Abstract
SARS-CoV-2 mainly infects the respiratory tract, and presents significantly higher active replication in the upper airways. To remain viable and infectious, the SARS-CoV-2 virion must be complete and integral, which is not easily demonstrated in the environment by positive reverse transcriptase PCR results. Real-life conditions in healthcare settings may be conducive to SARS-CoV-2 RNA dissemination in the environment but without evidence of its viability and infectiveness in air. Theoretically, SARS-CoV-2 shedding and dissemination nonetheless appears to be air-mediated, and a distinction between “air” and “droplet” transmission is too schematic to reflect the reality of the respiratory particles emitted by patients, between which a continuum exists. Airborne transmission is influenced by numerous environmental conditions that are not transposable between different viral agents and situations in healthcare settings or in the community. Even though international guidelines on “droplet” versus “air” precautions and personal protective equipment (surgical versus respirator masks) are under discussion, the existing literature underscores the effectiveness of “droplet” precautions as a means of protecting healthcare workers. Differentiation in guidelines between healthcare venues, community settings and, more generally, confined environments is of paramount importance, especially insofar as it underlines the abiding pandemic-related need for systematic mask wearing by the general population.
Keywords: COVID-19, Infection control, Pandemic, Aerosol, Mask
1. Introduction
SARS-CoV-2, which brought about the coronavirus infectious disease 2019 (COVID-19) pandemic, is an enveloped non-segmented virus presenting a positive-sense single-stranded RNA genome consisting of about 30,000 nucleotides. The virion (around 0.125 μm) presents a nucleocapsid containing genomic RNA and phosphoriled nucleocapsid (N) protein, which is buried inside phospholipid bilayers and covered by spike glycoprotein trimmer (S). The membrane (M) and envelope (E) proteins are inserted in the virus envelope among S proteins [1]. To be infective, the viral particle must contain all of these constitutive elements, which condition its integrity. Since the first report of SARS-CoV-2, its genome has evolved and displayed mutations leading to the emergence of numerous variants, some of them “of concern” (VOC) due to significant mutations conferring selective advantage to their transmission, virulence and/or immune escape [2].
The currently recommended method to diagnose COVID-19 is based on real-time reverse transcriptase polymerase chain reaction (rRT-PCR) aimed at detecting SARS-CoV-2 in biologic samples by amplifying at least 2 or 3 different targets for a sensitive diagnosis (https://www.who.int/publications/i/item/10665-331501). Positive rRT-PCR denotes a positive rRT-PCR signal for 2 or 3 portions of the SARS-CoV-2 genome [3] but does not guarantee its viability and infectivity, even when the viral genome is complete [4]. Because human samples contain biologic fluids and organic substances, positive rRT-PCR may signal the presence of viable viral particles, especially in respiratory samples where the active replication of SARS-CoV-2 is demonstrated [5]. However, a positive rRT-PCR result and related infectiousness must always take into consideration the following: disease evolution, nature of the samples [5], laboratory protocol and sensitivity of the methods [6], [7]. Regarding environmental specimens, a positive rRT-PCR result is difficult to interpret insofar as no active replication occurs in an inanimate environment.
Based on theoretical data and recent studies on SARS-CoV-2 environmental contamination in healthcare settings, the open letter by Morowoska & Milton [8], which was signed by an international collective of healthcare professionals, urged the WHO to reclassify SARS-CoV-2 as an airborne pathogen. To date, the WHO has recommended the implementation of “contact” and “droplet” precautions for healthcare workers (HCWs) (https://apps.who.int/iris/handle/10665/331695), according to which a medical mask is worn most of the time, while N95 or Filtering Facepiece (FFP2) respirator are reserved for invasive care procedures and aerosol-generating procedures (AGPs) [9].
More generally, the COVID-19 pandemic has occasioned a remarkable amount of scientific literature in record time. As Sosnowski et al. pointed out, “a vast amount of data on this subject were gathered and published in 2020, resulting in a kind of ‘information chaos’ created by a mix of essential with unimportant or even false conclusions” [10].
The present literature review briefly outlines current knowledge on the microbiological and pathophysiological characteristics, as well as the transmission routes, of SARS-CoV-2. Environmental dissemination and persistence of SARS-CoV-2 are interpreted on the basis of theoretical data, and also in real-life conditions in healthcare settings. Lastly, the effectiveness of surgical and respirator masks and the risks incurred by HCWs of being contaminated by SARS-CoV-2 are discussed with regard to current reports in scientific literature.
2. SARS-CoV-2: From the contamination to the shedding
Even though SARS-CoV-2 cannot replicate outside of a host cell, it may be internalized after liaison of the S protein to the angiotensin-conversion enzyme II (ACE2) receptor. Briefly, the binding allows the attachment and entry of the virus prior to the release of RNA genome, its replication, and the synthesis of proteins constitutive of the virion in human cells. Given that the genome is “minimalist” and does not contain all the enzymes necessary to the replication cycle, the virus requires the involvement of cell machinery. Once the viral RNA genome has been replicated and the viral proteins synthesized and conformed, the virions are assembled, and exocytosis and release in the extracellular compartment can occur [1].
After which, SARS-CoV-2 may be disseminated in the human body; ACE2 receptors are expressed in a decreasing gradient from the upper to the lower airwaves, which induces a gradient of infectivity of SARS-CoV-2 from the proximal to the distal respiratory tract [11].
Analyses of clinical samples from COVID-19 patients have shown that SARS-CoV-2 is primarily present in respiratory samples, rarely isolated in blood and urine, and that it can also be excreted at high concentrations over a long period of time in feces [5], [12], [13]. However, detection of SARS-CoV-2 RNA by rRT-PCR does not necessarily mean that the viral particles are viable and infective; while Wölfel et al. [5] have postulated the existence of active viral replication in digestive tract, they failed to cultivate the virus from feces. If fecal-oral transmission seems possible, SARS-CoV-2 viability and infectivity in feces is not demonstrated, and inhalation of infectious particles remains the principal route of contamination [5], [12], [13], [14].
