Abstract
The Zonule of Zinn, or ciliary zonule, is the elaborate system of extracellular fibers that centers the lens in the eye. In humans, the fibers transmit forces that flatten the lens during the process of disaccommodation, thereby bringing distant objects into focus. Zonular fibers are composed almost entirely of 10–12 nm-wide microfibrils, of which polymerized fibrillin is the main component. The thickest fibers have a fascicular organization, where hundreds or thousands of microfibrils are gathered into micrometer-wide bundles. Many such bundles are aggregated to form a fiber. Dozens of proteins comprise the zonule. Most are derived from cells of the non-pigmented ciliary epithelium in the pars plana region, although some are probably contributed by the lens and perhaps other tissues of the anterior segment. Zonular fibers are viscoelastic cables but their component microfibrils are relatively stiff structures. Thus, the elastic properties of the fibers likely stem from lateral interactions between microfibrils. Rupture of zonular fibers and subsequent lens dislocation (ectopia lentis) can result from blunt force trauma or be a sequela of other eye diseases, notably exfoliation syndrome. Ectopia lentis is also a feature of inherited diseases caused typically by mutations in microfibril-associated genes. The resulting ocular phenotypes raise the possibility that the zonule regulates lens size and shape, globe size, and even corneal topology, in addition to its well-recognized role in accommodation.
Keywords: Microfibril, fibrillin-1, LTBP-2, proteome, elastic modulus, zonule, Zinn, ectopia lentis, luxation
1. INTRODUCTION
1.1. Hiding in plain sight
The Zonule of Zinn (known also as the ciliary zonule or the suspensory ligament of the lens) is located immediately behind the iris. Consequently, the delicate fibers of the zonule can be seen only if the lens is subluxated (as may occur, for example, in Marfan syndrome) or if a coloboma of the iris is present.
Ophthalmologists pay close attention to the zonule. For cataract surgeons in particular, the integrity of the zonule is critical; it plays a role in determining whether the procedure will be “uneventful” or “challenging”. Weakened zonular fibers may rupture during surgery, necessitating use of capsular tensioning rings to spare the fibers undue stress. If the zonule is too fragile, the intraocular lens implant or capsular tension ring may need to be secured to the wall of the eye (rather than simply inserted into the capsular bag). For these reasons, cataract surgeons examine the eye for tell-tale signs of zonular problems ahead of surgery. A shallowing of the anterior chamber, or tremors of the lens with eye movement (phacodonesis) might signify partial lens dislocation. Similarly, tremulousness of the iris (iridodonesis), or visible deepening of the space between the iris and lens may indicate that the lens has become unstable due to zonule damage. Unfortunately, problems often become apparent only after the procedure has begun when, for example, the surgeon may notice difficulty in penetrating the lens capsule with the cystotome, or decreased resistance when pulling around the anterior capsular flap during the capsulorhexis, consequences of reduced capsular tension secondary to zonular dialysis.
Under the microscope, the most notable attribute of the zonule is a tendency to hide in plain sight. Paraffin sections of the eye invariably show the lens suspended in the center of the globe, with no apparent means of support (Fig. 1A). This reflects an extreme reluctance on the part of the zonular fibers to absorb conventional histological stains. However, the use of antibodies against specific components of the zonule reveals its exquisite three-dimensional organization (Figure 1B, C). Perhaps the technical challenges in visualizing the zonule explain why this intriguing structure has not received more attention. This is unfortunate because the zonule plays an important role in ocular biology and, as a model structure, has provided insights into the biology and pathobiology of other tissues, including the aorta, skin, and lung.
Figure 1.

Hiding in plain sight. (A) Hematoxylin and eosin-stained section of an eye from a 3-week-old mouse. Note the large, centrally-located lens and the apparent absence of any suspensory system. (B, C) Slice preparation of 1 one-month-old mouse eye following incubation with anti-fibrillin-1 (green) to visualize the zonular fibers. Three dimensional reconstructions provide en face (B) or sagittal views (C) of the zonular fibers near the lens surface.
Scholarly reviews of the zonule have appeared at (very) well-spaced intervals. A number were published at the end of the nineteenth century, when the anatomy of the zonule was finally clarified (see, for example, (Merkel 1870)). In the second half of the twentieth century, two notable reviews appeared in print, the first by J. Clement McCulloch (McCulloch, 1954) and the second by Barbara Streeten (Streeten, 1982). Readers are directed towards those publications for careful appraisals of the early literature. In the decades since the publication of Dr. Streeten’s review, much has been learned about the zonule, not least the identity of its major constituent, fibrillin-1. It seems timely, therefore, to revisit the anatomy and physiology of Zinn’s Zonule.
1.2. The discovery and naming of the zonule
Kepler was the first to describe the process of accommodation accurately, but was unsure about the underlying mechanism (Kepler, 1611). He proposed that by squeezing the eye, contraction of the ciliary muscle might regulate the distance between lens and retina (Mark, 1971). An astronomer by training, and in later life a telescope designer, Kepler habitually worked with glass lenses and probably found it hard to conceive of an optical system in which lens shape might be adjusted. By the early 1700s, however, ocular anatomists were increasingly of the opinion that the role of the ciliary body might not be to change the position of the lens but rather its form (an hypothesis which was to be eventually confirmed by Thomas Young (Young, 1801)). As a result, there was heightened interest in the spatial relationships between the ciliary body, the lens, and the anterior face of the vitreous humor (the so-called anterior hyaloid). The existence of the zonule as a discrete, physical structure was not suspected until much later. The practical challenge was that many of the structures in question were transparent and prone to liquefaction in the poorly preserved, post-mortem material available for study.
In 1726, a French anatomist and surgeon, Jean Louis Petit, asserted that the lens was suspended by a membranous layer derived from the anterior hyaloid (Petit, 1726). He reached this conclusion by blowing air through a thin tube inserted into the dissected eye. In this fashion, he probed for spaces between and among the gelatinous structures. Petit described a canal that ran around the lens, visible when inflated as a long, silvered tube. Petit’s canal is now the anatomical term for the narrow space between the hyaloid and the posterior fibers of the equatorial zonule (see Fig. 2). It is arguable whether such a canal exists and even more debatable, from his description, whether that was the pocket he had filled with air.
Figure 2.
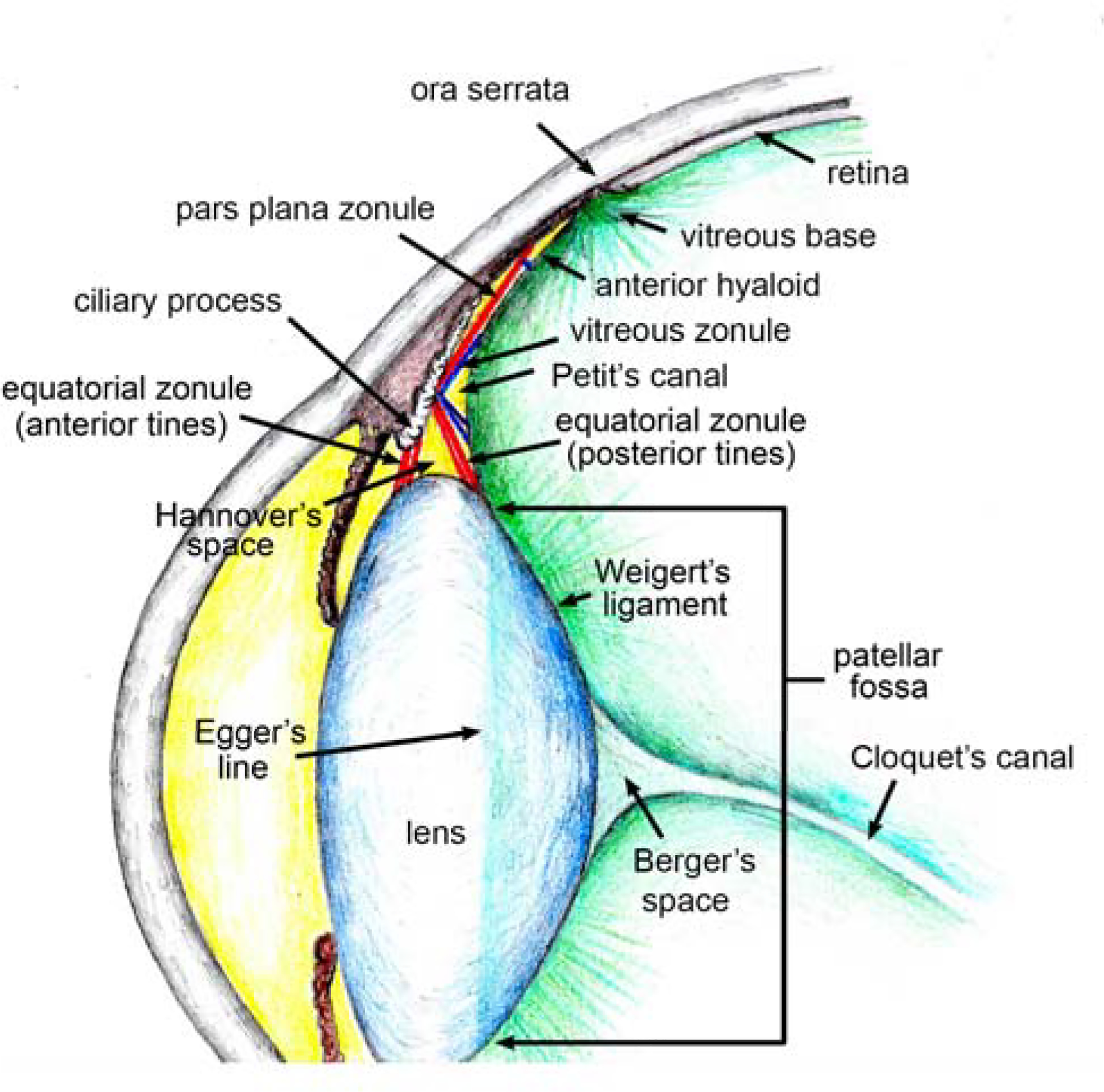
The relationship between the zonule and adjacent structures in the human eye.
A few decades later, Johann Zinn returned to the subject of the lens suspensory system, first in a brief monograph (Zinn, 1753) and then in his masterwork “Descriptio Anatomica Oculi Humani Iconibus Illustrata” (Zinn, 1755), written when he was just 29 years old. Descriptio, the first comprehensive anatomical atlas of the eye, is a beautifully illustrated and hugely influential work whose careful descriptions were based entirely on Zinn’s own observations. The book includes careful drawings of now eponymous structures, including the Zinn-Haller arterial circle (part of the blood supply to the optic nerve head) and the anulus of Zinn (the fibrous ring near the optic nerve head that serves as the posterior attachment point for the four rectus muscles). Alas, Zinn neglected to include a drawing of the suspensory system of the lens and we are left to gauge his understanding of zonule anatomy from his written description only. An English translation of the appropriate section of Descriptio is included as Supplemental Data. Interested readers can judge whether Zinn fully grasped the structure of the zonule. On balance, his description hews rather closely to that of Petit (with whose work he was familiar); that the lens is held in place through its association with one or more folds of the hyaloid membrane (this is certainly how later authorities interpreted Zinn’s description (Strickler 1889)).
Zinn, writing in Latin, used the term “zonule” to describe the suspensory system of the lens. Zonule is the diminutive form of the Latin word zona, meaning belt or girdle. Thus, a “zonule” is simply a little belt or girdle. In contemporary medicine the word is used rarely but has been retained in the field of cell biology. Specifically, it is used in reference to tight junctions (zonula occludens) or adhesion junctions (zonula adherens), components of the belt-like junctional complexes of epithelial cells. Given the etymology, it is not grammatically correct to refer to individual fibers as “zonules” (although this is commonly done). Rather, the entire belt-like fibrous system is the zonule. For Zinn, the word “zonule” was a generic anatomical term; he used it also when describing belt-like elements of the iris.
Almost a century after Zinn’s work, the idea that the lens is secured via one or more extensions of the anterior hyaloid was given further credence by Adolf Hannover (Hannover, 1845) who promulgated the notion that four folds of hyaloid membrane (rather than the two suggested by Petit and Zinn) might be required. Consequently, another dubious anatomical void, the “space of Hannover”, is shown in many text books (see also Fig. 2), usually positioned somewhat hopefully between the anterior and posterior tines of the equatorial zonule.
By the end of the nineteenth century, with improvements in histological techniques, a consensus was reached regarding the anatomical arrangement of the zonule. It had become clear that the lens was suspended not from folds of the hyaloid but rather from a system of radial fibers projecting from the ciliary body and attaching near the lens equator (Czermak, 1885). In the 1970’s, with the introduction of scanning electron microscopy (SEM), the definitive images that anatomists had sought for almost three hundred years finally arrived. The SEM micrographs revealed a zonular architecture more intricate than any the early anatomists might have imagined.
