Abstract
Aims
Non-steroidal anti-inflammatory drugs (NSAIDs), both non-selective and selective cyclooxygenase-2 (COX-2) inhibitors, are among the most widely prescribed drugs worldwide, but associate with increased blood pressure (BP) and adverse cardiovascular (CV) events. PRECISION-ABPM, a substudy of PRECISION was conducted at 60 sites, to determine BP effects of the selective COX-2 inhibitor celecoxib vs. the non-selective NSAIDs naproxen and ibuprofen.
Methods and results
In this double-blind, randomized, multicentre non-inferiority CV-safety trial, 444 patients (mean age 62 ± 10 years, 54% female) with osteoarthritis (92%) or rheumatoid arthritis (8%) and evidence of or at increased risk for coronary artery disease received celecoxib (100–200 mg bid), ibuprofen (600–800 mg tid), or naproxen (375–500 mg bid) with matching placebos in a 1: 1: 1 allocation, to assess the effect on 24-h ambulatory BP after 4 months. The change in mean 24-h systolic BP (SBP) in celecoxib, ibuprofen and naproxen-treated patients was -0.3 mmHg [95% confidence interval (CI), −2.25, 1.74], 3.7 (95% CI, 1.72, 5.58) and 1.6 mmHg (95% CI, −0.40, 3.57), respectively. These changes resulted in a difference of − 3.9 mmHg (P = 0.0009) between celecoxib and ibuprofen, of − 1.8 mmHg (P = 0.12) between celecoxib and naproxen, and of − 2.1 mmHg (P = 0.08) between naproxen and ibuprofen. The percentage of patients with normal baseline BP who developed hypertension (mean 24-h SBP ≥ 130 and/or diastolic BP ≥ 80 mmHg) was 23.2% for ibuprofen, 19.0% for naproxen, and 10.3% for celecoxib (odds ratio 0.39, P = 0.004 and odds ratio 0.49, P = 0.03 vs. ibuprofen and naproxen, respectively).
Conclusions
In PRECISION-ABPM, allocation to the non-selective NSAID ibuprofen, compared with the COX-2 selective inhibitor celecoxib was associated with a significant increase of SBP, and a higher incidence of new-onset hypertension.
ClinicalTrials
gov number NCT00346216
Keywords: Hypertension, Non-steroidal anti-inflammatory drugs, Cardiovascular risk, Selective cyclooxygenase-2 (COX-2) inhibitors, Osteoarthritis, Pain
Introduction
More than 70 million prescriptions for non-steroidal anti-inflammatory drugs (NSAIDs) are written each year in the USA.1 With over-the-counter use included, more than 30 billion doses of NSAIDs are consumed annually in the USA alone.1 , 2 On 9 July 2015, the US Food and Drug Administration (FDA) strengthened a warning on all prescription and over-the-counter non-selective NSAIDs and cyclooxygenase 2 (COX-2) selective inhibitors, stating that this class of agents can increase adverse cardiovascular outcomes. The uncertainty concerning the cardiovascular safety of NSAIDs presents practitioners with difficult management decisions,3 particularly for the 19% of the population in the USA who use at least one NSAID on a regular basis, including 30 million Americans with osteoarthritis,2 , 4 of whom more than 40% also have hypertension.5 , 6 Moreover, current hypertension guidelines only scarcely mention the use of analgesics in this particular population.7
Non-selective NSAIDs and selective inhibitors of COX-2 can increase blood pressure (BP) or interfere with BP control,8 , 9 and even small differences in BP may impact cardiovascular morbidity and mortality.10–13 Hence, there is a particular need to investigate the differential effects on BP with these NSAIDs. While effects on BP may in part explain the cardiovascular risks of NSAIDs, few prospective, long-term, placebo-controlled trials in patients with arthritis have specifically assessed the differential effects of non-selective and selective COX-2 inhibitors on ambulatory BP.
The recently published Prospective Randomized Evaluation of Celecoxib Integrated Safety vs. Ibuprofen Or Naproxen (PRECISION),14 a double-blind, triple-dummy, randomized, three-arm parallel group design multicentre cardiovascular safety trial mandated by the FDA in patients with arthritis with or at increased risk for cardiovascular disease, demonstrated distinctly different safety profiles amongst alternative NSAIDs. In view of concerns with regard to the contribution of BP elevations to increased cardiovascular events within this group of drugs, PRECISION-ABPM, a pre-specified substudy of PRECISION,15 aimed to delineate differential BP effects and the relationship between changes in ambulatory BP of the selective COX-2 inhibitor celecoxib vs. the non-selective NSAIDs naproxen and ibuprofen.
Methods
Detailed methods for the PRECISION trial have been published previously15 and both the protocol and statistical analysis plan for PRECISION-ABPM are available as Supplementary files.
Study design and oversight
PRECISION-ABPM is a pre-specified substudy of PRECISION, a randomized, multicentre, double-blind, non-inferiority trial conducted in patients with rheumatoid arthritis (RA) or osteoarthritis (OA) who had pre-existing or were at relatively high risk for cardiovascular disease. Randomization was stratified by the primary diagnosis (OA or RA), aspirin use, and geographic region. Institutional review boards approved the study and patients provided written informed consent. In PRECISION, a multidisciplinary executive committee supervised the trial and an independent data monitoring committee reviewed unblinded data for safety. Executive committee members agreed not to accept any financial payments related to NSAIDs from any manufacturer of NSAIDs throughout the duration of the trial, including from the trial’s sponsor. The sponsor participated in the design of the trial and writing of the protocol in collaboration with the executive committee and consultation with the FDA, assisted with data collection and maintained the trial database. The sponsor shared operational roles with the Cleveland Clinic Coordinating Center for Clinical Research (C5Research) and several contract research organizations. The academic authors wrote the articles for PRECISION and PRECISION-ABPM. The sponsor was allowed to review and comment on the article, but the decision to publish and final contents were determined by the academic authors with no limits on the right to publish. All authors had access to the final results, approved the article and take responsibility for its accuracy, completeness, and adherence to the study protocol.
Inclusion and exclusion criteria
PRECISION enrolled patients ≥18 years of age who, as determined by the patient and physician, required daily treatment with NSAIDs for arthritis pain. Inclusion required established cardiovascular disease or increased risk for development of cardiovascular disease (defined in the Supplementary material online, Appendix). The protocol and a prior publication describe other inclusion and exclusion criteria.15
Treatments
Following randomization, patients received either celecoxib, 100 mg bid, ibuprofen, 600 mg tid, or naproxen, 375 mg bid with matching placebos in a 1: 1: 1 allocation. At subsequent visits for RA patients, investigators could increase the dose to celecoxib 200 mg bid, ibuprofen 800 mg tid, or naproxen 500 mg bid for treatment of symptoms. For patients with OA, upward titration of ibuprofen and naproxen was permitted; however, regulatory dosing restrictions allowed dose escalation for celecoxib in RA patients but not OA patients. Esomeprazole (20–40 mg) was provided for gastric protection to all patients. Investigators were encouraged to provide optimal cardiovascular preventive management as recommended by current guidelines. Patients receiving low-dose aspirin (≤325 mg daily) were permitted to continue this therapy.
