Abstract
Digital health technology (DHT) has the potential to revolutionize healthcare delivery but its uptake has been low in clinical and research settings. The factors that contribute to the limited adoption of DHT, particularly in cardiovascular settings, are unclear. The objective of this review was to determine the barriers and facilitators of DHT uptake from the perspective of patients, clinicians, and researchers. We searched MEDLINE, EMBASE, and CINAHL databases for studies published from inception to May 2020 that reported barriers and/or facilitators of DHT adoption in cardiovascular care. We extracted data on study design, setting, cardiovascular condition, and type of DHT. We conducted a thematic analysis to identify barriers and facilitators of DHT uptake. The search identified 3075 unique studies, of which 29 studies met eligibility criteria. Studies employed: qualitative methods (n = 13), which included interviews and focus groups; quantitative methods (n = 5), which included surveys; or a combination of qualitative and quantitative methods (n = 11). Twenty-five studies reported patient-level barriers, most common of which were difficult-to-use technology (n = 7) and a poor internet connection (n = 7). Six studies reported clinician-level barriers, which included increased workload (n = 4) and a lack of integration with electronic medical records (n = 3).Twenty-four studies reported patient-level facilitators, which included improved communication with clinicians (n = 10) and personalized technology (n = 6). Four studies reported clinician-level facilitators, which included approval and organizational support from cardiology departments and/or hospitals (n = 3) and technologies that improved efficiency (n = 3). No studies reported researcher-level barriers or facilitators. In summary, internet access, user-friendliness, organizational support, workflow efficiency, and data integration were reported as important factors in the uptake of DHT by patients and clinicians. These factors can be considered when selecting and implementing DHTs in cardiovascular clinical settings.
Keywords: Digital Health Technology, Barriers, Facilitators, Cardiology, Cardiovascular Disease
Graphical Abstract
Introduction
Cardiovascular disease (CVD) places a considerable burden on patients and healthcare systems and is the leading cause of global mortality and a major cause of reduced quality of life.1–3 By 2030, the total global cost of CVD is estimated to increase from approximately $860 billion in 2010 to $1044 billion.4 Interventions that reduce costs attributed to CVD and improve the quality of patient care are a priority across health systems.5
Digital health technology (DHT)—the convergence of digital technologies with health, health care, and society—has the potential to revolutionize CVD healthcare delivery by streamlining operations, improving patient outcomes and decreasing healthcare costs.6 DHT has six key applications in cardiovascular care; facilitate patient self-care through the use of self-monitoring and self-management applications on mobile devices;7 provide clinicians with the ability to monitor patients remotely;7 provide decision support to clinicians at the point of care;7 facilitate virtual care encounters between clinicians and patients via digital platforms;8 enable education of clinicians and patients via educational modules, applications, and social networking platforms;9 and facilitate research through recruitment, randomization, data collection, and implementation.10
The SARS-CoV-2 2019 (COVID-19) pandemic has accelerated the development of DHT.11,12 Healthcare systems and policy makers have prioritized DHT as a strategy to flatten the COVID-19 curve, and to deliver care to patients in need.11,12 Despite the abundance of DHT innovations, however, uptake beyond telecare has been slow. Concerns about cost, effectiveness, and safety may be barriers, and current healthcare system processes may not readily facilitate the digital transformation.11–13 A recent position paper identified barriers impeding large-scale digital health uptake in Europe,13 but the barriers and facilitators of DHT adoption in cardiovascular care have not been systematically assessed.
The purpose of this systematic scoping review was to provide a comprehensive summary of barriers and facilitators of the uptake of DHT in cardiovascular care, among patients, clinicians, and researchers.
Methods and analysis
Study design
We conducted a systematic scoping review using the methodological framework developed by Arksey and O’Malley:14 identifying the research question, identifying relevant studies, selecting studies, charting the data, collating summarizing, and reporting the results. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses modified statement for scoping reviews (PRISMA-ScR) was used to inform conduct and reporting.15
The research question guiding this review was: What are the barriers and facilitators of the uptake of DHT in cardiovascular care?
Identifying relevant studies
With the aid of a professional information specialist, we conducted a systematic search of the literature for articles published in MEDLINE, EMBASE, and CINAHL. We developed our main search strategy in MEDLINE. The search strategy for MEDLINE is available in the Supplementary material online, Appendix. The search strategy was not limited by study design or language, and included the terms ‘cardiology’, ‘digital health technology’, ‘mHealth’, ‘telemedicine’, ‘barriers’, and ‘facilitators’. We supplemented the systematic literature search by hand searching the reference lists of included studies and relevant systematic reviews.
Study selection
We included studies published from inception of the databases to 7 May 2020. We included studies that focused on CVD management and reported on patient-, clinician-, and/or researcher-level barriers and/or facilitators of the uptake of DHT. For the purpose of this review, DHT was defined as a broad scope of tools that engage patients for clinical purposes; collect, organize, interpret, and use clinical data; and manage outcomes and other measures of care quality.16 Barriers and facilitators were defined as factors that hindered or enabled implementation of DHT in clinical practice.17,18 We excluded conference abstracts, case reports, commentaries, editorials, reviews, and protocols.
Two authors (S.W. and D.M.P.) independently screened all titles and abstracts acquired from the systematic search. Full-text versions of all studies that appeared to meet the inclusion criteria or those where there was insufficient information in the title and abstract to make a decision were obtained. S.W. and D.M.P. then independently screened all full-text versions of the studies for inclusion. Disagreements were resolved through discussion, and when required, by consulting a third author (H.G.C.V.).
Charting the data
Two authors (S.W. and D.M.P.) independently extracted the following information in duplicate: publication year, cardiovascular condition, sample size, description of the technology, method of data collection, and method of data analysis. We categorized the DHTs used in the including studies according to the American Medical Association (AMA)’s classification of digital health tools (Box 1).16 Any disagreements were resolved by discussion and consultation with a third author (H.G.C.V.).
Box 1.