2. COMPOSITION
2.1. Glycans
In 1957, while trying to remove a longstanding vitreous hemorrhage from a patient’s eye, the Spanish ophthalmologist Joaquin Barraquer made a surprising discovery. The dilute solution of α-chymotrypsin that he had instilled into the eye with the hope of dissolving the clot had instead caused the complete dissolution of the zonule (Barraquer, 1958). Chymotrypsin was employed for many years thereafter in intracapsular cataract procedures, especially in young patients, where the zonule is typically robust and difficult to detach.
Sensitivity to chymotrypsin (a protease) implies that the zonule is a proteinaceous structure. However, early histochemical studies showed that it reacts also with the Periodic Acid-Schiff (PAS) procedure, indicating the presence of a significant polysaccharide component (Bock, 1978; Streeten, 1982; Wislocki, 1952). Using panels of lectins, several authors have identified some of the sugar moieties present in the zonule. The human (Streeten et al., 1986), rat (Chan et al., 1999) and rabbit (Rhodes, 1983) zonules exhibit complex lectin staining, reflecting the presence of N-acetylglucosamine, N-acetylgalactosamine, β-galactose, and α-fucose, among other sugars.
The positive PAS and lectin staining reactions signify the presence of proteoglycans within the zonular fibers or, more likely, at their surface. Proteoglycans can also be visualized by electron microscopy, following reaction with cuprolinic blue. Stained in this fashion, proteoglycans (visible in the micrographs as electron dense “rodlets”) can be seen projecting from the surface of the zonular fibers (Chan and Choi, 1995). The bimodal rodlet size distribution suggests the presence of two or more types of proteoglycan. Based on antibody labeling and sensitivity to chondroitinase treatment, the zonule appears to be enriched in chondroitin sulfate proteoglycan(s) (Chan and Choi, 1995). Further, hyaluronan (HA), an unbranched, glycosaminoglycan (GAG) composed of two monosaccharides (ß(1,4)-N-acetyl-D-glucosamine and ß(1,3)-D-glucuronic acid), has been localized to the distal ends of the rodlets (Chan et al., 1997). HA is found at unusually high concentration in the nearby vitreous humor (from where it was first isolated (Meyer and Palmer, 1934)).
While the molecular identity of the fiber-associated proteoglycan(s) is not known, a promising candidate is versican, a large chondroitin sulfate proteoglycan and member of the lectican family. Mass spectrometric analysis has identified both versican and HA link proteins (HAPLN1 and 3) in the zonule (De Maria et al., 2017; Eckersley et al., 2018). In other settings, link proteins mediate interactions between lecticans and HA. Moreover, versican has been shown to interact directly with fibrillin microfibrils (Isogai et al., 2002) and high molecular weight fibrillin-versican-HA complexes have been isolated from the ciliary body (Ohno-Jinno et al., 2008). Together, the data suggest a model in which zonular fibers are coated with a layer of proteoglycan. The thickness of this glycan coat is difficult to estimate. The rodlets formed after cuprolinic blue labeling are 60–170 nm long (Chan et al., 1997) but the fixation and dehydration steps required for EM processing do not preserve GAG structure adequately. In view of the large molecular masses of both versican and HA (Le Goff and Bishop, 2008), the glycan coat could extend for hundreds of nanometers from the fiber surface. Although the function of the glycan coat is not known, it may protect zonular fibers from proteases present in the ocular medium. Alternatively, it could play a role in determining fiber diameters, as suspected for collagen fibers (Moorehead et al., 2019).
2.2. Proteins
Before cataloging the proteins that are demonstrably present in the zonule, is it worth mentioning one that is conspicuously absent: elastin. Zonular fibers are elastic structures that can be stretched to almost four times their original length without breaking (Canals et al., 1996). Nevertheless, elastin, the quintessential elastic protein (and core component of “elastic fibers”), is not present in the zonule. From early on, zonular fibers were classified as “oxytalan fibers”, specifically to distinguish them from collagen or elastic fibers. Oxytalan fibers are so named because they are not hydrolyzed at low pH (oxytalan means “acid resisting”) and take up stains only when oxidized by pretreatment with peracetic acid (Fullmer and Lillie, 1958). They are commonly found in tissues under stress, such as ligaments and tendons. Unsurprisingly, the zonule stains strongly with the oxytalan reaction (Bock, 1978). Oxytalan staining is believed to reflect the presence of abundant disulfide groups. This was confirmed for the zonule when amino acid analysis identified non-collagenous acidic glycoprotein with a high cysteine content (Streeten et al., 1983).
The composition of the zonule has been studied using both immunocytochemical and mass spectrometric approaches. The former offers the advantage of exquisite spatial resolution, while the latter provides a more comprehensive and potentially more quantitative analysis. The main disadvantage of the mass spectrometric approach is that the zonule must first be isolated mechanically, raising the concern that the sample may be contaminated by proteins derived from neighboring tissues. In practice, the two approaches are complementary.
As shown in Section 3, the zonule is composed almost entirely of fibrillin-based microfibrils. Accordingly, all three fibrillins (FBN1–3) have been detected in human samples by both mass spectrometry and immunocytochemistry (Table 1). Similarly, three well-characterized microfibril-associated proteins, LTBP-2, MFAP2, and EMILIN-1, are abundant in the zonule and their existence has been verified using both techniques.
Table 1.
Known microfibril components identified in the human (H), mouse (M), bovine (B), and chick (Ch) ciliary zonule by both mass spectrometry and immunocytochemistry.
| PROTEIN | Mass spectrometry | immunocytochemistry |
|---|---|---|
| Fibrillin-1 (FBN1) | 1–3 H, 1B, | 1,4 H, 5,6M, 7B, |
| Fibrillin-2 (FBN2) | 1H, 1B | 4H, 5,6M, |
| Fibrillin-3 (FBN3) | 1H, 1B | 4H |
| Latent TGFβ-binding protein-2 (LTBP-2) | 1H, 1B | 1H, 8,9M |
| Microfibril associated protein 2 (MFAP2 aka MAGP1) | 1–3H, 1B | 1,4H, 5M, |
| Elastin microfibril interface located protein-1 (EMILIN1) | 1,3H | 1H, 10Ch |
Cain zonule (Cain et al., 2006)
Working with human and bovine samples, De Maria et al. identified hundreds of zonular proteins. The 52 proteins that were common to both samples accounted for >95% of the protein present (De Maria et al., 2017), suggesting that although the accommodative mechanism differs between the species, the composition of the zonule is conserved. In both samples, the most abundant proteins were glycoproteins. These included fibrillin-1 (FBN1), microfibril-associated protein 2 (MFAP2), latent TGFβ-binding protein-2 (LTBP-2), emilin-1 (EMIL1), hemicentin-1 (HMCN1), A disintegrin and metalloproteinase with thrombospondin domain-like-6 (ADAMTSL6), and fibrillin-2 (FBN2; see Figure 3). Based on their abundance (together they accounted for about 80% of the protein), these proteins likely constitute important structural elements. Fibrillin-1 was by far the most abundant individual component, comprising 64% of the human zonule and 76% of the bovine zonule. A number of signaling molecules were also detected, including TGFβ−2, VEGFB, and Wnt pathway components (SFRP2, Wnt7A and Wnt2B, etc.) but it remains to be determined whether the zonule is a physiological repository for such molecules. Lysyloxidase-like 1 (LOXL1), a protein with a demonstrated role in elastin maturation, was detected in the zonule of both species, being especially abundant in the bovine sample. The presence of LOXL1 is of interest because sequence variants in LOXL1 are linked to development of exfoliation syndrome (XFS) in humans (Thorleifsson et al., 2007), a condition characterized by aggregation of microfibril components (Aboobakar et al., 2017) and weakening of zonular fibers.
Figure 3.
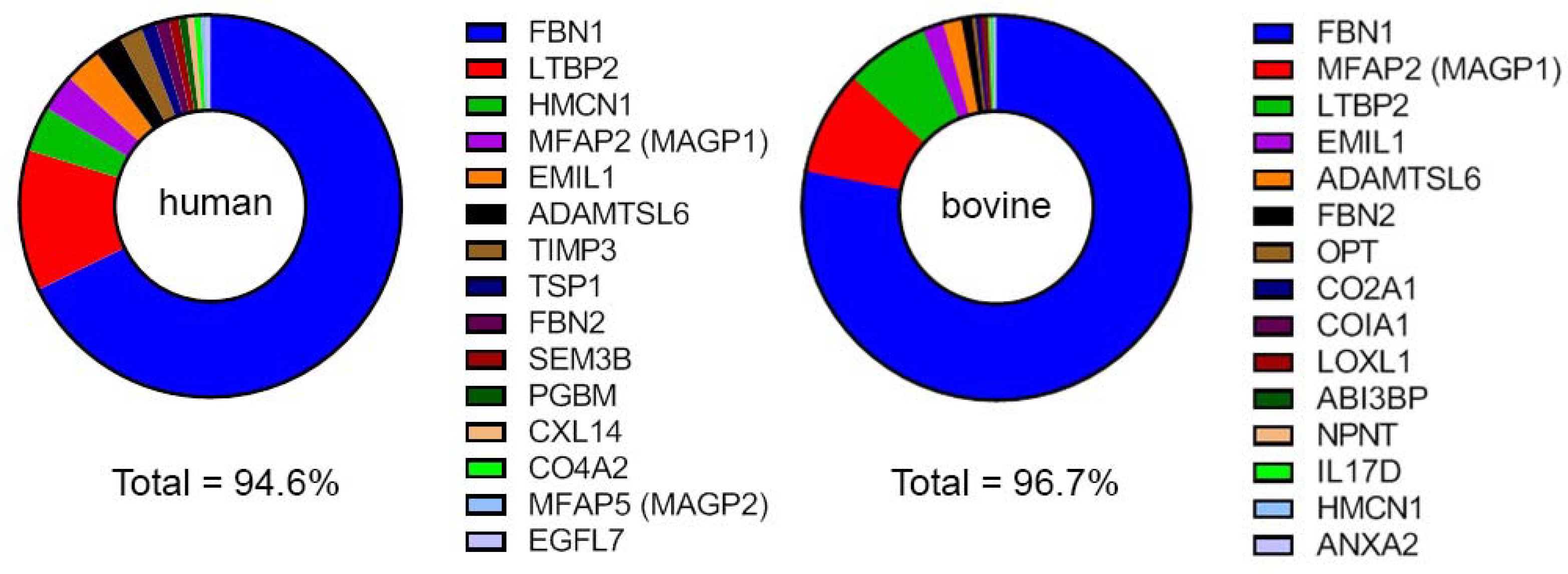
The fifteen most abundant proteins in zonular samples, as detected by mass spectrometric analysis of the human or bovine zonule (De Maria et al., 2017).
3. STRUCTURE
3.1. Architecture of the zonule
The zonule is an untidy structure, particularly in the mouse eye, where fibers of various diameters and orientations are present (Figure 4). It can be difficult to discern its overall organization but an analogy with a bridge may be helpful. Like a bridge, the zonule has a clear engineering purpose (lens centration and, in humans, accommodation) and a well-defined list of parts (see Figure 3) from which load-bearing elements, stabilizing cross struts, anchorage points, etc., are to be assembled. Like a bridge, it is erected across a sizeable physical gap (about 0.15 mm in mice (Bassnett, 2019) and 1.07 to 0.65 mm (depending on age) in the human eye (Kasthurirangan et al., 2011)) and must be maintained properly, if it is to be durable. In the following section, drawing on data from mouse and human eyes, zonular structure is considered at two levels of resolution: macroscopic organization and the substructure of the micrometer-wide fibers that comprise the zonule.
Figure 4.

Scanning electron micrograph of a portion of the ciliary zonule in the eye of a 1-month-old mouse. Scale bar = 5 μm.
3.11. Mouse zonule
In mammals, the ciliary epithelium is divided into two parts, the pars plicata region and the pars plana region. The flattened pars plana is situated between the folded pars plicata and the anterior border of the retina. In the mouse eye, the pars plicata is 0.2–0.3 mm wide (May et al., 2009) and consists of irregular, anastomosing ciliary processes with a loose, approximately radial orientation (See Figure 5). The pars plana region is only 40–50 μm wide (Napier and Kidson, 2005). Nevertheless, it is from this narrow region that most of the zonular fibers project.
Figure 5.