SARS-CoV-2 respiratory shedding seems higher during the pre-symptomatic and early stages of COVID-19, and it progressively decreases along with the disease evolution. Furthermore, it is significantly higher in patients presenting severe compared to mild forms of COVID-19 [5], [15]. While the median incubation period is estimated at 5.1 days (95% CI, 4.5 to 5.8 days), it varies widely according to several parameters (patient age…) [16], and 97.5% of symptomatic patients present symptoms within 11.5 days (95% CI, 8.2 to 15.6 days) of infection [17]. Considering these elements, the rate of silent transmission approximates 50%, with a peak of contagiousness 2 or 3 days before first symptoms and up to 8 days after their occurrence [18], [19].
Key-points: ACE-2 receptors are expressed in a decreasing gradient from the upper to the lower respiratory tract. Active replication of SARS-CoV-2 occurs more significantly in the upper than in the lower airwaves. SARS-CoV-2 load in respiratory samples is maximal during the pre-symptomatic and early stages of COVID-19 and progressively decreases according to disease evolution.
3. Theoretical definitions of air-mediated transmission: the differences between “air” and “droplets”
Several terms define air-mediated transmission and can lead to confusion, especially insofar as definitions vary between clinicians, scientists and the general population, as illustrated in Table 1 [20]. Respiratory activities (exhaling, speaking, singing, coughing, sneezing…) can emit both liquid (“droplets”, according to scientists) and solid (“droplet nuclei”) particles in aerosols, and their size covers a spectrum ranging from 1 μm to 100 μm. According to clinicians, droplets rapidly fall by gravity, or may desiccate in droplet nuclei, whereas “aerosols” remain suspended in the air. The duration of air suspension and distance of dissemination are conditioned by environmental conditions and particle size, with an artificial cut-off at 5 μm distinguishing large-size particles (> 5 μm) traveling over short distances (< 1 m), from fine particles (< 5 μm), which remain in suspension in the air and may travel over long distances (> 3 m) for a number of hours [21], [22]. Space-time models of particle dissemination show that the respiratory tract emits particles in highly variable sizes and loads according to the peculiarities of activities and individuals [23]:
-
•
Breathing emits 10 to 104 particles per liter of exhaled air, including 95% of particles of less than 1 μm;
-
•
Speaking produces 5000 particles of around 60 μm a minute;
-
•
Coughing emits 103 to 104 particles in sizes ranging from 0.5 to 30 μm;
-
•
Sneezing induces the shedding of 106 particles of 0.5 to 16 μm in size.
Table 1.
Differences between clinicians, aerosol scientists and the general public in the understanding of airborne terminology, adapted from Tang et al. [17].
| Terminology | Clinicians | Aerosol scientists | General population |
|---|---|---|---|
| Airborne | Long-distance transmission that requires a N95/FFP respirator for infection control (for example Measles) | Anything in the air | Anything in the air |
| Aerosol | Particle < 5 μm that mediates airborne transmission; produced during aerosol-generating procedures: requiring a N95/FFP respirator for infection control | Collection of solid and/or liquid particles of any size suspended in a gas | Hair spray or other personal/cleaning products |
| Droplet | Particle > 5 μm that falls rapidly to the ground within a distance of 1-2 m from source; requires a surgical mask for infection control | Liquid particle | What comes out of an eyedropper |
| Droplet nuclei | Residue of a droplet that has evaporated to < 5 μm; synonymous with aerosol | A related term, ‘cloud condensation nuclei’, refers to small particles on to which water condenses to form cloud droplets | Never heard of! |
| Particle | Virion | Tiny solid or liquid ‘blop’ in the air | Like soot or ash |
The smallest respiratory particles arise primarily from the lower respiratory tract (exhaled breath) while the larger particles are emitted from the upper respiratory tract [24]. Furthermore, particle deposition within the respiratory tract depends on their size, with a decreasing gradient from the nasopharyngeal fosses to the lungs [24]. All in all, drawing a distinction between “air” and “droplet” transmission appears too schematic to reflect the realty of air-mediated transmission.
Key-points: Various definitions of airborne-related terminology generate confusion. An aerosol can contain both liquid (droplets) and solid (droplet nuclei) particles of different sizes. Respiratory activities can produce a wide spectrum of droplets and aerosols in highly variable sizes and loads. A distinction between “droplets” and “air” transmission is too schematic to reflect the complexity of respiratory pathogen transmission.
4. SARS-CoV-2 air-mediated dissemination
Considering that SARS-CoV-2 active replication occurs primarily in the upper respiratory tract, one can hypothesize that while SARS-CoV-2 disseminates predominantly on large-size particles, it may also be borne on all particles larger than its own size (around 0.125 μm), including exhaled breath. Once emitted, the size of particles evolves according to environmental conditions (temperature, hygrometry…) and the presence of respiratory mucus [25]. Indeed, virus dissemination is likewise influenced by environmental factors including temperature, UV radiation, relative humidity, and air flows [26]. A speculative study assumes that even if some particle matter (PM)-related viruses were to remain intact and infectious, their viral load would be very low [27]. However, risk of viral infection would increase in cases of irritation and ulceration of the nasal epithelium, especially in individuals suffering from reduced mucociliary clearance occasioned by tobacco, asthma, or ARDS. Furthermore, lengthy exposure to air pollution can increase SARS-CoV-2 transmission and severe forms of COVID-19 by favoring systemic inflammation and affecting the innate immune system [28], [29], [30]. In data drawn from studies on non-specific forms of SARS-CoV-2, the role of particles themselves (not simply the viral load of which they are carriers) has been interrogated. For example, PM (especially PM2.5) can serve as a vector for SARS-CoV-2 virions and facilitate their spread over a wider perimeter and/or their introduction in lower airways. However, SARS-CoV-2 viability and infectiousness have not been documented outside an experimental context. In theory, with an enveloped virus SARS-CoV-2 may remain viable and infectious in the environment for a few hours to a few days, depending on the presence of biologic fluid and initial viral load. While in experimental aerosols, SARS-CoV remains viable for 3 hours [31], as a means of assessing the risk of airborne transmission, experimental demonstration is not transposable to real-life conditions. Lastly, most environmental studies do not consider other factors that may influence COVID-19 incidence: host susceptibility, demography, health system and access to care, epidemic containment measures [29], [30].
