresholded at a percentage difference equivalent to a chi-square test significant at p < .05 (55.56%), the nine patients who were disproportionately impaired on AP given their performance on AR had greater lesion overlap in IFG, S1, and SMG, whereas the six patients who were disproportionately impaired on AR given their performance on AP had greater lesion overlap in LTO (Figure 7). Thus, the results of these analyses support those of the residualized VLSM analyses. Additionally, to ensure that the behavioral differences between these two groups of patients cannot be attributed to differential language comprehension deficits, we compared their verbal comprehension scores (as measured by the Western Aphasia Battery) and found them not to differ [t(13) = 0.73, p = .48].
Figure 7.
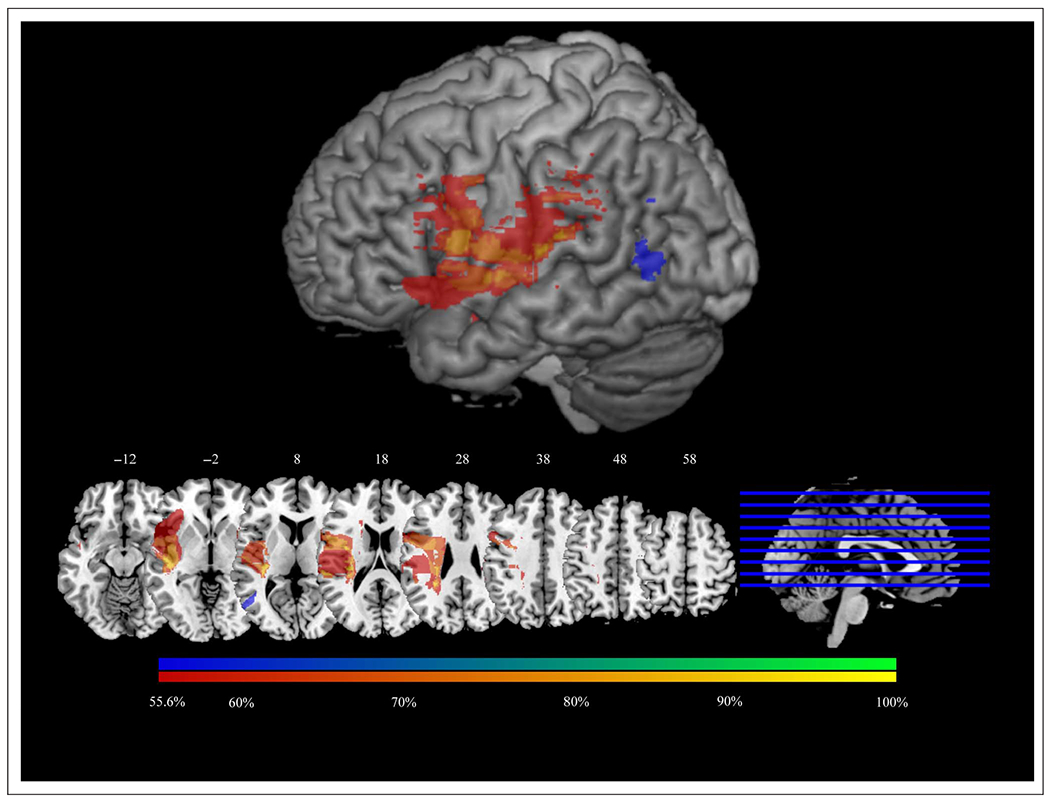
Subtraction of percentage of lesions belonging to patients with disparities between AP and AR that are more than 2 standard deviations from the group means for this relationship. Voxels with greater lesion overlap among patients with disproportionately low scores on AP given AR are colored red–yellow. Voxels with greater lesion overlap among patients with disproportionately low scores on AR given AP are colored blue–green. Subtraction maps are displayed at a minimum difference of 55.6% between groups (i.e., a difference of at least 55.6%, p < .05, in the proportion of patients in each group having lesions in each voxel). Surface renderings are displayed at a search depth of 8 mm. Z coordinates of axial slices are listed in MNI standardized space.
DISCUSSION
Assessment of the cognitive and neuroanatomic relationships between AP and AR revealed three major findings. First, performance on both tasks was significantly associated, and relied upon, common left hemisphere brain regions. Second, there were nevertheless dissociations between the tasks and the brain regions supporting each one. Third, we observed a mosaic-like organization in the posterior temporal lobe in which closely located regions are critical for each task. Below, we will discuss each of these findings and their implications for theories of action representation in the brain.
Common Substrates of AP and AR
Much of our data suggest common cognitive substrates for production and recognition of action. We observed a moderately strong correlation between patients’ performance on these tasks, even after controlling for overall lesion volume. This control ensures that the relationship is not due to lesion severity causing global deficits in cognitive processing. This result is consistent with prior data from our laboratory, indicating that AR and AP abilities are reliably linked (Buxbaum, Kyle, et al., 2005).
