Kids armed with anti-coronavirus B cells
It remains unclear whether B cell repertoires against coronaviruses and other pathogens differ between adults and children and how important these distinctions are. Yang et al. analyzed blood samples from young children and adults, as well as tissues from deceased organ donors, characterizing the B cell receptor (BCR) repertoires specific to six common pathogens and two viruses that they had not seen before: Ebola virus and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Children had higher frequencies of B cells with convergent BCR heavy chains against previously encountered pathogens and higher frequencies of class-switched convergent B cell clones against SARS-CoV-2 and related coronaviruses. These findings suggest that encounters with coronaviruses in early life may produce cross-reactive memory B cell populations that contribute to divergent COVID-19 susceptibilities.
Science, this issue p. 738
Blood taken from children before the COVID-19 pandemic contains memory B cells that bind SARS-CoV-2.
Abstract
Vaccination and infection promote the formation, tissue distribution, and clonal evolution of B cells, which encode humoral immune memory. We evaluated pediatric and adult blood and deceased adult organ donor tissues to determine convergent antigen-specific antibody genes of similar sequences shared between individuals. B cell memory varied for different pathogens. Polysaccharide antigenspecific clones were not exclusive to the spleen. Adults had higher clone frequencies and greater class switching in lymphoid tissues than blood, while pediatric blood had abundant class-switched convergent clones. Consistent with reported serology, prepandemic children had class-switched convergent clones to severe acute respiratory syndrome coronavirus 2 with weak cross-reactivity to other coronaviruses, while adult blood or tissues showed few such clones. These results highlight the prominence of early childhood B cell clonal expansions and cross-reactivity for future responses to novel pathogens.
The clonal identity of a B cell can be traced by the sequence of its B cell receptor (BCR), which determines its antigen specificity (1). Immunoglobulin (Ig) sequences are formed via irreversible variable, diversity, and joining (VDJ) gene segment rearrangement and can be diversified through somatic hypermutation (SHM) and class-switch recombination (CSR) (2). Convergent BCRs with high sequence similarity in individuals exposed to the same antigen reflect antigen-driven clonal selection and form shared immunological memory between individuals (3,5). It is still unclear, however, how B cell memory to different antigens distributes in human tissues and changes during an individuals life span.
Humoral immune responses can differ between children and adults; for example, children use more B cell clones to achieve neutralizing antibody breadth to HIV-1 (6). Children usually have milder disease following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection than adults do (7,10), potentially owing to differences in viral receptor expression and immune responses (11, 12). SARS-CoV-2infected children, in contrast to adults, show lower antibody titers and more IgG specific for the spike (S) protein over the nucleocapsid (N) protein. The faster viral clearance and lower viral antigen loads in children have been attributed to these differences (13). Whether B cell clones specific for coronaviruses and other pathogens differ between children and adults is unclear. Blood-based studies survey only a fraction of an individuals BCR repertoire. The lymph nodes, spleen, and gastrointestinal tract harbor greater numbers of B cells and are major sites for SHM and CSR (14, 15). Specialized responses in particular tissues have been reported, such as for polysaccharide antigenspecific B cells in functional splenic tissue (16, 17).
To study changes in antigen-specific B cell memory over the human life span and across tissues, we characterized convergent Ig heavy chain (IGH) repertoires specific to six common pathogens as well as two viruses not encountered by the participants, Ebola virus (EBOV) and SARS-CoV-2, in pre-COVID-19 pandemic individuals. We analyzed 12 cord blood (CB) samples; 93 blood samples from 51 children aged 1 to 3 years (18); 114 blood samples from healthy human adults aged 17 to 87 years (18); and blood, lymph node, and spleen samples from eight deceased organ donors (table S1). Children were vaccinated against Haemophilus influenzae type b (Hib), Pneumococcus pneumoniae (PP), and tetanus toxoid (TT) at 2, 4, 6, and 12 to 15 months, had influenza virus (flu) vaccination, and were very likely exposed to respiratory syncytial virus (RSV) but were not vaccinated against Neisseria meningitidis (NM) (19). Adult vaccination histories were unknown. Convergent IGHs were identified by clustering with pathogen-specific reference IGH (table S2) sharing IGH variable domain (IGHV) and joining region (IGHJ) gene segment usage, complementarity-determining region H3 (CDR-H3) length, and minimum 85% CDR-H3 amino acid sequence identity.
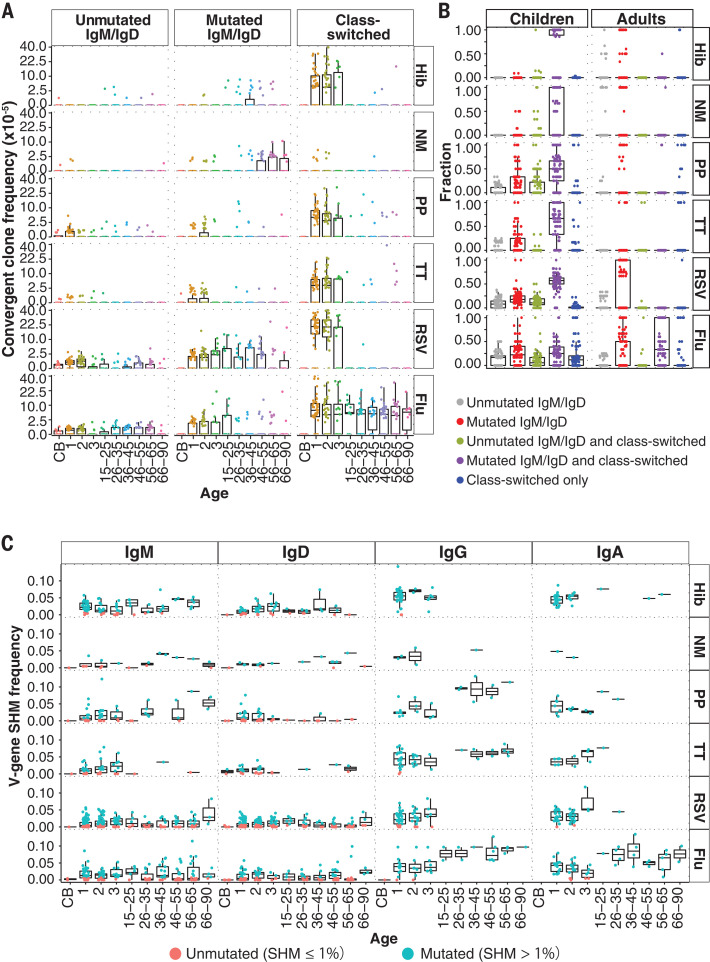
B cell clones fell into three groups: (i) nave clones containing only unmutated IgM or IgD (hereafter, unmutM/D); (ii) antigen-experienced IgM or IgD with median SHM over 1% and without class-switched members (hereafter, mutM/D); and (iii) antigen-experienced clones with class-switched members (hereafter, CS). As we hypothesized, CB samples showed the lowest convergent IGH frequencies, consistent with limited fetal pathogen or vaccine exposures (Fig. 1A). Convergent clones in children and adults were largely mutM/D or CS (Fig. 1A and fig. S1). In adult blood, convergent clones for Hib, NM, and RSV were predominantly mutM/D clones, whereas PP, TT, and flu clones were predominantly CS (fig. S2A). Adults over 45 years of age had elevated mutM/D B cell clone frequencies to NM, potentially from exposures preceding widespread NM vaccination (20). Unexpectedly, children had higher frequencies than adults of CS convergent clones for Hib, PP, TT, and RSV (fig. S2B), with mutated IgM or IgD also found in these clones (Fig. 1B). Convergent clone frequency in childrens blood was not significantly associated with vaccination timing (figs. S3 to S5 and table S3), indicating persistently elevated frequencies. Flu-specific convergent clone frequencies were comparable in children and adults (Fig. 1A), with age-related increases in IgG SHM potentially due to frequent exposures via vaccination or infection (Fig. 1C) (18).