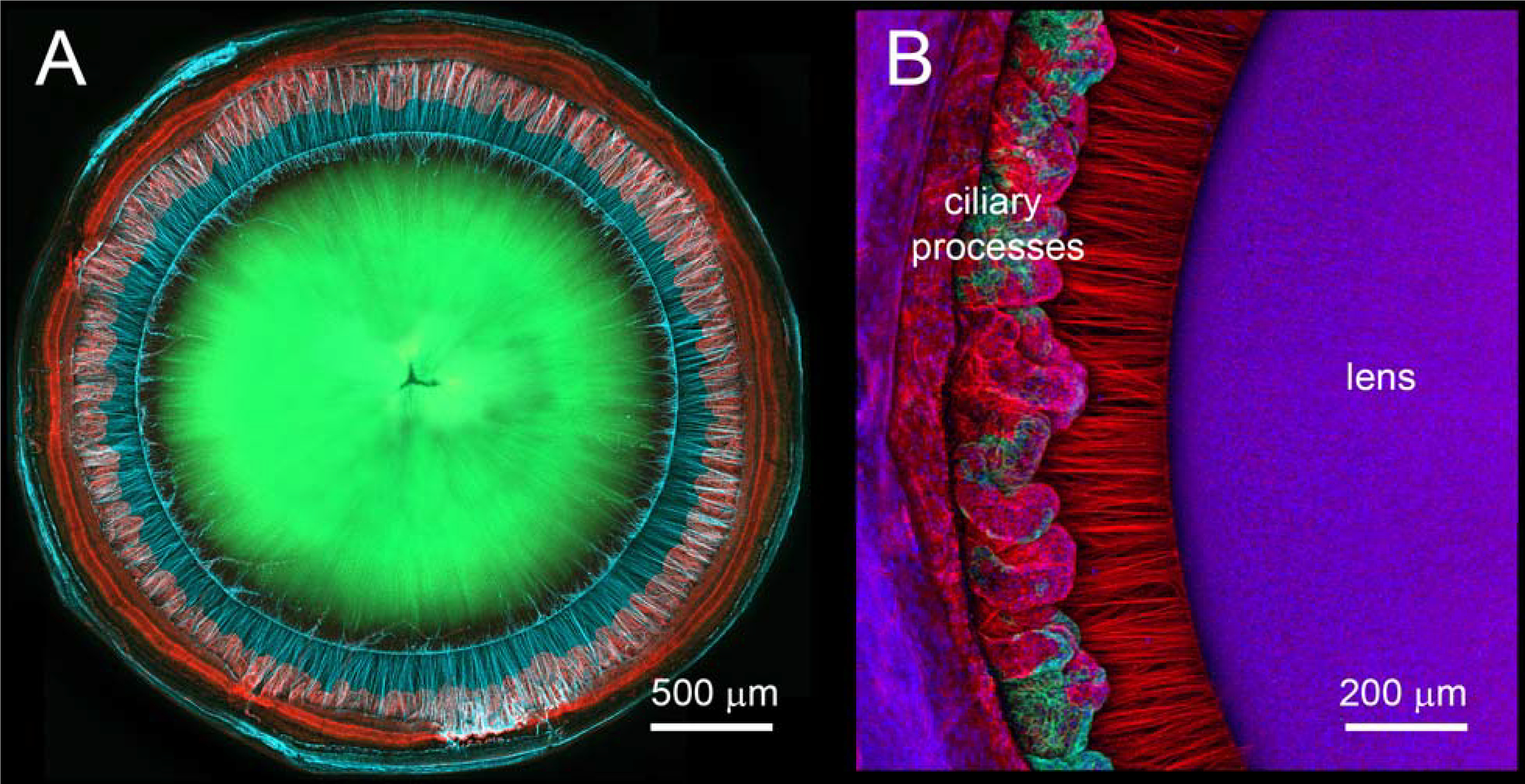
Structure of the mouse zonule. From the posterior aspect (A), the zonular fibers (light blue, here immunolabeled for fibrillin-1) project radially from the pars plana region of the ciliary epithelium to the lens (green) equator. A higher magnification image from the anterior aspect (B) after removal of the iris, shows individual zonular fibers (red, here immunolabeled for LTBP-2) and the irregular organi2) and the irregular organization of the ciliary processes. Images from (Jones et al. 2019).
The ciliary body in the mouse has a wedge-shaped cross section. It is probably supported by the underlying network of zonular fibers because in their absence, the forward tilt of the ciliary body is lost (Jones et al., 2019). While the majority of zonular fibers are anchored to the inner limiting membrane of the pars plana ciliary epithelium, some arise from the valleys between the folds in the posterior pars plicata. The fibers project directly to the lens, passing between the ciliary folds, fanning out as they approach the equatorial lens surface. The fibers diverge at an angle of about 70o (see Fig. 1C).
The zonule is usually visualized from the anterior or posterior aspect (Fig. 5). From these perspectives broken fibers are easily detected. However, to count the fibers and determine their cross-sectional shapes or areas, equatorial preparations such as shown in Fig 1B are the most useful. This orientation provides en face views of the equatorial lens surface and associated fibers (see Fig. 1B). From this perspective, it is clear that the mouse lens is suspended by tens of thousands of zonular fibers. Because the fibers branch repeatedly (Figure 6), the exact number depends on where along the length of a fiber the count is made. Measured 50 μm above the lens surface, for example, there are 25–30,000 fibers in a one-month-old mouse (Shi et al., 2020). The fibers attach to the lens capsule across an equatorial zone 330 μm wide. The attachment zone encompasses most of the germinative region of the lens (Shi et al., 2014). The posterior-most fibers attach to the lens surface above the transition zone at the epithelial margin.
Figure 6.
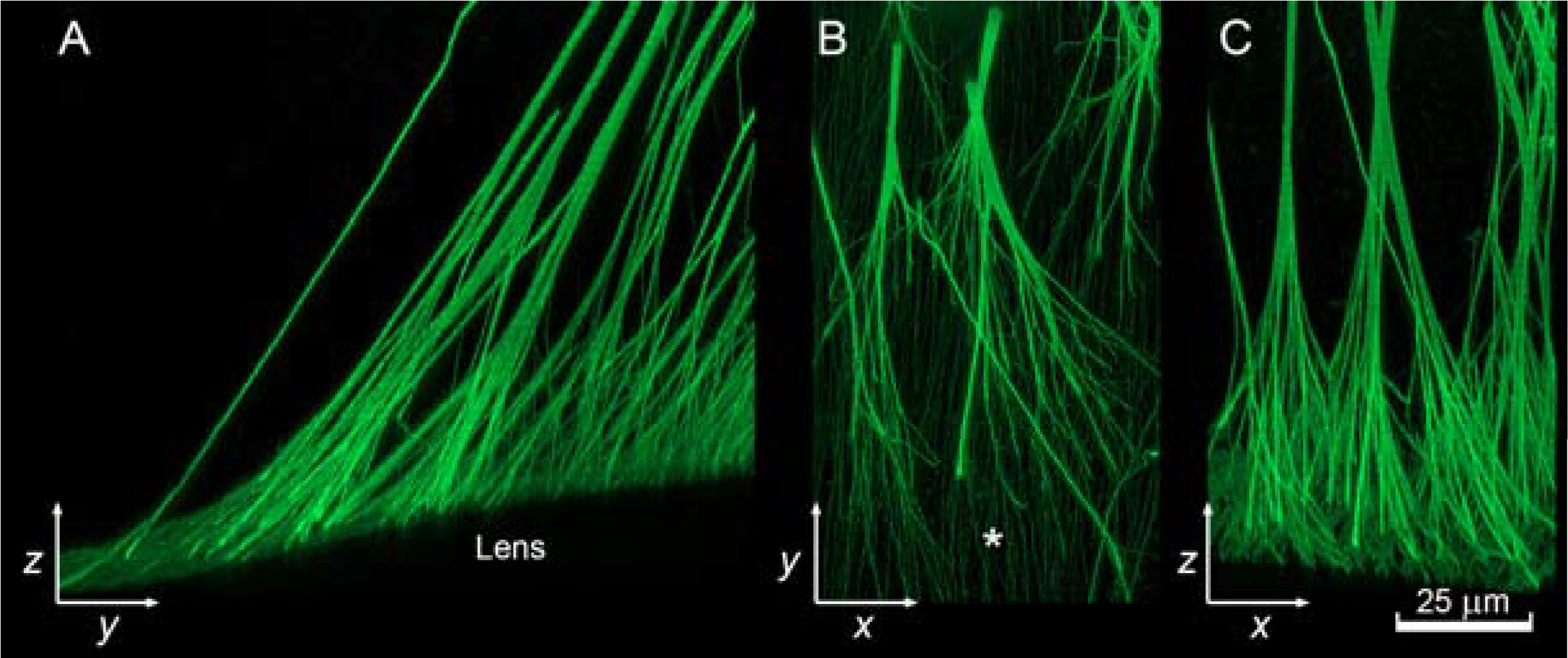
Branching patterns of zonular fibers in the mouse eye visualized in the yz (A), xy (B), and xz (C) planes. Longitudinal fibers of the fibrillar girdle indicated with * in B. Images from (Bassnett, 2019).
Branching of zonular fibers has been described (Bassnett, 2019). Cross sectional areas of fibers are conserved above and below branching points. Thus, the zonule follows the same branching behavior (known as Da Vinci’s rule) as natural branching systems like trees or rivers. However, the fibers do not all branch to the same degree or in the same pattern. Nodal points are evenly distributed along the length of equatorial fibers, whereas they cluster nearer the eye wall for anterior fibers and nearer the lens surface for posterior fibers.
The anterior-most zonular fibers appear to insert directly into the lens capsule. In contrast, the equatorial and posterior zonular fibers connect to a system of longitudinally-oriented fibers at the capsular surface (indicated by * in Fig. 6B). This layer of fibers encircles the lens and has been termed the fibrillar girdle (Shi et al., 2013). An analogous structure, the zonula lamellar, is present at the surface of the human lens (see below). As a result of branching, a single zonular fiber connects to multiple fibers in the fibrillar girdle (see Figure 6B, C).
Within the equatorial region, there are clearly demarcated areas of the lens surface to which no fibers attach. These regions cover about 30% of the available attachment zone and take the form of meandering channels (60–70 per eye). The fiber-free channels correspond to the original location of capillaries of the tunica vasculosa lentis (TVL), the temporary blood supply that nourishes the embryonic lens. The vessels appear to block the establishment of zonular connections, so that following their regression (which occurs in the second or third postnatal week (Ito and Yoshioka, 1999)) their silhouettes are imprinted in the field of fibers. These spaces constitute tunnels through the zonule and may provide a direct connection between the aqueous and vitreous humors.
3.12. Human zonule
The human lens is located in a shallow depression in the anterior face of the vitreous humor called the patellar (or hyaloid) fossa (Figure 2). Although it is not a true membrane, the face of the vitreous (often referred to as the anterior hyaloid) behaves much like one. It is firmly affixed to the posterior lens capsule at Wieger’s ligament, near the rim of the patellar fossa, approximately 1 mm behind the lens equator. The outermost edge of the attachment region, visible by slit lamp, is called Egger’s line and serves as a useful anatomical landmark. The vitreous is also attached firmly to the inner wall of the eye in the vicinity of the ora serrata (the scalloped anterior border of the retina). This attachment point is called the vitreous base.
Several anatomically distinct sets of zonular fibers are present in primate eyes (see Figure 2). There is no accepted naming convention, leading to some unfortunate confusion among authors when describing the various systems. One prominent set of fibers (here called the pars plana zonule) originates in the bays of the pars plana (McCulloch, 1954) and courses forward, running parallel to the surface of the pars plana before entering the valleys between the major and minor ciliary processes (Figure 7A). For much of their length, the fibers of the pars plana zonule are quite thin but they begin to coalesce as they approach the ciliary processes. They eventually form substantial, belt-like cables. These larger fibers pass through the valleys between the processes. To connect to the anterior and posterior surfaces of the lens, the fibers must deviate somewhat in their course (this is particularly true of the posterior fibers). This change in direction implies that the fibers are anchored to the ciliary process at the deflection point. Some authors have suggested that short “tensioning fibers” (Rohen, 1979) might secure the fibers to the walls of the ciliary processes, although this is disputed (Canals et al., 1996). In this region, referred to as the zonular plexus (Rohen, 1979), the fibers diverge, forming the anterior and posterior tines of the equatorial zonule (Figure 7B). The number of fibers in the human zonule (a few hundred (Kaczurowski, 1964)) is far fewer than in the mouse (20–30,000 (Bassnett, 2019)), but with diameters of >50 μm, the individual fibers are much more substantial. The zonular fibers insert into the lens capsule in a 1.5 mm-wide zone spanning the lens equator. This zone is characterized by the presence of a dense layer of longitudinally-oriented fibers at the capsular surface. This material is called the zonular lamellar and was first described by Berger (Berger, 1882). Some zonular fibers terminate in the center of the insertion zone, near the geometric equator of the lens. Most, however, terminate at anterior or posterior attachment zones located near the distal edges of the zonular lamellar. The zonular lamellar of the human lens is analogous to the fibrillar girdle of the mouse lens (see 3.11). However, in contrast to the mouse, some of the zonular fibers of the primate eye (particularly those of the posterior tines) appear to penetrate the lens capsule (Wheatley et al., 1995), terminating near the basal membrane surface of the lens fiber cells (Cohen, 1965; Raviola, 1971).