Key-points: Environmental factors condition the risk of transmission of the pathogens present in aerosols but are not transposable between pathogens. PM2.5 can serve as a vector for SARS-CoV-2 virions and facilitate its spread and/or introduction in the lower airways. However, SARS-CoV-2 viability and infectiousness have not been documented outside an experimental context. Air pollution and host susceptibility are major factors conditioning SARS-CoV-2 contamination and COVID-19 severity.
5. SARS-CoV-2 environmental contamination in healthcare settings
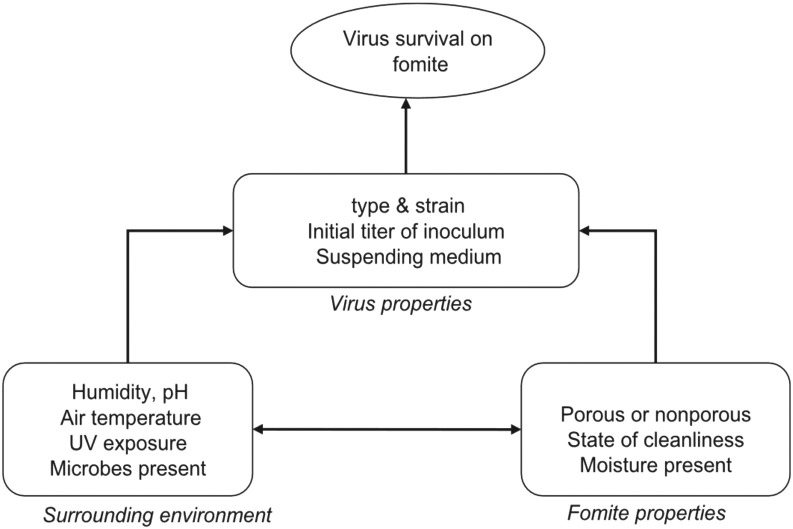
There is a direct link between surface and air contamination, as the passive vectors (fomites) carrying particles and microorganisms are resuspended through the airflows generated by movements. Viral contamination of surfaces may arise from viruses initially suspended in the air before settling on surfaces for an indeterminate time. Virus survival in fomites and its transmission to a susceptible host is conditioned by a number of factors specific to virus, host, and environmental conditions (temperature, humidity…) (Fig. 1 )[32].
Fig. 1.
Factors influencing virus survival on fomites (adapted from Boone et al., 2007).
Several studies have assessed SARS-CoV-2 environmental contamination in healthcare settings, using rRT-PCR to detect viral RNA in air or surface samples [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]. The regions amplified by rRT-PCR differ according to studies, which have targeted either 1 [36] or 2 genes [33], [34], [35], [37], [38], [39], [40], and two studies did not specify the genes having been amplified [41], [42](Table 2 ). When known, the portion of SARS-CoV-2 genome analyzed by PCR has ranged from 0.4% to 0.7%, and 7.7% to 57.7% of hospital surfaces were found to be positive for SARS-CoV-2, with average viral load ranging from 10 to 1.5 × 105 copies per sample. On the other hand, 16.3% to 66.7% of air samples have been found to be positive, with the exception of two studies, in which all of them were negative [37], [42](Table 2). When positive, the average SARS-CoV-2 viral load ranged from 10 to 104 copies per m3 of air. Two studies were performed in vitro cell culture but failed to demonstrate the viability and infectiousness of SARS-CoV-2 insofar as no cytopathogenic effect was detected [36], [38](Table 2). The authors assumed that this non-viability was linked to low viral loads in samples, whereas Zhou et al. [36] proposed a cycle threshold cut-off of 30 (around 5 log10 copies/mL) as the limit of detection (LOD) that would enable SARS-CoV-2 culturing from surface samples.
Table 2.
Summary of studies assessing SARS-CoV-2 environmental contamination in healthcare settings.
| Study | Environmental samples | rRT-PCR targets | Culture | Results |
|---|---|---|---|---|
| Zhou et al. 2020 | 218 surface samples 31 air samples (3 to 4 m3) |
E genea | Yes, Vero E6 (African Green monkey kidney) and Caco2 (human colon carcinoma) cells | Surface samples: 114/218 (52.3%) positive in rRT-PCR (10 to 104 copies per swab) Air samples: 14/31 (38.7%) positive in rRT-PCR (10 to 103 copies per m3). All culture-negative |
| Chia et al. 2020 | 245 surface samples 3 air samples (around 5 m3) |
E genea and ORF1ab gene | Not performed | Surface samples: 56/245 (22.9%) positive in rRT-PCR (viral load not specified) Air samples: 2/3 positive in rRT-PCR (1.84 × 103 to 3.38 × 103 copies per m3) for particles > 1 μm in size |
| Liu et al. 2020 | 35 air samples | ORF1ab and N genes in ddPCRb | Not performed | Air samples: 21/35 (60%) positive in ddPCR up to 40 copies per m3 for particles of < 1 μm in size and up to 10 copies per m3 for particles of > 1 μm in size |
| Guo et al. 2020 | 161 surface samples 80 air samples (9 m3) |
ORF1ab and N genes | Not performed | Surface samples: 41/161 (25.5%) positive in rRT-PCR (2.9 × 103 to 1.5 × 105 copies) Air samples: 13/80 (16.3%) positive in rRT-PCR (0.52 × 103 to 3.8 × 103 copies per m3) |
| Ong et al. 2020 | 78 surface samples 6 air samples (1.5 m3) |
RdRp and E genesa | Not performed | Surface samples: 45/78 (57.7%) positive in rRT-PCR (average of 103 to 104 copies) Air samples: all negative |
| Colinari et al. 2020 | 26 surface samples | RdRp and E genesa | Yes, Vero E6 cells | Surface samples: 2/26 (7.7%) positive in rRT-PCR (viral load not specified) All culture-negative |
| Faridi et al. 2020 | 10 air samples (9 m3) | RdRp and E genesa | Not performed | Air samples: all negative |
| Razzini et al. 2020 | 37 surface samples 5 air samples (2 m3) |
Not specified | Not performed | Surface samples: 9/37 (24.3%) positive in rRT-PCR (21.5 and 23.9 Ct value) Air samples: 2/5 (40%) positive in rRT-PCR (22.6 and 31.1 Ct value) |
| Li et al. 2020 | 135 surface samples 90 air samples (2,4 m3) |
Not specified | Not performed | Surface samples: 2/135 (1.5%) positive in rRT-PCR (viral load not specified) Air samples: all negative |
| Wei et al. 2020 | 112 surface samples 6 air samples (1.5 m3) |
ORF1ab and N genes | Not performed | Surface samples: 44/112 (39.3%) positive in rRT-PCR (viral load not specified) Air samples: all negative |
E: envelope (112 nucleotides); N: nucleocapsid; ORF: open reading frame; RdRp: RNA-dependent RNA polymerase (99 nucleotides).