Ambulatory blood pressure measurements
ABP measurements were obtained from all participants using a SpaceLabs 90207 monitor. A central ABPM reading laboratory performed the ABPM data collection, reading, and quality evaluation for the PRECISION trial database. ABP was measured every 20 min during daytime (06: 00–21: 59 h), and every 30 min during night-time (22: 00–05: 59 h). If informed by the central ABPM reading laboratory that quality criteria were not met, the investigators asked the patient to return to the clinical site to repeat the study within 3 days from notification by the central ABPM reading laboratory.
Outcomes
The primary ABPM substudy end point was the change from baseline in 24-h mean systolic BP (SBP) at Month 4. Secondary end points were the change from baseline in 24-h mean SBP at Month 2, change from baseline in 24-h average diastolic BP (DBP) at Months 2 and 4, 24-h pulse pressure (PP = SBP−DBP) change from baseline at Months 2 and 4, the mean awake (06: 00–21: 59 h) and sleep (22: 00–05: 59 h) SBP and DBP and mean arterial pressure change from baseline at Months 2 and 4. In addition, the relationship between change in BP (ABPM) and subsequent cardiovascular events, the composite of cardiovascular (CV) death, non-fatal myocardial infarction or nonfatal stroke, were analysed. An independent committee of multidisciplinary specialists at C5Research, blinded to treatment allocation, reviewed and adjudicated events.
Statistical analysis
The primary end point for the substudy was the change from baseline in 24-h mean SBP at Month 4. Assuming a standard deviation of approximately 7.5 mmHg and using a Bonferroni adjustment for multiple treatment comparisons a sample size of 117 evaluable patients per arm allowed detection of a 3 mmHg difference between any two treatment groups, with 80% power and at the 0.0167 (=0.05/3) level of significance. Assuming a 35% dropout rate, the study required randomization of 180 patients per arm (for a total of 540) to obtain 117 evaluable patients. In case the dropout rate was lower than 35%, the study design allowed enrolment to stop once the number of evaluable patients per arm was reached.
The ABPM analyses were based on the substudy modified intention-to-treat (MITT) population, consisting of all randomized patients who had valid ambulatory BP data for analyses thus excluding subjects with missing ABPM recording at baseline or subjects with a baseline ABPM but with no follow-up ABPM recordings. For patients who discontinued study drug prematurely prior to Month 2, measurements taken at time of discontinuation were used as the Month 2 measurement. Similarly, measurements taken at time of discontinuation at or after Month 2 were used as the Month 4 measurement. The primary analysis used an analysis of covariance (ANCOVA) model with treatment and region as factors, and the baseline 24-h average SBP and BMI as covariates. The least squares (LS) mean for each of the three treatment groups, the difference between each pair of the LS means, and the P-values for these differences were presented. Each of the three comparisons was considered statistically significant if the P-value was less than 0.0167. 95% confidence intervals (CIs) were presented for the primary analysis to allow for comparisons to other studies utilizing unadjusted intervals. Additionally, a sensitivity analysis of the primary end point was conducted to evaluate the potential effect of missing data, the primary analysis was repeated based on a mixed model repeated measurement (MMRM) model which included baseline SBP, and BMI as covariates, and factors for treatment, region, visit, and treatment by visit interaction. All secondary end points of changes in BP were analysed similarly to the primary end point using an ANCOVA model based on the MITT population. A significance level of 0.05 was used for these secondary analyses, with no adjustments for multiple comparisons.
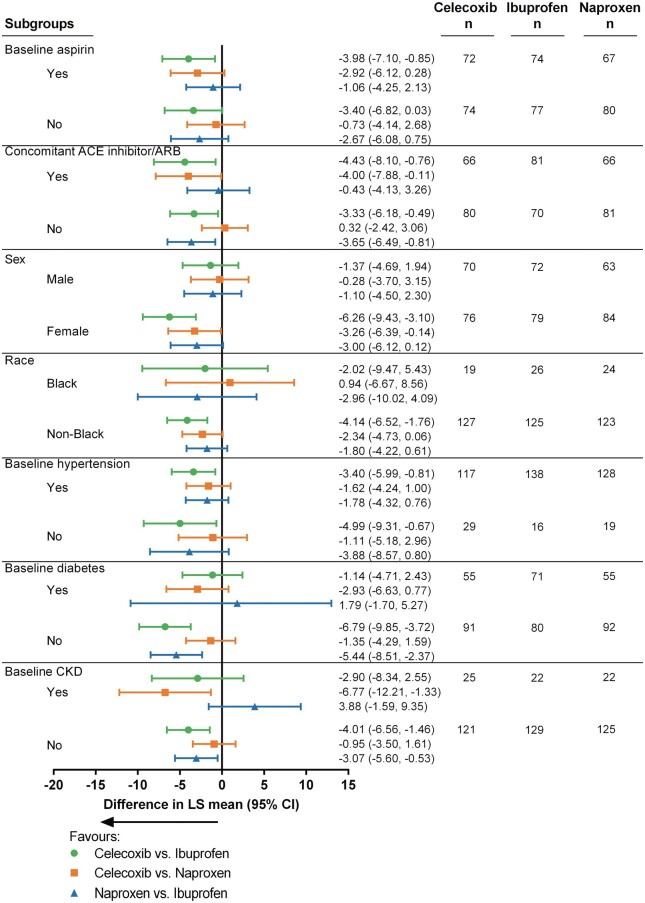
To evaluate the effect of subgroups on change in BP at Month 4, an ANCOVA model was used within each subgroup, with change in BP at Month 4 as the dependent variable, treatment, and region as factors, and baseline BP and BMI as covariates. Additionally treatment-by-subgroup interactions were determined to assess consistency of treatment effect across the subgroups. Prespecified subgroup analyses including chronic kidney disease (CKD) defined as a CKD-Epi16 eGFR < 60 and patients with hypertension at baseline. The remaining subgroup analyses [gender, race, diabetes, baseline use of aspirin, and (angiotensin-converting enzyme) ACE/angiotensin receptor blocker (ARB) concomitant use] were post hoc.
Additionally, a post hoc analysis was conducted for treatment comparison of the percent of normotensive patients (24-h SBP <130 mmHg and DBP <80 mmHg) who became hypertensive at Month 4 using Cochran–Mantel–Haenszel (CMH) test with adjustment for region. The proportion of patients with <0, 0–10, >10–20, and >20 mmHg increase from baseline to Month 4 in 24-h SBP was also compared between the three treatments using CMH stratified by region.