Figure 7.

Organization of the human zonule. (A) Fibers of the pars plana zonule originate in the bays of the pars plana close to the ora serrata and pass forward between the major and minor ciliary processes (from McCulloch, 1954; reprinted with permission from The American Ophthalmological Society). (B) The zonular fibers change direction as they pass between the processes, separating into anterior and posterior tines (from Rohen, 1979). Z, zonule, CP, ciliary processes, CB, ciliary body, L, lens, S, Sclera, and SC, Schlemm’s canal.
Additional sets of fibers connect to the anterior hyaloid (see Figure 2). One such grouping connects the ciliary processes to the anterior hyaloid adjacent to Wieger’s ligament (Bernal et al., 2006; Lutjen-Drecoll et al., 2010). Another originates at the ciliary processes and runs posteriorly along the face of the hyaloid parallel to but separated from the pars plana zonule. Finally, near the ora serrata, short fibers interlink the anterior hyaloid with the pars plana zonule. Collectively, these hyaloid-associated fibers (shown in blue in Figure 2) have been called the “vitreous zonule” (Lutjen-Drecoll et al., 2010). The vitreous zonule presumably ensures that the lens, zonule, ciliary body, and vitreous humor function as an integrated unit during accommodation. The presence of the vitreous zonule may also explain the great difficulty that early anatomists, such as Petit, Zinn, and Hannover, had in discriminating the hyaloid from the zonule (see Section 1.2).
The relative complexity of the human zonule (in comparison to the mouse zonule) and the robust nature of the zonule and its anchorage points is presumably attributable to its role in accommodation. The detailed accommodative mechanism is beyond the scope of this paper. Briefly, however, according to the widely held theory of Helmholtz (Helmholtz, 1855), the lens of the eye is held in a state of dynamic tension. During accommodation, the ciliary muscle contracts and in so doing, causes the ciliary body to move forward and inward. This translation reduces tension in the equatorial zonule, while increasing tension in the pars plana zonule. Energy stored in the elastic lens capsule molds the lens substance into a more spherical shape, causing an increase in optical power. When the ciliary muscle relaxes (during disaccommodation), energy stored in the pars plana zonule (and elastic elements of the muscle insertion) causes retraction of the ciliary body, simultaneously stretching the equatorial zonular fibers, flattening the lens and reducing its diopteric power. In contrast, the mouse eye (lacking a well-developed ciliary muscle) is not believed capable of significant accommodation and in that species the main role of the zonule is presumably lens centration.
3.2. Substructure of a zonular fiber
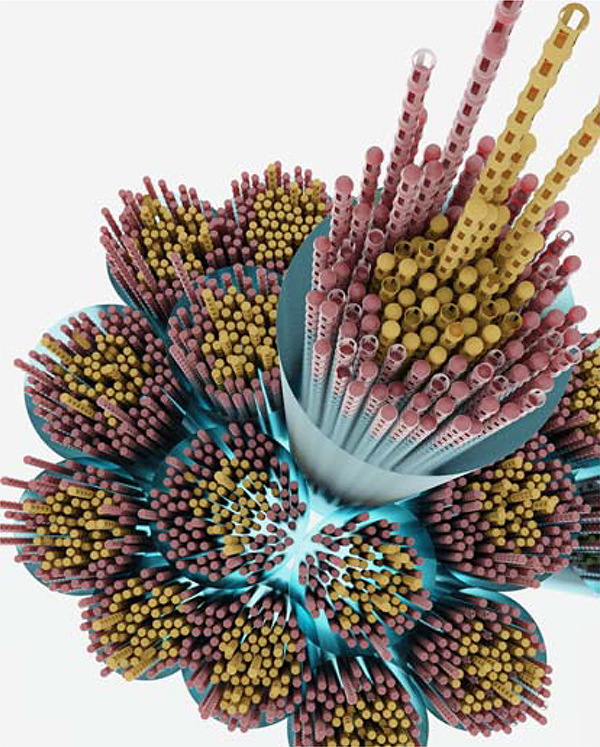
In mice, zonular fibers range in diameter from 0.1 – 4.0 μm, with a median value of about 0.5 μm (Jones et al., 2019). Generally, thinner fibers have circular cross sections, while those of larger fibers are elliptical. A zonular fiber is essentially a bundle of hundreds or thousands of individual microfibrils. The backbone of each microfibril is a fibrillin polymer, 10–12 nm in diameter and of indeterminate length. Two fibrillins, fibrillin-1 and −2, are present in abundance in the mouse zonule (Figure 3). A third fibrillin, fibrillin-3, is additionally present in the human zonule (Hubmacher et al., 2014), albeit at trace levels (De Maria et al., 2017). Work in mice suggests that zonular fibers have a coaxial internal organization (Shi et al., 2020). Thus, microfibrils in the interior of a fiber are composed predominantly of fibrillin-2, while those near its surface are enriched in fibrillin-1. This distribution may reflect the sequence in which fibrillins appear during development, with fibrillin-2 the first to be expressed (see section 4.1). While most fibers in the mouse zonule are < 1 μm in diameter, human zonular fibers are much more substantial, with diameters ranging from 20–60 μm for equatorial fibers (Flugel-Koch et al., 2016; Streeten, 1977). Evidence from serial block-face scanning EM of the bovine zonule suggests that large fibers have a fascicular organization reminiscent of skeletal muscle (Godwin et al., 2018). Thus, thousands of microfibrils are gathered into micrometer-wide bundles (as in the mouse zonule) and hundreds of such bundles are then aggregated to produce the large fibers (Figure 8). In the bovine eye, the bundles of fibers appear to be tied together by circumferential “wrapping fibers” (Godwin et al., 2018) but such an arrangement has not been reported in other species.
Figure 8.
Illustration showing the fascicular organization of a large diameter zonular fiber. Large fibers are formed from bundles of smaller diameter fibers, each of which contains many individual microfibrils. Bundles are shown surrounded by a glycan coat (blue, see section 2.1) and with a core of fibrillin-2-rich microfibrils (yellow) surrounded by a cortical layer of fibrillin-1-rich fibrils (red). Note that it has yet to be confirmed that the coaxial organization of fibrillins found in the mouse zonule is a universal feature.
Transmission EM has revealed the presence of a transverse banding pattern within zonular fibers (Figure 9A). This suggests a degree of registration between neighboring microfibrils in the fiber. Cross bridges between microfibrils have been identified in samples of bovine zonule prepared by the quick-freeze deep etch technique (Davis et al., 2002). The molecular composition of the bridges is unresolved but LTBP-2 (the second most abundant protein in the zonule proteome; Figure 3) has been localized to the base of putative bridging structures by immuno-EM (Inoue et al., 2014). The association between neighboring microfibrils is important because the viscoelastic behavior of the zonule probably depends on such interactions (see section 5).
Figure 9.
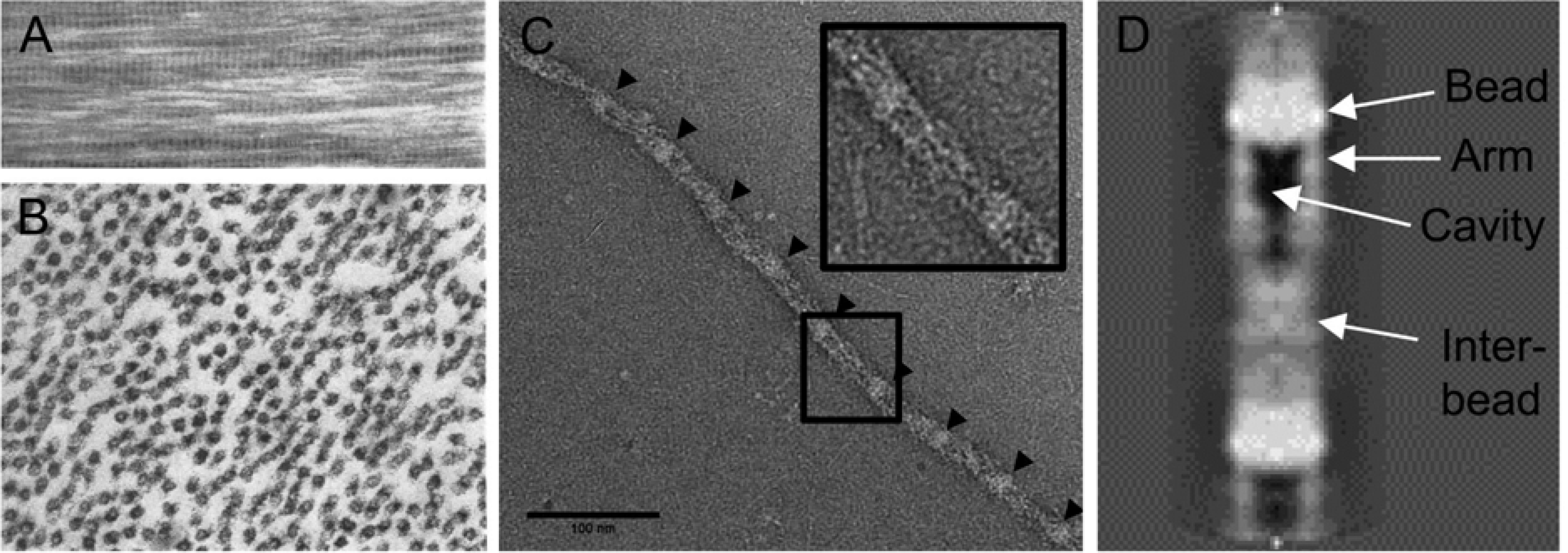
Microfibril ultrastructure. (A) Faint transverse banding is observed in longitudinally sectioned zonular fibers. (B) Transverse sections through a zonular fiber reveal the presence of many microfibrils in cross section (arrows). The microfibrils have electron-lucent cores. (C) An individual microfibril isolated from the bovine ciliary zonule (Godwin et al., 2018). Arrow heads indicate the position of “beads”. (D) High resolution reconstruction of a microfibril in the interbead region. Attributions: A and B, with permission from Raviola 1971, C and D from Godwin et al., 2018 (licensed under a Creative Commons CC-BY license).
The transverse banding pattern has a periodicity of 50–60 nm. Individual microfibrils, negatively stained and viewed by TEM, have a characteristic “beads-on-a-string” appearance, where the interbead distance is ≈56 nm. Thus, the banding pattern observed in EM images of zonular fibers probably reflects the underlying periodic structure of component microfibrils. In transverse section (Figure 9B), microfibrils generally appear as hollow structures (Raviola, 1971), reflecting the presence of a central cavity in the interbead region (Godwin et al., 2018). There is continuing uncertainty about the precise arrangement of fibrillin monomers within the repeating microfibril unit. Fibrillins are large molecules which, linearized, would extend for ≈ 150 nm. How such a molecule is accommodated in a polymer with a ≈56 nm repetitive structure is presently unclear. Mass measurements and 3D modeling suggest that eight fibrillin molecules (arranged perhaps as four dimers) contribute to each repeating unit, extending from the bead region, somewhat like staves in a barrel (Godwin et al., 2018; Sherratt et al., 1997; Wang et al., 2009). Another uncertainty is how the assemblage of microfibril-associated proteins (Table 1 and Figure 3) associate with the fibrillin polymer and each other. MAGP1 has been localized to the bead region of the microfibril by immuno-EM (Henderson et al., 1996). Similarly, LTBP-2 has been shown to bind to the N-terminus of fibrillin-1 (Hirani et al., 2007), which most models also place in bead region (Reinhardt et al., 1996).
4. SYNTHESIS, DEVELOPMENT AND AGING
4.1. Synthesis
The zonule is flanked by several tissues that could contribute to its synthesis or maintenance. These include the iris epithelium, lens epithelial and fiber cells, the inner, non-pigmented layer of the ciliary epithelium (NPCE), and the hyalocytes (Qiao et al., 2005) that populate the vitreous cortex. Messenger RNA transcripts encoding various zonular proteins have been localized to ocular tissues using in situ hybridization techniques. This approach has revealed a surprisingly complex pattern of spatial and temporal gene expression.