According to Corman et al., 2020.
Droplet Digital PCR (ddPCR) method described as more sensitive than rRT-PCR to detect SARS-CoV-2, according to Suo et al., 2020.
In clinical specimens, Huang et al. [4] highlighted a linear correlation between the SARS-CoV-2 viral load detected by rRT-PCR targeting both structural (E and N genes) and non-structural (nsp12 gene), regions, and infectivity was assessed by culture. In their study, the lowest copy number in rRT-PCR required for virus isolation in culture ranged from 5.4 to 6.0 log10 copies/mL sample, demonstrating that specimen cultivability of requires high copy numbers, regardless of whether structural or non-structural regions are being targeted. The results indicate that when evaluating the infectivity of clinical SARS-CoV-2 specimens, in addition to the copy number the integrity of the viral genome should be taken into consideration, targeting both the structural and the non-structural portions of the genome [4]. Genome integrity assessment is even more necessary with regard to environmental samples, for which, as long as no active replication occurs in the environment, a positive rRT-PCR cannot be interpreted in the same manner as biologic samples.
A negative viral culture could consequently mean that viral load is too low to be cultured, or absent from the sample, or that in vitro cell culture in laboratory is not sufficiently sensitive and effective [7]. While SARS-CoV-2 may remain on surfaces for several hours to several days, the viral load decreases rapidly and its infectiousness has rarely been demonstrated [31], [36]. The risk of SARS-CoV-2 transmission by contact is therefore evaluated as low [43], and it is easily controlled by regularly scheduled surface disinfection and scrupulous respect of hand hygiene [44].
Key points: rRT-PCR detection of SARS-CoV-2 in environmental samples should amplify 2 or 3 targets, in the same manner as biologic samples. Results should be interpreted carefully as long as no active replication occurs in the environment. SARS-CoV-2 infectiousness from environmental samples in healthcare settings (excluding experimental context) has not been demonstrated to date.
6. Are surgical or N95/FFP masks the most adapted for HCWs?
In addition to the issue of SARS-CoV-2 airborne transmission, questions remain on the effectiveness of PPE, especially masks, as means of protection for HCWs.
A recent literature review provided an update on the type of mask required to ensure HCW protection [45] and concluded that a medical facemask is as effective as a N95 respirator as a means of protecting HCWs from Influenza virus or MERS-CoV. However, another systematic literature review and meta-analysis concluded that N95 respirator seemed non-statistically significantly superior to medical mask [46] (P = 0.09, Odds ratio 0.14 (95% CI, 0.02 to 1.05)). The SARS-CoV-2-specific studies included in this review scored between 3 and 4 on the Newcastle-Ottawa Scale, reflecting a risk of bias. Up until now, no randomized, unbiased studies have compared the effectiveness of N95 respirator versus medical facemask for HCW protection against SARS-CoV-2 infection.
A recent literature review comparing the relative effectiveness of surgical and N95/FFP masks in the prevention of respiratory infections excluded experimental articles not transposable in real-life conditions and underlined the following [47]:
-
•
The majority of previously reported systematic reviews do not provide clear evidence that N95/FFP2 respirators are more effective than surgical masks in preventing respiratory infections, particularly viral respiratory infections, in HCWs;
-
•
One source of uncertainty concerns the time lapse during which a study participant carries the assigned device (a point not verified in the methodology of the review studies). The authors point out that wearing a N95/FFP2 mask is cumbersome and possibly troublesome. The surgical mask is more likely to be worn continuously throughout the period during which a risky contact may occur. The supposed superiority of N95/FFP2 over surgical masks for protection against airborne infections is based on the fact that these respirators are tested for their ability to filter aerosols smaller than the aerosols used to test surgical masks (0.1 vs. 3 μm). However, this does not take into account the fact that the microorganisms emitted by infected persons are absorbed in particles of diameter larger than the microorganisms themselves, which would explain why N95/FFP masks do not better prevent airborne viral infections than surgical masks in clinical conditions;
-
•
A meta-analysis on the interest of mask wearing to prevent SARS-CoV-2 airborne transmission included studies in which RR (95% CI) for the association between masks wearing and COVID-19 occurrence was obtained [48]. The risk of study bias was assessed by the Newcastle-Ottawa scale and out of the 7688 references obtained with initial search equations, only 4 articles were included, among which 3 assessed the effectiveness of mask wearing versus no mask. The findings showed that use of a facemask was linked to a decreased risk of SARS-CoV-2 infection, with a statistically significant association (combined RR 0.12; CI 95% [0.06, 0.27] (P < 0.000)). Study heterogeneity was minimal (I2 - 43.3% and P - 0.152)[48].
