Abstract
Background
Numerous studies investigate the association between the ABO blood groups and the occurrence of COVID-19 infection; discordant findings were reported. Therefore, the purpose of this meta-analysis was to evaluate the existing evidence on the susceptibility of the ABO blood group to COVID-19 infection.
Methods
Systematically searched published articles in PubMed, Google Scholar, Scopus, and EMBASE between 1 st January 2020 and 21 st March 2021. After quality control and the exclusion of irrelevant studies, 16 studies were included in the final analysis.
Results
Although the random-effect meta-analysis revealed a large heterogeneity among studies, I 2 = 99.197 %. The pooled event rates and (95 % CIs) for A, O, B, and AB blood group were 0.459 (95 %CI: 0.358–0.441), 0.342 (95 %CI: 0.298–0.374), 0.180 (95 %CI: 0.150–0.214), and 0.076 (95 %CI: 0.055–0.127), respectively. These results indicated that the COVID-19 infection rate was higher in persons with blood group A > O > B > AB. Overall, the ABO blood group's vulnerability to COVID-19 infection was statistically significant (pooled p -value<0.001).
Conclusion
This meta-analysis offers a further indication of blood group A individuals' vulnerability to COVID-19 infection, and blood type AB are linked to a lower risk of COVID-19 infection.
Keywords: ABO blood group system, COVID-19, Infection, Susceptibility, Systematic review and meta-analysis
1. Introduction
Since the last century, humanity has not faced a pandemic like COVID-19 that has a stunning global rhythm of infectiously, morbidity, and mortality [1]. In late January 2020, the World Health Organization (WHO) announced that the COVID-19 outbreak in Wuhan was an international concern and called for a public health emergency; after that, the spread of the virus increased across countries, and a global pandemic was announced in March 2020. The WHO has urged all countries worldwide to take restricted measures to reduce the spread of COVID-19 and increase population protection, including applying social distancing and quarantine. [2,3]. Despite those efforts to halt COVID-19 spreading since its discovery in Wuhan last December 2019, as of 21 st March 2021, approximately 126,727,456 infected cases were confirmed, including 2,780,162 deaths and 102,186,956 recovered [4].
Several symptoms have been manifested with COVID-19 positive individuals, ranging from asymptomatic/mild symptoms to severe illness and death. Common symptoms include cough, fever, and shortness of breath. Other reported symptoms are weakness, malaise, respiratory distress, muscle pain, sore throat, loss of taste and smell, multi-organ failure, septic shock, and blood clots [[5], [6], [7]]. Besides, longer-term damage to organs (particularly the lungs and the heart) was observed [8,9]. The spreading of the COVID-19 is under investigation, and several mechanisms were reported that include but limited to the droplets, aerosols, and direct contact [10,11]. It is usually diagnosed by real-time reverse transcription-polymerase chain reaction (RRT-PCR) [12].
With the emerged evidence and report about the association between the blood groups and diseases, including infectious diseases, several studies have reported the risks of diseases among ABO groups [[13], [14], [15], [16]]. The recently observed evidence continued to suggest that blood type may play a role in COVID-19 susceptibility. A couple of preprint reports and published articles found an association between the ABO blood groups and COVID-19 [17,18]. Nonetheless, other studies found no evidence of such connotation [19,20]. Therefore, this study was conducted to provide summary estimates on the link of the susceptibility of the ABO blood groups to COVID-19 infection to reduce the gap in the literature and support the evidence-based decision making as there is a better recognition of systematic review and meta-analysis findings in health policy and decision-making processes.
2. Methods
2.1. Data sources and literature search strategy
This meta‐analysis followed the PRISMA recommendations (Appendix A) and was registered with The International Prospective Register of Systematic Reviews (PROSPERO, registration No. CRD42020221025). Two authors independently, comprehensively, and systematically searched published articles in PubMed and Google Scholar, Scopus, and EMBASE between 1st January 2020 and 21st March 2021. The language restriction was English without limitation for the region, and the search MESH (medical subject heading) terms were “ABO blood group” and “COVID-19”. A free-text search also was conducted to increase the search scale. The PubMed search strategy using a combination of MeSH terms and free texts was presented as a sample of the applied search strategy (Appendix B).
2.2. Selection criteria (inclusion/exclusion)
After eliminating duplicates, two authors (SK and AK) individualistically assessed study eligibility by evaluating the titles, abstracts, and the retrieved full texts. Disagreements were resolved by consensus. The inclusion criteria were as follows: studies reported the number of confirmed patients with COVID-19 infection and their frequency distribution according to each blood group. Moreover, the exclusion criteria were as follows: (1) reviews, qualitative studies, case reports, letters, comments, non-human studies or symposium, and conference proceedings; (2) language other than English; (3) articles in which the outcome measure was not the frequency of infection with COVID-19 in each blood group (A, B, Ab, and O); (4) studies lacking relevant data; and (4) citations without full text.
2.3. Study screening and data extraction
EndNote V. × 8 software was used for removing duplicates and managing the screening processes. Additionally, two authors (SK and AK) handled data carefully and manually to minimize duplication risk. Details of the first author’s name, publication date, study title, country of origin, study design, sample size, gender, and mean age of participants and frequency and percentage of COVID-19 cases according to each blood group category (A, B, AB, and O-blood group) were extracted and recorded independently using a standardized data collection form, which was developed according to the sequence of variables required from the primary studies.
2.4. Quality assessment of the studies
The selected studies were assessed for quality sing “The Quality Assessment Tool For Quantitative Studies (QATFQS)," which was developed by the "Effective Public Health Practice Project (EPHPP)". This assessment tool was applied because it is comprehensive and enables an exhaustive assessment of the included studies' quality [21]. The assessment tool contains eight components that evaluate the quality of the study: "Selection bias, Study design, Confounders, Blinding, Data collection methods, withdrawals and dropouts, Intervention integrity, and Analysis." Each element is evaluated alone in three categories: "1 = Strong, 2 = Moderate, 3 = Weak," and for the evaluation for a complete study: "1 = STRONG (no WEAK ratings), 2 = MODERATE (one WEAK rating), 3 = WEAK (two or more WEAK ratings)" [22]. Each study's ratings were compared between the two investigators (SK and AK); any disagreement was resolved by joint discussion.
