Abstract
The paraventricular nucleus of the hypothalamus (PVH) is a heterogeneous collection of neurons that play important roles in modulating feeding and energy expenditure. Abnormal development or ablation of the PVH results in hyperphagic obesity and defects in energy expenditure whereas selective activation of defined PVH neuronal populations can suppress feeding and may promote energy expenditure. Here, we characterize the contribution of calcitonin receptor–expressing PVH neurons (CalcRPVH) to energy balance control. We used Cre-dependent viral tools delivered stereotaxically to the PVH of CalcR2Acre mice to activate, silence, and trace CalcRPVH neurons and determine their contribution to body weight regulation. Immunohistochemistry of fluorescently-labeled CalcRPVH neurons demonstrates that CalcRPVH neurons are largely distinct from several PVH neuronal populations involved in energy homeostasis; these neurons project to regions of the hindbrain that are implicated in energy balance control, including the nucleus of the solitary tract and the parabrachial nucleus. Acute activation of CalcRPVH neurons suppresses feeding without appreciably augmenting energy expenditure, whereas their silencing leads to obesity that may be due in part due to loss of PVH melanocortin-4 receptor signaling. These data show that CalcRPVH neurons are an essential component of energy balance neurocircuitry and their function is important for body weight maintenance. A thorough understanding of the mechanisms by which CalcRPVH neurons modulate energy balance might identify novel therapeutic targets for the treatment and prevention of obesity.
Keywords: paraventricular nucleus of the hypothalamus, calcitonin receptor, obesity, food intake, energy expenditure, energy homeostasis
The paraventricular nucleus of the hypothalamus (PVH) is a brain region that is critical for normal feeding and energy expenditure. The PVH serves as a hypothalamic node that receives ascending neuronal and humoral energy balance inputs, integrates this information, and then relays output signals to regions such as the hindbrain and spinal cord to drive physiologic responses to maintain energy homeostasis (1-3). The essential role of the PVH is revealed by mechanical lesions of this region that produce hyperphagic obesity and glucose dysregulation in both rodent models and humans (4, 5). Whereas complete inactivation of SIM1, a transcription factor required for PVH development, is incompatible with life, Sim1 haploinsufficiency disrupts PVH formation and results in profound hyperphagic obesity (6-9). Postnatal ablation of SIM1-expressing neurons leads to obesity with hyperphagia and a reduction in energy expenditure, revealing a critical role for PVH cell function in the regulation of both feeding and energy expenditure (10). Indeed, chemogenetic activation of SIM1PVH neurons acutely suppresses feeding and increases oxygen consumption and locomotor activity (11), while their inhibition leads to a robust increase in food intake (12). Given the importance of SIM1PVH neurons in energy balance and metabolic control, defining the energy balance contributions of subpopulations of SIM1PVH neurons may yield novel approaches to modulating discrete aspects of energy homeostasis.
The unique roles of several PVH neuronal subsets in energy balance have been identified using cell-specific manipulations with Cre-LoxP technology (11, 13-15). We have shown that activation of PVH neurons expressing nitric oxide synthase-1 (NOS1PVH) suppresses feeding and increases oxygen consumption (11). Importantly, activation of oxytocin (OXT)-expressing PVH neurons, which are a subpopulation of the NOS1PVH neurons, has no effect on feeding but does promote energy expenditure albeit to a lesser degree than NOS1 neurons (11). In contrast to the NOS1PVH neurons, acute activation of melanocortin-4 receptor (MC4R)-expressing PVH neurons suppresses feeding but has no effect on energy expenditure (15). These experiments underscore the anatomical and functional differences between diverse PVH neuronal populations and suggest that the modulation of feeding and energy expenditure may be compartmentalized to subsets of PVH neurons. Therefore, manipulation of genetically defined PVH cells uncovers the physiological roles of these neuronal subpopulations and delineates the neural circuitries responsible for discrete aspects of energy balance control.
Peripheral application of the polypeptide calcitonin suppresses feeding in several species (16-19). Calcitonin receptors (CalcR) are expressed in the PVH and direct application of salmon calcitonin (sCT) to the PVH dramatically suppresses feeding in rats, suggesting that the activity of CalcRPVH neurons may contribute to energy balance control (20, 21). To determine the role of CalcRs in PVH neurons in the anorectic actions of peripherally applied sCT, we deleted CalcRs selectively from the PVH using Cre-loxP technology. We found that loss of CalcRs from the PVH did not alter energy balance at baseline or attenuate the anorectic response to peripherally applied sCT (22). Although CalcR expression in the PVH is not necessary for the sCT-induced anorexia, the dramatic suppression of feeding following direct PVH application of calcitonin suggests that the PVH neurons that express CalcR likely play an important in energy homeostasis (21). To understand the contribution of CalcRPVH neurons to energy balance control, we used our CalcR2ACre transgenic mouse line and viral vectors to selectively target and manipulate CalcRPVH cells in male mice. This approach uses CalcR expression as a means to genetically access CalcRPVH neurons and is not a surrogate for calcitonin signaling in the PVH. Acute activation of CalcRPVH neurons suppresses feeding and promotes locomotor activity. To test the necessity of CalcRPVH neurons in the regulation of energy balance, we chronically silenced CalcRPVH neurons using Cre-dependent expression of tetanus toxin. Silencing CalcRPVH neurons leads to obesity due to increased food intake and a possible alteration in energy expenditure. We find that CalcR and MC4R expression overlap in the PVH, suggesting that the obesity seen with CalcRPVH silencing may be in part due to loss of melanocortin action in the PVH. Overall, this study demonstrates that CalcRPVH neurons lie within energy balance neurocircuitry and their activity is important for body weight maintenance. Moreover, selectively modulating CalcRPVH neuronal activity may represent a potential novel therapeutic approach to treating and preventing obesity and its comorbidities.
Material and Methods
Experimental Animals
CalcR-2a-Cre (or CalcR2ACre) mice were previously generated and validated as described (23). Adult male CalcRcre2ACre mice (7-16 weeks old) were used for activation and silencing studies. Adult male mice (8-17 weeks old) expressing Cre-recombinase in MC4R neurons (MC4RCre, JAX stock 030759; kindly provided by Dr. Brad Lowell) (24, 25) were used for the silencing studies. As preliminary data revealed similar responses to CalcRPVH neuron manipulation in both sexes, we conducted our studies in male mice to avoid potential confounding issues of hormonal cyclicity and to align our approach with previously published data (14). Immunohistochemical analysis of CalcRPVH neurons was performed using brains from CalcR2ACre mice crossed to a Cre-dependent green fluorescent protein (GFP) reporter (CalcR2ACre+GFP mice) (11, 26). In accordance with the Association for the Assessment and Approval of Laboratory Animal Care and National Institutes of Health guidelines, the procedures performed were approved by the University of Michigan Committee on the Care and Use of Animals. Animals were bred and housed within our colony, with ad libitum access to food and water provided (unless specified otherwise) in temperature-controlled rooms that followed a 12-hour light/dark cycle.
