Abstract
Ethnopharmacological relevance
Lianhuaqingwen (LHQW) is a Chinese medicine, developed from appropriate addition and reduction of combined traditional Chinese medicine (TCM) Yinqiao San and Maxing Shigan decoction. LHQW has been used in routine influenza treatment for decades and plays a role in a broad-spectrum therapy on various influenza viruses.
Aims of the study: The therapeutic effects of LHQW in coronavirus disease 2019 (COVID-19) have not been fully elucidated. A retrospective study was conducted in patients with COVID-19 to evaluate the influence of LHQW on laboratory results related to the disease, and to provide evidence for the clinical practice of TCM.
Materials and methods
We retrospectively collected 248 patients who met the moderate type COVID-19 diagnostic criteria, and received treatment in Tongji Hospital. Patients were divided into control (158 cases, standard treatment) and LHQW treatment (90 cases, standard treatment combined with LHQW) groups according to the different treatments administered. All laboratory data were obtained after 5–7 days’ treatment.
Results
In this study, the average patient age was 58.95 years and 131 patients were male. The two groups were comparable in demographic characteristics, symptoms, and treatment. Compared with in the control group, D-dimer and erythrocyte sedimentation rate were significantly lower in the LHQW treatment group (2.47 ± 4.67 vs. 1.68 ± 3.61; 44.47 ± 30.24 vs. 35.39 ± 27.43; both P < 0.05). Lymphocyte counts, albumin and hemoglobin levels were higher in the LHQW treatment group than those in the control group (1.00 ± 0.46 vs. 1.13 ± 0.5; 34.39 ± 5.2 vs. 35.71 ± 4.76; 127.03 ± 16.58 vs. 131.11 ± 14.66; both P < 0.05).
Conclusion
The study showed that LHQW significantly improved laboratory results of patients with COVID-19 and could be effectively applied alongside standard treatment of patients with moderate type COVID-19, providing preliminary clinical research evidence for the use of TCM in treatment of this disease.
Keywords: Traditional Chinese medicine, COVID-19, Lianhuaqingwen, Lymphocyte, Albumin
Abbreviations: LHQW, Lianhuaqingwen; COVID-19, Coronavirus disease 2019; TCM, traditional Chinese medicine
Graphical abstract
1. Introduction
Coronavirus disease (COVID-19) has become a major epidemic that seriously endangers human health and public safety all over the world. A newly emerged coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been recognized as being responsible for the occurrence of this disease (Pascarella et al., 2020). Similar to the other two highly pathogenic coronaviruses, SARS-CoV and MERS-CoV, SARS-CoV-2 can cause severe respiratory disorders and high mortality (Nkengasong, 2020). By 1st May 2021, more than 150 million confirmed cases of COVID-19 had been reported globally, including 2 million deaths, and a continuing increase is predicted. This disease inflicts enormous health, economic, and social crises, with no country escaping its effects.
At present, it is important to develop potential antiviral agents; however, these treatment strategies will take months, even years, to become available (Qiu et al., 2020). In 2003, patients with SARS who received traditional Chinese medicine (TCM) treatment had shorter hospital stays and fewer steroid-related side effects (Huang et al., 2020). The application of TCM can contribute to effective treatment as a complementary and alternative measure, reducing the rate of severe illness and death. From the start of the COVID-19 outbreak in Wuhan, Chinese physicians have adopted a combination of TCM and Western medicine (Ling, 2020), and the application of Lianhuaqingwen granules (LHQW) combined with standard treatment appears to be beneficial. LHQW is a Chinese medicine comprising 13 herbs, developed from appropriate addition and deletion of combined Yinqiao San and Maxing Shigan decoctions. Yinqiao San, derived from the traditional Wenbing Tiaobian, was mainly administered in the treatment of “warm disease” characterized by clinical symptoms including cough, fever and headache. Maxing Shigan decoction, stemming from the classical monograph Shanghan Lun, was typically used against diseases including measles, influenza, bronchitis, and pneumonia (Hsieh et al., 2012). LHQW has been used in routine influenza treatment for decades, and plays a role in broad-spectrum curative therapy for various influenza viruses, such as H7N9 and H1N1 (Chen et al., 2011; Ding et al., 2017). Lin et al. reported that LHQW significantly decreased the Visual Analog Scale and Sino-Nasal Outcome Test-22 scores in chronic rhinosinusitis patients in comparison with placebo group. Histologic changes in nasal mucosa were significantly improved only in LHQW group, including alveolar septum thickening, capillary congestion, interstitial edema, which might be associated with the levels of malonaldehyde, glutathione peroxidase, and super oxide dismutase, played significant roles in pathogenesis of lung injury. CD4+ and CD8+ T cells were decreased in the LHQW treatment group, but not in placebo group (Lin et al., 2020). In a prospective randomized controlled trial, the recovery rate was significantly higher in the LHQW therapy group compared with the control group (91.5% vs. 82.4%) in patients with COVID-19 (Hu et al., 2020). In a system review of COVID-19 (Liu et al., 2021), patients treated by LHQW combined with standard treatment have a higher overall effective rate and CT recovery rate compared with others treated by standard methods. Also, patients treated by LHQW combined with standard treatment have a lower incidence of diarrhea.
