Abstract
Bone serves to maintain the shape of the human body due to its hard and solid nature. A loss or weakening of bone tissues, such as in case of traumatic injury, diseases (e.g., osteosarcoma), or old age, adversely affects the individuals quality of life. Although bone has the innate ability to remodel and regenerate in case of small damage or a crack, a loss of a large volume of bone in case of a traumatic injury requires the restoration of bone function by adopting different biophysical approaches and chemotherapies as well as a surgical reconstruction. Compared to the biophysical and chemotherapeutic approaches, which may cause complications and bear side effects, the surgical reconstruction involves the implantation of external materials such as ceramics, metals, and different other materials as bone substitutes. Compared to the synthetic substitutes, the use of biomaterials could be an ideal choice for bone regeneration owing to their renewability, non-toxicity, and non-immunogenicity. Among the different types of biomaterials, nanocellulose-based materials are receiving tremendous attention in the medical field during recent years, which are used for scaffolding as well as regeneration. Nanocellulose not only serves as the matrix for the deposition of bioceramics, metallic nanoparticles, polymers, and different other materials to develop bone substitutes but also serves as the drug carrier for treating osteosarcomas. This review describes the natural sources and production of nanocellulose and discusses its important properties to justify its suitability in developing scaffolds for bone and cartilage regeneration and serve as the matrix for reinforcement of different materials and as a drug carrier for treating osteosarcomas. It discusses the potential health risks, immunogenicity, and biodegradation of nanocellulose in the human body.
Keywords: nanocellulose, bone diseases, tissue engineering, biodegradation, medical applications
Introduction
Nanocellulose refers to the cellulosic materials with dimensions in the nanoscale range, which combines the important properties of cellulose with the features of nanomaterials (Mazhar et al., 2021). It is obtained from plants (de Amorim et al., 2020), produced by different microorganisms predominantly from acetic acid bacteria (Andriani et al., 2020; Khan et al., 2020b), algae (Liu et al., 2017), and animals (Bacakova et al., 2019), as well as synthesized enzymatically such as by the cell-free enzyme systems (Ullah et al., 2015; Kim et al., 2019). There exist different types of nanocellulose, including cellulose nanofibers (CNFs), cellulose nanocrystals (CNCs), and microorganisms-derived cellulose knows as bacterial nanocellulose (BNC) or bacterial cellulose (BC) (Klemm et al., 2018). The CNFs and CNCs are extracted from different plants through complex extraction procedures, involving physico-mechanical degradation and chemical de-structuring such as acid hydrolysis (Dufresne, 2013; Ullah et al., 2016c). BNC is chemically similar to plant-derived cellulose; nevertheless, it represents the purest form of cellulose, as unlike plant cellulose, it does not contain hemicellulose, lignin, and other minerals (Bacakova et al., 2019; Ul-Islam et al., 2019a).
Bacterial nanocellulose is the most prominent biopolymer synthesized by specific bacterial species through the fermentation of sugars and plant carbohydrates. It possesses unique structural, physico-chemical, mechanical, thermal, and biological features and is a Generally Recognized As Safe (GRAS) biological product (Yang et al., 2018; Zhong, 2020). It has been extensively used for various biomedical and other applications in the form of hydrogels, membranes, particles, and three-dimensional (3D) scaffolds, where its properties are tuned according to the target applications (Ul-Islam et al., 2015). Its innate features are modulated through the development of its composites with different materials like natural and synthetic polymers (Shah et al., 2013), nanomaterials (Ul-Islam et al., 2014; Khan et al., 2015; Ullah et al., 2016b), peptides (Curvello et al., 2019), ceramics (DeMello, 2012), clays (Ul-Islam et al., 2013a), plant extracts (Fatima et al., 2021; Ul-Islam et al., 2021a), and others (Mbituyimana et al., 2021). Similarly, its biological synthesis is regulated to produce BNC with controlled features in the form of mechanically and thermally stable hydrogels and form simple to complex 3D structures (Shi et al., 2016). Moreover, its structural features are modified through different pre- and post-synthesis processing approaches (Ul-Islam et al., 2013b). Although the harvesting procedures of BNC hydrogels from the production medium are simple, cost-effective, and time-efficient, the drying procedures such as air-drying, oven-drying, spray-drying, press-drying, critical point drying, and freeze-drying which ultimately determine the characteristic features of BNC, are time-consuming (Lin and Dufresne, 2014; Sharma and Bhardwaj, 2019), and may cause unwanted changes to the innate structure of BNC. For example, air-drying causes the loss of the 3D network structure of BNC due to strong shrinkage-mediated wrinkling, fiber aggregation, and superficial hornification and ultimately results in reduced porosity. On the other hand, critical point drying and freeze-drying, although gentle procedures and retain the original nanofibrous structure (Sharma and Bhardwaj, 2019), may result in complete drying and causes brittleness. BNC is a non-toxic biopolymer that has been found with no severe signs of inflammation and toxicity on the genetic and cellular levels, even in long-term application. Currently, more attention is given to developing BNC-based scaffolds for various medical applications. For instance, the development of BNC-based wound dressings is currently on the market, while some BNC implants are in the phase of clinical studies as a novel solution to yet unresolved complications in regenerative medicine (Klemm et al., 2018; Sharma and Bhardwaj, 2019; Zhong, 2020). Studies have shown that the BNC-based scaffolds play vital roles in the regeneration of bone and cartilage (Khan et al., 2020a), as the drug delivery systems (Pachuau, 2017; Li et al., 2018), biosensing platforms (Jasim et al., 2017; Farooq et al., 2020), diagnostic tools (Ul-Islam et al., 2019b), and several other areas (Islam et al., 2015; Derakhshanfar et al., 2018; McCarthy et al., 2019a, b, Wang et al., 2020a, 2021). The impregnation of nanomaterials to BNC and its use as the wound dressing biomaterial, broad-spectrum antimicrobial, surface disinfectant, and nanodrugs for the treatment of different diseases (Lin and Dufresne, 2014; Di et al., 2017; Wang et al., 2020b) could be an effective approach for addressing several clinical issues.
Bone and cartilage encounter different defects associated with diseases, injuries, and aging. Some of these defects are conventionally treated by using autografts, allografts, and xenografts; however, their general use is hindered by immune rejection. Similarly, osteosarcoma, the malignant bone-tumor accounting for 20% of primary bone cancers (Carina et al., 2019), is another class of bone diseases. The widespread application of currently used strategies for treating osteosarcoma such as surgery (e.g., amputations and bone reconstruction) combined with chemotherapy is limited by the low efficacy of different therapeutic options as well as some chemotherapeutic drugs cause adverse effects and lead to severe complications (Carina et al., 2019). To overcome the adverse effects of surgery and chemotherapy, various biophysical approaches such as sono- and photodynamic therapies, low-intensity pulsed ultrasound, high-intensity focused ultrasound, and hyperthermia have been developed (Sengupta and Balla, 2018; Carina et al., 2019). To date, the knowledge about the efficacy of biophysical therapies against osteosarcoma is limited, and there are only limited reports available about the usage of nanotechnology and nano-delivery systems for the treatment of osteosarcomas (Gu et al., 2013).
The applications of nanotechnology could be expanded toward the treatment of bone and cartilage defects. For example, nanoparticles possess unique properties such as low toxicity, biodegradability, selective tumor cell targeting, higher deposition and retention in tumor tissue, and prolonged blood circulation time (Panday et al., 2018; Zhang et al., 2018b). Although there are also unclear aspects of nanotechnology restricting its broad-spectrum applications, the inherent unique properties of nanomaterials could help in improving their therapeutic efficacy against osteosarcomas (Turnbull et al., 2018; Zhang et al., 2018b). In addition to different nanoparticles, nanocellulose could also be used to replace the affected bone, like in the form of bone substitutes, as a matrix for impregnation of nanomaterials, and as a drug carrier (Khan et al., 2019) for treating different bone-related diseases. For instance, bisphosphonate-modified nanocellulose (pNC) could be used as a bone substitute for bone regeneration. The pNC is a bioresponsive injectable bone material that suppresses the formation of osteoclasts and enhances the differentiation of osteoblasts. Considering the regulation of osteoclast/osteoblast activity, pNC has enormous potential for treating bone diseases (Nishiguchi and Taguchi, 2019). Although the mechanical strength of nanocellulose is quite low compared to that of the bone tissues, it can be improved, such as through crosslinking. In a study, mechanically stable CNCs-based scaffolds were developed through sulfuric acid hydrolysis crosslinking, which showed high compressive strength and porosity. The scaffolds demonstrated in vivo osteoinductivity and bone formation across the damaged site (Osorio et al., 2019a). Besides, nanocellulose could also be modified with anti-osteosarcoma metals or nanoparticles, for instance with selenium, strontium, and arsenic nanoparticles, where nanocellulose could not only function as a carrier for different drugs but also serve as a substitute for the damaged bone tissues (Khan et al., 2019; Luz et al., 2020). Both selenium and arsenic have been widely studied for their anticancer effects, where selenium has been found effective against osteosarcoma when doped with hydroxyapatite (Wang et al., 2016; Khan et al., 2019). Therefore, combining these metals with nanocellulose may further increase their utilization in treating bone diseases. Besides bone, nanocellulose could also be used as a substitute for the cartilage that is a smooth elastic tissue and covers the ends of long bones at the joints and nerves and is commonly present in the ear, nose, rib cage, intervertebral disks, and several other parts of the body. Cartilage is softer than bone but much stiffer than muscles and contains chondrocytes which produce a collagenous extracellular matrix. In a study, a highly porous hydrogel comprised of interpenetrating networks of sodium alginate and gelatin reinforced with cellulose nanocrystals was prepared. The composite hydrogel supported the adhesion, growth, penetration, and differentiation of mesenchymal stem cells (MSCs), thus could be used as a substitute for cartilage (Naseri et al., 2016). In another study, a mitogenic hydrogel ink comprised of alginate sulfate with nanocellulose was printed that supported the adhesion, spreading, and proliferation of chondrocyte and produced collagen II (Mller et al., 2017). The printed 3D scaffold could be used as a potential substitute for cartilage.
Literature survey shows that there exist few reviews on using different forms of nanocellulose for their tissue engineering and other biomedical applications. For example, a couple of reviews specifically discusses the tissue engineering applications of BC (Torgbo and Sukyai, 2018) and CNCs (Murizan et al., 2020), while others overview the use of different forms of nanocellulose for bone tissue engineering along with other biomedical applications (Bacakova et al., 2019; Luo et al., 2019; Pang et al., 2020; Subhedar et al., 2021). However, there is no comprehensive review discussing not only the tissue engineering applications but also the role of nanocellulose as a matrix for nanoparticles and drugs for treating osteosarcomas. Moreover, this review discusses the important structural, physiological, and biological features of nanocellulose from the perspective of developing scaffolds suitable for tissue engineering and carriers for drugs and nanoparticles for treating bone defects and cancers. Most importantly, it discusses the immunogenicity and complications associated with the introduction of nanocellulose to the human body.
Natural Sources of Nanocellulose
Plants
Plants represent the most abundant source of nanocellulose. It is extracted from different plant sources such as trees, roots, vegetables, grasses, shrubs and herbs, succulents, flowers, and different other plant-derived sources. Trees are the main source of nanocellulose, and it is mostly obtained from coniferous (Pinus radiata) (Powell et al., 2016) and leaved trees (birch) (Bacakova et al., 2019). Various other trees, for example, Khaya senegalensis (Adewuyi et al., 2018), Banana pseudostem (Faradilla et al., 2017), palm and balsa (Bacakova et al., 2019), citrus trees (Matharu et al., 2018), Syzygium cumini (Singla et al., 2017), and Acacia mangium (Jasmani and Adnan, 2017), are also good sources of nanocellulose. The nanocellulose extracted from coniferous and leaved trees is referred to as the softwood and hardwood-derived nanocellulose, respectively. Hibiscus and cotton are the shrub sources of nanocellulose (Poonguzhali et al., 2017). Various other plant sources of nanocellulose include the corn leaf, carrot, triticale straw, sisal (Agave sisalana), Miscanthus giganteus (grass), bamboo, pineapple leaf, rice husk, and soybean straw (Song et al., 2018).
Microorganisms
The microorganism-derived nanocellulose (i.e., BNC or BC) (Yuen et al., 2017) is mainly produced by the Gram-negative acetic acid bacteria belonging to genera Gluconacetobacter, Acetobacter, Sarcina, Salmonella, Achromobacter, Agrobacterium, Alcaligenes, Rhodobacter, Azotobacter, Pseudomonas, Aerobacter, and Rhizobium. Among the different BNC-producing bacterial genera, Gluconacetobacter xylinum and Gluconacetobacter hansenii are the most widely studied bacterial species (Ahrem et al., 2014; Ullah et al., 2017). Besides, Gluconacetobacter kombuchae and Komagataeibacter medellinensis (low pH-resistant strain) (Bacakova et al., 2019) are also known for high-quality BNC production. BNC is typically synthesized as pure cellulose by the BNC-producing bacteria, which, unlike the plant-derived cellulose, does not require intensive processing for removal of unwanted impurities or contaminants (Lin et al., 2013). To date, different strategies have been developed for increasing the yield and productivity, minimizing the production cost, and enhancing the structural features of BNC (Ullah et al., 2019c; Ul-Islam et al., 2021b). For example, the addition of yeast extract to the bacterial growth medium enhances bacterial growth, thus leading to high productivity and yield (Saska et al., 2017; Kaminagakura et al., 2019). A study also reported enhanced BNC production through the symbiotic co-cultivation of M. gisevii with BNC-producing bacterium (Zharikov et al., 2018). The addition of different supplements to the growth medium not only improves the production but also enhances the structural features of BNC (Ul-Islam et al., 2017, 2020). Different reactors have also been designed for improving yield and productivity (Ullah et al., 2019c). Besides, extensive efforts have been made to explore low-cost substrates and utilize different wastes for cost-effective BNC production (Ul-Islam et al., 2017, 2020).
Cell-Free Enzyme System
Nanocellulose production by the cell-free enzyme systems represents a relatively new and previously uncharacterized approach. A cell-free enzyme system represents the state-of-the-art conversion of a substrate into the product through a series of enzymatic reactions, each catalyzed by specific enzymes and regulatory proteins (i.e., cofactors) (Khattak et al., 2014; Ullah et al., 2016a). Park and co-workers developed a G. hansenii-based cell-free enzyme system through a simple bead beating approach and demonstrated in vitro nanocellulose production (Ullah et al., 2015; Kim et al., 2019). The developed system contained all essential enzymes required for the synthesis of cellulose as characterized by the LC-MS/MS-LTQ Orbitrop and SDS-PAGE analyses. The system was further supplemented with external cofactors (i.e., ATP and NAD) to boost the nanocellulose-production (Ullah et al., 2015). The developed cell-free enzyme system effectively produced cellulose at a much higher yield (58%) as compared to the microbial cell system (i.e., 37%) under the same experimental conditions. Moreover, the synthesized cellulose demonstrated superior morphological structure, physiological features, and thermal and mechanical properties, as compared to the cellulose produced by the microbial cells (Ullah et al., 2016c). The cell-free enzyme system represents an in vitro and energy-efficient approach for nanocellulose synthesis that can be easily controlled, and the synthesized nanocellulose could be tuned for the desired structural features.
Algae
Cladophora and Cystoseria myrica are the major algal species that produce nanocellulose. Cladophora-derived nanocellulose is a potential biomedical material due to the presence of several useful materials in its structure, including endotoxins, glucans, and heavy metals (Liu et al., 2017). Its adsorption capacity for Congo-Red-dye (Ruan et al., 2018), hemocompatibility (Rocha et al., 2018), and suitability as the scaffold for cell adhesion and proliferation (Hua et al., 2016) have already been evaluated. Similarly, the Cystoseria myrica-derived nanocellulose together with Fe3O4 has been evaluated for the removal of mercury (Zarei et al., 2018).