Stereotaxic Injections
Stereotaxic injections were conducted on CalcR2Acre, MC4RCre, and nontransgenic wild-type (WT) mice as described previously (23, 24). Following induction of isoflurane anesthesia and application of presurgical analgesia (carprofen), mice were placed in a digital stereotaxic frame (Model 1900, Kopf Instruments). A pressurized picospritzer was used to perform viral injections into the PVH (coordinates relative to bregma: A/P = −0.500, M/L = ± 0.200, D/V = −4.77) through a pulled glass micropipette. For the anterograde tracing experiments, 50 nL of the adeno-associated virus with a Cre-dependent synaptophysin-GFP (rAAV8-flex Syn-EGFP, titer: 5.69 × 1014 vg/mL, produced by the Viral Vector Core at the University of Michigan) terminal tracer was injected unilaterally into the PVH of CalcR2ACre mice. The synaptophysin-GFP anterograde tracer traffics preferentially to the synaptic terminals of the neurons, which aids in the identification of downstream targets. To allow temporal and spatial control of CalcRPVH neural activity, we used Cre-dependent expression of the stimulatory Designer Receptors Exclusively Activated by Designer Drugs (DREADDs; hM3Dq). hM3Dq is a modified human muscarinic receptor coupled to the stimulatory Gq-protein which activates neurons upon binding the synthetic ligand clozapine N-oxide (CNO) (27, 28). Cre-dependent AAV-Flex-hM3Dq-mCherry (AAV-Flex-hM3Dq, titer: 4 × 1012 virus molecules/mL, purchased from UNC Vector Core) was bilaterally injected (50 nL/side) into the PVH of CalcR2ACre mice to specifically manipulate these cells. Mice for the tracing and acute activation studies were given at least 21 days to recover and ensure sufficient viral expression in the cell bodies and their terminals. For CalcRPVH neuron silencing studies, Cre-dependent tetanus toxin was used. Tetanus toxin cleaves synaptobrevin, a component of the SNARE core and prevents release of synaptic vesicles from targeted neurons (29, 30). Then, 50 nL of AAV-Flex-TetTox (titer: 1.2 × 1012 virus molecules/mL, generated at the UNC vector viral core based on a plasmid kindly provided by Dr. Wei Xu, UTSW) or control viruses were injected bilaterally in CalcR2ACre mice. For the MC4RPVH studies, MC4RCre and WT mice were bilaterally injected in the PVH with AAV-Flex-TetTox or a Cre-dependent GFP reporter virus (AAV-Flex-GFP).
Effects of CalcRPVH Neuronal Activation on Feeding Behavior and Energy Expenditure
Feeding and energy expenditure studies were performed at least 3 weeks after surgery to allow for surgical recovery and viral expression in CalcR2ACre and control mice (WT mice and CalcR2ACre mice injected with a control virus). For the acute feeding studies, mice received daily intraperitoneal (i.p.) injections of vehicle 3 days prior to the experiment. On the day of the feeding studies, the mice were fasted from 10 am to 6 pm and they received i.p. injection of CNO or vehicle at the onset of the dark cycle with the presentation of food. Food intake was then measured 1, 2, and 4 hours after the injection and the experiment was repeated at least 4 days later with the treatments switched (CalcR2ACre + AAV-Flex-hM3Dq mice, N = 12; controls, N = 7). To measure energy expenditure, CalcR2ACre + AAV-Flex-hM3Dq mice were acclimated to daily saline injections for 3 days prior to entering a Comprehensive Laboratory Animal Monitoring System (CLAMS, Columbus Instruments) in the University of Michigan’s Small Animal Phenotyping Core for multiparameter assessments. Mice were given 24 hours to acclimate to the CLAMS unit. On the second day of CLAMS, food was removed between 9 am and 5 pm and the mice received i.p. injection of either vehicle (0.9% sodium chloride) or CNO (0.3 mg/kg in saline) at 12:00 pm. On the fourth day of CLAMS, the mice received the alternate treatment (saline or CNO). Measurements were taken continuously during the experiment; energy expenditure measurements were then averaged over the course of 4 hours following the injection of either CNO or vehicle (CalcR2ACre + AAV-Flex-hM3Dq, N = 10). To demonstrate that the activation of the CalcRPVH neurons required both DREADDs expression and CNO injection, we quantified nuclear FOS expression in the PVH of CalcR2ACre Flex-hM3Dq and control mice injected with either CNO or saline. Counts were conducted by summing the FOS-positive cells in the PVH across 6 sections between bregma −0.47mm and −1.07mm (CalcR2ACre + AAV-Flex-hM3Dq + Saline, N = 4; CalcR2ACre + AAV-Flex-hM3Dq + CNO, N = 8; Control + Saline, N = 4; Control + CNO, N = 3).
Long-Term Body Weight, Food Intake, and Energy Expenditure Measurements
Mice injected with AAV-Flex-TetTox or control viruses were weighed just prior to surgery and were given 7 days to recover before weekly food intake and body weight measurements were taken for the CalcR2ACre (CalcR2ACre + AAV-Flex-TetTox, N = 13; controls, N = 25) and MC4RCre (MC4RCre + AAV-Flex-TetTox, N = 11; MC4RCre + GFP, N = 6; WT + AAV-Flex-TetTox, N = 10) experiments. CLAMS was used to measure oxygen consumption, carbon dioxide production, and locomotor activity via optical beam breaks between 8 and 10 weeks after injection for CalcR2Acre mice (CalcR2ACre + AAV-Flex-TetTox, N = 9; controls N = 20). MC4RCre mice had CLAMS conducted between 3 to 4 weeks after injection (MC4RCre + AAV-Flex-TetTox N = 8, MC4RCre + GFP N = 5, WT + AAV-Flex-TetTox N = 7). Mice were placed in CLAMS for 4 days; data collected from the second and third days were used for analysis to allow acclimatization to occur during the first day. Body composition analysis was conducted just prior to CLAMS for the CalcR2Acre mice (CalcR2ACre + AAV-Flex-TetTox, N = 9; controls, N = 20) and between 7 and 8 weeks after injection for the MC4RCre mice (Minispec LF90 II, Bruker Optics) (MC4RCre + AAV-Flex-TetTox, N = 11; MC4RCre + GFP, N = 6; WT + AAV-Flex-TetTox, N = 10). Nine weeks following viral injection, CalcR2ACre mice that received AAV-Flex-TetTox injections and the controls were acclimated to daily saline injections for 3 days prior to the day of the feeding experiment. They were then fasted during the light cycle between 10 am and 6 pm and at the beginning of the dark cycle (6 pm), mice were given ad libitum access to food and either an i.p. injection of salmon calcitonin at 100 ug/kg (sCT, Bachem, 4033011) or 0.9% sodium chloride. Food intake was then measured 2 and 4 hours following the injection. At least 4 days later, the injection treatments were switched, and food intake was measured (CalcR2ACre + AAV-Flex-TetTox, N = 9; controls, N = 20).
In Situ Hybridization
Mice were overdosed with the inhaled anesthetic isoflurane, then the brains were removed and flash-frozen in 2-methylbutane. The brains were cut using a cryostat (Leica CM 1950) into 16-μm sections onto glass slides and were immediately stored at −80 oC. The assays were conducted according to the protocol provided by the RNAscope manufacturer (Advanced Cell Diagnostics). Sections were fixed for 1 hour in cold 10% formalin and then dehydrated in 50% and 75% ethanol, each for 5 minutes, followed by 2 times in 100% ethanol. The sections were then dried for 30 minutes at 40 oC. To determine the PVH expression of Mc4r and Calcr mRNAs in the PVH, chromogen staining was performed with an RNAscope 2.5 HD Duplex Reagent Kit (ACD 322430). Sections were treated for 10 minutes with H2O2 and then incubated for 30 minutes with protease K IV. Following hybridization of Mc4r and Calcr mRNA probes, the sections were washed twice and then underwent amplification with Amp1 to Amp2 with 2 washes in between. The red signal component was detected through diluting Red-B 1:60 in component Red-A, which was then incubated on the tissue at room temperature for 10 minutes. The slides were rinsed 2 times in wash buffer to conclude the chromogen reaction. Amplification proceeded with Amp7 through Amp10, after which the green signal was detected by diluting Green-B 1:50 in component Green-A and incubating at room temperature for 10 minutes. The tissue was then counter stained by immersing the slides into 50% hematoxylin for 30 seconds, after which they were dried and mounted in VectaMount mounting medium (Vector Laboratories, Inc). Imaging for the chromogen-stained tissue was conducted with an Olympus BX-51 microscope with a DP80 camera (Olympus). To quantify cells expressing probe signal, images from coronal sections were processed in a uniform manner with Photoshop (Adobe) in order to remove the background and better visualize the individual probes. Hematoxylin-stained cells that were determined to be positive for each probe were quantified using ImageJ and overlapping expression was compared to the expression of a singular probe within each of the mouse groups (N = 3).
