Abstract
Background
The Gambia initiated a control programme for schistosomiasis in 2015. In light of this, recent and comprehensive data on schistosomiasis is required to effectively guide the control programme. This study aimed to evaluate the prevalence and associated risk factors of schistosomiasis among primary school children in The Gambia.
Methods
We utilised data from a previous study conducted in 2015 in 4 regions of The Gambia: North Bank Region (NBR), Lower River Region (LRR), Central River Region (CRR) and Upper River Region (URR). In the parent study, ten schools were selected randomly from each region. Urine and stool samples collected from 25 boys and 25 girls (7–14 years) in each school were examined for urinary schistosomiasis (Schistosoma haematobium infection) and intestinal schistosomiasis (Schistosoma mansoni infection) using urine filtration, dipstick and Kato-Katz methods.
Principal findings
Urinary schistosomiasis had an overall prevalence of 10.2% while intestinal schistosomiasis had a prevalence of 0.3% among the sampled school children. Prevalence of urinary schistosomiasis was significantly different among regions (χ 2 = 279.958, df = 3, p < 0.001), with CRR (27.6%) being the most endemic region, followed by URR (12.0%), then LRR (0.6%), and NBR (0.0%). Prevalence of intestinal schistosomiasis was also significantly variable among regions, with 4 of the 5 positive cases detected in CRR and 1 case in URR. Every school sampled in CRR had at least one student infected with S. haematobium, 50% of schools in URR had S. haematobium infection, and just one school in LRR had S. haematobium infection. While S. haematobium infection was significantly higher in boys (χ 2 = 4.440, df = 1, p = 0.035), no significant difference in infection rate was observed among age groups (χ 2 = 0.882, df = 2, p = 0.643). Two of the 5 students infected with S. mansoni were boys and 3 were girls. Four of these 5 students were in the 10–12 years age group and 1 was in the 7–9 years age group. Macrohaematuria and microhaematuria were found to be statistically associated with presence of S. haematobium eggs in urine. Being a male was a risk factor of S. haematobium infection. Bathing, playing and swimming in water bodies were found to pose less risk for S. haematobium infection, indicating that the true water contact behaviour of children was possibly underrepresented.
Conclusion
The findings of this study provide invaluable information on the prevalence of schistosomiasis in The Gambia. This was useful for the schistosomiasis control efforts of the country, as it guided mass drug administration campaigns in eligible districts in the study area. More studies on S. mansoni and its intermediate snail hosts are required to establish its true status in The Gambia. As children sometimes tend to provide responses that potentially please the research or their teacher, data collection frameworks and approaches that ensure true responses in studies involving children should be devised and used.
Author summary
In this study, we utilised data collected from a previous study that investigated schistosomiasis among primary school children in four regions (NBR, LRR, CRR and URR) of The Gambia, to assess the prevalence and risk factors of the disease. Our analysis revealed that urinary schistosomiasis was the most prevalent (10.2%) form of the disease and intestinal schistosomiasis was very rare (0.3%) among the school children. Urinary schistosomiasis was found in all study regions except NBR. CRR had the highest prevalence of urinary schistosomiasis and all the schools sampled in the region had at least on student with the infection. Fifty percent of schools in URR had urinary schistosomiasis infection and just one school in LRR had the infection. Presence of blood in urine was a good indicator of urinary schistosomiasis. While being male increases the chance of getting urinary schistosomiasis, playing, bathing and swimming in water bodies unexpedly turned out to pose less risk for infection, indicating the true water contact activities of the children were probably underrepresented in the data. The study provides vital information for the schistosomiasis control efforts of The Gambia. Further studies are required to know the real status of intestinal schistosomiasis in the country.
Introduction
Schistosomiasis is a parasitic neglected tropical disease that affects nearly 192 million people in over 40 countries in Sub-Saharan Africa [1]. It is caused by flatworms of the genus Schistosoma. Of the six Schistosoma species known to cause disease in humans [2], two are common in Sub-Saharan Africa: Schistosoma haematobium, the causative agent of urogenital schistosomiasis and Schistosoma mansoni, the causative agent of intestinal schistosomiasis [3]. Approximately two-thirds of all schistosomiasis cases in Sub-Saharan Africa are caused by S. haematobium [4,5]. Both S. haematobium and S. mansoni use molluscan snails as intermediate hosts to reach the infective larval stage in freshwater bodies, which become sources of infection to humans that come in contact with these sites [6,7].
Water contact activities such as playing, bathing, swimming, and irrigation farming have been found to predispose people to schistosome infections, and male children are reported to be at higher risk of being infected than female children [8–11]. As school age children are more likely to make contact with these freshwater bodies, they have an increased risk of getting infected with schistosomiasis [12,13]; hence most studies target this age group. The WHO recommended methods for mapping and field diagnosis of schistosomiasis are the urine filtration technique for detecting S. haematobium eggs in urine [14,15] and the Kato-Katz thick smear method [16] for detecting S. mansoni eggs in stool [2]. Urine dipstick is also often used alongside the filtration technique to detect blood in urine (haematuria), which is a good indicator of S. haematobium infection [13,17].
Since the London Declaration, made on the 30th January 2012, which called for the control and elimination of schistosomiasis [18,19], The Gambia has initiated a neglected tropical diseases (NTDs) control programme for schistosomiasis. However, there was a lack of current and comprehensive information on the prevalence and endemicity of schistosomiasis in the country to effectively guide this control programme. Some early studies on schistosomiasis in The Gambia focused on prevalence and distribution of the disease in some communities in the country [20,21] while others looked at its transmission and vectors [22,23]. The later studies shifted from observational to more interventional approaches with one study [24] using mollusciciding to drastically reduce Bulinus senegalensis snail populations in seasonal pools, such that transmission to humans was halted for three years in the intervention communities. As the latest of these studies were over three decades ago, and the current clinical data on schistosomiasis from hospitals was neither reliable nor adequate [25], there is a need for more up to date and inclusive information on the endemicity of schistosomiasis to effectively guide control strategies. In light of the above, this study evaluated the prevalence and associated risk factors of schistosomiasis in four selected regions of The Gambia.