LHQW is usually used as a supplementary therapeutic agent, which is considered to relieve symptoms, improve drug efficacy, shorten the course of the disease, and reduce the proportion of severe cases. LHQW can be used alone in some diseases with chronic airway inflammation and mild type of COVID-19 or other acute respiratory inflammation. A study demonstrated that LHQW treatment alone control the inflammation in nasal mucosa and result in the improvement of chronic rhinosinusitis (Lin et al., 2020). In this study, a retrospective study was performed to provide evidence for treatment of moderate COVID-19 with LHQW.
2. Methods and materials
2.1. Participants
Moderate COVID-19 pneumonia was diagnosed in accordance with the New Coronavirus Pneumonia Diagnosis and Treatment Program (5th version) published by the National Health Commission of China. This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China) (Approved No. TJ-C20200124).
2.2. Inclusion criteria
2.2.1. Inclusion criteria consisted of following
-
(1)
suspected cases with one of the following etiological evidences: a) RT-PCR assay for SARS-CoV-2 RNA; b) viral gene sequence is highly homologous to SARS-CoV-2;
-
(2)
moderate severity of disease, indicated by fever and respiratory symptoms with radiological findings consistent with pneumonia;
-
(3)
patients age over 18 years.
2.3. Exclusion criteria
Exclusion criteria consisted of the following:
-
(1)
patients with severe or critical COVID-19, requiring mechanical ventilation;
-
(2)
patients with acute respiratory disease not caused by SARS-CoV-2, but resulting from basic diseases such as primary immunodeficiency or acquired immunodeficiency disease, abnormal lung development, severe pulmonary interstitial disease, bronchiectasis, congenital respiratory tract malformation, congenital heart disease and gastroesophageal reflux disease;
-
(3)
patients with asthma requiring daily treatment, respiratory bacterial infections, including acute tracheobronchitis, sinusitis, and other respiratory tracts diseases that may affect outcome;
-
(4)
previous or present disorders that may affect outcome, including: malignant disease, severe liver disease, severe renal insufficiency or continuous renal replacement therapy, blood disease, and organ transplantation;
-
(5)
pregnant or lactating women;
-
(6)
allergy to the ingredients of LHQW;
-
(7)
participation in other clinical trials within the last 6 months;
-
(8)
patients who received other medications, in addition to standard treatment and LHQW.
2.4. Standard and LHQW treatments
The standard treatment comprised the following: (1) patients were restricted to bed rest and adequate nutrient intake, especially in terms of calories, was provided along with careful monitoring of water and electrolyte balance to maintain internal environment stability; (2) closely monitoring of vital signs and oxygen saturation, with provision of effective oxygen therapy; (3) according to patients’ conditions, monitoring of blood, and urine tests, c-reactive protein (CRP), coagulation function, biochemical indicators (liver enzyme, renal function, myocardial enzyme), arterial blood gas analysis, and chest CT-imaging; (4) antiviral therapy: arbidol, 200 mg, thrice daily.
LHQW granules were provided by Shijiazhuang Yiling Pharmaceutical Co., Ltd (Shijiazhuang, China). The major ingredients of LHQW consist of Forsythia suspensa, Isatis indigotica, Ephedra sinica, Dryopteris crassirhizoma, Lonicera japonica, Pogostemon cablin, Houttuynia cordata, Glycyrrhiza uralensis, Rhodiola crenulata, Prunus sibirica, Rheum palmatum, and gypsum (see Supplementary File 1 for full details of LHQW granules). The LHQW treatment consisted of standard treatment plus LHQW granules (6 g/bag, 1 bag, thrice daily; Z20100040).
