The Slovakian test case
Toward the end of 2020, Slovakia decided that it would test and then isolate positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases among its entire population of 5.5 million, and more than 50,000 positive cases were found during a rapid antigen testing campaign. Pavelka et al. analyzed the data and found that in 41 counties before and after the two rounds of testing, infection prevalence declined by about 80% (see the Perspective by Garca-Fiana and Buchan). They also used the data to test a microsimulation model for one county. Quarantine of the whole household after a positive test was essential to achieving a large reduction in prevalence. Since Autumn 2020, transmission in Slovakia has rebounded, despite other interventions, because high-intensity testing was not sustainable.
Science, this issue p. 635; see also p. 571
A nationwide testing effort in Slovakia during late 2020 reduced infection prevalence by more than 80% in 2 weeks but could not be sustained.
Abstract
Slovakia conducted multiple rounds of population-wide rapid antigen testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2020, combined with a period of additional contact restrictions. Observed prevalence decreased by 58% (95% confidence interval: 57 to 58%) within 1 week in the 45 counties that were subject to two rounds of mass testing, an estimate that remained robust when adjusting for multiple potential confounders. Adjusting for epidemic growth of 4.4% (1.1 to 6.9%) per day preceding the mass testing campaign, the estimated decrease in prevalence compared with a scenario of unmitigated growth was 70% (67 to 73%). Modeling indicated that this decrease could not be explained solely by infection control measures but required the addition of the isolation and quarantine of household members of those testing positive.
Nonpharmaceutical interventions have been extensively used worldwide to limit the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). These have included travel restrictions, mandating of face masks, closure of schools and nonessential businesses, and nationwide stay-at-home orders. All the measures were aimed at mitigating ill-health due to COVID-19 (2, 3); however, they also place an unprecedented economic and social burden on the majority of uninfected people (4, 5). Testing of reported symptomatic cases and tracing their contacts aims to provide a more targeted measure but, in many settings, has proven insufficient for containing transmission (6).
Mass testing campaigns are an alternative way to identify infectious individuals and allow the targeting of interventions without much added burden to those not infectious. However, the polymerase chain reaction (PCR) for the diagnosis of a SARS-CoV-2 infection is not suitable for mass use. Although laboratory capacities have been upscaled in record time, PCR testing remains expensive and often has turnaround times of more than 1 day, diminishing its utility (7). The PCR detection window also typically extends to the postinfectious period by detecting RNA fragments, hence identifying as infected those who are no longer infectious (8).
By contrast, rapid antigen tests are cheap and can be quickly produced in large quantities, offering results on site in 15 to 30 min without the need for a laboratory. They are less sensitive in detecting infections with low viral load that are less likely to transmit, but can detect over 70% of likely infectious cases. A recent observational study estimated the sensitivity of lateral flow devices in detecting infectious individuals to be as high as 83 to 91% (9). This makes mass testing a viable part of the portfolio of nonpharmaceutical interventions (10, 11).
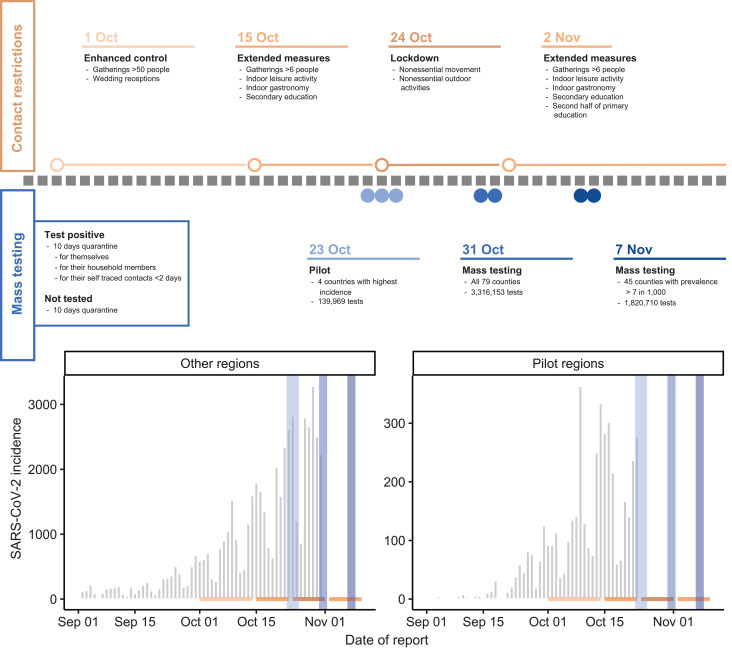
In October and November 2020, Slovakia used rapid antigen tests in a campaign that targeted the whole population to identify infectious cases at scale, rapidly reduce transmission, and thus allow easing of lockdown measures (12). A pilot took place between 23 and 25 October in the four most affected counties, followed by a round of national mass testing on 31 October and 1 November (round 1). High prevalence counties were again targeted with a subsequent round of testing on 7 and 8 November (round 2) (Fig. 1).
In total, 5,276,832 SD-Biosensor Standard Q rapid antigen tests were conducted by trained medical personnel during the mass testing campaigns, with 65% of the respective populations tested in the pilot, 66% in mass testing round 1 and 62% in round 2. This corresponded to 87, 83, and 84% of the age-eligible population (10 to 65 years and older adults in employment) in each round, respectively. It does not include residents who were quarantining at the time of the campaign or the 534,300 tests that were conducted on medical, military, and governmental personnel who were not included in geographical county data.
A total of 50,466 participants tested positive, indicating the presence of currently infectious SARS-CoV-2. The proportion of positive tests was 3.91% (range across counties: 3.12 to 4.84%) in the pilot, 1.01% (range: 0.13 to 3.22%) in round 1, and 0.62% (range: 0.28 to 1.65%) in round 2 (Fig. 2, C and D).
The potential for large numbers of false-positive tests has been a point of criticism for mass testing campaigns. Although multiple studies have found high specificity for the Biosensor test kit, they are not sufficiently powered to exclude specificity levels that at a population level would yield an overwhelming amount of false positives (13). From the low test-positive rates in some counties, we estimate with 95% certainty that the specificity of the SD Biosensor Standard Q antigen test exceeded 99.85%, and the occurrence false positives was therefore not of major concern in this study.