2.5. Data synthesis and statistical analysis
All statistical analyses were undertaken using Comprehensive Meta-Analysis Software (CMA, version 3 BioStat, USA). The Fail-Safe N method was applied to estimate the number of studies that should be added to the meta-analysis in order to reset the effect size value attained from the meta-analysis. The mean effect size of the studies in the meta-analysis was calculated. The ABO blood group susceptibilities to COVID-19 infection were pooled and examined using a random-effect model, and data are presented in the forest plots. Events rate and their corresponding 95 % confidence intervals and the p-value were calculated using the extracted data. The I2 statistic was used to ascertain the heterogeneity between the included studies, and I2 values of 0–40 %, 25–50 %, 50–75 %, and >75 % indicated insignificant, low, moderate, and substantial heterogeneity, respectively [23]. A non-substantial level of statistical heterogeneity was assumed when p < 0.1 or I2 < 50 %. A random-effect model was chosen due to the high heterogeneity [24]. Publication bias was assessed using a funnel plot, and Begg’s and Mazumdar’s rank correlation tests were applied to detect the potential evidence of publication bias between included studies.
3. Results
3.1. Search outcomes
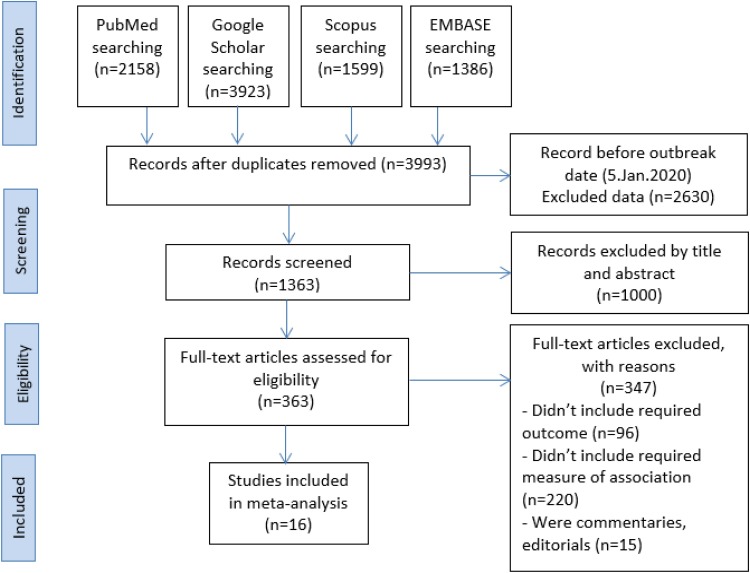
The search yielded in a total of 9066 articles from four databases: PubMed (n = 2158), Google Scholar (n = 3923), Scopus (n = 1599) and EMBASE (n = 1386). Afterwards, the duplicates were deleted, and 3993 studies remained. After removing 2630 due to the publication date before the outbreak date, we excluded 1000 irrelevant studies out of 1250 by screening title and abstract. Then we reviewed the full-text of the remaining 1363 articles and excluded 347 studies for not meeting our inclusion criteria. Eventually, a total of 16 studies were included in the qualitative synthesis and meta-analysis. The process of article screening and selection was presented in Fig. 1 .
Fig. 1.
The PRISMA flowchart of study identification and study selection process.
3.2. Characteristics of the included studies
Sixteen studies were identified [[17], [18], [19], [20],[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]] that met our meta-analysis inclusion and exclusion criteria; their general characteristics were presented in Table 1 .
Table 1.
The main characteristic of the included studies.
| The first author (year) | Country | Study design | Gender (M/F) |
Mean age | Sample n |
O n (%) |
A n (%) |
B n (%) |
AB n (%) |
Prevalence |
|---|---|---|---|---|---|---|---|---|---|---|
| Sunny Dzik et al. (2020) | United State | Retrospective | NR | NR | 957 | 465 (48.6) | 311 (32.5) | 140 (14.6) | 41 (4.3) | O > A > B > AB |
| Jiao Zhao et al. (2020) | China | Retrospective | 1030/858 | NR | 2173 | 486 (25.7) | 715 (37.9) | 494 (26.2) | 193 (10.2) | A > B>O > AB |
| Michael Zietz et al. (2020) | United Stat | Retrospective | NR | NR | 682 | 312 (45.7) | 233 (34.2) |
116 (17.0) | 21(3.1) | O > A > B > AB |
| Juyi Li et al. (2020) | China | Retrospective | 1143/1010 | NR | 2153 | 554 (25.7) | 819 (38.0) |
561 (26.1) | 219 (10.2) | A > B>O > AB |
| AKAN GÖKER et al. (2020) | Turkey | Retrospective | 100/86 | 42 | 186 | 46 (24.7) | 106 (57.0) |
20 (10.8) | 14 (7.5) | A > O>B > AB |
| Ghada Ali Aljanobi et al. (2020) | Saudi Arabia | Retrospective | 24/48 | 50 | 72 | 24 (33.3) | 17 (23.6) |
24 (33.3) | 7 (9.7) | O = B > A > AB |
| Yuqin Wu et al. (2020) | China | Retrospective | 97/90 | NR | 187 | 41(21.9) | 69 (36.9) | 63(33.7) | 14 (7.5) | A > B>O > AB |
| Rebecca K. Leaf et al. (2020) | United State | Prospective | 1297/736 | 62 | 2033 | 950 (46.7) | 666 (32.8) | 328 (16.1) | 89 (4.4) | O > A > B > AB |
| Falah Hasan Obayes AL-Khikani et al. (2020) | Iraq | Retrospective | NR | NR | 1775 | 458 (25.8) | 670 (37.7) | 469 (26.4) | 178 (10.0) | A > B>O > AB |
| Christopher A. Latz et al. (2020) | United State | Retrospective | 1207/872 | 56.6 | 1289 | 587 (45.5) | 440 (34.1) | 201 (15.6) | 61 (4.7) | O > A > B > AB |
| Michael Hultström et al. (2020) | Sweden | Retrospective | NR | NR | 64 | 28 (43.8) | 27 (42.2) | 6 (9.4) | 3 (4.7) | O > A > B > AB |
| Michael Zietz et al. (2020) | United Stat | Retrospective | 5024/365 | NR | 5389 | 2541 (47.2) | 1755 (32.6) | 879 (16.3) | 214 (4.0) | O > A > B > AB |
| Marion Kibler et al. (2020) | France | Retrospective | 7/15 | 82 | 22 | 4 (18.2) | 18 (81.8) | 0 (0) | 0 (0) | A > O |