Fig. 1. Frequency, class switching, and SHM of pathogen-specific convergent clones in children and adults.
(A) Convergent clone frequencies for each pathogen, plotted on a square root scale. Ages given in years. CB, cord blood. (B) Fractions of convergent clones expressing unmutated IgM or IgD, mutated IgM or IgD, class-switched, or combinations of these. Children have significantly larger fractions of class-switched convergent clones with mutated IgM/IgD clone members (colored in purple) than do adults [P = 5.08 1032, 6.66 1029, 2.39 1029, 3.45 1034, and 1.71 1041 for Hib, NM, PP, TT, and RSV, respectively, by Wilcoxon-Mann-Whitney (WMW) test]. (C) Median IGHV gene SHM frequencies of each convergent clone in participants of different ages indicated in years. SHM frequencies of convergent clones expressing IgG or IgA were lower in children than in adults (P = 6.50 1013 and 1.96 108, respectively; WMW test).
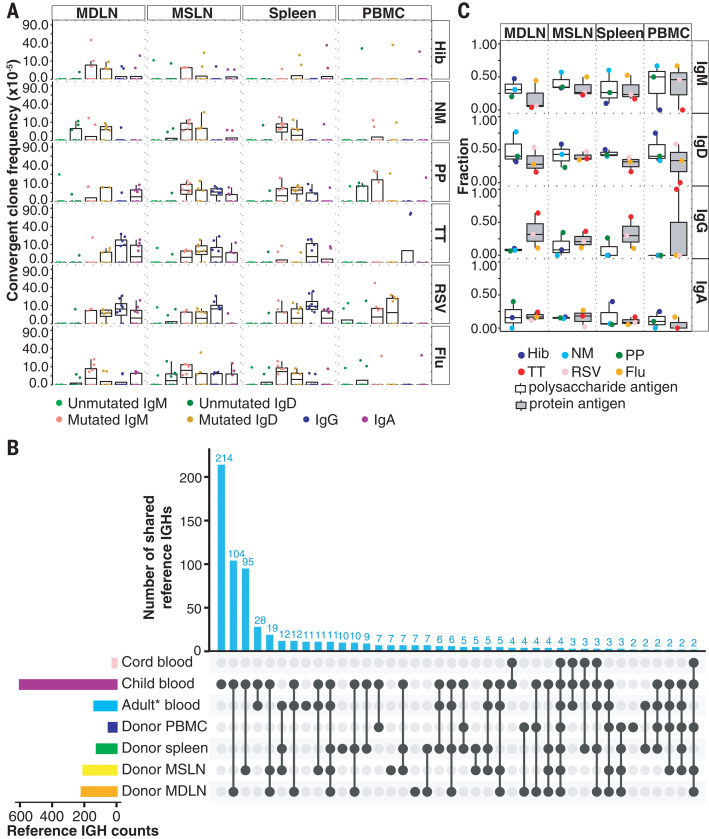
To test whether low frequencies of CS convergent clones in adult blood reflect preferential localization of clones in lymphoid tissues, we analyzed the blood, spleen, mediastinal lymph nodes (MDLN), and mesenteric lymph nodes (MSLN) of eight adult deceased organ donors. Lymph nodes and spleen showed greater clonal sharing with each other than with blood (fig. S6A), suggesting larger clone sizes in lymphoid tissues and limited recirculation. Each tissue was dominated by different clones (fig. S6B), and SHM correlated with the number of tissues a clone occupied (fig. S7), consistent with greater prior antigen exposure leading to wider tissue distribution (21). Convergent clone frequencies for Hib, NM, PP, TT, RSV, and flu were higher in adult lymph nodes and spleen than in blood (Fig. 2A). Adult lymph nodes and child blood shared more convergent clones than did adult and child blood, showing differing distributions of these clones in children and adults (Fig. 2B and fig. S8; P = 0.0001181, Fishers exact test). B cells specific for bacterial capsular polysaccharides are reported to be enriched in the spleen, and splenectomized patients are vulnerable to these bacteria (16, 17). However, frequencies of convergent clones for Hib, NM, and PP are similar or higher in lymph nodes than in the spleen. Moreover, estimated B cell numbers are greater in human lymph nodes than the spleen (22, 23), indicating that the spleen is not the sole reservoir of these clones. Convergent IGH for polysaccharides were usually IgM or IgD, with some CS clones for PP in lymph nodes and spleen (Fig. 2C). Thus, memory to these antigens spans a diversity of both lymphoid tissues and isotype expression.
Fig. 2. Convergent B cell clone distribution in tissues.
(A) Convergent clone frequencies in adult blood (PBMC), MDLN, MSLN, and spleen. Frequencies are on a square root scale. Frequencies in tissues were higher than in blood (P = 0.00049, 0.0037, 0.016, 6.71 107, 0.012, and 0.00017 for Hib, NM, PP, TT, RSV, and flu, respectively; WMW test). (B) Convergent antigen-specific IGH in CB and blood of children; healthy adults (Adult* blood); deceased organ donors (Donor PBMC); and donor spleen, MSLN, and MDLN. Vertical bars: reference antigenspecific IGH sequences per specimen combination. Left bars: total convergent IGH unique sequences per tissue. (C) Fraction of convergent clones containing the indicated isotypes in tissues. Some clones contain multiple isotypes. Compared with protein antigenspecific clones, polysaccharide-specific clones more frequently express IgM/D and less often express IgG (P = 0.035 and 0.0058, respectively; WMW test).
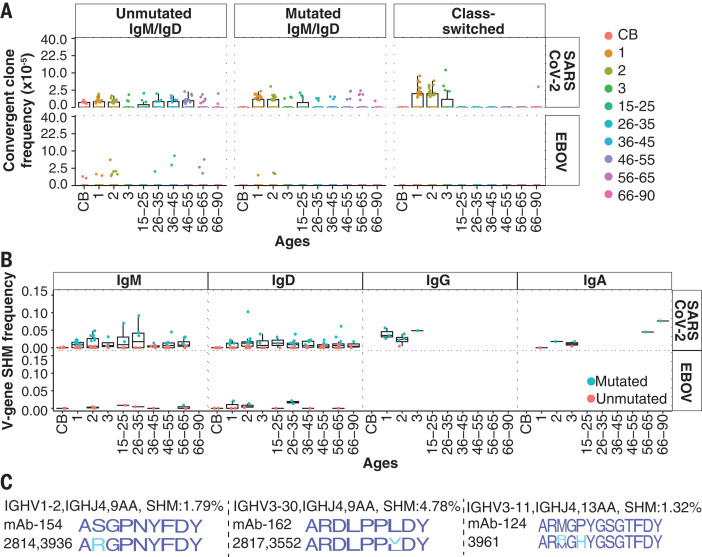
Recent reports describe SARS-CoV-2binding antibodies in prepandemic childrens blood (12, 24). Such antibodies and other physiological distinctions are under investigation in adults and children (25) and could contribute to the generally milder COVID-19 disease in children. SARS-CoV-2 S-binding B cells in unexposed individuals have been analyzed in a former SARS-CoV patient (26), nave B cells of healthy individuals (27), and memory B cells in prepandemic donors (26, 28). We detected rare convergent clones for EBOV, as unmutM/D in blood or tissues (Fig. 3A and fig. S9A). By contrast, convergent clones for SARS-CoV-2 (table S4) were more common in childrens blood. In 37 of 51 children, these clones displayed SHM with or without CS, indicating prior antigen experience (Fig. 3, A and B). Adult frequencies of SARS-CoV-2 convergent clones were lower in blood and lymphoid tissues compared with childrens blood, with few CS examples (Fig. 3A and fig. S9). Convergent clones specific for SARS-CoV-2 receptor binding domain (RBD) and other S domains showed similar distributions (fig. S10). Reference antibodies for SARS-CoV-2, EBOV, and the pathogens in Fig. 1 used a wide diversity of IGHV genes (fig. S11).