Methods
Ethics statement
Ethical approval was obtained for the parent study [26] and informed consent was obtained from students and their parents and guardians. Written consent forms were provided and signed by heads of schools on behalf of all students whose parents, guardians or carers gave consent, after being contacted by the heads of schools prior to the study exercise. Furthermore, information about the study was provided by the data collection teams to each of the participants—after which informed verbal consent was obtained from each student as they were enrolled in the study.
Data source
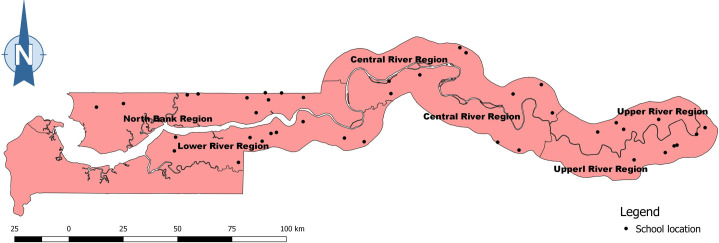
In this study, we extracted and utilised data from the following study: Field evaluation of a schistosome circulating cathodic antigen rapid test kit at point-of-care for mapping of schistosomiasis endemic districts in The Gambia [26], which was conducted as a sub study during The Gambia NTD mapping survey [27] in 2015. The study [26] was prospective and cross-sectional in design. The investigations were carried out in 4 regions of The Gambia in 2015: North Bank Region (NBR), Lower River Region (LRR), Central River Region (CRR), and Upper River Region (URR). Ten schools were selected at random from each region for the study (Fig 1). Following the WHO guidelines for participant selection in schistosomiasis mapping studies, 50 students (25 boys and 25 girls) between the ages of 7 and 14 years were randomly selected from each school. However, in some schools more than 50 students were sampled to make up for lost or dodgy samples. The total number of students sampled was 2,018 students distributed as follow; 501 in NBR, 500 in LRR, 515 in CRR, and 502 in URR [26]. The sample size determination followed the WHO recommended guidelines (with modifications) for NTD mapping in the WHO AFRO Region [28].
Fig 1. A map of The Gambia showing the locations of the 40 schools sampled across the 4 regions of the country.
Link to map base layer used in creating this figure: https://data.humdata.org/dataset/gambia-administrative-boundaries.
The methods of data collection are described in Sanneh et al [26]. Demographic information and water contact behaviour were recorded for each participant (S1 Questionnaire). In the field, each urine sample upon collection from the participant, was observed for visible blood in urine (macrohaematuria). A urine dipstick (Hemastix) was then dip in the samples to investigate microhaematuria levels. After the haematuria testing, each urine sample was well shaken, and 10 ml of urine passed through the urine filtration kit and the filter transferred to a slide for the examination of terminally spined S. heamatobium eggs, using field microscopes. Back in the laboratory, two Kato-Katz slides were prepared from each stool sample and were microscopically examined for laterally spined S. mansoni eggs [26].
We extracted the infection, demographic and water contact data of all the 2,018 participants and included them in the analysis of the current study.
Data analysis
SPSS statistical software for windows, version 24 (SPSS, Chicago, IL, USA) was used for all statistical analysis and computation. Descriptive statistics were used to show the distribution of demographic variables, and cross-tabulation was used to demonstrate infection prevalence, with 95% confidence intervals estimated using the Wilson Score Method. Chi-square test was used to compare infection rates among regions, gender, and age categories. Since the egg count data were not normally distributed, non-parametric tests were used to compare infection intensity means for gender (Mann-Whitney Test) and age categories (Kruskal-Wallis Test). Chi-square was further used to test for association between haematuria (both macrohaematuria and microhaematuria) and S. haematobium infection while logistic regression was employed to assess relationships between S. haematobium infection status and presence of water bodies around schools. Logistic regression was further used to assess relationships between S. haematobium infection status and water contact behaviours of participants. A generalised linear model with negative binomial log link function was used to assess association between presence of water bodies around schools and egg intensity. Spearman’s correlation was employed to assess correlations between prevalence of schistosomiasis and egg intensity in schools. All maps were created using QGIS version 3.0 (Girona Open Source Geospatial Foundation).
Results
Demography
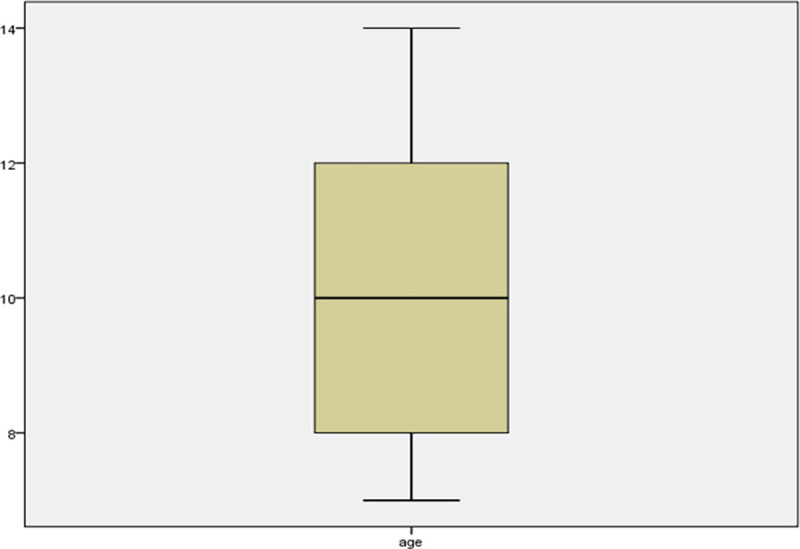
The study enrolled 2,018 primary school children, of which 1,010 were male, and 1,008 were female. Their ages ranged from 7 to 14 years. The median age (interquartile range) of the participants was 10 years (8 to 12 years). Fig 2 is a box/whisker plot showing the age distribution of the students enrolled in the study.