Results
Patient population
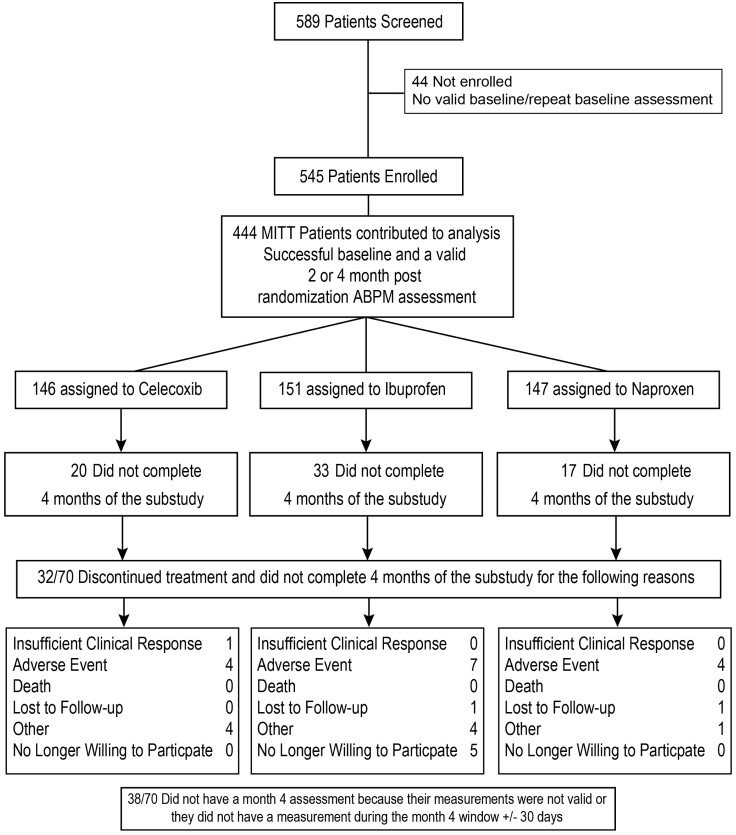
Five hundred eighty-nine patients were screened and 545 enrolled from 60 centres in the USA between 18 September 2008 and 25 March 2013; 101 patients were excluded from analysis leaving 444 analysable participants with successful baseline, 2 or 4 months post-randomization ABPM assessments (Figure 1). There were 146 patients assigned to celecoxib (mean daily dose 208 ± 34 mg), 147 to naproxen (852 ± 98 mg), and 151 to ibuprofen (2031 ± 237 mg). The groups had similar baseline characteristics (Table 1), including BP, serum creatinine, plasma glucose, and glycosylated haemoglobin concentrations. Sixty-two percent of the patients were treated with ACE inhibitors or ARBs, 35% with a diuretic and 22% with a calcium channel blocker, while 53% received multiple antihypertensive therapies.
Figure 1.
Patient disposition in the PRECISION-ABPM trial (consort diagram).
Table 1.
Baseline characteristics of the patients
| Characteristics | Celecoxib (100–200 mg bid) | Ibuprofen (600–800 mg tid) | Naproxen (375–500 mg bid) |
|---|---|---|---|
| N = 146 | N = 151 | N = 147 | |
| Age, years | 62.1 ± 10.1 | 61.9 ± 9.7 | 61.4 ± 10.3 |
| Sex (m/f) | 70/76 | 72/79 | 63/84 |
| Race, n (%) | |||
| White | 118 (80.8) | 120 (79.5) | 119 (81.0) |
| Black | 19 (13.0) | 26 (17.2) | 24 (16.3) |
| Other | 9 (6.2) | 5 (3.3) | 4 (2.7) |
| Weight (kg) | 91.4 ± 22.4 | 93.0 ± 22.3 | 90.5 ± 21.6 |
| BMI (kg/m2) | 32.6 ± 7.0 | 32.7 ± 6.9 | 31.9 ± 6.6 |
| Primary diagnosis, n (%) | |||
| Rheumatoid arthritis | 12 (8.2) | 13 (8.6) | 9 (6.1) |
| Osteoarthritis | 134 (91.8) | 138 (91.4) | 138 (93.9) |
| Baseline aspirin, n (%) | 72 (49.3) | 74 (49.0) | 67 (45.6) |
| Blood pressure | |||
| SBP, mmHg | 125.1 ± 9.41 | 125.5 ± 10.63 | 125.3 ± 9.93 |
| DBP, mmHg | 74.6 ± 7.43 | 74.2 ± 8.72 | 74.8 ± 7.52 |
| Laboratory characteristics | |||
| Cholesterol, mg/dL | 184.7 ± 39.33 | 183.1 ± 41.69 | 191.3 ± 46.14 |
| HDL, mg/dL | 49.1 ± 15.79 | 51.1 ± 13.22 | 52.9 ± 17.31 |
| LDL, mg/dL | 101.6 ± 38.23 | 102.0 ± 34.55 | 105.5 ± 37.73 |
| TG, mg/dL | 171.2 ± 107.79 | 150.8 ± 97.98 | 169.2 ± 156.83 |
| Hb, g/dL | 13.9 ± 1.37 | 13.8 ± 1.57 | 14.0 ± 1.38 |
| HbA1c, % | 7.6 ± 1.92 | 7.4 ± 1.63 | 7.5 ± 2.08 |
| Glucose, mg/dL | 119.1 ± 56.94 | 121.9 ± 57.50 | 116.9 ± 46.33 |
| Creatinine, mg/dL | 0.9 ± 0.21 | 0.9 ± 0.23 | 0.9 ± 0.20 |
| eGFR, ml/min/1.73m2 | 79.8 ± 18.28 | 79.8 ± 18.25 | 79.6 ± 18.16 |
| HAQ disability index | 1.0 ± 0.57 | 1.1 ± 0.61 | 1.0 ± 0.56 |
| Number (%) of patients with concomitant medication (below) | 124 (84.9) | 134 (88.7) | 128 (87.1) |
| Agents acting on the RAAS, n (%) | 86 (58.9) | 102 (67.5) | 86 (58.5) |
| Beta-blocker | 42 (28.8) | 53 (35.1) | 50 (34.0) |
| Ca channel blockers, n (%) | 34 (23.3) | 33 (21.9) | 32 (21.8) |
| Diuretics, n (%) | 47 (32.2) | 62 (41.1) | 47 (32.0) |
| Peripheral vasodilators | 12 (8.2) | 5 (3.3) | 8 (5.4) |
| Others | 19 (13.0) | 17 (11.3) | 19 (12.9) |
For baseline HDL, the P-value was statistically significantly different for the celecoxib vs. naproxen comparison (P = 0.0435), for all other baseline characteristics, showed no statistically significant differences between treatments.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic BP.
A total of 374 (84%) of 444 patients completed 4 months of the substudy, which included the primary outcome and ABP assessment. The remaining 70 patients (20 celecoxib, 33 ibuprofen, and 17 naproxen) did not have a valid Month 4 ambulatory BP assessment; 15 of these 70 patients were withdrawn from the study or treatment due to an adverse event prior to Month 4: 4 (2.7%) of the patients had been randomized to celecoxib, 7 (4.6%) to ibuprofen, and 4 (2.7%) to naproxen (Figure 1).