2.5. Study design and treatment procedure
We enroll a total of 248 patients with moderate type COVID-19, all receiving treatment in Tongji Hospital, between 5th March and March 15, 2020. After diagnosis, all patients were offered two treatment options (standard treatment and standard treatment plus LHQW treatment). Patients chose one of the treatment options and signed the informed consent form. The patients in the control group (n = 158) received standard treatment and the LHQW treatment group (n = 90) received standard treatment plus LHQW granules for 5–7 days. Nurses supervised patients taking medications on time, followed up every day, and paid close attention to changes in their condition during the treatment.
2.6. Data collection and evaluation parameters
We retrospectively reviewed medical records and clinical data for enrolled patients with moderate severity COVID-19 pneumonia from Tongji Hospital. All laboratory data were obtained after 5–7 days’ treatment, including blood cell count, biochemical parameters, inflammation parameters, and coagulation function.
Blood cell counts were detected by flow cytometry, while white blood cell (WBC) count, neutrophil count (NC), lymphocyte count (LC), eosinophil count (EC), hemoglobin (HGB), and platelet (PLT) count were also collected for analysis. Blood biochemical parameters including alanine aminotransferase (ALT), globulin (GLB), aspartate aminotransferase (AST), albumin (ALB), total protein (TP), lactate dehydrogenase (LDH), creatinine (CR), urea nitrogen (UREA), estimated glomerular filtration rate (eGFR) and bicarbonate (HCO3) were assessed using colorimetric kits (Roche, Switzerland). Myohemoglobin (MB) and creatine kinase-muscle/brain (CK-MB) were examined by chemiluminescence microparticle immunoassay (Abbott, Chicago). N-terminal prohormone brain natriuretic peptide (NT-proBNP) was detected by electro-chemiluminescence immunoassay (Roche, Switzerland).
For inflammation parameters, erythrocyte sedimentation rate (ESR) was measured by the Wei method. High-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6) and procalcitonin (PCT) were examined by electro-chemiluminescence (Roche). IL-1β, IL-2 receptor (IL-2R), IL-8, IL-10 and tumor necrosis factor α (TNF-α) were detected by chemiluminescence (Siemens, Munich Germany). Ferroprotein was tested by granule-enhanced immunoturbidimetry (Roche, Switzerland). Coagulation function, prothrombin time (PT), thrombin time (TT), activated partial prothrombin time (APTT) and fibrinogen (FIB) were detected by magnetic bead coagulation assay (Stago, USA); D-dimer was tested by immunoturbidimetry, and prothrombin activity (PTA) was tested by the chromophore substrate method. Internationally standardized ratio (INR) was calculated with PT ratio and International Standard Index. For urine protein, urine sediment dry chemistry was carried out by tape assay (GE Healthcare, Chicago, IL).
2.7. Statistical analysis
Differences in demographic characteristics (except age) between the two groups were analyzed by Chi square test; age was analyzed by T test. The Mann-Whitney U test was applied to assess differences in blood cell count, biochemical parameters, inflammation parameters and coagulation function between the two groups. All data analyses were performed with SPSS version 16.0(SPSS Inc. Chicago, IL). P < 0.05 was considered statistically significant.
3. Results
3.1. Demographic characteristics of enrolled patients
As shown in Table 1 , 248 patients (117 females and 131 males) with laboratory confirmed COVID-19 were enrolled. Of these, 45.16% (112/248) had at least one chronic disorder, such as cardiovascular and cerebrovascular diseases, or diabetes. All patients received standard treatment after COVID-19 diagnosis. The two treatment groups were comparable in terms of demographic characteristics, symptoms, and previous treatment (P > 0.05).
Table 1.