American Medical Association’s classification of digital health tools
| Remote monitoring for efficiency:16 Smart versions of common clinical devices such as thermometers, blood pressure cuffs, and scales that automatically record readings in the patient record, so you do not have to type it. |
| Remote monitoring and management for improved care: Apps and devices for use by chronic disease patients for daily measurement of vital signs such as weight, blood pressure, blood glucose, etc. Readings are visible to patients and transmitted to the physician’s office. Alerts are generated as appropriate for missing or out of range readings. |
| Clinical decision support: Modules used in conjunction with the EHR or apps that integrate with the EHR that highlight potentially significant changes in patient data (e.g. gain or loss of weight, change in blood chemistry). |
| Patient engagement: Solutions to promote patient wellness and active participation in their care for chronic diseases (e.g. adherence to treatment regimens). |
| Tele-visits/virtual visits: An audio/video connection used to see patients remotely (i.e. simple acute illness, adjusting therapy, etc.). |
| Point of care/workflow enhancement: Communication and sharing of electronic clinical data to consult with specialists, make referrals and/or transitions of care. |
| Consumer access to clinical data: Secure access allowing patients to view clinical information such as routine lab results, receive appointment reminders and treatment prompts, and to ask for prescription refills, appointments and to speak with their physician. |
Collating, summarizing, and reporting the findings
We followed the six-phase process developed by Braun and Clark to conduct our thematic analysis of the barriers and facilitators of DHT in CVD care: familiarizing oneself with the data, searching for the themes, reviewing themes, defining and naming themes, and producing the report.18
One author (S.W.) read all of the studies, annotated them, and identified broad barrier and facilitator categories. As additional studies were read, they were mapped to previously identified categories, based on the topics discussed in them, and more categories were added as new barrier and facilitators emerged. Barrier and facilitator categories were not mutually exclusive and each study could be mapped to multiple categories based on its content. The same author (S.W.) re-read all of the studies listed under each barrier and/or facilitator category, reviewed the annotations from the previous round, and compared and contrasted the various studies’ findings to identify recurrent themes, discrepancies and unique findings. A second author (D.M.P.) independently performed this phase of the analysis for the studies listed under the barrier and facilitator categories. S.W. and D.M.P. then compared the analyses and discussed disagreements between them, in order to reach a consensus. Any disagreements were resolved by discussion and consultation with a third author (H.G.C.V.). We performed descriptive analyses and presented the occurrences and frequencies of the barriers and facilitators using numbers and percentages.
Results
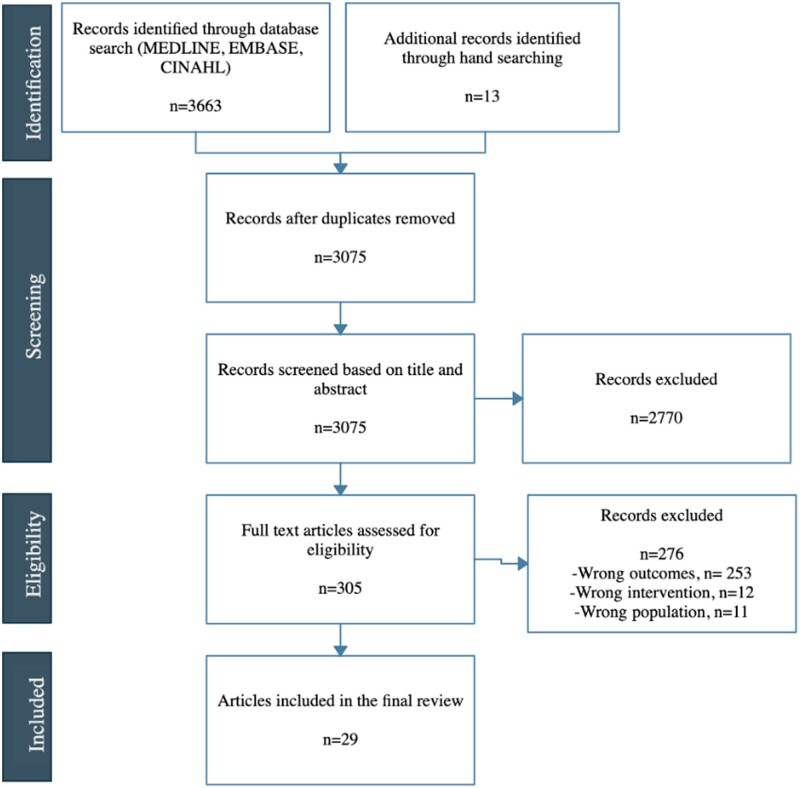
A total of 3062 titles and abstracts produced from the systematic search and 13 hand-selected articles from the supplementary literature search were assessed for eligibility after removing duplicates. Of the 3075 articles, 2770 were excluded on the basis of title and/or abstract review. We assessed 305 full-text articles, of which 29 met eligibility criteria (Figure 1; Table 1). The references to the included studies are included in the Supplementary material online, Appendix.
Figure 1.
PRISMA diagram of studies included in the systematic scoping review.
Table 1.
Characteristics of included studies (n = 29)
| Reference and country | Study period | Digital health tool | Description of tool | Participants | Method of data collection | Analysis |
|---|---|---|---|---|---|---|
| Allemann 2019 (Sweden) | 2015–2017 | Tele-visits/virtual visits | Information communication technologies designed to support family members in caring for patients. | Family members of patients with heart failure who received care from a heart function clinic (n = 8) | Focus groups | Content analysis |
| Alnosayan 2017 (USA) | 2012 | Remote monitoring and management | A mHealth system which includes a Bluetooth-enabled blood pressure monitor, weight scale, glucose metre, and a mobile phone loaded with the MyHeart application programmed to alert clinicians upon patient decompensation. | Patients with heart failure who had recently been discharged from the hospital (n = 8) and nurses who worked in heart failure clinics (n = 6) | Focus groups | Grounded theory |
| Ancker 2015 (USA) | 2015 | Patient engagement | Personal health information tracking facilitated through digital technology to monitor patient’s health conditions. | Patients with at least one chronic medical condition (n = 22) and clinicians from internal medicine clinics (n = 7) | Semi-structured interviews | Grounded theory and thematic analysis |
| Anttilla 2019 (Finland) | 2015–2016 | Tele-visits/virtual visits | A remote cardiac rehabilitation programme delivered through video conferencing software. | Patients who had undergone coronary angioplasty or coronary artery bypass (n = 39) and were prescribed cardiac rehabilitation | Focus groups | Grounded theory |
| Buck 2017 (USA) | 2015 | Patient engagement | An internet-based tablet where participants recorded their daily medication intake, weight, and time spent exercising and could access disease-specific educational videos. | Patients with heart failure (n = 12) who had recently been discharged from the hospital | Semi-structured interviews | Inductive analysis |
| Cajita 2017 (USA) | 2016 | Remote monitoring and management | A mHealth monitoring system composed of a weight scale, blood pressure monitor, pulse oximeter, and a mobile device. | Patients with heart failure (n = 129) who had recently been discharged from the hospital | Survey | Quantitative analysis |
| Chantler 2016 (UK) | 2013–2014 | Remote monitoring and management | A mHealth monitoring system that included a tablet and a Bluetooth enabled blood pressure monitor and weight scale. | Patients with heart failure (n = 45) | Semi-structured interviews | Thematic analysis |
| Chen 2018 (China) | 2015 | Patient engagement | A mobile application that administered messages to patients to improve medication adherence and encourage lifestyle modifications. | Patients with myocardial infarction or coronary heart disease (n = 24) and physicians who cared for cardiovascular disease patients (n = 10) | Surveys, focus groups and interviews | Quantitative and thematic analysis |
| Dang 2017 (USA) | 2011 | Patient engagement | A mobile phone monitoring system facilitated through website-based messaging. | Patients with heart failure (n = 34) who attended a heart failure clinic | Surveys and interviews | Quantitative and thematic analysis |
| Guo 2019 (China) | 2017 | Remote monitoring and management | A tablet-based platform that collected and integrated patient data from monitoring devices, electronic health records, and laboratory investigations with secure messaging and video conferencing capabilities. | Patients with heart failure (n = 66) who attended a specialty cardiology clinic and physicians (n = 23) | Surveys and interviews | Quantitative and thematic analyses |