We also found evidence that AP and AR share neural resources; specifically, impairments for both tasks were associated with lesions to the left pTC, IFG, and MFG. The pTC is an important locus of the semantic system (e.g., Wei et al., 2012; Noppeney et al., 2007; Dronkers & Wilkins, 2004; Chao, Haxby, & Martin, 1999) and the action semantic system, in particular (Amoruso et al., 2013; Watson et al., 2013; Kalénine et al., 2010; Mahon et al., 2007; Caramazza & Mahon, 2006). In fact, some researchers have recently claimed that pMTG acts as a semantic “hub” for tools and tool actions (Martin, Kyle Simmons, Beauchamp, & Gotts, 2014; van Elk, van Schie, & Bekkering, 2014). The MFG is frequently activated in functional neuroimaging studies when participants view familiar tools in a functional context (Bellebaum et al., 2013; Yang, Shu, Bi, Liu, & Wang, 2011; Bach, Peelen, & Tipper, 2010; Kaplan & Iacoboni, 2007), suggesting that it may implement high-level representations that link action goals with their proper use (Bach et al., 2010). Conversely, given that activation in MFG is not restricted to action-related tasks (e.g., Leung, Gore, & Goldman-Rakic, 2002), this region and IFG may support more general executive processes necessary to access tool use knowledge (Kouneiher, Charron, & Koechlin, 2009; Badre & Wagner, 2007; see also Kalénine et al., 2010).
Given that IFG has been implicated as a “mirror” region (e.g., Rizzolatti & Craighero, 2004), it is also possible that motor simulation within this region is necessary to both tasks. However, neither MFG nor pTC have been identified as parts of the mirror system, and these regions comprised the majority of significant voxels in a conjunction analysis of AR and AP. As we discuss below, shared reliance on regions outside the putative mirror system suggests that these tasks may not rely strongly on motor simulation.
Distinct Neural Substrates for AP and AR
Consistent with the findings of Negri et al. (2007), a proportion of our patients performed abnormally on AP but not AR, and a substantially smaller number of patients performed abnormally on AR but not AP. Furthermore, our data indicate that patients with impaired AR but spared AP tend to share lesions in left pTC (specifically, LTO), whereas patients with impaired AP but spared AR tend to share lesions in more anterior regions, including left M1, IPL, and thalamus. This pattern of results is reminiscent of that reported in a pioneering study by Heilman, Rothi, and Valenstein (1982), who found that apraxic patients with posterior lesions, including SMG and angular gyrus, showed greater impairments on tasks of AR than apraxic patients with lesions anterior to SMG. Although their results did not include the posterior temporal lobe, they were based on a very small sample (n = 20) with unclear coverage in that region.
In the current study, several frontoparietal areas were critical for production when controlling for recognition; thus, the odds of a lesion in this broad area are high, explaining the frequency of this pattern. By contrast, a relatively focal portion of pTC (LTO) was critical for recognition when controlling for production. Because this region is slightly outside the most frequent loci of most middle cerebral artery strokes, the odds of this lesion and pattern of apraxia are low. Moreover, patients with selectively impaired AR did not exhibit differences between the two tasks as large as those observed among patients with selectively impaired AP. Together, these results suggest that it is difficult to dramatically impair AR without also affecting production. However, it is also important to note that AR requires abilities that are not required for AP (e.g., AR requires visual perception of dynamic actions) and, as we will discuss below, implicates brain regions that are not merely a subset of those involved in AP.
The Role of the Posterior Temporal Lobe in AR and AP
In the pTC, we found a nuanced pattern of relationships between tool AR and AP: The majority of voxels associated with disproportionate recognition impairments were located posteriorly in LTO, whereas voxels associated with impairments on both tasks were located more anteriorly in pMTG. Many neuroimaging (e.g., Kellenbach, Brett, & Patterson, 2003; Chao et al., 1999; see Watson et al., 2013, for a meta-analysis) and neuropsychological (e.g., Kemmerer et al., 2012; Kalénine et al., 2009, 2010; Tranel, Kemmerer, Adolphs, Damasio, & Damasio, 2003) studies have found left pTC to be involved in recognizing tools and their actions. Some researchers have suggested that this region represents knowledge about actions specific to or derived from visual motion information (Kemmerer, 2015; Watson & Chatterjee, 2011; Kemmerer, Castillo, Talavage, Patterson, & Wiley, 2008; Kable, Lease-Spellmeyer, & Chatterjee, 2002). pTC has also been suggested as a region responsible for the multimodal integration of sensory (Fernandino et al., 2015) or semantic information (Visser, Jefferies, Embleton, & Ralph, 2012; Binder, Desai, Graves, & Conant, 2009; Willems, Özyürek, & Hagoort, 2009), especially information pertaining to actions and tools (Martin et al., 2014; van Elk et al., 2014; Noppeney, Josephs, Kiebel, Friston, & Price, 2005). However, recent evidence suggests that the pTC also participates in the preparation of tool actions. In particular, Gallivan, McLean, et al. (2013) used multi-voxel pattern analyses of fMRI data and found that hand- and tool-related action plans could be decoded from preparatory signals in LTO (see also Gallivan, Johnsrude, & Flanagan, 2015; Gallivan, Chapman, McLean, Flanagan, & Culham, 2013; Singhal, Monaco, Kaufman, & Culham, 2013). Furthermore, a recent study from our laboratory showed that the pTC is critically required for the production of the postural aspects of tool use actions (Buxbaum et al., 2014). Together, these results indicate that pTC is involved in the perception and understanding of tool-directed actions, as well as their preparation.