Perfusion and Immunohistochemistry
To confirm the accuracy of each viral injection, at the conclusion of all surgical experiments, mice were deeply anesthetized with inhaled isoflurane and then transcardially perfused with sterile PBS and then 10% buffered formalin. Brains were removed, post-fixed in 10% buffered formalin and dehydrated in 30% sucrose. Brains were then sectioned into 4 representative series of 30-μm coronal slices using a freezing microtome (Leica) and stored at −20 oC. For immunohistochemistry (IHC), we blocked brain tissue using 3% normal donkey serum, followed by incubation in primary and then secondary antibodies. For the AAV-Flex-hM3Dq-mCherry experiments, mCherry expression was detected using a primary antibody for dsRed (rabbit 1:1000, Clontech, 632496) (31), followed by secondary immunofluorescence detection with donkey anti-rabbit-Alexa 568 (1:200, Invitrogen) (32). For cFos imaging of CalcR2ACre + AAV-Flex-hM3Dq mouse brains, primary antibodies for cFos (rabbit 1:1000, Cell Signaling, 2250S) (33) and tdTomato (rat 1:1000, Kerafast, Inc EST203, which also recognizes mCherry) (34) were used, followed by donkey anti-rabbit-Alexa 488 (1:200, Invitrogen) (35) and goat anti-rat-Alexa 568 (1:200, Invitrogen) (36) secondary antibodies. For the anterograde tracing and TetTox-GFP experiments, a primary antibody for GFP (chicken 1:1000, Aves GFP–1020) (37) was used followed by a goat anti-chicken-Alexa 488 (1:200, Invitrogen) (38) secondary antibody. For the colocalization experiments (quantification took place in the PVH across 6 sections between bregma −0.47mm and −1.07mm, N = 3 CalcR2ACre+GFP mice were used per peptide investigated), we used primary antibodies for GFP (chicken 1:1000, Aves GFP–1020 or rabbit 1:1000, Invitrogen A6455) (37, 39), nNos1 (sheep 1:1000, kindly provided by Dr. Vincent Prevot), OXT (rabbit 1:4000, Peninsula Laboratories, LLC T-4084) (40), and AVP (rabbit 1:4000, Millipore AB1565) (41). To trap thyrotropin-releasing hormone (TRH) and corticotropin-releasing hormone (CRH) in PVH cell bodies for IHC visualization, we utilized intracerebral injections of colchicine (coordinates relative to bregma: third ventricle A/P = −0.340, M/L = −1.000, D/V = −2.400) into CalcR2ACre+GFP mice (42). We delivered 250 nL of colchicine solution (0.040 mg/mL colchicine in water) to the third ventricle of the mice and perfused 2 days later. For these mice, primary antibodies for Pro-TRH (rabbit 1:1000, Millipore ABN1658) (43) and CRH (rabbit 1:1000, Peninsula Laboratories, LLC T-4037) (44) were used. Secondary immunofluorescence for the colocalization experiments was conducted using goat anti-chicken-Alexa 488 (38), donkey anti-goat-Alexa 488 (45), donkey anti-rabbit-Alexa 488 (35), donkey anti-sheep-Alexa 594, and donkey anti-rabbit-Alexa 568 (1:200, Invitrogen) (46).
Data and Statistical Analysis
For experiments utilizing mice with the stereotaxic viral injections, data analysis was only performed using mice in which the viral expression was constrained to the PVH (as determined by post hoc IHC) in order to avoid confounding effects from viral transduction of cells outside the PVH. Unpaired t-tests, paired t-tests, 1-way and 2-way analysis of variance (ANOVA) with Sidak’s or Tukey’s multiple comparisons test post hoc tests (when appropriate), mixed model analyses, and simple linear regression and correlation analysis were conducted using GraphPad Prism 8, as noted in the figure legends. Significance is indicated by P < 0.05.
Results
Activation of CalcRPVH Neurons Regulates Feeding but Not Energy Expenditure
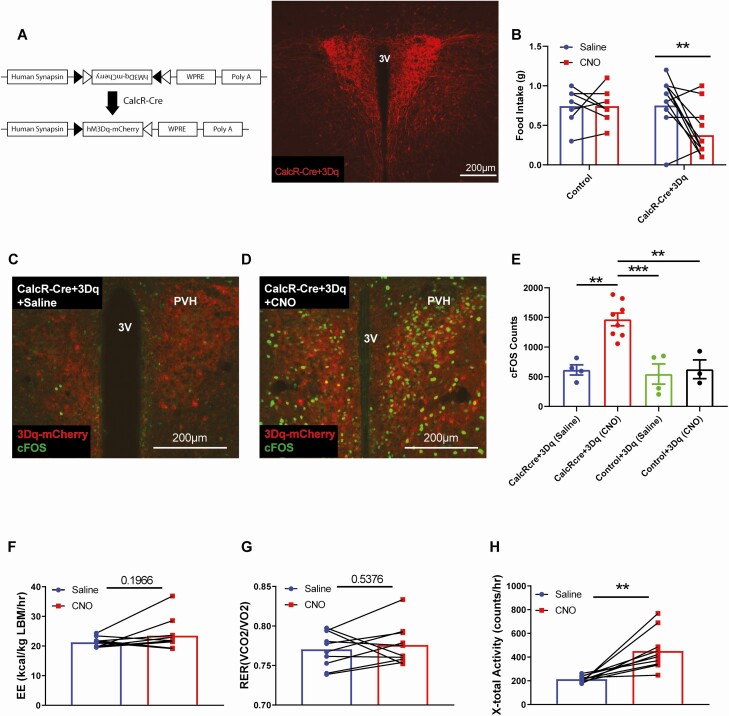
The anorectic effect of sCT delivered directly to the PVH suggests that activation of CalcRPVH cells may suppress feeding. To determine the contribution of CalcRPVH neurons to energy balance control, we used chemogenetics to acutely activate CalcRPVH neurons and examine the effects on food intake and energy expenditure (27, 28). We bilaterally injected a Cre-dependent activating DREADD (designer receptors exclusively activated by designer drugs) virus (AAV-Flex-hM3Dq) into the PVH of CalcR2ACre mice (Fig. 1A). After at least 3 weeks to allow for surgical recovery and viral expression, CalcR2ACre Flex-hM3Dq mice were fasted during the day and were then injected with either vehicle or CNO at the beginning of the dark cycle, the time at which mice typically start to feed. CalcRPVH neuron activation significantly decreased food intake over the first 2 hours of feeding (Fig. 1B). By 16 hours following the administration of CNO to the CalcR2ACre Flex-hM3Dq mice, cumulative food intake was comparable to controls, indicating that compensatory food intake occurred after the activating effects of the CNO dissipated (data not shown). Wild-type mice and CalcR2ACre mice injected with a control virus had no response to CNO (data were pooled as a single control group) demonstrating that neither CNO nor its metabolites alter food intake in this paradigm. CalcR2ACre Flex-hM3Dq mice injected with CNO expressed significantly more nuclear FOS in the PVH (Fig. 1D and 1E) than CalcR2ACre Flex-hM3Dq mice injected with saline (Fig. 1C and 1E) and control mice injected with CNO or saline (Fig. 1E) indicating a CNO + DREADD-dependent effect on neuron activity. Thus, activation of CalcRPVH neurons is sufficient to suppress feeding behavior and demonstrates that CalcRPVH neurons lie within the neurocircuitry that modulates feeding.
Figure 1.
Effect of acute activation of CalcR PVH neurons using DREADDs on food intake and energy expenditure. A) Diagram of Cre-dependent hM3Dq DREADDs expression vector. IHC was used to detect expression of AAV-Flex-hM3Dq bilaterally injected into the PVH of CalcR2ACre mice (CalcR-Cre + 3Dq). B) Two-hour food intake following i.p. injection of CNO (0.3 mg/kg) at start of the dark cycle. CalcR2ACre + Flex-hM3Dq and control mice were fasted for 90 minutes prior to i.p. injection of CNO or saline, and were perfused 2 hours following the injection. IHC for 3Dq-mCherry and FOS expression was conducted on CalcR2ACre + Flex-hM3Dq mice injected with saline (C), CalcR2ACre + Flex-hM3Dq mice injected with CNO (D), control mice injected with saline and control mice injected with CNO (not shown). FOS expression in the PVH was quantified and compared among the 4 groups (E). CLAMS measurements of energy expenditure (F), the respiratory exchange ratio (G) and total X-activity (H) following DREADD-mediated CalcRPVH activation. Average values ± SEM are shown. P values for feeding behavior and FOS quantification were determined by 2-way ANOVA followed by a Sidak’s multiple comparisons test; CLAMS measures, were compared using a paired t test. **P < 0.01, ***P < 0.001. Abbreviations: 3V, third ventricle, PVH, paraventricular nucleus of the hypothalamus.