Primary outcomes
The hourly ambulatory SBP curves over 24 h at baseline and at Month 4 for the 3 treatment groups are shown in Figure 2A–C. A consistent increase from baseline in SBP was observed in the ibuprofen group (P-value for change in 24-h SBP < 0.001). The change from baseline to Month 4 in 24-h SBP was not statistically significant for celecoxib and naproxen (P = 0.801 and 0.117, respectively). The change in mean 24-h SBP in celecoxib, ibuprofen, and naproxen-treated patients was −0.3 mmHg (95% CI, −2.25, 1.74), 3.7 (95% CI, 1.72, 5.58), and 1.6 mmHg (95% CI, −0.40, 3.57), respectively (Figure 3). These changes resulted in a statistically significant difference of −3.9 mmHg (95% CI, −6.19, −1.61; P ≤ 0.001) between celecoxib and ibuprofen; differences of −1.8 mmHg (95% CI, −4.15, 0.47; P= 0.12) between celecoxib and naproxen, and of −2.1 mmHg (95% CI, −4.36, 0.23; P= 0.08) (Table 2) between naproxen and ibuprofen were noted as well. Results from the MMRM results were consistent with the primary analysis, where at Month 4, the change from baseline in 24-h SBP was −0.3 ± 1.02, 3.7 ± 1.03, 1.9 ± 1.00 for celecoxib, ibuprofen, and naproxen respectively. P-values were 0.002, 0.07, 0.16 for celecoxib vs. ibuprofen, celecoxib vs. naproxen, and naproxen vs. ibuprofen respectively.
Figure 2.
(A–C) The hourly ambulatory SBP curves over 24 h (median and first and third quartiles) at baseline and at Month 4 for the 3 treatment groups (P for change in 24-h SBP for ibuprofen <0.001; for celecoxib and naproxen P = 0.801 and 0.117, respectively).
Figure 3.
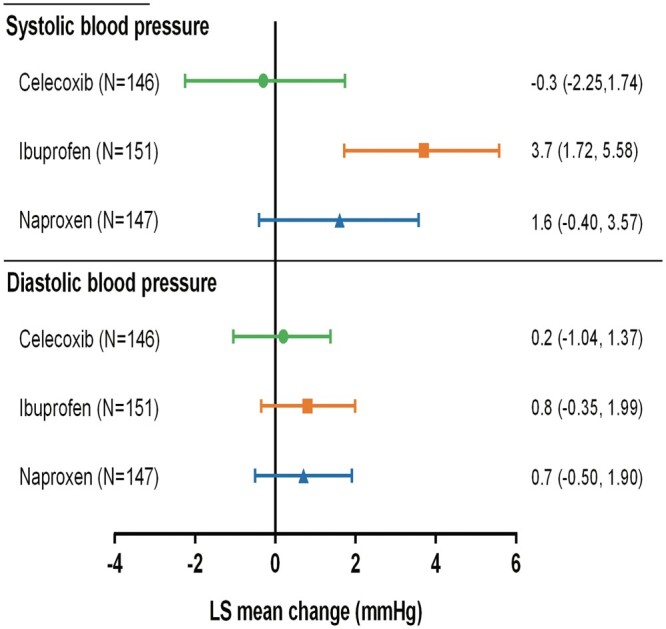
Change in ambulatory 24-h systolic and diastolic BP from baseline at 4 months. These changes resulted in difference of − 3.9 mmHg (95% CI, −6.19, −1.61; P ≤0.001) between celecoxib and ibuprofen; differences of − 1.8 mmHg (95% CI, −4.15, 0.47; P = 0.12) between celecoxib and naproxen, and of − 2.1 mm Hg (95% CI, −4.36, 0.23; P = 0.08) between naproxen and ibuprofen.
Table 2.
Effects of celecoxib, ibuprofen, and naproxen on 24-h ambulatory blood pressure
| Parameter | Celecoxib 100–200 mg BID | P-value | Ibuprofen 600–800 mg TID | Naproxen 375–500 mg BID | P-value |
|---|---|---|---|---|---|
| n = 146 | n = 151 | n = 147 | |||
| Systolic blood pressure | |||||
| Baseline | 124.18 ± 12.351 | 125.24 ± 11.775 | 123.55 ± 11.00 | ||
| After 4 months | 124.00 ± 13.213 | 128.65 ± 13.542 | 125.46 ± 12.487 | ||
| Change from Baseline | −0.18 ± 9.400 | 3.42 ± 12.259 | 1.91 ± 9.796 | ||
| Change from BL vs. Ibuprofen (Difference in LS Mean (CI)) | −3.9 (−6.19, −1.61) | 0.0009 | −2.06 (−4.36, 0.23) | 0.08 | |
| Change from BL vs. Naproxen (Difference in LS mean (CI)) | −1.84 (−4.15, 0.47) | 0.12 | |||
| Diastolic blood pressure | |||||
| Baseline | 70.88 ± 8.00 | 70.53 ± 8.457 | 70.12 ± 7.399 | ||
| After 4 months | 70.87 ± 8.770 | 71.26 ± 9.002 | 70.85 ± 7.922 | ||
| Change from baseline | −0.01 ± 5.933 | 0.74 ± 6.878 | 0.74 ± 6.294 | ||
| Change from BL vs. Ibuprofen (difference in LS Mean (CI)) | −0.65 (−2.04, 0.74) | 0.36 | −0.12 (−1.51, 1.27) | 0.87 | |
| Change from BL vs. Naproxen (Difference in LS mean (CI)) | −0.53 (−1.94, 0.87) | 0.46 | |||
| Mean blood pressure | |||||
| Baseline | 89.65 ± 8.454 | 89.86 ± 8.806 | 88.87 ± 7.475 | ||
| After 4 months | 89.69 ± 9.481 | 91.56 ± 9.295 | 90.26 ± 8.470 | ||
| Change from Baseline | 0.04 ± 6.972 | 1.71 ± 8.742 | 1.39 ± 7.357 | ||
| Change from BL vs. Ibuprofen (Difference in LS Mean (CI)) | −1.75 (−3.4, −0.10) | 0.04 | −0.69 (−2.34, 0.96) | 0.41 | |
| Change from BL vs. Naproxen (Difference in LS mean (CI)) | −1.06 (−2.72, 0.61) | 0.21 | |||
| Pulse pressure | |||||
| Baseline | 53.31 ± 9.920 | 54.71 ± 10.087 | 53.43 ± 9.833 | ||
| After 4 months | 53.13 ± 9.871 | 57.39 ± 11.804 | 54.60 ± 10.334 | ||
| Change from baseline | −0.17 ± 4.884 | 2.68 ± 7.018 | 1.17 ± 5.348 | ||
| Change from BL vs. Ibuprofen (Difference in LS Mean (CI)) | −2.99 (−4.3, −1.68) | <0.0001 | −1.71 (−3.02, −0.40) | 0.01 | |
| Change from BL vs. Naproxen (Difference in LS mean (CI)) | −1.28 (−2.60, 0.04) | 0.06 |
Mean ± SD are provided for treatment means.