Fig. 3. Convergent clones for SARS-CoV-2 and EBOV.
(A) Convergent clone frequencies on a square root scale. CS and mutM/D convergent clone frequencies for SARS-CoV-2 are higher in children than in adults (P = 1.22 1013 and 0.0089, respectively; WMW test). (B) SHM frequencies of convergent clones for each isotype in participants of different ages (x axis). (C) CDR-H3 amino acid sequences of convergent IGH cross-reactive to SARS-CoV-2 and other HCoVs. Top row: CDR-H3 sequence logos for reported antigen-specific clones. Second row: sequence logos for convergent clones from children (blue indicates a match, cyan indicates sequence differences).
Three convergent clones from five children in this study, but none from adults, had IGH sequences highly similar to SARS-CoV-2 S-binding clones isolated from a prepandemic donor that were reported to weakly bind other human coronavirus (HCoV) spikes (26) (Fig. 3C). Three other clones from six children had IGHs identical to known SARS-CoV-2 binders (fig. S12). We expressed 19 monoclonal antibody (mAb) clones for SARS-CoV-2 (table S5) with IGH from participants in this study and reference light chains, and we identified 17 binders for SARS-CoV-2 S and S domains (Table 1). Four RBD binders showed >90% blocking of angiotensin-converting enzyme 2 (ACE2) binding to SARS-CoV-2 S (table S6). mAb FY11H1 showed evidence of S2 binding and did not block ACE2 binding. We characterized the breadth of mAb binding using a panel of HCoV spikes and SARS-CoV-2 viral variant RBDs and spikes. Three child-derived mAbs (FY7H1, FY7H2, and FY1H2) and one adult mAb (FY4H1) showed the strongest binding to B.1.1.7, B.1.351, and P.1 S and RBD variants (table S7). Cross-reactive binding to endemic HCoV spikes was very weak to absent for all mAbs, as previously noted for reference mAb-154 (similar to mAb FY13H1) isolated from a sorted cross-reactive B cell (26). The child-derived mAbs FY13H1 and FY9H2 had a higher, although still weak, signal for binding HKU1. Thus, childrens convergent coronavirus-binding B cells may have greater cross-reactivity than those of adults, in addition to having higher frequencies.
Table 1. Convergent mAb binding data for SARS-CoV-2 spike, RBD, and nucleocapsid (N) and endemic HCoV spikes.
Testing by electrochemiluminescence immunoassay in duplicate wells, with the average arbitrary unit per milliliter (AU/ml) values displayed in the table. Antibodies with binding signal at least five standard deviations above the average of negative control antibodies (Neg1 to Neg5) are listed.
| mAb | CoV-2 S | CoV-2 RBD | CoV-2 S2 | CoV-2 N | CoV S | HKU1 S | OC43 S | NL63 S | 229E S | Source |
| FY1H3 | 161.84 | 132.42 | 0.12 | 2.42 | 4.62 | 0.22 | 0.30 | 0.30 | 0.22 | Children |
| FY3H1 | 158.05 | 130.83 | 0.08 | 0.89 | 1.00 | 0.10 | 0.17 | 0.17 | 0.11 | Both |
| FY3H3 | 153.27 | 123.26 | 0.26 | 6.33 | 2.91 | 0.38 | 0.69 | 0.61 | 0.45 | Children |
| FY3H2 | 150.57 | 127.35 | 0.24 | 0.87 | 1.18 | 0.10 | 0.16 | 0.14 | 0.09 | Adults |
| FY7H1 | 149.78 | 125.82 | 0.12 | 1.41 | 4.21 | 0.10 | 0.19 | 0.19 | 0.13 | Children |
| FY1H2 | 148.44 | 119.56 | 2.39 | 1.92 | 18.00 | 0.47 | 0.63 | 0.58 | 0.50 | Children |
| FY13H1 | 147.29 | 119.22 | 0.15 | 3.55 | 0.39 | 1.41 | 0.47 | 0.29 | 0.25 | Children |
| FY7H2 | 146.59 | 120.11 | 0.06 | 1.94 | 4.29 | 0.24 | 0.15 | 0.16 | 0.12 | Children |
| FY4H1 | 131.23 | 116.83 | 0.19 | 2.27 | 1.32 | 0.25 | 0.39 | 0.27 | 0.23 | Adults |
| FY8H1 | 114.20 | 107.56 | 0.03 | 0.80 | 2.08 | 0.12 | 0.09 | 0.07 | 0.04 | Adults |
| FY9H1 | 91.02 | 94.74 | 1.39 | 3.62 | 5.87 | 0.75 | 0.56 | 0.31 | 0.30 | Children |
| FY11H1 | 79.65 | 41.41 | 13.59 | 0.78 | 44.62 | 0.13 | 0.09 | 0.06 | 0.12 | Both |
| FY6H1 | 79.09 | 71.33 | 0.54 | 5.22 | 2.88 | 0.45 | 0.27 | 0.21 | 0.24 | Both |
| FY14H1 | 78.73 | 63.10 | 3.91 | 2.43 | 9.56 | 0.30 | 0.45 | 0.35 | 0.29 | Adults |
| FY5H1 | 69.60 | 45.71 | 0.33 | 3.27 | 1.71 | 0.49 | 0.50 | 0.25 | 0.23 | Children |
| FY9H2 | 53.53 | 13.86 | 3.83 | 2.42 | 10.03 | 1.46 | 0.35 | 0.23 | 0.25 | Children |
| FY10H1 | 9.96 | 8.69 | 0.02 | 0.18 | 0.27 | 0.02 | 0.08 | 0.02 | 0.02 | Adults |
| Neg5 | 0.52 | 0.01 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | Controls |
| Neg4 | 1.63 | 0.02 | 0.00 | 0.90 | 0.09 | 0.01 | 0.00 | 0.00 | 0.00 | Controls |
| Neg3 | 1.22 | 0.02 | 0.00 | 0.03 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | Controls |
| Neg2 | 1.37 | 0.02 | 0.00 | 0.02 | 0.08 | 0.01 | 0.00 | 0.00 | 0.00 | Controls |
| Neg1 | 0.87 | 0.02 | 0.00 | 0.01 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | Controls |
Childhood immune responses are particularly important in an individuals life, as they form the initial memory B cell pool that shapes future responses (29). We find that in comparison to adults, children have higher frequencies of convergent B cell clones in their blood for pathogens they have encountered. Notably, prepandemic children also had class-switched convergent clones to SARS-CoV-2 and its viral variants, but not EBOV, at higher frequencies than adults. We hypothesize that previous HCoV exposures may stimulate cross-reactive memory, and that such clonal responses may have their highest frequencies in childhood. The caveats of our analysis are that convergent clones may not fully represent the properties of all pathogen-specific clones in an individual and that binding affinities for cross-reactivity that would be relevant in vivo are not known. Further study of the role of cross-reactive memory B cell populations in primary immune responses to related but divergent viruses as well as better understanding of the determinants of long-lived B cell memory and plasma cell formation will be important for ongoing improvement of vaccines to SARS-CoV-2, its viral variants, and other pathogens.