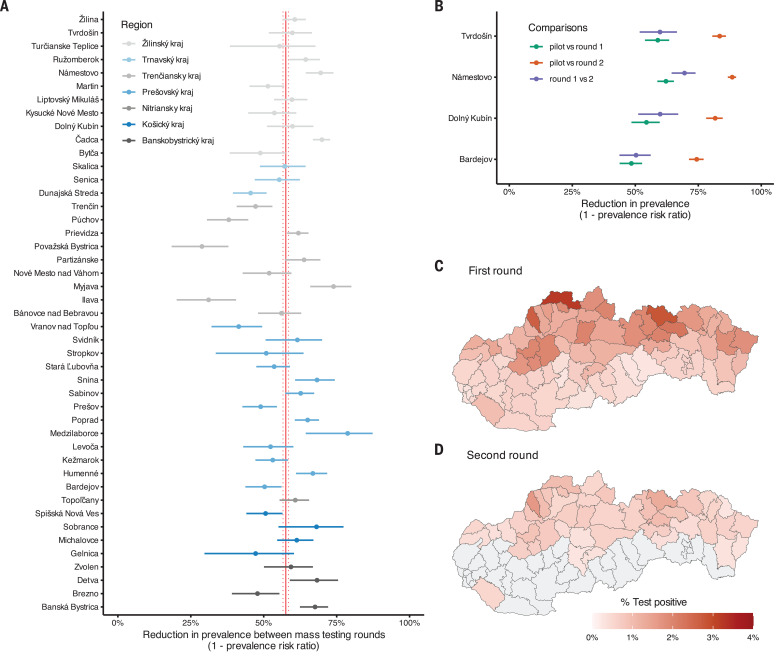
The counties with the highest prevalence were found in the Northern part of the country, whereas the two main Slovakian cities of Bratislava and Koice had some of the lowest observed prevalences (Fig. 1C). Reflecting this pattern, we found that high county-level prevalence was associated with a younger average population age and a lower population density (fig. S8). Given that prevalence varied at a much smaller than county scale (14), such associations may be clearer at the individual or community level, as observed in other countries.
Fig. 1. Overview of interventions and premass testing epidemiology.
(Top) Description of timing and extent of national contact restriction in Slovakia (color intensity indicates intensity of the measures) and timing and extent of the mass testing campaigns. Open circles and lines in respective colors indicate the start and duration of the contact restrictions, and the blue solid circles indicate the days on which mass testing was conducted, although the highest turnout was usually on the first day. (Left) Box illustrating contact-reducing measures for those testing positive and those who chose not to be tested. (Bottom) SARS-CoV-2 infection incidence as reported by the Slovak Ministry of Health and collected through passive symptomtriggered PCR testing. Using the same color coding as at the top, contact interventions are indicated by horizontal lines, and mass testing campaigns are indicated by vertical lines. Data from the passive surveillance subsequent to the respective first mass testing campaign are omitted to clearly illustrate the trends in infection rates that led up to the mass testing and because mass testing is likely to have changed the sensitivity of the passive surveillance, thereby distorting the observation of infection trends that followed mass testing.
In the four counties where the pilot was conducted, observed infection prevalence decreased by 56% [95% confidence interval (CI): 54 to 58%] between the pilot and round 1 of the mass testing campaign and a further 60% (95% CI: 56 to 63%) between rounds 1 and 2, totaling a decrease of 82% (95% CI: 81 to 83%) over 2 weeks. There was little heterogeneity between counties (Fig. 2B).
Fig. 2. The change in test positivity between mass testing campaigns.
(A) Change in test positivity [1 crude prevalence ratio (cPR)] observed from mass testing round 1 to round 2 in the 45 counties that were eligible for both rounds of mass testing. Counties are grouped and color coded into regions. The crude pooled estimate and its 95% confidence bounds are shown as red vertical lines. The confidence intervals were estimated using a normal approximation (Wald interval). (B) Change in test positivity (1 cPR) observed from the pilot mass testing round to either the first (green) or the second (orange) national round and from the first to the second mass testing round (blue) in the four counties that were included in the pilot. The confidence intervals were estimated using a normal approximation (Wald interval). (C and D) County-level test positivity in the (C) first and (D) second round of mass testing. Gray areas indicate counties that were not part of the second round because their test positivity rate was less than 7 per 1000 and hence have no estimates.
Among the 45 counties that were included in round 2 of the mass testing campaign, observed infection prevalence decreased by 58% (95% CI: 57 to 58%) in 1 week. Combining the pilot results with the ones from the two rounds of testing in 45 counties, each round of mass testing was estimated to have reduced observed infection prevalence by 56% (95% CI: 52 to 59%) when adjusted for attendance rates, reproduction number, and prevalence in previous rounds. The estimated reduction between rounds varied considerably by county, from 29% in county Povask Bystrica to 79% in county Medzilaborce, but although heterogeneous showed no regional pattern (Fig. 2A). Neither region, attendance rates, prevalence in round 1, nor the estimated growth rate before mass testing showed any significant impact on the observed county-specific reductions.
At the time of round 1 of the mass testing campaign, the incidence of confirmed cases reported through the syndromic surveillance system was rising in nonpilot counties, with an estimated infection growth rate of 4.4% (1.1% to 6.9%) per day. When adjusting for this growth trend, we estimated a self-adjusted prevalence ratio (saPR) of 0.30 (0.27 to 0.33). In the pilot counties, reported infection incidence showed signs of leveling in the week before the mass testing campaign, with an estimated infection growth rate of 1.3% (7.4 to 7.8%), yielding a respective saPR of 0.31 (0.26 to 0.33).
Because we used the test positivity rate of the subsequent round to estimate the impact of the previous one, we were unable to observe the impact of the last round in each county and hence the full effect of the campaign.
However, we found that the reduction achieved per round of testing was 56% (52 to 59%), indicating that the 41 counties with two rounds of testing likely reduced infection prevalence by 81% (77 to 83%) within 2 weeks and that the four counties included into the pilot testing reduced infection prevalence by 91% (89 to 93%) within 3 weeks.