| Haldane 2019 (Singapore) | Not reported | Patient engagement | Mobile text messages to promote medication adherence. | Patients with coronary artery disease, ischaemic stroke, peripheral artery disease, or atherosclerotic aortic disease (n = 20) | Surveys and semi-structured interviews | Quantitative and constant comparison analyses |
| Holender 2018 (UK) | 2015 | Patient engagement | Mobile phones, smart watches, and ingestible sensor systems to monitor medication adherence. | Patients (n = 12) taking cardiovascular medications | Focus groups | Thematic analysis |
| Hunting 2015 (Canada) | 2012–2014 | Remote monitoring and management | Telehomecare programme to increase self-management skills and to improve the monitoring of patients via remote health status monitoring. | Patients (n = 39) with heart failure and/or informal caregivers, nurses (n = 16), physicians (n = 7), technicians (n = 2), administrators (n = 12), and decision makers (n = 13) | Semi-structured interviews, ethnographic observations and document review | Grounded theory |
| Jiang 2019 (China) | 2016 | Patient engagement | A mobile health system to support disease-specific self-management. | Patients (n = 231) taking long-term medications for hypertension, coronary heart disease, heart failure, or arrhythmia | Survey | Quantitative analysis |
| Kerr 2010 (UK) | Not reported | Patient engagement | A web-based platform that provided educational information, behaviour change support, and peer and expert support. | Patients with coronary heart disease (n = 168) | Surveys and semi-structured interviews | Quantitative and thematic analyses |
| Lefler 2018 (USA) | Not reported | Remote monitoring and management | A mHealth monitoring system that included a tablet and a Bluetooth-enabled blood pressure monitor and weight scale to provide real-time monitoring and alert clinicians upon decompensation. | Patients with heart failure (n = 21) who attended specialist cardiology clinics | Surveys and semi-structured interviews | Quantitative and content analyses |
| Li 2017 (China) | 2011–2013 | Patient engagement | A short message service to encourage disease-specific self-management. | Patients admitted to the hospital with heart failure (n = 540) | Survey | Quantitative analysis |
| Nahm 2008 (USA) | 2006 | Patient engagement | A web-based eHealth management programme to support self-management. | Patients with heart failure (n = 44) | Survey | Quantitative analysis |
| Nguyen 2017 (Canada) | 2014 | Patient engagement | Digital health technology to promote self-care after hospital discharge. | Patients with heart failure (n = 18) who attended a heart function clinic after being discharged from the hospital and their informal caregivers (n = 10) | Surveys and semi-structured interviews | Quantitative and thematic analyses |
| Pfaeffli Dale 2015 (New Zealand) | 2011–2012 | Patient engagement | Mobile text messages and an interactive website with exercise prescriptions tailored to participants fitness level. | Patients with ischaemic heart disease (n = 17) | Surveys and interviews | Quantitative and thematic analyses |
| Pekmezaris 2016 (USA) | Not reported | Tele-visits/virtual visits | A telemonitoring system that combined monitoring of patients’ vital signs through a glucometer, blood pressure monitor, weight scale, pulse oximeter, and stethoscope with video visits from clinicians. | Patients with heart failure, caregivers, clinicians, and health policy representatives (n = 14) | Focus groups | Thematic analysis |
| Rief 2017 (USA) | 2010–2011 | Consumer access to clinical data | A patient accessible personal health record system that included medication lists, information about allergies and immunizations, medical histories, laboratory results, health reminders, and secure messaging. | Patients with coronary artery disease, heart failure, hypertension, or hyperlipidaemia (n = 41) | Focus groups | Thematic analysis |
| Sanders 2012 (UK) | 2008–2009 | Remote monitoring and management | A telehealth monitoring system that included a blood pressure monitor, glucometer, pulse oximeter, and weight scale and alerted clinicians upon patient decompensation. | Patients with diabetes, chronic obstructive pulmonary disease, or heart failure (n = 22) | Semi-structured interviews | Thematic analysis, grounded theory and constant comparison |
| Smith 2015 (India) | Not reported | Remote monitoring and management | The expanded use of mobile phones to improve cardiovascular disease management. | Accredited social health activists (n = 5), physicians (n = 5), and patients with cardiovascular disease (n = 5) | Semi-structured interviews | Thematic analysis |
| Treskes 2019 (Netherlands) | 2017 | Remote monitoring and management | The broad use of information technology to deliver health care in cardiology. | Physicians (n = 255) specializing in cardiology | Survey | Quantitative analysis |
| Wallin 2018 (Sweden) | 2015–2016 | Patient engagement | An internet-based cognitive behavioural therapy programme that included educational and therapeutic modules and secure messaging capabilities aimed to improve depression and anxiety symptoms. | Patients who were recently hospitalized for a myocardial infarction (n = 117) and were experiencing depression and/or anxiety | Semi-structured interviews | Content analysis |
| Walsh 2018 (Ireland) | 2016–2017 | Patient engagement | An internet-enabled, sensor-based home exercise cardiac rehabilitation programme to encourage exercise participation and self-management. | Patients with cardiovascular disease (n = 33) and healthcare stakeholders (n = 21) | Semi-structured interviews | Thematic analysis |
| Ware 2019 (Canada) | 2016–2018 | Remote monitoring and management | A mobile application where patients recorded their weight, blood pressure, heart rate, and symptoms to generate an algorithm that alerted clinicians upon decompensation. | Patients with heart failure (n = 24) who attended a heart function clinic | Surveys and semi-structured interviews | Quantitative and thematic analyses |
| Woods 2019 (Australia) | Not reported | Patient engagement | A mobile application designed to encourage self-management that included educational material, reminders and symptom tracking. | Patients with heart failure (n = 8) who had recently been discharged from the hospital | Surveys and semi-structured interviews | Quantitative and thematic analyses |
Characteristics of included studies
The studies in this review were published between 2008 and 2019, with a majority (n = 26) published after 2014. Studies were conducted in the following countries: Australia (n = 1), Canada (n = 3), China (n = 4), Finland (n = 1), India (n = 1), Ireland (n = 1), Netherlands (n = 1), New Zealand (n = 1), Singapore (n = 1), Sweden (n = 2), UK (n = 4), and the USA (n = 9). DHTs included in the review were classified into the following AMA categories:21 consumer access to clinical data (n = 1), patient engagement (n = 15), remote monitoring and management (n = 10), and tele-visits/virtual visits (n = 3). Most studies included patients with CVD (n = 27), heart failure (n = 19), in particular; other studies included informal caregivers (n = 5), healthcare clinicians (n = 7), and stakeholders and/or other participants (n = 3). Most of the studies (n = 13) employed qualitative methods of data collection, which included interviews and focus groups, some (n = 5) studies used quantitative methods, which included surveys, and the remaining studies (n = 11) used a combination of qualitative and quantitative methods. The data was analysed using qualitative methods, which included content analysis (n = 2), grounded theory (n = 3), inductive analysis (n = 1), thematic analysis (n = 6), quantitative analysis (n = 5), and mixed methods (n = 11) (Table 2).