Our results elucidate the nature of the relationship between AR and AP in pTC. Specifically, there is mounting evidence for a rough posterior–anterior gradient of abstraction of action representations within these areas (see Watson & Chatterjee, 2011; Chatterjee, 2008, for reviews). In a meta-analysis of functional neuroimaging studies, Watson and colleagues found that viewing both action words and action images consistently activated a portion of pMTG anterior to areas responding only to action images (Watson et al., 2013). They hypothesized that this anterior region represents information about actions derived from visual motion information in hMT+, but sufficiently abstract to be insensitive to the modality of input (see also Kable et al., 2002, 2005; Noppeney et al., 2005). More broadly, Thompson-Schill and colleagues (Rugg & Thompson-Schill, 2013; Yee, Chrysikou, & Thompson-Schill, 2013; Thompson-Schill, 2003) have suggested that such an “anterior shift” also applies to other aspects of object knowledge, like color and form. Specifically, Rugg and Thompson-Schill (2013) argue that a given aspect of semantic memory is represented at multiple levels of abstraction, and recruitment of regions along this abstraction continuum can vary depending on the task. Although more posterior regions often participate in modality-specific (e.g., perceptual) tasks, anterior regions integrate information across modalities (Yee et al., 2013; see Rogers et al., 2004; Plaut, 2002, for computational evidence regarding how such an organization might arise).
To our knowledge, our findings offer the only lesion-deficit evidence suggestive of this anterior shift hypothesis within pTC. We found that disproportionate recognition impairments were associated with lesions to LTO, overlapping with hMT+ (e.g., Watson et al., 2014). On the other hand, impairments to both production and recognition tasks were primarily associated with lesions in pMTG, anterior to hMT+ and LTO. This pattern is consistent with a rough, mosaic-like posterior-to-anterior gradient of abstraction in pTC. In particular, LTO (including regions specialized for visual motion) is especially critical for tasks that place strong demands on visual perception of actions. We also speculate that more anterior parts of pTC (pMTG) integrate information across modalities, with action representations that are sufficiently abstract to be recruited by different kinds of input (i.e., action images and words; Watson et al., 2013) and tasks (i.e., AR and AP). This shared reliance on multimodal representations of actions offers an alternative explanation for the linkage between AR and AP that does not invoke mechanisms of motor simulation.
The Role of Anterior Brain Regions in AP
Damage to several anterior brain regions impaired patients’ ability to produce tool-related actions. These areas included, unsurprisingly, primary motor and sensory areas, as well as SMG and IFG, echoing previous findings of frontoparietal activation during pantomime (Bohlhalter et al., 2009; Johnson-Frey, 2004; Rumiati et al., 2004). These latter regions comprise important nodes of the tool use network (e.g., Buxbaum et al., 2014; Lewis, 2006). We and others have suggested that IPL and SMG, in particular, implement biased competition between possible tool-related actions during AP but not recognition (Watson & Buxbaum, 2015; see also Cisek & Kalaska, 2010; Cisek, 2007, for related accounts). Similarly, a recent neuroimaging study showed that activation within SMG (as well as premotor cortex and the insula) “increase[s] with the competition load between object-evoked action options” (Schubotz, Wurm, Wittmann, & Von Cramon, 2014, p. 10). IFG may be critical for biasing action competition within SMG (Watson & Buxbaum, 2015). Neuroimaging work has implicated this region in the inhibition of intentional action (Brass & Haggard, 2007; Wager et al., 2005), and so its role in action selection may be to inhibit the selection of a competing motor response in favor of the correct one.
Implications for Theories of Action Representation and Simulation
Several accounts suggest that AP and AR both recruit the process of “simulation,” a reenactment of sensory and motor states acquired during experience with the world (Decety & Grèzes, 2006; Buccino et al., 2001; Jeannerod, 2001; Barsalou, 1999). Yet, as noted by Grafton (2009), “…defining the level of simulation becomes a central issue for interpreting both behavioral and physiological studies” (p. 98). For instance, when someone observes an action, they may simulate a simple visual trajectory, a complex sequence of muscle movements, or an abstracted action “engram.” Furthermore, it is important to identify the processing stage at which a patient’s impairments lie. For example, selective AP deficits may arise from damage at several points along the processing stream, from retrieval of stored knowledge about tool use to execution of a motor command. Not all of these impairments are informative for assessing the cognitive and neural overlap between AP and AR: for example, some patients with impaired AP and relatively intact recognition may successfully access action representations shared by the two tasks but perform poorly on production due to impairments at later processing stages. Vagueness regarding what information is being simulated, and when, has led some to adopt all-or-nothing approaches: that any evidence for shared neural resources between AP and AR indicates that recognition relies solely upon motor resonance (e.g., Gallese, 2005) or instead that any evidence for distinct brain regions involved in AP and AR implies a total lack of simulation during action understanding (e.g., Stasenko, Garcea, & Mahon, 2013; Negri et al., 2007). We suggest that the truth lies somewhere in between. Our data suggest a limited role of simulation in action understanding; that is, AR shares neural resources with AP at only one stage of processing, specifically, when drawing upon pTC and MFG to access stored knowledge about tool actions (Kalénine et al., 2010; Kellenbach et al., 2003). Thus, recognizing the actions of others does not entail a total recruitment of regions involved in AP.
