Abstract
-
•
Four B cell–depleted non-Hodgkin lymphoma (NHL) patients with SARS-CoV-2 pneumonia after rituximab therapy were initially treated with a 5-day remdesivir course and steroids. After transient virologic and clinical response, they all experienced early relapse and subsequent prolonged disease course, with rapid and significant response to convalescent hyperimmune plasma in association with an extended course of remdesivir. The clinical observations here reported suggest that the immunological effects of Rituximab treatment in NHL patients should be taken into account for the proper choice and interpretation of SARS-CoV-2 laboratory tests and to guide the appropriate therapeutical approach.
Keywords: SARS-CoV-2, non-Hodgkin lymphoma, Rituximab, B cell depletion convalescent hyperimmune plasma remdesivir
Introduction
Coronavirus disease 2019 (COVID-19) is an emerging pandemic disease caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) first identified in December 2019.
Few treatment options are currently available, one of which is remdesivir, an inhibitor of the viral RNA-dependent RNA polymerase, recently approved for the treatment of COVID-19 pneumonia requiring hospitalization;1 , 2 another is convalescent hyperimmune plasma collected from recovered COVID-19 patients.3, 4, 5
Cancer patients, including malignant lymphoma patients, are at higher risk of developing a severe form of COVID-196than patients without cancer. The safety of anti-CD20 monoclonal antibody (rituximab) in the treatment of malignant B cell lymphoma in the context of COVID-19 is unclear, and how B cell depletion could affect antiviral immunity, including development of SARS, is a matter of study.
This article reports the clinical courses and therapeutic responses to convalescent plasma in association with an extended course of remdesivir in 4 B cell–depleted non-Hodgkin lymphoma (NHL) patients with COVID-19 disease, who relapsed after a 5-day course of remdesivir and steroid therapy.
The patients had recently been treated with rituximab in association with chemotherapy or were receiving maintenance treatment with rituximab alone and were admitted to our Infectious Disease Unit between December 2020 and January 2021 owing to SARS-CoV-2 pneumonia (Table 1 ).
Table 1.
Patient Characteristics, Treatments, COVID-19–Related Outcomes, and Additional Laboratory and Clinical Data
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Characteristics and NHL treatments | ||||
| Sex | Male | Male | Male | Male |
| Age (y) | 53 | 50 | 49 | 53 |
| NHL subtype | Diffuse large B cell lymphoma | Follicular lymphoma | Follicular lymphoma | Diffuse large B cell lymphoma |
| Previous lines of NHL treatment | MATILDE + rituximab | CHOP + rituximab | CHOP + rituximab | CHOP + rituximab + HD methotrexate |
| Current NHL treatment | Rituximab maintenance | Rituximab maintenance | ||
| Doses of rituximab | 6 | 17 | 15 | 6 |
| Time from last anti-NHL therapy (d) | 90 | 18 (rituximab maintenance dose, 11°) | 10 (rituximab maintenance dose, 9°) | 38 |
| Lymphoma disease status | Complete remission | Complete remission | Complete remission | Complete remission |
| Comorbidities | Hepatitis B, diabetes | |||
| Laboratory values at verification of COVID-19 | ||||
| IgG (mg/dL) | 384 | 708 | 957 | 816 |
| IgA (mg/dL) | 9 | 96 | <5 | 101 |
| IgM (mg/dL) | 49 | 5 | 158 | 70 |
| Absolute lymphocyte count (B ± T cells) (/mm3) | 370.0 | 943.36 | 365.18 | 568.45 |
| B cells (/mm3) | NE | 0.1 | 0.08 | 0.0 |
| T cells (/mm3) | NE | 943.26 | 365.1 | 568.45 |
| CD4+/CD8+T cells (/mm3)a |
NE | 1.9 | 0.4 | 0.5 |
| Neutrophil count (×103/µL) | 2650 | 2540 | 3050 | 2150 |
| SARS-CoV-2 viremia, PCR, and specific IgG response | ||||
| SARS-CoV-2 viremia | Negative | Negative | Positive | Negative |
| SARS-CoV-2 anti-spike IgG (U/mL) | NE | 0.07 (day 40 from symptom onset) | 0.01 (day 40 from symptom onset) | 0.05 (day 74 from symptom onset) |
| Time to negative PCR, days | 49 | 33 | 48 | 77 |
| Clinical course of COVID-19 and hospitalization | ||||
| Oxygen requirement (1st/2nd admission) | High/low flow rate via nasal cannula | High/low flow rate via nasal cannula | Low/low flow rate via nasal cannula | Low/low flow rate via nasal cannula |
| Severity according to WHO | Severe | Severe | Severe | Severe |
| Complications | Left distal lower extremity DVT | Right upper extremity DVT | ||
| Total (1st ± 2nd) hospitalization time (d) | 33 (15 + 18) | 26 (16 + 10) | 17 (6 + 11) | 38 (15 + 23) |
| Death due to COVID-19 | No | No | No | No |
CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; DVT, deep vein thrombosis; Ig, immunoglobulin; MATILDE, methotrexate, cytarabine, thiotepa, idarubicine; NE, not evaluated; NHL, non-Hodgkin lymphoma; PCR, polymerase chain reaction; SARS, severe acute respiratory syndrome; WHO, World Health Organization.