Key-points: Current scientific evidence suggests that surgical and N95/FFP2 masks confer equivalent protection against airborne viral infections for HCWs during routine care. This can be explained by the better comfort of surgical masks, allowing continuous wear. Although the SARS-CoV-2 virion is a nanoparticle, it is usually carried by larger particles, and easily stopped and contained by a mask.
7. HCW contamination rate as a means of assessing the risk of airborne transmission
In the absence of direct scientific evidence, indirect evidence can help to determine whether HCWs are adequately protected from the risk of respiratory transmission. Contamination rates should be analyzed by considering several possible confounding factors, including their over-representation, especially during lockdown periods. Given the proportion of the overall population in lockdown, HCWs are among the most exposed individuals, which explains their being over-represented. Another confounding factor is screening strategy, which tends to systematically include HCWs, even if they are asymptomatic. Nevertheless, and as shown in a rapid response by Alberta Public Health Services [49], the international literature does not reveal high incidence of SARS-CoV-2 contamination in the HCW population. More specifically, risk of infection by occupational transmission has been estimated at 0.01%, while community transmission risk seems higher than in the general population (0.14% versus 0.10%). Risk was also found to be 9 to 11 times higher for HCWs versus the general population in areas with very high incidence and prevalence of SARS-CoV-2 infection. A study conducted in Madrid on HCWs in a public hospital concluded that there was no significant difference in PCR-detected infection among HCWs in direct contact with COVID-19 patients versus staff of the same facility without contact with patients, a finding suggesting that many HCW infections result from community transmission [50]. A Chinese publication also highlighted a lower rate of contamination of front-line HCWs compared to less exposed HCWs [51], a finding providing reassurance about the effectiveness of barrier measures aimed at ensuring HCW protection during contact with infected patients. That said, a risk of HCW contamination during social interactions (out of care) was suggested in a German preprinted study [52]. On the other hand, a South Korean retrospective cohort study indicated that cases of occupational HCW contamination were correlated with defective application of barrier measures (especially facemask wearing) and insufficient COVID-19 case quarantine [53]. On another score, a rapid review concluded that the main risk factors for HCW contamination were: lack of and/or inadequate PPE, exposure to infected patients, work overload, poor infection control, and preexisting risk factors [54]. Furthermore, in their letter to the editor, Wang et al. highlighted that proper HCW preparedness and appropriate use of PPE help to lower infection risk [55].
Epidemiological investigation following the unexpected identification of cases in a 12-bed common room concluded that SARS-CoV-2 was not transmitted by air [56]. An index patient was symptomatic and received 8L/min oxygen therapy delivered through facemask before being diagnosed with COVID-19. Preventive measures in the facility included systematic facemask wearing by HCWs and the monitoring of visitors and patients in the common room. The authors concluded that the absence of secondary cases was likely related to systematic facemask wearing, high adherence to hand hygiene guidelines and regular environmental cleaning [56]. Lastly, a case report published by a Chinese team underlined the absence of HCW contamination following unexpected identification of SARS-CoV-2 infection in a patient whose condition had necessitated aerosol-generating procedures [54], [57].
Recently, Cheng et al. published a case report on a cluster involving 9 HCWs and 12 patients from the palliative medicine unit at a Hong Kong hospital [58]. The index case was a 91-year-old patient in a 4-bed room. Cases were defined as any patient or caregiver tested positive for SARS-CoV-2 and who had been present in the same unit as the index patient during the 14 days prior to and after identification. Environmental epidemiological investigation showed higher rates of SARS-CoV-2 RNA on ventilation grids located more than 2 m from the patient and on surfaces close to patients (36.4%, 8/22 vs. 3.4%, 1/29, P = 0.003, respectively). The authors concluded that airborne contamination is possible and suggested a need for reflection on the design of ventilation systems. In this particular case, however, investigation was only environmental. Care practices, compliance with PPE wearing strictures and interactions between patients were not assessed, nor was the difficulty of applying appropriate measures in a 4-bed room. Implementation of barrier measures for older populations is complicated, especially in dementia settings. It would have been interesting to explore this point in view of the elderliness of patients in the ward (median age of 84 years [20–92]). At the end of the survey, the attack rate was 15% for patients (12/78) and 8.1% for caregivers (7/86). By comparison, in the literature the attack rate of the influenza virus (which is transmitted by droplets) ranges from 1% to 38% [59].
A recent report raised the question of airborne transmission in a description of community contaminations among persons respecting physical distancing of more than one meter (buses, concerts)[60]. It remains difficult to determine transmission routes in these settings, and findings are not transposable to environments where guidelines impose continuous use of facemasks to protect HCWs. It also bears mentioning that up until now, the scientific literature has highlighted the importance of applying the measures currently recommended to protect HCWs from SARS-CoV-2 contamination: systematic use of facemask, compliance with hand hygiene indications, and regular environmental cleaning. Indeed, HCW contamination appeared greater at the beginning of the epidemic, before the systematic implementation of preventive measures; according to currently available studies, a majority of cases resulted from community transmission. Once barrier measures were recommended, the risk of HCWs contamination, even among HCWs on duty in the units most exposed to COVID-19, did not appear significantly higher compared to the general population, provided, once again, that good practice guidelines were respected [61], [62].
A recent publication proposed risk management strategy calibrated on pandemic evolution [63]. Many countries are currently facing active virus circulation in the community. HCWs are at risk of exposure to both positive patients and co-workers, due especially to the large number of asymptomatic carriers. The risk of transmission from asymptomatic carriers can vary according to several parameters: distance, room aeration and case activity; Jones et al. (2020) drafted a risk assessment table taking these parameters into account [64]. Poorly ventilated environments with high occupancy are conducive to a high risk of transmission [65]; they can include break rooms, meeting rooms or locker rooms. These at-risk areas bring together several factors favorable to contamination, and may be responsible of superspreading events [66]; awareness campaigns are called for.