The change in 24-h blood pressure values was analysed using analysis of covariance with treatment and region as factors and baseline 24-h blood pressure and body mass index as covariates.
BP, blood pressure; BL, baseline; CI, confidence interval; LS least squares.
Secondary outcomes and subgroup analyses
Average 24-h mean arterial BP (MABP = DBP + 1/3 × (SBP−DBP) at Month 4 was increased in the ibuprofen group, but not in patients receiving celecoxib or naproxen (Table 2). Correspondingly, the change from baseline at Month 4 of awake (06: 00–21: 59 h) SBP, as well as the sleep (22: 00–05: 59 h) SBP and therefore the average 24-h pulse pressure (PP = SBP−DBP) significantly increased in the ibuprofen group compared with celecoxib, as 24-h DBP remained unchanged throughout the course of the study. The results of the clinic SBP measurements for the population of this sub-study, paralleled the ambulatory BP results. Of note, at Month 4, clinic SBP increased by 5.2 ± 1.41 mmHg in the ibuprofen group, by 3.2 ± 1.41 in the naproxen group and by 1.0 ± 1.41 mmHg in celecoxib patients; (P = 0.007 for the ibuprofen vs. celecoxib comparison and P = 0.17 for the celecoxib vs. naproxen comparison).
The percentage of patients with baseline 24-h SBPs lower than 130 mmHg and 24-h DBP lower than 80 mmHg (normotension) who developed hypertension (defined as mean 24-h SBP ≥ 130 and/or DBP ≥ 80 mmHg) was significantly greater for both ibuprofen and naproxen compared with celecoxib: OR 0.39 (95% CI, 0.21, 0.75) for celecoxib vs. ibuprofen and OR 0.49 (95% CI, 0.25, 0.96) for celecoxib vs. naproxen (Figure 4A). Compared with celecoxib, the ibuprofen treatment groups had larger proportions of patients whose 24-h SBP increased (P = 0.003), while the difference between celecoxib and naproxen was not significant (P = 0.07) (Figure 4B). During a mean follow-up of 2.49 years, 22 Anti-platelet Trialist Collaboration (APTC) events17 (composite of CV death, non-fatal myocardial infarction, or non-fatal stroke) occurred, nine in the ibuprofen, six in the naproxen, and seven in the celecoxib groups.
Figure 4.
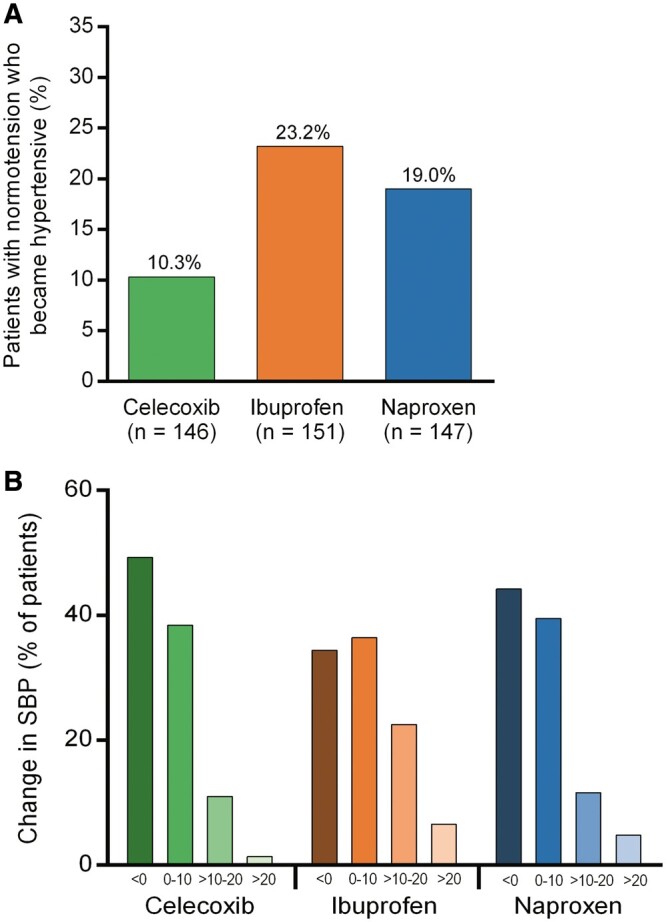
(A) The percentage of patients with baseline normotension who developed hypertension was significantly greater for both ibuprofen and naproxen than for celecoxib (P = 0.004 and P = 0.035, respectively). (B) Compared with celecoxib, the ibuprofen treatment groups had larger proportions of patients whose 24-h SBP increased (P = 0.003). There was no significant difference between celecoxib and naproxen (P = 0.07).
In PRECISION,14 the risk for hospitalization with hypertension increased by 69% with ibuprofen compared with celecoxib (see Supplementary material online, Figure S1). Furthermore, in PRECISION, celecoxib was associated with the lowest rate of investigator reported increase in office BP (2.3%) compared with ibuprofen (3.1%) and naproxen (2.5%).14
Sub-group Forest plots examining effects of comorbidities, patient characteristics and medications are shown in Figure 5. There was no statistical heterogeneity by aspirin use in the present study. Patients receiving a higher dose of ibuprofen (1800 mg vs. 2400 mg) did not show a higher BP. Indeed, the change in mean SBP for ibuprofen was 2.96 ± 1.603 mmHg in 79 patients with dose titration, and 4.04 ± 1.312 mmHg for the 72 patients without. In contrast, the adjusted mean change in SBP at Month 4 for Naproxen was 2.23 ± 1.639 mmHg with (n = 80), and 0.62 ± 1.346 mmHg without dose titration (n = 67). Since regulatory restrictions precluded a dose escalation for celecoxib, only 9 of the 146 patients receiving celecoxib had the dose increased during the course of the study and the mean change in 24-h SBP for these patients was 3.3 + 3.77, vs. a mean change of −0.3 + 1.08 for celecoxib patients who did not titrate. For patients who did not titrate, P-values for mean change in 24-h SBP were 0.002, 0.51, 0.04 for celecoxib vs. ibuprofen, celecoxib vs. naproxen, and naproxen vs. ibuprofen respectively.
Figure 5.
Sub-group forest plots examining effects of comorbidities, patient characteristics, and medications.
Importantly, pain control was similar according to the different treatment groups. The reduction from baseline in the visual analogue scale for pain was 12.4 ± 2.41, 9.4 ± 2.37, 7.9 ± 2.41 for celecoxib, ibuprofen, and naproxen respectively (all P-values were not significant).
Discussion
In the PRECISION-ABPM trial, the use of the non-selective NSAID ibuprofen, compared with celecoxib, associated with a significant increase in ambulatory SBP. In view of the established continuous relationship between BP and both cardiovascular and cerebrovascular events, 10 – 13 the pressor response of more than 3 mm Hg associated with the use of ibuprofen, along with a higher incidence of de novo hypertension and worsening of BP control could impact clinical outcomes for patients chronically using NSAIDs.