Animals
Nanocellulose has also been obtained from some animals such as Styela clava and Halocynthia roretzi Drasche (tunicates from phylum Chordata) (Bacakova et al., 2019). Styela clavaderived nanocellulose has been used in wound dressings and other biomedical applications, including scaffolds-based tissue engineering and the development of absorbable hemostats and hemodialysis membranes and stitching fibers (Song et al., 2014, 2017). There are also various industrial and technological applications of nanocellulose from animals. For example, Halocynthia roretzi Drasche (composite)-derived nanocellulose together with TiO2 nanoparticles was used for the removal of oil from wastewater (Zhan et al., 2018).
Nanocellulose-Related Health Complications in Humans
Nanocellulose, mainly BNC, is applied in different forms to humans, such as skin substitute (Khan et al., 2018), wound dressing materials (Sajjad et al., 2020), synthetic blood vessels (Scherner et al., 2014), artificial cornea (Wang et al., 2010), bone and cartilage (Basu et al., 2018), synthetic heart valves (Mohammadi, 2011), gastrointestinal tract (Lamboni et al., 2019), tympanic membrane (Kim et al., 2013), dental implants (Jinga et al., 2014), neural implants (Yang et al., 2017), urinary conduit replacement (Huang et al., 2015), contact lenses (Cavicchioli et al., 2015), and several others, and therefore, it is necessary to identify the associated potential health risks (Endes et al., 2016). Several studies have reported brown lung, alveo-bronchiolitis, fibrosis, and granulomatous inflammation in vivo following the exposure to cellulose (Ttrai et al., 1995). Similarly, studies have reported the persistence of cellulose, both in vitro and in vivo, alveolitis, granulomata, and higher cellulose fiber durability in the lungs (Endes et al., 2016). A study has also reported the cytotoxicity of high doses of nanocellulose crystals (Yanamala et al., 2014). The high doses of cellulose can significantly increase the radical formation (Stefaniak et al., 2014) and generation of oxidative stress during long-term exposure, which can ultimately lead to serious consequences in the human body. Currently, few studies have investigated the genotoxic effects of nanocellulose in the human body, including potential for mutagenicity, formation of micronuclei, and measurement of DNA strand breaks (Endes et al., 2016). Although no studies have reported any effects on the formation of micronuclei or alteration in DNA quality after exposure to nanocellulose exposure (Kovacs et al., 2010; Cataln et al., 2015), a study by de Lima et al. (2012) found chromosomal aberration in the animal cells after exposure to nanocellulose. Surprisingly, the chronic exposure to nanocellulose via inhalation increased the susceptibility to cytotoxicity in males and was associated with higher inflammatory and oxidative stress. These biochemical alterations further cause significant genotoxicity (Shvedova et al., 2016), and the genotoxic effects are extremely detrimental to the male reproductive system (Farcas et al., 2016). However, a detailed long-term mechanistic toxicological assessment study to investigate the potential biological effects on human health is still lacking. These assessments are essential and are considered as the landmarks of nanotoxicological research strategies (Camarero-Espinosa et al., 2016).
Attractions in Nanocellulose-Based Scaffolds to Substitute Bone Tissues
As bone primarily maintains the shape of the body, the biological scaffolds serving as the bone substitute must demonstrate the desired mechanical strength and biocompatibility. Nanocellulose possesses unique structural, physico-chemical, mechanical, thermal, and biological features to meet the desired features of biological scaffolds. In addition, its 3D fibrous and porous structure and the presence of free hydroxyl (OH) groups on its surface provide an excellent platform for the development of biomaterials with tuned properties.
Abundance and Renewability
Nanocellulose is the most abundant and renewable material on Earth. As described earlier (see section Natural Sources of Nanocellulose), it is obtained from a variety of sources like plants, microorganisms, algae, and animals and synthesized enzymatically. The abundance, renewability, and easy availability, along with other properties, make nanocellulose an ideal choice for the development of biological scaffolds for applications in bone tissue engineering (Table 1).
TABLE 1.
Applications of BNC-based biomaterials in bone regeneration.
| Reinforcement material | Synthesis method | Model system for analysis | Enhanced properties | References |
| HAp | Post-synthesis phosphorylation | Ca/P ratio | High Ca/P ratio | DeMello, 2012 |
| Loading of HAp to BNC | HAp loading | High loading of HAp in phosphorylated BNC | Wan et al., 2007 | |
| Post-synthesis loading | Ca/P ratio, in vivo inflammatory tests | Ca/P ratio similar to natural bone and no in vitro inflammation | Saska et al., 2011 | |
| Biomimetic synthesis | hBMSC | Enhanced cell adhesion and biological activity | Fang et al., 2009 | |
| Post-synthesis loading | XPS analysis, ALP activity, osteoblast growth, and formation of bone nodule | Presence of Ca2+ and PO42, enhanced adhesion growth of osteoblast, and osteoconductivity on membranes | Tazi et al., 2012 | |
| HAp and magnetic nanoparticles | Ca/P ratio, crystallinity, magnetic field response, in vitro MC3T3-E1 cells | High porosity, decreased crystallinity and swelling, decreased saturation magnetization, and enhanced biocompatibility | Torgbo and Sukyai, 2019 | |
| HAp and graphene oxide | Wet chemical precipitation | ALP activity, growth of MG-63 and NIH-3T3 cells | Water uptake, in vitro degradation, cell adhesion and growth, and ALP activity | Ramani and Sastry, 2014 |
| HAp and gelatin | Laser patterning | Porosity and In vitro C5.18 cells | Enhanced adhesion and proliferation of chondrogenic rat cells, high porosity | Jing et al., 2013 |
| HAp and strontium | Oxidation of BNC, ex situ mineralization | In vitro cytotoxicity and hemocompatibility | Guided bone regeneration, in vivo biocompatibility, in vitro degradation, bioactivity, non-cytotoxicity, low inflammation, swelling, thermal stability, enhanced desorption | Luz et al., 2020 |
| HAp or Col with and without OGP | Post-synthesis loading | CHO-K1 cells, CBMN assay, comet assay, XTT assay, and clonogenic assay | Cell adhesion and proliferation, and no mutagenic, genotoxic, or cytotoxic effects on the cells | Raquel Mantuaneli Scarel-Caminaga, 2014 |
| HAp and poly (vinyl pyrrolidone) | Biomimetic mineralization | Ca/P ratio | Enhanced mineralization | Yin et al., 2011 |
| Agarose, gelatin, HAp, and procyanidins | Post synthesis crosslinking | Mechanical strength, pore size distribution, in vitro hGMSCs, in vitro and in vivo bone formation | Porosity, mechanical strength, cell viability, in vitro bone formation in mice and in vivo bone repair in rabbit | Huang et al., 2017 |
| GO, Hap, and -glucan | Free radical polymerization and freeze-drying | Surface morphology, porosity, and mechanical strength, hydrophobicity, aqueous degradation, in vitro MC3T3-E1 | High stability, hydrophobicity, aqueous degradation, spongy morphology, porosity, and mechanical strength, antibacterial activity, biocompatibility, hemocompatibility | Umar Aslam Khan et al., 2020 |
| 2-chloro-N, N-dimethyl ethylamine hydrochloride, glycidyl trimethyl ammonium chloride, and monochloro acetic acid sodium salt | Post-synthesis chemical reaction | In vitro EqMSCs | Enhanced in vitro adhesion, proliferation, and osteogenic differentiation of EqMSCs | Favi, 2014 |
| Gelatin | Post-synthesis loading | Crystallinity index, mechanical strength, in vitro adhesion of NIH 3T3 cells | Crystallinity index, enhanced mechanical strength and thermal stability, improved in vitro cell adhesion | Cai and Kim, 2010 |
| PVA and boron nitride | 3D printing | Mechanical strength, swelling, in vitro osteoblast cell line | Decreased tensile strength and increased elongation strain, enhanced cell adhesion and viability, improved swelling | Aki et al., 2020 |
| Plant-derived recombinant human osteopontin (p-rhOPN), and RGD-containing biomolecule | In vitro grafting | Quantification of p-rhOPN immobilization, in vitro mineralization, and in vitro hPDLSCs | Enhanced osteogenic differentiation of hPDLSCs, cytocompatibility, in vitro calcification | Klinthoopthamrong et al., 2020 |
| Bone morphogenic protein (BMP-2) | Post-synthesis loading | In vitro mouse fibroblast-like C2C12 cells, in vivo bone formation | Differentiation of C2C12 cells into osteoblasts and in vivo formation of bone with high calcium content | Shi et al., 2012a, b |
| 3D scaffolds, ECM-mimicking | Low dose treatment of BMB-2, micro- and nano-porosity, in vitro C3H10T1/2 cells | Enhanced cell adhesion, growth, and infiltration, bone matrix secretion and maturation, biomineralization, osteoinduction | Dubey et al., 2020 | |
| Collage and BMP-2 | Malaprade and Schiff-base reactions, template method combined with reverse-phase suspension regeneration | Porosity, in vitro MC3T3-E1, ALP activity | Biocompatibility, 3D porous microspheres with multiple structures, thermal stability, increased crystallinity, osteoblast differentiation | Zhang et al., 2020 |
| Otoliths and collagen | Post-synthesis loading | Histological examination | In vivo regeneration of bone tissue with higher osteoblast activity, degree of regularity, and osteo-reabsorption activity | Olyveira et al., 2011 |
| Col1 | Post-synthesis crosslinking | Tensile strength, elastic modulus, and morphology and proliferation of osteogenic cells | Decreased tensile strength and elastic modulus of BNC-Col1, a slight increase in strain at break, cell viability and proliferation, and maintenance of cell morphology on the scaffold | Saska et al., 2012 |
| Paraffin wax particles | In situ loading of particles | MC3T3-E1 osteoprogenitor cells, confocal microscopy, and histology | Enhanced clustering of MC3T3-E1 osteoprogenitor cells in the porous composite | Zaborowska et al., 2010 |
An overview of different reinforcement materials, synthesis methods, model systems used for analysis, and enhanced properties of BNC and its composites.
Surface Chemistry
Nanocellulose has a high surface area and aspect ratio (length to diameter) and contains abundant OH groups. The cellulose chain is asymmetric, with one end having reducing functionality due to the presence of the OH group while the other end is non-reducing (Habibi et al., 2010; Islam et al., 2018). On average, each glucose monomer in cellulose contains three OH groups, which can be easily accessed for surface modification (Phanthong et al., 2018). For example, TEMPO-oxidation at the surface of nanocellulose creates a negative charge by replacing the OH groups with carboxylic (COOH) and aldehyde (CHO) groups, which allows the repulsion of nanofibrils and causes fibrillation (Saito et al., 2007; Isogai et al., 2011), thus greatly contributes to addressing the aggregation issue of cellulose fibrils. This aggregation issue could also be addressed by introducing the anionic sulfate groups onto the surface of nanocellulose that produces electrostatic repulsion among the fibers in the aqueous dispersion and yield stable CNCs (Eyley and Thielemans, 2014). It is worth mentioning here that not all OH groups are modified; a large number of reactive OH groups are preserved to allow the grafting or doping of other molecules to impart additional structural and functional characteristics to nanocellulose. A study reported the uptake of non-aggregated softwood pulp sheet-derived nanocellulose by different cells (Hosseinidoust et al., 2015), thus could be used as a carrier for nanodrugs.
Biocompatibility
Biocompatibility refers to the ability of a material to interact with living tissues without provoking the immunogenic response, inflammation, or allergy and remain non-toxic (Morais et al., 2010; Ul-Islam et al., 2015). According to Williamss definition, biocompatibility is the ability of a material to perform with an appropriate host response in a specific application (Williams, 2019). In general, all types of nanocelluloses are considered biocompatible materials in that these are non-toxic, non-immunogenic, non-inflammatory, non-allergic, and somehow supports the adhesion, proliferation, growth, migration, and differentiation of cells, either alone or in the form of composites with other materials (Eslahi et al., 2020). A study reported the use of bleached birch pulp-derived nanofibrillated cellulose for wound dressing, which effectively adhered and detached from the skin after completion of wound healing (Hakkarainen et al., 2016). Similarly, BNC is considered a moderate to highly biocompatible biomaterial due to its non-toxic nature and ability to support cell growth, proliferation, and infiltration owing to its highly porous and fibrous structure (Klemm et al., 2001; Schumann et al., 2009). An in vivo study demonstrated the subcutaneous implantation of BNC in rats up to 12 weeks that did not show any signs of immunogenicity, inflammation, or formation of exudates around the implant (Helenius et al., 2006). Although non-toxic, pristine BNC lacks cell adhesion sites and its biocompatibility is not up to the desired levels in some cases; thus, different strategies like a surface modification to introduce bioactive functional groups such as peptides antimicrobial peptides (Frsatz et al., 2018) and formation of composites with compatible polymers like gelatin (Khan et al., 2018), chitosan (Ul-Islam et al., 2019b), collagen (Zhang et al., 2020; Li et al., 2021), and other materials, have been developed to achieve the desired biocompatibility for specific applications (Phanthong et al., 2018; Ullah et al., 2019b; Table 1).
Biodegradability/Bioresorbability
Biodegradation or bioresorbability is an important feature of nanocellulose for its application in bone tissue engineering (Ullah et al., 2019b). The nanocellulose-based scaffolds degrade both in vitro and in vivo; nevertheless, these sometimes require additional pre-treatment and modification, such as oxidation (Faradilla et al., 2017), and must degrade at a controlled rate of resorption for specific bone tissue engineering applications. Moreover, the degradation products of nanocellulose are also biologically safe due to their innate non-toxic nature; however, a careful selection of the reinforcement materials during the development of nanocellulose-based scaffolds must be ensured for the safety of the degradation products. A study reported that the architecture of biological scaffolds changes with the degradation, where the produced byproducts could interfere with other in vivo biological processes (Dorozhkin, 2013).
Compared to other forms of nanocellulose, BNC is rarely biodegradable due to its crystalline nature and compact fibrils arrangement. Moreover, human lacks the cellulose-degrading cellulase enzyme. The biodegradation of BNC has been enhanced through several methods. For example, Li et al. (2009) carried out the periodate oxidation of BNC, which led to its enhanced in vitro oxidation in water, phosphate buffer saline, and simulated body fluid. The periodate oxidation of BNC allows specific cleavage of C2C3 of the glucopyranoside and produces two aldehyde molecules from a single glucose unit as well as the biodegradable and biocompatible 2,3-dialdehyde cellulose (DAC) (Fan et al., 2001; Li et al., 2009). The DAC is biodegradable at physiological pH, both in vitro and in vivo, and produces 2,4-dihydroxybutyric acid and glycolic acid (Singh et al., 1982), where the former participates in the metabolism of L-homoserine in the liver while the latter is either excreted through urine or enters the Krebss cycle (RoyChowdhury and Kumar, 2006). Similarly, a study by Yang et al. (2016) reported the development of carboxymethylated chitosan and hairy nanocrystalline cellulose-based biodegradable aerogels.
Immunogenicity
Immunogenicity refers to the potential of a substance, referred to as antigen, to provoke an immune response when it enters the body. Although nanocellulose is generally perceived as non-immunogenic, very little knowledge about the immunogenic impact of nanocellulose on the immune cells such as macrophages and dendritic cells is available. The evaluation of immunogenicity is particularly important when nanocellulose-based scaffolds are used for in vivo tissue engineering applications or when these come in direct contact with the blood. A very recent review provides the current-state of knowledge of the immunological aspects of nanocellulose against the immune cells (oli et al., 2020). A study reported that CNCs induce inflammation upon internalization by macrophages; nevertheless, this immunogenic response by the macrophages can be suppressed by introducing special functional groups on nanocellulose. For example, the introduction of carboxyl groups onto the surface of CNFs shifted the tolerogenic potential of dendritic cells toward the induction of regulatory CD8+ T cells. On the other hand, the introduction of phosphonates onto the surface of CNFs enabled the dendritic cells to induce both the regulatory CD8+ T cells and type I regulatory cells (Tomi et al., 2018). These and several other studies show that nanocellulose, especially CNCs, can potentially induce an immunogenic response; nevertheless, this response and its level are highly dependent on the source, preparation methods, morphology and size, agglomeration, presence of contaminants in nanocellulose as well as the type of interacting cells.