The observational nature of this study made it difficult to separate the effects of the mass testing campaigns from that of the other nonpharmaceutical interventions introduced over the same period that aimed to reduce contacts and mobility, although much less than during the spring lockdown (fig. S4). Nevertheless, a greater than 50% decline in infection prevalence within 1 week (or 80% in 2 weeks) is noteworthy, particularly while primary schools and workplaces were mostly open. For comparison, a month-long lockdown in November in the UK resulted in just a 30% decrease in prevalence (15). This, alongside the inability in December to control the rebounding spread of SARS-CoV-2 in Slovakia through even more stringent contact restrictions, indicates that the mass testing campaigns were responsible for a large share of case reduction in the previous months.
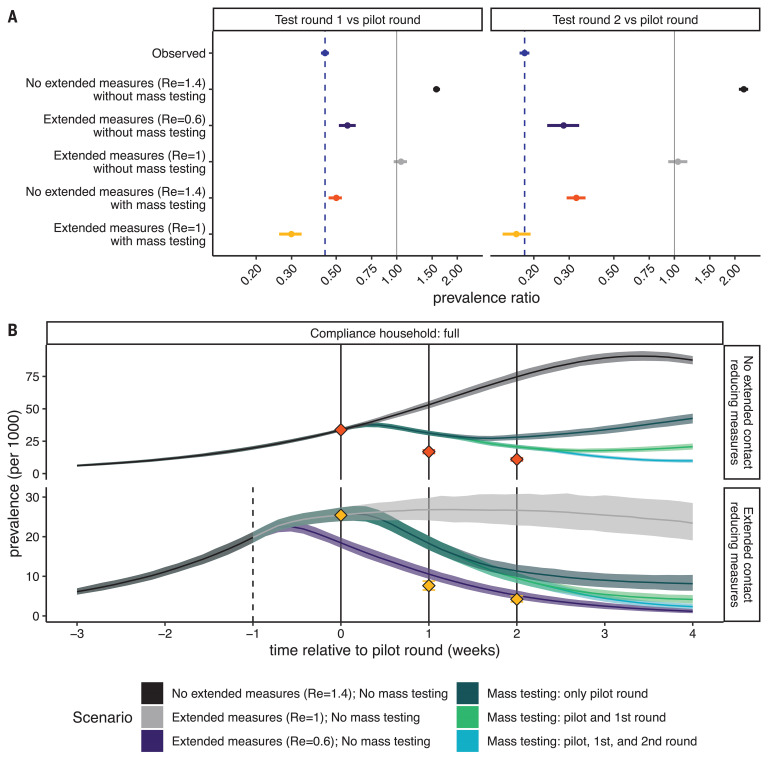
To further investigate the relationship between the reduction in prevalence, mass testing, and nonpharmaceutical interventions, we used a microsimulation model for fine-scale SARS-CoV-2 transmission in a representative county included in the pilot phase of the mass testing. Among the multiple intervention scenarios tested, only the scenario that assumed a substantial impact of both the additional contact reducing measures and the mass testing campaigns was able to generate reductions in test positivity rates between testing rounds that were similar to those observed (Fig. 3). Thus, the requirement for quarantine for the whole household after a positive test was essential for the combined effect of mass testing and contact reduction measures. The model predicted a prevalence ratio between the first two testing rounds of 0.30 (0.26 to 0.34) with household quarantine and 0.78 (0.72 to 0.84) without household quarantine.
Fig. 3. Simulated relative effectiveness of the extended contact-reducing measures and the mass testing.
(A) The change in observed prevalence of infectious nonquarantining individuals between 10 and 65 years of age as predicted by the microsimulation model. For comparison, the observed test-positivity rate is shown in blue. The facets show changes (left) from the pilot to the first round of mass testing and (right) from the pilot to the second round of mass testing. Shown scenarios compare the effect of (top to bottom) no additional interventions that limit the growth rate of reproduction number (Re) = 1.4, the extended contact-reduction measures drastically reducing the growth rate to Re = 0.6 and no mass testing being conducted, the extended contact-reduction measures reducing the growth rate to Re = 1.0 and no mass testing being conducted, no change in growth rate but mass testing, and the extended contact reduction measures reducing the growth rate to Re = 1 and mass testing. In scenarios without mass testing, we compared prevalence of infectious individuals on the same days as testing occurred in scenarios with mass testing. CIs around the modeled values in each scenario are calculated as the 2.5 and 97.5% percentiles across 500 model iterations, with the point estimate representing the median. The CI around the observed value is its binomial CI. (B) Simulated infection incidence of alternative intervention strategies. Simulations are aligned by the date of the first mass test [time (t) = 0]. The dashed line indicates the timing of the extended contact-reducing measures, and the solid lines indicate the timing of the mass testing campaigns. Colors indicate the simulations stratified into whether no mass testing or one, two, or three testing rounds were performed and the effectiveness of the extended contact-reduction measures on the growth rate. Red and yellow diamonds indicate the prevalence of infectiousness observed among the tested nonquarantining age-eligible population, corresponding to the scenarios in (A).
Despite a reduction of more than 50% in test positivity between mass testing campaigns, standard syndromic surveillance did not report a rapid collapse in test-positive cases corresponding to drastic reductions in prevalence. This may be explained by a variety of reasons. Foremost, the national mass testing campaigns are likely to have a major disruptive effect on routine passive syndromic surveillance. Also, the ability of PCR to detect viral RNA well beyond the infectious period will partially mask a sudden drop in infectious cases. In addition, starting mid-September incidence surveillance has been operating at capacity with long waiting lists for testing and stricter eligibility criteria, which reduced substantially in the period after mass testing and hence may have artificially reduced the observable change in these data. By contrast, data on hospital bed occupancy shows a sudden flattening from mid-November, indicating a sharp decrease in new admissions that is consistent with a sizable reduction in new infections when the mass testing campaigns occurred (fig. S6).
Executing a large-scale mass testing campaign comes with several challenges. The need to mobilize sufficient medical personnel to conduct the nasopharyngeal swabs proved to be a major obstacle. Also, the logistics of mobilizing large numbers of assisting army personnel and vast amounts of testing and personal protective equipment (PPE) material proved challenging. Some of the challenges could be overcome by using other rapid antigen tests with similarly high sensitivity but that are also licensed for use with nasal swabs (16, 17). Nasal swabs can be self-administered and reduce demand on trained personnel and transmission risk in the process of sample collection and can even enable testing at home. Self-administered swabs are also less intrusive and can be better suited for children and mass testing at schools. However, these benefits must be weighed against the potential loss of sensitivity if self-administered swabs are not conducted appropriately (18). The details of the Slovak mass testing experience need to be studied carefully before considering potential replication elsewhere (19).