Acknowledgments
We thank all staff members of the California Transplant Donor Network (now Donor Network West), especially S. Swain. We thank A. Z. Fire, E. A. Hope, and J. D. Merker for helpful discussions and contributions to the research. We thank Meso Scale Diagnostics for helpful collaboration and material support in this study. Funding: This work was supported by NIH/NIAID R01AI127877, R01AI130398, U19AI057229, and U19AI090019 and NIH/NCI U54CA260517 (S.D.B.); NIH R01 HD063142 (J.P.); and an endowment from the Crown Family Foundation (S.D.B.). Author contributions: F.Y. and S.D.B. conceived of the project. F.Y., S.C.A.N., K.J.L.J., Y.L., and K.M.R. performed data analyses. F.Y., S.C.A.N., and S.D.B. verified the analyses. F.Y., S.C.A.N., R.A.H., K.R., E.H., O.F.W., G.H.J., R.S.O., E.M.O., J.-Y.L., K.N., and T.D.P. contributed to sample preparation and carried out the experiments. T.D.P., K.C.N., C.U.N., J.P., and S.D.B. provided samples and supported the project. F.Y., S.C.A.N., and S.D.B. wrote the initial manuscript. All authors provided critical feedback and contributed to the final manuscript. Competing interests: S.D.B.: consulting for Regeneron, stock ownership in AbCellera Biologics, and collaboration with Meso Scale Diagnostics. K.N.: director of the World Allergy Organization (WAO) Center of Excellence at Stanford; advisor at Cour Pharma; co-founder of Before Brands, Alladapt, Latitude, and IgGenix; national scientific committee member at Immune Tolerance Network (ITN) and the National Institutes of Health (NIH) clinical research centers; and data and safety monitoring board member for NHLBI. Data and materials availability: All data are available in the main text or the supplementary materials. Previously generated IGH repertoire data are available under BioProject numbers PRJNA503602 (child dataset) and PRJNA491287 [114 healthy human adult dataset was previously reported (18)]. The IGH sequences for mAbs tested in this study were deposited in GenBank (MW821491 to MW821509). The IGH repertoire data for the deceased organ donors and the cord blood infant samples are available under BioProject number PRJNA674610. This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/. This license does not apply to figures/photos/artwork or other content included in the article that is credited to a third party; obtain authorization from the rights holder before using such material.
Supplementary Materials
science.sciencemag.org/content/372/6543/738/suppl/DC1
Materials and Methods
Figs. S1 to S12
Tables S1 to S9
MDAR Reproducibility Checklist
References and Notes
- 1.Boyd S. D., Crowe J. E. Jr., ., Deep sequencing and human antibody repertoire analysis. Curr. Opin. Immunol. 40, 103–109. (2016). 10.1016/j.coi.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley D. D., Chaudhuri J., Bassing C. H., Alt F. W., Mechanism and control of V(D)J recombination versus class switch recombination: Similarities and differences. Adv. Immunol. 86, 43–112. (2005). 10.1016/S0065-2776(04)86002-4 [DOI] [PubMed] [Google Scholar]
- 3.Jackson K. J., Liu Y., Roskin K. M., Glanville J., Hoh R. A., Seo K., Marshall E. L., Gurley T. C., Moody M. A., Haynes B. F., Walter E. B., Liao H.-X., Albrecht R. A., Garca-Sastre A., Chaparro-Riggers J., Rajpal A., Pons J., Simen B. B., Hanczaruk B., Dekker C. L., Laserson J., Koller D., Davis M. M., Fire A. Z., Boyd S. D., Human responses to influenza vaccination show seroconversion signatures and convergent antibody rearrangements. Cell Host Microbe 16, 105–114. (2014). 10.1016/j.chom.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trck J., Ramasamy M. N., Galson J. D., Rance R., Parkhill J., Lunter G., Pollard A. J., Kelly D. F., Identification of antigen-specific B cell receptor sequences using public repertoire analysis. J. Immunol. 194, 252–261. (2015). 10.4049/jimmunol.1401405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setliff I., McDonnell W. J., Raju N., Bombardi R. G., Murji A. A., Scheepers C., Ziki R., Mynhardt C., Shepherd B. E., Mamchak A. A., Garrett N., Karim S. A., Mallal S. A., Crowe J. E. Jr.., Morris L., Georgiev I. S., Multi-donor longitudinal antibody repertoire sequencing reveals the existence of public antibody clonotypes in HIV-1 infection. Cell Host Microbe 23, 845–854.e6. (2018). 10.1016/j.chom.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goo L., Chohan V., Nduati R., Overbaugh J., Early development of broadly neutralizing antibodies in HIV-1-infected infants. Nat. Med. 20, 655–658. (2014). 10.1038/nm.3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gtzinger F., Santiago-Garca B., Noguera-Julin A., Lanaspa M., Lancella L., Cal Carducci F. I., Gabrovska N., Velizarova S., Prunk P., Osterman V., Krivec U., Lo Vecchio A., Shingadia D., Soriano-Arandes A., Melendo S., Lanari M., Pierantoni L., Wagner N., LHuillier A. G., Heininger U., Ritz N., Bandi S., Krajcar N., Rogli S., Santos M., Christiaens C., Creuven M., Buonsenso D., Welch S. B., Bogyi M., Brinkmann F., Tebruegge M.; ptbnet COVID-19 Study Group , COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc. Health 4, 653–661. (2020). 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X., Zhang L., Du H., Zhang J., Li Y. Y., Qu J., Zhang W., Wang Y., Bao S., Li Y., Wu C., Liu H., Liu D., Shao J., Peng X., Yang Y., Liu Z., Xiang Y., Zhang F., Silva R. M., Pinkerton K. E., Shen K., Xiao H., Xu S., Wong G. W. K.; Chinese Pediatric Novel Coronavirus Study Team , SARS-CoV-2 infection in children. N. Engl. J. Med. 382, 1663–1665. (2020). 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiehao C., Jin X., Daojiong L., Zhi Y., Lei X., Zhenghai Q., Yuehua Z., Hua Z., Ran J., Pengcheng L., Xiangshi W., Yanling G., Aimei X., He T., Hailing C., Chuning W., Jingjing L., Jianshe W., Mei Z., A case series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin. Infect. Dis. 71, 1547–1551. (2020). 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liguoro I., Pilotto C., Bonanni M., Ferrari M. E., Pusiol A., Nocerino A., Vidal E., Cogo P., SARS-COV-2 infection in children and newborns: A systematic review. Eur. J. Pediatr. 179, 1029–1046. (2020). 10.1007/s00431-020-03684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carsetti R., Quintarelli C., Quinti I., Piano Mortari E., Zumla A., Ippolito G., Locatelli F., The immune system of children: The key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolesc. Health 4, 414–416. (2020). 10.1016/S2352-4642(20)30135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng K. W., Faulkner N., Cornish G. H., Rosa A., Harvey R., Hussain S., Ulferts R., Earl C., Wrobel A. G., Benton D. J., Roustan C., Bolland W., Thompson R., Agua-Doce A., Hobson P., Heaney J., Rickman H., Paraskevopoulou S., Houlihan C. F., Thomson K., Sanchez E., Shin G. Y., Spyer M. J., Joshi D., OReilly N., Walker P. A., Kjaer S., Riddell A., Moore C., Jebson B. R., Wilkinson M., Marshall L. R., Rosser E. C., Radziszewska A., Peckham H., Ciurtin C., Wedderburn L. R., Beale R., Swanton C., Gandhi S., Stockinger B., McCauley J., Gamblin S. J., McCoy L. E., Cherepanov P., Nastouli E., Kassiotis G., Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 370, 1339–1343. (2020). 10.1126/science.abe1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisberg S. P., Connors T. J., Zhu Y., Baldwin M. R., Lin W.-H., Wontakal S., Szabo P. A., Wells S. B., Dogra P., Gray J., Idzikowski E., Stelitano D., Bovier F. T., Davis-Porada J., Matsumoto R., Poon M. M. L., Chait M., Mathieu C., Horvat B., Decimo D., Hudson K. E., Zotti F. D., Bitan Z. C., La Carpia F., Ferrara S. A., Mace E., Milner J., Moscona A., Hod E., Porotto M., Farber D. L., Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 22, 25–31. (2021). 10.1038/s41590-020-00826-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berek C., Milstein C., Mutation drift and repertoire shift in the maturation of the immune response. Immunol. Rev. 96, 23–41. (1987). 10.1111/j.1600-065X.1987.tb00507.x [DOI] [PubMed] [Google Scholar]