Our results also reveal that AP and AR jointly rely on regions outside the putative human mirror neuron system (i.e., pTC and MFG). Although some researchers have suggested that the involvement of the mirror system during recognition depends on action familiarity (Calvo-Merino, Grézes, Glaser, Passingham, & Haggard, 2006) and transitivity (Agnew, Wise, & Leech, 2012), we failed to find shared involvement of the mirror system in spite of the fact that the actions used in the current study were tool-directed, goal-oriented, and familiar. Instead, based on prior studies of the nature of information within pTC, we suggest that simulation during AR may involve reenactment of information derived from vision rather than the motor system. Nevertheless, the present results do not allow us to definitively determine the format of information within regions shared by production and recognition.
We note that simulation processes may also play a role in action preparation, enabling one to model the outcome of a movement before executing it. Such a mechanism fits well within a model of the brain that uses an internal forward model to continuously generate motor predictions to minimize errors in both perception and action. The brain’s action observation network has been shown to utilize forward models involving activity in the inferior frontal cortex to generate “anticipatory motor simulation[s]” (Avenanti, Annella, Candidi, Urgesi, & Aglioti, 2013). The ability to generate such predictions has been found to be deficient in apraxic patients with left parietal lobe lesions (Buxbaum, Johnson-Frey, & Bartlett-Williams, 2005; see also Schwoebel, Buxbaum, & Coslett, 2004). However, these predictive simulation mechanisms appear to rely on frontoparietal regions, whereas we found evidence for action simulation in pTC, a region not typically associated with action simulation (but see Gallivan, McLean, et al., 2013).
VLSM offers a significant advancement to earlier localization methods in cognitive neuropsychology. However, some limitations remain. First, although lesioned gray and white matter voxels were included in our VLSM analyses, and previous VLSM studies have revealed that damage to known white matter pathways also contributes to behavioral (Mirman et al., 2015; Schwartz et al., 2012; Chechlacz et al., 2010) and action impairments (Watson & Buxbaum, 2015), we did not directly assess the intactness of structural and/or functional connectivity between action-related brain regions. More research is needed to understand the way in which white matter tracts that underlie normal tool use knowledge (e.g., Hoeren et al., 2013; Ramayya, Glasser, & Rilling, 2010) contribute to recognition and production impairments when damaged (see Bi et al., 2015, for a recent study addressing this very question). Second, statistical tests in VLSM are performed independently at each voxel; such a “mass univariate” approach, however, is not well suited to discovering relationships among combinations of voxels. As a result, impairments that rely on a pattern of damage across multiple areas may be difficult to uncover. Similarly, VLSM does not allow us to determine the “vascular association” between voxels—that is, whether the vascular architecture of the brain causes lesions in different areas to frequently co-occur. We addressed concerns regarding vascular association by controlling for total lesion volume in our VLSM analyses. However, to further address the univariate limitations of the VLSM approach, future studies may profit from using brain–behavior methods that consider multivariate patterns among lesioned voxels (e.g., Mirman et al., 2015; Mah, Husain, Rees, & Nachev, 2014).
Conclusion
We compared performance on tests of AP and AR in 131 left-hemisphere stroke patients and used VLSM and lesion subtraction analyses to determine the neural underpinnings of these abilities. We found that, although MFG and pTC were involved in both tasks, each task also relied disproportionately on distinct neural substrates. Our results suggest that simulation during action understanding recruits only a subset of left-hemisphere brain areas involved in AP and, moreover, that the regions critical to both tasks lie outside the putative human mirror neuron system. These findings elucidate a long debate within the literature and frame new questions about the functional relationships between the pTC and more anterior regions. In particular, given that predictive mechanisms entailing simulation may be used throughout the brain (e.g., Adams, Shipp, & Friston, 2013; Kilner, Friston, & Frith, 2007), future research may fruitfully explore potentially distinguishable loci of visual, somatosensory, proprioceptive, and motor simulations in the action system and the role of simulation in single and multiple modalities across different high-level action tasks. Finally, given that univariate lesion-based analyses are limited in their ability to account for effects of vascular association or structural connectivity, we hope that future work will utilize tools such as DTI, resting-state fMRI, and the analysis of multivariate lesion patterns to complement our findings.
Acknowledgments
This research was supported by NIH grant RO1-NS065049 awarded to Laurel J. Buxbaum. We are grateful to the patients who participated in this research and the research assistants who collected and processed the data. Finally, we thank Dr. H. Branch Coslett, whose assistance with lesion segmentation was essential to our study.
Footnotes
Throughout the article, we use pTC to refer broadly to the region that includes lateral temporo-occipital cortex and pMTG; both areas are consistently implicated in studies of action cognition. When there is a distinction between the two, we refer to each subregion individually.
Many studies of action abilities in stroke use pantomime tasks—in addition to or instead of object use tasks—due to their sensitivity in demonstrating subtle spatiotemporal movement deficits. Pantomime and actual object use are strongly correlated (e.g., Osiurak, Jarry, Lesourd, Baumard, & Le Gall, 2013; Negri et al., 2007), and movement errors are of a similar type in both tasks (Hermsdörfer, Li, Randerath, Roby-Brami, & Goldenberg, 2013; Goldenberg, Hentze, & Hermsdörfer, 2004).