The findings of PRECISION-ABPM concur with the primary outcome results of the overall PRECISION trial14 that showed that ibuprofen-treated patients, compared with those who received naproxen and celecoxib, experienced numerically more cardiovascular and renal events. In PRECISION, celecoxib was associated with the lowest rate in reported increase in office BP (2.3%) vs. ibuprofen (3.1%) and naproxen (2.5%), while the rate of hospitalization for hypertension was 69% higher with ibuprofen compared with celecoxib.14 Investigator-reported adverse effects also showed a similar pattern with a higher reported incidence of hypertension.14
PRECISION-ABPM demonstrates that celecoxib and naproxen induce either a slight decrease (celecoxib) or a relatively small increase (naproxen) in BP and lower rates of development of hypertension compared with ibuprofen. A widely cited hypothesis has proposed that the adverse effects of NSAIDs relate directly to the effects of these drugs on platelets and endothelial cells.18 The current findings provide evidence that elevated cardiovascular risk with NSAIDs may not only depend on effects on the vascular endothelium but also agent-specific increases in BP. Given the widespread use of NSAIDs, even a small rise in SBP among hypertensive patients with osteoarthritis could substantially increase cardiovascular events in a population. Indeed, maintaining or achieving BP control in these patients could avoid an estimated >70 000 deaths from stroke and 60 000 deaths from coronary heart disease, resulting in 449 000 patient-years of life saved and 3.8 billion dollars in direct health care cost savings.19
The current results support a distinct heterogeneity with respect to BP elevations and increased cardiovascular events within the group of non-selective and selective NSAIDs. Indeed, previous head-to-head studies and meta-analyses already questioned whether all NSAID and coxibs have similar effects on BP.20–27 In controlled hypertensive patients with osteoarthritis in TARGET, 28 patients treated with ibuprofen had a 2.2 mm Hg increase in 24 h SBP, compared with a reduction of 2.7 mm Hg in patients randomized to the selective COX-2 inhibitor lumiracoxib. In contrast, in CRESCENT, rofecoxib, particularly at higher doses, significantly increased BP compared with celecoxib or naproxen.26 Although comparison across different randomized clinical trials requires caution, hypertension was more frequently adjudicated in clinical trials with rofecoxib, as in APPROVe29 and VIGOR,30 when compared with placebo, than in the CLASS,31 APC,32 and PreSAP33 trials with celecoxib.
If a ‘class’ effect is not evident for COX-2 selective inhibitors, what might explain the observed differences on BP and cardiovascular risk between the different coxibs and other NSAIDs? While all COX-2 selective inhibitors may disrupt the balance between prostacyclin and thromboxane, multiple and opposing cardiovascular influences might also have contributed to our findings including differences in disposition, metabolites, effects on intrarenal prostaglandin production, distinctions in molecular structure, differences in membrane permeability,34 and differential effects on endothelium-dependent relaxation which may influence BP.35 , 36 Moreover, the substantial pharmacological heterogeneity among the different NSAIDs and coxibs requires consideration, as these drugs have distinct chemical structures, pharmacokinetic properties and subsequent metabolism (cytosol reductase vs. cytochrome P450).37–39 In previous studies with patients receiving NSAIDs on a background therapy with aspirin,40 plasma- and urinary- concentrations of prostacyclin and thromboxane remained unchanged, thus rendering a potential COX-2 inhibiting effect unlikely to explain fully the more pronounced hypertensive effects of ibuprofen compared with celecoxib and naproxen under the conditions of the present study. Although some data suggest that ibuprofen and naproxen interfere with the antiplatelet effects of aspirin, this study showed no heterogeneity of BP based on aspirin consumption.
The PRECISION-ABPM trial has limitations. Regulatory restrictions limited the dose of celecoxib to 200 mg daily for osteoarthritis patients who comprised the majority enrolled, which may have provided a safety advantage for celecoxib. Per protocol, RA patients could increase the celecoxib dose to 200 mg twice daily if needed. However, mean doses for both non-selective NSAIDs were also submaximal. Indeed, the three study drugs showed similar analgesic efficacy. For ethical reasons, a placebo comparison arm was not feasible since the protocol required all patients and physicians to document that patient had required NSAID treatment for at least 6 months for adequate symptom relief. Therefore potential changes in BP vs. no treatment are unknown. Acetaminophen was not selected as a comparator because prior studies had demonstrated its ineffectiveness in patients with NSAID-dependent arthritis. Notably, even acetaminophen increases ambulatory BP and heart rate in patients with coronary artery disease.41 Furthermore, extrapolations to occasional intake of these drugs for pain flares require considerable caution. In a recent very large patient data meta-analysis (almost a half-million individuals assembled from Canadian and European health care databases), use of all NSAIDs associated with increased risk of myocardial infarction.42
Although 16% of patients (14% celecoxib, 12% naproxen, and 22% ibuprofen) did not have Month 4 measurements, the current observations represent the largest ABPM comparison of these agents. The blinded study design and the objective nature of the study outcome variable support the reliability of our findings. Of note, ABPM improves the accuracy of the diagnosis, predicts cardiovascular morbidity and mortality much better than conventional clinic BP measurements, and identifies and prevents unnecessary treatment of patients with white-coat hypertension, which occurs in 15–30% of patients with an elevated office BP.43 , 44 A painful arthritis flare may elevate BP transiently leading to misclassification of hypertension. The generally similar analgesic efficacy of the active therapies suggests that this possible confounder should not affect the head-to-head comparisons in this study.
In conclusion, the PRECISION-ABPM trial reveals differential BP effects of treatment with celecoxib vs. the non-selective NSAID ibuprofen. These results support and extend the findings of the PRECISION Trial demonstrating non-inferiority for the primary cardiovascular outcomes for moderate doses of celecoxib compared with naproxen or ibuprofen. These findings may have the greatest clinical significance in the elderly, who have a high prevalence of arthritis and hypertension. Since PRECISION-ABPM demonstrates differential effects of NSAIDs on BP, clinicians need to weigh the potential hazards of worsening BP control and its clinical sequelae as well as the risks to gastrointestinal safety when considering the use of these agents, particularly ibuprofen.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was funded by Pfizer Inc. Grant support to institution from Abbott, AstraZeneca, Bayer Health Care, Biotronik, Boston Scientific, Eli Lilly, Medtronic, Novartis, Servier and St. Jude [to T.F L.]; grant support to institution from Pfizer [to D.H.S, E.H; V.M., S.E.N.]; and Pfizer for the PRECISION trial [to D.A.D., L.M.W.].