The CDC and the WHO [67], [68] have stated in their respective guidelines that during close contacts, the main mode of transmission is droplet-mediated, while a risk of airborne transmission may occur under specific circumstances:
-
•
Closed spaces in which susceptible individuals are exposed to an infectious person;
-
•
Prolonged exposure to respiratory particles, often generated by exhalation (e.g., screaming, singing, physical exercise) that increase the concentration of suspended respiratory droplets;
-
•
Inadequate ventilation or air treatment favoring the accumulation of small droplets and suspended respiratory particles.
Many healthcare facilities are equipped with a ventilation system or air treatment that limits the risk of accumulation of small droplets. However, a higher risk of airborne transmission may occur in the community, where high-density viral situations are more frequent (transports, offices, home…)[65].
Key-points: When barrier measures are correctly applied, the risk of HCW contamination, including in COVID-19 units, do not appear significantly higher compared to the general population. Risk of airborne transmission seems to arise only under specific circumstances with high viral density, which is more common in community than in healthcare facilities, where HCWs are fully aware of the need to implement guidelines aimed at preventing SARS-CoV-2 transmission (droplet precautions, contact limitations, routine mask wearing, aeration…).
8. Conclusion
Current knowledge on SARS-CoV-2, and more broadly on respiratory pathogens, both theoretical or under real-life conditions in healthcare settings, shows that based on approximate particle size limits, a dichotomy between “air” and “droplets” is too schematic to reflect the reality, which corresponds to a continuum [69]. Based on conventional definitions of airborne and droplet transmission, in 2015 Jones et al. proposed the concept of aerosol transmission as a means of unraveling the relevant frontiers [70]. In this perspective, infective aerosol would represent a combination of particles of different sizes that carry pathogens in the air; they can settle onto a person or be inhaled. Aerosol transmission is biologically plausible when:
-
•
infectious aerosols are generated by or from an infective person;
-
•
the pathogen remains viable in the environment during a sufficient amount of time;
-
•
the target tissues in which the pathogen develops the infection are accessible to the aerosol.
Jones et al. went on to propose a scale of evidence for each of these three circumstances as a means of assessing the biological plausibility of aerosol transmission [70]. As regards the specific case of SARS-CoV-2, while the level of evidence for points #1 and #3 is moderate, for point #2 it is high, with a final score of 7/9. Some experts have contended that in this context, the data from theoretical studies should be interpreted with caution; their transposition into real-life conditions remains problematic, and proof of the airborne transmission of SARS-CoV-2 is incomplete [21], [71]. To more accurately assess and measure the risk of the latter, Carducci et al. have proposed the determination of “minimal dose and dose-response relations”; “ways and amount of exposure for susceptible people in different settings” (community, healthcare working environments) and “estimated reduction in exposure of different preventive measures” (use of different masks, ventilation systems, etc.) [71].
Current scientific evidence suggests that surgical and N95/FFP2 masks provide equivalent protection against airborne viral infections, excluding aerosol-generating procedures. Although the wearing of N95/FFP respirator presents a higher theoretical filtration capacity than a surgical mask, it is more restrictive in practice. Many healthcare facilities possess a limited number of respirator models [72], and it is not possible to provide all HCWs with a respirator tested and adapted to their facial morphology. Moreover, while viruses are nanoparticles, they are carried in larger droplets and particles, which may explain the non-superiority of N95/FFP on surgical masks. In the final analysis, SARS-CoV-2 transmission is not limited to the respiratory route; it may also include contact transmission, which should be taken into close account [73], [74].
Even if SARS-CoV-2 airborne transmission is possible, particularly in confined environments and in the absence of systematic mask wearing, SARS-CoV-2 is predominantly transmitted through respiratory droplets during close contact. In healthcare settings, “droplets” and “contact” precautions remain efficient as means of protecting the HCWs caring for COVID-19 patients, while mask wearing and barrier measures are to be systematically recommended in indoor environments or when it is impossible to maintain physical distancing in external environments [75].
Funding
None to declare.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
We gratefully acknowledge the contributions of all the members of the Scientific Committee of the French Society for Hospital Hygiene.
References
- 1.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., et al. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung K., Shum M.H., Leung G.M., Lam T.T., Wu J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill. 2021;26(1) doi: 10.2807/1560-7917.ES.2020.26.1.2002106. [2002106] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younes N., Al-Sadeq D.W., Al-Jighefee H., Younes S., Al-Jamal O., Daas H.I., et al. Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2. Viruses. 2020;12(6):582. doi: 10.3390/v12060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C.G., Lee K.M., Hsiao M.J., Yang S.L., Huang P.N., Gong Yn, et al. Culture-Based Virus Isolation To Evaluate Potential Infectivity of Clinical Specimens Tested for COVID-19. J Clin Microbiol. 2020;58(8):e01068–e1120. doi: 10.1128/JCM.01068-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 6.Romano C.M., Felix A.C., Pannuti C.S. Viable SARS-CoV-2 Shedding. N Engl J Med. 2021;384(17) doi: 10.1056/NEJMc2102494. [DOI] [PubMed] [Google Scholar]
- 7.Min-Chul K., Man-Seong P., Jin-Won C. Viable SARS-CoV-2 Shedding. Reply. N Engl J Med. 2021;384(17) doi: 10.1056/NEJMc2102494. [DOI] [PubMed] [Google Scholar]