In a prior study (Buxbaum et al., 2014), we reported VLSM data relevant to poor scores on these action components. Because our interest in this study was the question of the relationship between AP and AR, we focused our analyses on total scores.
REFERENCES
- Adams RA, Shipp S, & Friston KJ (2013). Predictions not commands: Active inference in the motor system. Brain Structure and Function, 218, 611–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aglioti SM, Cesari P, Romani M, & Urgesi C (2008). Action anticipation and motor resonance in elite basketball players. Nature Neuroscience, 11, 1109–1116. [DOI] [PubMed] [Google Scholar]
- Agnew ZK, Wise RJ, & Leech R (2012). Dissociating object directed and non-object directed action in the human mirror system; implications for theories of motor simulation. PloS One, 7, e32517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoruso L, Gelormini C, Aboitiz F, González MA, Manes F, Cardona JF, et al. (2013). N400 ERPs for actions: Building meaning in context. Frontiers in Human Neuroscience, 7, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, & Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis, 12, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A, Annella L, Candidi M, Urgesi C, & Aglioti SM (2013). Compensatory plasticity in the action observation network: Virtual lesions of STS enhance anticipatory simulation of seen actions. Cerebral Cortex, 23, 570–580. [DOI] [PubMed] [Google Scholar]
- Bach P, Peelen MV, & Tipper SP (2010). On the role of object information in action observation: An fMRI study. Cerebral Cortex, 20, 2798–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, & Wagner AD (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45, 2883–2901. [DOI] [PubMed] [Google Scholar]
- Balser N, Lorey B, Pilgramm S, Stark R, Bischoff M, Zentgraf K, et al. (2014). Prediction of human actions: Expertise and task-related effects on neural activation of the action observation network. Human Brain Mapping, 35, 4016–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsalou LW (1999). Perceptions of perceptual symbols. Behavioral and Brain Sciences, 22, 637–660. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Tettamanti M, Marchetta E, Della Rosa P, Rizzo G, Daum I, et al. (2013). Neural representations of unfamiliar objects are modulated by sensorimotor experience. Cortex, 49, 1110–1125. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B, Methodological, 57, 289–300. [Google Scholar]
- Bi Y, Han Z, Zhong S, Ma Y, Gong G, Huang R, et al. (2015). The white matter structural network underlying human tool use and tool understanding. The Journal of Neuroscience, 35, 6822–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, & Conant LL (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19, 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Hattori N, Wheaton L, Fridman E, Shamim EA, Garraux G, et al. (2009). Gesture subtype–dependent left lateralization of praxis planning: An event-related fMRI study. Cerebral Cortex, 19, 1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, & Haggard P (2007). To do or not to do: The neural signature of self-control. Journal of Neuroscience, 27, 9141–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. (2001). Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. European Journal of Neuroscience, 13, 400–404. [PubMed] [Google Scholar]
- Buxbaum LJ (2001). Ideomotor apraxia: A call to action. Neurocase, 7, 445–458. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Johnson-Frey SH, & Bartlett-Williams M (2005). Deficient internal models for planning hand–object interactions in apraxia. Neuropsychologia, 43, 917–929. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, & Menon R (2005). On beyond mirror neurons: Internal representations subserving imitation and recognition of skilled object-related actions in humans. Cognitive Brain Research, 25, 226–239. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Shapiro AD, & Coslett HB (2014). Critical brain regions for tool-related and imitative actions: A componential analysis. Brain, 137, 1971–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, & Haggard P (2006). Seeing or doing? Influence of visual and motor familiarity in action observation. Current Biology, 16, 1905–1910. [DOI] [PubMed] [Google Scholar]
- Caramazza A, & Mahon BZ (2006). The organisation of conceptual knowledge in the brain: The future’s past and some future directions. Cognitive Neuropsychology, 23, 13–38. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, & Eickhoff SB (2010). ALE meta-analysis of action observation and imitation in the human brain. Neuroimage, 50, 1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebolla AM, Palmero-Soler E, Dan B, & Cheron G (2014). Modulation of the N30 generators of the somatosensory evoked potentials by the mirror neuron system. Neuroimage, 95, 48–60. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, & Martin A (1999). Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience, 2, 913–919. [DOI] [PubMed] [Google Scholar]
- Chatterjee A (2008). The neural organization of spatial thought and language. Seminars in Speech and Language, 29, 226–238. [DOI] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Bickerton WL, Hansen PC, Deb S, & Humphreys GW (2010). Separating neural correlates of allocentric and egocentric neglect: Distinct cortical sites and common white matter disconnections. Cognitive Neuropsychology, 27, 277–303. [DOI] [PubMed] [Google Scholar]
- Cisek P (2007). Cortical mechanisms of action selection: The affordance competition hypothesis. Philosophical Transactions of the Royal Society, Series B, Biological Sciences, 362, 1585–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, & Kalaska JF (2010). Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience, 33, 269–298. [DOI] [PubMed] [Google Scholar]