Conflict of interest statement: F.R.: personal fees from St. Jude Medical, Servier, ZOLL Medical, AstraZeneca, HeartWare, Sanofi, Cardiorentis, Novartis, Amgen, and Bristol-Myers Squibb, and grant support from St. Jude Medical; J.S.B.: fees for serving on data and safety monitoring boards from Cardiorentis, Novartis, Celladon, GlaxoSmithKline, and Pfizer, fees for serving on executive committees from Servier and Biotronik, fees for serving on event adjudication committees from AstraZeneca and Takeda, fees for serving on an advisory board from ARMGO Pharma, consulting fees from Boehringer Ingelheim, Abbott Laboratories, Sarepta Therapeutics, Amgen, Servier, and Gilead Sciences, and holding stock in BioMarin Pharmaceutical; A.J.F.: Personal fees from Bayer, Novartis, Bristol-Myers Squibb, Orion, Amgen, all outside the submitted work; N.D.Y.: will receive a fee for an advisory panel from Pfizer; P.L.: is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Merck & Co. Inc., Novartis, Pfizer, Sanofi-Regeneron, Takeda Pharmaceuticals and XBiotech, Inc. Member of scientific advisory board for Amgen, Athera biotechnologies, Corvidia Therapeutics, DalCor Pharmaceuticals, Interleukin Genetics, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune and Novartis. Dr. Libby’s laboratory has received research funding in the last 2 years from Novartis; T.F L.: Personal fees for Adjudication Committee ARIVE trial, Bayer Health Care; E.H.: fees for serving on advisory boards from AbbVie, Bristol-Myers Squibb, Amgen, UCB Pharma, Regeneron, and Janssen, and grant support from Sanofi–Genzyme; D.Y.G.: is a consultant for RedHill Biopharma regarding novel H. pylori therapies and has received research support for culture of Helicobacter pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies; R.F.: full-time employee of Pfizer and holds stock options with Pfizer; B.B.: full-time employee of Pfizer and holds stock options with Pfizer; D.I.: full-time employee of Pfizer and holds stock options with Pfizer; and M.L.: receiving fees for serving on advisory panels for the Food and Drug Administration from Abbott, Amgen, and Sarepta, and grant support to his institution from Eli Lilly, Roche, CSL Behring, Esperion Therapeutics, and AstraZeneca.
Supplementary Material
Contributor Information
Frank Ruschitzka, Cardiology, University Heart Center, University Hospital Zurich, Switzerland.
Jeffrey S Borer, Cardiovascular Medicine, Schiavone Cardiovascular Translational Research Institute, State University of New York, Downstate College of Medicine, New York, NY, USA.
Andreas J Flammer, Cardiology, University Heart Center, University Hospital Zurich, Switzerland.
Neville D Yeomans, Cardiovascular Medicine, Western Sydney University, Campbelltown, NSW, Australia.
Peter Libby, Cardiovascular Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Thomas F Lüscher, Cardiology, University Heart Center, University Hospital Zurich, Switzerland.
Daniel H Solomon, Cardiovascular Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
M Elaine Husni, Department of Rheumatic and Immunologic Diseases, Cleveland Clinic, Cleveland, OH, USA.
David Y Graham, Cardiovascular Medicine, Baylor College of Medicine, Veterans Affairs Medical Center, Houston, TX, USA.
Deborah A Davey, Department for Cleveland Clinic, Cleveland Clinic, Cleveland, OH, USA.
Lisa M Wisniewski, Department for Cleveland Clinic, Cleveland Clinic, Cleveland, OH, USA.
Venu Menon, Department for Cleveland Clinic, Cleveland Clinic, Cleveland, OH, USA.
Rana Fayyad, Cardiovascular Medicine, Pfizer, New York, NY, USA.
Bruce Beckerman, Cardiovascular Medicine, Pfizer, New York, NY, USA.
Dinu Iorga, Cardiovascular Medicine, Pfizer, New York, NY, USA.
A Michael Lincoff, Cardiovascular Medicine, Baylor College of Medicine, Veterans Affairs Medical Center, Houston, TX, USA.
Steven E Nissen, Cardiovascular Medicine, Baylor College of Medicine, Veterans Affairs Medical Center, Houston, TX, USA.
References
- 1. Kumar B, Swee ML. Nonsteroidal anti-inflammatory drug use in a patient with hypertension: a teachable moment. JAMA Intern Med 2015;175:892–893. [DOI] [PubMed] [Google Scholar]
- 2. Forman JP, Rimm EB, Curhan GC. Frequency of analgesic use and risk of hypertension among men. Arch Intern Med 2007;167:394–399. [DOI] [PubMed] [Google Scholar]
- 3. MacDonald TM, Hawkey CJ, Ford I, McMurray JJ, Scheiman JM, Hallas J, Findlay E, Grobbee DE, Hobbs FD, Ralston SH, Reid DM, Walters MR, Webster J, Ruschitzka F, Ritchie LD, Perez-Gutthann S, Connolly E, Greenlaw N, Wilson A, Wei L, Mackenzie IS. Randomized trial of switching from prescribed non-selective non-steroidal anti-inflammatory drugs to prescribed celecoxib: the standard care vs. celecoxib outcome trial (SCOT). Eur Heart J 2017;38:1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41:778–799. [DOI] [PubMed] [Google Scholar]
- 5. Harley C, Wagner S. The prevalence of cardiorenal risk factors in patients prescribed nonsteroidal anti-inflammatory drugs: data from managed care. Clin Ther 2003;25:139–149. [DOI] [PubMed] [Google Scholar]
- 6. Houston MC. Nonsteroidal anti-inflammatory drugs and antihypertensives. Am J Med 1991;90:42S–47S. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De BG, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de BP, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De BM, De GS, Derumeaux GA, Erdine S, Farsang C, Funck BC, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker BH, Tsioufis C, van de BP, Vlachopoulos C, Volpe M, Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 8. Warner TD, Mitchell JA. COX-2 selectivity alone does not define the cardiovascular risks associated with non-steroidal anti-inflammatory drugs. Lancet 2008;371:270–273. [DOI] [PubMed] [Google Scholar]
- 9. Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med 1994;121:289–300. [DOI] [PubMed] [Google Scholar]
- 10. SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265: 3255–3264. [PubMed] [Google Scholar]
- 11. Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O'brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 12. MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990;335:765–774. [DOI] [PubMed] [Google Scholar]
- 13. Gurwitz JH, Avorn J, Bohn RL, Glynn RJ, Monane M, Mogun H. Initiation of antihypertensive treatment during nonsteroidal anti-inflammatory drug therapy. JAMA 1994;272:781–786. [PubMed] [Google Scholar]
- 14. Nissen SE, Yeomans ND, Solomon DH, Luscher TF, Libby P, Husni ME, Graham DY, Borer JS, Wisniewski LM, Wolski KE, Wang Q, Menon V, Ruschitzka F, Gaffney M, Beckerman B, Berger MF, Bao W, Lincoff AM, Investigators PT. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 2016;375:2519–2529. [DOI] [PubMed] [Google Scholar]