In mice, the first of the fibrillins to be expressed in the eye is fibrillin-2 (Shi et al., 2013), transcripts for which are already abundant at embryonic day 12.5 (E12.5) in the periocular mesenchyme and blood vessels surrounding the lens. By E16.5, Fbn2 is strongly expressed in the anterior lip of the optic cup, in tissue that will become the NPCE. However, Fbn2 expression in the NPCE begins to wane before birth and by P30 has fallen to near background levels (Jones et al., 2019). Fbn1 shows a reciprocal expression pattern, with low expression in the embryonic eye but strong and sustained postnatal expression. A similar pattern has been noted in the guinea pig eye (Hanssen et al., 2001). At birth, Fbn1 is expressed at comparable levels in equatorial lens cells and NPCE cells. However, the NPCE probably makes the more consequential contribution to the nascent zonule because lens-specific knockout of Fbn1 does not affect zonular development, whereas targeting the Fbn1 locus in NPCE results in production of fewer and thinner fibers and, ultimately, rupture of the zonule (Jones et al., 2019). Expression of Ltbp2 is restricted largely to the NPCE and does not begin until near the end of the first postnatal week, at which point LTBP-2 protein is detectable in the proximal portion of the zonular fibers and circumferential fibers spanning the tips of the ciliary processes (Shi et al., 2020). Unlike Fbn1, Fbn2, and Ltbp2, which are all strongly expressed in the NPCE, another abundant zonular protein, MFAP2 (aka MAGP1) is broadly expressed in tissues flanking the zonule (Jones et al., 2019). Finally, Adamtsl4, mutations in which cause isolated ectopia lentis in humans (see section 6.1), is expressed predominantly by cells of the peripheral lens epithelium, where it may play a role in the anchorage of zonular fibers (Collin et al., 2015). The sites of synthesis of EMILIN-1, ADAMTSL6 and other major zonule components identified in the proteome have yet to be determined. Together, the in situ hybridization data suggest that no single tissue expresses all the principal zonule genes and that production of the mature zonule requires contributions from several tissues of the posterior chamber. Further, the temporal expression sequence implies that regions of the zonule produced early in development will be relatively enriched in fibrillin-2, whereas elements produced later will be enriched in fibrillin-1 and LTBP-2. This is a plausible explanation for the coaxial fibrillin labeling pattern observed in murine zonular fibers (section 3.2 and Figure 8).
4.2. Development
Microfibrils are near ubiquitous features of the ECM throughout the body but little is known about their assembly or subsequent incorporation into the matrix. After preprocessing steps within the cell, fibrillin is secreted and assembled into microfibrils at or near the cell surface. Cell culture studies have identified a requirement for accessory proteins, such as fibronectin (Kinsey et al., 2008) or syndecan-4 (Baldwin et al., 2014) in microfibril formation. The rate of microfibril production may also be influenced by tension, as shown by experiments in which stretching of fibroblasts from the periodontal ligament (a microfibril-rich tissue) resulted in a significant increase in fibrillin incorporation into the ECM (Tsuruga et al., 2009).
Although the mature zonule bridges the circumlental space, the initial structure is assembled early in development, when the lens is still in direct contact with the inner wall of the optic cup. At that stage, the lens is enveloped by the TVL. Capillaries of the TVL are embedded in a temporary matrix at the lens surface, rich in fibrillin-2 microfibrils (Shi et al., 2013). It has been suggested that the TVL capillaries, some of which project to the adjacent wall of the optic cup, may constitute a scaffold upon which the zonule is laid down (Hubmacher et al., 2014). This is an attractive hypothesis but at present remains speculative.
The mouse eye is markedly ellipsoidal until P2 but becomes spherical shortly thereafter. This change in shape of the globe may signify the initial pressurization of the eyeball. Inflation of the eye is expected to cause the separation of the lens from the inner wall of the eye (thereby creating the circumlental space), in the process placing the fibers of the nascent zonule under tension. The ciliary epithelium is thus responsible for both the secretion of aqueous humor (to inflate the eye) and production of the zonule (the fibers of which, placed under tension, will oppose further inflation). It is possible that these two phenomena form part of a feedback mechanism helping govern globe size in the developing animal.
4.3. Aging
The anecdotal experiences of cataract surgeons would suggest that the zonule becomes more fragile with age. This is consistent with semi-quantitative measurements of elasticity which have found a linear decrease in the distance that zonular fibers can be stretched before breaking (Assia et al., 1991). Similarly, the force needed to rupture the zonule was reduced by ≈30% in zonular samples from elderly donors (Nishikawa and Okisaka, 1992). Increased fragility of the aging zonule might suggest an inability to replace worn components. There have been no direct measurements of fibrillin half-life in the eye. However, based on the ratio of carbon isotopes and the racemization rate for D-aspartate, an estimate of 70 years was obtained for the half-life of elastin (Shapiro et al., 1991). In that study, which was carried out on pulmonary tissue, the authors made the incidental finding that microfibrils (an unavoidable contaminant in the elastin preparations) have a similarly long half-life.
5. BIOMECHANICAL PROPERTIES
5.1. Elastic modulus of the zonule
The zonule is composed of radial fibers that maintain the lens in a state of dynamic tension, rather like springs on a trampoline. A number of studies have set out to measure the material properties of zonular fibers and the individual microfibrils that comprise them. In principle, such studies are straightforward. One simply has to measure the change in fiber length in response to a given stretching force (the stress) to calculate the spring constant of the zonule (units of N/m). If the proportional increase in fiber length (the strain) is determined, then the slope of the resulting stress/strain curve gives the system modulus. Further, if one can measure accurately the number and cross-sectional areas of the zonular fibers, then the elastic modulus (i.e., Young’s modulus) of the zonule can be computed (units of N/m2 or Pa). The difficulty lies in isolating and then measuring the properties of fibers that vary in diameter from 0.2 to 50 μm (depending on the species in question). Due to the challenge in handling individual fibers, investigators have resorted to measuring the properties of ensembles of fibers. Such measurements are usually made on segments of dissected eyes using a variety of ingenious stretching devices. These approaches, which necessarily include the zonule as an in-series element (along with the ciliary body, lens, and sclera) generate data that are not always straightforward to interpret. Nevertheless, estimates for the zonule modulus have been reported for several species.
Reported values for the elastic modulus of zonular fibers are in the range of 0.2–1.50 MPa (Table 2). This is comparable to the modulus of elastin-containing fibers (0.3–1.2 MPa (Green et al., 2014) but much lower that that of Type I collagen fibers (0.2–0.8 GPa (van der Rijt et al., 2006).
Table 2.
Measurements of the zonular elastic modulus
| Species | Elastic modulus | Reference |
|---|---|---|
| Human | 0.35 MPa | (Fisher, 1986) |
| 0.27–0.34 MPa | (Michael et al., 2012) | |
| 1.5 MPa | (van Alphen and Graebel, 1991) | |
| Porcine | 0.2–0.25 MPa | (Bocskai et al., 2014) |
| Bovine | 0.18–0.25 MPa | (Wright et al., 1999) |
| Mouse | 0.11–0.23 MPa | (Shi et al., 2020) |
The studies by Wright et al (Wright et al., 1999) and Shi et al (Shi et al., 2020) are of particular interest because they examine the stress/relaxation behavior of the zonular fibers. In both cases, the modulus computed immediately after loading was greater than that measured tens of seconds later. This time-dependent behavior indicates that zonular fibers are viscoelastic structures. The physical basis of viscoelastic behavior of fibrous structures is not well understood in any system. In the case of the zonule, an improved understanding of the fiber interior, the possible contribution of glycans, and the nature of the interactions between neighboring microfibrils will be required to properly model the viscoelastic behavior.
5.2. Properties of individual microfibrils
The biomechanical properties of individual microfibrils have been measured using a “molecular combing” technique, in which a drying front is allowed to pass across partially tethered microfibrils. The operant forces at the drying front can be computed and the resulting increase in interbead distance used to calculate an effective elastic modulus. Values ranging from 78–96 MPa have been reported using this approach (Sherratt et al., 2003). Note that the modulus values for individual microfibrils are almost two orders of magnitude greater than for zonular fibers (Table 2). Thus, microfibrils are much stiffer structures than the fibers they comprise. The properties of zonular fibers cannot be readily extrapolated from those of the component microfibrils and are presumably governed by interactions between microfibrils.
5.3. Insights from mouse models
The tensile properties of fibrillin-1-depleted zonules have been measured in mice (Jones et al., 2019). Surprisingly, a zonule with a relatively normal appearance is produced in the absence of its most abundant protein, fibrillin-1 (Beene et al., 2013). However, the tensile strength of the fibrillin-depleted mouse zonule is only ≈ 10% of wild type values and lens luxation occurs within a few months (Jones et al., 2019). Closer inspection of fibrillin-1 depleted zonules showed that both the number of zonular fibers and their average diameters were reduced in the absence of fibrillin-1. An ectopia lentis phenotype has also been described in mice lacking LTBP-2 (Inoue et al., 2014). In that case, biomechanical measurements indicate that the tensile strength of the zonule was reduced by approximately 50% (Shi et al., 2020). In contrast to Fbn1-null animals, the number and thickness of the zonular fibers in Ltbp2-null mice was indistinguishable from wild type, implying that LTBP-2 may have a direct mechanical role within the fiber rather than, for example, promoting microfibril synthesis. It is noteworthy that in both LTBP-2 and fibrillin-1-deficient mice, the zonule does not fail immediately. Rather, the ectopia lentis phenotype manifests in the period between 2 and 4 months of age. The eventual failure of the zonule may be due to the presence of rapid movements of the eye called saccades. During saccadic eye movement in mice, the globe can accelerate to speeds of >2500o/sec (Sakatani and Isa, 2007). The forces produced in the zonular fibers during such a movement may be sufficient to rupture the weakened mutant fibers.
6. ZONULOPATHIES & GENETICS
6.1. Ectopia lentis
In ectopia lentis, the lens becomes untethered. If the dislocated lens remains substantially within the patellar fossa (see Figure 2), it is described as subluxated. An entirely dislodged lens is said to be luxated. Usually, ectopia lentis is accompanied by frank rupture of the zonular fibers but this is not always the case. In Marfan syndrome, for example, zonular fibers often appear intact but hyperextended (Maumenee, 1981).
The lens is denser than the humors that surround it. As a result, zonular fibers in the superior quadrant presumably bear the greatest mechanical load and might be expected to break first under pathological conditions. If such were the case, ectopic lenses would be displaced inferiorly. While this is observed in conditions such as Weill-Marchesani syndrome or homocystinuria, it is demonstrably not the case for Marfan syndrome where, curiously, the lens generally dislocates in the superotemporal direction (Nelson and Maumenee, 1982).
Most cases of lens luxation are the result of direct trauma to the eye or blunt force injuries to the head. For example, in a study of 166 consecutive patients presenting at Johns Hopkins Ophthalmology Department with ectopia lentis, more than half were due to trauma (Jarrett, 1967). However, fragmentation of zonular fibers, and subsequent destabilization of the lens, is also a feature of some common eye diseases. The most notable example is exfoliation syndrome (XFS). XFS is characterized by the deposition of insoluble fibrillar aggregates on the surface of tissues throughout the anterior segment, including lens, iris, corneal endothelium, and zonule (Aboobakar et al., 2017). XFS is a major risk factor for glaucoma, due to the presumed proclivity of XFS aggregates to block the outflow pathway. Interestingly, XFS aggregates contain a number of microfibril-associated proteins (Challa and Johnson, 2018), including fibrillins, MFAP2, LTBP-1 and −2 and LOXL1 (see Table 1 and Fig. 2). While some of these components could be derived from disintegrating zonular fibers, much of the material appears to be produced locally. Electron micrographs of the equatorial lens surface, for example, show upwellings of fibrous material, emanating from small pits in the basal membranes of lens epithelial cells. XFS material penetrates through the capsular matrix and accumulates on the lens surface (Ashton et al., 1965). It has been proposed that production of XFS material beneath the inner limiting membrane of the NPCE and the equatorial lens capsule destabilizes the two basement membranes, leading to detachment of the zonular fibers and lens subluxation (Schlotzer-Schrehardt and Naumann, 1994).
6.11. Heritable forms
Ectopia lentis is a key feature of several inherited conditions. It is sufficiently common in Marfan syndrome, for example, to be included as one of two cardinal clinical features in the disease nosology (the other being aortic root aneurysm [Loeys et al., 2010]). Most cases of inherited ectopia lentis are attributable to mutations in a relatively restricted set of genes (see Table 3). Of note, in a retrospective population study of congenital ectopia lentis in Denmark, almost one third of cases were of unknown etiology (Fuchs and Rosenberg, 1998). Of the remainder, 68% of patients were diagnosed with Marfan Syndrome, 21% had ectopia lentis et pupillae, 8% had ectopia lentis, and 1% had homocystinuria. Sulphite oxidase deficiency and Weill-Marchesani syndrome each accounted for 0.7%.