- 15.Jacob J., Kelsoe G., Rajewsky K., Weiss U., Intraclonal generation of antibody mutants in germinal centres. Nature 354, 389–392. (1991). 10.1038/354389a0 [DOI] [PubMed] [Google Scholar]
- 16.Vinuesa C. G., de Lucas C., Cook M. C., Clinical implications of the specialised B cell response to polysaccharide encapsulated pathogens. Postgrad. Med. J. 77, 562–569. (2001). 10.1136/pmj.77.911.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLennan I. C., Liu Y. J., Marginal zone B cells respond both to polysaccharide antigens and protein antigens. Res. Immunol. 142, 346–351. (1991). 10.1016/0923-2494(91)90089-2 [DOI] [PubMed] [Google Scholar]
- 18.Nielsen S. C. A., Roskin K. M., Jackson K. J. L., Joshi S. A., Nejad P., Lee J.-Y., Wagar L. E., Pham T. D., Hoh R. A., Nguyen K. D., Tsunemoto H. Y., Patel S. B., Tibshirani R., Ley C., Davis M. M., Parsonnet J., Boyd S. D., Shaping of infant B cell receptor repertoires by environmental factors and infectious disease. Sci. Transl. Med. 11, eaat2004 (2019). 10.1126/scitranslmed.aat2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn S. R., Ryder A. B., Tollefson S. J., Xu M., Saville B. R., Williams J. V., Seroepidemiologies of human metapneumovirus and respiratory syncytial virus in young children, determined with a new recombinant fusion protein enzyme-linked immunosorbent assay. Clin. Vaccine Immunol. 20, 1654–1656. (2013). 10.1128/CVI.00750-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broderick M. P., Faix D. J., Hansen C. J., Blair P. J., Trends in meningococcal disease in the United States military, 19712010. Emerg. Infect. Dis. 18, 1430–1437. (2012). 10.3201/eid1809.120257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng W., Zhang B., Schwartz G. W., Rosenfeld A. M., Ren D., Thome J. J. C., Carpenter D. J., Matsuoka N., Lerner H., Friedman A. L., Granot T., Farber D. L., Shlomchik M. J., Hershberg U., Luning Prak E. T., An atlas of B-cell clonal distribution in the human body. Nat. Biotechnol. 35, 879–884. (2017). 10.1038/nbt.3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganusov V. V., De Boer R. J., Do most lymphocytes in humans really reside in the gut? Trends Immunol. 28, 514–518. (2007). 10.1016/j.it.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 23.Langeveld M., Gamadia L. E., ten Berge I. J., T-lymphocyte subset distribution in human spleen. Eur. J. Clin. Invest. 36, 250–256. (2006). 10.1111/j.1365-2362.2006.01626.x [DOI] [PubMed] [Google Scholar]
- 24.Anderson E. M., Goodwin E. C., Verma A., Arevalo C. P., Bolton M. J., Weirick M. E., Gouma S., McAllister C. M., Christensen S. R., Weaver J., Hicks P., Manzoni T. B., Oniyide O., Ramage H., Mathew D., Baxter A. E., Oldridge D. A., Greenplate A. R., Wu J. E., Alanio C., DAndrea K., Kuthuru O., Dougherty J., Pattekar A., Kim J., Han N., Apostolidis S. A., Huang A. C., Vella L. A., Kuri-Cervantes L., Pampena M. B., Betts M. R., Wherry E. J., Meyer N. J., Cherry S., Bates P., Rader D. J., Hensley S. E.; UPenn COVID Processing Unit , Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 184, 1858–1864.e10. (2021). 10.1016/j.cell.2021.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunyavanich S., Do A., Vicencio A., Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 323, 2427–2429. (2020). 10.1001/jama.2020.8707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wec A. Z., Wrapp D., Herbert A. S., Maurer D. P., Haslwanter D., Sakharkar M., Jangra R. K., Dieterle M. E., Lilov A., Huang D., Tse L. V., Johnson N. V., Hsieh C.-L., Wang N., Nett J. H., Champney E., Burnina I., Brown M., Lin S., Sinclair M., Johnson C., Pudi S., Bortz R. 3rd, Wirchnianski A. S., Laudermilch E., Florez C., Fels J. M., OBrien C. M., Graham B. S., Nemazee D., Burton D. R., Baric R. S., Voss J. E., Chandran K., Dye J. M., McLellan J. S., Walker L. M., Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science 369, 731–736. (2020). 10.1126/science.abc7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S. I., Noh J., Kim S., Choi Y., Yoo D. K., Lee Y., Lee H., Jung J., Kang C. K., Song K.-H., Choe P. G., Kim H. B., Kim E. S., Kim N.-J., Seong M.-W., Park W. B., Oh M. D., Kwon S., Chung J., Stereotypic neutralizing VH antibodies against SARS-CoV-2 spike protein receptor binding domain in patients with COVID-19 and healthy individuals. Sci. Transl. Med. 13, eabd6990 (2021). 10.1126/scitranslmed.abd6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.G. Song, W.-T. He, S. Callaghan, F. Anzanello, D. Huang, J. Ricketts, J. L. Torres, N. Beutler, L. Peng, S. Vargas, J. Cassell, M. Parren, L. Yang, C. Ignacio, D. M. Smith, J. E. Voss, D. Nemazee, A. B. Ward, T. Rogers, D. R. Burton, R. Andrabi, Cross-reactive serum and memory B cell responses to spike protein in SARS-CoV-2 and endemic coronavirus infection. bioRxiv 2020.09.22.308965 [Preprint]. 23 September 2020. 10.1101/2020.09.22.308965. [DOI] [PMC free article] [PubMed]
- 29.Arevalo C. P., Le Sage V., Bolton M. J., Eilola T., Jones J. E., Kormuth K. A., Nturibi E., Balmaseda A., Gordon A., Lakdawala S. S., Hensley S. E., Original antigenic sin priming of influenza virus hemagglutinin stalk antibodies. Proc. Natl. Acad. Sci. U.S.A. 117, 17221–17227. (2020). 10.1073/pnas.1920321117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roskin K. M., Simchoni N., Liu Y., Lee J.-Y., Seo K., Hoh R. A., Pham T., Park J. H., Furman D., Dekker C. L., Davis M. M., James J. A., Nadeau K. C., Cunningham-Rundles C., Boyd S. D., IgH sequences in common variable immune deficiency reveal altered B cell development and selection. Sci. Transl. Med. 7, 302ra135 (2015). 10.1126/scitranslmed.aab1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye J., Ma N., Madden T. L., Ostell J. M., IgBLAST: An immunoglobulin variable domain sequence analysis tool. Nucleic Acids Res. 41, W34–W40. (2013). 10.1093/nar/gkt382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefranc M. P., IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 31, 307–310. (2003). 10.1093/nar/gkg085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C., Liu Y., Cavanagh M. M., Le Saux S., Qi Q., Roskin K. M., Looney T. J., Lee J.-Y., Dixit V., Dekker C. L., Swan G. E., Goronzy J. J., Boyd S. D., B-cell repertoire responses to varicella-zoster vaccination in human identical twins. Proc. Natl. Acad. Sci. U.S.A. 112, 500–505. (2015). 10.1073/pnas.1415875112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lefranc M. P., Giudicelli V., Duroux P., Jabado-Michaloud J., Folch G., Aouinti S., Carillon E., Duvergey H., Houles A., Paysan-Lafosse T., Hadi-Saljoqi S., Sasorith S., Lefranc G., Kossida S., IMGT, the international ImMunoGeneTics information system 25 years on. Nucleic Acids Res. 43, D413–D422. (2015). 10.1093/nar/gku1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucas A. H., McLean G. R., Reason D. C., OConnor A. P., Felton M. C., Moulton K. D., Molecular ontogeny of the human antibody repertoire to the Haemophilus influenzae type B polysaccharide: Expression of canonical variable regions and their variants in vaccinated infants. Clin. Immunol. 108, 119–127. (2003). 10.1016/S1521-6616(03)00094-9 [DOI] [PubMed] [Google Scholar]