- Crawford JR, & Garthwaite PH (2005). Testing for suspected impairments and dissociations in single-case studies in neuropsychology: Evaluation of alternatives using Monte Carlo simulations and revised tests for dissociations. Neuropsychology, 19, 318. [DOI] [PubMed] [Google Scholar]
- Cross KA, & Iacoboni M (2014). To imitate or not: Avoiding imitation involves preparatory inhibition of motor resonance. Neuroimage, 91, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, & Grèzes J (2006). The power of simulation: Imagining one’s own and other’s behavior. Brain Research, 1079, 4–14. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, & Wilkins DP (2004). Lesion analysis of the brain areas involved in language comprehension. Cognition, 92, 145–177. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, LeGoualher G, Pike GB, et al. (2000). A new anatomical landmark for reliable identification of human area V5/MT: A quantitative analysis of sulcal patterning. Cerebral Cortex, 10, 454–463. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Arnold SL, Fitzgibbon BM, Hoy KE, Susilo DA, & Fitzgerald PB (2012). Transcranial direct current stimulation (tDCS) of the inferior frontal gyrus disrupts interpersonal motor resonance. Neuropsychologia, 50, 1628–1631. [DOI] [PubMed] [Google Scholar]
- Fellows L, Heberlein A, Morales D, Shivde G, Waller S, & Wu D (2005). Method matters: An empirical study of impact in cognitive neuroscience. Journal of Cognitive Neuroscience, 17, 850–858. [DOI] [PubMed] [Google Scholar]
- Fernandino L, Binder J, Desai R, Pendl S, Humphries C, Gross W, et al. (2015). Concept representation reflects multimodal abstraction: A framework for embodied semantics. Cerebral Cortex. doi: 10.1093/cercor/bhv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Gallese V (2005). Embodied simulation: From neurons to phenomenal experience. Phenomenology and the Cognitive Sciences, 4, 23–48. [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, & Rizzolatti G (1996). Action recognition in the premotor cortex. Brain, 119, 593–609. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, Chapman CS, McLean DA, Flanagan JR, & Culham JC (2013). Activity patterns in the category-selective occipitotemporal cortex predict upcoming motor actions. European Journal of Neuroscience, 38, 2408–2424. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, Johnsrude IS, & Flanagan JR (2015). Planning ahead: Object-directed sequential actions decoded from human frontoparietal and occipitotemporal networks. Cerebral Cortex, doi: 10.1093/cercor/bhu302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Valyear KF, & Culham JC (2013). Decoding the neural mechanisms of human tool use. Elife, 2, e00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G, Hentze S, & Hermsdörfer J (2004). The effect of tactile feedback on pantomime of tool use in apraxia. Neurology, 63, 1863–1867. [DOI] [PubMed] [Google Scholar]
- Grafton ST (2009). Embodied cognition and the simulation of action to understand others. Annals of the New York Academy of Sciences, 1156, 97–117. [DOI] [PubMed] [Google Scholar]
- Grézes J, & Decety J (2001). Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta analysis. Human Brain Mapping, 12, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJ, & Valenstein E (1982). Two forms of ideomotor apraxia. Neurology, 32, 342–346. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J, Li Y, Randerath J, Roby-Brami A, & Goldenberg G (2013). Tool use kinematics across different modes of execution. Implications for action representation and apraxia. Cortex, 49, 184–199. [DOI] [PubMed] [Google Scholar]
- Hickok G (2009). Eight problems for the mirror neuron theory of action understanding in monkeys and humans. Journal of Cognitive Neuroscience, 21, 1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G (2014). The myth of mirror neurons: The real neuroscience of communication and cognition. New York: WW Norton & Company. [Google Scholar]
- Hoeren M, Kaller CP, Glauche V, Vry MS, Rijntjes M, Hamzei F, et al. (2013). Action semantics and movement characteristics engage distinct processing streams during the observation of tool use. Experimental Brain Research, 229, 243–260. [DOI] [PubMed] [Google Scholar]
- Hoeren M, Kümmerer D, Bormann T, Beume L, Ludwig V, Vry M, et al. (2014). Neural bases of imitation and pantomime in acute stroke patients: Distinct streams for praxis. Brain, 137, 2796–2810. [DOI] [PubMed] [Google Scholar]
- Jeannerod M (2001). Neural simulation of action: A unifying mechanism for motor cognition. Neuroimage, 14, S103–S109. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH (2004). The neural bases of complex tool use in humans. Trends in Cognitive Sciences, 8, 71–78. [DOI] [PubMed] [Google Scholar]
- Kable JW, Kan IP, Wilson A, Thompson-Schill SL, & Chatterjee A (2005). Contraceptual representations of action in the lateral temporal cortex. Journal of Cognitive Neuroscience, 17, 1855–1870. [DOI] [PubMed] [Google Scholar]
- Kable J, Lease-Spellmeyer J, & Chatterjee A (2002). Neural substrates of action event knowledge. Journal of Cognitive Neuroscience, 14, 795–805. [DOI] [PubMed] [Google Scholar]
- Kalénine S, Buxbaum LJ, & Coslett HB (2010). Critical brain regions for action recognition: Lesion symptom mapping in left hemisphere stroke. Brain, 133, 3269–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalénine S, Peyrin C, Pichat C, Segebarth C, Bonthoux F, & Baciu M (2009). The sensory-motor specificity of taxonomic and thematic conceptual relations: A behavioral and fMRI study. Neuroimage, 44, 1152–1162. [DOI] [PubMed] [Google Scholar]
- Kaplan JT, & Iacoboni M (2007). Multimodal action representation in human left ventral premotor cortex. Cognitive Processing, 8, 103–113. [DOI] [PubMed] [Google Scholar]