Table 3.
Partial listing of inherited conditions leading to ectopia lentis
| Condition | GENE/protein | Inheritance | OMIM # | Remarks |
|---|---|---|---|---|
| Conditions with systemic associations | ||||
| Marfan Syndrome (MFS) | FBN1/Fibrillin-1 | AD | 154700 | Lens is usually dislocated in the superior temporal direction |
| Homocystinuria | CBS/cystathionine β synthase | AR | 236200 | Lens usually dislocated toward inferior nasal quadrant |
| Weill-Marchesani syndrome (WMS) | Type 1 ADAMTS10 Type 2 FBN1 Type 3 LTBP2 Type 4 ADAMTS17 |
AR AD AR AR |
277600 608328 614819 613195 |
WMS Types1–4 are considered clinically indistinguishable but subtle genotype/phenotype may emerge as more samples are examined |
| Isolated Sulfite Oxidase Deficiency (ISOD) | SUOX/Sulfite Oxidase | AR | 272300 | |
| Molybdenum Cofactor Deficiency (MOCOD) | MOCS1 | AR | 252150 | Sulfite oxidase utilizes molybdenum cofactor. Consequently, MOCOD tends to phenocopy ISOD. |
| Knobloch Syndrome (KNO) | KNO1: COL18A1 KNO2 KNO3 |
AR | 267750 | Genes underlying KNO2 and 3 have not been identified. |
| Traboulsi Syndrome | ASPH/Aspartate β hydroxylase | AR | 601552 | ASPH may be involved in hydroxylation of calcium binding-EGF domains in fibrillin-1(Siggs et al., 2019) |
| Conditions with ocular associations only | ||||
| Isolated Ectopia Lentis 2 (ECTOL2) | ADAMTSL4 | AR | 225100 | ECTOL1, caused by a dominant mutation in FBN1, is now more often viewed as a mild form of MFS |
| Ectopia Lentis et Pupillae | ADAMTSL4 | AR | 225200 | The lens and the pupil are displaced (usually in opposite directions) |
| Microspherophakia (MSPKA) | LTBP2 | AR | 251750 | Microspherophakia present at birth, whereas ectopia lentis usually develops later |
6.2. Microspherophakia.
Microspherophakia is a relatively rare condition in which the lens is smaller and more spherical than usual. Because the radius of curvature of the lens surfaces is reduced, patients with microspherophakia are usually highly myopic. It is typically a congenital condition with an autosomal recessive inheritance pattern. It can occur in isolation (Ben Yahia et al., 2009; Kumar et al., 2010) but is more often one of a constellation of ocular symptoms in syndromic disorders such as Marfan Syndrome or Weill-Marchesani Syndrome.
The undersized lens is probably due not to an autonomous lens growth defect but rather secondary to structural problems in the zonular system. Genes implicated in microspherophakia (such as LTBP2 and FBN1) are expressed strongly in the NPCE but only weakly or not at all in the lens. FBN1 and LTBP2 encode the two major components of the zonule (Fig. 3). In their absence, its tensile strength is measurably reduced (Jones et al., 2019; Shi et al., 2020), at least in mouse models. A failure to place the lens under appropriate tension may be the direct cause of the microspherophakia phenotype. As part of the normal emmetropization process in humans, the lens changes shape significantly during the first few years of life, from a relatively spherical structure to the oblate spheroid characteristic of the adult lens. Forces applied via the zonule may play an important role in specifying the size and shape of the lens during this critical early period. Thus, mutations in zonular proteins may manifest as a lens geometry more typical of the fetal period.
6.3. Long Anterior Zonule Trait
The anterior tines of the equatorial zonule usually attach to the capsule just above the lens equator. Consequently, most of the anterior surface of the lens is free from zonular fibers. However, in some people, zonular fibers are unusually long, projecting far beyond their usual insertion point and often becoming coated with pigment (Sturrock and Tripathi, 1976). This condition has been termed the long anterior zonule trait (LAZ). It has an estimated prevalence of about 2%. Most cases are idiopathic but a subset of individuals with the LAZ trait carry a serine to arginine (S163R) substitution in the complement 1q tumor necrosis factor-related protein 5 (C1QTNF5) gene. This mutation is associated with late-onset retinal degeneration (L-ORD) (Ayyagari et al., 2005). For people with the LAZ trait who do not carry the S163R mutation, a statistically significant association with mildly elevated IOP has been reported (Roberts et al., 2018). The mechanistic basis of this association is currently unknown.
6.4. Animal models
Several thousand mutations have been implicated in the development of human Marfan syndrome (http://www.umd.be/FBN1/) and a range of animal models have been developed to gain insight into the disease mechanism. An autosomal dominant form of bovine Marfan syndrome exhibits many of the ocular symptoms of human Marfan syndrome, including ectopia lentis and megaloglobus (Pessier and Potter, 1996). Interestingly, mice carrying missense mutations in Fbn1 develop aortic dilatation but not ocular disease (Sakai et al., 2016). Mice in which Fbn1 is knocked out in the germline generally die from aortic aneurysm in the first few weeks of life. To circumvent this problem, the Cre-Lox technique was used to target the Fbn1 locus specifically in the NPCE layer of the eye (Jones et al., 2019). Absence of fibrillin-1 from the zonule was associated with a reduction in the number of fibers and their diameters, loss of zonular integrity, and lens luxation by 8 weeks of age. At later time points, a megaloglobus phenotype developed, reminiscent of the human and bovine phenotypes.
Certain types of dog (particularly terriers and related breeds) are prone to lens luxation, which often occurs in association with primary glaucoma (Curtis, 1990). Three independent mutations in ADAMTS17 have been linked to this condition (Farias et al., 2010; Forman et al., 2015; Oliver et al., 2018). In humans, mutations in ADAMTS17 are responsible for WMS 4 (see Table 3) where, in addition to ectopia lentis and microspherophakia, the phenotype includes short stature. It has been suggested that selective breeding for small body size in certain dog breeds may have inadvertently selected for ADAMTS17 mutations, leading to their surprisingly high prevalence in the general dog population (Jeanes et al., 2019).
Four genes (FBN1, LTBP2, ADAMTS10, ADAMTS17) are associated with the development of WMS in humans (WMS1–4). The four WMS subtypes are considered to be clinically indistinguishable, suggesting that the four genes may function in a common pathway. While fibrillin-1 and LTBP-2 are major components of the zonule, little is known about the roles of the two metalloproteinases, ADAMTS10 and ADAMTS17, in the zonule or elsewehere. In particular, their physiological substrates are not well defined. No ocular phenotype has been reported for Adamts17-null mice (Oichi et al., 2019), although the mice exhibit some of the musculoskeletal and thick skin features of WMS as well as showing increased incorporation of fibrillin-2 into the ECM. Interestingly, Adamts10-null mice do not develop ectopia lentis, although this a characteristic feature of human WMS. In fact, the zonular fibers are thicker than usual in the knockout mice and show increased amounts of fibrillin-2 (Mularczyk et al., 2018; Wang et al., 2019). Fibrillin-2 serves as a substrate for ADAMTS10 (Wang et al., 2019), at least in vitro, so the increased fibrillin-2 signal in the eyes of knockout mice might indicate that ADAMTS10 normally removes fibrillin-2 in the course of ocular development. Alternatively, ADAMTS10 may promote microfibril production independent of its proteolytic activity.
LTBP2 null mutations in humans are associated with WMS3 and isolated microspherophakia (Table 3). As with human patients, germline inactivation of Ltbp2 in mice results in ectopia lentis (Inoue et al., 2014). Initially, the LTBP-2 depleted zonule is morphologically indistinguishable from wild type but the tensile strength of the zonular fibers is reduced and ectopia lentis develops by four months of age (insert Shi et al). In humans, mutations in LTBP2 often results in microspherophakia but this has not been observed in mice. Similarly, mutations in LTBP2 are linked to elevated IOP and primary congenital glaucoma in humans, but no evidence of raised IOP or glaucomatous damage to the optic nerve head has been reported in the knockout mice (Inoue et al., 2014).
Human mutations in ADAMTSL4 underlie isolated ectopia lentis 2 and ectopia lentis et pupillae. In mice, Adamtsl4 is expressed strongly by the equatorial lens epithelium (Collin et al., 2015). A nonsense mutation in Adamtsl4 was identified after chemical mutagenesis with N-ethyl-N-nitrosourea. The mutant mice exhibited a progressive ectopia lentis phenotype and dedifferentiation of RPE in the inferior region of the eye. The pupils were correctly positioned (in contrast to human patients with ectopia lentis et pupillae, where the lens and pupil are usually shifted in different directions) but a mild increase in axial length (similar to that seen in mice deficient in fibrillin-1 (Jones et al., 2019)) was recorded.
Homocystinuria is an inherited disorder of methionine metabolism that results in increased levels of homocysteine and methionine in the blood and urine. A deficiency in cystathionine β synthase (CBS) is the most common cause of the condition. Mouse models of homocystinuria have been developed that replicate many of the features of the human disease, including damage to the ciliary zonule (Majtan et al., 2018). Enzyme replacement strategies have recently been shown to prevent or correct clinical symptoms of CBS deficiency and hold promise for preventing lens luxation in this disease (Bublil and Majtan, 2020).
7. SUMMARY AND FUTURE DIRECTIONS
Syndromic conditions that negatively impact the zonule often affect the cardiovascular and musculoskeletal systems, where microfibrils play similarly critical roles. An armamentarium of mouse models is available for the study of systemic microfibril disease. As our ability to visualize the mouse zonule and measure its mechanical properties improves, it should be possible to exploit this rich genetic resource. This approach will facilitate structure-function studies on microfibrils and a clearer understanding of the contribution of microfibril-associated proteins to the overall properties of the fibers. Mouse models have already proved useful. Mice deficient in fibrillin-1 or LTBP-2 develop ectopia lentis at predictable ages and represent excellent models in which to test therapies to preserve or restore the zonule. Development of enzyme replacement strategies for the treatment of homocystinuria constitute an encouraging template for such studies (Majtan et al., 2018).
Much remains to be learned about zonule structure, particularly the nature of lateral interactions between microfibrils. The existence of cross-bridging structures has yet to be confirmed and their molecular identity remains elusive. Preliminary data suggest that, in mice at least, the composition of the zonular fibers is not uniform. It varies from fiber-to-fiber, along the length of a fiber, from the surface to the core of a fiber, and even with radial position. The functional consequence (if any) of this intrinsic heterogeneity is not yet understood.
Zonular fibers exert a tensile force on the lens equator and the ciliary epithelium. In the human eye, such forces presumably fluctuate during accommodation. It is tempting to speculate that the tissues at either end of the zonule might sense and respond to such fluctuations. In vitro studies of lens have shown that epithelial cell proliferation is sensitive to zonular tension (Kumar et al., 2019), perhaps explaining why microspherophakia is commonly observed in syndromes that affect zonular integrity (Table 3). Flow of water through the internal circulation system of the lens also appears to be sensitive to zonular tension, via activation of pressure-sensitive TRPV channels (Chen et al., 2019). It has been proposed that the circulation system helps establish the index gradient of the lens (Donaldson et al., 2017). If so, then the zonule has a role in the correction of spherical aberration. Whether the tissue at the other end of the zonule, the ciliary epithelium, is similarly responsive to zonular tension, modulating the rate of aqueous humor secretion accordingly, is currently unknown.
Tensile forces in the zonule oppose the force on the eye wall exerted by the IOP. It is noteworthy that in the eye the most characteristic histopathological feature of Marfan Syndrome (a condition in which the zonule is weakened) is megaloglobus (Maumenee, 1981). Microfibrils are distributed throughout the choroid, sclera, and cornea. It could be argued that megaloglobus is the result of generalized weakening of the eye wall due to depletion of microfibrils, rather than a specific defect in the zonule. Experiments in mice, however, imply that zonular weakening may be sufficient to cause globe enlargement. In those studies, conditional deletion of Fbn1 specifically in the NPCE was associated with a significant increase in globe size (Jones et al., 2019). In human Marfan patients, zonular fibers in the inferior nasal quadrant are most affected. Analysis of corneal astigmatism in such patients suggest that the steepest axis of corneal curvature is aligned with the lens displacement axis (Maumenee, 1981). Together, these data imply that Zinn’s zonule, in addition to centering the lens on the visual axis and transmitting the forces required for accommodation, may indirectly influence the external dimensions of the eye and even the sphericity of the corneal surface.