Fig 2. A box/whisker plot showing the age distribution of the study participants.
Infection prevalence and intensity
The study revealed an overall prevalence of 10.2% (95% CI: 8.9–11.6%) for S. haematobium infection, with Schistosoma mansoni accounting for infection in just five students giving a prevalence of 0.3% (95% CI: 0.1–0.6%) among the sampled primary school children. A significant difference was observed in infection rate among regions for both S. haematobium (χ 2 = 279.958, df = 3, p < 0.001) and S. mansoni (χ 2 = 8.627, df = 3, p = 0.035). For S. haematobium, CRR was the most endemic of the 4 regions with a prevalence of 27.6% among the school children. URR, LRR and NBR had a prevalence of 12, 0.6, and 0%, respectively (Table 1). Four of the five students infected with S. mansoni were in CRR, and the fifth infected student was in URR. The rate of S. haematobium infection in boys (11.6%) was significantly higher than the rate of infection in girls (8.7%) across the four regions (χ 2 = 4.440, df = 1, p = 0.035). For S. mansoni, two males and three female students were infected with the intestinal worm. Prevalence of infection with S. haematobium was highest (10.9%) among the 10 to 12 years age category, followed by the 13 to 14 years age group (10.2%), then the 7 to 9 years category (9.5%) of the school children, see Table 1. However, these differences in infection rate among age groups were not statistically significant (χ 2 = 0.882, df = 2, p = 0.643). Four of the five individuals infected with S. mansoni were in the 10 to 12 years age group while the fifth person was in the youngest age group (7 to 9 years).
Table 1. Prevalence of urinary schistosomiasis (S. haematobium infection) among primary school children in 4 regions of The Gambia, 2015.
| Variable | No. tested | No. positive | Prevalence, % (95% CI) |
|---|---|---|---|
| Region | |||
| NBR | 501 | 0 | 0.0 (0.0–0.8) |
| LRR | 499 | 3 | 0.6 (0.2–1.8) |
| CRR | 514 | 142 | 27.6 (23.9–31.7) |
| URR | 502 | 60 | 12.0 (9.4–15.1) |
| Gender | |||
| Male | 1010 | 117 | 11.6 (9.8–13.7) |
| Female | 1006 | 88 | 8.7 (7.2–10.7) |
| Age category | |||
| 7–9 | 953 | 91 | 9.5 (7.8–11.6) |
| 10–12 | 750 | 82 | 10.9 (8.9–13.4) |
| 13–14 | 313 | 32 | 10.2 (7.3–14.1) |
| Total | 2,016* | 205 | 10.2 (8.9–11.6) |
*Two of the study participants have missing data for S. haematobium infection and were not included in this analysis, making the total number 2,016 participants instead of 2018.
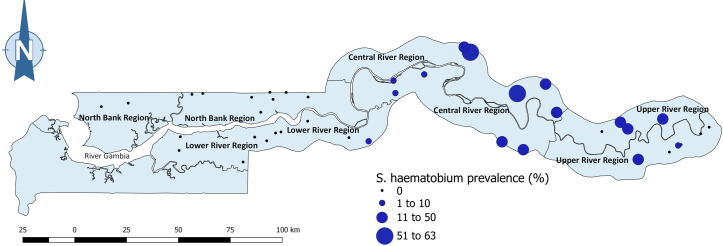
In terms of school prevalence, at least one student was infected with S. haematobium in every school sampled in CRR (Fig 3). Schools in CRR had S. haematobium prevalence ranging from 5.7% to 63.0%, and the only two schools with an infection rate more than 50% (51.0% and 63.0%) were in CRR (Fig 3). Half of the schools sampled in URR had S. haematobium infection, with infection prevalence ranging from 1.9% to 38.0%. In LRR, S. haematobium infection was found in just one school with a prevalence of 6.0%, while no S. haematobium infection was found in any of the ten schools sampled in NBR (Fig 3). See S1 Table for S. haematobium prevalence of each school. Two of the four S. mansoni-infected students in CRR were in the same school, while the other two infected cases were in two different schools (one case each). The only school with S. mansoni infection in URR had just one student infected.
Fig 3. Prevalence of S. haematobium infection by school in 4 regions of The Gambia.
Ten schools were sampled in each region. Link to map base layer used in creating this figure: https://data.humdata.org/dataset/gambia-administrative-boundaries.
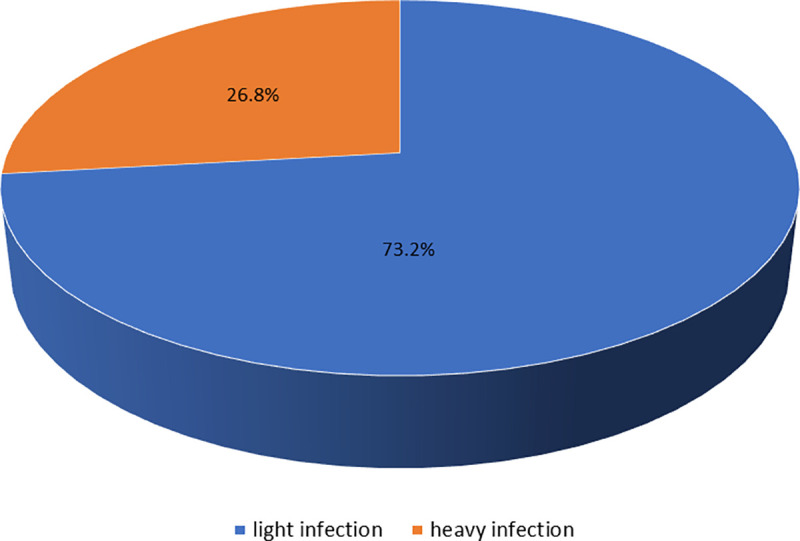
The mean (SD) egg count for S. haematobium was 6.15 (40.955). Of the 205 individuals infected with S. haematobium, 150 (73.2%) had ‘light infection’ (1–49 eggs/10 ml of urine), and 55 (26.8%) manifested ‘heavy infection’ (≥ 50 eggs/10 ml of urine), see Fig 4. All the participants infected with S. mansoni, presented ‘light infection’ intensity (< 400 eggs/g of faeces). Males had significantly higher infection intensity than females for S. haematobium (Mann-Whitney Test = 493170.0, p = 0.030). There was no significant difference in S. haematobium egg counts among age groups (Kruskal-Wallis Test, chi-square = 1.016, df = 2, p = 0.602). A Spearman’s correlation analysis found a positive correlation between prevalence of S. haematobium infection and egg intensity (r = 0.995, p < 0.001).