aReference value, 1.2 to 2/mm3.
SARS-CoV-2 RNA was detected from nasopharyngeal swabs (NS) in all the patients. Chest x-ray and computed tomography (CT) scans documented bilateral pulmonary consolidation and peripheral ground-glass infiltrates consistent with SARS-CoV-2 pneumonia. On hospital admission, they all required oxygen supplementation, at low or high flow rate via nasal cannula.
A 5-day course of remdesivir in association with steroid therapy was administered, with resolution of fever and progressive improvement of respiratory failure. They were discharged afebrile and in good clinical condition on steroid taper. SARS-CoV-2 in NS turned out to be negative on discharge in all cases.
On the same day of steroid discontinuation or the day after, all patients experienced relapse of fever and were readmitted to the hospital for clinical worsening after a median of 13 days (range 5 to 31) from the first discharge. Chest X-ray or CT scans showed worsened bilateral pulmonary ground-glass opacities. Low-flow oxygen support via nasal cannula was required in all cases. SARS-CoV-2 turned out to be positive in NS in only 1 patient. In the other 3 patients, viral RNA was detected from low respiratory tract samples (sputum or bronchoalveolar lavage [BAL]).
Neutralizing SARS-CoV-2 antibodies were undetectable in all cases by rapid immunochromatographic assays. After obtaining informed consent and medical authorization, 3 doses (300 ml each) of convalescent plasma were infused in association with remdesivir therapy, which was extended for 10 days.
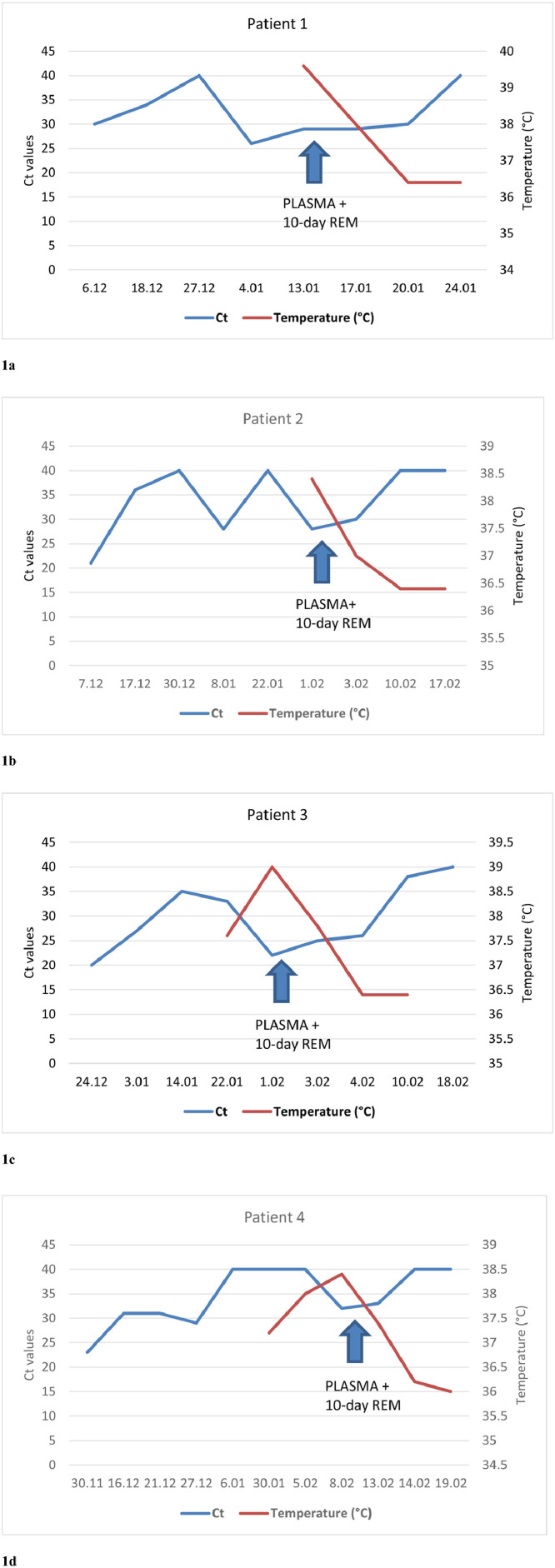
As shown in Fig. 1 , defervescence was observed after a median of 1.5 days (range 1 to 2) after administration of convalescent plasma in the absence of steroid therapy. SARS-CoV-2 RNA in NS or BAL samples progressively declined and turned negative after a median of 10.6 days (range 10 to 12) after treatment. C-reactive protein levels consistently decreased significantly over time.
Figure 1.
Time course of viral clearance on respiratory samples and longitudinal evolution of temperature in relation to convalescent hyperimmune plasma infusion and a 10-day course of remdesivir therapy for patients 1–4 (a–d, respectively). RT-PCR testing was performed using the Korea Ministry of Food and Drug Safety approved Seegene Allplex SARS-CoV-2 Assay (Arrow Diagnostics, Genova, Italy), a single-tube assay targeting 4 viral genes (E, RdRP/S, and N). Ct, cycle threshold; REM, remdesivir; RT-PCR, reverse transcription polymerase chain reaction; SARS, severe acute respiratory syndrome.
No clinical relapse was observed after a median follow-up of 34.5 days (range 24 to 55).
Discussion
The 4 patients described here exhibited similar and peculiar clinical and serological courses.