- 15. Becker MC, Wang TH, Wisniewski L, Wolski K, Libby P, Luscher TF, Borer JS, Mascette AM, Husni ME, Solomon DH, Graham DY, Yeomans ND, Krum H, Ruschitzka F, Lincoff AM, Nissen SE, Investigators P. Rationale, design, and governance of Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen Or Naproxen (PRECISION), a cardiovascular end point trial of nonsteroidal antiinflammatory agents in patients with arthritis. Am Heart J 2009;157:606–612. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 18. Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med 2004;351:1709–1711. [DOI] [PubMed] [Google Scholar]
- 19. Grover SA, Coupal L, Zowall H. Treating osteoarthritis with cyclooxygenase-2-specific inhibitors: what are the benefits of avoiding blood pressure destabilization? Hypertension 2005;45:92–97. [DOI] [PubMed] [Google Scholar]
- 20. Aw TJ, Haas SJ, Liew D, Krum H. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med 2005;165:490–496. [DOI] [PubMed] [Google Scholar]
- 21. Izhar M, Alausa T, Folker A, Hung E, Bakris GL. Effects of COX inhibition on blood pressure and kidney function in ACE inhibitor-treated blacks and hispanics. Hypertension 2004;43:573–577. [DOI] [PubMed] [Google Scholar]
- 22. MacDonald TM, Reginster JY, Littlejohn TW, Richard D, Lheritier K, Krammer G, Rebuli R. Effect on blood pressure of lumiracoxib versus ibuprofen in patients with osteoarthritis and controlled hypertension: a randomized trial. J Hypertens 2008;26:1695–1702. [DOI] [PubMed] [Google Scholar]
- 23. Morgan TO, Anderson A, Bertram D. Effect of indomethacin on blood pressure in elderly people with essential hypertension well controlled on amlodipine or enalapril. Am J Hypertens 2000;13:1161–1167. [DOI] [PubMed] [Google Scholar]
- 24. Polonia J, Boaventura I, Gama G, Camoes I, Bernardo F, Andrade P, Nunes JP, Brandao F, Cerqueira-Gomes M. Influence of non-steroidal anti-inflammatory drugs on renal function and 24h ambulatory blood pressure-reducing effects of enalapril and nifedipine gastrointestinal therapeutic system in hypertensive patients. J Hypertens 1995;13:925–931. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz JI, Thach C, Lasseter KC, Miller J, Hreniuk D, Hilliard DA, Snyder KM, Gertz BJ, Gottesdiener KM. Effects of etoricoxib and comparator nonsteroidal anti-inflammatory drugs on urinary sodium excretion, blood pressure, and other renal function indicators in elderly subjects consuming a controlled sodium diet. J Clin Pharmacol 2007;47:1521–1531. [DOI] [PubMed] [Google Scholar]
- 26. Sowers JR, White WB, Pitt B, Whelton A, Simon LS, Winer N, Kivitz A, van IH, Brabant T, Fort JG, Celecoxib Rofecoxib E , Safety in Comorbidities Evaluation Trial I. The effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-h blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med 2005;165:161–168. [DOI] [PubMed] [Google Scholar]
- 27. White WB, Kent J, Taylor A, Verburg KM, Lefkowith JB, Whelton A. Effects of celecoxib on ambulatory blood pressure in hypertensive patients on ACE inhibitors. Hypertension 2002;39:929–934. [DOI] [PubMed] [Google Scholar]
- 28. Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, Gitton X, Krammer G, Mellein B, Gimona A, Matchaba P, Hawkey CJ, Chesebro JH, Group TS. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet 2004;364:675–684. [DOI] [PubMed] [Google Scholar]
- 29. Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA; Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 2005;352:1092–1102. [DOI] [PubMed] [Google Scholar]
- 30. Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ; VIGOR Study Group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 2000; 343: 1520–1528. [DOI] [PubMed] [Google Scholar]
- 31. Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000;284:1247–1255. [DOI] [PubMed] [Google Scholar]
- 32. Solomon SD, Pfeffer MA, McMurray JJ, Fowler R, Finn P, Levin B, Eagle C, Hawk E, Lechuga M, Zauber AG, Bertagnolli MM, Arber N, Wittes J. Apc, Pre SAPTI. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation 2006;114:1028–1035. [DOI] [PubMed] [Google Scholar]
- 33. Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, Rosenstein RB, Macdonald K, Bhadra P, Fowler R, Wittes J, Zauber AG, Solomon SD, Levin B; PreSAP Trial Investigators. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 2006;355:885–895. [DOI] [PubMed] [Google Scholar]
- 34. Walter MF, Jacob RF, Day CA, Dahlborg R, Weng Y, Mason RP. Sulfone COX-2 inhibitors increase susceptibility of human LDL and plasma to oxidative modification: comparison to sulfonamide COX-2 inhibitors and NSAIDs. Atherosclerosis 2004;177:235–243. [DOI] [PubMed] [Google Scholar]
- 35. Widlansky ME, Price DT, Gokce N, Eberhardt RT, Duffy SJ, Holbrook M, Maxwell C, Palmisano J, Keaney JF Jr, Morrow JD, Vita JA. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension 2003;42:310–315. [DOI] [PubMed] [Google Scholar]
- 36. Chenevard R, Hurlimann D, Bechir M, Enseleit F, Spieker L, Hermann M, Riesen W, Gay S, Gay RE, Neidhart M, Michel B, Luscher TF, Noll G, Ruschitzka F. Selective COX-2 inhibition improves endothelial function in coronary artery disease. Circulation 2003;107:405–409. [DOI] [PubMed] [Google Scholar]
- 37. Steffel J, Hermann M, Greutert H, Gay S, Luscher TF, Ruschitzka F, Tanner FC. Celecoxib decreases endothelial tissue factor expression through inhibition of c-Jun terminal NH2 kinase phosphorylation. Circulation 2005;111:1685–1689. [DOI] [PubMed] [Google Scholar]
- 38. FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 2001;345:433–442. [DOI] [PubMed] [Google Scholar]
- 39. White WB, Campbell P. Blood pressure destabilization on nonsteroidal antiinflammatory agents: acetaminophen exposed? Circulation 2010;122:1779–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bippi H, Frolich JC. Effects of acetylsalicylic acid and paracetamol alone and in combination on prostanoid synthesis in man. Br J Clin Pharmacol 1990;29:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sudano I, Flammer AJ, Periat D, Enseleit F, Hermann M, Wolfrum M, Hirt A, Kaiser P, Hurlimann D, Neidhart M, Gay S, Holzmeister J, Nussberger J, Mocharla P, Landmesser U, Haile SR, Corti R, Vanhoutte PM, Luscher TF, Noll G, Ruschitzka F. Acetaminophen increases blood pressure in patients with coronary artery disease. Circulation 2010;122:1789–1796. [DOI] [PubMed] [Google Scholar]
- 42. Bally M, Dendukuri N, Rich B, Nadeau L, Helin-Salmivaara A, Garbe E, Brophy JM. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ 2017;357:j1909.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Staessen JA, Thijs L, Fagard R, O'brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. JAMA 1999; 282: 539–546. [DOI] [PubMed] [Google Scholar]
- 44. O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y. European Society of Hypertension Working Group on Blood Pressure M. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.