Hemocompatibility
Blood compatibility, known as hemocompatibility, refers to the ability of a biomaterial to interact with the blood without causing toxic effects. Hemocompatibility is an important property of biomaterials for the development of blood-contacting artificial organs, such as artificial heart valves (Mohammadi, 2011) and blood vessels (Scherner et al., 2014). The scaffold, which needs to be implanted into the human body, must be hemocompatible as non-hemocompatible scaffolds may cause toxic effects at the site of implantation such as inflammation, provoking an immune response, or infection. The nanocellulose-based scaffolds are generally hemocompatible (Andrade et al., 2011; Leito et al., 2013) and allow proper osteoconduction, integration, and induction (Pang et al., 2020). The hemocompatible nature of BNC was first reported by Andrade et al. (2011), who determined the plasma recalcification time and whole blood clotting of RGD-modified BNC. The findings of this study showed the successful deposition of plasma protein and prevention of platelet adhesion both on pristine and RGD-modified BNC scaffolds, indicting the hemocompatible nature of BNC (Andrade et al., 2011). In another study, Leito et al. (2013) evaluated hemocompatibility of BNC and polyvinyl alcohol (PVA) nanocomposite by determining the whole blood clotting time, plasma recalcification, Factor XII activation, platelet adhesion and activation, and hemolytic index. The findings of the study showed low activation of platelets and Factor XII, indicating the hemocompatible nature of BNC/PVA composite (Leito et al., 2013). In a more recent study, Osorio et al. (2019b) compared the in vitro and in vivo hemocompatibility of 3D BNC scaffold with the 2D BNC architecture. The findings of the study showed antihemolytic and anti-thrombogenic effects and only a mild acute inflammatory response of BNC (Osorio et al., 2019b). The hemocompatible BNC-based scaffolds promote neo-vascularization, gaseous exchange, and diffusion of nutrients and minerals in the newly formed or regenerated bones (Seyednejad et al., 2012; Torgbo and Sukyai, 2018). A study showed that the oral administration of TEMPO-oxidized cellulose nanofibers in mice effectively reduced the concentrations of postprandial glucose-dependent insulinotropic-polypeptide, plasma insulin, triglycerides, and blood glucose, suggesting the occurrence of cellulose-induced significant biological and hemocompatible activities (Lin and Dufresne, 2014).
Mechanical Strength
The cellulose fibers containing strong hydrogen bonding networks and various OH groups offer unique surface properties (Dufresne, 2017; Klemm et al., 2018). The cellulose fibrils contain the crystalline (highly ordered regions) and amorphous (disordered regions) structures (Cheng et al., 2009). The chain molecules at the crystalline regions are packed in such ways that these significantly enhance the stiffness and strength of cellulose, while the amorphous regions confer flexibility (Klemm et al., 2018). Besides, the low density (1.6 g/cm3), lightweight, and other strength properties make nanofibers highly stiff of 220 GPa of elastic modulus (greater than Kevlar fiber) and high tensile strength of 10 GPa (greater than cast iron), and the strength ratio to weight is eight times greater than the stainless steel. The mechanical strength of nanocellulose is further enhanced by developing its composites with mechanically strong reinforcement materials such as ceramics, nanoparticles, and polymers. The nanocellulose-based composites possess excellent mechanical properties due to their transparent nature and lightweight (Abdul Khalil et al., 2012; Abitbol et al., 2016). For example, the soybean-derived nanocellulose impregnated with three different types of synthetic polymers showed excellent mechanical properties, including stiffness and tensile strength as compared to the pristine nanocellulose (Wang and Sain, 2007). In another study, Nogi et al. (2009) studied the fabrication of wood flour-derived nanocellulose-based transparent paper and found a high modulus (13 GPa) and strength (223 MPa) and minimal thermal expansion (8.5 ppm K1) of the optically transparent nanocellulose paper.
Porosity
Porosity is one of the most important properties of biomaterials, specifically for their applications in bone tissue engineering. A porous material promotes the effective release of biofactors such as cells, drugs, nanomaterials, proteins, and others from the scaffold. From the biomedical perspective, porosity is one of the most important features of nanocellulose as it allows the impregnation of different minerals, particles, ceramics, polymer solutions, and viable cells (Li et al., 2021).
Bacterial nanocellulose is porous in nature, and its level of porosity is largely dependent on its synthesis method, chemical composition of the medium, microbial strain, pre- and post-synthesis processing, and drying methods. The porous geometry of BNC provides an ideal environment for the impregnation of a variety of materials into its matrix, including solid particles of different shapes as well as liquid solutions (Shah et al., 2013). The innate porous nature of BNC could be advantageous from the perspective that it would prevent the invasion of microbial cells; however, the small pore size could be a limitation in that it may prevent the impregnation of large size particles and infiltration and migration of mammalian cells (Hutchens et al., 2006). The porous morphology of nanocellulose is also advantageous for the targeted drug delivery, as well as it serves as an efficient physical barrier against external infections (Kowalska-Ludwicka et al., 2013). Although the innate properties of BNC favor its application as a scaffold for bone, cartilage, and connective tissue formation, its small pore size prevents the infiltration of cells deep into its matrix, thus limiting its direct application. The pore size of BNC could be controlled at micro, nano, and mesoscales to meet the desired features for bone tissue engineering application. One such strategy is the introduction of various materials, called porogens (Khan et al., 2016). Examples of porogens include different salts, paraffin particles, ice crystals, gelatin, and different sugars, which are added to the network structure of BNC as the space holders and subsequently removed, leaving behind pores of desired shape and size (Bckdahl et al., 2008). The porogens should be adsorbed only physically and not chemically, whose removal should not alter the fibrous morphology of BNC. The porogens of the desired shape and size are selected according to the size of the cells and the end application of nanocellulose. In one study, Bckdahl et al. (2008) used the potato starch and paraffin wax particles as the porogens for the development of interconnected BNC tubes. They achieved partial particle fusion through heat treatment of paraffin wax particles at specific temperatures. The developed BNC tubes supported the growth of smooth muscle cells inside the pores (Bckdahl et al., 2008). In another study, gelatin was added as porogen to BNC to regenerate 3D microporous regenerated scaffolds. The developed scaffolds supported not only the adhesion and proliferation of cells but also their impregnation deep into the matrix of the scaffold, thus suggesting the development of 3D scaffolds. The developed porous scaffolds also showed considerable in vivo results in the mice model (Khan et al., 2018). In a study, a highly porous nanocomposite of BNC was developed through the incorporation of Fe3O4 and hydroxyapatite (BNC/Fe3O4/HAp) (Torgbo and Sukyai, 2019). The porosity of the nanocomposite was comparable to that of the trabecular/cancellous bone. Moreover, the BNC/Fe3O4/HAp nanocomposite demonstrated high mechanical strength and cytocompatibility, thus demonstrating its ability to promote bone tissue regeneration.
Electric Charge and Wettability
The electric charge and wettability, as well as the charge density of nanocellulose, play an important role in the biotechnological properties of biomaterials for their applications in tissue engineering. Nanocellulose can be desirably modulated by adding different chemical groups, and its structural properties could be tuned to meet the desired features of bone tissues. Generally, moderate wettability modulates the cell growth and adhesion or adsorption (Bacakova et al., 2011), while the charge density modulates the roughness and morphology of nanocellulose films (Ahola et al., 2008) and their interaction with the cells to improve the cell adhesion, growth, and susceptibility for transfection with DNA constructs (Liu et al., 2016).
Applications
Nanocellulose as a Substitute for Bone and Cartilage Regeneration
The repairing of cartilage damage resulting from trauma or degeneration has been a serious clinical concern (Das et al., 2019). The available treatments for small cartilage defect repair include multiple drilling, abrasion arthroplasty, mosaicplasty, and autogenous and allogeneic chondrocyte transplantation (Cao et al., 2014). There are several limitations associated with the use of allografts, such as disease transmission, immune rejection, and slower remodeling. Likewise, autografts have limitations for their requirements of the patient to undergo many surgeries (Baldwin et al., 2019). The rise of tissue engineering that utilizes cells, biodegradable scaffolds, and growth factors provides a new avenue for the repair of articular cartilage (De Witte et al., 2018). In cartilage tissue engineering, scaffolds provide a 3D structure for cartilage cells and support cell adhesion and proliferation (De Witte et al., 2018; Kabir et al., 2020). The scaffolds are further supplemented with different growth factors or antimicrobial agents, which help in encountering microbial and other infections (Ul-Islam et al., 2014). The structure of a cartilage scaffold is required to mimic the native articular cartilage, which is an oriented structure associated with its mechanical function. The oriented ECM-derived scaffolds enhance the biomechanical property of tissue-engineered cartilage, while the oriented poly(lactide-co-glycolide) (PLGA) scaffolds efficiently promote cell migration, thus probably contributes to improving tissue regeneration. The physical and biochemical properties are crucial for the scaffolds during the entire cartilage repair process (Dorati et al., 2017; Kabir et al., 2020).
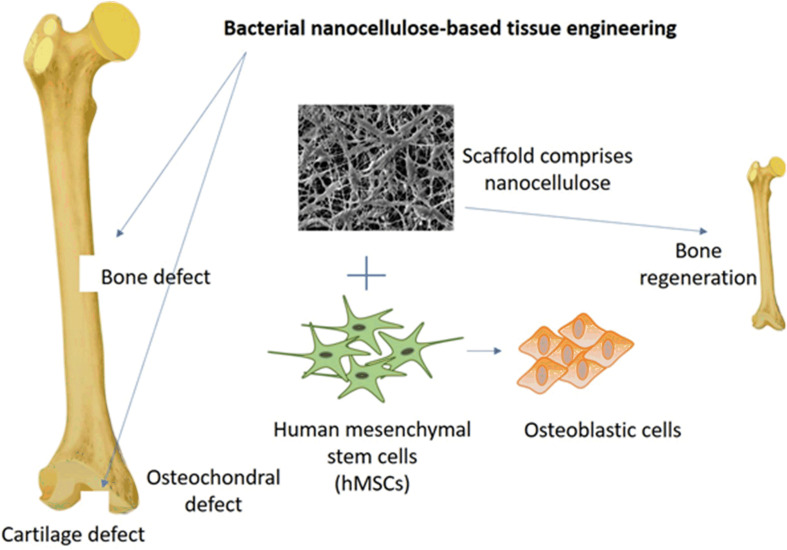
The scaffolds comprised of nanocellulose have a great efficacy for bone and cartilage regeneration and allows various types of cells to adhere and develop into the desired tissues and organs (Figure 1; Halib et al., 2017). A study reported that a membrane comprised of BNC and HAp was used to regenerate bone by significantly enhancing the adhesion and proliferation of osteoblastic cells with elevated bone nodules due to higher activity of phosphatase (Tazi et al., 2012). Studies have also reported the development of BNC and collagen composite. The developed scaffold supported the in vitro adhesion, growth, and migration of cells. Further, the developed scaffolds showed the regeneration of pig meniscus. The in vitro and in vivo studies show the potential use of BNC/collagen scaffolds in meniscus transplantation (Bodin et al., 2007) and artificial cartilage (Lopes et al., 2011) for treating articular joints. In a recent study, BNC was used to design an ear-shaped structure that could be utilized to develop a complete human ear with a specific size and shape (Nimeskern et al., 2013). In vivo evaluation of non-critical bone defects in rat tibiae on BNC membrane showed no inflammatory reactions with defects completely filled by the new bone tissues after 4 weeks (Saska et al., 2011). Similar results were obtained from studies on the bone regeneration effect of BNC membrane on rat skulls, which showed new bone formation on the margin and center of the bone defect after 8 weeks of the implant (Lee et al., 2017b). Another study conducted using healthy male beagle dogs showed healing of implant sites with no evidence of inflammatory reactions and implant failure (Lee et al., 2017a). The evaluation of BNC as a barrier membrane for guided bone regeneration on rat calvarial defect did not induce any inflammation and maintained adequate space for bone regeneration (Lee et al., 2015). Similarly, no foreign body reaction was observed when BNC was grafted to correct the nasal dorsum of rabbits, which showed a positive sign of BNC integration by fragmentation after 6 months (Amorim et al., 2009). Mineralized bone formation was observed both on the outer and inner surface of femoral cortical bone in dogs on day 14 of the implant (Yuan et al., 2006). In a study, BNC was used as a guided tissue regeneration membrane to repair maxillary canine periodontal defects in beagle dogs, which showed enhanced periodontal tissue regeneration and formation of new bone (Zhang et al., 2018a). It could be concluded from the above studies that the different nanocellulose-based scaffolds could be efficiently used in in vivo regeneration of different types of bones, including tibiae, skulls bones, calvarial defected bone, nasal dorsum bone, femoral cortical bone, tendon, and periodontal tissue regeneration through the formation of new bone (Jiang et al., 2020; Zhu et al., 2020). Typical bone regeneration by using BNC-based scaffold as the repair material is illustrated in Figure 1.
FIGURE 1.
Bone regeneration concept conducted by the synergistic effect of hMSCs cells and BNC-based scaffold. The scaffold allowed the differentiation of hMSCs into the osteoblasts.
Nanocellulose as a Matrix for Doping of Minerals and Ceramics
In order to enhance the potency, accessibility, and stability of nanomaterials, such as HAp, silica, calcium carbonate (CaCO3), calcium chloride (CaCl2), and nanoclay, for their application in bone tissue engineering, these are impregnated into the fibrous network or blended with different types of nanocellulose whether these interact chemically with the free OH group or adsorb physically between the fibers. For this purpose, various strategies have been developed, such as in situ addition of nanomaterials to the culture medium (Shah et al., 2013), ex situ vigorous stirring of nanocellulose with the nanoparticles suspension (Zhang et al., 2015), and solvo (hydrothermal) approach where cation adsorption of cellulose is carried out (Shahmohammadi Jebel and Almasi, 2016). The addition of CaCO3 to the bacterial growth culture helps the binding of nano metallic oxides to bind with BNC (Mohammadkazemi et al., 2016). Similarly, the soaking of BNC in an ethanol solution of silicon ethoxide enhanced the uptake of silica nanoparticles and the development of BNC-SiO2 nanocomposite (Barud et al., 2008; Sai et al., 2013). To produce iron oxide containing BNC nanocomposite, the solvothermal technique was applied by autoclaving urea, BNC, and iron nitrate (Wan et al., 2015a, b). The solvothermal technique using ethanol or hot water was used to develop 30 nm sized CdS/BNC nanomaterials (Yang et al., 2012). To develop pure metallic nanoparticles and BNC/metal nanocomposites, two different approaches are used: either readymade metallic nanoparticles are mixed with BNC, or BNC itself is used as the reducing and capping agent for the generation of metallic nanoparticles from metallic salts. This approach has been effectively used for the production of Pd, Au, and Ag nanoparticles (Li et al., 2008; Johnson et al., 2011). BNC can easily cause a reduction of silver or gold salt solutions at 55100C and produce BNC-capped silver and gold nanoparticles with enhanced availability and stability when exploited for variable applications. Although the ex situ impregnation of already prepared metallic nanoparticles into the BNC matrix can be achieved through simple stirring, the in situ uptake by the microbial cells requires chemical or physical treatment such as ultrasonic activation (Cai et al., 2011).
Hydroxyapatite, obtained from various sources, including eggshells and bones of animals or codfish, is considered as the efficient carrier for the nanodrugs due to its biocompatibility and hydrophilic nature and has been extensively used in bone tissue regeneration applications (Wang et al., 2011; Luo et al., 2014; Ramani and Sastry, 2014; Kong et al., 2016; Ullah et al., 2018, 2019a, 2020; Table 1). An earlier study reported the formation of calcium phosphate in the BNC matrix, which supported the adhesion and proliferation of osteoblast cells as well as the differentiation of mesenchymal stem cells into bone cells in the absence of external markers (Fang et al., 2009). A study by Zimmermann et al. (2011) reported the development of calcium-deficient HAp composite, which supported the adhesion of osteoprogenitor cells. Although the surface bioactivity of HAp causes the lack of biofunctionality of the implanted drug (Meagher et al., 2016), several studies have concluded that HAp takes the drug into the bloodstream instead of delivering it to the target site (Kundu et al., 2013; Sun et al., 2018). The drug released in the bloodstream can cause adverse effects and activate the immune cells, which eliminate the drug from the body prior to reaching the target site. Other limitations include low stability, high pH sensitivity, diverse chemical composition, and ionic surface, which may cause alteration in the drug composition or poor delivery of a drug (Huang et al., 2017; Veerla et al., 2019).