Table 2.
Summary of characteristics of included studies (n = 29)
| Number of studies (%) | |
|---|---|
| Country | |
| Australia | 1 (3.4) |
| Canada | 3 (10.3) |
| China | 4 (13.8) |
| Finland | 1 (3.4) |
| India | 1 (3.4) |
| Ireland | 1 (3.4) |
| Netherlands | 1 (3.4) |
| New Zealand | 1 (3.4) |
| Singapore | 1 (3.4) |
| Sweden | 2 (6.9) |
| UK | 4 (13.8) |
| USA | 9 (31.0) |
| Participants | |
| Caregivers | 5 (17.2) |
| Patients | 27 (93.1) |
| Clinicians | 7 (24.1) |
| Stakeholders and other | 3 (10.3) |
| Digital health tool | |
| Consumer access to clinical data | 1 (3.4) |
| Patient engagement | 15 (51.7) |
| Remote monitoring and management | 10 (34.5) |
| Tele-visits/virtual visits | 3 (10.3) |
| Cardiovascular condition | |
| Arrhythmia | 1 (3.4) |
| Atherosclerotic aortic disease | 1 (3.4) |
| Coronary artery bypass graft | 1 (3.4) |
| Coronary artery disease | 6 (20.7) |
| Heart failure | 19 (65.5) |
| Myocardial infarction | 2 (6.9) |
| Unspecified | 5 (17.2) |
| Data collection | |
| Focus groups | 6 (20.7) |
| Interviews | 7 (24.1) |
| Mixed methods | 11 (37.9) |
| Surveys | 5 (17.2) |
| Analysis method | |
| Content analysis | 2 (6.9) |
| Grounded theory | 3 (10.3) |
| Inductive analysis | 1 (3.4) |
| Mixed methods analysis | 12 (41.4) |
| Quantitative analysis | 5 (17.2) |
| Thematic analysis | 6 (20.7) |
| Year of publication | |
| 2008 | 1 (3.4) |
| 2010 | 1 (3.4) |
| 2012 | 1 (3.4) |
| 2015 | 4 (13.8) |
| 2016 | 2 (6.9) |
| 2017 | 7 (24.1) |
| 2018 | 5 (17.2) |
| 2019 | 8 (27.6) |
Patient-level barriers and facilitators
We identified 14 distinct patient-level barriers and a total of 13 facilitators (Table 3). Of the 29 included studies, 25 (86.2%) studies reported patient-level barriers. The most frequent barriers in the literature were difficulty using technology (n = 7), poor internet connection (n = 7) and fear of using technology (n = 6), followed by feeling that the DHT delivered care was impersonal (n = 5), older age (n = 5) and lack of interest in technology (n = 5).
Table 3.
Patient-level barriers and facilitators of the uptake of digital health technology (29 studies)
| Studies, n (%) | |
|---|---|
| Facilitators | |
| Improved connection and communication with clinicians | 10 (34.5) |
| Personalized components | 6 (20.7) |
| Easy to use technology | 5 (17.2) |
| Previous experience with technology | 5 (17.2) |
| Perceived usefulness | 5 (17.2) |
| Empowerment | 5 (17.2) |
| Education and training sessions | 3 (10.3) |
| Support from family and/or caregivers | 2 (6.9) |
| Home internet access | 2 (6.9) |
| Technological support | 2 (6.9) |
| Willingness to learn | 1 (3.4) |
| Higher education | 1 (3.4) |
| Improved sense of security | 1 (3.4) |
| Barriers | |
| Difficult to use technology | 7 (24.1) |
| Poor internet connection | 7 (24.1) |
| Fear of using technology | 6 (20.7) |
| Impersonal care delivery | 5 (17.2) |
| Older age | 5 (17.2) |
| Lack of interest in technology | 5 (17.2) |
| Cognitive impairment | 4 (13.8) |
| Technical problems | 4 (13.8) |
| Time consuming | 4 (13.8) |
| Emotional and/or moral implications | 3 (10.3) |
| Financial concerns | 3 (10.3) |
| Language barriers | 3 (10.3) |
| Anxiety and/or other mental health conditions | 1 (3.4) |
| Mobility limitations | 1 (3.4) |
Of the 29 included studies, 24 (82.7%) studies reported patient-level facilitators. The most frequent facilitator was improved connection and communication with clinicians (n = 10), followed by personalized components within the technology (n = 6), previous experience with technology (n = 5), easy to use technology (n = 5), and perceived usefulness of the technology (n = 5).
Clinician-level barriers and facilitators
We identified 10 distinct clinician-level barriers and a total of 5 facilitators (Table 4). Of the 29 included studies, 6 (20.7%) studies reported clinician-level barriers. The most frequent barriers we identified were increased work and responsibilities (n = 4) and unreliable technologies and/or lack of evidence supporting the use of technology (n = 3), lack of integration with electronic medical records (n = 3), followed by financial concerns (n = 2), data privacy and security (n = 2), and feeling that DHT delivered care was impersonal (n = 2).
Table 4.