An initial infection, ranging from mild to moderate in severity, was followed by transient negativization of PCR in NS and subsequent relapse/exacerbation of symptoms concomitant with steroid withdrawal. Molecular tests turned back to positive 8 to 20 days after the first negative result; of note, viral RNA was detected in NS in 1 case and in low respiratory tract samples (bronchoalveolar lavage fluid or sputum) in the other 3 patients.
Reverse transcription polymerase chain reaction (RT-PCR) on NS is the established routine method for the diagnosis of SARS-CoV-2 infection. Of note, while nasopharyngeal viral load is high during the first days of symptoms and decreases thereafter, viral load in the low respiratory tract may remain higher for longer periods and decrease more slowly, making bronchoscopy with BAL a significantly more sensitive test in the eventuality of persistent clinical suspicion despite NS-negative results.
Impairment of adaptive immunity in rituximab-treated patients translates into transient or inefficient control of viral infection, resulting in early relapse or exacerbation of symptoms even after early negativization of PCR on NS. On the other hand, impaired antibody production might be responsible for a mild/moderate, although prolonged, clinical course. Anti-SARS-CoV-2 antibodies and immune complexes have in fact been recognized as playing a role in monocyte or alveolar macrophage activation, thereby contributing to sustained secretion of proinflammatory cytokines and the development of pulmonary disease.
A prolonged COVID-19 course and initial false-negative test results were previously reported in a patient with marginal-zone NHL and B cell depletion after rituximab maintainance.7 We postulate that the negative results reported 10 to 20 days after previous positive tests might represent the effect of an actual response to remdesivir in the context of partial immunological control, rather than actual false-negative results. This is supported by observation of early remission of symptoms, although transient and restricted to steroid therapy duration.
Time to ultimate negativization of PCR appears to be significantly prolonged compared with the general population, ranging from 33 to 77 days. Total hospitalization duration was also considerably longer (range 17 to 38 days) compared with the general population (median 12 days).8
None of the patients required ICU admission or mechanical ventilation; severe complications such as stroke, myocardial infarction, and liver failure were not reported. Of note, 2 of 4 patients were diagnosed with deep vein thrombosis, representing relapsing thrombotic events in both cases. The high incidence of thrombotic complications in this setting might be related to hypercoagulability secondary to cancer treatment or lymphoproliferative disease itself; pre-existent congenital thrombophilic conditions were not assessed and cannot be ruled out.