- 8.Morawska L., Milton D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa939. [ciaa939] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepelletier D., Grandbastien B., Romano-Bertrand S., Aho S., Cidiac C., Géhanno J.F., et al. What face mask for what use in the context of the COVID-19 pandemic? The French guidelines. J Hosp Infect. 2020;105(3):414–418. doi: 10.1016/j.jhin.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosnowski T.R. Inhaled aerosols: their role in COVID-19 transmission including biophysical interactions in the lungs. Curr Opin Colloid Interface Sci. 2021;54 doi: 10.1016/j.cocis.2021.101451. [101451] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182(2):429–446. doi: 10.1016/j.cell.2020.05.042. [e14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1443. [m1443] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai J., Yang L., Zhao J. Probable Longer Incubation Period for Elderly COVID-19 Cases: Analysis of 180 Contact Tracing Data in Hubei Province, China. Risk Manag Healthc Policy. 2020;13:1111–1117. doi: 10.2147/RMHP.S257907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 19.Santé publique France . 2020. Synthèse rapide des connaissances - Part des formes asymptomatiques et transmission du SARS-CoV-2 en phase pré-symptomatique. [Online]. Available: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/synthese-rapide-des-connaissances/part-des-formes-asymptomatiques-et-transmission-du-sars-cov-2-en-phase-pre-symptomatique.-synthese-rapide-covid-19. [Google Scholar]
- 20.Tang J.W., Bahnfleth W.P., Bluyssen P.M., Buonanno G., Jimenez J.L., Kurnitski J., et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Hosp Infect. 2021;110:89–96. doi: 10.1016/j.jhin.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization, Pandemic and Epidemic Diseases . WHO guidelines; 2014. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. [PubMed] [Google Scholar]
- 22.Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agence française de sécurité sanitaire de l’environnement et du travail . 2006. Avis relatif à « l’évaluation du risque sanitaire pour l’homme lié à la présence de virus Influenza pandémique dans l’air des bâtiments et sa diffusion éventuelle par les dispositifs de ventilation ». [Online]. Available: (https://www.anses.fr/fr/system/files/AIR2006et0003Ra.pdf) [Google Scholar]
- 24.Roy C.J., Milton D.K. Airborne Transmission of Communicable Infection – The Elusive Pathway. N Engl J Med. 2004;350(17):1710–1712. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- 25.Romano-Bertrand S., Aho-Glele L.S., Grandbastien B., Gehanno J.F., Lepelletier D. Sustainability of SARS-CoV-2 in aerosols: should we worry about airborne transmission? J Hosp Infect. 2020;105(4):601–603. doi: 10.1016/j.jhin.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ather B., Mirza T.M., Edemekong P.F. StatPearls Publishing; Treasure Island (FL): 2021. Airborne Precautions. [PubMed] [Google Scholar]
- 27.Farhangrazi Z.S., Sancini G., Hunter A.C., Moghimi S.M. Airborne Particulate Matter and SARS-CoV-2 Partnership: Virus Hitchhiking, Stabilization and Immune Cell Targeting - A Hypothesis. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.579352. [579352] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatkin J.M., Godoy I. Are smoking, environmental pollution, and weather conditions risk factors for COVID-19? J Bras Pneumol. 2020;46(5) doi: 10.36416/1806-3756/e20200183. [e20200183] [DOI] [PubMed] [Google Scholar]
- 29.Domingo J.L., Marquès M., Rovira J. Influence of airborne transmission of SARS-CoV-2 on COVID-19 pandemic. A review. Environ Res. 2020;188 doi: 10.1016/j.envres.2020.109861. [109861] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carraturo F., Del Giudice C., Morelli M., Cerullo V., Libralato G., Galdiero E., et al. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ Pollut. 2020;265(Pt B) doi: 10.1016/j.envpol.2020.115010. [115010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boone S.A., Gerba C.P. Significance of Fomites in the Spread of Respiratory and Enteric Viral Disease. Appl Environ Microbiol. 2007;73(6):1687–1696. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K., et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 2020;11(1):2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 35.Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C., et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26(7):1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J., Otter J.A., Price J.R., Cimpeanu C., Garcia D.M., Kinross J., et al. Investigating SARS-CoV-2 surface and air contamination in an acute healthcare setting during the peak of the COVID-19 pandemic in London. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa905. [ciaa905] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colaneri M., Seminari E., Novati S., Asperges E., Biscarini S., Piralla A., et al. Severe acute respiratory syndrome coronavirus 2 RNA contamination of inanimate surfaces and virus viability in a health care emergency unit. Clin Microbiol Infect. 2020;26(8) doi: 10.1016/j.cmi.2020.05.009. [1094.e1-1094.e5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faridi S., Niazi S., Sadeghi K., Naddafi K., Yavarian J., Shamsipour M., et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138401. [138401] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei L., Lin J., Duan X., Huang W., Lu X., Zhou J., et al. Asymptomatic COVID-19 Patients Can Contaminate Their Surroundings: an Environment Sampling Study. mSphere. 2020;5(3) doi: 10.1128/mSphere.00442-20. [e00442-20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razzini K., Castrica M., Menchetti L., Maggi L., Negroni L., Orfeo N.V., et al. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140540. [140540] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y.H., Fan Y.Z., Jiang L., Wang H.B. Aerosol and environmental surface monitoring for SARS-CoV-2 RNA in a designated hospital for severe COVID-19 patients. Epidemiol Infect. 2020;148:e154. doi: 10.1017/S0950268820001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mondelli M.U., Colaneri M., Seminari E.M., Baldanti F., Bruno R. Low risk of SARS-CoV-2 transmission by fomites in real-life conditions. Lancet Infect Dis. 2021;21(5):e112. doi: 10.1016/S1473-3099(20)30678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sommerstein R., Fux C.A., Vuichard-Gysin D., Abbas M., Marschall J., Balmelli C., et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9(1):100. doi: 10.1186/s13756-020-00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Violante T., Violante F.S. Surgical masks vs respirators for the protection against coronavirus infection: state of the art. Med Lav. 2020;111(5):365–371. doi: 10.23749/mdl.v111i5.9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabatabaeizadeh S.A. Airborne transmission of COVID-19 and the role of face mask to prevent it: a systematic review and meta-analysis. Eur J Med Res. 2021;26(1):1. doi: 10.1186/s40001-020-00475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alberta Health Services . 2020. COVID-19 Scientific Advisory Group Rapid Response Report. [Available: https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-hcw-risk-rapid-review.pdf] [Google Scholar]