Clinician-level barriers and facilitators of the uptake of digital health technology (29 studies)
| Studies, n (%) | |
|---|---|
| Facilitators | |
| Approval and organizational support from cardiology departments and/or hospitals | 3 (10.3) |
| Improved efficiency | 3 (10.3) |
| Perceived usefulness | 2 (6.9) |
| Increased communication with patients and clinicians | 2 (6.9) |
| Training programmes | 1 (3.4) |
| Barriers | |
| Increased work and responsibilities | 4 (13.8) |
| Unreliable technologies and/or lack of evidence supporting the use of technology technology | 3 (10.3) |
| Lack of integration with electronic medical records | 3 (10.3) |
| Data privacy and security concerns | 2 (6.9) |
| Financial concerns | 2 (6.9) |
| Impersonal care delivery | 2 (6.9) |
| Lack of customizable features | 1 (3.4) |
| Negative pressure from department and/or hospitals | 1 (3.4) |
| Healthcare reimbursement issues | 1 (3.4) |
| Time consuming | 1 (3.4) |
Of the 29 included studies, 4 (13.8%) studies reported clinician-level facilitators. The most frequent facilitators were approval and organizational support from cardiology departments and/or hospitals (n = 3), technologies that improved efficiency (n = 3), technologies that clinicians perceived as useful (n = 2) and technologies that improved communication between patients and clinicians (n = 2).
Researcher-level barriers and facilitators
Of the 29 included studies, no studies reported researcher-level barriers or facilitators.
Discussion
This systematic scoping review included 29 studies highlighting factors that impede or facilitate the uptake of DHT in cardiovascular care. We found that common barriers of DHT among patients included difficult-to-use technology, a poor internet connection, and fear of using technology. Common facilitators of DHT use among patients included improved connection and communication with clinicians, personalized components within the DHT, and user-friendliness. The most frequent barriers to DHT uptake by clinicians included increased work related to the technology, unreliable technology or limited evidence supporting its use, and lack of integration with electronic medical records. The most common facilitators among clinicians were institutional approval and organizational support, improved efficiency, and DHTs that were perceived to be useful. We did not identify any researcher-level barriers or facilitators of DHT.
To the best of our knowledge, this is the first systematic review to report barriers and facilitators of DHT adoption in cardiovascular care. There is limited published literature on barriers to and facilitators of DHT adoption across the medical field. A systematic review of 101 studies assessed factors influencing the uptake of DHT by clinicians across multiple diseases.19 Consistent with our findings, this review found that the most common facilitating factor for clinicians was perceived usefulness of DHT, and that lack of reimbursement and unreliable technologies were barriers to DHT adoption by clinicians.19 However, this review did not report on cardiovascular care. A recent scoping review of 36 studies assessed factors associated with DHT adoption for hypertension management.20 Also consistent with our findings, this review found that impersonal and difficult to use technologies were barriers to, and easy to use technologies and enhanced patient-clinician communication were facilitators of DHT uptake among patients.20 This review also did not report on cardiovascular care. We did not identify any publications that reported researcher-level barriers or facilitators in CVD, or any other disease state.
A number of frameworks have been developed to describe the acceptance and usage of DHTs. These frameworks include the Theory of Reasoned Action,21 Theory of Planned Behaviour,22 Technology Acceptance Model,23 Unified Theory of Acceptance and Use of Technology,24 Innovation Diffusion Theory,25 and the Social Cognitive Theory.26 A recent literature review examined the conceptual models and identified similarities between the constructs used to explain DHT adoption and use.27 We found that the results of our thematic analysis aligned with many of the common constructs. For example, perceived usefulness of DHTs, facilitating conditions such as internet access and technological support, and easy to use DHTs were common constructs amongst the frameworks,27 all of which we identified to be facilitating factors for DHT adoption. The consistencies between our findings and the pre-existing frameworks suggest that there may be common barriers and facilitators that underlie DHT uptake. Thus, targeted efforts to address these factors may improve DHT adoption, both in CVD care and across medicine as a whole.
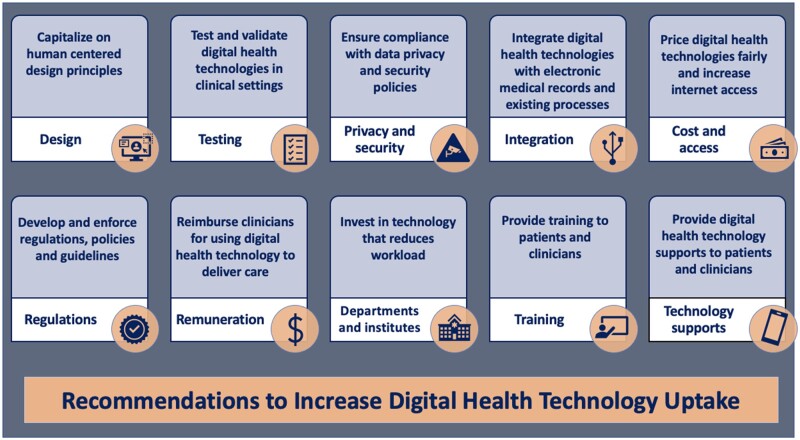
Recommendations to increase the uptake of digital health technologies
As we navigate the COVID-19 era and beyond, urgent actions are required if we are to realize the potential of DHT.11,12 Focused strategies should be implemented to address the identified barriers and facilitators, and to promote DHT uptake in cardiovascular care (Table 5).
Table 5.