| Eduardo Muñiz-Diaz et al. (2021) | Cyprus | Retrospective | 338/516 | 45 | 854 | 345 (40.8) | 403 (47.7) | 65 (7.7) | 32 (3.8) | A > O>B > AB |
| İhsan Solmaz et al. (2020) | Turkey | Cross-sectional | NR | NR | 1667 | 447 (26.8) | 753 (45.2) | 311 (18.7) | 156 (9.4) | A > O>B > AB |
| Qian Fan et al. (2020) | China | Case-control | 55/20 | 57 | 105 | 23 (21.9) | 45 (42.9) | 28 (26.7) | 9 (8.6) | A > O>B > AB |
M/F: male/female, NR: Not reported.
According to the quality assessment for the selected studies, five were considered in strong quality, seven in moderate quality, and four in week quality. The week quality results are related to two items, the selection bias and confounders, which were the most common weaknesses (Appendix C).
The studies were published in 2020 and 2021; five of them were conducted in the United States, four in China, two in Turkey, one in Saudi Arabia, one in Iraq, one in Sweden, one in France, and one in Cyprus. 13 out of the 16 included studies were retrospective in the design; one was a case-control study, one was a cross-sectional study, and the remaining one was a prospective study. In this prospective study, consecutive adults (aged ≥18 years) with laboratory-confirmed COVID-19 admitted to participating intensive care units (ICUs) between 4 March and 11 April 2020 at 67 hospitals across the United States were enrolled. Patients were followed until the first of hospital discharge, death or 8 May 2020, when the database for the analysis was locked. All patients who remained hospitalized at the time of analysis had a minimum of 28-day follow-up.
The study's participants' summation is 14938, of whom 69.1 % were males and 30.9 % females. The sample size of the studies ranged from 22 to 5389 individuals. Only seven studies mentioned the mean age of their participants, which ranged between 42–82 years. All studies reported the number of confirmed patients with COVID-19 infection and the frequency distribution according to each blood group category (A, B, AB, and O-blood group). We calculated the percentage of COVID-19–infected people in each blood group.
3.3. Distribution of ABO blood groups by country
The studies conducted in the United States, China, Saudi Arabia, and Iraq stated that individuals with O blood types are at increased risk for COVID-19. In contrast, individuals with AB blood types are at a decreased risk for COVID-19. The studies from Turkey, Sweden, France, and Cyprus showed that individuals with A blood types are at increased risk for COVID-19 while individuals with AB blood types are at a decreased risk for COVID-19.
The prevalence of ABO blood groups among COVID-19 infected patients was evaluated and demonstrated in Table 2 . This distribution pattern revealed a different pattern between countries; for instance, O > A > B > AB was observed among studies conducted in the United States and Sweden. Moreover, the A > B>O > AB pattern was recognized in China and Iraq. However, in China, a different pattern was demonstrated (A > O>B > AB) that was also observed in Cyprus and Turkey. In Saudi Arabia, a unique pattern was observed (O = B > A > AB). Comparing the distribution pattern of COVID-19 patents' ABO blood group to the country-specific ABO blood distribution revealed that the ABO patterns in COVID-19 studies followed the country-specific pattern in United States, China, Turkey, Sweden and Cyprus. Except for Saudi Arabia, Iraq, and France, where they have different distributions (Table 2).
Table 2.
Distribution of ABO blood groups by country.
| Country | O | A | B | AB | Prevalence |
|---|---|---|---|---|---|
| Unites State | 44 % | 42 % | 10 % | 5 % | O > A > B > AB |
| China | 48 % | 38 % | 19 % | 5 % | O > A > B > AB |
| Turkey | 34 % | 43 % | 16 % | 8 % | A > O>B > AB |
| Saudi Arabia | 52 % | 26 % | 18 % | 4 % | O > A > B > AB |
| Iraq | 36 % | 28 % | 28 % | 8 % | O > A=B > AB |
| Sweden | 38 % | 44 % | 12 % | 6 % | A > O>B > AB |
| France | 42 % | 44 % | 10 % | 4 % | A > O>B > AB |
| Cyprus | 39 % | 44 % | 12 % | 5 % | A > O>B > AB |
3.4. Fail-Safe N method
The Fail-Safe N method was applied to estimate the number of studies that should be added to the meta-analysis in order to reset the effect size value attained from the meta-analysis [38]. The obtained considerably high N value by the classic Fail-Safe N was 6095, indicating that the effect value achieved by our meta-analysis is exceptionally resistant to publication bias (Table 3 ).
Table 3.