The combination of nationwide restrictions and mass testing with quarantining of household contacts of test positives rapidly reduced the prevalence of infectious residents in Slovakia. Although it was impossible to disentangle the precise contribution of control measures and mass testing, the latter is likely to have had a substantial effect in curbing the pandemic in Slovakia and may provide a valuable tool in future containment of SARS-CoV-2 elsewhere.
Acknowledgments
We thank all health care workers, Slovak armed forces, and countless volunteers who helped with the execution of the mass testing campaign. Further, we thank all participants who contributed their time to help curb the pandemic and particularly those who had to quarantine as a result of their or their households or contacts test result. Funding: This work was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society 208812/Z/17/Z (to S.Fl.); Wellcome Trust Senior Research Fellowship (to S.Fu., S.A., and K.S.); and Elrhas Research for Health in Humanitarian Crises (R2HC) Programme funded by the UK Government (DFID), the Wellcome Trust, and the UK National Institute for Health Research (NIHR) (K.V.-Z.). The following funding sources are acknowledged as providing funding for the working group authors: BBSRC LIDP BB/M009513/1 (to D.S.); Bill & Melinda Gates Foundation INV-001754 (to M.Q.); INV-003174 (to K.P., M.J., and Y.L.); OPP1157270 (to K.A.); NTD Modelling Consortium OPP1184344 (to C.A.B.P. and G.F.M.); OPP1180644 (to S.R.P.); OPP1183986 (to E.S.N.); DFID/Wellcome Trust Epidemic Preparedness Coronavirus research program 221303/Z/20/Z: (to C.A.B.P.); EDCTP2 RIA2020EF-2983-CSIGN (to H.P.G.); ERC #757699 (to M.Q.); The European Unions Horizon 2020 research and innovation programproject EpiPose 101003688 (to K.P., M.J., P.K., R.C.B., W.J.E., and Y.L.); The Global Challenges Research Fund (GCRF) project RECAP managed through RCUK and ESRC ES/P010873/1 (to A.G., C.I.J., and T.J.); HDR UK MR/S003975/1 (to R.M.E.); MRC MR/N013638/1 (to N.R.W.); Nakajima Foundation (to A.E.); The National Institute for Health Research NIHR 16/136/46 (to B.J.Q.); 16/137/109 (to B.J.Q., F.Y.S., M.J., and Y.L.); Health Protection Research Unit for Immunisation NIHR200929 (to N.G.D.); Health Protection Research Unit for Modelling Methodology HPRU-2012-10096 (to T.J.); NIHR200908 (to R.M.E.); NIHR200929 (to F.G.S. and M.J.); PR-OD-1017-20002 (to A.R. and W.J.E.); The Royal Society Dorothy Hodgkin Fellowship (to R.L.); RP\EA\180004 (to P.K.); UK DHSC/UK Aid/NIHR PR-OD-1017-20001 (to H.P.G.); UK MRC MC_PC_19065Covid 19: Understanding the dynamics and drivers of the COVID-19 epidemic using real-time outbreak analytics (to A.G., N.G.D., R.M.E., S.C., T.J., W.J.E., and Y.L.); MR/P014658/1 (to G.M.K.); The UK Public Health Rapid Support Team funded by the United Kingdom Department of Health and Social Care (to T.J.); Wellcome Trust 206250/Z/17/Z (to A.J.K. and T.W.R.); 206471/Z/17/Z (to O.J.B.); 208812/Z/17/Z (to S.C.); 210758/Z/18/Z (to J.D.M., J.H., N.I.B., S.A., and S.R.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Author contributions: The following authors were part of the Centre for Mathematical Modelling of Infectious Disease (CMMID) COVID-19 Working Group. Each contributed in processing, cleaning, and interpretation of data; interpreted findings; contributed to the manuscript; and approved the work for publication: G. M. Knight, N. R. Waterlow, C. A. B. Pearson, F. Y. Sun, S. R. Procter, A. Showering, R. M. Eggo, Y.-W. Desmond Chan, E. S. Nightingale, D. Simons, O. Brady, B. J Quilty, P. Klepac, A. Gimma, H. P. Gibbs, W. J. Edmunds, A. J. Kucharski, S. Abbott, J. Williams, K. Prem, R. C. Barnard, T. Jombart, G. Medley, K. E. Atkins, S. Clifford, N. G. Davies, K. Abbas, M. Jit, T. W. Russell, F. G. Sandmann, D. C. Tully, J. D. Munday, A. M. Foss, A. Rosello, S. R. Meakin, J. Hellewell, C. J. Villabona-Arenas, C. I. Jarvis, R. Lowe, A. Endo, M. Quaife, N. I. Bosse, and Y. Liu. The following authors were part of the Intitt Zdravotnch Analz. Each contributed in processing, cleaning, and interpretation of data; interpreted findings; contributed to the manuscript; and approved the work for publication: M. Mik, K. ufliarsky, A. Kluka, E. Kofira, P. iduliak, S. Slobodnkov, and J. erven. Conceptualization: M.P., M.M., P.J., M.K., S.Fu., and S.Fl. Methodology: K.V.-Z., S.A., M.M., S.Fu., and S.Fl. Software: K.V.-Z., M.M., S.Fu., and S.Fl. Validation: K.V.-Z., S.A., K.S., P.J., M.K., S.Fu., and S.Fl. Formal analysis: M.P., K.V.-Z., S.A., K.S., M.M., S.Fu., and S.Fl. Investigation: M.P., K.V.-Z., P.J., S.Fu., and S.Fl. Resources: M.P., P.J., and M.K. Data curation: M.P., K.V.-Z., P.J., M.K., S.Fu., and S.Fl.. Writing, original draft: M.P., K.V.-Z., and S.Fl.. Writing, review and editing: M.P., K.S., M.M., P.J., M.K., S.Fu., and S.Fl. Visualization: M.P., K.V.-Z., S.Fu., and S.Fl. Supervision: P.J., M.K., S.Fu., and S.Fl.. Project administration: M.P., S.Fu., and S.Fl.. Funding acquisition: Table 1: M.P. and S.Fu.; Fig. 1: M.P., K.V.-Z., S.Fu., and S.Fl.; Fig. 2: M.P., K.V.-Z., S.Fu., and S.Fl.; Fig. 3: M.P., K.V.-Z., S.Fu., and S.Fl.; table S1: M.P., K.V.-Z., S.Fu., and S.Fl.; fig. S1: S.Fu.; fig. S2: K.V.-Z.; fig. S3: K.V.-Z.; fig. S4: S.Fu.; fig. S5: K.V.-Z.; fig. S6: M.P., S.Fu., and S.Fl.; fig. S7: M.P., S.Fu., and S.Fl.; fig. S8: M.P., S.Fu., and S.Fl.; table S2: S.Fu. and S.Fl.; table S3: M.P., S.Fu., and S.Fl.. S.Fl. and S.Fu. contributed equally to this work, and their order in the author list was determined with a coin toss. Competing interests: M.P. is employed as epidemiologist and public health data analyst at the Slovak Ministry of Health but had no involvement in the design of the mass testing campaigns. M.K. is the current Slovak Minister of Health and was involved in the design of the mass testing campaigns. S.Fu. advises the UK government as a member of the Scientific Pandemic Influenza Group on Modelling (SPI-M) in an unpaid role. All other authors declare that they have no conflicts of interest. Data and materials availability: Daily incidence of positive COVID-19 test reports and the results of the mass testing are available through governmental websites (12, 20). All analyses were conducted in R (21) and can be found at (22). This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/. This license does not apply to figures/photos/artwork or other content included in the article that is credited to a third party; obtain authorization from the rights holder before using such material.