Fig 4. Infection intensity of S. haematobium (light against heavy infection) among children from 40 primary schools in 4 regions of The Gambia.
Macro and microhaematuria
Overall, 10 (0.5%) individuals had visible blood in their urine or macrohaematuria, with 9 (90%) of them positive for S. haematobium eggs. Of the 2,006 participants negative for macrohaematuria, only 9.8% (196) had eggs in their urine (Table 2). Fisher’s Exact Test showed a significant association between macrohaematuria and the presence of S. haematobium eggs in urine (OR = 83.11, 95% Cl 10.48–659.46, p<0.001). For the dipstick method (microhaematuria), 363 (18.0%) individuals were positive overall. One hundred and fifty-three (74.6%) of the 205 individuals infected with S. haematobium were positive for microhaematuria, and 88.8% of the total 1,811 students that were not infected with urinary schistosomiasis showed no presence of microhaematuria in their urine (Table 2).
Table 2. Frequency of macro and microhaematuria among the studied school children, 2015.
| S. haematobium infection | |||
|---|---|---|---|
| Positive (%) | Negative (%) | Total (%) | |
| Macrohaematuria | |||
| Yes | 9 (90.0%) | 1 (10.0%) | 10 (100%) |
| No | 196 (9.8%) | 1810 (90.2%) | 2,006 (100%) |
| Total | 205 | 1811 | 2016 |
| Microhaematuria | |||
| Yes | 153 (42.1%) | 210 (57.9%) | 363 (100%) |
| No | 52 (3.1) | 1601 (96.9) | 1,653 (100%) |
| Total | 205 | 1811 | 2,016* |
*Two of the study participants have missing data for S. haematobium infection status, which brings the total number to 2,016 instead of 2,018 participants.
Risk factors
The univariate analysis showed that males were more likely to get infected with S. haematobium than females (OR = 1.37, 95% CI 1.02–1.83, p = 0.036), and no association was found between age category and urinary schistosomiasis infection (Table 3).
Table 3. Demographic variables as risk factors of urinary schistosomiasis infection among school children in 4 regions of The Gambia, 2015.
| Variable | S. haematobium infection status | OR (95% CI) | p-value | ||
|---|---|---|---|---|---|
| Total (%) | Positive (%) | Negative (%) | |||
| Gender | |||||
| Male | 1,010 (100) | 117 (11.6) | 893 (88.4) | 1.37 (1.02–1.83) | 0.036* |
| Female | 1,006 (100) | 88 (8.7) | 918 (91.3) | 1 | |
| Age category | |||||
| 7–9 | 953 (100) | 91 (9.5) | 862 (90.5) | 1 | |
| 10–12 | 750 (100) | 82 (10.9) | 668(89.1) | 1.16 (0.85–1.59) | 0.348 |
| 13–14 | 313 (100) | 32 (10.2) | 281 (89.8) | 1.08 (0.71–1.65) | 0.727 |
*Statistical significance at p<0.05
OR = Odd Ratio
A generalised linear model with negative binomial log link function found a significant association between egg intensity and proximity of schools to water bodies (b = 0.429, 95% Cl = 0.318–0.541, p < 0.001). Egg intensity was higher in schools that had a fresh water body close by 15 minutes round-trip. Logistic regression found no significant association between S. haematobium infection status and presence of water bodies close by the schools (OR = 1.07, 95% Cl 0.76–1.50, p = 0.719. Water-contact behavioural risk factors such as washing clothes/dishes, fishing, fetching water, and crossing water bodies were not significantly associated with S. haematobium infection (see Table 4). Of the 205 individuals positive for S. haematobium, only 25 (12.2%) responded yes for bathing in freshwater bodies, 22 (10.7%) said yes for playing in freshwater bodies, and 15 (7.3%) said yes for swimming in freshwater bodies. Binary logistic regression analyses showed an unexpected association between urinary schistosomiasis infection and water-contact activities like bathing, playing, and swimming in water bodies. Instead of being risk for infection, bathing (OR = 0.59, 95% Cl 0.39–0.92, p = 0.019), playing (OR = 0.55, 95% Cl 0.35–0.88, p = 0.012) and swimming (OR = 0.52, 95% Cl 0.30–0.898, p = 0.019) were found to be less risk for S. haematobium infection, i.e. they were found to reduce the risk of infection with S. haematobium (Table 4). See S1 Data for the infection information and water contact responses of participants.