The mineralized tissues also contain CaCO3 and CaCl2. Earlier studies reported the deposition of CaCO3 into the BNC matrix by using sodium carbonate and CaCl2 as the reagents and in the presence of microwave irradiation (Stoica-Guzun et al., 2012, 2013). In another study, Saska et al. (2011) developed a BNC composite with CaCl2 and sodium hydrogen phosphate that effectively regenerated a defect in rat tibial bone by completely regenerating the bone within 4 weeks of implantation and did not show any inflammation.
Nanocellulose as a Drug Carrier for Treating Bone-Related Diseases
Various novel approaches have been developed to treat bone-related diseases, among which the use of nanodrugs is receiving great attention due to their broad-spectrum antimicrobial and antitumor activities. For example, the CaCO3, copper, silica, gold, magnesium-oxide, silver, and boron nanoparticles have been reported to effectively treat various bone-related diseases, such as multiple myeloma, rheumatoid arthritis, microbial infections, and osteoporosis (Bari et al., 2017; Hassani Besheli et al., 2017; Qadri et al., 2017; Gisbert-Garzarn et al., 2020; Cmara-Torres et al., 2021). In a study, the administration of gold nanoparticles in the ankle of rats effectively reduced collagen-induced arthritis and inhibited angiogenesis by blocking the key factors, such as vascular endothelial growth factor (VEGF), synovial fluid, and cell proliferation (Tsai et al., 2007). Besides the broad-spectrum therapeutic ability, nanodrugs have some limitations. For example, metallic nanoparticles might activate the immune system and immune responses and lead to the removal of nanodrug by the mononuclear phagocytic system, Kupffer cells, and phagocytic macrophages (Dong et al., 2012). The macrophage and phagocytosis removal of metallic nanoparticles could be avoided if their size is equal to or more than 100 nm and these are hydrophilic in character (Dong et al., 2012).
After injecting in humans, the nanodrugs are often taken up either by the Kupffer cells or hepatocytes. In Kupffer cells, the nanodrugs are degraded, while hepatocytes eliminate them through the hepatic biliary duct. For example, the PLGA nanoparticles can deliver the drugs; however, their retention time is very low in the bloodstream, and the drug is either eliminated by the Kupffer cells or hepatocytes due to their hydrophobic nature (Adjei et al., 2016). Even if the PLGA nanoparticles escape the Kupffer and hepatic cells and are delivered to the target site, these cause acidification in cells after degradation and give rise to the generation of reactive oxygen species (ROS) (Dos Reis et al., 2019). In a study, Yan et al. (2013) investigated the toxic effect of PLGA nanoparticles on the retinal pigment epithelium (RPE) cells and indicated that PLGA nanoparticles caused 20% apoptosis of RPE cells, and the apoptosis frequency reached 50% at higher concentration.
The free OH groups in BNC possess strong hydrogen bonding due to their hydrophilic nature; therefore, these serve as the ideal candidate for interaction with drugs and their controlled delivery (Ptzinger et al., 2017). The encapsulation of nanodrug in BNC can increase the size of nanoparticles and, due to the hydrophilic nature of cellulose, can skip the immune response. Various cell lines, such as HBMEC, bEnd, RAW 264, MCF-10A, MDA-MB-231, MDA-MB-468, KB, PC-3, and C6 were assessed for the cytotoxic effect of nanocellulose, where none of the cell lines were affected after 48 h (Pachuau, 2017). The fluorescein-5-isothiocyanate technique was used to label the nanocellulose to elucidate the site-specific uptake that resulted in minimal imprecise cellular receive, indicating nanocellulose a useful candidate for drug delivery (Dong et al., 2012; Pachuau, 2017). The coating of BNC on tablets using the spray technique enhanced the mechanical properties of tablets with the prolonged drug release (Amin et al., 2012). In a similar study, berberine hydrochloride and berberine sulfate coated with BNC resulted in drug protection with increased drug release duration (Huang et al., 2013). The boding of cell-specific antigen and receptor with nanocellulose and nanodrugs can further enhance the cell-specific uptake ability of the drug (Tan et al., 2019). In a recent study, Li et al. (2018) reported the formation of a sandwiched structure of BNC with polyaniline that showed sustained drug release at varying pH and under electrical stimulation (Li et al., 2018). These studies demonstrate the potential use of different forms of nanocellulose and their composites as drug delivery systems for treating bone-related diseases.
Challenges
Challenges Associated With Nanocellulose Production and Purification
Nanocellulose from plants and algae is not pure and contains impurities like unwanted toxic heavy metals, glucan, endotoxins, lignin, alkaloids, hemicelluloses, and pectin. Therefore, the plant and algal-derived nanocellulose require purification through chemical and physical approaches prior to their use for different applications (Sano et al., 2010; Picheth et al., 2017). The common methods used to extract nanocellulose from a cellulosic material contain several problems, such as the higher amount of acid wastewater generation for the acid hydrolysis, high energy utilization for the mechanical process, and elevated reaction duration for enzymatic hydrolysis (Phanthong et al., 2018; Skiba et al., 2020). As compared to the large-scale production of CNF and CNC, the BNC production is only limited to laboratory-scale due to the high cost of production medium and growth maintenance of BNC-producing microbial strains as well as the low yield (Ul-Islam et al., 2020). Regardless of the development of various bioreactors for BNC production, the highest productivity with aerosol bioreactor is only 0.38 g/(L/) (Lee et al., 2014). Although pristine nanocellulose is non-toxic and non-genotoxic, the chemically modified nanocellulose might cause complications when used for biomedical applications (Chinga-Carrasco, 2018). For example, an earlier study reported that the addition of the dialdehyde group to nanocellulose enhanced the gene expression of tumor necrosis factor (TNF-) and induced inflammation at the target site (Kollar et al., 2011). Moreover, the bacteria-derived nanocellulose might contain contamination of unwanted lipopolysaccharides, which may cause inflammation at the target site (Chinga-Carrasco, 2018).
In vivo Biodegradation of Nanocellulose
Cellulose has excellent mechanical and biocompatible properties; however, it is not degradable or degrades very slowly in animals due to a lack of cellulase enzyme (Lin and Dufresne, 2014); nevertheless, slow degradation of nanocellulose could be advantageous by favoring a continuous drug release, which could be further optimized (Patel et al., 2019). Furthermore, the innate features of nanocellulose such as hydration, swelling, and crystallinity may affect not only the degree of degradation but also the absorption and immune response (Lin and Dufresne, 2014). For example, in canine, cellulose, and cellulose derivatives, the rate of degradation depends mainly on the cellulose chemical derivation and crystalline form. The oxidized cellulose is more vulnerable to hydrolysis and, therefore, could be degraded by the human body (Luo et al., 2013). Mineral acids, such as phosphoric acid, sulfuric acid, and hydrochloric acid, hydrolyze the nanocellulose crystals (Endes et al., 2016). The composites of nanocellulose with metal oxide (Fe2O3, graphene oxide, ZnO, and TiO2) have been utilized for improving the degradation rate (Shak et al., 2018). The degradation of nanocellulose could be enhanced by introducing N-acetylglucosamine residues and by incorporating cellulase enzymes, making it applicable for several medical purposes (Bacakova et al., 2019).
Conclusion and Prospects of Using Nanocellulose in Treating Bone-Related Diseases
Over the past couple of decades, extensive efforts have been devoted to nanocellulose research. The nanocellulose research is primarily focused on exploring low-cost substrates, developing advanced and facile preparation strategies, and exploring new application areas. To date, different forms of nanocellulose, especially BNC, have been extensively explored for various biomedical applications, especially for developing tissue engineering scaffolds. Nanocellulose is not only suitable for the development of soft tissue scaffolds such as skin, neural, and other tissues, it can be effectively utilized for the development of hard tissues such as bone and cartilage when combined with other materials to impart it compatible compression and shape.
Advanced therapeutic strategies for treating bone diseases could be developed by using nanocellulose both as the scaffold and a matrix or carrier. Due to its high mechanical strength and biocompatible nature, nanocellulose could be used as a suitable biomaterial for scaffolding and bone regeneration and development. The deposition of metallic nanoparticles on nanocellulose would further impart antimicrobial activity to the scaffold. Nanocellulose could also be used as an immobilizer for macromolecules, such as DNA, protein, and enzymes. It can also be utilized to enhance the production of secondary metabolites in highly medicinal plants by activating the key genes. The loading of luminescent nanomaterial on nanocellulose could be a novel approach for its use as a fluorescent probe and for photoelectric and photothermal applications. Similarly, nanocellulose could serve as a matrix or carrier for the immobilization of drugs and nanoparticles for avoiding the immune response and for the controlled release of advanced therapeutic molecules and nanomaterials. For targeted delivery of therapeutic molecules/metallic components to treat bone diseases, there must be an appropriate nanocarrier that could be used to encapsulate the therapeutic entities in order to avoid the immune response and early elimination from the body. Certain nanocarriers that protect nanodrugs and deliver to a specific target site encounter several limitations; thus, an alternative approach is required. Nanocellulose could be a suitable nanocarrier for metallic nanoparticles to treat bone diseases. For example, nanocellulose biocompatible and works well as the stabilizing agent for metallic nanoparticles by preventing their agglomeration and maintaining their morphological stability. Nanocellulose can be implanted over metallic nanodrugs through chemical reduction or physical adsorption (electrostatic association). Furthermore, the attachment of osteoblastic antigen on the surface of nanocellulose can enhance the potency of targeted delivery of metallic nanodrugs and can become a novel approach to treat damaged bone or bone-related diseases. Similarly, a controlled drug release is an important phenomenon that gains high importance in cases where the timely release of drug is required. For instance, an anticancer drug bendamustine requires controlled release after oral delivery; therefore, nanocellulose can be helpful to regulate the drug release inside the body. Although nanocellulose is largely considered to be used for conventional FDA approved drugs, it can also be used for the delivery of more advanced therapeutic entities such as siRNAs. The siRNAs-based therapies are considered very effective; however, their poor stability in the biological environment is a serious concern. Therefore, nanocellulose-based materials can be used to provide protection from degradation and enhancing cellular uptake.
Although the above discussion justifies the importance and potential use of nanocellulose in treating bone-related diseases, further investigation on cell-scaffold interaction, in vivo degradation, and resorption of degradation products is required. Moreover, immunological analysis of nanocellulose-based scaffolds is required for a complete understanding of their effects on both innate and adaptive immunity. To this end, a detailed investigation on the identification of molecular mechanisms present in the immune system for recognition of nanocellulose and the activation of associated downstream signaling pathways will pave the way to rationally develop biologically safe nanocellulose-based scaffolds for clinical applications.
Author Contributions
SK and RS wrote the original draft. SK, DH, MAS, GN, QB, SM, and MWU revised and modified the manuscript. MX, MWU, and HB supervised and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the China Postdoctoral Science Foundation (grant nos. 2020M672291 and 2016M602291), the operating grant support from the National Natural Science Foundation of China (grant nos. 81870942, 81471174, and 81520108011), Key R&D and promotion projects (Science and Technology Project) from Henan Science and Technology Department, No: 212102310127, and Henan Middle-aged Youth Health Technology Innovation Talent Project, No: YXKC2020059.