As activation of some subsets of PVH neurons promotes oxygen consumption and locomotor activity (11, 47), we next assessed whether CalcRPVH neuron activation could alter energy expenditure. CalcR2ACre Flex-hM3Dq mice were placed into CLAMS to measure oxygen consumption and locomotor activity following CNO activation. In the absence of food, activation of CalcRPVH neurons did not significantly change energy expenditure or respiratory exchange ratio (Fig. 1F and 1G). Although acute activation of CalcRPVH neurons increases locomotor activity slightly (Fig. 1H), this behavioral change is not sufficient to alter overall energy expenditure, at least as measured by changes in oxygen consumed or carbon dioxide produced in the CLAMS. Taken together, these findings indicate that activation of CalcRPVH neurons suppresses feeding without an appreciable effect on energy expenditure.
CalcRPVH Neurons Project to Regions of the Hindbrain and Display Unique Neuropeptide Expression
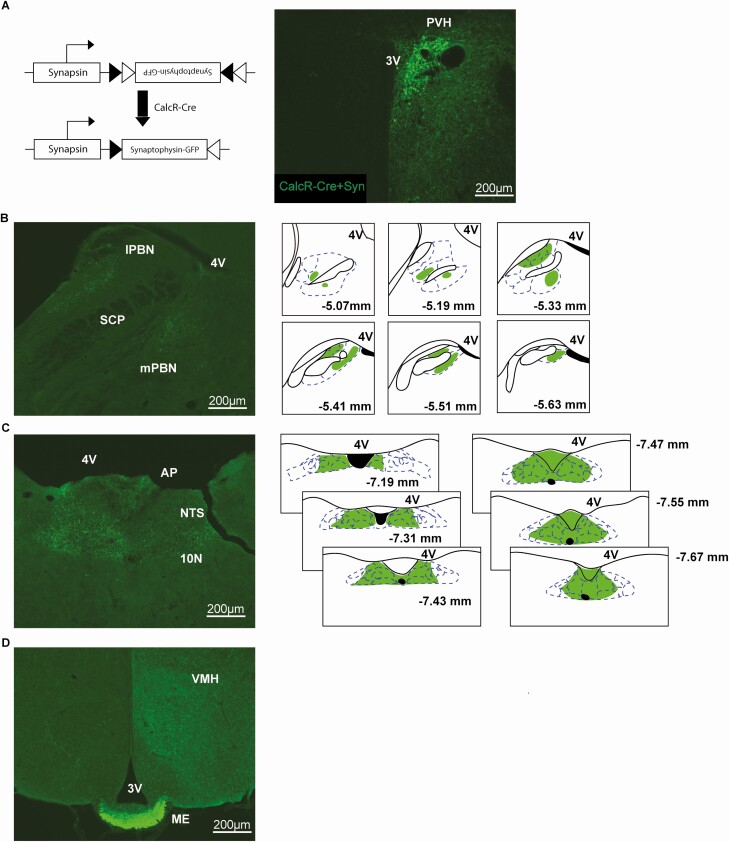
Having established a potential role for CalcRPVH neurons in energy balance control, we sought to characterize the neural circuitry in which CalcRPVH neurons reside. A Cre-dependent viral anterograde tracer, AAV-Flex-synaptophysin-GFP was unilaterally injected into the PVH of CalcR2ACre mice (Fig 2A). Dense projection terminals from CalcRPVH neurons were found in hindbrain regions such as the parabrachial nucleus (PBN) and the nucleus of the solitary tract (NTS) (Fig. 2B and 2C) brain regions known to be involved in feeding and energy expenditure regulation. Projections to the PBN range between −5.07 and −5.63 mm relative to bregma, with prominent signal in the medial PBN (MPBN) as well as the lateral PBN (LPBN) and its subnuclei including the central, external, lateral crescent, dorsal, internal lateral, and ventral lateral regions of the LPBN (Fig. 2B). NTS projections were found between bregma −7.19 and −7.67 mm, with signal in the gelatinous subnucleus, dorsolateral subnucleus, central subnucleus, central subnucleus, intermediate tract nucleus, medial subnucleus, dorsomedial subnucleus, commissural subnucleus, interstitial subnucleus, and limited signal in the parasolitary nucleus. Signal was also found in the area postrema (AP) and the dorsal motor nucleus of vagus nerve (10N) (Fig. 2C). CalcRPVH neurons also project to the external zone of the median eminence indicating engagement with the hypophyseal portal system and a potential role in pituitary regulation (Fig. 2D) (48). These tracing data demonstrate that CalcRPVH neurons are anatomically positioned to alter energy balance through projections to critical brain regions.
Figure 2.
Projection targets of CalcR PVH neurons using an anterograde tracer. A) Diagram of the Cre-dependent synaptophysin-GFP vector. The synaptophysin-GFP virus was unilaterally injected into the PVH of CalcR2ACre mice (CalcR-Cre + Syn). CalcRPVH axonal terminals were observed in the medial PBN (mPBN) and lateral PBN (lPBN), with an illustration showing signal between Bregma 5.07 and −5.63 mm (B). Projections were observed in the NTS between Bregma −7.19 and −7.67 mm, with signal also shown in the AP and 10N (C). Projections were also found in the external zone of the ME (D). Illustrations are based on coronal images from the atlas Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates 4th Edition. Abbreviations: 3V, third ventricle; 4V, fourth ventricle; 10N, dorsal motor nucleus of vagus nerve; AP, area postrema; lPBN, lateral parabrachial nucleus; ME, median eminence; mPBN, medial parabrachial nucleus; NTS, nucleus of the solitary tract; PVH, paraventricular nucleus of the hypothalamus; SCP, superior cerebellar peduncle; VMH, ventromedial nucleus of the hypothalamus.