Table 4. Behavioural risk factors of S. haematobium infection in 4 regions of The Gambia, 2015.
| Variable | S. haematobium infection status | OR (95% Cl) | p-value | ||
|---|---|---|---|---|---|
| Total (%) | Positive (%) | Negative (%) | |||
| Water bodies | |||||
| Yes | 452 (100) | 48 (10.6) | 404 (89.4) | 1.07 (0.76–1.50) | 0.179 |
| No | 1564 (100) | 157 (10.0) | 1407 (90.0) | 1 | |
| Bathing | |||||
| Yes | 368 (100) | 25 (6.8) | 343 (93.2) | 0.59 (0.39–0.92) | 0.019* |
| No | 1,648 (100) | 180 (10.9) | 1468 (89.1) | 1 | |
| Washing clothes/dishes | |||||
| Yes | 60 (100) | 6 (10.0) | 54 (90.0) | 0.97 (0.42–2.31) | 0.965 |
| No | 1,956 (100) | 199 (10.2) | 1,757 (89.8) | 1 | |
| Fishing | |||||
| Yes | 69 (100) | 5 (7.2) | 64 (92.8) | 0.68 (0.27–1.71) | 0.417 |
| No | 1,947 (100) | 200 (10.3) | 1,747 (89.7) | 1 | |
| Crossing water bodies | |||||
| Yes | 33 (100) | 2 (6.1) | 31 (93.9) | 0.57 (0.13–2.38) | 0.437 |
| No | 1,983 (100) | 203 (10.2) | 1,781 (89.8) | 1 | |
| Fetching water | |||||
| Yes | 32 (100) | 2 (6.3) | 30 (93.8) | 0.59 (0.14–2.47) | 0.465 |
| No | 1,984 (100) | 203 (10.2) | 1,781 (89.8) | 1 | |
| Playing | |||||
| Yes | 345 (100) | 22 (6.4) | 323 (93.6) | 0.55 (0.35–0.88) | 0.012* |
| No | 1,671 (100) | 183 (11.0) | 1,488 (89.0) | 1 | |
| Swimming | |||||
| Yes | 253 (100) | 15 (5.9) | 238 (94.1) | 0.52 (0.30–0.898) | 0.019* |
| No | 1763 (100) | 190 (10.8) | 1573 (89.2) | 1 | |
*Statistical significance at p<0.05
Discussion
Human schistosomiasis was first reported in The Gambia in 1945 [29], but a more detailed account was given in 1954 by Duke and McCullough [20] on the distribution of the disease in Central River Region (CRR), then called McCarthy Island Division. Several studies on the disease followed over the next three decades until the mid-1980s [21,24,30,31]. After this period, efforts geared towards the control of schistosomiasis in the country (including prevalence studies and interventions) became limited. In 2014 when The Gambia took its first steps in setting up a schistosomiasis control programme, a knowledge gap on the distribution of the disease was found [25]. This study was conducted to provide information on the endemicity of the disease to help fill that knowledge gap.
The study assessed the prevalence and associated risk factors of schistosomiasis among primary school children in four of the five regions of the country. As reported by previous studies [20,21], urinary schistosomiasis was the predominant form of schistosomiasis seen in this study, with intestinal schistosomiasis observed in just 0.3% of the sampled school children investigated for infection. Of the numerous past studies on schistosomiasis in The Gambia, only one study reported the occurrence of intestinal schistosomiasis in the country [32]. Additionally, few or no cases of intestinal schistosomiasis have presented at hospitals and health facilities in recent times [25]. This led to the notion that S. mansoni does not exist in the country and that the few cases encountered in health facilities and studies might be imported cases. In this study, a follow-up on 2 of the five participants positive for S. mansoni, revealed they had a recent travel history. Prior to the study, the two students who are twins from the same household travelled to Guinea Conakry where S. mansoni is endemic [33,34].
The study found the highest prevalence of schistosomiasis among the school children in CRR and URR. This corroborates previous studies that found the eastern regions (represented by CRR and URR) to be the parts of the country endemic for urinary schistosomiasis [20,23,30,31]. In this study, CRR had a higher prevalence of S. haematobium than URR, with at least one student found infected in each of the ten schools sampled in the region. The River Gambia is fresh along both regions and has many tributaries (streams), locally called ‘bolongs,’ along these regions, which serve as sources of infection in the dry season [23]. Further away from the River Gambia, the landscape is a sandstone plateau with rocky, laterite iron pan [35]. When depressions formed in this laterite plateau, they hold rainwater and give rise to seasonal pools that become sources of infection in the rainy season [24,36,37]. Additionally, CRR has a huge irrigation project under the Ministry of Agriculture of The Gambia, with an irrigated area that stretches along many communities in the region engaged in rice farming. These factors might explain why the school children from these regions had the highest prevalence of urinary schistosomiasis. Conversely, the River Gambia is brackish in NBR and LRR, and there is no laterite plateau in these regions, which might be the reason for the zero and low infection rates among the school children in these regions.
Observations for both visible blood in urine (macrohaematuria) and non-visible blood in urine (microhaematuria) were found associated with presence of S. haematobium eggs in urine. Previous studies also found presence of blood in urine to correlate with presence of S. haematobium eggs, and it has been demonstrated as a good indicator of S. haematobium infection [13,17]. While not all haematuria are related to S. haematobium infection, presence of blood in urine provides an early defining indication of infection. The presence of blood in urine is caused by granulomatous inflammation resulting from the lodging of S. haematobium eggs in the bladder and urogenital system [2], and is increasingly becoming an important criteria for assessing S. haematobium infection in studies. Self reported blood in urine is even used to identify potential S. haematobium infection in people living in endemic areas [38]. Prevalence of S. haematobium was found to increase with increase in egg intensity. Egg intensity was also higher in schools that had a water body close by 15 minutes round-trip.
Previous studies have shown schistosomiasis prevalence to be higher in males than females [6,8,10,11,39], with males up to 16 times more likely of getting infected [6]. In this study, male students had a higher infection rate and had higher risk of getting urinary schistosomiasis than females. The context here is that ’male children’ are more likely to play in freshwater bodies and/or be responsible for carrying out important designated tasks such as watering livestock and other domesticated animals. Geleta et al. [11] found ‘having a parent as a farmer’ to be a predisposition for schistosomiasis while Amuta and Houmsou [8] found ‘being involved with irrigation’ as a risk factor for the disease. This study found no significant difference in S. haematobium infection prevalence among the different age groups and no association between age groups and S. haematobium infection. Many previous studies that involved school aged children made similar observations [9–11], while other studies reported the opposite [40,41].