- 36.Adderson E. E., Shackelford P. G., Quinn A., Wilson P. M., Cunningham M. W., Insel R. A., Carroll W. L., Restricted immunoglobulin VH usage and VDJ combinations in the human response to Haemophilus influenzae type b capsular polysaccharide. Nucleotide sequences of monospecific anti-Haemophilus antibodies and polyspecific antibodies cross-reacting with self antigens. J. Clin. Invest. 91, 2734–2743. (1993). 10.1172/JCI116514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucas A. H., Larrick J. W., Reason D. C., Variable region sequences of a protective human monoclonal antibody specific for the Haemophilus influenzae type b capsular polysaccharide. Infect. Immun. 62, 3873–3880. (1994). 10.1128/IAI.62.9.3873-3880.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry J. D., Boese D. J., Law D. K., Zollinger W. D., Tsang R. S., Molecular analysis of monoclonal antibodies to group variant capsular polysaccharide of Neisseria meningitidis: recurrent heavy chains and alternative light chain partners. Mol. Immunol. 42, 335–344. (2005). 10.1016/j.molimm.2004.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smithson S. L., Srivastava N., Hutchins W. A., Westerink M. A., Molecular analysis of the heavy chain of antibodies that recognize the capsular polysaccharide of Neisseria meningitidis in hu-PBMC reconstituted SCID mice and in the immunized human donor. Mol. Immunol. 36, 113–124. (1999). 10.1016/S0161-5890(99)00024-3 [DOI] [PubMed] [Google Scholar]
- 40.Hutchins W. A., Adkins A. R., Kieber-Emmons T., Westerink M. A., Molecular characterization of a monoclonal antibody produced in response to a group C meningococcal polysaccharide peptide mimic. Mol. Immunol. 33, 503–510. (1996). 10.1016/0161-5890(96)00012-0 [DOI] [PubMed] [Google Scholar]
- 41.Chen Z., Cox K. S., Tang A., Roman J., Fink M., Kaufhold R. M., Guan L., Xie A., Boddicker M. A., Mcguinness D., Xiao X., Li H., Skinner J. M., Verch T., Retzlaff M., Vora K. A., Human monoclonal antibodies isolated from a primary pneumococcal conjugate Vaccinee demonstrates the expansion of an antigen-driven Hypermutated memory B cell response. BMC Infect. Dis. 18, 613 (2018). 10.1186/s12879-018-3517-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolibab K., Smithson S. L., Rabquer B., Khuder S., Westerink M. A., Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: Analysis of the variable heavy chain repertoire. Infect. Immun. 73, 7465–7476. (2005). 10.1128/IAI.73.11.7465-7476.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith K., Muther J. J., Duke A. L., McKee E., Zheng N.-Y., Wilson P. C., James J. A., Fully human monoclonal antibodies from antibody secreting cells after vaccination with Pneumovax23 are serotype specific and facilitate opsonophagocytosis. Immunobiology 218, 745–754. (2013). 10.1016/j.imbio.2012.08.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adler A. S., Mizrahi R. A., Spindler M. J., Adams M. S., Asensio M. A., Edgar R. C., Leong J., Leong R., Roalfe L., White R., Goldblatt D., Johnson D. S., Rare, high-affinity anti-pathogen antibodies from human repertoires, discovered using microfluidics and molecular genomics. MAbs 9, 1282–1296. (2017). 10.1080/19420862.2017.1371383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeKosky B. J., Ippolito G. C., Deschner R. P., Lavinder J. J., Wine Y., Rawlings B. M., Varadarajan N., Giesecke C., Drner T., Andrews S. F., Wilson P. C., Hunicke-Smith S. P., Willson C. G., Ellington A. D., Georgiou G., High-throughput sequencing of the paired human immunoglobulin heavy and light chain repertoire. Nat. Biotechnol. 31, 166–169. (2013). 10.1038/nbt.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Kruif J., Kramer A., Visser T., Clements C., Nijhuis R., Cox F., van der Zande V., Smit R., Pinto D., Throsby M., Logtenberg T., Human immunoglobulin repertoires against tetanus toxoid contain a large and diverse fraction of high-affinity promiscuous VH genes. J. Mol. Biol. 387, 548–558. (2009). 10.1016/j.jmb.2009.02.009 [DOI] [PubMed] [Google Scholar]
- 47.Frlich D., Giesecke C., Mei H. E., Reiter K., Daridon C., Lipsky P. E., Drner T., Secondary immunization generates clonally related antigen-specific plasma cells and memory B cells. J. Immunol. 185, 3103–3110. (2010). 10.4049/jimmunol.1000911 [DOI] [PubMed] [Google Scholar]
- 48.Poulsen T. R., Jensen A., Haurum J. S., Andersen P. S., Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J. Immunol. 187, 4229–4235. (2011). 10.4049/jimmunol.1000928 [DOI] [PubMed] [Google Scholar]
- 49.Gilman M. S., Castellanos C. A., Chen M., Ngwuta J. O., Goodwin E., Moin S. M., Mas V., Melero J. A., Wright P. F., Graham B. S., McLellan J. S., Walker L. M., Rapid profiling of RSV antibody repertoires from the memory B cells of naturally infected adult donors. Sci. Immunol. 1, eaaj1879 (2016). 10.1126/sciimmunol.aaj1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams J. V., Weitkamp J. H., Blum D. L., LaFleur B. J., Crowe J. E. Jr., ., The human neonatal B cell response to respiratory syncytial virus uses a biased antibody variable gene repertoire that lacks somatic mutations. Mol. Immunol. 47, 407–414. (2009). 10.1016/j.molimm.2009.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang A., Chen Z., Cox K. S., Su H.-P., Callahan C., Fridman A., Zhang L., Patel S. B., Cejas P. J., Swoyer R., Touch S., Citron M. P., Govindarajan D., Luo B., Eddins M., Reid J. C., Soisson S. M., Galli J., Wang D., Wen Z., Heidecker G. J., Casimiro D. R., DiStefano D. J., Vora K. A., A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat. Commun. 10, 4153 (2019). 10.1038/s41467-019-12137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cortjens B., Yasuda E., Yu X., Wagner K., Claassen Y. B., Bakker A. Q., van Woensel J. B. M., Beaumont T., Broadly reactive anti-respiratory syncytial virus G antibodies from exposed individuals effectively inhibit infection of primary airway epithelial cells. J. Virol. 91, e02357-16 (2017). 10.1128/JVI.02357-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodwin E., Gilman M. S. A., Wrapp D., Chen M., Ngwuta J. O., Moin S. M., Bai P., Sivasubramanian A., Connor R. I., Wright P. F., Graham B. S., McLellan J. S., Walker L. M., Infants infected with respiratory syncytial virus generate potent neutralizing antibodies that lack somatic hypermutation. Immunity 48, 339–349.e5. (2018). 10.1016/j.immuni.