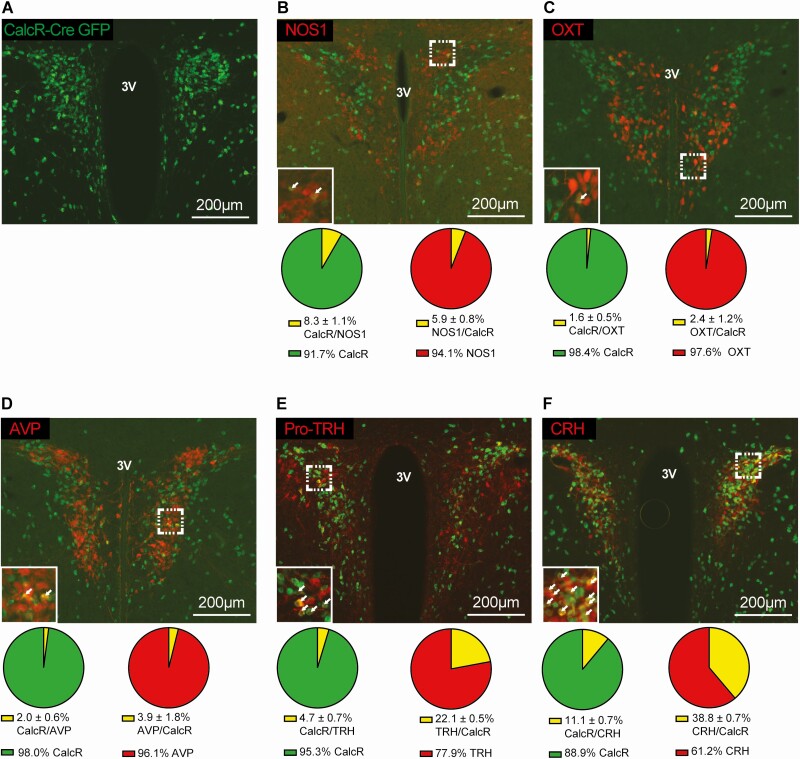
DREADD-mediated control of CalcRPVH neuron activity is not a simple surrogate for calcitonin signaling but enforces activation of CalcRPVH neurons and presumably their neurotransmitter contents. To determine if CalcRPVH neuron manipulations may impact other previously described PVH cell systems, we employed immunohistochemistry (IHC) to examine co-expression of several neuropeptides in CalcRPVH neurons. To identify CalcRPVH cells, we used the CalcR2ACre mouse to drive Cre-dependent GFP expression in all CalcR-expressing cells (CalcR2ACre +eGFP) and then co-stained for GFP in conjunction with primary antibodies that specifically bind to NOS1, OXT, AVP, CRH, and TRH. As CRH and TRH are quickly trafficked to the synaptic terminals of their neurons, we treated CalcR2ACre+GFP mice with colchicine (intracerebroventricularly) to trap these peptides in the cell bodies. Co-expression of GFP with each antigen was performed using brain sections from 3 mice for quantification. We found that CalcRPVH neurons are largely distinct from NOS1 (8.3% ± 1.1% of CalcR neurons express NOS1 and 5.9% ± 0.8% of NOS1 neurons express CalcR), OXT (1.6% ± 0.5% of CalcR neurons express OXT and 2.4% ± 1.2% of OXT neurons express CalcR), and AVP (2.0% ± 0.6% of CalcR neurons express AVP and 3.9% ± 1.8% of AVP neurons express CalcR) neurons in the PVH (Fig. 3B-3D). A small amount of overlap is observed with the TRHPVH neuronal population (4.7% ± 0.7% of CalcR neurons express TRH and 22.1% ± 0.5% of TRH neurons express CalcR) and there is a greater degree of overlap with the CRHPVH population (11.1% ± 0.7% of CalcR neurons express CRH and 38.8% ± 0.7% of CRH neurons express CalcR) (Fig. 3E and 3F).
Figure 3.
Colocalization of CalcR PVH neurons with other PVH populations implicated in energy homeostasis. Representative immunofluorescent images taken of the PVH of CalcR2ACre+GFP mice. Mice were stained for Cre-dependent GFP expression (A-F) shown in green. Sections were also stained using primary antibodies for NOS1 (B), OXT (C), AVP (D), Pro-TRH (E), and CRH (F) shown in red. Percent expression of the overlap (yellow) relative to the individual cell populations (green/red) were quantified in corresponding pie charts below their image. Abbreviation: 3V, third ventricle.
CalcRPVH Neurons Are Required for Normal Feeding and Body Weight Maintenance
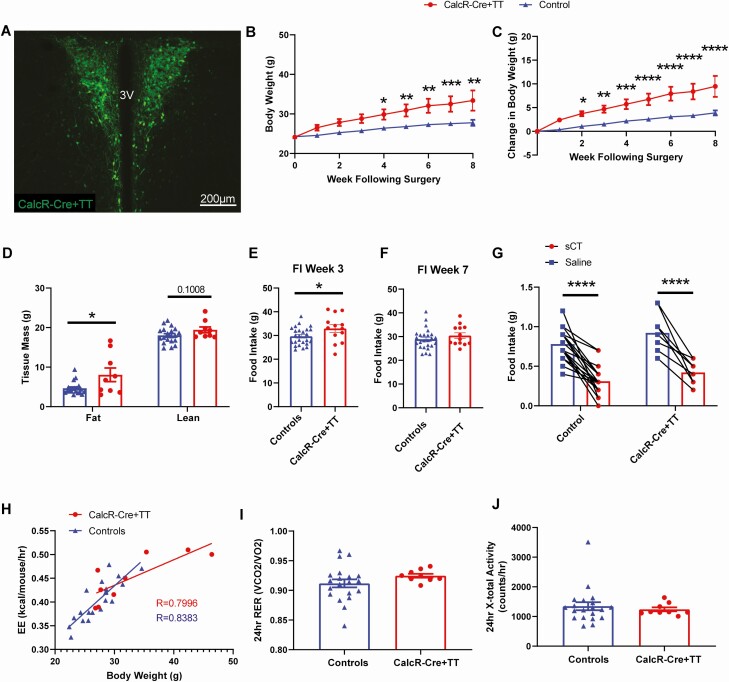
Although chemogenetic manipulation of neurons reveals their capacity to alter energy balance, this approach is not informative regarding the contribution of a neuronal subset to basal physiology. To determine the importance of CalcRPVH neurons in maintaining energy homeostasis, we chronically silenced CalcRPVH neurons using Cre-dependent viral expression of tetanus toxin (AAV-Flex-TetTox). AAV-Flex-TetTox or a Cre-dependent AAV control virus was bilaterally injected into the PVH of CalcR2ACre mice (Fig. 4A). Following the surgery, CalcR2ACre -Flex-TetTox mice gained more weight compared to the CalcR2Acre mice injected with the AAV control virus (Fig. 4B and 4C). Weight gain in the CalcR2ACre -Flex-TetTox was associated with increased fat mass and a trend toward increased lean mass relative to controls (Fig. 4D). The obesity in CalcR2ACre -Flex-TetTox mice was potentially driven by increased caloric intake, as food intake was significantly higher in CalcR2ACre -Flex-TetTox mice compared with controls when measured 3 weeks following the injection (Fig. 4E). This difference in food intake between the 2 groups is no longer discernable by week 7 (Fig. 4F), suggesting that compensatory responses to blunt persistent hyperphagia may have occurred as obesity developed. As was expected from our previous PVH-specific CalcR deletion studies (22), the anorectic effect of sCT was preserved in CalcR2ACre-Flex-TetTox mice (Fig. 4G) demonstrating that the anorectic effect of peripheral sCT is not dependent on PVH CalcR expression or CalcRPVH neuronal function.
Figure 4.
Physiological effects of silencing CalcR PVH neurons. A) Bilateral expression of AAV-TetTox-GFP in the PVH of CalcR2ACre mice (CalcR-Cre + TT). Effect of CalcRPVH silencing on body weight (BW) (B), body weight gain (C), fat and lean mass (D) and food intake during week 3 (E) and week 7 (F). Two-hour food intake following i.p. injection of sCT (100 µg/kg) at the start of the dark cycle (G). CLAMS measurements of energy expenditure over a 24-hour period were compared to respective body weights (H). CLAMS measurements of respiratory exchange rate (I) and X-total activity (J). Average values ± SEM are shown. P values for BW, change in BW and sCT food intake were determined using a 2-way ANOVA followed by Sidak’s multiple comparisons test, and for body composition, food intake and CLAMS measurements, an unpaired t test was used. For the energy expenditure measurements over a 24-hour period comparison, a simple linear regression and correlation analysis was used to determine the Pearson correlation (R) values. * P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Energy expenditure was also examined in CalcR2ACre - Flex-TetTox mice 8 to 10 weeks following injection of virus. To avoid the confounds of energy expenditure assessments of animals with different body weights, we plotted 24-hour energy expenditure as a function of body weight. While the change in energy expenditure as a function of body weight in this cohort of CalcR2ACre - Flex-TetTox mice was slightly less than the controls, this observed trend is strongly influenced by the 2 heaviest animals in the dataset (Fig. 4H). Respiratory exchange ratio and locomotor activity levels (measuring X-total activity) over a 24-hour period were not significantly different between the CalcR2ACre -Flex-TetTox mice and controls (Fig. 4I and 4J). Taken together, CalcRPVH silencing studies suggest that CalcRPVH neurons likely modulate feeding with at most a small contribution to energy expenditure control.