Table 1. Overview of county-specific test numbers and reductions for the 79 counties in Slovakia.
R, median estimate of the reproduction number on 22 October, based on test-positive cases from syndromic surveillance up to 30 October and estimated by using a renewal process model on back-calculated estimates of infection incidence; %, proportion positive out of those attending mass testing.
| Pilot | Round 1 | Round 2 | ||||||||||
| County | Region | Population | R | Attendance | Positive | % | Attendance | Positive | % | Attendance | Positive | % |
| Bnovce nad Bebravou | Treniansky kraj | 36281.5 | 1.4 | 23264 | 457 | 1.96 | 22248 | 192 | 0.86 | |||
| Bansk Bystrica | Banskobystrick kraj | 110828.5 | 1.2 | 64127 | 687 | 1.07 | 66544 | 231 | 0.35 | |||
| Bansk tiavnica | Banskobystrick kraj | 16086.0 | 0.7 | 11725 | 33 | 0.28 | ||||||
| Bardejov | Preovsk kraj | 77771.0 | 0.7 | 48320 | 1569 | 3.25 | 44197 | 740 | 1.67 | 43983 | 366 | 0.83 |
| Bratislava I | Bratislavsk kraj | 44798.0 | 1.2 | 29047 | 108 | 0.37 | ||||||
| Bratislava II | Bratislavsk kraj | 108139.0 | 1.2 | 80958 | 345 | 0.43 | ||||||
| Bratislava III | Bratislavsk kraj | 61418.0 | 1.2 | 49788 | 175 | 0.35 | ||||||
| Bratislava IV | Bratislavsk kraj | 93058.0 | 1.2 | 63857 | 81 | 0.13 | ||||||
| Bratislava V | Bratislavsk kraj | 141259.0 | 1.2 | 68139 | 268 | 0.39 | ||||||
| Brezno | Banskobystrick kraj | 61449.5 | 1.4 | 37339 | 450 | 1.21 | 38515 | 242 | 0.63 | |||
| Byta | ilinsk kraj | 30917.0 | 1.6 | 21419 | 328 | 1.53 | 20931 | 164 | 0.78 | |||
| adca | ilinsk kraj | 90080.0 | 1.0 | 53907 | 1736 | 3.22 | 52304 | 506 | 0.97 | |||
| Detva | Banskobystrick kraj | 32051.0 | 1.3 | 19704 | 211 | 1.07 | 23255 | 79 | 0.34 | |||
| Doln Kubn | ilinsk kraj | 39456.5 | 1.0 | 29347 | 916 | 3.12 | 24251 | 345 | 1.42 | 24170 | 138 | 0.57 |
| Dunajsk Streda | Trnavsk kraj | 122358.0 | 1.3 | 87329 | 840 | 0.96 | 110083 | 577 | 0.52 | |||
| Galanta | Trnavsk kraj | 94076.0 | 1.3 | 71243 | 349 | 0.49 | ||||||
| Gelnica | Koick kraj | 31868.0 | 1.3 | 18331 | 131 | 0.71 | 19087 | 72 | 0.38 | |||
| Hlohovec | Trnavsk kraj | 45012.5 | 1.4 | 28892 | 171 | 0.59 | ||||||
| Humenn | Preovsk kraj | 61985.5 | 1.1 | 32962 | 598 | 1.81 | 32750 | 197 | 0.60 | |||
| Ilava | Treniansky kraj | 59187.5 | 1.4 | 37604 | 442 | 1.18 | 35931 | 291 | 0.81 | |||
| Kemarok | Preovsk kraj | 75235.0 | 1.4 | 43959 | 845 | 1.92 | 43252 | 390 | 0.90 | |||
| Komrno | Nitriansky kraj | 101711.5 | 1.5 | 61268 | 343 | 0.56 | ||||||
| Koice - okolie | Koick kraj | 129543.5 | 1.2 | 32849 | 196 | 0.60 | ||||||
| Koice I | Koick kraj | 67513.0 | 1.2 | 39314 | 295 | 0.75 | ||||||
| Koice II | Koick kraj | 82287.5 | 1.2 | 11109 | 41 | 0.37 | ||||||
| Koice III | Koick kraj | 28748.5 | 1.2 | 26992 | 135 | 0.50 | ||||||
| Koice IV | Koick kraj | 60126.0 | 1.2 | 80426 | 487 | 0.61 | ||||||
| Krupina | Banskobystrick kraj | 22182.0 | 1.4 | 13388 | 66 | 0.49 | ||||||
| Kysuck Nov Mesto | ilinsk kraj | 32914.0 | 1.6 | 20605 | 384 | 1.86 | 20491 | 177 | 0.86 | |||
| Levice | Nitriansky kraj | 110824.0 | 1.4 | 70155 | 375 | 0.53 | ||||||
| Levoa | Preovsk kraj | 33702.0 | 1.0 | 18344 | 373 | 2.03 | 17747 | 172 | 0.97 | |||
| Liptovsk Mikul | ilinsk kraj | 72260.5 | 1.2 | 47172 | 667 | 1.41 | 46827 | 267 | 0.57 | |||
| Luenec | Banskobystrick kraj | 73466.0 | 1.0 | 40655 | 213 | 0.52 | ||||||
| Malacky | Bratislavsk kraj | 74323.0 | 1.3 | 54657 | 285 | 0.52 | ||||||
| Martin | ilinsk kraj | 96338.0 | 1.5 | 56533 | 771 | 1.36 | 57513 | 381 | 0.66 | |||
| Medzilaborce | Preovsk kraj | 11841.5 | 1.1 | 6980 | 91 | 1.30 | 6142 | 17 | 0.28 | |||
| Michalovce | Koick kraj | 110705.0 | 1.0 | 58929 | 512 | 0.87 | 62790 | 211 | 0.34 | |||
| Myjava | Treniansky kraj | 26356.0 | 0.9 | 17753 | 249 | 1.40 | 18599 | 68 | 0.37 | |||
| Nmestovo | ilinsk kraj | 62663.5 | 0.9 | 40052 | 1910 | 4.77 | 37029 | 668 | 1.80 | 37659 | 207 | 0.55 |