Recommendations to increase the uptake of digital health technology in cardiovascular care
| Design | Developers should capitalize on human-centred design principles and engage patients, clinicians and stakeholders throughout the entirety of the technology development process. |
| Testing |
Digital health technologies should be tested and validated with cardiology patients and clinicians. Digital health technologies should be routinely evaluated to account for data errors as a result of user error and/or misuse. Evaluation of all digital health technologies should adhere to quality improvement guidelines. |
| Data privacy and security | Digital health technologies should comply with all required data governance, privacy, and security regulations. |
| Personalized care | Digital health technology should supplement in-person care (blended model). |
| Integration | Digital health technologies should be integrated with electronic medical records and existing health system processes. |
| Cost and access |
Digital health technologies should be tailored to the target regions. Federal and private sectors should invest in technology and infrastructure to increase internet/broadband access. Digital health technologies should be priced fairly to the target users. Patients should be able to claim digital health technologies through insurance plans. Subsidized smartphone plans, free Wi-Fi hotspots and technology renting programmes should be available for those who need it. Automated applications and devices that do not require continuous internet access should be implemented in regions without sufficient infrastructure and funds to support cellular and data coverage. |
| Regulations |
Regulatory models should be developed to permit clinicians to obtain credentialing for digital health technologies. Regulations and guidelines should be established to define and communicate responsibilities and liabilities to technology vendors, clinicians and patients. All digital health technologies should be regulated and certified. |
| Reimbursement |
Clinicians should receive reimbursement for using digital health technologies. Guidelines, policies and procedures for using and reimbursing digital health technologies should be implemented. |
| Departments and institutions |
Departments and institutions should support and encourage the use of digital health technologies. Performance incentives and mandates should be implemented to encourage digital health technology uptake. |
| Patient supports |
Patients should receive education on the benefits of digital health technologies. Patients should be encouraged to participate in e-patient movements and advocacy. Patients should be provided with accessible translating features and accessible technologies to overcome language barriers and disabilities. Family members and informal caregivers should engage in digital health technologies with the patients. |
| Training |
Patients should be provided training when prescribed digital health technology. Medical students should receive education on digital health technology as part of their medical school curriculum. Practicing clinicians should be required to complete continuing education programmes or certification courses in digital health technology. |
| Technology support | Technology supports should be provided for both patients and clinicians. |
Design and testing
Developers should capitalize on human-centred design principles, which involve co-designing, testing and improving the technologies based on partnerships between industry, patients, and clinicians.28 The number of DHTs is continuously increasing, with more than 300 000 health applications and more than 200 being added daily.29 A recent review found that most DHTs were not evaluated in randomized controlled trials, despite this methodology being the gold standard for evaluating the effectiveness of healthcare interventions.30 Traditionally designed randomized controlled trials are unable to match the speed of DHT development, and must evolve to efficient, pragmatic designs.30 Innovative observational designs, such as simulation-based research, could also facilitate quality and timely evidence.30 The World Health Organization’s recommendations for DHTs and the United Kingdom’s Institute for Health and Care Excellence’s Evidence Standard Framework can be used to guide the evaluation of DHTs.31,32
Integration
DHTs should be integrated into the current clinician workflow in order to improve access for clinicians and to effectively scale up DHTs.33 A literature review concluded that increased quality of care, reduced medical errors, improved patient outcomes and reduced costs were among the benefits that occur when DHT is integrated into existing workflows.34 Medical record systems and DHTs are often proprietary and designed to function as stand-alone systems. Clinicians are less likely to adopt DHTs that do not reduce their workload and that require them to maintain multiple systems.31,33 Without proper integration and interoperability, DHT uptake will likely be limited to clinicians who are highly motivated to adopt technologies despite the inefficiencies in workflow that come from information siloes.31,33
Cost and access
Efforts should be directed towards increasing access to and affordability of DHTs. Although approximately 4 billion people used the internet worldwide in 2019, internet usage was significantly greater in high-income than low- and middle-income regions (82% in Europe vs. 28% in Africa).35 Federal and private sectors should invest in technology and infrastructure to increase internet access, and should work on negotiating fair pricing for DHTs with vendors similar to that done for drugs and implanted devices in single-payer systems.35,36 DHTs directed at patients in the form of mobile devices, applications, or wearables should be priced fairly, and should potentially be supported through insurance plans. A survey of 253 829 individuals in the USA found that higher income was a significant predictor of owning and/or utilizing DHTs compared to lower incomes.37 Subsidized smartphone plans and free Wi-Fi hotspots can also provide temporary solutions for accessibility barriers.12
Regulation
In fee-for-service systems, clinicians should be remunerated for delivery of healthcare through DHTs. Current policies in many jurisdictions require face-to-face encounters for remuneration of consults.38 A survey of 106 clinicians in Canada, the USA and Ireland found that regulatory and reimbursement disincentives, including the inability to bill for services delivered via telemedicine, were among the most prominent barriers to DHT adoption.39 To overcome these barriers, the credentialing process for provision of DHT-related services could be made easier so that it is easy for clinicians to begin to provide care using digital health platforms. Reimbursement schemes for clinician services should not be contingent on the use of approved but expensive proprietary platforms; for example, clinicians and patients could be encouraged to use videoconferencing platforms that are Health Insurance Portability and Accountability Act (HIPAA)-compliant but available on their smart phones or existing devices instead of investing in more expensive, vendor-specific platforms. Remuneration for services should extend to the range of care options that are possible using DHTs, including but not limited to remote monitoring, telephone advice to patients or clinicians, digital consultation to patients and clinicians, and electronic communications regarding patient care. DHTs should comply with required privacy and security regulations, which include storing data in secure, firewall-protected, access-controlled locations.40 DHTs should also follow robust data governance frameworks that include ethical oversight and informed consent processes, data protection and sustainability of ethical data use to reduce the risks of data breach or misuse.40
Institutional support
Institution should consider the long-term cost savings of DHTs through increased efficiencies and less reliance on bricks and mortar, and support their use among clinicians through training, financial investment in networks, and technical support. The long-term healthcare system benefits and cost savings from DHTs are often unclear to institutions and the upfront costs of DHTs are a barrier to uptake. A systematic review of 14 studies examined the cost-effectiveness of DHTs used in CVD care.41 This review found that 43% of studies reported an association between DHTs, higher quality-adjusted life years (QALYs), and cost savings.41 Among the remaining 57% of studies, costs were higher, but cost-effectiveness ratios were acceptable given the concomitant increase in QALYs.41 DHT use and mandates for DHT use are associated with increased DHT uptake.42 Although we did not identify the involvement of healthcare administrative staff or other stakeholders as barriers or facilitators of DHT uptake, the AMA found that engaging hospital executives and administrative staff in DHT decision processes improved DHT uptake.43 The engagement of administrative staff and stakeholders should be considered when implementing DHT.43
Training
Training programmes for patients and clinicians can facilitate uptake of DHTs and mitigate patients’ uneasiness and fear around the use of DHT. An analysis of 101 older adults (≥65 years) enrolled in the intervention arm of a randomized controlled trial found that training programmes and continued support were crucial to patient uptake.44 New approaches should also be applied in modern medical education to ensure that new clinicians have the skills required to work with DHTs. A mixed methods review investigated the impact that DHT-based lessons had on medical students.45 The review found that students that received DHT-based lessons were more familiar with available DHTs and felt more comfortable using DHTs in their future careers compared to students who did not receive the lessons.45 Other training approaches should be implemented to reach practicing clinicians, such as formally requiring DHT training as part of continuing education courses or certification programmes.