Classic and Orwin’s Fail-Safe N outcomes.
| Classic Fail-Safe N Method | Orwin’s Fail-Safe N Method | ||
|---|---|---|---|
| Z-value for observed studies | −99.866 | The event rate is observed in studies | 0.289 |
| The P-value for observed studies | 0.000 | The criterion for a "trivial" event rate | 0.500 |
| Alpha | 0.050 | Mean event rate in missing studies | 0.500 |
| Tails | 2.000 | ||
| Z for alphas | 1.959 | ||
| Number of observed studies | 64.000 | ||
| Number of missing studies that would bring the P-value to > alpha (N value) | 6095.000 | ||
3.5. Unified findings
The mean effect size of the studies in the meta-analysis was calculated. The points of estimate were 0.290 and 0.215 according to the fixed and mixed effects analysis, respectively. According to the study of the fixed and mixed effect, the 95 % confidence intervals were (0.286−0.293) and (0.185−0.249), respectively. Contrarily, the Q value calculated by the homogeneity test shows that distributions of blood group data have a heterogeneous structure (Q = 6993.202; p < 0.001). According to the random-effects model, we decided to conduct a meta-analysis to remove the illusions arising from the sample's heterogeneity. Accordingly, the between ABO blood group and risk of COVID-19 infection was studied by applying the random-effects model. The tau (T) value (0.750), the between-study variance, estimated standard deviation of underlying effects, and amount of true heterogeneity across studies.
The yielded heterogeneity measure, I-squared (I2), estimates the pooled proportion of variability between the included studies in a meta-analysis explained by differences rather than by sampling error to allow reliable determination of the accuracy of meta-analyses [39]. The I2 values obtained from the meta-analysis were higher than 99 %, indicating that the dispersion of effect sizes in the current meta-analysis is considerable, containing large amounts of heterogeneity. According to the studies' random effect analysis in the meta-analysis, the mean effect size and confidence intervals are given in Table 4 .
Table 4.
Meta-analyses effect analysis values of included studies, homogeneous distribution value, average effect size, and confidence intervals.
| Model | Effect size and 95 % interval |
Test of null (2-Tail |
Heterogeneity |
Tau-squared |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Number of Studies | Point of estimate | Lower limit | Upper limit | Z-value | P-value | Q-value | df (Q) | P-value | I-squared | Tau Squared | Standard error | Variance | Tau |
| Fixed | 64 | 0.290 | 0.286 | 0.293 | −101.757 | 0.000 | 6993.202 | 63 | 0.000 | 99.099 | 0.562 | 0.160 | 0.026 | 0.750 |
| Random | 64 | 0.215 | 0.185 | 0.249 | −13.238 | 0.000 | ||||||||
3.6. ABO blood group susceptibility to COVID-19 infection
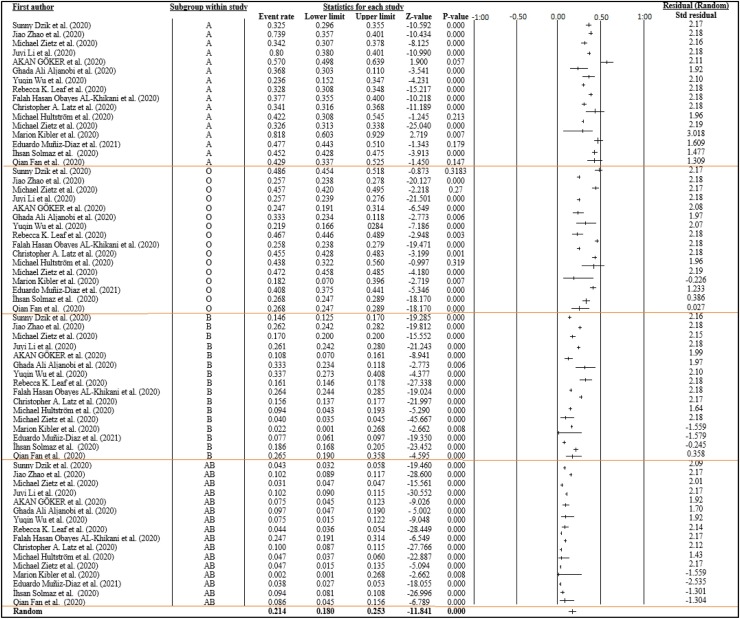
Although the random-effect meta-analysis revealed a large heterogeneity among studies, I2 = 99.099 %, the pooled event rates and (95 %CIs) for A, O, B, and AB blood group were 0.459 (95 %CI: 0.358–0.441), 0.342 (95 %CI: 0.298–0.374), 0.180 (95 %CI: 0.150–0.214), and 0.076 (95 %CI: 0.055–0.127), respectively. Results indicated that the COVID-19 infection rate is higher in persons with blood group A, followed by blood groups O, B, and AB, respectively. Overall, the vulnerability of the ABO blood group to COVID-19 infection was statistically significant (pooled p-value<0.001) (Fig. 2 ).
Fig. 2.
Forest plot from the random-effects analysis: ABO Blood group susceptibility to COVID-19 infection.