- 50.Folgueira M.D., Muñoz-Ruipérez C., Alonso-López M.A., Delgado R. SARS-CoV-2 infection in Health Care Workers in a large public hospital in Madrid, Spain, during March 2020. Infectious Diseases. 2020 doi: 10.1101/2020.04.07.20055723. [DOI] [Google Scholar]
- 51.Lai X., Wang M., Qin C., Tan L., Ran L., Chen D., et al. Coronavirus Disease 2019 (COVID-2019) Infection Among Health Care Workers and Implications for Prevention Measures in a Tertiary Hospital in Wuhan. China. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.9666. [e209666] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kluytmans-van den Bergh M.F.Q., Buiting A.G.M., Pas S.D., Bentvelsen R.G., van den Bijllaardt W., van Oudheusden A.J.G., et al. Prevalence and Clinical Presentation of Health Care Workers With Symptoms of Coronavirus Disease 2019 in 2 Dutch Hospitals During an Early Phase of the Pandemic. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.9673. [e209673] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong S.C.Y., Kwong R.T.S., Wu T.C., Chan J.W.M., Chu M.Y., Lee S.Y., et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020;105(2):119–127. doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mhango M., Dzobo M., Chitungo I., Dzinamarira T. COVID-19 Risk Factors Among Health Workers: A Rapid Review. Saf Health Work. 2020;11(3):262–265. doi: 10.1016/j.shaw.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J., Zhou M., Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID-19) in China. J Hosp Infect. 2020;105(1):100–101. doi: 10.1016/j.jhin.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang M.C., Hur J., Park D. Strategies for the Prevention of the Intra-Hospital Transmission of COVID-19: A Retrospective Cohort Study. Healthcare (Basel) 2020;8(3):195. doi: 10.3390/healthcare8030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ng K., Poon B.H., Kiat Puar T.H., Shan Quah J.L., Loh W.J., et al. COVID-19 and the Risk to Health Care Workers: A Case Report. Ann Intern Med. 2020;172(11):766–767. doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng V.C.C., Fung K.S.C., Siu G.K.H., Wong S.C., Cheng L.S.K., Wong M.S., et al. Nosocomial outbreak of COVID-19 by possible airborne transmission leading to a superspreading event. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab313. [ciab313] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung N.H.L. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 2021:1–18. doi: 10.1038/s41579-021-00535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis D. Mounting evidence suggests coronavirus is airborne – but health advice has not caught up. Nature. 2020;583(7817):510–513. doi: 10.1038/d41586-020-02058-1. [DOI] [PubMed] [Google Scholar]
- 61.Tang L.H., Tang S., Chen X.L., Zhang S., Xiong Y., Chen R., et al. Avoiding health worker infection and containing the coronavirus disease 2019 pandemic: Perspectives from the frontline in Wuhan. Int J Surg. 2020;79:120–124. doi: 10.1016/j.ijsu.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nienhaus A., Hod R. COVID-19 among Health Workers in Germany and Malaysia. Int J Environ Res Public Health. 2020;17(13):4881. doi: 10.3390/ijerph17134881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bielicki J.A., et al. Monitoring approaches for health-care workers during the COVID-19 pandemic. Lancet Infect Dis. 2020;20(10):e261–e267. doi: 10.1016/S1473-3099(20)30458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones N.R., Qureshi Z.U., Temple R.J., Larwood J.P.J., Greenhalgh T., Bourouiba L. Two metres or one: what is the evidence for physical distancing in Covid-19? BMJ. 2020;370 doi: 10.1136/bmj.m3223. [m3223] [DOI] [PubMed] [Google Scholar]
- 65.Allen J.G., Ibrahim A.M. Indoor Air Changes and Potential Implications for SARS-CoV-2 Transmission. JAMA. 2021 doi: 10.1001/jama.2021.5053. [DOI] [PubMed] [Google Scholar]
- 66.Adam D.C., Wu P., Wong J.Y., Lau E.H.Y., Tsang T.K., Cauchemez S., et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26(11):1714–1719. doi: 10.1038/s41591-020-1092-0. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization. Coronavirus disease (COVID-19): How is it transmitted? https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted. (accessed Apr. 21, 2021).
- 68.Centers for Diseases Control and Prevention. Science Brief: SARS-CoV-2 and Potential Airborne Transmission | CDC. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-sars-cov-2.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fmore%2Fscientific-brief-sars-cov-2.html. (accessed Apr. 21, 2021).
- 69.Bahl P., Doolan C., de Silva C., Chughtai A.A., Bourouiba L., MacIntyre C.R. Airborne or Droplet Precautions for Health Workers Treating Coronavirus Disease 2019? J Infect Dis. 2020 doi: 10.1093/infdis/jiaa189. [jiaa189] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones R.M., Brosseau L.M. Aerosol Transmission of Infectious Disease. J Occup Environ Med. 2015;57(5):501–508. doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 71.Carducci A., Federigi I., Verani M. Covid-19 Airborne Transmission and Its Prevention: Waiting for Evidence or Applying the Precautionary Principle? Atmosphere. 2020;11(7):710. doi: 10.3390/atmos11070710. [DOI] [Google Scholar]
- 72.Pellissier G., Liolom, Balty I., Simon L., Leroy M.G., Bayeux-Dunglas M.C. Appareils de protection respiratoire utilisés dans les établissements de santé français dans le cadre des précautions ‘air’ en 2018. INRS. 2020 [Online]. Available: https://www.inrs.fr/media.html?refINRS=TF%20278. [Google Scholar]
- 73.Centers for Diseases Control and Prevention . 2020. Coronavirus Disease 2019 (COVID-19) [https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html (accessed Apr. 21, 2021)] [Google Scholar]
- 74.Birgand G., Kerneis S., Lucet J.-C. Modes de transmission du SARS-CoV-2: Que sait-on actuellement? Revue Francophone d’Infectiologie. 2021;1:1–8. [Google Scholar]
- 75.Haut Conseil de la santé publique . 2020. Préconisations du Haut Conseil de la santé publique relatives à l’adaptation des mesures barrières et de distanciation sociale à mettre en œuvre en population générale, hors champs sanitaire et médico-social, pour la maîtrise de la diffusion du SARS-CoV-2. [Online]. Available: https://www.hcsp.fr/Explore.cgi/avisrapportsdomaine?clefr=806. [Google Scholar]