Support groups and engagement of caregivers
Patients and clinicians should be provided with technology support to increase DHT uptake. The support for patients could come from formal programmes or peer support groups and e-movements.46 Supports to overcome language barriers may further increase the uptake of DHT.47 A mobile application integrated with language translating features was found to improve the quality of communication between clinicians and patients with limited English proficiency.48 In addition, clinicians and industry stakeholders should recognize and respect that not all patients will want to engage with DHTs.49 Informal caregivers and family members are important participants in healthcare, and engaging them in DHT may also be a strategic way to increase uptake among patients. An observational study of 11 patients and 5 caregivers found that caregivers who engaged in DHTs alongside patients were able to better interpret health information, advocate for quality care and better manage their patient’s medical care compared to caregivers who did not engage in DHTs.50 To further support patients, DHT could be used to supplement in-person care as part of a blended model. A narrative review found that a blended model of care between usual in-person care and DHT delivered care helped patients with chronic diseases better manage their conditions, compared to DHT delivered care alone.51
Strengths and limitations
The strengths of our review included the comprehensive, evidence-based scoping review methodology,14 which provided the opportunity to develop an evidence base in the topic area.52 The screening, data extraction and thematic coding were completed independently and in duplicate, with high inter-rater agreeability, which reduced the likelihood of single reviewer bias. We utilized a broad search strategy that did not restrict studies by language or publication date, reducing the likelihood of language and selection bias. Our review also identified a significant gap in the literature, as we did not find any published primary literature on researcher-level barriers or facilitators of DHT uptake. Researcher-level barriers and facilitators of DHT uptake should be a focus of future research to further increase DHT adoption in CVD care.
Limitations of our review must be acknowledged. In keeping with the scoping review methodology,14,52 our search and study inclusion process were broader than a standard systematic review but also more limited in terms of databases. There is a possibility that eligible studies may have been missed. To mitigate this risk, we hand searched the reference lists of all articles that underwent full text screening. Ascertaining barriers and facilitators of DHTs was not the primary aim of all of our included studies. We used our best judgement to identify and classify themes but recognize that our personal biases may have influenced the thematic analysis. The DHTs described in the included studies were multifaceted. This may have led to misclassification errors in the thematic analysis. However, we anticipate this error being small as all stages of the review were completed in duplicate and consensus was reached for each classification. Lastly, we did not include grey literature, which may have biased our results as the field of DHT is moving rapidly and novel interventions may not have been published. We recommend that researchers aiming to build on this work use a wider range of databases, search unpublished and grey literature and incorporate direct feedback from relevant stakeholders, researchers, clinicians, and patient partners.
Conclusion
DHT has the potential to revolutionize CVD healthcare delivery. Our results demonstrate that there are a multitude of barriers and facilitators to the uptake of DHT in cardiovascular care. The findings of this study can be used to inform and guide clinicians, and stakeholders who wish to develop and implement DHTs that meet the needs of clinicians and patients in the COVID-19 era and beyond.
Supplementary material
Supplementary material is available at European Heart Journal Digital Health online.
Funding
H.G.C.V. receives research funding from the Canadian Institutes of Health Research and research salary support from McMaster University Department of Medicine and Women As One Escalator Award.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflict of interest: none declared.
Supplementary Material
References
- 1.World Health Organization. The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (6 July 2020).
- 2.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Heart Association. Heart and stroke statistics 2017 at a glance. https://healthmetrics.heart.org/wp-content/uploads/2017/06/Heart-Disease-and-Stroke-Statistics-2017-ucm_491265.pdf (6 July 2020).
- 5.World Health Organization. 2013-2020 Action plan for the global strategy for the prevention and control of noncommunicable diseases. https://apps.who.int/iris/bitstream/handle/10665/94384/9789241506236_eng.pdf;jsessionid=5CB55F0DCAAB17E003D31C6CDD674A7D?sequence=1 (6 July 2020).
- 6. Mitchell M, Kan L.. Digital technology and the future of health systems. Health Syst Reform 2019;5:113–120. [DOI] [PubMed] [Google Scholar]
- 7. Lopez Perales CR, Van Spall HGC, Maeda S, Jimenez A, Latcu DG, Milman A, Kirayoya-Samadoulougou F, Mamas MA, Muser D, Arroyo RC. Mobile health applications for the detection of atrial fibrillation: a systematic review. Europace 2020;23:11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oseran AS, Wasfy JH.. Early experiences with cardiology electronic consults: a systematic review. Am Heart J 2019;215:139–146. [DOI] [PubMed] [Google Scholar]
- 9. Eliya Y, Pellegrini D, Gevaert A, Code J, Van Spall HGC.. Social media in heart failure: a mixed methods systematic review. Curr Cardiol Rev 2019. https://pubmed.ncbi.nlm.nih.gov/31820703/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inan OT, Tenaerts P, Prindiville SA, Reynolds HR, Dizon DS, Cooper-Arnold K, Turakhia M, Pletcher MJ, Preston KL, Krumholz HM, Marlin BM, Madl KD, Klasnja P, Spring B, Iturriaga E, Campo R, Desvigne-Nickens P, Rosenberg Y, Steinhuble SR, Califf RM.. Digitizing clinical trials. NPJ Digit Med 2020;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keesara S, Jones A, Schulman K.. Covid-19 and health care’s digital revolution. N Engl J Med 2020;382:e82. [DOI] [PubMed] [Google Scholar]
- 12. Whitelaw S, Mamas MA, Topol E, Van Spall HGC.. Applications of digital technology in COVID-19 pandemic planning and response. Lancet Digit Health 2020;2:e435–e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frederix I, Caiani EG, Dendale P, Anker S, Bax J, Bohm A, Cowie M, Crawford J, de Groot N, Dilaveris P, Hansen T, Koehler F, Krstacic G, Lambrinou E, Lancellotti P, Meier P, Neubeck L, Parati G, Piotrowicz E, Tubaro M, van der Velde E.. ESC e-Cardiology working group position paper: overcoming challenges in digital health implementation in cardiovascular medicine. Eur J Prev Cardiol 2019;26:1166–1177. [DOI] [PubMed] [Google Scholar]
- 14. Arksey H, O’Malley L.. Scoping studies: towards a methodological framework. Int J Soc Res 2005;8:19–32. [Google Scholar]
- 15. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA,, Chang C,, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tuncalp O, Straus SE.. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–473. [DOI] [PubMed] [Google Scholar]
- 16.American Medical Association. Physicians’ motivations and requirements for adopting digital health Adoption and attitudinal shifts from 2016 to 2019. https://www.ama-assn.org/system/files/2020-02/ama-digital-health-study.pdf (7 July 2020).