Schistosomiasis is known to infect humans during water contact behaviours. Direct contact with open freshwater bodies through bathing, swimming, and wading is reported to be a risk factor of schistosomiasis [6,8,9,13]. In this study, however, for each water contact activity, less than 13% of the children infected with S. haematobium indicated that they were engaged in such water contact behaviour. From experience and our broad observations, this seemed an underrepresentation of their true water contact behaviours. Regression analysis of this data further indicated that children that engaged in some water contact activities (bathing, playing, and swimming) were less likely to get S. haematobium infection. A possible explanation is that the responses provided by the children are not a true representation of their water contact behaviour and activity, although the field teams ensured the children were comfortable throughout the interviews and were given gifts. While this finding did raise some questions–notably about response quality [42] around contact with water bodies, we are confident that it represented the response of the children interviewed. As most parents and teachers in these regions know about the disease, they will have advised their children against playing or bathing in these water bodies. For this reason, the children would naturally be uncomfortable disclosing contact with waterbodies for fear that they will be reprimanded by the teachers, parents or even us the researchers. The children may therefore have given inaccurate responses about their water contact behaviours.
Furthermore, if we explore the case of the children who are likely to be exposed to the water bodies by virtue of assumed responsibilities for carrying out activities such as watering livestock and other domesticated animals, an important potential risk factor is the proximity to the water source. Indeed, in a study conducted in Sudan by Hajissa and colleagues in 2018 [39], the authors identified the distance between children’s place of residence and the water source as an important determinant to the risk of schistosomiasis infection in children. This factor was not assessed in the present study and is one of the limitations of the study. We however assessed the proximity of children’s schools to water bodies but found no significant association with schistosomiasis disease outcome. Children spend far more time away from school than in school. Additionally, a major source of schistosomiasis infection in The Gambia is seasonal pools which were non-existent during this study as the study was conducted in the dry season. These may explain the non association between proximity of schools to water bodies and S. haematobium infection status. Other potential important cofounders which could have shed more light on risk factors of infection if they were assessed in this study include parents’ occupation and snail density.
More innovative, collaborative Frameworks and approaches that ensure true response to questionnaires in studies involving children need to be developed and used, as children in early childhood tend to please the researcher or teacher [43]. Moreover, children are sometimes afraid to say something wrong or undesirable, especially in school-like settings [44]. We suggest that, to avoid anxiety in children, questions formulated for questionnaires and interviews should not resemble a school test, and children should be reassured that there is no wrong answer and that they will not be reprimanded for giving any particular answer. The questions should take into account cultural nuances which may serve as ‘unaccounted for’ confounders in researches involving children. Doing so will hopefully encourage honest feedback from children, who in some cases by virtue of associating researchers with ‘local authority’ are potentially affected by a ‘perceived power-dynamic hierarchy’.
Another point to make is that the The Taskforce for Global Health developed questionnaires we used to collect water contact data did not include activities like ‘being involved with irrigation farming’ and ‘watering livestock or domesticated animals in freshwater bodies.’ Yet, in rural Gambia, many children assist their parents in irrigation farming, as well as watering livestock and other domesticated animals. The lesson to draw from this is that protocols/frameworks should be tailored to country-specific or local needs as much as possible.
The findings of the study revealed information that was vital for the follow up interventions in the national schistosomiasis control programme of The Gambia. The prevalence data of the study revealed that nine districts (five in CRR, three in URR and one in LRR) in the study area were endemic for schistosomiasis and required chemotherapy. A follow up mass drug administration campaign was conducted in 2017 in these districts found eligible for chemotherapy, and 43, 013 people that included pre-school age children, school age children and adults were treated with 600mg single dose of praziquantel [45]. Although this study provided important information that guided mass chemotherapeutic interventions in 2017, new and continuous studies are still required to provide up to date prevalence estimates to guide subsequent rounds of MDA and other interventions of the control programme. These future studies should employ more advance diagnostic methods such as CCA and PCR, which are more sensitive than the traditional parasitological techniques used in this study. The studies should also focus on all aspects of the population, as the present study has a limitation that it sampled only primary school children. More studies on S. mansoni and its intermediate snail hosts are also required to describe intestinal schistosomiasis status in The Gambia.
Conclusion
The study evaluated the prevalence and associated risk factors of schistosomiasis in primary school children in 4 regions of The Gambia (NBR, LRR, CRR, and URR) in 2015. Urine and stool samples were collected from 2,018 students and examined for S. haematobium and S. mansoni infections. 10.2% had S. haematobium infection, while 0.3% had S. mansoni infection. CRR was the most endemic region for S. haematobium infection, followed by URR, then LRR, and NBR had no infection. The prevalence of S. haematobium infection in boys was more than the prevalence in girls. No significant difference in infection rate was observed among the different age groups involved in the study. Boys had a higher risk of getting infected than girls, and presence of blood in urine was a good indicator of urinary schistosomiasis infection. Water contact activities such as bathing, playing and swimming were found to pose less risk for urinary schistosomiasis infection, suggesting untrue responses from children about their water contact behaviours. The findings of this study provide invaluable information on the endemicity of schistosomiasis in The Gambia that guided mass durg administration campaigns in the study area. More studies on S. mansoni, including investigation of its intermediate snail hosts, are required to establish the true status of intestinal schistosomiasis in the country.