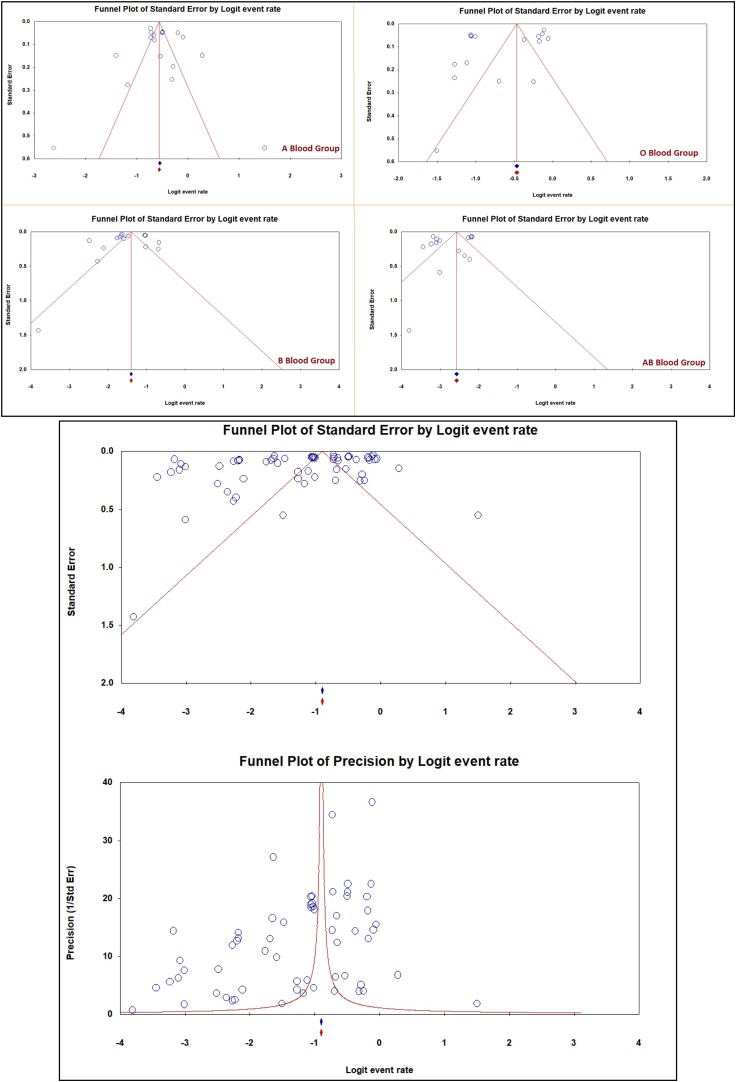
The result of Begg’s and Mazumdar’s rank correlation test did not indicate the presence of a significant level of publication bias. The Kendall’s tau without continuity and Kendall’s tau with continuity p-values (2-tailed) were 0.108 and 0.109, respectively (Table 5 ). The funnel plot of publication bias of the ABO blood group infection for COVID-19 is shown in Fig. 3 .
Table 5.
Begg and Mazumdar rank correction.
| Kendall’s S statistic (P-Q) | −277.000 |
|---|---|
| Kendall’s tau without continuity correction | |
| Tau | −0.137 |
| z-value for tau | 1.604 |
| P-value (1-tailed) | 0.054 |
| P-value (2-tailed) | 0.108 |
| Kendall’s tau with continuity correction | |
| Tau | −0.137 |
| z-value for tau | 1.599 |
| P-value (1-tailed) | 0.054 |
| P-value (2-tailed) | 0.109 |
Fig. 3.
Publication bias of the infection of ABO blood group for COVID-19.
4. Discussion
COVID-19 imposed global concerns to the level that it was declared a global pandemic by the world health organization. Several vaccines are on their way to the final FDA approval process and will be available soon worldwide. A unique way to restrict the spread of the disease is through determining risk factors. There are well-known factors associated with the worst prognosis, morbidity, and mortality, including hypertension, chronic cardiovascular disease, diabetes, and smoking. Even with the vaccine’s availability, determining factors that increase infection susceptibility is essential for the implication of control and protective measures. Several studies linked ABO blood groups with COVID-19 infection. Thus, the analysis helped determine the risk of COVID-19 on pooled event rated 16 studies published between 1st January 2020 and 21st March 2021that met the research’s inclusion criteria.
The current study found that the pooled event rates and (95 %CIs) for A, O, B, and AB blood group were 0.459 (95 %CI: 0.358–0.441), 0.342 (95 %CI: 0.298–0.374), 0.180 (95 %CI: 0.150–0.214), and 0.076 (95 %CI: 0.055–0.127), respectively. That demonstrates that COVID-19 infects all ABO blood groups at different rates. These findings also indicated that a greater proportion of people with blood group A and group O were among the most COVID-19 infected patients. In contrast, a person with blood group AB was less infected by COVID-19. Our finding is consistent with another meta-analysis publication, published in December 2020 [40]. Their findings indicated that group O had a protective advantage and group A had unfavourable outcomes than other blood groups' odd ratios. They did not find any significant association between the AB group and COVID infection. This due to the fact that distribution patterns in COVID-19 studies were aligned with the country-specific pattern as in the United State, China, Turkey, Sweden, and Cyprus. Nevertheless, some studies observed a unique distribution pattern, such as in Saudi Arabia, Iraq, and France.
The finding is incomplete consistent with the previous meta-analysis finding from other publication [41,42]; Wu et al. (2020) and Golinelli et al. (2020) concluded that individuals with blood type A were at a higher risk of infection, whereas individuals with blood type O linked to a lower risk to COVID-19 infection. In the current study, the AB blood group had a lower risk of COVID-19 infection. Consistent with these findings, the meta-analysis conducted by Golinelli and his research team showed a considerable amount of heterogeneity among the included studies [42]. Scientists have proved that ethnic groups can differ in their prevalence of blood groups and blood antigens [43]. In the current research, the studies conducted in the United States, China, Saudi Arabia, and Iraq stated that individuals with O blood types are at increased risk for COVID-19.
In contrast, individuals with AB blood types are at a decreased risk for COVID-19. The other studies from Turkey, Sweden, France, and Cyprus showed that individuals with A blood types are at increased risk for COVID-19 while individuals with AB blood types are at a decreased risk for COVID-19. This result could be attributed to the variability of studies regions, races, sample size, and the difference in participation proportion of COVID-19 positive participants according to their ABO blood group [17,29]. The discrepancy from the previous meta-analysis publications was due to the data quality and reliability. The difference in sample size was demonstrated by the higher heterogenicity and publication bias in Fig. 3. To overcome such discrepancy, it is essential to access more reliable data from newer publications with higher sample sizes. The impact of race difference in blood group distribution among COVID-19 patients cannot be assessed due to heterogeneity in the current study.
Additionally, Theoretically, race could affect the susceptibility of COVID-19 infection and contribute to the ABO blood group distribution in the populations. Due to lack of publications, until submitting this work, from Africa and Australia. Therefore, future work should consider more publications, if ever made available from various publications, to assess the relationship between suitability to COVID-19 infection in different populations worldwide.