- 17. Busetto L, Luijkx K, Calciolari S, Ortiz LGG, Vrijhoef HJM.. Barriers and facilitators to workforce changes in integrated care. Int J Integr Care 2018;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Braun V, Clarke V.. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. [Google Scholar]
- 19. Gagnon MP, Desmartis M, Labrecque M, Car J, Pagliari C, Pluye P, Fremont P, Gagnon J, Tremblay N, Legare F.. Systematic review of factors influencing the adoption of information and communication technologies by healthcare professionals. J Med Syst 2012;36:241–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palacholla RS, Fischer N, Coleman A, Agboola S, Kirley K, Felsted J, Katz C, Lloyd S, Jethwani K.. Provider- and patient-related barriers to and facilitators of digital health technology adoption for hypertension management: scoping review. JMIR Cardio 2019;3:e11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fishbein M, Ajzen I.. Belief, Attitude, Intention, and Behavior Reading. MA: Addison-Wesley; 1975. p913–927. [Google Scholar]
- 22. Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179–211. [Google Scholar]
- 23. Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quarterly 1989;13:319–340. [Google Scholar]
- 24. Venkatesh V, Morris MG, Davis GB, Davis FD.. User acceptance of information technology: Toward a unified view. MIS Quarterly 2003;27:425–478. [Google Scholar]
- 25. Rogers EM. Diffusion of Innovations, 5th edition. Toronto: Simon and Schuster; 2010. [Google Scholar]
- 26. Bandura A, Walters RH.. Social Learning Theory. Englewood Cliffs, NJ: Prentice-Hall; 1977. [Google Scholar]
- 27. Kim Y, Crowston K.. Technology adoption and use theory review for studying scientists' continued use of cyber‐infrastructure. Proc Am Soc Inf Sci Technol 2011;48:1. [Google Scholar]
- 28.UID. Design thinking—new old creativity. https://www.uid.com/en/news/design-thinking-revolutionize-human-centred-design (14 July 2020).
- 29.IQVIA. Institute for human data science study: impact of digital health grows as innovation, evidence and adoption of mobile health apps accelerate. https://www.iqvia.com/newsroom/2017/11/impact-of-digital-health-grows-as-innovation-evidence-and-adoption-of-mobile-health-apps-accelerate/ (7 January 2021).
- 30. Guo C, Ashrafian H, Ghafur S, Fontana G, Garder C, Prime M.. Challenges for the evaluation of digital health solutions—a call for innovative evidence generation approaches. NPJ Digit Med 2020;3:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Recommendations on digital interventions for health system strengthening. https://apps.who.int/iris/bitstream/handle/10665/311941/9789241550505-eng.pdf?ua=1 (7 January 2021). [PubMed]
- 32.National Institute for Health and Care Excellence. Evidence standards framework for digital health technologies. https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/evidence-standards-framework/digital-evidence-standards-framework.pdf (7 January 2021).
- 33. Topol E. Preparing the Healthcare Workforce to Deliver the Digital Future: The Topol Review: An Independent Report on Behalf of the Secretary of State for Health and Social Care. London, UK: NHS Health Education; 2019. [Google Scholar]
- 34. Miller RH, Sim I.. Physicians’ use of electronic medical records: barriers and solutions. Health Aff 2004;23:116–126. [DOI] [PubMed] [Google Scholar]
- 35.GSMA. State of mobile internet connectivity. https://www.gsma.com/mobilefordevelopment/wp-content/uploads/2019/07/GSMA-State-of-Mobile-Internet-Connectivity-Report-2019.pdf (21 May 2020). [Google Scholar]
- 36. Liu P, Astudillo K, Velez D, Kelley L, Cobbs-Lomax D, Spatz ES.. Use of mobile health applications in low-income populations: a prospective study of facilitators and barriers. Circ Cardiovasc Qual Outcomes 2020;13:e007031. [DOI] [PubMed] [Google Scholar]
- 37. Mahajan S, Lu Y, Spatz ES, Nasir K, Krumholz HM.. Trends and predictors of use of digital health technology in the United States. Am J Med 2020;134:129–134. [DOI] [PubMed] [Google Scholar]
- 38. Stanistreet K, Verma J, Kirvan K, Drimer N, Liddy C.. Physician remuneration for remote consults: an overview of approaches across Canada. Healthc Q 2017;20:12–15. [DOI] [PubMed] [Google Scholar]
- 39. Rogove HJ, McArthur D, Demaerschalk BM, Vespa PM.. Barriers to telemedicine: survey of current users in acute care units. Telemed J E Health 2012;18:48–53. [DOI] [PubMed] [Google Scholar]
- 40. Filkins BL, Kim JY, Roberts B, Armstrong W, Miller MA, Hultner ML, Castillo AP, Ducome JC, Topol EJ, Steinhubl SR.. Privacy and security in the era of digital health: what should translational researchers know and do about it? Am J Transl Res 2016;8:1560–1580. [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang X, Ming WK, You JH.. The cost-effectiveness of digital health interventions on the management of cardiovascular diseases: systematic review. J Med Internet Res 2019;21:e13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Christodoulakis C, Asgarian A, Easterbrook S. Barriers to adoption of information technology in healthcare. In: Marcellus M. (ed.) Proceedings of the 27th Annual International Conference on Computer Science and Software Engineering. New Jersy, United States. 2017;66–75.
- 43.American Medical Association. Digital health implementation playbook. https://www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf (7 January 2021).
- 44. Tsai HY, Rikard RV, Cotten SR, Shillair R.. Senior technology exploration, learning, and acceptance (STELA) model: from exploration to use–a longitudinal randomized controlled trial. Educ Gerontol 2019;45:728–743. [Google Scholar]
- 45. Waseh S, Dicker AP.. Telemedicine training in undergraduate medical education: mixed-methods review. JMIR Med Educ 2019;5:e12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chu A, Huber J, Mastel-Smith B, Cesario S.. “Partnering with seniors for better health”: computer use and internet health information retrieval among older adults in a low socioeconomic community. J Med Libr Assoc 2009;97:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang DTS, Thyer IA, Hayne D, Katz DJ.. Using mobile technology to overcome language barriers in medicine. Ann R Surg Engl 2014;96:e23–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Müller F, Chandra S, Furaijat G, Kruse S, Waligorski A, Simmenroth A, Kleinert E.. A Digital Communication Assistance Tool (DCAT) to obtain medical history from foreign-language patients: development and pilot testing in a primary health care center for refugees. Int J Environ Res Public Health 2020;17:1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Knowles B, Hanson VL.. The wisdom of older technology (non)users. Commun ACM 2018;61:72. [Google Scholar]
- 50. Tieu L, Sarkar U, Schillinger D, Ralston JD, Ratanawongsa N, Pasick R, Lyles CR.. Barriers and facilitators to online portal use among patients and caregivers in a safety net health care system: a qualitative study. J Med Internet Res 2015;17:e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Talboom-Kamp EP, Verdijk NA, Kasteleyn MJ, Numans ME, Chavannes NH.. From chronic disease management to person-centered eHealth; a review on the necessity for blended care. Clin eHealth 2018;1:3–7. [Google Scholar]
- 52. Pham MT, Rajic A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA.. A scoping review of scoping reviews: advancing the approach and enhancing consistency. Res Synth Methods 2014;5:371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflict of interest: none declared.