Characteristics of COVID-19 patients.
| Characteristic | The control group n x±sd or % | The LHQW group n x±sd or % | pa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, years | 158 | 59.08 ± 15.55 | 90 | 58.73 ± 15.60 | 0.865b | ||||
| Gender | Male | 82 | 51.9 | 49 | 54.4 | 0.699 | |||
| Female | 76 | 48.1 | 41 | 45.6 | |||||
| Basic disease | Yes | 74 | 46.8 | 38 | 42.2 | 0.483 | |||
| No | 84 | 53.2 | 52 | 57.8 | |||||
| Influenza IgM | Negative | 78 | 56.9 | 38 | 49.4 | 0.285 | |||
| Positive | 59 | 43.1 | 39 | 50.6 | |||||
| MP/Cpn -IgM | Negative | 127 | 92.7 | 67 | 89.3 | 0.266 | |||
| MP-IgM Positive | 8 | 5.8 | 8 | 10.7 | |||||
| Cpn-IgM Positive | 2 | 1.5 | 0 | 0 | |||||
Abbreviation: MP-IgM, mycoplasma pneumoniae IgM; Cpn-IgM, Chlamydia pneumoniae IgM.
Difference between groups was tested by Chi-square test except difference of age.
Difference between groups was tested by T test.
3.2. Blood cell count
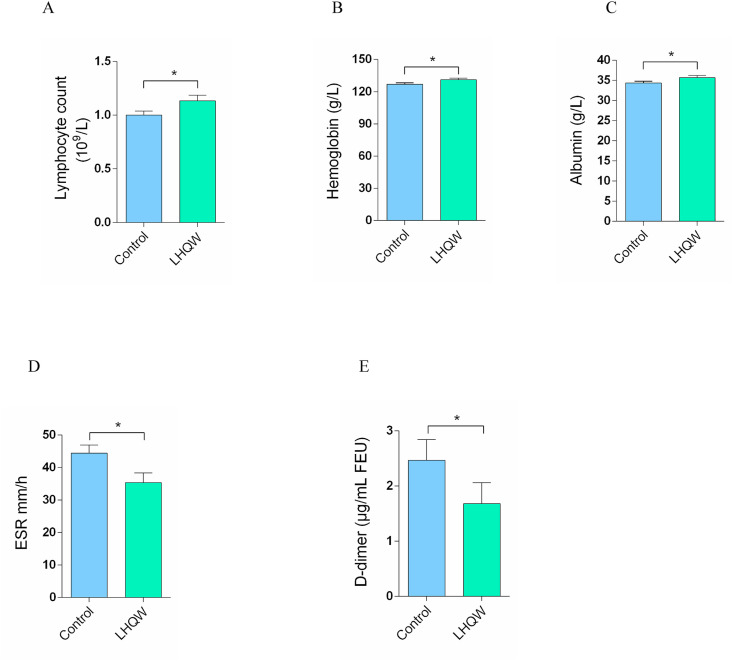
As shown in Table 2 , compared with in the control group, LC (Fig. 1 A) and HGB (Fig. 1B) in blood routine analysis were significantly increased in the LHQW treatment group (P < 0.05), while no significant difference was found between the two groups in terms of NC, PLT, EC, and WBC (P > 0.05).
Table 2.
Blood cell count.
| Blood cell count | The control group n x±sd | The LHQW group n x±sd | p | ||
|---|---|---|---|---|---|
| WBC (∗10^9/L) | 158 | 6.29 ± 3.13 | 90 | 6.09 ± 2.92 | 0.669 |
| NC (∗10^9/L) | 158 | 4.78 ± 3.17 | 90 | 4.44 ± 2.75 | 0.593 |
| LC (∗10^9/L) | 158 | 1.00 ± 0.46 | 90 | 1.13 ± 0.5* | 0.022 |
| EC (∗10^9/L) | 158 | 0.04 ± 0.08 | 90 | 0.05 ± 0.15 | 0.571 |
| HGB (g/L) | 158 | 127.03 ± 16.58 | 90 | 131.11 ± 14.66* | 0.037 |
| PLT (∗10^9/L) | 158 | 231.06 ± 95.83 | 90 | 216.87 ± 90.09 | 0.263 |
Difference between groups was tested by Mann-Whitney U test.
Abbreviation: WBC, white blood cell; NC, neutrophil count; LC, lymphocyte count; EC, eosinophil count; HGB, hemoglobin; PLT, platelets.
p < 0.05 is considered statistically significant.
Fig. 1.