MC4R Signaling May Be an Essential Component of CalcRPVH Neuron–Mediated Energy Balance
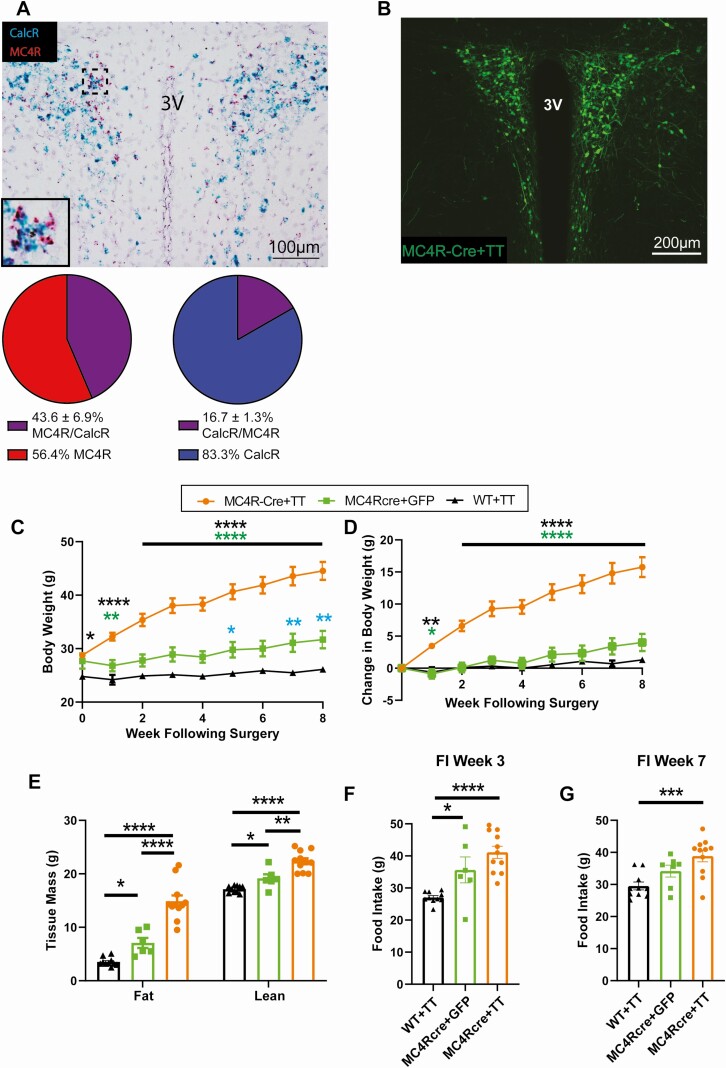
MC4R expression in PVH neurons is critical for normal feeding and body weight regulation with loss of MC4R signaling in the PVH characterized by hyperphagia and significant obesity (15, 26, 49). Given the increased food intake and obesity seen with silencing of CalcRPVH neurons, we examined the overlap of Mc4r and Calcr transcripts in the PVH. In situ hybridization revealed that almost half of MC4RPVH neurons express Calcr mRNA (43.6% ± 6.9%, n = 3 mice) whereas less than a quarter of CalcRPVH neurons express Mc4r mRNA (16.7% ± 1.3%, n = 3 mice) (Fig. 5A). Therefore, CalcRPVH neuron silencing might significantly impact MC4RPVH neuron function. In an effort to estimate the contribution of abrogated MC4R-signaling to the obesity phenotype provoked by CalcRPVH silencing, we selectively silenced MC4RPVH neurons and examined the effect on feeding and body weight regulation. We bilaterally injected 50 nL of the AAV-Flex-TetTox into the PVH of MC4R-Cre mice (MC4RCre -Flex-TetTox) (Fig. 5B). For controls, WT mice were injected with the AAV-Flex-TetTox and MC4RCre mice were injected with an AAV-Flex-GFP virus. In line with a prior study (24), silencing of MC4RPVH neurons resulted in rapid development of obesity (Fig. 5C and 5D). Fat mass and lean mass increased significantly in the MC4RCre-Flex-TetTox mice compared to the controls. Fat and lean mass also increased slightly in the MC4RCre-Flex-GFP mice control group relative to WT controls which may be inherent to the genetic targeting of the Mc4r locus for Cre expression (24, 50) (Fig. 5E). MC4RCre-Flex-TetTox mice consumed more food than WT controls on week 3 and week 7 after injection (Fig. 5F and 5G). It is important to note that MC4RCre -Flex-GFP mice also exhibited increased food intake relative to WT controls; the effect on feeding was similar to that seen for MC4RCre-Flex-TetTox mice, at least at 3 weeks following virus injection. Thus, the significant weight gain seen with MC4RCre-Flex-TetTox relative to MC4RCre-Flex-GFP mice control group (Fig. 5C and 5D) cannot be attributed solely to hyperphagia. Given the overlap of Calcr and Mc4r expression in the PVH, the silencing of CalcRPVH neurons would be expected to silence a significant fraction of MC4RPVH neurons which likely contributes to the CalcR2ACre-Flex-TetTox obesity phenotype. These findings indicate that the CalcR+/MC4R+ subpopulation of the PVH is potentially responsible for some of the obesity observed when all CalcRPVH neurons are silenced.
Figure 5.
Physiological effects of silencing MC4R PVH neurons. A) In situ hybridization in WT mouse for CalcR (Blue) and Mc4R RNAs (red). Percent expression of CalcR in MC4R positive cells and percent expression of MC4R in CalcR positive cells. B) Bilateral expression of AAV-TetTox-GFP in the PVH of MC4RCre mice (MC4R-Cre + TT). Effect of MC4RPVH silencing on body weight (BW) (C), body weight gain (D), fat and lean mass (E). Food intake in MC4RPVH silenced mice during week 3 (F) and during week 7 (G) following injection. Average values ± SEM are shown for BW, change in BW, body composition, and food intake. P values for BW and change in BW were determined using a 2-way ANOVA followed by Tukey’s multiple comparisons test, and for body composition and food intake, a 1-way ANOVA followed by a Tukey’s multiple comparisons test was conducted. * P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
Body weight maintenance is largely determined by actions of the central nervous system. Successfully targeting the central nervous system for obesity treatment or prevention will require a deeper understanding of the mechanisms and neurocircuitry that modulate feeding and energy expenditure (1). CalcR agonists, such as calcitonin and amylin, promote anorexia and weight loss in both humans and rodents (16, 18, 51, 52). CalcR is expressed in many regions of the central nervous system known to regulate energy balance suggesting that the site(s) of action for CalcR agonists may be distributed. Recently, we used Cre-loxP technology to delete CalcR from different regions of the brain in order to identify the neurocircuitry mediating the anorexic effects of peripherally administered CalcR agonists (22). Despite the significant expression of CalcR in the PVH, a brain region known to play critical roles in the energy balance control, deletion of CalcR from the PVH does not perturb physiologic feeding or body weight, and mice lacking CalcRs in the PVH have a normal anorectic response to peripherally applied sCT. These findings indicate that CalcR expression in the PVH is not required for body weight maintenance or sCT-induced anorexia. Nevertheless, the anorexia induced by the direct application of sCT to the PVH suggests that the CalcR-expressing PVH neurons lie within feeding neural circuits and that CalcRPVH neuron activity may regulate feeding behavior (21). It is for this reason that we used site-directed, temporal manipulations to specifically investigate the contribution of the CalcR-expressing neurons of the PVH to energy homeostasis.
Acute activation of CalcRPVH neurons using chemogenetic tools was sufficient to suppress feeding, thereby demonstrating that CalcRPVH neurons engage neural circuits that modulate feeding behavior. We and others have found that chemogenetic activation of a variety of genetically tagged PVH neurons is sufficient to alter feeding (11, 24). It is important to note, however, that this is not a ubiquitous property of PVH cells, as direct activation of oxytocin expressing PVH neurons does not alter feeding, thus highlighting the compartmentalization of PVH cell function (11). Chemogenetic activation can reveal the functional potential of a group of neurons but does not elucidate its role in normal physiological states. To probe the contribution of CalcRPVH neurons to body weight regulation, we chronically silenced these neurons in adult animals and found that their activity was necessary for restraining feeding and body weight gain. In line with our previous findings that demonstrated that mice lacking CalcRs in the PVH respond normally to peripheral application of sCT, the anorectic properties of sCT are still present even when CalcRPVH neurons are silenced. The contribution of CalcRPVH neurons on feeding and body weight regulation are revealed by both activating and silencing manipulations, demonstrating that these neurons lie within and modulate neural circuitry that is crucial to the regulation of energy homeostasis.