References
- Abdul Khalil H. P. S., Bhat A. H., Ireana Yusra A. F. (2012). Green composites from sustainable cellulose nanofibrils: a review. Carbohydr. Polym. 87 963–979.. 10.1016/j.carbpol.2011.08.078 [DOI] [Google Scholar]
- Abitbol T., Rivkin A., Cao Y., Nevo Y., Abraham E., Ben-Shalom T., et al. (2016). Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 39 76–88.. 10.1016/j.copbio.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Adewuyi A., Otuechere C. A., Adebayo O. L., Anazodo C., Pereira F. V. (2018). Renal toxicological evaluations of sulphonated nanocellulose from Khaya sengalensis seed in Wistar rats. Chem. Biol. Interact. 284 56–68.. 10.1016/j.cbi.2018.02.015 [DOI] [PubMed] [Google Scholar]
- Adjei I. M., Sharma B., Peetla C., Labhasetwar V. (2016). Inhibition of bone loss with surface-modulated, drug-loaded nanoparticles in an intraosseous model of prostate cancer. J. Control. Release 232 83–92.. 10.1016/j.jconrel.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahola S., Salmi J., Johansson L. S., Laine J., sterberg M. (2008). Model films from native cellulose nanofibrils. preparation, swelling, and surface interactions. Biomacromolecules 9 1273–1282.. 10.1021/bm701317k [DOI] [PubMed] [Google Scholar]
- Ahrem H., Pretzel D., Endres M., Conrad D., Courseau J., Mller H., et al. (2014). Laser-structured bacterial nanocellulose hydrogels support ingrowth and differentiation of chondrocytes and show potential as cartilage implants. Acta Biomater. 10 1341–1353.. 10.1016/j.actbio.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Aki D., Ulag S., Unal S., Sengor M., Ekren N., Lin C. C., et al. (2020). 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater. Des. 196:109094. 10.1016/j.matdes.2020.109094 [DOI] [Google Scholar]
- Amin M. C. I. M., Abadi A. G., Ahmad N., Katas H., Jamal J. A. (2012). Bacterial cellulose film coating as drug delivery system: physicochemical, thermal and drug release properties. Sains Malaysiana 41 561–568.. [Google Scholar]
- Amorim W. L., Costa H. O., De Souza F. C., De Castro M. G., Da Silva L. (2009). Experimental study of the tissue reaction caused by the presence of cellulose produced. Braz. J. Otorhinolaryngol. 75 200–207.. 10.1016/S1808-8694(15)30779-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade F. K., Silva J. P., Carvalho M., Castanheira E. M. S., Soares R., Gama M. (2011). Studies on the hemocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 98A 554–566.. 10.1002/jbm.a.33148 [DOI] [PubMed] [Google Scholar]
- Andriani D., Apriyana A. Y., Karina M. (2020). The optimization of bacterial cellulose production and its applications: a review. Cellulose 27 6747–6766.. 10.1007/s10570-020-03273-3279 [DOI] [Google Scholar]
- Bacakova L., Filova E., Parizek M., Ruml T., Svorcik V. (2011). Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 29 739–767.. 10.1016/j.biotechadv.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Bacakova L., Pajorova J., Bacakova M., Skogberg A., Kallio P., Kolarova K., et al. (2019). Versatile application of nanocellulose: from industry to skin tissue engineering and wound healing. Nanomaterials 9:164. 10.3390/nano9020164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bckdahl H., Esguerra M., Delbro D., Risberg B., Gatenholm P. (2008). Engineering microporosity in bacterial cellulose scaffolds. J. Tissue Eng. Regen. Med. 2 320–330.. 10.1002/term.97 [DOI] [PubMed] [Google Scholar]
- Baldwin P., Li D. J., Auston D. A., Mir H. S., Yoon R. S., Koval K. J. (2019). Autograft, allograft, and bone graft substitutes: clinical evidence and indications for use in the setting of orthopaedic trauma surgery. J. Orthop. Trauma 33 203–213.. 10.1097/BOT.0000000000001420 [DOI] [PubMed] [Google Scholar]
- Bari A., Bloise N., Fiorilli S., Novajra G., Vallet-Reg M., Bruni G., et al. (2017). Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 55 493–504.. 10.1016/j.actbio.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Barud H. S., Assuno R. M. N., Martines M. A. U., Dexpert-Ghys J., Marques R. F. C., Messaddeq Y., et al. (2008). Bacterial cellulosesilica organicinorganic hybrids. J. Sol-Gel Sci. Technol. 46 363–367.. 10.1007/s10971-007-1669-1669 [DOI] [Google Scholar]
- Basu P., Saha N., Alexandrova R., Andonova-Lilova B., Georgieva M., Miloshev G., et al. (2018). Biocompatibility and biological efficiency of inorganic calcium filled bacterial cellulose based hydrogel scaffolds for bone bioengineering. Int. J. Mol. Sci. 19:3980. 10.3390/ijms19123980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin A., Concaro S., Brittberg M., Gatenholm P. (2007). Bacterial cellulose as a potential meniscus implant. J. Tissue Eng. Regen. Med. 1 406–408.. 10.1002/term.51 [DOI] [PubMed] [Google Scholar]
- Cai Z., Hou C., Yang G., Kim J. (2011). Bacterial cellulose as a template for the formation of polymer/nanoparticle nanocomposite. J. Nanotechnol. Eng. Med. 2:031006. 10.1115/1.4004361 [DOI] [Google Scholar]
- Cai Z., Kim J. (2010). Preparation and characterization of novel bacterial cellulose/gelatin scaffold for tissue regeneration using bacterial cellulose hydrogel. J. Nanotechnol. Eng. Med. 1:021002. 10.1115/1.4000858 [DOI] [Google Scholar]
- Cmara-Torres M., Duarte S., Sinha R., Egizabal A., lvarez N., Bastianini M., et al. (2021). 3D additive manufactured composite scaffolds with antibiotic-loaded lamellar fillers for bone infection prevention and tissue regeneration. Bioact. Mater. 6 1073–1082.. 10.1016/j.bioactmat.2020.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero-Espinosa S., Endes C., Mueller S., Petri-Fink A., Rothen-Rutishauser B., Weder C., et al. (2016). Elucidating the potential biological impact of cellulose nanocrystals. Fibers 4:21. 10.3390/fib4030021 [DOI] [Google Scholar]
- Cao Z., Dou C., Dong S. (2014). Scaffolding biomaterials for cartilage regeneration. J. Nanomater. 2014 1–8.. 10.1155/2014/489128 [DOI] [Google Scholar]
- Carina V., Costa V., Sartori M., Bellavia D., Luca A., De, et al. (2019). Adjuvant biophysical therapies in osteosarcoma. Cancers (Basel) 11:348. 10.3390/cancers11030348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataln J., Ilves M., Jrventaus H., Hannukainen K. S., Kontturi E., Vanhala E., et al. (2015). Genotoxic and immunotoxic effects of cellulose nanocrystals in vitro. Environ. Mol. Mutagen. 56 171–182.. 10.1002/em.21913 [DOI] [PubMed] [Google Scholar]
- Cavicchioli M., Corso C. T., Coelho F., Mendes L., Saska S., Soares C. P., et al. (2015). Characterization and cytotoxic, genotoxic and mutagenic evaluations of bacterial cellulose membranes incorporated with ciprofloxacin: a potential material for use as therapeutic contact lens. World J. Pharm. Pharm. Sci. 4 1626–1647.. [Google Scholar]
- Cheng Q., Wang S., Harper D. P. (2009). Effects of process and source on elastic modulus of single cellulose fibrils evaluated by atomic force microscopy. Compos. Part A Appl. Sci. Manuf. 40 583–588.. 10.1016/j.compositesa.2009.02.011 [DOI] [Google Scholar]
- Chinga-Carrasco G. (2018). Potential and limitations of nanocelluloses as components in biocomposite inks for three-dimensional bioprinting and for biomedical devices. Biomacromolecules 19 701–711.. 10.1021/acs.biomac.8b00053 [DOI] [PubMed] [Google Scholar]
- oli M., Tomi S., Beki M. (2020). Immunological aspects of nanocellulose. Immunol. Lett. 222 80–89.. 10.1016/j.imlet.2020.04.004 [DOI] [PubMed] [Google Scholar]
- Curvello R., Raghuwanshi V. S., Garnier G. (2019). Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 267 47–61.. 10.1016/j.cis.2019.03.002 [DOI] [PubMed] [Google Scholar]
- Das P., Singh Y. P., Joardar S. N., Biswas B. K., Bhattacharya R., Nandi S. K., et al. (2019). Decellularized caprine conchal cartilage toward repair and regeneration of damaged cartilage. ACS Appl. Bio Mater. 5 2037–2049.. 10.1021/acsabm.9b00078 [DOI] [PubMed] [Google Scholar]
- de Amorim J. D. P., de Souza K. C., Duarte C. R., da Silva, Duarte I., de Assis Sales, et al. (2020). Plant and bacterial nanocellulose: production, properties and applications in medicine, food, cosmetics, electronics and engineering. a review. Environ. Chem. Lett. 18 851–869.. 10.1007/s10311-020-00989-989 [DOI] [Google Scholar]
- de Lima R., Feitosa L. O., Maruyama C. R., Barga M. A., Yamawaki P. C., Vieira I. J., et al. (2012). Evaluation of the genotoxicity of cellulose nanofibers. Int. J. Nanomed. 7 3555–3565.. 10.2147/IJN.S30596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witte T. M., Fratila-Apachitei L. E., Zadpoor A. A., Peppas N. A. (2018). Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen. Biomater. 5 197–211.. 10.1093/rb/rby013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMello J. A. (2012). Bacterial Cellulose Templates for Nano-Hydroxyapatite Fibre Synthesis. Ph.D. Thesis, London: The University of Western Ontario. [Google Scholar]
- Derakhshanfar S., Mbeleck R., Xu K., Zhang X., Zhong W., Xing M. (2018). 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact. Mater. 3 144–156.. 10.1016/j.bioactmat.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Z., Shi Z., Ullah M. W., Li S., Yang G. (2017). A transparent wound dressing based on bacterial cellulose whisker and poly(2-hydroxyethyl methacrylate). Int. J. Biol. Macromol. 105 638–644.. 10.1016/j.ijbiomac.2017.07.075 [DOI] [PubMed] [Google Scholar]
- Dong S., Hirani A. A., Colacino K. R., Lee Y. W., Roman M. (2012). Cytotoxicity and cellular uptake of cellulose nanocrystals. Nano Life 2:1241006. 10.1142/s1793984412410061 [DOI] [Google Scholar]
- Dorati R., DeTrizio A., Modena T., Conti B., Benazzo F., Gastaldi G., et al. (2017). Biodegradable scaffolds for bone regeneration combined with drug-delivery systems in osteomyelitis therapy. Pharmaceuticals 10:96. 10.3390/ph10040096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorozhkin S. V. (2013). Calcium orthophosphate-based bioceramics. Materials (Basel) 6 3840–3942.. 10.3390/ma6093840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Reis L. G., Lee W. H., Svolos M., Moir L. M., Jaber R., Windhab N., et al. (2019). Nanotoxicologic effects of PLGA nanoparticles formulated with a cell-penetrating peptide: searching for a safe pDNA delivery system for the lungs. Pharmaceutics 11:12. 10.3390/pharmaceutics11010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey S., Mishra R., Roy P., Singh R. P. (2020). 3-D macro/microporous-nanofibrous bacterial cellulose scaffolds seeded with BMP-2 preconditioned mesenchymal stem cells exhibit remarkable potential for bone tissue engineering. Int. J. Biol. Macromol. 167 934–946.. 10.1016/j.ijbiomac.2020.11.049 [DOI] [PubMed] [Google Scholar]
- Dufresne A. (2013). Nanocellulose: a new ageless bionanomaterial. Mater. Today 16 220–227.. 10.1016/j.mattod.2013.06.004 [DOI] [Google Scholar]
- Dufresne A. (2017). Nanocellulose: From Nature to High Performance Tailored Materials, 2. Edn. Berlin: De gruyter. 10.1515/9783110480412 [DOI] [Google Scholar]
- Endes C., Camarero-Espinosa S., Mueller S., Foster E. J., Petri-Fink A., Rothen-Rutishauser B., et al. (2016). A critical review of the current knowledge regarding the biological impact of nanocellulose. J. Nanobiotechnol. 14:78. 10.1186/s12951-016-0230-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslahi N., Mahmoodi A., Mahmoudi N., Zandi N., Simchi A. (2020). Processing and properties of nanofibrous bacterial cellulose-containing polymer composites: a review of recent advances for biomedical applications. Polym. Rev. 60 144–170.. 10.1080/15583724.2019.1663210 [DOI] [Google Scholar]
- Eyley S., Thielemans W. (2014). Surface modification of cellulose nanocrystals. Nanoscale 65 45–55.. 10.1039/c4nr01756k [DOI] [PubMed] [Google Scholar]
- Fan Q. G., Lewis D. M., Tapley K. N. (2001). Characterization of cellulose aldehyde using fourier transform infrared spectroscopy. J. Appl. Polym. Sci. 82 1195–1202.. 10.1002/app.1953 [DOI] [Google Scholar]
- Fang B., Wan Y.-Z., Tang T.-T., Gao C., Dai K.-R. (2009). Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. Part A 15 1091–1098.. 10.1089/ten.tea.2008.0110 [DOI] [PubMed] [Google Scholar]
- Faradilla R. H. F., Lee G., Arns J. Y., Roberts J., Martens P., Stenzel M. H., et al. (2017). Characteristics of a free-standing film from banana pseudostem nanocellulose generated from TEMPO-mediated oxidation. Carbohydr. Polym. 174 1156–1163.. 10.1016/j.carbpol.2017.07.025 [DOI] [PubMed] [Google Scholar]
- Farcas M. T., Kisin E. R., Menas A. L., Gutkin D. W., Star A., Reiner R. S., et al. (2016). Pulmonary exposure to cellulose nanocrystals caused deleterious effects to reproductive system in male mice. J. Toxicol. Environ. Heal. - Part A Curr. Issues. 79 984–997.. 10.1080/15287394.2016.1211045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq U., Ullah M. W., Yang Q., Aziz A., Xu J., Zhou L., et al. (2020). High-density phage particles immobilization in surface-modified bacterial cellulose for ultra-sensitive and selective electrochemical detection of Staphylococcus aureus. Biosens. Bioelectron. 157:112163. 10.1016/j.bios.2020.112163 [DOI] [PubMed] [Google Scholar]
- Fatima A., Yasir S., Khan M. S., Manan S., Ullah M. W., Ul-Islam M. (2021). Plant extract-loaded bacterial cellulose composite membrane for potential biomedical applications. J. Bioresour. Bioprod. 6 26–32.. 10.1016/j.jobab.2020.11.002 [DOI] [Google Scholar]
- Favi P. M. (2014). Engineering Bacterial Cellulose Scaffold and its Biomimetic Composites for Bone and Cartilage Tissue Regeneration. PhD disseration, Knoxville, TE: University of Tennessee. [Google Scholar]
- Frsatz M., Skog M., Sivlr P., Palm E., Aronsson C., Skallberg A., et al. (2018). Functionalization of bacterial cellulose wound dressings with the antimicrobial peptide -poly-L-Lysine. Biomed. Mater. 13:025014. 10.1088/1748-605X/aa9486 [DOI] [PubMed] [Google Scholar]
- Gisbert-Garzarn M., Manzano M., Vallet-Reg M. (2020). Mesoporous silica nanoparticles for the treatment of complex bone diseases: bone cancer, bone infection and osteoporosis. Pharmaceutics 12:83. 10.3390/pharmaceutics12010083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W., Wu C., Chen J., Xiao Y. (2013). Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int. J. Nanomed. 8 2305–2317.. 10.2147/IJN.S44393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi Y., Lucia L. A., Rojas O. J. (2010). Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem. Rev. 110 3479–3500.. 10.1021/cr900339w [DOI] [PubMed] [Google Scholar]
- Hakkarainen T., Koivuniemi R., Kosonen M., Escobedo-Lucea C., Sanz-Garcia A., Vuola J., et al. (2016). Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 244(Pt B), 292–301.. 10.1016/j.jconrel.2016.07.053 [DOI] [PubMed] [Google Scholar]