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krause J. C., Tsibane T., Tumpey T. M., Huffman C. J., Briney B. S., Smith S. A., Basler C. F., Crowe J. E. Jr., ., Epitope-specific human influenza antibody repertoires diversify by B cell intraclonal sequence divergence and interclonal convergence. J. Immunol. 187, 3704–3711. (2011). 10.4049/jimmunol.1101823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moody M. A., Zhang R., Walter E. B., Woods C. W., Ginsburg G. S., McClain M. T., Denny T. N., Chen X., Munshaw S., Marshall D. J., Whitesides J. F., Drinker M. S., Amos J. D., Gurley T. C., Eudailey J. A., Foulger A., DeRosa K. R., Parks R., Meyerhoff R. R., Yu J.-S., Kozink D. M., Barefoot B. E., Ramsburg E. A., Khurana S., Golding H., Vandergrift N. A., Alam S. M., Tomaras G. D., Kepler T. B., Kelsoe G., Liao H.-X., Haynes B. F., H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLOS ONE 6, e25797 (2011). 10.1371/journal.pone.0025797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wrammert J., Koutsonanos D., Li G.-M., Edupuganti S., Sui J., Morrissey M., McCausland M., Skountzou I., Hornig M., Lipkin W. I., Mehta A., Razavi B., Del Rio C., Zheng N.-Y., Lee J.-H., Huang M., Ali Z., Kaur K., Andrews S., Amara R. R., Wang Y., Das S. R., ODonnell C. D., Yewdell J. W., Subbarao K., Marasco W. A., Mulligan M. J., Compans R., Ahmed R., Wilson P. C., Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208, 181–193. (2011). 10.1084/jem.20101352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ellebedy A. H., Jackson K. J. L., Kissick H. T., Nakaya H. I., Davis C. W., Roskin K. M., McElroy A. K., Oshansky C. M., Elbein R., Thomas S., Lyon G. M., Spiropoulou C. F., Mehta A. K., Thomas P. G., Boyd S. D., Ahmed R., Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 17, 1226–1234. (2016). 10.1038/ni.3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neu K. E., Guthmiller J. J., Huang M., La J., Vieira M. C., Kim K., Zheng N.-Y., Cortese M., Tepora M. E., Hamel N. J., Rojas K. T., Henry C., Shaw D., Dulberger C. L., Pulendran B., Cobey S., Khan A. A., Wilson P. C., Spec-seq unveils transcriptional subpopulations of antibody-secreting cells following influenza vaccination. J. Clin. Invest. 129, 93–105. (2019). 10.1172/JCI121341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Tan H.-X., Koutsakos M., Jegaskanda S., Esterbauer R., Tilmanis D., Aban M., Kedzierska K., Hurt A. C., Kent S. J., Wheatley A. K., Cross-lineage protection by human antibodies binding the influenza B hemagglutinin. Nat. Commun. 10, 324 (2019). 10.1038/s41467-018-08165-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirano D., Ohshima N., Kubota-Koketsu R., Yamasaki A., Kurosawa G., Okuno Y., Yoshida S., Kurosawa Y., Three types of broadly reacting antibodies against influenza B viruses induced by vaccination with seasonal influenza viruses. J. Immunol. Res. 2018, 7251793 (2018). 10.1155/2018/7251793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasuhara A., Yamayoshi S., Soni P., Takenaga T., Kawakami C., Takashita E., Sakai-Tagawa Y., Uraki R., Ito M., Iwatsuki-Horimoto K., Sasaki T., Ikuta K., Yamada S., Kawaoka Y., Diversity of antigenic mutants of influenza A(H1N1)pdm09 virus escaped from human monoclonal antibodies. Sci. Rep. 7, 17735 (2017). 10.1038/s41598-017-17986-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joyce M. G., Wheatley A. K., Thomas P. V., Chuang G.-Y., Soto C., Bailer R. T., Druz A., Georgiev I. S., Gillespie R. A., Kanekiyo M., Kong W.-P., Leung K., Narpala S. N., Prabhakaran M. S., Yang E. S., Zhang B., Zhang Y., Asokan M., Boyington J. C., Bylund T., Darko S., Lees C. R., Ransier A., Shen C.-H., Wang L., Whittle J. R., Wu X., Yassine H. M., Santos C., Matsuoka Y., Tsybovsky Y., Baxa U., Mullikin J. C., Subbarao K., Douek D. C., Graham B. S., Koup R. A., Ledgerwood J. E., Roederer M., Shapiro L., Kwong P. D., Mascola J. R., McDermott A. B.; NISC Comparative Sequencing Program , Vaccine-induced antibodies that neutralize group 1 and group 2 influenza A viruses. Cell 166, 609–623. (2016). 10.1016/j.cell.2016.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu Y., Zhang Z., Sheehan J., Avnir Y., Ridenour C., Sachnik T., Sun J., Hossain M. J., Chen L.-M., Zhu Q., Donis R. O., Marasco W. A., A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat. Commun. 7, 12780 (2016). 10.1038/ncomms12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li G. M., Chiu C., Wrammert J., McCausland M., Andrews S. F., Zheng N.-Y., Lee J.-H., Huang M., Qu X., Edupuganti S., Mulligan M., Das S. R., Yewdell J. W., Mehta A. K., Wilson P. C., Ahmed R., Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc. Natl. Acad. Sci. U.S.A. 109, 9047–9052. (2012). 10.1073/pnas.1118979109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watanabe A., McCarthy K. R., Kuraoka M., Schmidt A. G., Adachi Y., Onodera T., Tonouchi K., Caradonna T. M., Bajic G., Song S., McGee C. E., Sempowski G. D., Feng F., Urick P., Kepler T. B., Takahashi Y., Harrison S. C., Kelsoe G., Antibodies to a conserved influenza head interface epitope protect by an IgG subtype-dependent mechanism. Cell 177, 1124–1135.e16. (2019). 10.1016/j.cell.2019.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCarthy K. R., Watanabe A., Kuraoka M., Do K. T., McGee C. E., Sempowski G. D., Kepler T. B., Schmidt A. G., Kelsoe G., Harrison S. C., Memory B cells that cross-react with group 1 and group 2 influenza A viruses are abundant in adult human repertoires. Immunity 48, 174–184.e9. (2018). 10.1016/j.immuni.2017.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andrews S. F., Joyce M. G., Chambers M. J., Gillespie R. A., Kanekiyo M., Leung K., Yang E. S., Tsybovsky Y., Wheatley A. K., Crank M. C., Boyington J. C., Prabhakaran M. S., Narpala S. R., Chen X., Bailer R. T., Chen G., Coates E., Kwong P. D., Koup R. A., Mascola J. R., Graham B. S., Ledgerwood J. E., McDermott A. B., Preferential induction of cross-group influenza A hemagglutinin stem-specific memory B cells after H7N9 immunization in humans. Sci. Immunol. 2, eaan2676 (2017). 10.1126/sciimmunol.aan2676 [DOI] [PubMed] [Google Scholar]