Improved clinical indicators between the control group and the LHQW treatment group: (A) lymphocyte count; (B) hemoglobin; (C) albumin; (D) erythrocyte sedimentation rate (ESR); (E) D-dimer. The values shown are expressed as mean ± SEM. *p < 0.05.
3.3. Biochemical parameters
As shown in Table 3 , blood biochemistry demonstrated that ALB (Fig. 1C) was higher in the LHQW treatment group compared with in the control group (P < 0.05). However, no significant differences in ALT, AST, LDH, GLB, CR, UREA, MB, CK-MB, eGFR, HCO3, and NT-proBNP were found between the two groups.
Table 3.
Biochemical parameters.
| Biochemical parameters | The control group n x±sd | The LHQW group n x±sd | p | |||
|---|---|---|---|---|---|---|
| ALT (U/L) | 158 | 36.25 ± 68.25 | 90 | 33.21 ± 27.34 | 0.091 | |
| AST (U/L) | 158 | 42.17 ± 71.55 | 90 | 33.6 ± 18.21 | 0.514 | |
| TP (g/L) | 158 | 69.01 ± 6.58 | 90 | 70.04 ± 5.82 | 0.313 | |
| ALB (g/L) | 158 | 34.39 ± 5.2 | 90 | 35.71 ± 4.76* | 0.03 | |
| GLB (g/L) | 158 | 34.79 ± 5.1 | 90 | 34.33 ± 5.04 | 0.270 | |
| LDH (U/L) | 158 | 321.71 ± 157.17 | 90 | 321.03 ± 170.38 | 0.905 | |
| CR (μmol/L) | 158 | 80.61 ± 78.06 | 90 | 87.68 ± 107.41 | 0.076 | |
| UREA (mmol/L) | 158 | 5.14 ± 3.43 | 90 | 5.31 ± 4.37 | 0.835 | |
| eGFR (ml/min) | 158 | 90.34 ± 23.71 | 90 | 88.98 ± 23.49 | 0.514 | |
| HCO3 (mmol/L) | 158 | 23.67 ± 2.76 | 90 | 23.5 ± 3.12 | 0.842 | |
| cTnI (pg/mL) | 151 | 33.8 ± 174.13 | 82 | 24.17 ± 131.43 | 0.205 | |
| MB (ng/mL) | 43 | 135.32 ± 216.84 | 29 | 118.76 ± 218.85 | 0.954 | |
| CK-MB (ng/mL) | 39 | 2.46 ± 6.63 | 27 | 1.15 ± 1.19 | 0.556 | |
| NT-proBNP (pg/mL) | 95 | 617.09 ± 1708.98 | 51 | 387.25 ± 1373.6 | 0.070 | |
Difference between groups was tested by Mann-Whitney U test.
Abbreviation: ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein; ALB, albumin; GLB, globulin; LDH, lactate dehydrogenase; CR, creatinine; UREA, urea nitrogen; eGFR, estimated glomerular filtration rate; MB, myohemoglobin; CK-MB, creatine kinase-muscle/brain; NT-proBNP, N-terminal prohormone brain natriuretic peptide.
*p < 0.05 is considered statistically significant.
3.4. Inflammation parameters
As shown in Table 4 , differences in hs-CRP, IL-1β, TNF-α, IL-2R, IL-6, IL-8, IL-10, ferroprotein, and PCT between the two groups were not statistically significant (P > 0.05). However, ESR was significantly higher in the control group compared with in the LHQW treatment (P < 0.05) (Fig. 1D).
Table 4.
Inflammation parameters.
| Inflammation parameters | The control group n x±sd | The LHQW group n x±sd | p | ||
|---|---|---|---|---|---|
| ESR (mm/H) | 143 | 44.47 ± 30.24 | 84 | 35.39 ± 27.43* | 0.031 |
| hs-CRP (mg/L) | 156 | 59.88 ± 62.1 | 89 | 54.36 ± 66.95 | 0.324 |
| IL-1β(pg/mL) | 19 | 5.25 ± 0.96 | 11 | 16.22 ± 25.88 | 0.466 |
| IL-2R (U/mL) | 110 | 860.58 ± 574.22 | 67 | 757.27 ± 476.73 | 0.301 |
| IL-6 (pg/mL) | 110 | 38.6 ± 68.5 | 67 | 38.01 ± 52.86 | 0.272 |
| IL-8 (pg/mL) | 110 | 25.48 ± 36.75 | 67 | 22.43 ± 22.53 | 0.422 |
| IL-10 (pg/mL) | 109 | 8.68 ± 9.61 | 67 | 6.76 ± 3.3 | 0.462 |
| TNF-α (pg/mL) | 109 | 9.97 ± 5.08 | 67 | 9.49 ± 4.55 | 0.588 |
| Ferroprotein (μg/mL) | 114 | 842.15 ± 806.11 | 70 | 747.09 ± 543.15 | 0.995 |
| PCT (ng/mL) | 151 | 0.33 ± 1.09 | 87 | 0.34 ± 1.28 | 0.423 |
Difference between groups was tested by Mann-Whitney U test.