- Halib N., Perrone F., Cemazar M., Dapas B., Farra R., Abrami M., et al. (2017). Potential applications of nanocellulose-containing materials in the biomedical field. Materials (Basel) 10:977. 10.3390/ma10080977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani Besheli N., Mottaghitalab F., Eslami M., Gholami M., Kundu S. C., Kaplan D. L., et al. (2017). Sustainable release of vancomycin from silk fibroin nanoparticles for treating severe bone infection in rat tibia osteomyelitis model. ACS Appl. Mater. Interfaces 9 5128–5138.. 10.1021/acsami.6b14912 [DOI] [PubMed] [Google Scholar]
- Helenius G., Bckdahl H., Bodin A., Nannmark U., Gatenholm P., Risberg B. (2006). In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. - Part A 76 431–438.. 10.1002/jbm.a.30570 [DOI] [PubMed] [Google Scholar]
- Hosseinidoust Z., Alam M. N., Sim G., Tufenkji N., Van De Ven T. G. M. (2015). Cellulose nanocrystals with tunable surface charge for nanomedicine. Nanoscale 7 16647–16657.. 10.1039/c5nr02506k [DOI] [PubMed] [Google Scholar]
- Hua K., Rocha I., Zhang P., Gustafsson S., Ning Y., Strmme M., et al. (2016). Transition from bioinert to bioactive material by tailoring the biological cell response to carboxylated nanocellulose. Biomacromolecules 17 1224–1233.. 10.1021/acs.biomac.6b00053 [DOI] [PubMed] [Google Scholar]
- Huang J. W., Lv X. G., Li Z., Song L. J., Feng C., Xie M. K., et al. (2015). Urethral reconstruction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model. Biomed. Mater. 10:055005. 10.1088/1748-6041/10/5/055005 [DOI] [PubMed] [Google Scholar]
- Huang L., Chen X., Nguyen T. X., Tang H., Zhang L., Yang G. (2013). Nano-cellulose 3D-networks as controlled-release drug carriers. J. Mater. Chem. B 1 2976–2984.. 10.1039/c3tb20149j [DOI] [PubMed] [Google Scholar]
- Huang Y., Wang J., Yang F., Shao Y., Zhang X., Dai K. (2017). Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C. 75 1034–1041.. 10.1016/j.msec.2017.02.174 [DOI] [PubMed] [Google Scholar]
- Hutchens S. A., Benson R. S., Evans B. R., ONeill H. M., Rawn C. J. (2006). Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 27 4661–4670.. 10.1016/j.biomaterials.2006.04.032 [DOI] [PubMed] [Google Scholar]
- Islam M. S., Chen L., Sisler J., Tam K. C. (2018). Cellulose nanocrystal (CNC)-inorganic hybrid systems: synthesis, properties and applications. J. Mater. Chem. B. 6 864–883.. 10.1039/c7tb03016a [DOI] [PubMed] [Google Scholar]
- Islam M. U., Khan S., Khattak W. A., Park J. K. (2015). Synthesis, chemistry, and medical application of bacterial cellulose nanocomposite, in Eco-friendly Polymer Nanocomposites. Advanced Structured Materials, Vol. 74 eds Thakur V., Thakur M. (New Delhi: Springer; ). [Google Scholar]
- Isogai A., Saito T., Fukuzumi H. (2011). TEMPO-oxidized cellulose nanofibers. Nanoscale 3 71–85.. 10.1039/c0nr00583e [DOI] [PubMed] [Google Scholar]
- Jasim A., Ullah M. W., Shi Z., Lin X., Yang G. (2017). Fabrication of bacterial cellulose/polyaniline/single-walled carbon nanotubes membrane for potential application as biosensor. Carbohydr. Polym. 163 62–69.. 10.1016/j.carbpol.2017.01.056 [DOI] [PubMed] [Google Scholar]
- Jasmani L., Adnan S. (2017). Preparation and characterization of nanocrystalline cellulose from Acacia mangium and its reinforcement potential. Carbohydr. Polym. 161 166–171.. 10.1016/j.carbpol.2016.12.061 [DOI] [PubMed] [Google Scholar]
- Jiang X., Wu S., Kuss M., Kong Y., Shi W., Streubel P. N., et al. (2020). 3D printing of multilayered scaffolds for rotator cuff tendon regeneration. Bioact. Mater. 5 636–643.. 10.1016/j.bioactmat.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing W., Chunxi Y., Yizao W., Honglin L., Fang H., Kerong D., et al. (2013). Laser patterning of bacterial cellulose hydrogel and its modification with gelatin and hydroxyapatite for bone tissue engineering. Soft Mater. 11 173–180.. 10.1080/1539445X.2011.611204 [DOI] [Google Scholar]
- Jinga S. I., Voicu G., Stoica-Guzun A., Stroescu M., Grumezescu A. M., Bleotu C. (2014). Biocellulose nanowhiskers cement composites for endodontic use. Dig. J. Nanomater. Biostructures. 9 543–550.. [Google Scholar]
- Johnson L., Thielemans W., Walsh D. A. (2011). Synthesis of carbon-supported Pt nanoparticle electrocatalysts using nanocrystalline cellulose as reducing agent. Green Chem. 13 1686–1693.. 10.1039/c0gc00881h [DOI] [Google Scholar]
- Kabir W., Di Bella C., Jo I., Gould D., Choong P. F. M. (2020). Human stem cell based tissue engineering for in vivo cartilage repair: a systematic review. Tissue Eng. Part B Rev. 27 74–93.. 10.1089/ten.teb.2020.0155 [DOI] [PubMed] [Google Scholar]
- Kaminagakura K. L. N., Sue Sato S., Sugino P., Kataki, de Oliveira Veloso L., dos Santos D. C., et al. (2019). Nanoskin to treat full thickness skin wounds. J. Biomed. Mater. Res. - Part B Appl. Biomater. 107 724–732.. 10.1002/jbm.b.34166 [DOI] [PubMed] [Google Scholar]
- Khan A., Wang B., Ni Y. (2020a). Chitosan-Nanocellulose composites for regenerative medicine applications. Curr. Med. Chem. 27 4584–4592.. 10.2174/0929867327666200127152834 [DOI] [PubMed] [Google Scholar]
- Khan H., Kadam A., Dutt D. (2020b). Studies on bacterial cellulose produced by a novel strain of Lactobacillus genus. Carbohydr. Polym. 229:115513. 10.1016/j.carbpol.2019.115513 [DOI] [PubMed] [Google Scholar]
- Khan S., Ul-Islam M., Ikram M., Islam S. U., Ullah M. W., Israr M., et al. (2018). Preparation and structural characterization of surface modified microporous bacterial cellulose scaffolds: a potential material for skin regeneration applications in vitro and in vivo. Int. J. Biol. Macromol. 117 1200–1210.. 10.1016/j.ijbiomac.2018.06.044 [DOI] [PubMed] [Google Scholar]
- Khan S., Ul-Islam M., Ikram M., Ullah M. W., Israr M., Subhan F., et al. (2016). Three-dimensionally microporous and highly biocompatible bacterial cellulose-gelatin composite scaffolds for tissue engineering applications. RSC Adv. 6 110840–110849.. 10.1039/C6RA18847H [DOI] [Google Scholar]
- Khan S., Ul-Islam M., Khattak W. A., Ullah M. W., Park J. K. (2015). Bacterial cellulose-titanium dioxide nanocomposites: nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 22 565–579.. 10.1007/s10570-014-0528-524 [DOI] [Google Scholar]
- Khan S., Ullah M. W., Siddique R., Liu Y., Ullah I., Xue M., et al. (2019). Catechins-modified selenium-doped hydroxyapatite nanomaterials for improved osteosarcoma therapy through generation of reactive oxygen species. Front. Oncol. 9:499. 10.3389/fonc.2019.00499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak W. A., Ullah M. W., Ul-Islam M., Khan S., Kim M., Kim Y., et al. (2014). Developmental strategies and regulation of cell-free enzyme system for ethanol production: a molecular prospective. Appl. Microbiol. Biotechnol. 98 9561–9578.. 10.1007/s00253-014-6154-6150 [DOI] [PubMed] [Google Scholar]
- Kim J., Kim S. W., Park S., Lim K. T., Seonwoo H., Kim Y., et al. (2013). Bacterial cellulose nanofibrillar patch as a wound healing platform of tympanic membrane perforation. Adv. Healthc. Mater. 2 1525–1531.. 10.1002/adhm.201200368 [DOI] [PubMed] [Google Scholar]
- Kim Y., Ullah M. W., Ul-Islam M., Khan S., Jang J. H., Park J. K. (2019). Self-assembly of bio-cellulose nanofibrils through intermediate phase in a cell-free enzyme system. Biochem. Eng. J. 142 135–144.. 10.1016/j.bej.2018.11.017 [DOI] [Google Scholar]
- Klemm D., Cranston E. D., Fischer D., Gama M., Kedzior S. A., Kralisch D., et al. (2018). Nanocellulose as a natural source for groundbreaking applications in materials science: todays state. Mater. Today 21 720–748.. 10.1016/j.mattod.2018.02.001 [DOI] [Google Scholar]
- Klemm D., Schumann D., Udhardt U., Marsch S. (2001). Bacterial synthesized cellulose - artificial blood vessels for microsurgery. Prog. Polym. Sci. 26 1561–1603.. 10.1016/S0079-6700(01)00021-21 [DOI] [Google Scholar]
- Klinthoopthamrong N., Chaikiawkeaw D., Phoolcharoen W., Rattanapisit K., Kaewpungsup P., Pavasant P., et al. (2020). Bacterial cellulose membrane conjugated with plant-derived osteopontin: preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 149 51–59.. 10.1016/j.ijbiomac.2020.01.158 [DOI] [PubMed] [Google Scholar]
- Kollar P., Zvalov V., Hoek J., Havelka P., Sopuch T., Karpek M., et al. (2011). Cytotoxicity and effects on inflammatory response of modified types of cellulose in macrophage-like THP-1 cells. Int. Immunopharmacol. 11 997–1001.. 10.1016/j.intimp.2011.02.016 [DOI] [PubMed] [Google Scholar]
- Kong L., Mu Z., Yu Y., Zhang L., Hu J. (2016). Polyethyleneimine-stabilized hydroxyapatite nanoparticles modified with hyaluronic acid for targeted drug delivery. RSC Adv. 6 101790–101799.. 10.1039/c6ra19351j [DOI] [Google Scholar]
- Kovacs T., Naish V., OConnor B., Blaise C., Gagn F., Hall L., et al. (2010). An ecotoxicological characterization of nanocrystalline cellulose (NCC). Nanotoxicology 4 255–270.. 10.3109/17435391003628713 [DOI] [PubMed] [Google Scholar]
- Kowalska-Ludwicka K., Cala J., Grobelski B., Sygut D., Jesionek-Kupnicka D., Kolodziejczyk M., et al. (2013). Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 9 527–534.. 10.5114/aoms.2013.33433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu B., Ghosh D., Sinha M. K., Sen P. S., Balla V. K., Das N., et al. (2013). Doxorubicin-intercalated nano-hydroxyapatite drug-delivery system for liver cancer: an animal model. Ceram. Int. 39 9557–9566.. 10.1016/j.ceramint.2013.05.074 [DOI] [Google Scholar]
- Lamboni L., Xu C., Clasohm J., Yang J., Saumer M., Schfer K. H., et al. (2019). Silk sericin-enhanced microstructured bacterial cellulose as tissue engineering scaffold towards prospective gut repair. Mater. Sci. Eng. C 102 502–510.. 10.1016/j.msec.2019.04.043 [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Buldum G., Mantalaris A., Bismarck A. (2014). More than meets the eye in bacterial cellulose: biosynthesis, bioprocessing, and applications in advanced fiber composites. Macromol. Biosci. 14 10–32.. 10.1002/mabi.201300298 [DOI] [PubMed] [Google Scholar]
- Li Z., Friedrich A., Taubert A. (2008). Gold microcrystal synthesis via reduction of HAuCl4 by cellulose in the ionic liquid 1-butyl-3-methyl imidazolium chloride. J. Mater. Chem. 18 1008–1014.. 10.1039/b716135m [DOI] [Google Scholar]
- Lee S. H., An S. J., Lim Y. M., Huh J. B. (2017a). The efficacy of electron beam irradiated bacterial cellulose membranes as compared with collagen membranes on guided bone regeneration in peri-implant bone defects. Materials (Basel) 10:1018. 10.3390/ma10091018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J., An S. J., Bae E., Bin, Gwon H. J., Park J. S., et al. (2017b). The effect of thickness of resorbable bacterial cellulose membrane on guided bone regeneration. Materials (Basel) 10:320. 10.3390/ma10030320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Lim Y. M., Jeong S. I., An S. J., Kang S. S., Jeong C. M., et al. (2015). The effect of bacterial cellulose membrane compared with collagen membrane on guided bone regeneration. J. Adv. Prosthodont. 7 484–495.. 10.4047/jap.2015.7.6.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leito A. F., Gupta S., Silva J. P., Reviakine I., Gama M. (2013). Hemocompatibility study of a bacterial cellulose/polyvinyl alcohol nanocomposite. Colloids Surfaces B Biointerfaces 111 493–502.. 10.1016/j.colsurfb.2013.06.031 [DOI] [PubMed] [Google Scholar]
- Li J., Wan Y., Li L., Liang H., Wang J. (2009). Preparation and characterization of 2,3-dialdehyde bacterial cellulose for potential biodegradable tissue engineering scaffolds. Mater. Sci. Eng. C 29 1635–1642.. 10.1016/j.msec.2009.01.006 [DOI] [Google Scholar]
- Li S., Jasim A., Zhao W., Fu L., Ullah M. W., Shi Z., et al. (2018). Fabrication of pH-electroactive bacterial cellulose/polyaniline hydrogel for the development of a controlled drug release system. ES Mater. Manuf. 1 41–49.. 10.30919/esmm5f120 [DOI] [Google Scholar]
- Li Z., Du T., Ruan C., Niu X. (2021). Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 6 1491–1511.. 10.1016/j.bioactmat.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N., Dufresne A. (2014). Nanocellulose in biomedicine: current status and future prospect. Eur. Polym. J. 59 302–325.. 10.1016/j.eurpolymj.2014.07.025 [DOI] [Google Scholar]
- Lin S. P., Loira Calvar I., Catchmark J. M., Liu J. R., Demirci A., Cheng K. C. (2013). Biosynthesis, production and applications of bacterial cellulose. Cellulose 20 2191–2219.. 10.1007/s10570-013-9994-9993 [DOI] [Google Scholar]
- Liu J., Cheng F., Grnman H., Spoljaric S., Seppl J., Eriksson J. E., et al. (2016). Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 148 259–271.. 10.1016/j.carbpol.2016.04.064 [DOI] [PubMed] [Google Scholar]
- Liu J., Willfr S., Mihranyan A. (2017). On importance of impurities, potential leachables and extractables in algal nanocellulose for biomedical use. Carbohydr. Polym. 172 11–19.. 10.1016/j.carbpol.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Lopes J. L., Machado J. M., Castanheira L., Granja P. L., Gama F. M., Dourado F., et al. (2011). Friction and wear behaviour of bacterial cellulose against articular cartilage. Wear 271 2328–2333.. 10.1016/j.wear.2010.12.042 [DOI] [Google Scholar]
- Luo H., Cha R., Li J., Hao W., Zhang Y., Zhou F. (2019). Advances in tissue engineering of nanocellulose-based scaffolds: a review. Carbohydr. Polym. 224:115144. 10.1016/j.carbpol.2019.115144 [DOI] [PubMed] [Google Scholar]
- Luo H., Xiong G., Hu D., Ren K., Yao F., Zhu Y., et al. (2013). Characterization of TEMPO-oxidized bacterial cellulose scaffolds for tissue engineering applications. Mater. Chem. Phys. 143 373–379.. 10.1016/j.matchemphys.2013.09.012 [DOI] [Google Scholar]
- Luo H., Zhang J., Xiong G., Wan Y. (2014). Evolution of morphology of bacterial cellulose scaffolds during early culture. Carbohydr. Polym. 111 722–728.. 10.1016/j.carbpol.2014.04.097 [DOI] [PubMed] [Google Scholar]
- Luz E. P. C. G., das Chagas B. S., de Almeida N. T., de Ftima Borges M., Andrade F. K., Muniz C. R., et al. (2020). Resorbable bacterial cellulose membranes with strontium release for guided bone regeneration. Mater Sci Eng C Mater Biol Appl. 116:111175. 10.1016/j.msec.2020.111175 [DOI] [PubMed] [Google Scholar]
- Matharu A. S., de Melo E. M., Remn J., Wang S., Abdulina A., Kontturi E. (2018). Processing of citrus nanostructured cellulose: a rigorous design-of-experiment study of the hydrothermal microwave-assisted selective scissoring process. ChemSusChem 11 1344–1353.. 10.1002/cssc.201702456 [DOI] [PubMed] [Google Scholar]
- Mazhar U.-I., Yasir S., Mombasawala L., Manan S., Wajid Ullah M. (2021). Bacterial cellulose: a versatile material for fabrication of conducting nanomaterials. Curr. Nanosci. 16 10.2174/1573413716999201005214832 [DOI] [Google Scholar]
- Mbituyimana B., Mao L., Hu S., Ullah M. W., Chen K., Fu L., et al. (2021). Bacterial cellulose/glycolic acid/glycerol composite membrane as a system to deliver glycolic acid for anti-aging treatment. J. Bioresour. Bioprod. 10.1016/j.jobab.2021.02.003 [DOI] [Google Scholar]
- McCarthy R. R., Ullah M. W., Booth P., Pei E., Yang G. (2019a). The use of bacterial polysaccharides in bioprinting. Biotechnol. Adv. 37:107448. 10.1016/j.biotechadv.2019.107448 [DOI] [PubMed] [Google Scholar]