Supplementary Material
8. ACKNOWLEDGEMENTS
This work was supported by NIH R01 EY024607, P30 EY002687, the Marfan Foundation, and an unrestricted grant to the Department of Ophthalmology and Visual Sciences at Washington University from Research to Prevent Blindness. The author is especially grateful to Ian McNeely, of the Department of Classics at Washington University for his generous help in translating portions of Zinn’s Descriptio Anatomica Oculi Humani (see Supplemental Data) and to Luka Bassnett and George Harocopos for their critical reading of the manuscript.
9. REFERENCES
- Aboobakar IF, Johnson WM, Stamer WD, Hauser MA, Allingham RR, 2017. Major review: Exfoliation syndrome; advances in disease genetics, molecular biology, and epidemiology. Exp Eye Res 154, 88–103. [DOI] [PubMed] [Google Scholar]
- Ashton N, Shakib M, Collyer R, Blach R, 1965. Electron Microscopic Study of Pseudo-Exfoliation of the Lens Capsule. I. Lens Capsule and Zonular Fibers. Invest Ophthalmol 4, 141–153. [PubMed] [Google Scholar]
- Assia EI, Apple DJ, Morgan RC, Legler UF, Brown SJ, 1991. The relationship between the stretching capability of the anterior capsule and zonules. Invest Ophthalmol Vis Sci 32, 2835–2839. [PubMed] [Google Scholar]
- Ayyagari R, Mandal MN, Karoukis AJ, Chen L, McLaren NC, Lichter M, Wong DT, Hitchcock PF, Caruso RC, Moroi SE, Maumenee IH, Sieving PA, 2005. Late-onset macular degeneration and long anterior lens zonules result from a CTRP5 gene mutation. Invest Ophthalmol Vis Sci 46, 3363–3371. [DOI] [PubMed] [Google Scholar]
- Baldwin AK, Cain SA, Lennon R, Godwin A, Merry CL, Kielty CM, 2014. Epithelial-mesenchymal status influences how cells deposit fibrillin microfibrils. J Cell Sci 127, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraquer J, 1958. Enzymatic zonulolysis: Contribution to the surgery of the crystalline lens (preliminary note). Acta Ophthalmologica 36, 803–806. [Google Scholar]
- Bassnett S, 2019. A method for preserving and visualizing the three-dimensional structure of the mouse zonule. Exp Eye Res 185, 107685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beene LC, Wang LW, Hubmacher D, Keene DR, Reinhardt DP, Annis DS, Mosher DF, Mecham RP, Traboulsi EI, Apte SS, 2013. Nonselective assembly of fibrillin 1 and fibrillin 2 in the rodent ocular zonule and in cultured cells: implications for Marfan syndrome. Invest Ophthalmol Vis Sci 54, 8337–8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Yahia S, Ouechtati F, Jelliti B, Nouira S, Chakroun S, Abdelhak S, Khairallah M, 2009. Clinical and genetic investigation of isolated microspherophakia in a consanguineous Tunisian family. J Hum Genet 54, 550–553. [DOI] [PubMed] [Google Scholar]
- Berger E, 1882. Beiträge zur Anatomie der Zonula Zinnii. Albrecht von Graefes Archiv für Ophthalmologie 28, 28–62. [Google Scholar]
- Bernal A, Parel JM, Manns F, 2006. Evidence for posterior zonular fiber attachment on the anterior hyaloid membrane. Invest Ophthalmol Vis Sci 47, 4708–4713. [DOI] [PubMed] [Google Scholar]
- Bock P, 1978. The distribution of disulfide-groups in Descemet’s membrane, lens capsule, and zonular fibers. Acta Histochem 63, 127–136. [DOI] [PubMed] [Google Scholar]
- Bocskai ZI, Sandor GL, Kiss Z, Bojtar I, Nagy ZZ, 2014. Evaluation of the mechanical behaviour and estimation of the elastic properties of porcine zonular fibres. J Biomech 47, 3264–3271. [DOI] [PubMed] [Google Scholar]
- Bressan GM, Daga-Gordini D, Colombatti A, Castellani I, Marigo V, Volpin D, 1993. Emilin, a component of elastic fibers preferentially located at the elastin-microfibrils interface. J Cell Biol 121, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublil EM, Majtan T, 2020. Classical homocystinuria: From cystathionine beta-synthase deficiency to novel enzyme therapies. Biochimie 173, 48–56. [DOI] [PubMed] [Google Scholar]
- Cain SA, Morgan A, Sherratt MJ, Ball SG, Shuttleworth CA, Kielty CM, 2006. Proteomic analysis of fibrillin-rich microfibrils. Proteomics 6, 111–122. [DOI] [PubMed] [Google Scholar]
- Canals M, Costa-Vila J, Potau JM, Merindano MD, Ruano D, 1996. Scanning electron microscopy of the human zonule of the lens (Zonula ciliaris). Acta Anat (Basel) 157, 309–314. [DOI] [PubMed] [Google Scholar]
- Challa P, Johnson WM, 2018. Composition of Exfoliation Material. J Glaucoma 27 Suppl 1, S29–S31. [DOI] [PubMed] [Google Scholar]
- Chan FL, Choi HL, 1995. Proteoglycans associated with the ciliary zonule of the rat eye: a histochemical and immunocytochemical study. Histochem Cell Biol 104, 369–381. [DOI] [PubMed] [Google Scholar]
- Chan FL, Choi HL, Underhill CB, 1997. Hyaluronan and chondroitin sulfate proteoglycans are colocalized to the ciliary zonule of the rat eye: a histochemical and immunocytochemical study. Histochem Cell Biol 107, 289–301. [DOI] [PubMed] [Google Scholar]
- Chan FL, Poon HK, Huang Y, Choi HL, 1999. Glycoconjugates of the rat ciliary body epithelium: a lectin histochemical and protein blotting study. Histochem J 31, 95–107. [DOI] [PubMed] [Google Scholar]
- Chen Y, Gao J, Li L, Sellitto C, Mathias RT, Donaldson PJ, White TW, 2019. The Ciliary Muscle and Zonules of Zinn Modulate Lens Intracellular Hydrostatic Pressure Through Transient Receptor Potential Vanilloid Channels. Invest Ophthalmol Vis Sci 60, 4416–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI, 1965. The Electron Microscopy of the Normal Human Lens. Invest Ophthalmol 4, 433–446. [PubMed] [Google Scholar]
- Collin GB, Hubmacher D, Charette JR, Hicks WL, Stone L, Yu M, Naggert JK, Krebs MP, Peachey NS, Apte SS, Nishina PM, 2015. Disruption of murine Adamtsl4 results in zonular fiber detachment from the lens and in retinal pigment epithelium dedifferentiation. Hum Mol Genet 24, 6958–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R, 1990. Lens luxation in the dog and cat. Vet Clin North Am Small Anim Pract 20, 755–773. [DOI] [PubMed] [Google Scholar]
- Czermak W, 1885. Zur zonulafrage Graefe’s Archive for Clinical and Experimental Ophthalmology 31, 79–138. [Google Scholar]
- Davis EC, Roth RA, Heuser JE, Mecham RP, 2002. Ultrastructural properties of ciliary zonule microfibrils. J Struct Biol 139, 65–75. [DOI] [PubMed] [Google Scholar]
- De Maria A, Wilmarth PA, David LL, Bassnett S, 2017. Proteomic Analysis of the Bovine and Human Ciliary Zonule. Invest Ophthalmol Vis Sci 58, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson PJ, Grey AC, Maceo Heilman B, Lim JC, Vaghefi E, 2017. The physiological optics of the lens. Prog Retin Eye Res 56, e1–e24. [DOI] [PubMed] [Google Scholar]
- Eckersley A, Mellody KT, Pilkington S, Griffiths CEM, Watson REB, O’Cualain R, Baldock C, Knight D, Sherratt MJ, 2018. Structural and compositional diversity of fibrillin microfibrils in human tissues. J Biol Chem 293, 5117–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias FH, Johnson GS, Taylor JF, Giuliano E, Katz ML, Sanders DN, Schnabel RD, McKay SD, Khan S, Gharahkhani P, O’Leary CA, Pettitt L, Forman OP, Boursnell M, McLaughlin B, Ahonen S, Lohi H, Hernandez-Merino E, Gould DJ, Sargan DR, Mellersh C, 2010. An ADAMTS17 splice donor site mutation in dogs with primary lens luxation. Invest Ophthalmol Vis Sci 51, 4716–4721. [DOI] [PubMed] [Google Scholar]
- Fisher RF, 1986. The ciliary body in accommodation. Trans Ophthalmol Soc U K 105 ( Pt 2), 208–219. [PubMed] [Google Scholar]
- Flugel-Koch CM, Croft MA, Kaufman PL, Lutjen-Drecoll E, 2016. Anteriorly located zonular fibres as a tool for fine regulation in accommodation. Ophthalmic Physiol Opt 36, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman OP, Pettitt L, Komaromy AM, Bedford P, Mellersh C, 2015. A Novel Genome-Wide Association Study Approach Using Genotyping by Exome Sequencing Leads to the Identification of a Primary Open Angle Glaucoma Associated Inversion Disrupting ADAMTS17. PLoS One 10, e0143546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J, Rosenberg T, 1998. Congenital ectopia lentis. A Danish national survey. Acta Ophthalmol Scand 76, 20–26. [DOI] [PubMed] [Google Scholar]
- Fullmer HM, Lillie RD, 1958. The oxytalan fiber: a previously undescribed connective tissue fiber. J Histochem Cytochem 6, 425–430. [DOI] [PubMed] [Google Scholar]
- Godwin ARF, Starborg T, Smith DJ, Sherratt MJ, Roseman AM, Baldock C, 2018. Multiscale Imaging Reveals the Hierarchical Organization of Fibrillin Microfibrils. J Mol Biol 430, 4142–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EM, Mansfield JC, Bell JS, Winlove CP, 2014. The structure and micromechanics of elastic tissue. Interface Focus 4, 20130058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannover A, 1845. Entdeckung des Baues des Glaskorpers. Archiv für Anatomie, Physiologie und Wissenschaftliche Medicin, 467–477. [Google Scholar]
- Hanssen E, Franc S, Garrone R, 2001. Synthesis and structural organization of zonular fibers during development and aging. Matrix Biol 20, 77–85. [DOI] [PubMed] [Google Scholar]
- Helmholtz H, 1855. Ueber die accommodation des auges. Archiv für Ophthalmologie 1, 1–74. [Google Scholar]
- Henderson M, Polewski R, Fanning JC, Gibson MA, 1996. Microfibril-associated glycoprotein-1 (MAGP-1) is specifically located on the beads of the beaded-filament structure for fibrillin-containing microfibrils as visualized by the rotary shadowing technique. J Histochem Cytochem 44, 1389–1397. [DOI] [PubMed] [Google Scholar]
- Hirani R, Hanssen E, Gibson MA, 2007. LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol 26, 213–223. [DOI] [PubMed] [Google Scholar]
- Hubmacher D, Reinhardt DP, Plesec T, Schenke-Layland K, Apte SS, 2014. Human eye development is characterized by coordinated expression of fibrillin isoforms. Invest Ophthalmol Vis Sci 55, 7934–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Ohbayashi T, Fujikawa Y, Yoshida H, Akama TO, Noda K, Horiguchi M, Kameyama K, Hata Y, Takahashi K, Kusumoto K, Nakamura T, 2014. Latent TGF-beta binding protein-2 is essential for the development of ciliary zonule microfibrils. Hum Mol Genet 23, 5672–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai Z, Aspberg A, Keene DR, Ono RN, Reinhardt DP, Sakai LY, 2002. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J Biol Chem 277, 4565–4572. [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshioka M, 1999. Regression of the hyaloid vessels and pupillary membrane of the mouse. Anat Embryol (Berl) 200, 403–411. [DOI] [PubMed] [Google Scholar]
- Jarrett WH II, 1967. Dislocation of the lens. A study of 166 hospitalized cases. Arch Ophthalmol 78, 289–296. [DOI] [PubMed] [Google Scholar]
- Jeanes EC, Oliver JAC, Ricketts SL, Gould DJ, Mellersh CS, 2019. Glaucoma-causing ADAMTS17 mutations are also reproducibly associated with height in two domestic dog breeds: selection for short stature may have contributed to increased prevalence of glaucoma. Canine Genet Epidemiol 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Rodriguez J, Bassnett S, 2019. Targeted deletion of fibrillin-1 in the mouse eye results in ectopia lentis and other ocular phenotypes associated with Marfan syndrome. Dis Model Mech 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczurowski MI, 1964. Zonular Fibers of the Human Eye. Am J Ophthalmol 58, 1030–1047. [DOI] [PubMed] [Google Scholar]
- Kasthurirangan S, Markwell EL, Atchison DA, Pope JM, 2011. MRI study of the changes in crystalline lens shape with accommodation and aging in humans. J Vis 11. [DOI] [PubMed] [Google Scholar]
- Kepler J, 1611. Dioptrice seu demonstratio eorum quae visui & visibilibus propter conspicilla non ita pridem inventa accidunt.
- Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM, 2008. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci 121, 2696–2704. [DOI] [PubMed] [Google Scholar]
- Kumar A, Duvvari MR, Prabhakaran VC, Shetty JS, Murthy GJ, Blanton SH, 2010. A homozygous mutation in LTBP2 causes isolated microspherophakia. Hum Genet 128, 365–371. [DOI] [PubMed] [Google Scholar]
- Kumar B, Chandler HL, Plageman T, Reilly MA, 2019. Lens Stretching Modulates Lens Epithelial Cell Proliferation via YAP Regulation. Invest Ophthalmol Vis Sci 60, 3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff MM, Bishop PN, 2008. Adult vitreous structure and postnatal changes. Eye (Lond) 22, 1214–1222. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, Pyeritz RE, Sponseller PD, Wordsworth P, De Paepe AM, 2010. The revised Ghent nosology for the Marfan syndrome. J Med Genet 47, 476–485. [DOI] [PubMed] [Google Scholar]
- Lutjen-Drecoll E, Kaufman PL, Wasielewski R, Ting-Li L, Croft MA, 2010. Morphology and accommodative function of the vitreous zonule in human and monkey eyes. Invest Ophthalmol Vis Sci 51, 1554–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majtan T, Jones W Jr., Krijt J, Park I, Kruger WD, Kozich V, Bassnett S, Bublil EM, Kraus JP, 2018. Enzyme Replacement Therapy Ameliorates Multiple Symptoms of Murine Homocystinuria. Mol Ther 26, 834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark HH, 1971. Johannes Kepler on the eye and vision. Am J Ophthalmol 72, 869–878. [DOI] [PubMed] [Google Scholar]
- Maumenee IH, 1981. The eye in the Marfan syndrome. Trans Am Ophthalmol Soc 79, 684–733. [PMC free article] [PubMed] [Google Scholar]
- May A, Nimtschke U, May CA, 2009. The architecture of the mouse ciliary processes and their changes during retinal degeneration. Exp Eye Res 88, 561–565. [DOI] [PubMed] [Google Scholar]
- McCulloch C, 1954. The zonule of Zinn: its origin, course, and insertion, and its relation to neighboring structures. Trans Am Ophthalmol Soc 52, 525–585. [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Palmer JW, 1934. The polysaccharide of the vitreous humor. Journal of Biological Chemistry 107, 629–634. [Google Scholar]
- Michael R, Mikielewicz M, Gordillo C, Montenegro GA, Pinilla Cortes L, Barraquer RI, 2012. Elastic properties of human lens zonules as a function of age in presbyopes. Invest Ophthalmol Vis Sci 53, 6109–6114. [DOI] [PubMed] [Google Scholar]
- Moorehead C, Prudnikova K, Marcolongo M, 2019. The regulatory effects of proteoglycans on collagen fibrillogenesis and morphology investigated using biomimetic proteoglycans. J Struct Biol 206, 204–215. [DOI] [PubMed] [Google Scholar]
- Mularczyk EJ, Singh M, Godwin ARF, Galli F, Humphreys N, Adamson AD, Mironov A, Cain SA, Sengle G, Boot-Handford RP, Cossu G, Kielty CM, Baldock C, 2018. ADAMTS10-mediated tissue disruption in Weill-Marchesani syndrome. Hum Mol Genet 27, 3675–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier HR, Kidson SH, 2005. Proliferation and cell shape changes during ciliary body morphogenesis in the mouse. Dev Dyn 233, 213–223. [DOI] [PubMed] [Google Scholar]
- Nelson LB, Maumenee IH, 1982. Ectopia lentis. Surv Ophthalmol 27, 143–160. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Okisaka S, 1992. [The tension of zonule and aging changes of ciliary bodies]. Nippon Ganka Gakkai Zasshi 96, 721–730. [PubMed] [Google Scholar]
- Ohno-Jinno A, Isogai Z, Yoneda M, Kasai K, Miyaishi O, Inoue Y, Kataoka T, Zhao JS, Li H, Takeyama M, Keene DR, Sakai LY, Kimata K, Iwaki M, Zako M, 2008. Versican and fibrillin-1 form a major hyaluronan-binding complex in the ciliary body. Invest Ophthalmol Vis Sci 49, 2870–2877. [DOI] [PubMed] [Google Scholar]
- Oichi T, Taniguchi Y, Soma K, Oshima Y, Yano F, Mori Y, Chijimatsu R, Kim-Kaneyama JR, Tanaka S, Saito T, 2019. Adamts17 is involved in skeletogenesis through modulation of BMP-Smad1/5/8 pathway. Cell Mol Life Sci 76, 4795–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JAC, Rustidge S, Pettitt L, Jenkins CA, Farias FHG, Giuliano EA, Mellersh CS, 2018. Evaluation of ADAMTS17 in Chinese Shar-Pei with primary open-angle glaucoma, primary lens luxation, or both. Am J Vet Res 79, 98–106. [DOI] [PubMed] [Google Scholar]
- Pessier AP, Potter KA, 1996. Ocular pathology in bovine Marfan’s syndrome with demonstration of altered fibrillin immunoreactivity in explanted ciliary body cells. Lab Invest 75, 87–95. [PubMed] [Google Scholar]
- Petit JL, 1726. Memoire sur plusieurs decouvertes faites dans les yeux de l’homme, des animaux a quatre pieds, des oiseaux & des poissons. Histoire de l’Académie royale des sciences, avec les mémoires de mathématique et de physique., 96–116.
- Qiao H, Hisatomi T, Sonoda KH, Kura S, Sassa Y, Kinoshita S, Nakamura T, Sakamoto T, Ishibashi T, 2005. The characterisation of hyalocytes: the origin, phenotype, and turnover. Br J Ophthalmol 89, 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola G, 1971. The fine structure of the ciliary zonule and ciliary epithelium. With special regard to the organization and insertion of the zonular fibrils. Invest Ophthalmol 10, 851–869. [PubMed] [Google Scholar]
- Reinhardt DP, Keene DR, Corson GM, Poschl E, Bachinger HP, Gambee JE, Sakai LY, 1996. Fibrillin-1: organization in microfibrils and structural properties. J Mol Biol 258, 104–116. [DOI] [PubMed] [Google Scholar]
- Rhodes RH, 1983. A comparative study of vitreous-body and zonular glycoconjugates that bind to the lectin from Ulex europaeus. Histochemistry 78, 349–360. [DOI] [PubMed] [Google Scholar]
- Roberts DK, Newman TL, Roberts MF, Teitelbaum BA, Winters JE, 2018. Long Anterior Lens Zonules and Intraocular Pressure. Invest Ophthalmol Vis Sci 59, 2015–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohen JW, 1979. Scanning electron microscopic studies of the zonular apparatus in human and monkey eyes. Invest Ophthalmol Vis Sci 18, 133–144. [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Engvall E, 1986. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol 103, 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai LY, Keene DR, Renard M, De Backer J, 2016. FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders. Gene 591, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakatani T, Isa T, 2007. Quantitative analysis of spontaneous saccade-like rapid eye movements in C57BL/6 mice. Neurosci Res 58, 324–331. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Naumann GO, 1994. A histopathologic study of zonular instability in pseudoexfoliation syndrome. Am J Ophthalmol 118, 730–743. [DOI] [PubMed] [Google Scholar]
- Shapiro SD, Endicott SK, Province MA, Pierce JA, Campbell EJ, 1991. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J Clin Invest 87, 1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt MJ, Baldock C, Haston JL, Holmes DF, Jones CJ, Shuttleworth CA, Wess TJ, Kielty CM, 2003. Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. J Mol Biol 332, 183–193. [DOI] [PubMed] [Google Scholar]
- Sherratt MJ, Holmes DF, Shuttleworth CA, Kielty CM, 1997. Scanning transmission electron microscopy mass analysis of fibrillin-containing microfibrils from foetal elastic tissues. Int J Biochem Cell Biol 29, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Shi Y, De Maria A, Lubura S, Sikic H, Bassnett S, 2014. The penny pusher: a cellular model of lens growth. Invest Ophthalmol Vis Sci 56, 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Jones W, Beatty W, Tan Q, Mecham R, Kumra H, Reinhardt D, Gibson M, Reilly M, Rodriguez J, Bassnett S, 2020. Latent-transforming growth factor beta-binding protein-2 (LTBP-2) is required for longevity but not for development of zonular fibers. Matrix Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Tu Y, De Maria A, Mecham RP, Bassnett S, 2013. Development, composition, and structural arrangements of the ciliary zonule of the mouse. Invest Ophthalmol Vis Sci 54, 2504–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggs OM, Souzeau E, Craig JE, 2019. Loss of ciliary zonule protein hydroxylation and lens stability as a predicted consequence of biallelic ASPH variation. Ophthalmic Genet 40, 12–16. [DOI] [PubMed] [Google Scholar]
- Streeten BW, 1977. The zonular insertion: a scanning electron microscopic study. Invest Ophthalmol Vis Sci 16, 364–375. [PubMed] [Google Scholar]
- Streeten BW, 1982. The nature of the ocular zonule. Trans Am Ophthalmol Soc 80, 823–854. [PMC free article] [PubMed] [Google Scholar]
- Streeten BW, Gibson SA, Li ZY, 1986. Lectin binding to pseudoexfoliative material and the ocular zonules. Invest Ophthalmol Vis Sci 27, 1516–1521. [PubMed] [Google Scholar]
- Streeten BW, Swann DA, Licari PA, Robinson MR, Gibson SA, Marsh NJ, Vergnes JP, Freeman IL, 1983. The protein composition of the ocular zonules. Invest Ophthalmol Vis Sci 24, 119–123. [PubMed] [Google Scholar]
- Sturrock GD, Tripathi RC, 1976. Pigmented lens striae. Br J Ophthalmol 60, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K, 2007. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 317, 1397–1400. [DOI] [PubMed] [Google Scholar]
- Tsuruga E, Nakashima K, Ishikawa H, Yajima T, Sawa Y, 2009. Stretching modulates oxytalan fibers in human periodontal ligament cells. J Periodontal Res 44, 170–174. [DOI] [PubMed] [Google Scholar]
- van Alphen GW, Graebel WP, 1991. Elasticity of tissues involved in accommodation. Vision Res 31, 1417–1438. [DOI] [PubMed] [Google Scholar]
- van der Rijt JA, van der Werf KO, Bennink ML, Dijkstra PJ, Feijen J, 2006. Micromechanical testing of individual collagen fibrils. Macromol Biosci 6, 697–702. [DOI] [PubMed] [Google Scholar]
- Wang LW, Kutz WE, Mead TJ, Beene LC, Singh S, Jenkins MW, Reinhardt DP, Apte SS, 2019. Adamts10 inactivation in mice leads to persistence of ocular microfibrils subsequent to reduced fibrillin-2 cleavage. Matrix Biol 77, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Lu Y, Baldock C, 2009. Fibrillin microfibrils: a key role for the interbead region in elasticity. J Mol Biol 388, 168–179. [DOI] [PubMed] [Google Scholar]
- Wheatley HM, Traboulsi EI, Flowers BE, Maumenee IH, Azar D, Pyeritz RE, Whittumhudson JA, 1995. Immunohistochemical Localization of Fibrillin in Human Ocular-Tissues - Relevance to the Marfan-Syndrome. Arch Ophthalmol-Chic 113, 103–109. [DOI] [PubMed] [Google Scholar]
- Wislocki GB, 1952. The anterior segment of the eye of the rhesus monkey investigated by histochemical means. Am J Anat 91, 233–261. [DOI] [PubMed] [Google Scholar]
- Wright DM, Duance VC, Wess TJ, Kielty CM, Purslow PP, 1999. The supramolecular organisation of fibrillin-rich microfibrils determines the mechanical properties of bovine zonular filaments. J Exp Biol 202, 3011–3020. [DOI] [PubMed] [Google Scholar]
- Zinn JG, 1753. De Ligamentis Ciliaribus, Gottingen. [Google Scholar]
- Zinn JG, 1755. Descriptio Anatomica Oculi Humani Iconibus Illustrata, Gottingen. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.