Abbreviation: ESR, erythrocyte sedimentation rate; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; PCT, procalcitonin; IL-2R, Interleukin-2 receptor; IL-8, interleukin-8; IL-10, interleukin-10; TNF-α, tumor necrosis factor α.
*p < 0.05 is considered statistically significant.
3.5. Coagulation function
As shown in Table 5 , D-dimer in the control group was significantly higher than that in the LHQW treatment group (P < 0.05) (Fig. 1E). However, no significant differences in PT, PTA, INR, APTT, FIB, and TT were found between the two groups.
Table 5.
Coagulation function.
| coagulation function | The control group n x±sd | The LHQW group n x±sd | p | ||
|---|---|---|---|---|---|
| PT (s) | 112 | 14.00 ± 1.02 | 64 | 13.86 ± 0.87 | 0.656 |
| PTA | 112 | 91.76 ± 12.98 | 64 | 93.33 ± 12.46 | 0.696 |
| INR | 112 | 1.15 ± 0.9 | 64 | 1.06 ± 0.08 | 0.605 |
| FIB (g/L) | 88 | 5.12 ± 1.4 | 54 | 5.03 ± 1.26 | 0.586 |
| APTT (s) | 88 | 40.71 ± 6.1 | 54 | 41.74 ± 5.95 | 0.306 |
| TT (s) | 88 | 16.34 ± 1.08 | 54 | 16.83 ± 1.65 | 0.219 |
| D-dimer (μg/mL FEU) | 154 | 2.47 ± 4.67 | 90 | 1.68 ± 3.61* | 0.019 |
Difference between groups was tested by Mann-Whitney U test.
Abbreviation: PT, prothrombin time; PTA, prothrombin activity; INR, Internationally standardized ratio; FIB, fibrinogen; APTT, activated partial prothrombin time; TT, thrombin time.
*p < 0.05 is considered statistically significant.
4. Discussion
In this study, we aimed to retrospectively identify differences in clinical indicators among patients receiving TCM in addition to standard Western treatments for COVID-19, to provide evidence for the efficacy of TCM in this disease. Overall, treatment with LHQW for 5–7 days resulted in significant improvements in LC, ESR, D-dimer, ALB and HGB in patients with COVID-19.
SARS-CoV-2 causes damage to the respiratory tract, heart, nervous system, esophagus, urinary bladder, kidney, and jejunum (Li and De Clercq, 2020; Zou et al., 2020). Susceptibility to SARS-CoV-2 infection may be related to efficiency of binding to angiotensin converting enzyme 2. Currently, the main therapeutic approach is symptomatic supportive and antiviral treatment. Although antiviral drug research and development have made great progress, there remain as yet no clinically validated specific antiviral agents.
In the present study, we found that D-dimer and ESR were significantly reduced in patients receiving standard treatment combined with LHQW granules. However, compared with in the control group, ALB, LC, and HGB were increased in the treatment group. It is reported that most patients with COVID-19 present lymphopenia and elevated levels of infection-related biomarkers, indicating an imbalance in the internal environment and poor condition. D-dimer is a commonly used prognostic indicator of pneumonia. Approximately 50% of patients with moderate COVID-19 had a slight increase in D-dimer; the observed significant increase of D-dimer in patients with severe COVID-19 may be associated with disseminated intravascular coagulation and secondary fibrinolysis due to the formation of extensive thrombus (Guan et al., 2020).