This study’s importance stems from expanding the range of human knowledge about the pathogenic factors that accompany the COVID-19 pandemic. According to blood types, recognizing people's susceptibility can help understand the pandemic dynamics and spread mechanisms to improve the ability to make appropriate health decisions that will limit the virus's spread. Although the precise mechanism of COVID-19 infection is still under investigation and the relationship with ABO antigens is one of the hypotheses, it can be concluded that there is a solid foundation for such a hypothesis and warrants further investigation. The differences in the ABO antigens could explain the difference in the suitability to COVID-19 infection. Dai (2020) demonstrated a spike protein of SARS-COV-2 that mimics the blood group's antigen by 80 %, an earlier virus that caused acute severe respiratory syndrome in 2003. The same study suggested that antigens' antibodies against the blood groups could supply a protective effect against the SARs-COV2 virus [44]. This could explain the lower risk of COVID-19 infection in the AB group and increased risk of infection in A and O groups. Several studies found an association between the risk of intubation or death among COVID-19 infected patients differed depending on the blood groups. The alive meta-analysis conducted by Pourali et al. (2020) to investigate the relationship between blood group and risk of infection and death in COVID-19 showed that individuals with blood group A are at higher risk of COVID-19 infection while those with blood group O are at lower risk [45]. Similarly, the population-based cohort study conducted in Ontario, Canada, to determine whether ABO and Rh blood groups are associated with risk for SARS-CoV-2 infection and severe coronavirus disease 2019 (COVID-19) illness showed that type O blood might be related to a lower risk for SARS-CoV-2 infection and severe COVID-19 illness or death [46].
Later research contradicted this nation and suggested publication bias and limitations in the methods used to analyze this relationship. Additionally, the researcher should evaluate their findings based on the country-specific ABO distribution. The investigation of the relationship between the outcomes of the diagnosed COVID-19 infected patients and ABO blood groups were not investigated in the current study due to the lack of details about the included studies' results. The assessment of the relationship between outcomes of the disease and ABO groups requires more studies in the analysis to draw any definite conclusion. Theoretically, this investigation is worth pursuing as evidence suggests; therefore, it is recommended for future work.
Growing evidence suggests that the A blood group is associated with increased susceptibility to and severity of COVID-19. The mechanisms beyond this relationship are still unknown; nevertheless, numerous hypotheses might be raised. Anti-A antibodies could probably lead to a decreased interaction of SARS-CoV-2 with its cellular receptor ACE-2 [47]. Interestingly, the A blood group has also been associated with an increased risk of cardiovascular diseases [48]. Numerous biological pathways have been proposed to account for the relationship between the A blood group and atherothrombosis, including increased production of soluble intercellular adhesion molecules [49] and/or von Willebrand factor (vWF) [50]. Other researchers have emphasized the consequence of vWF cleavage in subjects with the O blood group [15], an event that may decrease thrombotic risk in SARS-CoV-2-infected individuals [19]. Lately, a molecular genetic analysis of case/control data recognized two loci (3p21.31 and 9q34.2) as meaningfully related to severe COVID-19. Remarkably, the ABO gene resides on chromosome 9 at the band 9q34.2. Besides, this study stated an increased risk of severe COVID-19 in patients with the A blood group (OR: 1.45) while the O blood group had a protective effect (OR: 0.65) [51].
However, the current results should be interpreted considering the following limitations: First, this meta-analysis included articles affected by the pandemic emergency circumstance as those studies were carried out and published within a short period; therefore, we think that those articles are still preliminary, and more verification is needed to ensure their intrinsic quality. Second: although the inclusion criteria in our meta-analysis were stringent, substantial heterogeneity was found. Third: adjustment confounders were varied among the studies; therefore, we could not conduct a subgroup analysis. Fourth: the current study did not include studies from Africa. Finally, we think that the included studies' sample sizes were not representative of the original general populations.
5. Conclusion
We found that the COVID-19 infection rate in persons with blood group A > O > B > AB. Accordingly, this evidence-based meta-analysis study further indicates blood group A individuals' vulnerability to COVID-19 infection. Blood type AB is linked to a lower risk of COVID-19 infection. Nonetheless, the exact molecular and clinical mechanism by which ABO blood group's different susceptibility to COVID-19 infection is mostly unclear. Thus, further studies are warranted to understand better how ABO blood group influence COVID-19 infection and whether intensification of infection control measures by blood category could reduce the risk of infection with COVID-19. Moreover, it is essential to report blood types and subtypes for each infected individual in all populations to correlate with a more extensive data set in the future based on their demographic information, including gender, age, and ethnicity.
Funding
This work was supported by the Deanship of Scientific Research at UmmAl-Qura University, Makkah, Saudi Arabia [Grant Code: 20-MED-4-13-0013].
Declaration of Competing Interest
The authors declare no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
Acknowledgements
The authors would like to thank the Deanship of scientific research, Umm Al Qura University, Makkah, Saudi Arabia, for supporting this work by Grant Code: 20-MED-4-13-0013.
Appendix A. : PRISMA recommendations
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 3 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 3,4 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 3 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 3,4 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3,4 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 4,5 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 6,7 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 5 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 4,5 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 5 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 6 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 7,8 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 7,9 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 11 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 5−14 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 12 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 11,12,13 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 14 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 19 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 19 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 20 |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:https://doi.org/10.1371/journal.pmed1000097.
For more information, visit: www.prisma-statement.org.
