Abstract
This report describes a patient with severe acute respiratory syndrome coronavirus 2 infection and irreversible lung destruction who underwent successful lung transplantation after 138 days of bridging with extracorporeal membrane oxygenation support. The case exemplifies that lung transplantation may be a possibility after very long-term coronavirus disease 2019 care, even if the patient is initially an unsuitable candidate.
Coronavirus disease 2019 (COVID-19) is a pandemic disease with a risk for respiratory complications where extracorporeal membrane oxygenation (ECMO) may be initiated in severe cases. ECMO as a bridge to lung transplantation (LTx) is an established option in selected patients.1 We report a patient with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and irreversible lung destruction who underwent successful LTx after 138 days of bridging with ECMO support.
A previously healthy 55-year-old man presented to the emergency department with a fever (39.4°C), a high respiratory rate, and an arterial partial pressure of oxygen of 5.9 kPa despite noninvasive high-flow oxygen. He was intubated, and the diagnostic workup showed a positive polymerase chain reaction for SARS-CoV-2. After more than 48 hours on a ventilator, using prone ventilation, and with a fraction of inspired oxygen of 1.0 with increasing oxygenation difficulties, venovenous ECMO was initiated.
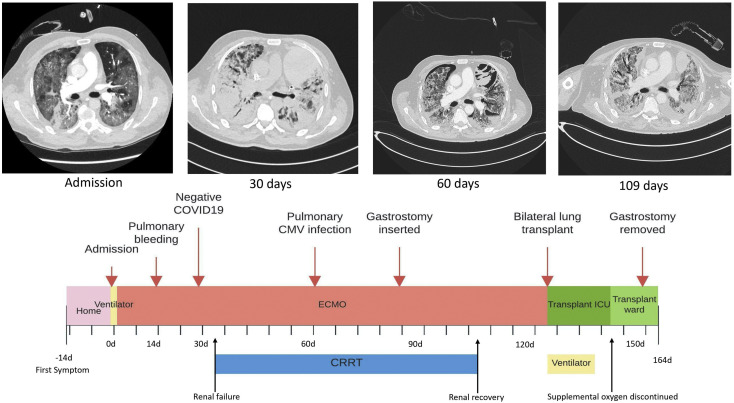
Cannulas were placed in the right internal jugular vein (23-F HLS cannula, Maquet, Rastatt, Germany) and the right common femoral vein (19-F Bio-Medicus NexGen cannula, Medtronic, Minneapolis, MN). The initial heparin infusion was 2000 U/h and was continued with a target partial thromboplastin time of 50 to 70 seconds. Significant complications during ECMO treatment are outlined in Figure 1 , and bleeding was managed conservatively.
Figure 1.
Timeline for significant events: computed tomography of the lung on admission showed generalized ground-glass opacities that progressed to almost completely opaque lungs at day 30. The opacities gradually regressed, but computed tomographic scans obtained later were dominated by generalized honeycombing and traction bronchiectasis, as shown in the image 109 days after initiation of extracorporeal membrane oxygenation (ECMO). (CMV, cytomegalovirus; COVID-19, coronavirus disease 2019; CRRT, chronic renal replacement therapy; ICU, intensive care unit.)
After 127 days of ECMO support, with the patient physically and mentally stable, without signs of improvement, and with single-organ failure, he was accepted for LTx. This procedure was undertaken after 138 days of ECMO support. Thoracosternotomy was performed, and venovenous ECMO was converted to venoarterial ECMO by connecting both venous cannulas as venous drainage and inserting a straight arterial cannula by the Seldinger technique into the ascending aorta. Bilateral LTx was performed, first on the right side and then on the left, without technical difficulties, and the patients was subsequently easily weaned from ECMO. Venous cannulas were left in place for an hour to allow venovenous ECMO to be restarted on desaturation, and the cannulas were then removed. The chest was left open as a result of coagulopathy and to prevent severe primary graft dysfunction because the lungs were deemed somewhat edematous.
The patient was bleeding moderately postoperatively and was brought back to the operating room on day 2. The blood clots were removed, and the chest was closed. He remained stable and was assessed as having primary graft dysfunction grades 1, 1, and 0 at 24, 48, and 72 hours, respectively. The patient was weaned from the ventilator on postoperative day 14, and he was discharged from the intensive care unit on day 20.
Besides transient atrial fibrillation directly after intensive care discharge, the patient’s further recovery was uneventful. He remained on the step-down unit until he was able to mobilize without supplemental oxygen and was then discharged to a rehabilitation facility on day 34 after LTx. Discharge spirometry showed a forced expiratory volume during the first second of 45% predicted, the bronchial anastomoses were well healed on bronchoscopy, and there was no evidence of acute transplant rejection in transbronchial biopsies.