- McCarthy R. R., Ullah M. W., Pei E., Yang G. (2019b). Antimicrobial inks: the anti-infective applications of bioprinted bacterial polysaccharides. Trends Biotechnol. 37 1153–1155.. 10.1016/j.tibtech.2019.05.004 [DOI] [PubMed] [Google Scholar]
- Meagher M. J., Weiss-Bilka H. E., Best M. E., Boerckel J. D., Wagner D. R., Roeder R. K. (2016). Acellular hydroxyapatite-collagen scaffolds support angiogenesis and osteogenic gene expression in an ectopic murine model: effects of hydroxyapatite volume fraction. J. Biomed. Mater. Res. - Part A. 104 2178–2188.. 10.1002/jbm.a.35760 [DOI] [PubMed] [Google Scholar]
- Mohammadi H. (2011). Nanocomposite biomaterial mimicking aortic heart valve leaflet mechanical behaviour. Proc. Institution Mechan. Eng. Part H: J. Eng. Med. 225 718–722.. 10.1177/0954411911399826 [DOI] [PubMed] [Google Scholar]
- Mohammadkazemi F., Faria M., Cordeiro N. (2016). In situ biosynthesis of bacterial nanocellulose-CaCO3 hybrid bionanocomposite: one-step process. Mater. Sci. Eng. C 65 393–399.. 10.1016/j.msec.2016.04.069 [DOI] [PubMed] [Google Scholar]
- Morais J. M., Papadimitrakopoulos F., Burgess D. J. (2010). Biomaterials/Tissue interactions: possible solutions to overcome foreign body response. AAPS J. 12 188–196.. 10.1208/s12248-010-9175-9173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mller M., ztrk E., Arlov , Gatenholm P., Zenobi-Wong M. (2017). Alginate sulfatenanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 45 210–223.. 10.1007/s10439-016-1704-5 [DOI] [PubMed] [Google Scholar]
- Murizan N. I. S., Mustafa N. S., Ngadiman N. H. A., Yusof N. M., Idris A. (2020). Review on nanocrystalline cellulose in bone tissue engineering applications. Polymers (Basel) 12 1–22.. 10.3390/polym12122818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseri N., Deepa B., Mathew A. P., Oksman K., Girandon L. (2016). Nanocellulose-Based Interpenetrating Polymer Network (IPN) hydrogels for cartilage applications. Biomacromolecules 17 3714–3723.. 10.1021/acs.biomac.6b01243 [DOI] [PubMed] [Google Scholar]
- Nimeskern L., Martnez vila H., Sundberg J., Gatenholm P., Mller R., Stok K. S. (2013). Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 22 12–21.. 10.1016/j.jmbbm.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Nishiguchi A., Taguchi T. (2019). Osteoclast-Responsive, injectable bone of bisphosphonated-nanocellulose that regulates osteoclast/osteoblast activity for bone regeneration. Biomacromolecules 20 1385–1393.. 10.1021/acs.biomac.8b01767 [DOI] [PubMed] [Google Scholar]
- Nogi M., Iwamoto S., Nakagaito A. N., Yano H. (2009). Optically transparent nanofiber paper. Adv. Mater. 21 1595–1598.. 10.1002/adma.200803174 [DOI] [Google Scholar]
- Olyveira G., Valido D. P., Costa L. M. M., Gois P. B. P., Xavier Filho L., Basmaji P. (2011). First otoliths/collagen/bacterial cellulose nanocomposites as a potential scaffold for bone tissue regeneration. J. Biomater. Nanobiotechnol. 2 239–243.. 10.4236/jbnb.2011.23030 [DOI] [Google Scholar]
- Osorio D. A., Lee B. E. J., Kwiecien J. M., Wang X., Shahid I., Hurley A. L., et al. (2019a). Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds. Acta Biomater. 87 152–165.. 10.1016/j.actbio.2019.01.049 [DOI] [PubMed] [Google Scholar]
- Osorio M., Caas A., Puerta J., Daz L., Naranjo T., Ortiz I., et al. (2019b). Ex vivo and in vivo biocompatibility assessment (blood and tissue) of three-dimensional bacterial nanocellulose biomaterials for soft tissue implants. Sci. Rep. 9:10553. 10.1038/s41598-019-46918-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachuau L. (2017). Application of nanocellulose for controlled drug delivery, in Nanocellulose and Nanohydrogel Matrices: Biotechnological and Biomedical Applications, eds Jawaid M., Mohammad F. (Hoboken, NJ: Wiley; ), 10.1002/9783527803835.ch1 [DOI] [Google Scholar]
- Panday R., Poudel A. J., Li X., Adhikari M., Ullah M. W., Yang G. (2018). Amphiphilic core-shell nanoparticles: synthesis, biophysical properties, and applications. Colloids Surfaces B Biointerfaces 172 68–81.. 10.1016/j.colsurfb.2018.08.019 [DOI] [PubMed] [Google Scholar]
- Pang M., Huang Y., Meng F., Zhuang Y., Liu H., Du M., et al. (2020). Application of bacterial cellulose in skin and bone tissue engineering. Eur. Polym. J. 122:109365. 10.1016/j.eurpolymj.2019.109365 [DOI] [Google Scholar]
- Patel D. K., Dutta S. D., Lim K. T. (2019). Nanocellulose-based polymer hybrids and their emerging applications in biomedical engineering and water purification. RSC Adv. 9 19143–19162.. 10.1039/c9ra03261d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phanthong P., Reubroycharoen P., Hao X., Xu G., Abudula A., Guan G. (2018). Nanocellulose: extraction and application. Carbon Resour. Convers. 1 32–43.. 10.1016/j.crcon.2018.05.004 [DOI] [Google Scholar]
- Picheth G. F., Pirich C. L., Sierakowski M. R., Woehl M. A., Sakakibara C. N., de Souza C. F., et al. (2017). Bacterial cellulose in biomedical applications: a review. Int. J. Biol. Macromol. 104 97–106.. 10.1016/j.ijbiomac.2017.05.171 [DOI] [PubMed] [Google Scholar]
- Poonguzhali R., Basha S. K., Kumari V. S. (2017). Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 105(Pt 1), 111–120.. 10.1016/j.ijbiomac.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Ptzinger Y., Kralisch D., Fischer D. (2017). Bacterial nanocellulose: the future of controlled drug delivery? Ther. Deliv. 8 753–761.. 10.4155/tde-2017-2059 [DOI] [PubMed] [Google Scholar]
- Powell L. C., Khan S., Chinga-Carrasco G., Wright C. J., Hill K. E., Thomas D. W. (2016). An investigation of Pseudomonas aeruginosa biofilm growth on novel nanocellulose fibre dressings. Carbohydr. Polym. 137 191–197.. 10.1016/j.carbpol.2015.10.024 [DOI] [PubMed] [Google Scholar]
- Qadri S., Haik Y., Mensah-Brown E., Bashir G., Fernandez-Cabezudo M. J., al-Ramadi B. K. (2017). Metallic nanoparticles to eradicate bacterial bone infection. Nanomedicine 13 2241–2250.. 10.1016/j.nano.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Ramani D., Sastry T. P. (2014). Bacterial cellulose-reinforced hydroxyapatite functionalized graphene oxide: a potential osteoinductive composite. Cellulose 21 3585–3595.. 10.1007/s10570-014-0313-314 [DOI] [Google Scholar]
- Raquel Mantuaneli, and Scarel-Caminaga. (2014). Nanocomposites based on bacterial cellulose in combination with osteogenic growth peptide for bone repair: cytotoxic, genotoxic and mutagenic evaluations. J. Appl. Biol. Biotechnol. 2 001–008.. [Google Scholar]
- Rocha I., Lindh J., Hong J., Strmme M., Mihranyan A., Ferraz N. (2018). Blood compatibility of sulfonated Cladophora nanocellulose beads. Molecules 23:601. 10.3390/molecules23030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RoyChowdhury P., Kumar V. (2006). Fabrication and evaluation of porous 2,3-dialdehydecellulose membrane as a potential biodegradable tissue-engineering scaffold. J. Biomed. Mater. Res. - Part A. 76 300–309.. 10.1002/jbm.a.30503 [DOI] [PubMed] [Google Scholar]
- Ruan C. Q., Strmme M., Lindh J. (2018). Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr. Polym. 181 200–207.. 10.1016/j.carbpol.2017.10.072 [DOI] [PubMed] [Google Scholar]
- Sai H., Xing L., Xiang J., Cui L., Jiao J., Zhao C., et al. (2013). Flexible aerogels based on an interpenetrating network of bacterial cellulose and silica by a non-supercritical drying process. J. Mater. Chem. A. 181 200–207.. 10.1039/c3ta11198a [DOI] [Google Scholar]
- Saito T., Kimura S., Nishiyama Y., Isogai A. (2007). Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 8 2485–2491.. 10.1021/bm0703970 [DOI] [PubMed] [Google Scholar]
- Sajjad W., He F., Ullah M. W., Ikram M., Shah S. M., Khan R., et al. (2020). Fabrication of bacterial cellulose-curcumin nanocomposite as a novel dressing for partial thickness skin burn. Front. Bioeng. Biotechnol. 8:553037. 10.3389/fbioe.2020.553037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M. B., Rojas A. D., Gatenholm P., Davalos R. V. (2010). Electromagnetically controlled biological assembly of aligned bacterial cellulose nanofibers. Ann. Biomed. Eng. 38 2475–2484.. 10.1007/s10439-010-9999-0 [DOI] [PubMed] [Google Scholar]
- Saska S., Barud H. S., Gaspar A. M. M., Marchetto R., Ribeiro S. J. L., Messaddeq Y. (2011). Bacterial cellulose-hydroxyapatite nanocomposites for bone regeneration. Int. J. Biomater. 2011 1–8.. 10.1155/2011/175362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saska S., Teixeira L. N., de Castro Raucci L. M. S., Scarel-Caminaga R. M., Franchi L. P., dos Santos R. A., et al. (2017). Nanocellulose-collagen-apatite composite associated with osteogenic growth peptide bone regeneration. Int. J. Biol. Macromol. 103 467–476.. 10.1016/j.ijbiomac.2017.05.086 [DOI] [PubMed] [Google Scholar]
- Saska S., Teixeira L. N., Tambasco, De Oliveira P., Minarelli Gaspar A. M., Lima Ribeiro S. J., et al. (2012). Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 22 22102–22112.. 10.1039/c2jm33762b [DOI] [Google Scholar]
- Scherner M., Reutter S., Klemm D., Sterner-Kock A., Guschlbauer M., Richter T., et al. (2014). In vivo application of tissue-engineered blood vessels of bacterial cellulose as small arterial substitutes: proof of concept? J. Surg. Res. 189 340–347.. 10.1016/j.jss.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Schumann D. A., Wippermann J., Klemm D. O., Kramer F., Koth D., Kosmehl H., et al. (2009). Artificial vascular implants from bacterial cellulose: preliminary results of small arterial substitutes. Cellulose 16 877–885.. 10.1007/s10570-008-9264-y [DOI] [PubMed] [Google Scholar]
- Sengupta S., Balla V. K. (2018). A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. J. Adv. Res. 14 97–111.. 10.1016/j.jare.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyednejad H., Gawlitta D., Kuiper R. V., De Bruin A., Van Nostrum C. F., Vermonden T., et al. (2012). In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(-caprolactone). Biomaterials 33 4309–4318.. 10.1016/j.biomaterials.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Shah N., Ul-Islam M., Khattak W. A., Park J. K. (2013). Overview of bacterial cellulose composites: a multipurpose advanced material. Carbohydr. Polym. 98 1585–1598.. 10.1016/j.carbpol.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Shahmohammadi Jebel F., Almasi H. (2016). Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 149 8–19.. 10.1016/j.carbpol.2016.04.089 [DOI] [PubMed] [Google Scholar]
- Shak K. P. Y., Pang Y. L., Mah S. K. (2018). Nanocellulose: recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 9 2479–2498.. 10.3762/bjnano.9.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C., Bhardwaj N. K. (2019). Bacterial nanocellulose: present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 104:109963. 10.1016/j.msec.2019.109963 [DOI] [PubMed] [Google Scholar]
- Shi Q., Li Y., Sun J., Zhang H., Chen L., Chen B., et al. (2012a). The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 33 6644–6649.. 10.1016/j.biomaterials.2012.05.071 [DOI] [PubMed] [Google Scholar]
- Shi Z., Zang S., Jiang F., Huang L., Lu D., Ma Y., et al. (2012b). In situ nano-assembly of bacterial cellulosepolyaniline composites. RSC Adv. 2 1040–1046.. 10.1039/C1RA00719J [DOI] [Google Scholar]
- Shi Z., Gao X., Ullah M. W., Li S., Wang Q., Yang G. (2016). Electroconductive natural polymer-based hydrogels. Biomaterials 111 40–54.. 10.1016/j.biomaterials.2016.09.020 [DOI] [PubMed] [Google Scholar]
- Shvedova A. A., Kisin E. R., Yanamala N., Farcas M. T., Menas A. L., Williams A., et al. (2016). Gender differences in murine pulmonary responses elicited by cellulose nanocrystals. Part. Fibre Toxicol. 13:28. 10.1186/s12989-016-0140-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Ray A. R., Vasudevan P. (1982). Biodegradation studies on periodate oxidized cellulose. Biomaterials 3 16–20.. 10.1016/0142-9612(82)90055-90052 [DOI] [PubMed] [Google Scholar]
- Singla R., Soni S., Patial V., Kulurkar P. M., Kumari A., Mahesh S., et al. (2017). Cytocompatible anti-microbial dressings of Syzygium cumini cellulose nanocrystals decorated with silver nanoparticles accelerate acute and diabetic wound healing. Sci. Rep. 7:10457. 10.1038/s41598-017-08897-8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiba E. A., Budaeva V. V., Ovchinnikova E. V., Gladysheva E. K., Kashcheyeva E. I., Pavlov I. N., et al. (2020). A technology for pilot production of bacterial cellulose from oat hulls. Chem. Eng. J. 383:123128. 10.1016/j.cej.2019.123128 [DOI] [Google Scholar]
- Song L., Li Y., Xiong Z., Pan L., Luo Q., Xu X., et al. (2018). Water-Induced shape memory effect of nanocellulose papers from sisal cellulose nanofibers with graphene oxide. Carbohydr. Polym. 179 110–117.. 10.1016/j.carbpol.2017.09.078 [DOI] [PubMed] [Google Scholar]
- Song S. H., Kim J. E., Lee Y. J., Kwak M. H., Sung G. Y., Kwon S. H., et al. (2014). Cellulose film regenerated from Styela clava tunics have biodegradability, toxicity and biocompatibility in the skin of SD rats. J. Mater. Sci. Mater. Med. 25 1519–1530.. 10.1007/s10856-014-5182-5188 [DOI] [PubMed] [Google Scholar]
- Song S. H., Seong K. Y., Kim J. E., Go J., Koh E. K., Sung J. E., et al. (2017). Effects of different cellulose membranes regenerated from Styela clava tunics on wound healing. Int. J. Mol. Med. 39 1173–1187.. 10.3892/ijmm.2017.2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniak A. B., Seehra M. S., Fix N. R., Leonard S. S. (2014). Lung biodurability and free radical production of cellulose nanomaterials. Inhal. Toxicol. 26 733–749.. 10.3109/08958378.2014.948650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica-Guzun A., Stroescu M., Jinga S., Jipa I., Dobre T., Dobre L. (2012). Ultrasound influence upon calcium carbonate precipitation on bacterial cellulose membranes. Ultrason. Sonochem. 19 909–915.. 10.1016/j.ultsonch.2011.12.002 [DOI] [PubMed] [Google Scholar]
- Stoica-Guzun A., Stroescu M., Jinga S. I., Jipa I. M., Dobre T. (2013). Microwave assisted synthesis of bacterial cellulose-calcium carbonate composites. Ind. Crops Prod. 50 414–422.. 10.1016/j.indcrop.2013.07.063 [DOI] [Google Scholar]
- Subhedar A., Bhadauria S., Ahankari S., Kargarzadeh H. (2021). Nanocellulose in biomedical and biosensing applications: a review. Int. J. Biol. Macromol. 166 587–600.. 10.1016/j.ijbiomac.2020.10.217 [DOI] [PubMed] [Google Scholar]
- Sun W., Fan J., Wang S., Kang Y., Du J., Peng X. (2018). Biodegradable drug-loaded hydroxyapatite nanotherapeutic agent for targeted drug release in tumors. ACS Appl. Mater. Interfaces 10 7832–7840.. 10.1021/acsami.7b19281 [DOI] [PubMed] [Google Scholar]
- Tan T. H., Lee H. V., Dabdawb W. A. Y., Hamid S. B. B. O. A. A. (2019). Chapter 5 - a review of nanocellulose in the drug-delivery system, in Materials for Biomedical Engineering, eds Holban A.-M., Grumezescu A. M. (Amsterdam: Elsevier; ), 131–164.. 10.1016/B978-0-12-816913-1.00005-2 [DOI] [Google Scholar]