Supporting information
(PDF)
(DOCX)
(XLSX)
Acknowledgments
We thank all the parent study investigation team members whose efforts generated the data utilised in this study. We are also grateful to all students (with their parents and teachers) that participated in the parent study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The study that generated the secondary data utilised in this paper was supported by the Taskforce for Global Health, INC (https://taskforce.org/) [grant number A992A78-CC70-4846-AF50BE65573CA71B to BS]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19:196–205. 10.1016/j.bjid.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet (London, England). 2014;383: 2253–64. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Working to overcome the global impact of neglected tropical diseases: First WHO report on neglected tropical diseases [Internet]. WHO. 2010. [Cited 2020 Aug 2]. Available from: 10.1177/1757913912449575 [DOI] [Google Scholar]
- 4.Van der Werf MJ, de Vlas SJ, Brooker S, Looman CWN, Nagelkerke NJD, Habbema JDF, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003; 86: 125–139. 10.1016/s0001-706x(03)00029-9 [DOI] [PubMed] [Google Scholar]
- 5.Brindley PJ, Hotez PJ. Break Out: Urogenital Schistosomiasis and Schistosoma haematobium Infection in the Post-Genomic Era. PLoS Negl Trop Dis. 2013; 7(3): e1961. 10.1371/journal.pntd.0001961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couto LD, Tibiriça SH, Pinheiro IO, Mitterofhe A, Lima AC, Castro MF, et al. Neglected tropical diseases: prevalence and risk factors for schistosomiasis and soil-transmitted helminthiasis in a region of Minas Gerais State, Brazil. Trans R Soc Trop Med Hyg. 2014;108(6):363–371. 10.1093/trstmh/tru054 [DOI] [PubMed] [Google Scholar]
- 7.Campbell SJ, Stothard JR, O’Halloran F, Sankey D, Durant T, Ombede DE, et al. Urogenital schistosomiasis and soil-transmitted helminthiasis (STH) in Cameroon: An epidemiological update at Barombi Mbo and Barombi Kotto crater lakes assessing prospects for intensified control interventions. Infect Dis Poverty. 2017;6(1):49. 10.1186/s40249-017-0264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amuta EU, Houmsou RS. Prevalence, intensity of infection and risk factors of urinary schistosomiasis in pre-school and school aged children in Guma Local Government Area, Nigeria. Asian Pac J Trop Med. 2014;7: 34–9. 10.1016/S1995-7645(13)60188-1 [DOI] [PubMed] [Google Scholar]
- 9.Ismail HA, Hong ST, Babiker AT, Hassan RM, Sulaiman MA, Jeong HG, et al. Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Parasit Vectors. 2014; 7:478. 10.1186/s13071-014-0478-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senghor B, Diallo A, Sylla SN, Doucoure S, Ndiath MO, Gaayeb L, et al. Prevalence and intensity of urinary schistosomiasis among school children in the district of Niakhar, region of Fatick, Senegal. Parasit Vectors. 2014; 7:5. 10.1186/1756-3305-7-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geleta S, Alemu A, Getie S, Mekonnen Z, Erko B. Prevalence of urinary schistosomiasis and associated risk factors among Abobo Primary School children in Gambella Regional State, southwestern Ethiopia: a cross sectional study. Parasit Vectors. 2015; 8:215. 10.1186/s13071-015-0822-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–18 10.1016/S0140-6736(06)69440-3 [DOI] [PubMed] [Google Scholar]
- 13.Zida A, Briegel J, Kabré I, Sawadogo MP, Sangaré I, Bamba S, et al. Epidemiological and clinical aspects of urogenital schistosomiasis in women, in Burkina Faso, West Africa. Infect Dis Poverty. 2016; 5(1): 81. 10.1186/s40249-016-0174-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. The control of schistosomiasis: second report of the WHO Expert Committee. Geneva: World Health Organization; 1991. [PubMed] [Google Scholar]
- 15.WHO. Human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Genava: World Health Organization; 2006. Preventive Chemotherapy. [Google Scholar]
- 16.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972; 14:397–400. [PubMed] [Google Scholar]
- 17.Hessler MJ, Cyrs A, Krenzke SC, Mahmoud ES, Sikasunge C, Mwansa J, et al. Detection of duo-schistosome infection from filtered urine samples from school children in Zambia after MDA. PLoS ONE. 2017; 12(12): e0189400. 10.1371/journal.pone.0189400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. The London Declaration. Uniting to combat neglected tropical diseases. Ending the Neglect & Reaching 2020 Goals. Table of commitments. 2012. Available from: http://www.who.int/neglected_diseases/NTD_London_Event_Table_Commitments.pdf [Google Scholar]
- 19.WHO. Accelerating work to overcome the global impact of neglected tropical diseases: a roadmap for implementation. Geneva: World Health Organization. 2012. Available from: http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf [Google Scholar]
- 20.Duke BOL, Mccullough FS. Schistosomiasis in The Gambia, II. The epidemiology and distribution of urinary schistosomiasis. Ann Trop Med Parasit. 1954; 48: 287–299. 10.1080/00034983.1954.11685626 [DOI] [PubMed] [Google Scholar]
- 21.Wilkins HA. Schistosoma haematobium in a Gambian community I. The intensity and prevalence of infection. Ann Trop Med Parasit. 1977; 71: 53–58 10.1080/00034983.1977.11687161 [DOI] [PubMed] [Google Scholar]
- 22.Mccullough FS, Duke BOL. Schistosomiasis in The Gambia, I. Observations on the potential snail vectors of Schistosoma haematobium and S. mansoni. Ann Trop Med Parasit. 1954; 48:227–286. [PubMed] [Google Scholar]
- 23.Smithers SR. On the ecology of schistosome vectors in The Gambia, with evidence of their role in transmission. Trans R Soc Trop Med Hyg. 1956; 50(4): 354–365. 10.1016/0035-9203(56)90044-x [DOI] [PubMed] [Google Scholar]
- 24.Goll PH, Wilkins HA, Marshall TFdeC. Dynamics of Schistosoma haematobium infection in a Gambia community. II. The effect on transmission of the control of Bulinus senegalensis by the use of niclosamide. Trans R Soc Trop Med Hyg. 1984; 78:222–226. 10.1016/0035-9203(84)90282-7 [DOI] [PubMed] [Google Scholar]
- 25.Ministry of Health and Social Welfare (MOHSW), The Gambia. National Master Plan for Neglected Tropical diseases 2015–2020. 2014 [Cited 2020 Aug 3]. Available from: https://espen.afro.who.int/system/files/content/resources/GAMBIA_NTD_Master_Plan_2015_2020.pdf.