- Kellenbach M, Brett M, & Patterson K (2003). Actions speak louder than functions: The importance of manipulability and action in tool representation. Journal of Cognitive Neuroscience, 15, 30–46. [DOI] [PubMed] [Google Scholar]
- Kemmerer D (2015). Does the motor system contribute to the perception and understanding of actions? Reflections on Gregory Hickok’s The myth of mirror neurons: The real neuroscience of communication and cognition. Language and Cognition, 7, 450–475. [Google Scholar]
- Kemmerer D, Castillo JG, Talavage T, Patterson S, & Wiley C (2008). Neuroanatomical distribution of five semantic components of verbs: Evidence from fMRI. Brain and Language, 107, 16–43. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Rudrauf D, Manzel K, & Tranel D (2012). Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex, 48, 826–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A (1982). Western aphasia battery test manual. San Antonio, TX: Psychological Corp. [Google Scholar]
- Kilner JM (2011). More than one pathway to action understanding. Trends in Cognitive Sciences, 15, 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, & Frith CD (2007). Predictive coding: An account of the mirror neuron system. Cognitive Processing, 8, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg DY, & Aguirre GK (2001). VoxBo: A flexible architecture for functional neuroimaging. In The Human Brain Project/NeuroInformatics Conference Abstract Bethesda, MD. [Google Scholar]
- Kouneiher F, Charron S, & Koechlin E (2009). Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience, 12, 939–945. [DOI] [PubMed] [Google Scholar]
- Leung HC, Gore J, & Goldman-Rakic P (2002). Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. Journal of Cognitive Neuroscience, 14, 659–671. [DOI] [PubMed] [Google Scholar]
- Lewis JW (2006). Cortical networks related to human use of tools. The Neuroscientist, 12, 211–231. [DOI] [PubMed] [Google Scholar]
- Liepelt R, Von Cramon DY, & Brass M (2008). How do we infer others’ goals from non-stereotypic actions? The outcome of context-sensitive inferential processing in right inferior parietal and posterior temporal cortex. Neuroimage, 43, 784–792. [DOI] [PubMed] [Google Scholar]
- Lingnau A, & Petris S (2013). Action understanding within and outside the motor system: The role of task difficulty. Cerebral Cortex, 23, 1342–1350. [DOI] [PubMed] [Google Scholar]
- Macuga KL, & Frey SH (2012). Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage, 59, 2798–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah YH, Husain M, Rees G, & Nachev P (2014). Human brain lesion-deficit inference remapped. Brain, doi: 10.1093/brain/awu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GA, Rumiati RI, Caramazza A, & Martin A (2007). Action-related properties shape object representations in the ventral stream. Neuron, 55, 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris S, & Urgesi C (2015). Neural underpinnings of superior action prediction abilities in soccer players. Social, Cognitive and Affective Neuroscience, 10, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Kyle Simmons W, Beauchamp MS, & Gotts SJ (2014). Is a single ‘hub’, with lots of spokes, an accurate description of the neural architecture of action semantics?: Comment on “Action semantics: A unifying conceptual framework for the selective use of multimodal and modality-specific object knowledge” by van Elk, van Schie and Bekkering. Physics of Life Reviews, 11, 261–262. [DOI] [PubMed] [Google Scholar]
- Mirman D, & Graziano K (2012). Individual differences in the strength of taxonomic versus thematic relations. Journal of Experimental Psychology, General, 141, 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Zhang Y, Wang Z, Coslett HB, & Schwartz MF (2015). The ins and outs of meaning: Behavioral and neuroanatomical dissociation of semantically-driven word retrieval and multimodal semantic recognition in aphasia. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, & Mattingley JB (2012). Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neuroscience & Biobehavioral Reviews, 36, 341–349. [DOI] [PubMed] [Google Scholar]
- Moore C, & Müller S (2014). Transfer of expert visual anticipation to a similar domain. Quarterly Journal of Experimental Psychology, 67, 186–196. [DOI] [PubMed] [Google Scholar]
- Negri GA, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, & Caramazza A (2007). What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cognitive Neuropsychology, 24, 795–816. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Josephs O, Kiebel S, Friston KJ, & Price CJ (2005). Action selectivity in parietal and temporal cortex. Cognitive Brain Research, 25, 641–649. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss H, Stamatakis EA, Bright P, et al. (2007). Temporal lobe lesions and semantic impairment: A comparison of herpes simplex virus encephalitis and semantic dementia. Brain, 130, 1138–1147. [DOI] [PubMed] [Google Scholar]
- Osiurak F, Jarry C, Lesourd M, Baumard J, & Le Gall D (2013). Mechanical problem-solving strategies in left-brain damaged patients and apraxia of tool use. Neuropsychologia, 51, 1964–1972. [DOI] [PubMed] [Google Scholar]
- Plaut DC (1995). Double dissociation without modularity: Evidence from connectionist neuropsychology. Journal of Clinical and Experimental Neuropsychology, 17, 291–321. [DOI] [PubMed] [Google Scholar]
- Plaut DC (2002). Graded modality-specific specialisation in semantics: A computational account of optic aphasia. Cognitive Neuropsychology, 19, 603–639. [DOI] [PubMed] [Google Scholar]