Appendix B. : PubMed search strategy
| Search | Query | Hits* |
|---|---|---|
| #1 | (COVID-19[MeSH Terms]) OR (ABO blood group[MeSH Terms]) | 15,055 |
| #2 | ((((((((((((COVID-19[Title/Abstract]) OR (COVID19[Title/Abstract])) OR (Coronavirus[Title/Abstract])) OR (sars cov 2[Title/Abstract])) OR (SARS-CoV-2[Title/Abstract])) OR (MERS-CoV[Title/Abstract])) OR (SARS-CoV[Title/Abstract])) OR (ABO[Title/Abstract])) OR (Blood groups[Title/Abstract])) OR (ABO blood groups[Title/Abstract])) OR (A, B, AB[Title/Abstract] AND O[Title/Abstract])) OR (Blood types[Title/Abstract])) OR (ABO group[Title/Abstract]) | 105,130 |
| #3 (#1 AND #2) | ((COVID-19[MeSH Terms]) OR (ABO blood group[MeSH Terms])) AND (((((((((((((COVID-19[Title/Abstract]) OR (COVID19[Title/Abstract])) OR (Coronavirus[Title/Abstract])) OR (sars cov 2[Title/Abstract])) OR (SARS-CoV-2[Title/Abstract])) OR (MERS-CoV[Title/Abstract])) OR (SARS-CoV[Title/Abstract])) OR (ABO[Title/Abstract])) OR (Blood groups[Title/Abstract])) OR (ABO blood groups[Title/Abstract])) OR (A, B, AB[Title/Abstract] AND O[Title/Abstract])) OR (Blood types[Title/Abstract])) OR (ABO group[Title/Abstract])) | 8,126 |
*= Date of search: September 20, 2020
Appendix C. : Quality assessment for the selected studies using The National Institute of Health quality assessment tool
| Sunny Dzik et al. (2020) | Jiao Zhao et al. (2020) | Michael Zietz et al. (2020) | Juyi Li et al. (2020) | AKAN GÖKER et al. (2020) | Ghada Ali Aljanobi et al. (2020) | Yuqin Wu et al. (2020) | Rebecca K. Leaf et al. (2020) | Falah Hasan Obayes AL-Khikani et al. (2020) | Christopher A. Latz et al. (2020) | Michael Hultström et al. (2020) | Michael Zietz et al. (2020) | Marion Kibler et al. (2020) | Eduardo Muñiz-Diaz et al. (2021) | İhsan Solmaz et al. (2020) | Qian Fan et al. (2020) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A SELECTION BIAS |
2 | 2 | 2 | 2 | 3 | 3 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 3 | 3 |
| B STUDY DESIGN |
2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 3 | 2 |
| C CONFOUNDERS |
2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 |
| D BLINDING |
2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 |
| E DATA COLLECTION METHOD |
1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 3 |
| F WITHDRAWALS AND DROPOUTS |
1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 1 |
| G WITHDRAWALS AND DROPOUTS |
1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| H ANALYSES |
1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total | 1 | 1 | 1 | 1 | 2 | 3 | 1 | 1 | 2 | 2 | 3 | 2 | 3 | 2 | 3 | 3 |
Note: Key answer: STRONG = 1 MODERATE = 2 WEAK = 3.
References
- 1.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica: Atenei Parmensis. 2020;91(1):157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.W.H. Organization . Vol. 182. 2020. (Coronavirus disease (COVID-19): situation report). [Google Scholar]
- 4.Worldmeter . 2020. COVID-19 CORONAVIRUS PANDEMIC. Last updated: November 30, 2020, 20:25 GMT. [Google Scholar]
- 5.Ye Q., Wang B., Mao J. Cytokine storm in COVID-19 and treatment. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin‐Loeches I., Browne P., et al. More on COVID‐19 coagulopathy in Caucasian patients. Br J Haematol. 2020 doi: 10.1111/bjh.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D., O’Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medicine T.L.R. COVID-19 transmission—up in the air. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karia R., Gupta I., Khandait H., Yadav A., Yadav A. COVID-19 and its modes of transmission. SN Compr Clin Med. 2020:1–4. doi: 10.1007/s42399-020-00498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J., Wu R., Huang H., Zheng W., Ren X., Wu N., et al. 2020. Computed tomographic imaging of 3 patients with coronavirus disease 2019 pneumonia with negative virus real-time reverse-transcription polymerase chain reaction test, clinical infectious diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins P.V., O’Donnell J.S. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion. 2006;46(10):1836–1844. doi: 10.1111/j.1537-2995.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 14.Dunne E., Qi Q.M., Shaqfeh E.S., O’Sullivan J.M., Schoen I., Ricco A.J., et al. Blood group alters platelet binding kinetics to von Willebrand factor and consequently platelet function. Blood. 2019;133(12):1371–1377. doi: 10.1182/blood-2018-06-855528. [DOI] [PubMed] [Google Scholar]
- 15.Bowen D.J. An influence of ABO blood group on the rate of proteolysis of von Willebrand factor by ADAMTS13. J Thromb Haemost. 2003;1(1):33–40. doi: 10.1046/j.1538-7836.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 16.Gill J.C., Endres-Brooks J., Bauer P.J., Marks W.J., Montgomery R.R. 1987. The effect of ABO blood group on the diagnosis of von Willebrand disease. [PubMed] [Google Scholar]
- 17.Zhao J., Yang Y., Huang H., Li D., Gu D., Lu X., et al. Relationship between the ABO blood group and the COVID-19 susceptibility. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zietz M., Zucker J., Tatonetti N.P. Associations between blood type and COVID-19 infection, intubation, and death. Nat Commun. 2020;11(1):1–6. doi: 10.1038/s41467-020-19623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J., Wang X., Chen J., Cai Y., Deng A., Yang M. Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia. Br J Haematol. 2020 doi: 10.1111/bjh.16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dzik S., Eliason K., Morris E.B., Kaufman R.M., North C.M. COVID‐19 and ABO blood groups. Transfusion. 2020 doi: 10.1111/trf.15946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armijo‐Olivo S., Stiles C.R., Hagen N.A., Biondo P.D., Cummings G.G. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18(1):12–18. doi: 10.1111/j.1365-2753.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- 22.Thomas H., Ciliska D., Dobbins M. Effective Public Health Practice Project McMaster University; Toronto: 2003. Quality assessment tool for quantitative studies. [Google Scholar]