- 68.Brouwer P. J. M., Caniels T. G., van der Straten K., Snitselaar J. L., Aldon Y., Bangaru S., Torres J. L., Okba N. M. A., Claireaux M., Kerster G., Bentlage A. E. H., van Haaren M. M., Guerra D., Burger J. A., Schermer E. E., Verheul K. D., van der Velde N., van der Kooi A., van Schooten J., van Breemen M. J., Bijl T. P. L., Sliepen K., Aartse A., Derking R., Bontjer I., Kootstra N. A., Wiersinga W. J., Vidarsson G., Haagmans B. L., Ward A. B., de Bree G. J., Sanders R. W., van Gils M. J., Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 369, 643–650. (2020). 10.1126/science.abc5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rogers T. F., Zhao F., Huang D., Beutler N., Burns A., He W. T., Limbo O., Smith C., Song G., Woehl J., Yang L., Abbott R. K., Callaghan S., Garcia E., Hurtado J., Parren M., Peng L., Ramirez S., Ricketts J., Ricciardi M. J., Rawlings S. A., Wu N. C., Yuan M., Smith D. M., Nemazee D., Teijaro J. R., Voss J. E., Wilson I. A., Andrabi R., Briney B., Landais E., Sok D., Jardine J. G., Burton D. R., Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 369, 956–963. (2020). 10.1126/science.abc7520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zost S. J., Gilchuk P., Chen R. E., Case J. B., Reidy J. X., Trivette A., Nargi R. S., Sutton R. E., Suryadevara N., Chen E. C., Binshtein E., Shrihari S., Ostrowski M., Chu H. Y., Didier J. E., MacRenaris K. W., Jones T., Day S., Myers L., Eun-Hyung Lee F., Nguyen D. C., Sanz I., Martinez D. R., Rothlauf P. W., Bloyet L.-M., Whelan S. P. J., Baric R. S., Thackray L. B., Diamond M. S., Carnahan R. H., Crowe J. E. Jr., ., Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat. Med. 26, 1422–1427. (2020). 10.1038/s41591-020-0998-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi R., Shan C., Duan X., Chen Z., Liu P., Song J., Song T., Bi X., Han C., Wu L., Gao G., Hu X., Zhang Y., Tong Z., Huang W., Liu W. J., Wu G., Zhang B., Wang L., Qi J., Feng H., Wang F.-S., Wang Q., Gao G. F., Yuan Z., Yan J., A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 584, 120–124. (2020). 10.1038/s41586-020-2381-y [DOI] [PubMed] [Google Scholar]
- 72.E. Seydoux, L. J. Homad, A. J. MacCamy, K. R. Parks, N. K. Hurlburt, M. F. Jennewein, N. R. Akins, A. B. Stuart, Y. H. Wan, J. Feng, R. E. Nelson, S. Singh, K. W. Cohen, M. J. McElrath, J. A. Englund, H. Y. Chu, M. Pancera, A. T. McGuire, L. Stamatatos, Characterization of neutralizing antibodies from a SARS-CoV-2 infected individual. bioRxiv 2020.05.12.091298 [Preprint]. 12 May 2020. 10.1101/2020.05.12.091298. [DOI]
- 73.Robbiani D. F., Gaebler C., Muecksch F., Lorenzi J. C. C., Wang Z., Cho A., Agudelo M., Barnes C. O., Gazumyan A., Finkin S., Hgglf T., Oliveira T. Y., Viant C., Hurley A., Hoffmann H. H., Millard K. G., Kost R. G., Cipolla M., Gordon K., Bianchini F., Chen S. T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A. W., Waltari E., Pak J. E., Huey-Tubman K. E., Koranda N., Hoffman P. R., West A. P. Jr.., Rice C. M., Hatziioannou T., Bjorkman P. J., Bieniasz P. D., Caskey M., Nussenzweig M. C., Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584, 437–442. (2020). 10.1038/s41586-020-2456-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pinto D., Park Y. J., Beltramello M., Walls A. C., Tortorici M. A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A., Peter A., Guarino B., Spreafico R., Cameroni E., Case J. B., Chen R. E., Havenar-Daughton C., Snell G., Telenti A., Virgin H. W., Lanzavecchia A., Diamond M. S., Fink K., Veesler D., Corti D., Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295. (2020). 10.1038/s41586-020-2349-y [DOI] [PubMed] [Google Scholar]
- 75.Kreer C., Zehner M., Weber T., Ercanoglu M. S., Gieselmann L., Rohde C., Halwe S., Korenkov M., Schommers P., Vanshylla K., Di Cristanziano V., Janicki H., Brinker R., Ashurov A., Krhling V., Kupke A., Cohen-Dvashi H., Koch M., Eckert J. M., Lederer S., Pfeifer N., Wolf T., Vehreschild M. J. G. T., Wendtner C., Diskin R., Gruell H., Becker S., Klein F., Longitudinal isolation of potent near-germline SARS-CoV-2-neutralizing antibodies from COVID-19 patients. Cell 182, 1663–1673. (2020). 10.1016/j.cell.2020.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis C. W., Jackson K. J. L., McElroy A. K., Halfmann P., Huang J., Chennareddy C., Piper A. E., Leung Y., Albario C. G., Crozier I., Ellebedy A. H., Sidney J., Sette A., Yu T., Nielsen S. C. A., Goff A. J., Spiropoulou C. F., Saphire E. O., Cavet G., Kawaoka Y., Mehta A. K., Glass P. J., Boyd S. D., Ahmed R., Longitudinal analysis of the human B cell response to Ebola virus infection. Cell 177, 1566–1582.e17. (2019). 10.1016/j.cell.2019.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Virtanen P., Gommers R., Oliphant T. E., Haberland M., Reddy T., Cournapeau D., Burovski E., Peterson P., Weckesser W., Bright J., van der Walt S. J., Brett M., Wilson J., Millman K. J., Mayorov N., Nelson A. R. J., Jones E., Kern R., Larson E., Carey C. J., Polat ., Feng Y., Moore E. W., VanderPlas J., Laxalde D., Perktold J., Cimrman R., Henriksen I., Quintero E. A., Harris C. R., Archibald A. M., Ribeiro A. H., Pedregosa F., van Mulbregt P.; SciPy 1.0 Contributors , SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272. (2020). 10.1038/s41592-019-0686-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fu L., Niu B., Zhu Z., Wu S., Li W., CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152. (2012). 10.1093/bioinformatics/bts565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nielsen S. C. A., Yang F., Jackson K. J. L., Hoh R. A., Rltgen K., Jean G. H., Stevens B. A., Lee J.-Y., Rustagi A., Rogers A. J., Powell A. E., Hunter M., Najeeb J., Otrelo-Cardoso A. R., Yost K. E., Daniel B., Nadeau K. C., Chang H. Y., Satpathy A. T., Jardetzky T. S., Kim P. S., Wang T. T., Pinsky B. A., Blish C. A., Boyd S. D., Human B cell clonal expansion and convergent antibody responses to SARS-CoV-2. Cell Host Microbe 28, 516–525.e5. (2020). 10.1016/j.chom.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.W. McKinney, Data structures for statistical computing in Python, Proceedings of the 9th Python in Science Conference, Austin, Texas, 28 June to 3 July 2010. [Google Scholar]
- 81.R Development Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2010); www.R-project.org.
- 82.R. A. Ehling, C. R. Weber, D. M. Mason, S. Friedensohn, B. Wagner, F. Bieberich, E. Kapetanovic, R. Vazquez-Lombardi, R. B. Di Roberto, K.-L. Hong, C. Wagner, C. J. Sheward, B. Murrell, A. Yermanos, A. P. Cuny, M. Savic, F. Rudolf, S. T. Reddy, Single-cell sequencing of plasma cells from COVID-19 patients reveals highly expanded clonal lineages produce specific and neutralizing antibodies to SARS-CoV-2. bioRxiv 2021.02.12.430940 [Preprint]. 12 February 2021. 10.1101/2021.02.12.430940. [DOI]
- 83.D. Li, R. J. Edwards, K. Manne, D. R. Martinez, A. Schfer, S. M. Alam, K. Wiehe, X. Lu, R. Parks, L. L. Sutherland, T. H. Oguin III, C. McDanal, L. G. Perez, K. Mansouri, S. M. C. Gobeil, K. Janowska, V. Stalls, M. Kopp, F. Cai, E. Lee, A. Foulger, G. E. Hernandez, A. Sanzone, K. Tilahun, C. Jiang, L. V. Tse, K. W. Bock, M. Minai, B. M. Nagata, K. Cronin, V. Gee-Lai, M. Deyton, M. Barr, T. Von Holle, A. N. Macintyre, E. Stover, J. Feldman, B. M. Hauser, T. M. Caradonna, T. D. Scobey, W. Rountree, Y. Wang, M. A. Moody, D. W. Cain, C. T. DeMarco, T. N. Denny, C. W. Woods, E. W. Petzold, A. G. Schmidt, I.-T. Teng, T. Zhou, P. D. Kwong, J. R. Mascola, B. S. Graham, I. N. Moore, R. Seder, H. Andersen, M. G. Lewis, D. C. Montefiori, G. D. Sempowski, R. S. Baric, P. Acharya, B. F. Haynes, K. O. Saunders, The functions of SARS-CoV-2 neutralizing and infection-enhancing antibodies in vitro and in mice and nonhuman primates. bioRxiv 2020.12.31.424729 [Preprint]. 18 February 2021. 10.1101/2020.12.31.424729. [DOI]
- 84.Raybould M. I. J., Kovaltsuk A., Marks C., Deane C. M., CoV-AbDab: the coronavirus antibody database. Bioinformatics 10.1093/bioinformatics/btaa739 (2020). 10.1093/bioinformatics/btaa739 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
science.sciencemag.org/content/372/6543/738/suppl/DC1
Materials and Methods
Figs. S1 to S12
Tables S1 to S9
MDAR Reproducibility Checklist