| Nitra | Nitriansky kraj | 161560.0 | 1.3 | 99175 | 674 | 0.68 | ||||||
| Nov Mesto nad Vhom | Treniansky kraj | 62553.5 | 1.5 | 40829 | 363 | 0.89 | 46269 | 198 | 0.43 | |||
| Nov Zmky | Nitriansky kraj | 139004.5 | 1.3 | 79234 | 478 | 0.60 | ||||||
| Partiznske | Treniansky kraj | 45596.5 | 1.5 | 26492 | 494 | 1.86 | 27585 | 186 | 0.67 | |||
| Pezinok | Bratislavsk kraj | 65145.0 | 1.3 | 45801 | 240 | 0.52 | ||||||
| Pieany | Trnavsk kraj | 62802.5 | 1.3 | 40122 | 183 | 0.46 | ||||||
| Poltr | Banskobystrick kraj | 21471.0 | 2.0 | 12455 | 71 | 0.57 | ||||||
| Poprad | Preovsk kraj | 104913.5 | 1.4 | 59072 | 1059 | 1.79 | 58098 | 364 | 0.63 | |||
| Povask Bystrica | Treniansky kraj | 62438.5 | 1.4 | 37822 | 505 | 1.34 | 36092 | 343 | 0.95 | |||
| Preov | Preovsk kraj | 175609.5 | 1.0 | 84781 | 724 | 0.85 | 108271 | 472 | 0.44 | |||
| Prievidza | Treniansky kraj | 133979.5 | 1.3 | 76457 | 1497 | 1.96 | 77170 | 576 | 0.75 | |||
| Pchov | Treniansky kraj | 44309.5 | 1.3 | 29455 | 782 | 2.65 | 28017 | 461 | 1.65 | |||
| Revca | Banskobystrick kraj | 39636.5 | 1.7 | 21419 | 58 | 0.27 | ||||||
| Rimavsk Sobota | Banskobystrick kraj | 84159.0 | 1.7 | 46872 | 197 | 0.42 | ||||||
| Roava | Koick kraj | 62208.5 | 1.2 | 34307 | 100 | 0.29 | ||||||
| Ruomberok | ilinsk kraj | 56702.0 | 1.6 | 34000 | 682 | 2.01 | 33056 | 236 | 0.71 | |||
| Sabinov | Preovsk kraj | 60518.5 | 1.4 | 35366 | 804 | 2.27 | 34757 | 295 | 0.85 | |||
| aa | Nitriansky kraj | 51685.0 | 1.2 | 31993 | 199 | 0.62 | ||||||
| Senec | Bratislavsk kraj | 89832.0 | 1.4 | 66052 | 314 | 0.48 | ||||||
| Senica | Trnavsk kraj | 60446.0 | 1.2 | 40675 | 384 | 0.94 | 46000 | 194 | 0.42 | |||
| Skalica | Trnavsk kraj | 47104.5 | 1.2 | 29223 | 368 | 1.26 | 31200 | 168 | 0.54 | |||
| Snina | Preovsk kraj | 36240.5 | 1.3 | 19122 | 345 | 1.80 | 19396 | 111 | 0.57 | |||
| Sobrance | Koick kraj | 22819.0 | 0.9 | 12986 | 135 | 1.04 | 12966 | 43 | 0.33 | |||
| Spisk Nov Ves | Koick kraj | 99765.0 | 1.3 | 54279 | 739 | 1.36 | 53712 | 361 | 0.67 | |||
| Star ubova | Preovsk kraj | 53953.5 | 1.2 | 28749 | 805 | 2.80 | 27234 | 354 | 1.30 | |||
| Stropkov | Preovsk kraj | 20532.0 | 1.1 | 10494 | 125 | 1.19 | 10764 | 63 | 0.59 | |||
| Svidnk | Preovsk kraj | 32564.0 | 1.1 | 16631 | 220 | 1.32 | 16705 | 85 | 0.51 | |||
| Topoany | Nitriansky kraj | 70131.5 | 1.4 | 44627 | 748 | 1.68 | 50253 | 330 | 0.66 | |||
| Trebiov | Koick kraj | 105353.0 | 0.9 | 68503 | 400 | 0.58 | ||||||
| Trenn | Treniansky kraj | 114523.0 | 1.2 | 73424 | 832 | 1.13 | 72546 | 434 | 0.60 | |||
| Trnava | Trnavsk kraj | 132454.5 | 1.2 | 92215 | 557 | 0.60 | ||||||
| Turianske Teplice | ilinsk kraj | 15884.0 | 1.7 | 11287 | 112 | 0.99 | 12210 | 54 | 0.44 | |||
| Tvrdon | ilinsk kraj | 36180.0 | 1.3 | 22250 | 1078 | 4.84 | 18541 | 369 | 1.99 | 20502 | 164 | 0.80 |
| Vek Krt | Banskobystrick kraj | 43473.0 | 1.2 | 24652 | 76 | 0.31 | ||||||
| Vranov nad Topou | Preovsk kraj | 80766.5 | 1.4 | 43552 | 460 | 1.06 | 45424 | 281 | 0.62 | |||
| arnovica | Banskobystrick kraj | 26152.5 | 1.4 | 16272 | 105 | 0.65 | ||||||
| iar nad Hronom | Banskobystrick kraj | 46861.5 | 0.8 | 26260 | 108 | 0.41 | ||||||
| ilina | ilinsk kraj | 158043.0 | 1.5 | 111155 | 1392 | 1.25 | 103898 | 512 | 0.49 | |||
| Zlat Moravce | Nitriansky kraj | 40572.5 | 0.9 | 26180 | 156 | 0.60 | ||||||
| Zvolen | Banskobystrick kraj | 68758.5 | 1.4 | 39422 | 276 | 0.70 | 47764 | 136 | 0.28 | |||
Supplementary Materials
science.sciencemag.org/content/372/6542/635/suppl/DC1
Materials and Methods
Supplementary Text
Figs. S1 to S8
Tables S1 to S3
CMMID COVID-19 Working Group Authors List
Intitt Zdravotnch Analz Authors List
MDAR Reproducibility Checklist
References and Notes
- 1.Guidelines for the implementation of non-pharmaceutical interventions against COVID-19. Eur. Cent. Dis. Prev. Control (2020); www.ecdc.europa.eu/en/publications-data/covid-19-guidelines-non-pharmaceutical-interventions.