- Ramayya AG, Glasser MF, & Rilling JK (2010). A DTI investigation of neural substrates supporting tool use. Cerebral Cortex, 20, 507–516. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, & Craighero L (2004). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, & Gallese V (2001). Neurophysiological mechanisms underlying the understanding and imitation of action. Nature Reviews Neuroscience, 2, 661–670. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, MacClelland JL, Hodges JR, et al. (2004). Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychological Review, 111, 205–235. [DOI] [PubMed] [Google Scholar]
- Rugg MD, & Thompson-Schill SL (2013). Moving forward with fMRI data. Perspectives on Psychological Science, 8, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumiati RI, Weiss PH, Shallice T, Ottoboni G, Noth J, Zilles K, et al. (2004). Neural basis of pantomiming the use of visually presented objects. Neuroimage, 21, 1224–1231. [DOI] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, & Thompson-Schill SL (2009). Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences U.S.A, 106, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, Wurm MF, Wittmann M, & Von Cramon DY (2014). Objects tell us what action we can expect: Dissociating brain areas for retrieval and exploitation of action knowledge during action observation in fMRI. Frontiers in Psychology, 5, 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Faseyitan O, Kim J, & Coslett HB (2012). The dorsal stream contribution to phonological retrieval in object naming. Brain, 135, 3799–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwoebel J, Buxbaum LJ, & Coslett HB (2004). Representations of the human body in the production and imitation of complex movements. Cognitive Neuropsychology, 21, 285–298. [DOI] [PubMed] [Google Scholar]
- Singhal A, Monaco S, Kaufman LD, & Culham JC (2013). Human fMRI reveals that delayed action re-recruits visual perception, Plos One, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, & Lieberman MD (2012). Dissociating modality-specific and supramodal neural systems for action understanding. Journal of Neuroscience, 32, 3575–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasenko A, Garcea FE, & Mahon BZ (2013). What happens to the motor theory of perception when the motor system is damaged?. Language and Cognition, 5, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL (2003). Neuroimaging studies of semantic memory: Inferring “how” from “where”. Neuropsychologia, 41, 280–292. [DOI] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D, Adolphs R, Damasio H, & Damasio AR (2003). Neural correlates of conceptual knowledge for actions. Cognitive Neuropsychology, 20, 409–432. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Calvo-Merino B, Haggard P, & Aglioti SM (2007). Transcranial magnetic stimulation reveals two cortical pathways for visual body processing. Journal of Neuroscience, 27, 8023–8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Ionta S, & Aglioti SM (2007). Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nature Neuroscience, 10, 30–31. [DOI] [PubMed] [Google Scholar]
- van Elk M, van Schie H, & Bekkering H (2014). The scope and limits of action semantics: Reply to comments on ‘Action semantics: A unifying conceptual framework for the selective use of multimodal and modality-specific object knowledge’. Physics of Life Reviews, 11, 273–279. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, & Baetens K (2009). Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage, 48, 564–584. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Embleton KV, & Ralph MAL (2012). Both the middle temporal gyrus and the ventral anterior temporal area are crucial for multimodal semantic processing: Distortion-corrected fMRI evidence for a double gradient of information convergence in the temporal lobes. Journal of Cognitive Neuroscience, 24, 1766–1778. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CYC, Lacey SC, Nee DE, Franklin M, & Jonides J (2005). Common and unique components of response inhibition revealed by fMRI. Neuroimage, 27, 323–340. [DOI] [PubMed] [Google Scholar]
- Watson CE, & Buxbaum LJ (2015). A distributed network critical for selecting among tool-directed actions. Cortex, 65, 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CE, Cardillo ER, Bromberger B, & Chatterjee A (2014). The specificity of action knowledge in sensory and motor systems. Frontiers in Psychology, 5, 494. doi:103389/fpsyg.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CE, Cardillo ER, Ianni GR, & Chatterjee A (2013). Action concepts in the brain: An activation likelihood estimation meta-analysis. Journal of Cognitive Neuroscience, 25, 1191–1205. [DOI] [PubMed] [Google Scholar]
- Watson CE, & Chatterjee A (2011). The functional neuroanatomy of actions. Neurology, 76, 1428–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei T, Liang X, He Y, Zang Y, Han Z, Caramazza A, et al. (2012). Predicting conceptual processing capacity from spontaneous neuronal activity of the left middle temporal gyrus. The Journal of Neuroscience, 32, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RM, Özyürek A, & Hagoort P (2009). Differential roles for left inferior frontal and superior temporal cortex in multimodal integration of action and language. Neuroimage, 47, 1992–2004. [DOI] [PubMed] [Google Scholar]
- Yang J, Shu H, Bi Y, Liu Y, & Wang X (2011). Dissociation and association of the embodied representation of tool-use verbs and hand verbs: An fMRI study. Brain and Language, 119, 167–174. [DOI] [PubMed] [Google Scholar]
- Yee E, Chrysikou EG, & Thompson-Schill SL (2013). Semantic memory. In Ochsner K & Kosslyn SJ (Eds.), The Oxford handbook of cognitive neuroscience volume 1: Core topics (pp. 353–374). Oxford, UK: Oxford University Press. [Google Scholar]
- Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, & Wang Z (2014). Multivariate lesion-symptom mapping using support vector regression. Human Brain Mapping, 35, 5861–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]