While we have demonstrated that CalcRPVH neurons modulate feeding and locomotor activity and are necessary for body weight maintenance, we have yet to determine the neurocircuitry through which these neurons alter feeding and energy balance. PVH neurons, such as the NOS1PVH neurons, project to hindbrain regions known to contribute to energy balance (11, 26, 53, 54). Similarly, CalcRPVH neurons send projection terminals to the PBN, a hindbrain region strongly associated with both appetitive and aversive feeding circuitry (15, 54). CalcRPVH neurons also send projections to the NTS, a region that integrates neuronal and hormone satiety signals that originate from the periphery (53). The NTS also influences sympathetic outflow through neurons with polysynaptic contact with brown adipose tissue and can both activate and inhibit brown adipose tissue thermogenesis (55-58). Our current studies do not distinguish the physiological responses driven by CalcRPVH neuronal projections to either the PBN or NTS specifically; terminal specific manipulations (eg, via optogenetics) will be necessary to discriminate these effects. As the PVH is largely glutamatergic and intra-PVH connections are known to exist, it remains possible that activation of a defined cell population could be cascaded through neighboring cells to effect physiological outputs (1-3, 59). In this regard, CalcRPVH neurons, when activated, may serve to transmit a signal within the PVH rather than directly to a downstream target. Technologies that manipulate discrete CalcRPVH outputs will be needed to better characterize the brain regions targeted by CalcRPVH neurons to modulate defined energy balance parameters.
The PVH is composed of a variety of neuronal subpopulations that have been implicated in energy balance regulation (2, 3, 60). Discriminating these subsets of neurons by receptor or neuropeptide expression is important for understanding PVH function. Neurochemical characterization of genetically tagged PVH neurons is a critical step in interpreting the mechanisms and potential novelty of functional manipulations and helps formulate hypotheses regarding the molecular mechanisms engaged to drive physiologic responses to changes in PVH activity. In the case of CalcRPVH neurons, we found them to be distinct from the NOS1PVH, OXTPVH, and AVPPVH neuronal subpopulations, each of which alter energy balance when acutely activated (11, 13, 61). This raises the intriguing possibility of the additive benefits of selectively activating combinations of these populations. Such studies would also reveal whether there is a threshold for the beneficial effects of PVH stimulation on feeding and energy expenditure.
Subsets of CalcRPVH neurons express corticotropin-releasing hormone (CRH) or thyrotropin-releasing hormone (TRH), neuropeptides implicated in feeding and energy expenditure (62, 63). Intracerebral injection of CRH in genetically obese and lean mice decreases feeding, decreases oxygen consumption and decreases activity level (62). Mice lacking CRH, however, display a normal body weight (64); whether developmental loss of CRH provokes compensation in body weight neural circuitry to prevent obesity is not known. CRHPVH neuron activity is rapidly reduced following refeeding after a fast, indicating that CRHPVH neuron activity may be more responsive to alterations in stress than feeding directly (24). In addition, chronic silencing of CRHPVH neurons is not associated with hyperphagia in contrast to what we find with CalcRPVH neuron silencing (24). There is a small amount of overlap between the CalcR and TRH neuronal populations of the PVH. TRHPVH neurons regulate the thyroid axis which directly contributes to resting energy expenditure (22, 63). Chemogenetic activation of TRHPVH neurons stimulates feeding through projections to AGRP neurons, which is opposite to the anorectic effect of activating CalcRPVH neurons (65). Taken together, it seems unlikely that CRHPVH or TRHPVH neurons contained within the CalcRPVH field are responsible for the physiologic effects on body weight and feeding following CalcRPVH neuron manipulations.
The central melanocortin system is a critical component of energy balance control and direct manipulations of MC4Rs in the PVH have profound effects on feeding and body weight (15, 26, 49). Since manipulation of CalcRPVH neurons altered feeding, we examined the overlap of MC4R and CalcRs in the PVH and found that nearly half of the MC4RPVH neurons co-express CalcR. Selective silencing of CalcRPVH neurons therefore effectively disables signaling from about half of the MC4RPVH neurons. Given the gene dosage effect associated with MC4R-mediated obesity, loss of ~1/2 of MC4RPVH through inactivation of CalcRPVH neurons would be predicted to produce a fraction of the overall obesity seen upon complete MC4RPVH inactivation. Consistent with this prediction, we find that the change in body weight in the MC4RPVH-silenced mice is nearly twice as much as compared to the CalcRPVH-silenced mice. This comparative approach is an indirect method of determining the necessity of MC4R expression in CalcRPVH neurons to mediate energy homeostasis. To definitively assess the contribution of melanocortin signaling CalcRPVH neurons to body weight control, specific deletion of MC4Rs from CalcRPVH neurons is required. Simply crossing the CalcR2ACre line to a loxP-flanked MC4R transgenic line would delete MC4R expression from all CalcR neurons, including those expressed outside the PVH, and confound the data interpretation.
There are important caveats associated with our experimental approach. Tethering Cre-recombinase activity to CalcR gene expression allows us to target CalcR-expressing neurons specifically, but subsequent activation or silencing of these neurons with chemogenetics or tetanus toxin expression impacts a variety of signaling systems within these cells. Indeed, manipulations of CalcRPVH neuron activity with viral vectors should not be viewed as a surrogate for calcitonin agonist signaling in these neurons. The interpretation of any physiologic effect attributed to CalcRPVH neuron activation or silencing must take into account that a range of signaling pathways and neurotransmitter release are being affected. This highlights the importance of identifying other neuropeptides (eg, CRH or TRH) and signaling systems (NOS1) that function within CalcRPVH neurons, as these systems likely contribute to the modulation of energy balance parameters revealed by our viral manipulations.
Through Cre-dependent viral tools and Cre-LoxP technology, we find that CalcRPVH neuron activity is required for maintaining normal body weight in mice which highlights an important role for CalcRPVH neurons in energy homeostasis. CalcRPVH neurons are largely distinct from other known PVH neurons and project to brain regions known to be involved in energy balance control. A thorough understanding of CalcRPVH neurons and their neurocircuitry will deepen our understanding of central energy balance control and may uncover novel approaches to targeting disordered eating and obesity.
Acknowledgments
Core support (Animal Phenotyping) was provided by the Michigan Diabetes Research and Training Center and Nutrition and Obesity Research Center. We also thank members of both the Olson and Myers labs for helpful discussions and technical support.
Financial Support: This work was supported by the US National Institutes of Health (F31 DK122753-01 to I.E.G., DK104999 to D.P.O.), the Systems and Integrative Biology Training Grant (T32-GM008322 to I.E.G.) and the Michigan Diabetes Research Center pilot and feasibility program (DK20572 to D.P.O.).
Author Contributions: D.P.O. and I.E.G. conceived the project and designed research. I.E.G., J.R.M, C.L., and A.Z. performed the experiments and analyzed the data. C.L. performed the in situ hybridization. D.P.O. and W.P. generated the CalcR2Acre mice. D.P.O. and M.G.M. provide the resources. D.P.O and I.E.G. wrote the manuscript. D.P.O., M.G.M., I.E.G., J.R.M, C.L., W.P., and A.Z. reviewed and edited the manuscript.