- 2.Davies N. G., et al., Lancet Public Health 10.1016/S2468-2667(20)30133-X (2020). [Google Scholar]
- 3.Flaxman S., Mishra S., Gandy A., Unwin H. J. T., Mellan T. A., Coupland H., Whittaker C., Zhu H., Berah T., Eaton J. W., Monod M., Ghani A. C., Donnelly C. A., Riley S., Vollmer M. A. C., Ferguson N. M., Okell L. C., Bhatt S.; Imperial College COVID-19 Response Team , Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 584, 257–261. (2020). 10.1038/s41586-020-2405-7 [DOI] [PubMed] [Google Scholar]
- 4.Everyone Included, Social Impact of COVID-19Dros. Inf. Serv. D; www.un.org/development/desa/dspd/everyone-included-covid-19.html.
- 5.Socio-economic impact of COVID-19. UNDP; www.undp.org/content/undp/en/home/coronavirus/socio-economic-impact-of-covid-19.html.
- 6.Kucharski A. J., Klepac P., Conlan A. J. K., Kissler S. M., Tang M. L., Fry H., Gog J. R., Edmunds W. J., Emery J. C., Medley G., Munday J. D., Russell T. W., Leclerc Q. J., Diamond C., Procter S. R., Gimma A., Sun F. Y., Gibbs H. P., Rosello A., van Zandvoort K., Hu S., Meakin S. R., Deol A. K., Knight G., Jombart T., Foss A. M., Bosse N. I., Atkins K. E., Quilty B. J., Lowe R., Prem K., Flasche S., Pearson C. A. B., Houben R. M. G. J., Nightingale E. S., Endo A., Tully D. C., Liu Y., Villabona-Arenas J., OReilly K., Funk S., Eggo R. M., Jit M., Rees E. M., Hellewell J., Clifford S., Jarvis C. I., Abbott S., Auzenbergs M., Davies N. G., Simons D., CMMID COVID-19 working group , Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: A mathematical modelling study. Lancet Infect. Dis. 20, 1151–1160. (2020). 10.1016/S1473-3099(20)30457-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kretzschmar M. E., Rozhnova G., Bootsma M. C. J., van Boven M., van de Wijgert J. H. H. M., Bonten M. J. M., Impact of delays on effectiveness of contact tracing strategies for COVID-19: A modelling study. Lancet Public Health 5, e452–e459. (2020). 10.1016/S2468-2667(20)30157-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wlfel R., Corman V. M., Guggemos W., Seilmaier M., Zange S., Mller M. A., Niemeyer D., Jones T. C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brnink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C., Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469. (2020). 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 9.Oxford University, An observational study of SARS-CoV-2 infectivity by viral load and demographic factors and the utility lateral flow devices to prevent transmission (Univ. of Oxford, 2021); www.ox.ac.uk/news/2021-01-21-lateral-flow-devices-detect-most-infectious-covid-19-cases-and-could-allow-safer.
- 10.Mina M. J., Parker R., Larremore D. B., Rethinking Covid-19 Test Sensitivity - A Strategy for Containment. N. Engl. J. Med. 383, e120 (2020). 10.1056/NEJMp2025631 [DOI] [PubMed] [Google Scholar]
- 11.Larremore D. B., Wilder B., Lester E., Shehata S., Burke J. M., Hay J. A., Tambe M., Mina M. J., Parker R., Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci. Adv. 7, eabd5393 (2021). 10.1126/sciadv.abd5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Som Zodpovedn; www.somzodpovedny.sk.
- 13.Z. Igli et al., medRxiv 20234104 [Preprint] 20 November 2020. 10.1101/2020.11.18.20234104 [DOI]
- 14.K. Boov, R. Kollr, medRxiv 20248808 [Preprint] 26 December 2020. 10.1101/2020.12.23.20248808 [DOI]
- 15.S. Riley et al., medRxiv 20239806 [Preprint] 2 December 2020. 10.1101/2020.11.30.20239806 [DOI]
- 16.Panbio COVID-19 Ag Rapid Test Device; www.globalpointofcare.abbott/en/product-details/panbio-covid-19-ag-antigen-test.html. [DOI] [PubMed]
- 17.Sofia SARS Antigen FIA, Quidel; www.quidel.com/immunoassays/rapid-sars-tests/sofia-sars-antigen-fia.