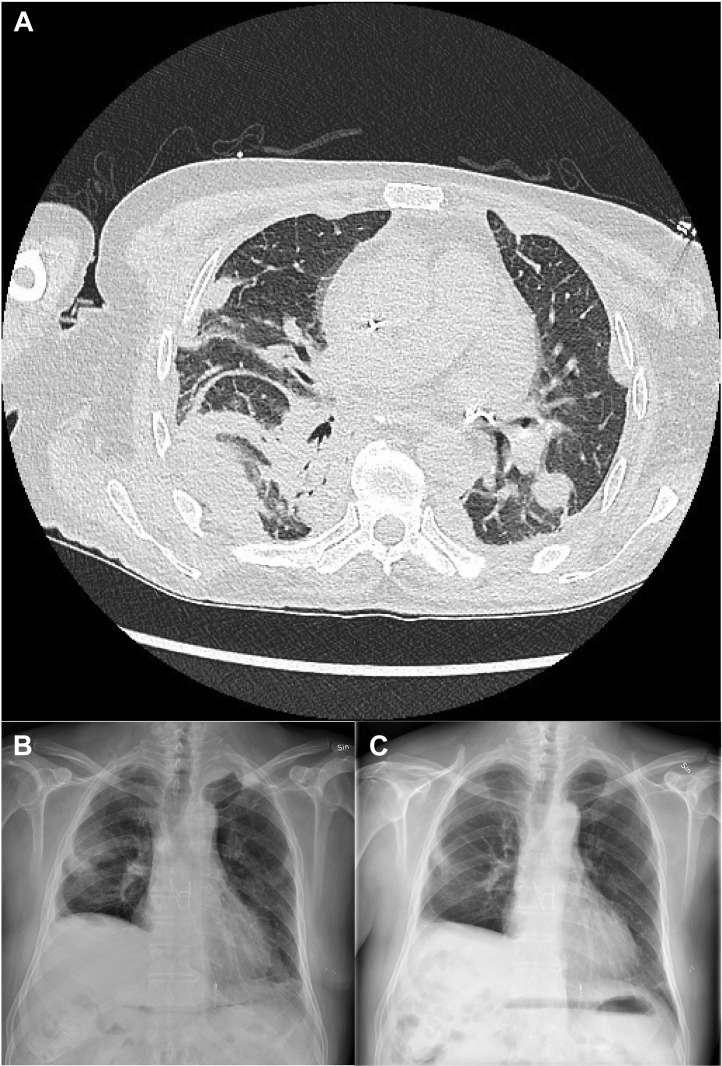
The most recent outpatient visit was 5 months after LTx. The patient was moving freely and had a forced expiratory volume during the first second on spirometry of 61% predicted. The infiltrates noted on the plain chest roentogenogram had receded but not disappeared (Figure 2 ). Moreover, the right phrenic palsy seen on the discharge roentogenogram had not improved. Bronchoscopy showed no signs of infection or acute or humoral transplant rejection. Results of surveillance testing for donor-specific antibodies were negative.
Figure 2.
(A) Computed tomography after transplantation. Remnants of the early bleeding can be seen, mainly dorsally on the right lung. (B) Discharge chest roentogenogram showing infiltrates secondary to the postoperative bleeding in the right lung, a basal pleural effusion in the left lung, and right-sided phrenic palsy. (C) Chest roentogenogram from the 5-month visit showing slightly diminished infiltrates in the right lung and no pleural effusion in the left lung. The right-sided phrenic palsy is unchanged.
Comment
We report that a patient with lungs destroyed by SARS-CoV-2 infection may undergo ECMO as a bridge to LTx and have a good outcome even after a prolonged duration of mechanical circulatory support. The patient was awake during ECMO and was able to perform physical training using bed cycling under careful monitoring, and this likely contributed to the excellent result. Patients with COVID-19 have been reported to undergo ECMO as a bridge to LTx,2 , 3 but data are insufficient on when to list a patient with acute respiratory distress syndrome caused by COVID-19. In addition, the inherent transplant dilemma with a limited donor pool provides further considerations.4 Previous data indicate that 70% of patients who underwent ECMO for COVID-19 were weaned from ECMO during the first wave of COVID-19.5 During subsequent waves of COVID-19, this figure may be lower. Our patient initially had multiple complications and was thus ineligible for LTx. However, the patient’s condition stabilized over time, and he eventually had true single organ failure. It is possible that the additional complications, such as lung bleeding and pulmonary cytomegalovirus infection, hindered the healing process in our patient because he otherwise improved physically. Although this patient is not the first to have undergone LTx from ECMO, he was treated with ECMO for longer than previous cases. We recognize that only a small proportion of patients will be suitable for undergoing an ECMO bridge and subsequent LTx. Moreover, patients should not be listed for LTx until there is a very high degree of certainty that the damage to the lungs is irreversible.
We conclude that very select patients with lungs destroyed by SARS CoV-2 infection may undergo ECMO as a bridge to LTx and have a good outcome even after prolonged ECMO care.
References
- 1.Dellgren G., Riise G.C., Sward K., et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: a long-term study. Eur J Cardiothorac Surg. 2015;47:95–100. doi: 10.1093/ejcts/ezu112. [DOI] [PubMed] [Google Scholar]
- 2.Lang C., Jaksch P., Hoda M.A., et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. 2020;8:1057–1060. doi: 10.1016/S2213-2600(20)30361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharat A., Querrey M., Markov N.S., et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cypel M., Keshavjee S. When to consider lung transplantation for COVID-19. Lancet Respir Med. 2020;8:944–946. doi: 10.1016/S2213-2600(20)30393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaro R.P., MacLaren G., Boonstra P.S., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]