Glossary
Abbreviations
- ANOVA
analysis of variance
- CalcR
calcitonin receptor
- CalcRPVH
calcitonin receptor–expressing PVH neurons
- CLAMS
Comprehensive Laboratory Animal Monitoring System
- CNO
clozapine N-oxide
- CRH
corticotropin-releasing hormone
- DREADD
Designer Receptors Exclusively Activated by Designer Drugs
- GFP
green fluorescent protein
- IHC
immunohistochemistry
- i.p.
intraperitoneal
- LPBN
lateral parabrachial nucleus
- MC4R
melanocortin-4 receptor
- MPBN
medial parabrachial nucleus
- NOS1
nitric oxide synthase-1
- NTS
nucleus of the solitary tract
- OXT
oxytocin
- PBN
parabrachial nucleus
- PVH
paraventricular nucleus of the hypothalamus
- sCT
salmon calcitonin
- TRH
thyrotropin-releasing hormone
- WT
wild-type
Additional Information
Disclosures: I.E.G., J.R.M., C.L., W.P., and A.Z. have nothing to declare. D.P.O. and M.G.M. receive research funding from Novo Nordisk and MedImmune.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
- 1. Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15(6):367-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sutton AK, Myers MG Jr, Olson DP. The Role of PVH Circuits in Leptin Action and Energy Balance. Annu Rev Physiol. 2016;78:207-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31(6):410-417. [DOI] [PubMed] [Google Scholar]
- 4. Sims JS, Lorden JF. Effect of paraventricular nucleus lesions on body weight, food intake and insulin levels. Behav Brain Res. 1986;22(3):265-281. [DOI] [PubMed] [Google Scholar]
- 5. Leibowitz SF, Hammer NJ, Chang K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol Behav. 1981;27(6):1031-1040. [DOI] [PubMed] [Google Scholar]
- 6. Duplan SM, Boucher F, Alexandrov L, Michaud JL. Impact of Sim1 gene dosage on the development of the paraventricular and supraoptic nuclei of the hypothalamus. Eur J Neurosci. 2009;30(12):2239-2249. [DOI] [PubMed] [Google Scholar]
- 7. Tolson KP, Gemelli T, Gautron L, Elmquist JK, Zinn AR, Kublaoui BM. Postnatal Sim1 deficiency causes hyperphagic obesity and reduced Mc4r and oxytocin expression. J Neurosci. 2010;30(10):3803-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12(20):3264-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holder JL Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9(1):101-108. [DOI] [PubMed] [Google Scholar]
- 10. Xi D, Gandhi N, Lai M, Kublaoui BM. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. Plos One. 2012;7(4):e36453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sutton AK, Pei H, Burnett KH, Myers MG Jr, Rhodes CJ, Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci. 2014;34(46):15306-15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol Metab. 2014;3(2):209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sutton AK, Gonzalez IE, Sadagurski M, et al. Paraventricular, subparaventricular and periventricular hypothalamic IRS4-expressing neurons are required for normal energy balance. Sci Rep. 2020;10(1):5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garfield AS, Li C, Madara JC, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18(6):863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perlow MJ, Freed WJ, Carman JS, Wyatt RJ. Calcitonin reduces feeding in man, monkey and rat. Pharmacol Biochem Behav. 1980;12(4):609-612. [DOI] [PubMed] [Google Scholar]
- 17. Plata-Salamán CR, Oomura Y. Calcitonin as a feeding suppressant: localization of central action to the cerebral III ventricle. Physiol Behav. 1987;40(4):501-513. [DOI] [PubMed] [Google Scholar]
- 18. Lutz TA, Tschudy S, Rushing PA, Scharrer E. Amylin receptors mediate the anorectic action of salmon calcitonin (sCT). Peptides. 2000;21(2):233-238. [DOI] [PubMed] [Google Scholar]
- 19. Bello NT, Kemm MH, Moran TH. Salmon calcitonin reduces food intake through changes in meal sizes in male rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2008;295(1):R76-R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheward WJ, Lutz EM, Harmar AJ. The expression of the calcitonin receptor gene in the brain and pituitary gland of the rat. Neurosci Lett. 1994;181(1-2):31-34. [DOI] [PubMed] [Google Scholar]
- 21. Chait A, Suaudeau C, De Beaurepaire R. Extensive brain mapping of calcitonin-induced anorexia. Brain Res Bull. 1995;36(5):467-472. [DOI] [PubMed] [Google Scholar]
- 22. Cheng W, Gonzalez I, Pan W, et al. Calcitonin Receptor Neurons in the Mouse Nucleus Tractus Solitarius Control Energy Balance via the Non-aversive Suppression of Feeding. Cell Metab. 2020;31(2):301-312.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan W, Adams JM, Allison MB, et al. Essential Role for Hypothalamic Calcitonin Receptor‒Expressing Neurons in the Control of Food Intake by Leptin. Endocrinology. 2018;159(4):1860-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li C, Navarrete J, Liang-Guallpa J, et al. Defined Paraventricular Hypothalamic Populations Exhibit Differential Responses to Food Contingent on Caloric State. Cell Metab. 2019;29(3): 681-694.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. RRID:IMSR_JAX:030759.
- 26. Shah BP, Vong L, Olson DP, et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci U S A. 2014;111(36):13193-13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89(4):683-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104(12):5163-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Link E, Edelmann L, Chou JH, et al. Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun. 1992;189(2):1017-1023. [DOI] [PubMed] [Google Scholar]
- 30. Humeau Y, Doussau F, Grant NJ, Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82(5):427-446. [DOI] [PubMed] [Google Scholar]
- 31. RRID:AB_10013483.
- 32. RRID:AB_2534017.
- 33. RRID:AB_2247211.
- 34. RRID:AB_2732803.
- 35. RRID:AB_2535792.
- 36. RRID:AB_141874.
- 37. RRID:AB_10000240.
- 38. RRID:AB_142924.
- 39. RRID:AB_221570.
- 40. RRID:AB_518524.
- 41. RRID:AB_90782.
- 42. Dubé D, Pelletier G. Effect of colchicine on the immunohistochemical localization of somatostatin in the rat brain: light and electron microscopic studies. J Histochem Cytochem. 1979;27(12):1577-1581. [DOI] [PubMed] [Google Scholar]
- 43. RRID:AB_2884885.
- 44. RRID:AB_2314240.
- 45.RRID:AB_2534102.
- 46. RRID:AB_2534083.
- 47. An JJ, Liao GY, Kinney CE, Sahibzada N, Xu B. Discrete BDNF Neurons in the Paraventricular Hypothalamus Control Feeding and Energy Expenditure. Cell Metab. 2015;22(1):175-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35(2):159-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Balthasar N, Dalgaard LT, Lee CE, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493-505. [DOI] [PubMed] [Google Scholar]
- 50. Xiao C, Liu N, Province H, Piñol RA, Gavrilova O, Reitman ML. BRS3 in both MC4R- and SIM1-expressing neurons regulates energy homeostasis in mice. Mol Metab. 2020;36:100969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tam CS, Lecoultre V, Ravussin E. Novel strategy for the use of leptin for obesity therapy. Expert Opin Biol Ther. 2011;11(12):1677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lutz TA, Rossi R, Althaus J, Del Prete E, Scharrer E. Amylin reduces food intake more potently than calcitonin gene-related peptide (CGRP) when injected into the lateral brain ventricle in rats. Peptides. 1998;19(9):1533-1540. [DOI] [PubMed] [Google Scholar]
- 53. Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16(3):296-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roman CW, Sloat SR, Palmiter RD. A tale of two circuits: CCKNTS neuron stimulation controls appetite and induces opposing motivational states by projections to distinct brain regions. Neuroscience. 2017;358:316-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276(6):R1569-R1578. [DOI] [PubMed] [Google Scholar]
- 56. Cao WH, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2010;299(1):R277-R290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fyda DM, Cooper KE, Veale WL. Modulation of brown adipose tissue-mediated thermogenesis by lesions to the nucleus tractus solitarius in the rat. Brain Res. 1991;546(2):203-210. [DOI] [PubMed] [Google Scholar]
- 58. Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R831-R843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu Y, Wu Z, Sun H, et al. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab. 2013;18(6):860-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Biag J, Huang Y, Gou L, et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520(1):6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yoshimura M, Nishimura K, Nishimura H, et al. Activation of endogenous arginine vasopressin neurons inhibit food intake: by using a novel transgenic rat line with DREADDs system. Sci Rep. 2017;7(1):15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Drescher VS, Chen HL, Romsos DR. Corticotropin-releasing hormone decreases feeding, oxygen consumption and activity of genetically obese (ob/ob) and lean mice. J Nutr. 1994;124(4):524-530. [DOI] [PubMed] [Google Scholar]
- 63. Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209-235. [DOI] [PubMed] [Google Scholar]
- 64. Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373(6513):427-432. [DOI] [PubMed] [Google Scholar]
- 65. Krashes MJ, Shah BP, Madara JC, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507(7491):238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.