Prolonged disease course is consistent with serological findings in the patients analyzed. Most immune-competent COVID-19 patients show seroconversion 8 to 14 days after diagnosis.9 In line with a previous reporting,7 our patients showed no immunoglobulin M (IgM) antibody response, evaluated by rapid immunochromatographic assay, and absent or inadequate response in terms of SARS-CoV-2 anti-spike IgG production (range 0.01 to 0.05 UI/ml) 40 to 74 days after initial symptoms.
Of note, clinical and serological courses were similar in the 4 patients independent of baseline Ig levels. Only 1 patient showed severe hypogammaglobulinemia involving IgG, IgA, and IgM; 1 patient had selective IgA deficiency with unmeasurable IgA levels preexistent to treatment; and in 1 patient, Ig levels were within normal limits. A common finding was a profound B cell depletion secondary to rituximab treatment on immunophenotyping of lymphocyte subpopulations; in addition, CD4+T cell counts and CD4+/CD8+T cell ratio were reduced in 2 of 3 patients analyzed. B cell lymphopenia translates into low protective antibody production by effector B cells, as well as impaired development of virus-specific memory B cells responsible for faster antibody production in subsequent infections. In the presence of severe humoral immune deficiency, a prominent role of T cell–mediated immune responses can be postulated in this specific setting of patients. Emerging studies suggest that a majority of patients develop a strong T response, both CD4+ and CD8+, and some have a memory phenotype, potentially responsible for longer-term immunity.10
Rituximab therapy and drug-related impairment of primary and secondary humoral response thus may increase the risk of early relapse of symptoms after apparent clinical and microbiological recovery as well as increase the risk of reinfection. This suggests a low threshold for repeat testing, including high-sensitivity methods (PCR on sputum, BAL, or blood; chest CT), to allow timely treatment of patients and guide protection measures for health care staff even shortly after or in the presence of initial negative results.
Consistently, assessment of infection resolution should rely on high-sensitivity methods such as PCR in low respiratory tract samples, especially in those patients experiencing a protracted course of disease, because of the low sensitivity of PCR in NS in this setting. As previously shown, serologic results are of limited use in B cell–depleted patients and have to be interpreted cautiously.
Circulating SARS-CoV-2 RNA was detected in 1 patient the day after onset of symptoms. A previous report of an immune-compromised AML patient11 illustrated that SARS-CoV-2 viremia inversely correlates with anti-SARS-CoV-2 antibody production and that antibody detection coincides with viral clearance in plasma. Viremia can be detected in an early phase of infection, even before the onset of symptoms and detection of SARS-CoV-2 by PCR in NS. Based on these observations, we advocate the potential of viremia detection as a diagnostic tool for early diagnosis in immunocompromised patients in whom delayed or inadequate antibody production is expected. Determination of SARS-CoV-2–specific T cells has also been proposed as an alternative to seroconversion in patients treated with rituximab.7
Adaptive immunity impairment correlates with dramatic responses in terms of viral clearance and improvement in clinical and laboratory inflammation markers to convalescent hyperimmune serum. Thus convalescent hyperimmune serum might represent a therapeutic option in this specific setting of immune-compromised patients developing severe COVID-19 disease.
A recent open-label, randomized, multicenter trial did not show a significant difference in terms of efficacy between a 5- or 10-day course of remdesivir in patients with severe COVID-19 disease not requiring mechanical ventilation.2
As shown in Figure 1, all patients analyzed experienced an initial clearance of the virus concurrent with resolution of symptoms, followed by virological and clinical relapse. Unlike immune-competent patients, none of the B cell–depleted patients actually achieved complete SARS-CoV-2 clearance after the 5-day course of remdesivir first administered. The infusion of convalescent plasma in association with a 10-day course of remdesivir finally led to complete viral clearance and COVID-19 disease resolution.
We therefore propose an extended course of antiviral therapy in B cell–depleted patients aimed at achieving sustained viral clearance.
In the lack of specific convalescent serum, high-dose IV Ig administration could also be beneficial, as previously reported by Kos et al.7 in a B cell–depleted NHL patient.