- Ttrai F., Adamis Z., Bhm U., Mertey K., Ungvry G. (1995). Role of cellulose in wood dust-induced fibrosing alveo-bronchiolitis in rat. J. Appl. Toxicol. 15 45–48.. 10.1002/jat.2550150110 [DOI] [PubMed] [Google Scholar]
- Tazi N., Zhang Z., Messaddeq Y., Almeida-Lopes L., Zanardi L. M., Levinson D., et al. (2012). Hydroxyapatite bioactivated bacterial cellulose promotes osteoblast growth and the formation of bone nodules. AMB Express 2:61. 10.1186/2191-0855-2-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomi S., Ili N., Kokol V., Gruden-Movsesijan A., Mihajlovi D., Beki M., et al. (2018). Functionalization-dependent effects of cellulose nanofibrils on tolerogenic mechanisms of human dendritic cells. Int. J. Nanomed. 13 6941–6960.. 10.2147/IJN.S183510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgbo S., Sukyai P. (2018). Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 11 34–49.. 10.1016/j.apmt.2018.01.004 [DOI] [Google Scholar]
- Torgbo S., Sukyai P. (2019). Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 237:121868. 10.1016/j.matchemphys.2019.121868 [DOI] [Google Scholar]
- Tsai C. Y., Shiau A. L., Chen S. Y., Chen Y. H., Cheng P. C., Chang M. Y., et al. (2007). Amelioration of collagen-induced arthritis in rats by nanogold. Arthritis Rheum. 56 544–554.. 10.1002/art.22401 [DOI] [PubMed] [Google Scholar]
- Turnbull G., Clarke J., Picard F., Riches P., Jia L., Han F., et al. (2018). 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 3 278–314.. 10.1016/j.bioactmat.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ul-Islam M., Ahmad F., Fatima A., Shah N., Yasir S., Ahmad M. W., et al. (2021a). Ex situ synthesis and characterization of high strength multipurpose bacterial cellulose-aloe vera hydrogels. Front. Bioeng. Biotechnol. 9:601988. 10.3389/fbioe.2021.601988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ul-Islam M., Ullah M. W., Khan T., Park J. K. (2021b). Bacterial cellulose: trends in synthesis, characterization, and applications, in Handbook of Hydrocolloids, eds Phillips G. O., Williams P. A. (Amsterdam: Elsevier; ), 923–974.. 10.1016/B978-0-12-820104-6.00010-3 [DOI] [Google Scholar]
- Ul-Islam M., Khan S., Ullah M. W., Park J. K. (2015). Bacterial cellulose composites: synthetic strategies and multiple applications in bio-medical and electro-conductive fields. Biotechnol. J. 10 1847–1861.. 10.1002/biot.201500106 [DOI] [PubMed] [Google Scholar]
- Ul-Islam M., Khan S., Ullah M. W., Park J. K. (2019a). Comparative study of plant and bacterial cellulose pellicles regenerated from dissolved states. Int. J. Biol. Macromol. 137 247–252.. 10.1016/j.ijbiomac.2019.06.232 [DOI] [PubMed] [Google Scholar]
- Ul-Islam M., Subhan F., Islam S. U., Khan S., Shah N., Manan S., et al. (2019b). Development of three-dimensional bacterial cellulose/chitosan scaffolds: analysis of cell-scaffold interaction for potential application in the diagnosis of ovarian cancer. Int. J. Biol. Macromol. 137 1050–1059.. 10.1016/j.ijbiomac.2019.07.050 [DOI] [PubMed] [Google Scholar]
- Ul-Islam M., Khan T., Khattak W. A., Park J. K. (2013a). Bacterial cellulose-MMTs nanoreinforced composite films: novel wound dressing material with antibacterial properties. Cellulose 20 589–596.. 10.1007/s10570-012-9849-9843 [DOI] [Google Scholar]
- Ul-Islam M., Khattak W. A., Kang M., Kim S. M., Khan T., Park J. K. (2013b). Effect of post-synthetic processing conditions on structural variations and applications of bacterial cellulose. Cellulose 20 253–263.. 10.1007/s10570-012-9799-9799 [DOI] [Google Scholar]
- Ul-Islam M., Khattak W. A., Ullah M. W., Khan S., Park J. K. (2014). Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose 21 433–447.. 10.1007/s10570-013-0109-y [DOI] [Google Scholar]
- Ul-Islam M., Ullah M. W., Khan S., Park J. K. (2020). Production of bacterial cellulose from alternative cheap and waste resources: a step for cost reduction with positive environmental aspects. Korean J. Chem. Eng. 37 925–937.. 10.1007/s11814-020-0524-523 [DOI] [Google Scholar]
- Ul-Islam M., Ullah M. W., Khan S., Shah N., Park J. K. (2017). Strategies for cost-effective and enhanced production of bacterial cellulose. Int. J. Biol. Macromol. 102 1166–1173.. 10.1016/j.ijbiomac.2017.04.110 [DOI] [PubMed] [Google Scholar]
- Ullah I., Gloria A., Zhang W., Ullah M. W., Wu B., Li W., et al. (2019a). Synthesis and characterization of sintered Sr/Fe-Modified hydroxyapatite bioceramics for bone tissue engineering applications. ACS Biomater. Sci. Eng. Acsbiomater. 6 375–388.. 10.1021/acsbiomaterials.9b01666 [DOI] [PubMed] [Google Scholar]
- Ullah M. W., Fu L., Lamboni L., Shi Z., Yang G. (2019b). Current trends and biomedical applications of resorbable polymers, in Materials for Biomedical Engineering, (Amsterdam: Elsevier; ), 41–86.. 10.1016/B978-0-12-818415-8.00003-6 [DOI] [Google Scholar]
- Ullah M. W., Manan S., Kiprono S. J., Ul-Islam M., Yang G. (2019c). Synthesis, structure, and properties of bacterial cellulose, in Nanocellulose, eds Huang J., Dufresne A., Lin N. (Hoboken, NJ: Wiley Online Library; ), 10.1002/9783527807437.ch4 [DOI] [Google Scholar]
- Ullah I., Li W., Lei S., Zhang Y., Zhang W., Farooq U., et al. (2018). Simultaneous co-substitution of Sr2+/Fe3+ in hydroxyapatite nanoparticles for potential biomedical applications. Ceram. Int. 44 21338–21348.. 10.1016/j.ceramint.2018.08.187 [DOI] [Google Scholar]
- Ullah I., Zhang W., Yang L., Ullah M. W., Atta O. M., Khan S., et al. (2020). Impact of structural features of Sr/Fe co-doped HAp on the osteoblast proliferation and osteogenic differentiation for its application as a bone substitute. Mater. Sci. Eng. C 110:110633. 10.1016/j.msec.2020.110633 [DOI] [PubMed] [Google Scholar]
- Ullah M. W., Khattak W. A., Ul-Islam M., Khan S., Park J. K. (2016a). Metabolic engineering of synthetic cell-free systems: strategies and applications. Biochem. Eng. J. 105 391–405.. 10.1016/j.bej.2015.10.023 [DOI] [Google Scholar]
- Ullah M. W., Ul-Islam M., Khan S., Kim Y., Jang J. H., Park J. K. (2016b). In situ synthesis of a bio-cellulose/titanium dioxide nanocomposite by using a cell-free system. RSC Adv. 6 22424–22435.. 10.1039/C5RA26704H [DOI] [Google Scholar]
- Ullah M. W., Ul-Islam M., Khan S., Kim Y., Park J. K. (2016c). Structural and physico-mechanical characterization of bio-cellulose produced by a cell-free system. Carbohydr. Polym. 136 908–916.. 10.1016/j.carbpol.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Ullah M. W., Ul Islam M., Khan S., Shah N., Park J. K. (2017). Recent advancements in bioreactions of cellular and cell-free systems: a study of bacterial cellulose as a model. Korean J. Chem. Eng. 34 1591–1599.. 10.1007/s11814-017-0121-122 [DOI] [Google Scholar]
- Ullah M. W., Ul-Islam M., Khan S., Kim Y., Park J. K. (2015). Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 132 286–294.. 10.1016/j.carbpol.2015.06.037 [DOI] [PubMed] [Google Scholar]
- Umar Aslam, Khan M., Haider S., Haider A., Rafiq Abdul, Kadir M., et al. (2020). Development of porous, antibacterial and biocompatible GO/n-HAp/Bacterial Cellulose/ -Glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem. 14:102924. 10.1016/j.arabjc.2020.102924 [DOI] [Google Scholar]
- Veerla S. C., Kim D. R., Kim J., Sohn H., Yang S. Y. (2019). Controlled nanoparticle synthesis of Ag/Fe co-doped hydroxyapatite system for cancer cell treatment. Mater. Sci. Eng. C 98 311–323.. 10.1016/j.msec.2018.12.148 [DOI] [PubMed] [Google Scholar]
- Wan Y., Yang Z., Xiong G., Guo R., Liu Z., Luo H. (2015a). Anchoring Fe3O4 nanoparticles on three-dimensional carbon nanofibers toward flexible high-performance anodes for lithium-ion batteries. J. Power Sources 294 414–419.. 10.1016/j.jpowsour.2015.06.057 [DOI] [Google Scholar]
- Wan Y., Yang Z., Xiong G., Luo H. (2015b). A general strategy of decorating 3D carbon nanofiber aerogels derived from bacterial cellulose with nano-Fe3O4 for high-performance flexible and binder-free lithium-ion battery anodes. J. Mater. Chem. A 3 15386–15393.. 10.1039/c5ta03688g [DOI] [Google Scholar]
- Wan Y. Z., Huang Y., Yuan C. D., Raman S., Zhu Y., Jiang H. J., et al. (2007). Biomimetic synthesis of hydroxyapatite/bacterial cellulose nanocomposites for biomedical applications. Mater. Sci. Eng. C 27 855–864.. 10.1016/j.msec.2006.10.002 [DOI] [Google Scholar]
- Wang B., Sain M. (2007). Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos. Sci. Technol. 67 2521–2527.. 10.1016/j.compscitech.2006.12.015 [DOI] [Google Scholar]
- Wang C., Huang W., Zhou Y., He L., He Z., Chen Z., et al. (2020a). 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 5 82–91.. 10.1016/j.bioactmat.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Hu S., Ullah M. W., Li X., Shi Z., Yang G. (2020b). Enhanced cell proliferation by electrical stimulation based on electroactive regenerated bacterial cellulose hydrogels. Carbohydr. Polym. 249:116829. 10.1016/j.carbpol.2020.116829 [DOI] [PubMed] [Google Scholar]
- Wang C., Lai J., Li K., Zhu S., Lu B., Liu J., et al. (2021). Cryogenic 3D printing of dual-delivery scaffolds for improved bone regeneration with enhanced vascularization. Bioact. Mater. 6 137–145.. 10.1016/j.bioactmat.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Gao C., Zhang Y., Wan Y. (2010). Preparation and in vitro characterization of BC/PVA hydrogel composite for its potential use as artificial cornea biomaterial. Mater. Sci. Eng. C 30 214–218.. 10.1016/j.msec.2009.10.006 [DOI] [Google Scholar]
- Wang J., Wan Y., Han J., Lei X., Yan T., Gao C. (2011). Nanocomposite prepared by immobilising gelatin and hydroxyapatite on bacterial cellulose nanofibres. Micro Nano Lett. 6:133. 10.1049/mnl.2010.0209 [DOI] [Google Scholar]
- Wang Y., Wang J., Hao H., Cai M., Wang S., Ma J., et al. (2016). In vitro and in vivo mechanism of bone tumor inhibition by selenium-doped bone mineral nanoparticles. ACS Nano 10 9927–9937.. 10.1021/acsnano.6b03835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. F. (2019). Biocompatibility in clinical practice: predictable and unpredictable outcomes. Prog. Biomed. Eng. 1:013001. 10.1088/2516-1091/ab22cc [DOI] [Google Scholar]
- Yan Y., Lin H., Matsumoto H., Bouzika P., Miller J., Vavvas D. (2013). Comparison of the toxicity of different drug delivery nanoparticles in RPE and photoreceptor cells. Invest. Ophthalmol. Vis. Sci. 54:1940. [Google Scholar]
- Yanamala N., Farcas M. T., Hatfield M. K., Kisin E. R., Kagan V. E., Geraci C. L., et al. (2014). In vivo evaluation of the pulmonary toxicity of cellulose nanocrystals: a renewable and sustainable nanomaterial of the future. ACS Sustainable Chem. Eng. 2 1691–1698.. 10.1021/sc500153k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Sheikhi A., Van De Ven T. G. M. (2016). Reusable green aerogels from cross-linked hairy nanocrystalline cellulose and modified chitosan for dye removal. Langmuir 32 11771–11779.. 10.1021/acs.langmuir.6b03084 [DOI] [PubMed] [Google Scholar]
- Yang J., Du M., Wang L., Li S., Wang G., Yang X., et al. (2018). Bacterial cellulose as a supersoft neural interfacing substrate. ACS Appl. Mater. Interfaces 10 33049–33059.. 10.1021/acsami.8b12083 [DOI] [PubMed] [Google Scholar]
- Yang J., Yang X., Wang L., Zhang W., Yu W., Wang N., et al. (2017). Biomimetic nanofibers can construct effective tissue-engineered intervertebral discs for therapeutic implantation. Nanoscale 9 13095–13103.. 10.1039/C7NR03944A [DOI] [PubMed] [Google Scholar]
- Yang Z., Chen S., Hu W., Yin N., Zhang W., Xiang C., et al. (2012). Flexible luminescent CdSe/bacterial cellulose nanocomoposite membranes. Carbohydr. Polym. 88 173–178.. 10.1016/j.carbpol.2011.11.080 [DOI] [Google Scholar]
- Yin N., Chen S., Ouyang Y., Tang L., Yang J., Wang H. (2011). Biomimetic mineralization synthesis of hydroxyapatite bacterial cellulose nanocomposites. Prog. Nat. Sci. Mater. Int. 21 472–477.. 10.1016/S1002-0071(12)60085-60089 [DOI] [Google Scholar]
- Yuan H., Van Blitterswijk C. A., De Groot K., De Bruijn J. D. (2006). A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods. J. Biomed. Mater. Res. - Part A. 78 139–147.. 10.1002/jbm.a.30707 [DOI] [PubMed] [Google Scholar]
- Yuen J. D., Walper S. A., Melde B. J., Daniele M. A., Stenger D. A. (2017). Electrolyte-sensing transistor decals enabled by ultrathin microbial nanocellulose. Sci. Rep. 7:40867. 10.1038/srep40867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborowska M., Bodin A., Bckdahl H., Popp J., Goldstein A., Gatenholm P. (2010). Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 6 2540–2547.. 10.1016/j.actbio.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Zarei S., Niad M., Raanaei H. (2018). The removal of mercury ion pollution by using Fe3O4-nanocellulose: synthesis, characterizations and DFT studies. J. Hazard. Mater. 344 258–273.. 10.1016/j.jhazmat.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Zhan H., Peng N., Lei X., Huang Y., Li D., Tao R., et al. (2018). UV-induced self-cleanable TiO2/nanocellulose membrane for selective separation of oil/water emulsion. Carbohydr. Polym. 201 464–470.. 10.1016/j.carbpol.2018.08.093 [DOI] [PubMed] [Google Scholar]
- Zhang G., Liao Q., Zhang Z., Liang Q., Zhao Y., Zheng X., et al. (2015). Novel piezoelectric paper-based flexible nanogenerators composed of BaTiO3 nanoparticles and bacterial cellulose. Adv. Sci. 3:1500257. 10.1002/advs.201500257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wang J., Wang K., Xu L. (2018a). A bilayered PLGA/multiwall carbon nanotubes/bacterial cellulose composite membrane for tissue regeneration of maxillary canine periodontal bone defects. Mater. Lett. 212 118–121.. 10.1016/j.matlet.2017.10.058 [DOI] [Google Scholar]
- Zhang Y., Wang F., Li M., Yu Z., Qi R., Ding J., et al. (2018b). Self-Stabilized hyaluronate nanogel for intracellular codelivery of doxorubicin and cisplatin to osteosarcoma. Adv. Sci. 5:1700821. 10.1002/advs.201700821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Wang X., Li X., Zhang L., Jiang F. (2020). A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 236:116043. 10.1016/j.carbpol.2020.116043 [DOI] [PubMed] [Google Scholar]
- Zharikov A. N., Lubyansky V. G., Gladysheva E. K., Skiba E. A., Budaeva V. V., Semyonova E. N., et al. (2018). Early morphological changes in tissues when replacing abdominal wall defects by bacterial nanocellulose in experimental trials. J. Mater. Sci. Mater. Med. 29:95. 10.1007/s10856-018-6111-z [DOI] [PubMed] [Google Scholar]
- Zhong C. (2020). Industrial-Scale production and applications of bacterial cellulose. Front. Bioeng. Biotechnol. 8:605374. 10.3389/fbioe.2020.605374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Cui Y., Zhang M., Zhao D., Liu G., Ding J. (2020). Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact. Mater. 5 584–601.. 10.1016/j.bioactmat.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K. A., LeBlanc J. M., Sheets K. T., Fox R. W., Gatenholm P. (2011). Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C 31 43–49.. 10.1016/J.MSEC.2009.10.007 [DOI] [Google Scholar]