Abstract
Purpose
High-grade patterns (micropapillary/solid/complex gland) are associated with a higher recurrence rate and shorter disease-free survival. Thus far, it remains unclear whether the efficacy of first-line anticancer therapy is different from that of the other adenocarcinoma subgroups for patients with high-grade patterns. The study aimed to investigate the association between an adenocarcinoma with high-grade patterns with the outcomes of first-line treatment in patients with lung cancer.
Patients and Methods
Patients with a high-grade pattern adenocarcinoma (more than 20% of micropapillary/solid components/complex glandular patterns) were retrospectively analyzed between June 2015 and June 2017. Patients’ clinical characteristics and treatment outcomes were compared with those of the remaining control adenocarcinoma subgroups.
Results
In total, 239 patients with adenocarcinoma, including 115 (48.1%) high-grade patterns and 124 (51.9%) control groups, were enrolled. Patients’ clinical characteristics such as age, sex, smoking status, and stage were similar between the two groups. Among them, 108 patients received first-line chemotherapy, and 131 received epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs). In the chemotherapy group, adenocarcinoma of high-grade patterns had a significantly lower objective response rate (ORR; 15.6% vs 36.4%, P=0.045), shorter progression-free survival (PFS; median 4.1 vs 5.4 months, P=0.007) and overall survival (OS, median 19.6 vs 23.8 months, P=0.048) compared with the control group. As for these treated with EGFR-TKIs, a similar ORR (70.7% vs 72.1%, P=0.703), PFS (median 11.3 vs 13.9 months, P=0.065) and OS (median 34.1 vs 29.6%, p=0.575) were observed between these two groups.
Conclusion
An adenocarcinoma with high-grade patterns is associated with inferior outcomes to first-line chemotherapy in relapsed lung cancer. Patients who received chemotherapy had a significantly shorter PFS and OS and lower ORR than control subjects, while there was no difference in the EGFR-TKI cohort. This study is the first to report the distribution of adenocarcinoma with high-grade patterns.
Keywords: adenocarcinoma, micropapillary, solid, chemotherapy, EGFR-TKIs
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide, and adenocarcinoma is the most common histological subtype. In 2015, based on the predominant histologic pattern, the World Health Organization (WHO) recommended a new classification and divided pulmonary adenocarcinoma into the following prognostic groups: lepidic, acinar, papillary, solid, and micropapillary predominant.1 Recently, complex glandular patterns (cribriform and fused glands) have been recognized and added as a subtype of pulmonary adenocarcinoma.2–6
Over the past decade, the landscape of adjuvant treatment in patients with lung cancer has dramatically changed.7–9 Evidence from previous literatures suggested that tumors with micropapillary, solid components (MS) and complex glandular patterns are associated with a higher recurrence rate and poorer prognosis.10–17 Thus, pathological subtype needs to be taken into consideration when performing adjuvant treatment. A new grading system showed that tumors with 20% or more of high-grade patterns (micropapillary and solid components and complex glandular patterns) behave in a more aggressive fashion similar to those with a predominant high-grade pattern; thus, these tumors should be classified as poorly differentiated.6 Although several clinical trials (ie, NCT03254004 and NCT03351842) are ongoing to evaluate the efficacy of adjuvant treatment in these settings, whether the efficacy of first line systemic therapy in patients with high-grade patterns is different from that of the other adenocarcinoma subgroups is still largely unknown.
Aimed to investigate the clinicopathological characteristics of high-grade pattern adenocarcinoma and its association with the efficacy of first-line treatment, we retrospectively collected 239 patients with adenocarcinoma in this study.
Patients and Methods
Patient Population and Study Design
From June 2015 to June 2017, 115 patients with high-grade pattern adenocarcinoma (any tumor with more than 20% solid, micropapillary, or complex glandular patterns) who underwent surgical resection and relapsed were collected from Shanghai Pulmonary Hospital, Tongji University School of Medicine. We randomly selected 124 patients with other adenocarcinoma subgroups in the same period as the comparator.6 The inclusion criteria were as follows: (1) previous surgery. (2) Pathologically diagnosed as an invasive adenocarcinoma. (3) Patients with recurrent IIIB/IV NSCLC (4) received standard first-line chemotherapy or first-generation epidermal growth factor receptor–tyrosine kinase inhibitors (EGFR-TKIs). Patients should have evaluable lesions according to the response evaluation criteria solid tumor criteria (RECIST) version 1.1.18 The study was approved by the ethics committee of Shanghai Pulmonary Hospital according to the Declaration of Helsinki, and informed consent was obtained from each patient.
Pathologic Evaluation
All resected specimens were fixed in 10% formalin and embedded in paraffin. Serial sections were stained with hematoxylin and eosin, and all cases using the semiquantitative estimation of all patterns of 5% increment as suggested by the current WHO classification of lung tumors.19 Five patterns recognized by WHO and nontraditional patterns, such as cribriform and fused glands (complex glandular patterns), were included in a total of 100%. All patients were re-staged according to the eighth edition of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) TNM classification for lung cancer.20,21 The grading system was adopted by IASLC.6 All samples were reanalyzed by experienced pathologists (DZ and WC) who were blinded to the clinical characteristics and prognosis of patients. All of the slides were reviewed independently by 2 pathologists. If there was a discrepancy in the results, a third pathologist reviewed the slide and reached a consensus after discussion.