ESR is a widely used clinical inflammatory marker. When an infection occurs in the body, the inflammatory response progresses and the ESR accelerates. ESR can reflect the severity of the infection. Patients with moderate severity COVID-19 had no serious organ damage or excessive inflammatory responses in the early stage of the disease; therefore, LHQW is likely to reduce D-dimer and ESR, thus delaying disease progression.
The mechanism behind the observed decrease in ALB in patients with COVID-19 remains unclear, but may be related to increased catabolism of ALB, decreased synthesis or insufficient intake, and abnormal distribution after viral infection. Hypoproteinemia observed in patients with viral pneumonia could also be related to the above factors (Liu et al., 2020). Hypoproteinemia caused by fever and insufficient protein intake during COVID-19 is closely related to the course of disease, with a longer course signifying, a greater the possibility of hypoproteinemia. Pathologic anatomy showed that COVID-19 mainly caused inflammatory reactions characterized by injury of the deep airways and alveoli. Substantial quantities of fluid, fibrin exudate, and transparent membrane formation were observed in alveoli. Pulmonary exudation and hypoproteinemia suggest the possibility of capillary leakage syndrome, but this is often accompanied by systemic edema, which is different from the presentation of COVID-19 and requires further study. LHQW plays a role in correcting ALB and HGB levels, which may be related to improving the airway microenvironment, regulating the immune response, and controlling symptoms such as fever and anorexia.
As a classic TCM prescription for treating respiratory diseases, LHQW is the only approved TCM used in the treatment of influenza and SARS. Studies have shown that honeysuckle and forsythia had therapeutic effects on COVID-19 by blocking multiple binding sites of angiotensin converting enzyme II and SARS-CoV-2 in humans (Niu et al., 2020). Rhodiola and its active ingredients could improve hypoxia and blood oxygen partial pressure, with significant positive effects on pulmonary hypoxia caused by pathological damage, relieving edema, and inhibiting inflammation of lung tissue. In a randomized, double-blind, multi-center clinical trial of influenza (H1N1) in 2009 (Duan et al., 2011), compared with the oseltamivir phosphate treatment group, no significant differences were found in the frequency of viral nucleotide transversion and remission of all influenza symptoms in patients treated with LHQW capsules. LHQW was superior to oseltamivir phosphate in reducing fever and relieving cough, muscle soreness, fatigue, and other symptoms. These findings provided evidence for the antiviral effects of LHQW.
The inflammatory cytokine storm has been identified as an over-defensive response to various viruses, which can cause diffuse lung injury and lead to severe disease (Xu et al., 2020). In a recent study (Runfeng et al., 2020), the authors found that LHQW significantly inhibited SARS-COV-2 replication and reduced excessive expression of pro-inflammatory cytokines (IL-6, TNF-α and macrophage chemokine protein-1). LHQW also effectively reduced the expression of IL-1β, IL -2, and IL-13 and attenuated lung injury associated with inflammatory cell infiltration. These findings suggest that LHQW protects against SARS-CoV-2 and may be a potentially effective agent for controlling COVID-19.
The development of Chinese herbal products is valuable for the treatment of COVID-19 because there are currently no existing antiviral medications other than convalescent plasma. After the outbreak of COVID-19, LHQW as a representative TCM prescription was recommended in the Guideline for the Diagnosis and Treatment of COVID-19 issued by National Health Commission of the People's Republic of China. This TCM provided both antiviral effects and symptomatic relief, thus demonstrating clinical benefits (Shaikh et al., 2019). The purpose of this retrospective study was to explore the effect of LHQW on routine blood, biochemical, and coagulation functions of patients with COVID-19 and provide evidence for further elucidating the mechanism of action of TCM. LHQW combined with antiviral treatment for moderate COVID-19 can improve therapeutic effects.
This study had some limitations. It should be noted that the sample size was small, and there was no comparison between the treatment efficiency and the rate of severe illness between the LHQW group and the control group. Finally, there is a lack of research regarding indicators and symptoms of LHQW side effects. In the future, prospective, double-blind, randomized controlled clinical studies are needed to further assess the clinical efficacy of LHQW.
5. Conclusion
LHQW has shown therapeutic effects in COVID-19 by improving LC, ALB, and HGB and reducing ESR and D-dimer. Considering the efficacy profiles, LHQW could be a potential agent for the treatment of COVID-19.
Declaration of competing interest
None.
Acknowledgements
This study was supported by National Natural Science Foundation of China [grant numbers 81803917, 81904024 and 81874423].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jep.2021.114220.