- 26.Sanneh B, Joof E, Sanyang AM, Renneker K, Camara Y, Sey AP, et al. Field evaluation of a schistosome circulating cathodic antigen rapid test kit at point-of-care for mapping of schistosomiasis endemic districts in The Gambia. PLoS ONE. 2017; 12(8): e0182003 10.1371/journal.pone.0182003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camara Y, Sanneh B. Assessment of the endemicity status of schistosomiasis and soil-transmitted helminthiasis in The Gambia. BMJ Glob Health. 2017; 2: A61 [Google Scholar]
- 28.WHO. Guide for Mapping Neglected Tropical Diseases Amenable to Preventive Chemotherapy in the African Region. 2014. Available from: https://www.eliminateschisto.org/sites/gsa/files/content/attachments/2020-02-13/AFRO%20NTD%20Mapping%20Guide%20vFeb2014_Revised%20March%202018.pdf
- 29.Jones WM, Thomas CC. A preliminary survey of the incidence of infection with Schistosoma haematobium in the Colony and Protectorate of The Gambia. 1945. Available from: https://scholar.google.com/scholar_lookup?hl=en&publication_year=1945&author=W.+M.+Jones&author=C.+C.+Thomas&title=A+preliminary+survey+of+the+incidence+of+infection+with+S.+haematobium+in+the+Colony+and+Protectorate+of+the+Gambia [Google Scholar]
- 30.Wilkins HA, Goll PH, Marshall TFdeC, Moore PJ. Dynamics of Schistosoma haematobium infection in a Gambian community. III. Acquisition and loss of infection. Trans R Soc Trop Med Hyg. 1984; 78:227–232. 10.1016/0035-9203(84)90283-9 [DOI] [PubMed] [Google Scholar]
- 31.Blumenthal UJ. Transmission of Schistosoma haematobium in seasonal pools in The Gambia, with particular reference to the role of human water contact. PhD thesis, University of Liverpool. 1985. Available from: https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.303695
- 32.Smithers SR. The Occurrence of Schistosoma mansoni in the Gambia. Ann Trop Med Parasit.1957; 51(4). 10.1080/00034983.1957.11685824 [DOI] [PubMed] [Google Scholar]
- 33.Koma M, Beer SA. Intestinal schistosomiasis in the Guinea People’s Revolution Republic (West Africa). Med Parazitol (Mosk). 1982; 60(2): 43–48. [PubMed] [Google Scholar]
- 34.Hodges M, Koroma MM, Baldé MS, Turay H, Fofanah I, Divall MJ, et al. Current status of schistosomiasis and soil-transmitted helminthiasis in Beyla and Macenta Prefectures, Forest Guinea, Trans R Soc Trop Med Hyg. 2011; 105(11): 672–674. 10.1016/j.trstmh.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 35.Hanrion C, Mercky P, Chauvel A. Projet pilote des Terres Neuves. Etudes pedologique du perimetre Sud Kompentoum. Centre ORSTOM de Dakar-Hann, Senegal. 1971. Available from: http://www.documentation.ird.fr/hor/fdi:04985 [Google Scholar]
- 36.Goll PH, Wilkins HA. Field studies on Bulinus senegalensis Muller and the transmission of Schistosoma haematobium infection in a Gambian community. Tropenmed Parasitol. 1984; 35(1):29–36 [PubMed] [Google Scholar]
- 37.Senghor B, Diaw OT, Doucoure S, Seye M, Talla I, Diallo A, et al. Study of the snail intermediate hosts of urogenital schistosomiasis in Niakhar, region of Fatick, West central Senegal. Parasit Vectors. 2015; 8: 410. 10.1186/s13071-015-1030-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. 10.1136/bmj.d2651 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajissa K, Muhajir A, Eshag HA, Alfadel A, Nahied E, Dahab R, et al. Prevalence of schistosomiasis and associated risk factors among school children in Um-Asher Area, Khartoum, Sudan. BMC Res Notes. 2018; 11(1): 779. 10.1186/s13104-018-3871-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivoke N, Ivoke ON, Nwani CD, Ekeh FN, Asogwa CN, Atama CI, et al. Prevalence and transmission dynamics of Schistosoma haematobium infection in a rural community of southwestern Ebonyi State, Nigeria. Trop Biomed. 2014; 31: 77–88. [PubMed] [Google Scholar]
- 41.Kabuyaya M, Chimbari MJ, Mukaratirwa S. Infection status and risk factors associated with urinary schistosomiasis among school-going children in the Ndumo area of Mkhanyakude district in KwaZulu-Natal, South Africa two years post-treatment. Int J Infect Dis. 2018; 71: 100–106. 10.1016/j.ijid.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 42.Borgers N, Hox J, Sikkel D. Response Effects in Surveys on Children and Adolescents: The Effect of Number of Response Options, Negative Wording, and Neutral Mid-Point. Qual Quant. 2004;38(1): 17–33. [Google Scholar]
- 43.de Leeuw ED. Improving data quality when surveying children and adolescents: Cognitive and social development and its role in questionnaire construction and pretesting. Paper presented at the Annual Meeting of the Academy of Finland: Research Programs Public Health Challenges and Health and Welfare of Children and Young People. May 10–12 Naantali, Finland. 2011 [Cited 2020 Aug 24]. Available at: https://www.aka.fi/globalassets/awanhat/documents/tiedostot/lapset/presentations-of-the-annual-seminar-10-12-may-2011/surveying-children-and-adolescents_de-leeuw.pdf
- 44.Delfos M. Are you listening to me? Communicating with children from four to twelve years old. 1st ed. Amsterdam: SWP Publishers; 2001. [Google Scholar]
- 45.Ministry of Health and Social welfare (MOHSW), The Gambia. Schistosomiasis and soil-transmitted helminthiasis mass drug administration report. 2017 [Cited 2020 Aug 3]. Available from: https://www.afro.who.int/news/gambia-launches-nationwide-mass-drug-administration-campaign-against-ntds
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.