- 18.Mahase E., BMJ 371, m4469 (2020). [Google Scholar]
- 19.K. Landokova, How Slovakia tested 3.6 million people for COVID-19 in a single weekend; www.bi.team/blogs/how-slovakia-tested-3-6-million-people-for-covid-19-in-a-single-weekend.
- 20.Koronavrus a Slovensko; https://korona.gov.sk.
- 21.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2019; www.R-project.org).
- 22.M. Pavelka et al., Data and R code accompanying the manuscript The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia. Zenodo (2021); doi: 10.5281/zenodo.4570939. 10.5281/zenodo.4570939 [DOI] [PMC free article] [PubMed]
- 23.Products: STANDARD Q COVID-19 Ag , ; http://sdbiosensor.com/xe/product/7672.
- 24.A. Berger et al., medRxiv 20235341 [Preprint] 23 November 2020. . 10.1101/2020.11.20.20235341 [DOI]
- 25.Prince-Guerra J. L., Almendares O., Nolen L. D., Gunn J. K. L., Dale A. P., Buono S. A., Deutsch-Feldman M., Suppiah S., Hao L., Zeng Y., Stevens V. A., Knipe K., Pompey J., Atherstone C., Bui D. P., Powell T., Tamin A., Harcourt J. L., Shewmaker P. L., Medrzycki M., Wong P., Jain S., Tejada-Strop A., Rogers S., Emery B., Wang H., Petway M., Bohannon C., Folster J. M., MacNeil A., Salerno R., Kuhnert-Tallman W., Tate J. E., Thornburg N. J., Kirking H. L., Sheiban K., Kudrna J., Cullen T., Komatsu K. K., Villanueva J. M., Rose D. A., Neatherlin J. C., Anderson M., Rota P. A., Honein M. A., Bower W. A., Evaluation of Abbott BinaxNOW Rapid Antigen Test for SARS-CoV-2 Infection at Two Community-Based Testing Sites - Pima County, Arizona, November 3-17, 2020. MMWR Morb. Mortal. Wkly. Rep. 70, 100–105. (2021). 10.15585/mmwr.mm7003e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbott S., Hellewell J., Thompson R. N., Sherratt K., Gibbs H. P., Bosse N. I., Munday J. D., Meakin S., Doughty E. L., Chun J. Y., Chan Y.-W. D., Finger F., Campbell P., Endo A., Pearson C. A. B., Gimma A., Russell T., Flasche S., Kucharski A. J., Eggo R. M., Funk S., Estimating the time-varying reproduction number of SARS-CoV-2 using national and subnational case counts. Wellcome Open Res. 5, 112 (2020). 10.12688/wellcomeopenres.16006.2 [DOI] [Google Scholar]
- 27.Statistics, Eurostat; https://ec.europa.eu/eurostat/databrowser/view/cens_01rhsize/default/table?lang=en.
- 28.Prem K., Cook A. R., Jit M., Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLOS Comput. Biol. 13, e1005697 (2017). 10.1371/journal.pcbi.1005697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United Nations, World Population prospects; www.google.com/url?q=https://population.un.org/wpp/Download/Files/1_Indicators%2520(Standard)/EXCEL_FILES/1_Population/WPP2019_POP_F01_1_TOTAL_POPULATION_BOTH_SEXES.xlsx&sa=D&source=editors&ust=1614209701533000&usg=AOvVaw0e_BV1Z-ofSu_U0Q5NeZBf.
- 30.European Commission, Eurostat Data Browser; https://ec.europa.eu/eurostat/databrowser/view/CENS_01RHSIZE__custom_214489/default/table?lang=en.
- 31.Lauer S. A., et al., Ann. Intern. Med. 10.7326/M20-0504 (2020). [Google Scholar]
- 32.L. Ferretti et al., medRxiv 20188516 [Preprint] 16 September 2020. (2020). 10.1101/2020.09.04.20188516 [DOI]
- 33.Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z., Liu X., Wei L., Truelove S. A., Zhang T., Gao W., Cheng C., Tang X., Wu X., Wu Y., Sun B., Huang S., Sun Y., Zhang J., Ma T., Lessler J., Feng T., Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: A retrospective cohort study. Lancet Infect. Dis. 20, 911–919. (2020). 10.1016/S1473-3099(20)30287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishiura H., Linton N. M., Akhmetzhanov A. R., Serial interval of novel coronavirus (COVID-19) infections. Int. J. Infect. Dis. 93, 284–286. (2020). 10.1016/j.ijid.2020.02.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K. S. M., Lau E. H. Y., Wong J. Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T. T. Y., Wu J. T., Gao G. F., Cowling B. J., Yang B., Leung G. M., Feng Z., Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382, 1199–1207. (2020). 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies N. G., Klepac P., Liu Y., Prem K., Jit M., Eggo R. M.; CMMID COVID-19 working group , Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 26, 1205–1211. (2020). 10.1038/s41591-020-0962-9 [DOI] [PubMed] [Google Scholar]
- 37.Liu Y., Funk S., Flasche S., Centre for Mathematical Modelling of Infectious Diseases nCoV Working Group , The contribution of pre-symptomatic infection to the transmission dynamics of COVID-2019. Wellcome Open Res. 5, 58 (2020). 10.12688/wellcomeopenres.15788.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He X., Lau E. H. Y., Wu P., Deng X., Wang J., Hao X., Lau Y. C., Wong J. Y., Guan Y., Tan X., Mo X., Chen Y., Liao B., Chen W., Hu F., Zhang Q., Zhong M., Wu Y., Zhao L., Zhang F., Cowling B. J., Li F., Leung G. M., Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 26, 672–675. (2020). 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 39.L. J. Krger et al., medRxiv 20203836 [Preprint] 4 October 2020. (2020). 10.1101/2020.10.01.20203836 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- M. Pavelka et al., Data and R code accompanying the manuscript The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia. Zenodo (2021); doi: 10.5281/zenodo.4570939. 10.5281/zenodo.4570939 [DOI] [PMC free article] [PubMed]
Supplementary Materials
science.sciencemag.org/content/372/6542/635/suppl/DC1
Materials and Methods
Supplementary Text
Figs. S1 to S8
Tables S1 to S3
CMMID COVID-19 Working Group Authors List
Intitt Zdravotnch Analz Authors List
MDAR Reproducibility Checklist