- 23.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Zietz M., Tatonetti N.P. Testing the association between blood type and COVID-19 infection, intubation, and death. MedRxiv. 2020 doi: 10.1038/s41467-020-19623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Göker H., Karakulak E.A., Demiroğlu H., Ceylan Ç.M.A., Büyükaşik Y., Inkaya A.Ç., et al. The effects of blood group types on the risk of COVID-19 infection and its clinical outcome. Turk J Med Sci. 2020;50(4):679–683. doi: 10.3906/sag-2005-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aljanobi G.A., Alhajjaj A.H., Alkhabbaz F.L., Al-Jishi J.M. The relationship between ABO blood group type and the COVID-19 susceptibility in Qatif Central Hospital, Eastern Province, Saudi Arabia: a retrospective cohort study. Open J Intern Med. 2020;10(2):232–238. [Google Scholar]
- 28.Wu Y., Feng Z., Li P., Yu Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin Chim Acta. 2020;509:220–223. doi: 10.1016/j.cca.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leaf R.K., Al‐Samkari H., Brenner S.K., Gupta S., Leaf D.E. ABO phenotype and death in critically ill patients with COVID‐19. Br J Haematol. 2020;190(4):e204–e208. doi: 10.1111/bjh.16984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.AL-Khikani F.H.O. The role of blood group in COVID-19 infection: more information is needed. J Nat Sci Med. 2020;3(3):225. [Google Scholar]
- 31.Latz C.A., DeCarlo C., Boitano L., Png C.M., Patell R., Conrad M.F., et al. Blood type and outcomes in patients with COVID-19. Ann Hematol. 2020;99(9):2113–2118. doi: 10.1007/s00277-020-04169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hultström M., Persson B., Eriksson O., Lipcsey M., Frithiof R., Nilsson B. Blood type A associates with critical COVID-19 and death in a Swedish cohort. Crit Care. 2020;24(1):1–2. doi: 10.1186/s13054-020-03223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Khikani F.O. COVID-19 and blood type: people with which group are more vulnerable? J Med Sci Res. 2020;3(2) 158-158. [Google Scholar]
- 34.Kibler M., Dietrich L., Kanso M., Carmona A., Marchandot B., Matsushita K., et al. Risk and severity of COVID-19 and ABO blood group in transcatheter aortic valve patients. J Clin Med. 2020;9:3769. doi: 10.3390/jcm9113769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muñiz-Diaz E., Llopis J., Parra R., Roig I., Ferrer G., Grifols J., et al. Relationship between the ABO blood group and COVID-19 susceptibility, severity and mortality in two cohorts of patients. Blood Transf. 2021;19:54. doi: 10.2450/2020.0256-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solmaz İ., Araç S. ABO blood groups in COVID‐19 patients; cross‐sectional study. Int J Clin Pract. 2020 doi: 10.1111/ijcp.13927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan Q., Zhang W., Li B., Li D.-J., Zhang J., Zhao F. Association between ABO blood group system and COVID-19 susceptibility in Wuhan. Front Cell Infect Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothstein H.R., Sutton A.J., Borenstein M. 2005. Publication bias in meta-analysis, publication bias in meta-analysis: prevention, assessment and adjustments; pp. 1–7. [Google Scholar]
- 39.Borenstein M., Higgins J.P., Hedges L.V., Rothstein H.R. Basics of meta‐analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8(1):5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 40.Liu N., Zhang T., Ma L., Zhang H., Wang H., Wei W., et al. The impact of ABO blood group on COVID-19 infection risk and mortality: a systematic review and meta-analysis. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu B.-B., Gu D.-Z., Yu J.-N., Yang J., Shen W.-Q. Association between ABO blood groups and COVID-19 infection, severity and demise: a systematic review and meta-analysis, Infection. Genet Evol. 2020;84 doi: 10.1016/j.meegid.2020.104485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golinelli D., Boetto E., Maietti E., Fantini M.P. The association between ABO blood group and SARS-CoV-2 infection: a meta-analysis. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volken T., Crawford R.J., Amar S., Mosimann E., Tschaggelar A., Taleghani B.M. Blood group distribution in Switzerland-a historical comparison. Transfus Med Hemother. 2017;44(4):210–216. doi: 10.1159/000479191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320922370. 2047487320922370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pourali F., Afshari M., Alizadeh-Navaei R., Javidnia J., Moosazadeh M., Hessami A. Relationship between blood group and risk of infection and death in COVID-19: a live meta-analysis. New Microbes New Infect. 2020;37 doi: 10.1016/j.nmni.2020.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ray J.G., Schull M.J., Vermeulen M.J., Park A.L. Association between ABO and Rh blood groups and SARS-CoV-2 infection or severe COVID-19 illness: a population-based cohort study. Ann Intern Med. 2020 doi: 10.7326/M20-4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guillon P., Clément M., Sébille V., Rivain J.G., Chou C.F., Ruvoën-Clouet N., et al. Inhibition of the interaction between the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18(2):1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu O., Bayoumi N., Vickers M.A., Clark P. ABO (H) blood groups and vascular disease: a systematic review and meta‐analysis. J Thromb Haemost. 2008;6(1):62–69. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 49.Paré G., Chasman D.I., Kellogg M., Zee R.Y., Rifai N., Badola S., et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4(7) doi: 10.1371/journal.pgen.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lugus J.J., Park C., Ma Y.D., Choi K. Both primitive and definitive blood cells are derived from Flk-1+ mesoderm. Blood, J Am Soc Hematol. 2009;113(3):563–566. doi: 10.1182/blood-2008-06-162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., et al. Genomewide association study of severe COVID-19 with respiratory failure. N Engl J Med. 2020;383(16):522–534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]