Author contributions
Funding acquisition: Chao Chen, Jing Li, Gang Chen.
Investigation: Pan Shen.
Methodology: Jing Li, Yongtiao Peng.
Software: Gang Chen.
Supervision: Chao Chen.
Writing – original draft: Pan Shen.
Writing – review & editing: Pan Shen, Yanran Wu, Shenghao Tu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Chen W., Lim C.E., Kang H.J., Liu J. Chinese herbal medicines for the treatment of type A H1N1 influenza: a systematic review of randomized controlled trials. PloS One. 2011;6(12) doi: 10.1371/journal.pone.0028093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Zeng L., Li R., Chen Q., Zhou B., Chen Q., Cheng P.L., Yutao W., Zheng J., Yang Z., Zhang F. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Compl. Alternative Med. 2017;17(1):130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z.P., Jia Z.H., Zhang J., Liu S., Chen Y., Liang L.C., Zhang C.Q., Zhang Z., Sun Y., Zhang S.Q., Wang Y.Y., Wu Y.L. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chinese Med J. 2011;124(18):2925–2933. [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.F., Lo C.W., Liu C.H., Lin S., Yen H.R., Lin T.Y., Horng J.T. Mechanism by which ma-xing-shi-gan-tang inhibits the entry of influenza virus. J. Ethnopharmacol. 2012;143(1):57–67. doi: 10.1016/j.jep.2012.05.061. [DOI] [PubMed] [Google Scholar]
- Hu K., Guan W.J., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., Zheng Q., Yang Z., Liang J., Han M., Ruan L., Wu C., Zhang Y., Jia Z.H., Zhong N.S. Efficacy and Safety of Lianhuaqingwen Capsules, a repurposed Chinese Herb, in Patients with Coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2020:153242. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Fu F., Ye H., Gao H., Tan B., Wang R., Lin N., Qin L., Chen W. Chinese herbal Huo-Gu Formula for treatment of steroid-associated osteonecrosis of femoral head: a 14-years follow-up of convalescent SARS patients. Journal of orthopaedic translation. 2020;23:122–131. doi: 10.1016/j.jot.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Lin L., Dai F., Ren G., Wei J., Chen Z., Tang X. Efficacy of lianhuaqingwen granules in the management of chronic rhinosinusitis without nasal polyps. Am. J. Otolaryngol. 2020;41(1):102311. doi: 10.1016/j.amjoto.2019.102311. [DOI] [PubMed] [Google Scholar]
- Ling C.Q. Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2) Journal of integrative medicine. 2020;18(2):87–88. doi: 10.1016/j.joim.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Gao Y., Yuan Y., Yang K., Shi S., Tian J., Zhang J. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: a systematic review and meta-analysis. Integrative medicine research. 2021;10(1):100644. doi: 10.1016/j.imr.2020.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M., Wang R.L., Wang Z.X., Zhang P., Bai Z.F., Jing J., Guo Y.M., Zhao X., Zhan X.Y., Zhang Z.T., Song X.A., Qin E.Q., Wang J.B., Xiao X.H. [Rapid establishment of traditional Chinese medicine prevention and treatment of 2019-nCoV based on clinical experience and molecular docking] Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica. 2020;45(6):1213–1218. doi: 10.19540/j.cnki.cjcmm.20200206.501. [DOI] [PubMed] [Google Scholar]
- Nkengasong J. China's response to a novel coronavirus stands in Stark contrast to the 2002 SARS outbreak response. Nat. Med. 2020;26(3):310–311. doi: 10.1038/s41591-020-0771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F., Scarlata S., Agro F.E. COVID-19 diagnosis and management: a comprehensive review. J. Intern. Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J., Kui Z., Shuxiang H., Jun D., Xiaobo L., Xiaotao H., Lin W., Nanshan Z., Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh F., Zhao Y., Alvarez L., Iliopoulou M., Lohans C., Schofield C.J., Padilla-Parra S., Siu S.W.I., Fry E.E., Ren J., Stuart D.I. Structure-Based in silico screening identifies a potent ebolavirus inhibitor from a traditional Chinese medicine library. J. Med. Chem. 2019;62(6):2928–2937. doi: 10.1021/acs.jmedchem.8b01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet. Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.