Another potentially relevant implication of rituximab-induced B cell depletion is impaired vaccine efficacy. Data on anti-SARS-CoV-2 vaccine efficacy, based on serological responses and incidence of infection or reinfection in vaccinated patients, are needed in this setting to define both the optimal timing of vaccine administration and the risk of failure. The observations we reported on clinical and serological course suggest a need for vaccination despite past symptomatic SARS-CoV-2 infection in this setting, to prevent reinfection.
Interestingly, one of the patients receiving rituximab maintenance had been recognized to have a primary selective IgA deficiency. This condition is known to be associated with low/absent IgA levels both in the circulatory and mucosal compartments. Mucosal secretory IgA in the upper respiratory trait exerts a protective effect in early stages of SARS-CoV-2 infection. IgA deficiency is thus expected to favor progression of viral infection, facilitating descent into the lower respiratory tract and development of advanced disease. The absence of anti-inflammatory control by IgA antibodies may translate into dysregulated inflammation, leading to a severe clinical picture.12
Another issue is the potential emergence of viral mutations. It is plausible that impaired viral clearance due to inadequate and delayed antibody response may favor the emergence of viral mutations. The incidence of mutations and their causative role in relapse and prolonged clinical course in immune compromised patients need to be explored.
In our small series, cancer activity in terms of persistent/progressive disease does not seem to have played a role in compromising immunity and affecting COVID-19 disease course, since all patients reported were demonstrated to be in a complete remission. Moreover, clinical behavior and microbiological findings were similar independently of NHL grade and subtype (follicular NHL had been diagnosed in 2 patients and diffuse large B cell lymphoma in 2) and independently of chemotherapy regimens administered in association with rituximab (cyclophosphamide, doxorubicin, vincristine, and prednisone [CHOP]; CHOP + methotrexate; MATILDE ± radiotherapy). Similar features were previously described in a patient diagnosed with marginal NHL treated with bendamustine plus rituximab followed by rituximab maintainance.7
Conclusion
We believe that the effects of adaptive immunity deficiency in patients treated with rituximab should be taken into account for the proper choice and interpretation of laboratory tests and to guide appropriate therapeutical approaches and protective measures.
Authors contributions
Anna Furlan and Gabriella Forner were major contributors in writing the manuscript and contributed equally. Conception of the work: Anna Furlan. Data collection: Anna Furlan, Gabriella Forner, Ludovica Cipriani (clinical data), Elisa Vian, Roberto Rigoli (diagnostic microbiologic tests). Data analysis and interpretation: Anna Furlan, Gabriella Forner. Drafting of manuscript: Anna Furlan. Critical revision: Anna Furlan, Gabriella Forner, Piergiorgio Scotton, Filippo Gherlinzoni. All authors read and approved the final manuscript.
References
- 1.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383:1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanza F, Seghatchian J. Reflection on passive immunotherapy in those who need most: some novel strategic arguments for obtaining safer therapeutic plasma or autologous antibodies from recovered COVID-19 infected patients. Br J Haematol. 2020;190:e27–e29. doi: 10.1111/bjh.16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130:4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salazar E, Perez KK, Ashraf M, et al. Treatment of Coronavirus Disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020;190:1680–1690. doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kos I, Balensiefer B, Roth S, et al. Prolonged course of COVID-19-associated pneumonia in a B-cell depleted patient after rituximab. Front Oncol. 2020;10:1578. doi: 10.3389/fonc.2020.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masià M, Telenti G, Fernandez M, et al. SARS-CoV-2 seroconversion and viral clearance in patients hospitalized with COVID-19: viral load predicts antibody response. Open Forum Infect Dis. 2021;8:ofab005. doi: 10.1093/ofid/ofab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Cristanziano V, Meyer-Schwickerath C, Eberhardt KA, et al. Detection of SARS-CoV-2 viremia before onset of COVID-19 symptoms in an allo-transplanted patient with acute leukemia. Bone Marrow Transplant. 2021;56:716–719. doi: 10.1038/s41409-020-01059-y. [DOI] [PubMed] [Google Scholar]
- 12.Russell MW, Moldoveanu Z, Ogra PL, Mestecky J. Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.611337. [DOI] [PMC free article] [PubMed] [Google Scholar]