EGFR Mutation Detection
All mutation analyses were performed at the Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China. Briefly, DNA from tissue was extracted using the DNeasy Blood and Tissue Kit or the QIAamp DNA FFPE tissue kit (Qiagen, Hilden, Germany). EGFR mutations were detected by ARMS, as described in our previous publications (Amoy Diagnostics Co. Ltd., Xiamen, China).22,23
Clinical Assessment
Clinical information was obtained from patient medical records. We screened the data, including sex, age, smoking history, stage, Eastern Cooperative Oncology Group Performance Status (ECOG-PS) status, histological subtype, adjuvant chemotherapy, and first-line therapy. The objective response rate (ORR) was calculated as the total percentage of patients with complete response (CR) or partial response (PR). A clinical evaluation of PFS was performed from the start of the first-line chemotherapy to the earliest identifiable sign of disease progression, as determined using computed tomography or magnetic resonance imaging using the RECIST or any cause of death. The overall survival (OS) was measured as the period from the start of first-line chemotherapy until death from any cause.
Statistical Analysis
The relationship between categorical parameters was tested using Pearson’s χ2 test (Fisher’ s exact test was used when n≤5). The Kaplan–Meier curve was used to estimate the distribution of survival, and the Log rank test was used to analyze the differences between the groups. We used Cox proportional hazards models for univariate and multivariate analyses to estimate clinicopathological features. Independent variables (P<0.10 in the univariate analysis were included in the multivariate analysis. P-values <0.05 were defined statistically significant. Confidence intervals (CIs) were calculated using 95% confidence interval (CI). Statistical tests were performed using SPSS software (version 20.0; IBM Corporation, Armonk, NY, USA).
Results
Patient Characteristics
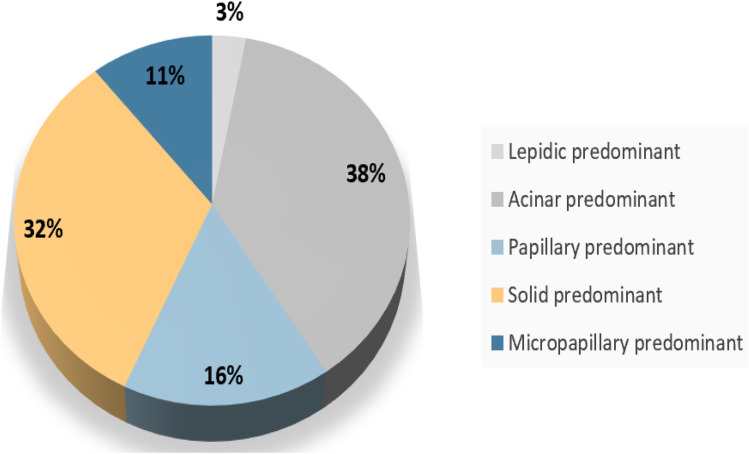
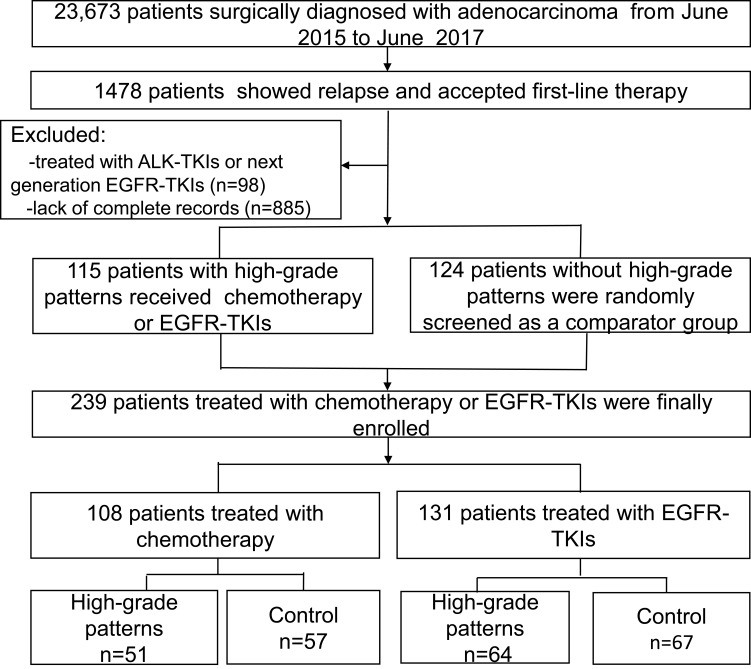
From June 1, 2015, to June 1, 2017, total of 1478 patients were relapsed after surgery. Patients with micropapillary, solid, acinar, papillary, and lepidic predominant patterns accounted for 7.9% (117/1478), 32.4% (479/1478), 38.0% (562/1478),15.7% (232/1478), and 3.0% (44/1478), respectively (Figure 1). Among them, 115 patients with high-grade tumors who received first-line chemotherapy or EGFR-TKIs and documented detailed clinicopathological features were enrolled for further analysis. We randomly matched 124 patients of the remaining adenocarcinoma subgroups as a comparator. Ultimately, 239 patients were included in this study. A flowchart describing the eligible study selection was shown in Figure 2. The detail Their baseline characteristics are presented in Table 1.
Figure 1.
Distribution of histological subtypes in relapsed patients.
Figure 2.
Flowchart of the patient selection process.
Table 1.
Baseline Characteristics of the Patients
| Characteristics | All Patients (n=239) | High-Grade Patterns (n=115) | Control (n=124) | P value |
|---|---|---|---|---|
| Age, n (%) | 0.943 | |||
| ≥70 | 42(17.6) | 20(17.4) | 22(17.7) | |
| <70 | 197(82.4) | 95(82.6) | 102(82.3) | |
| Sex, n (%) | 0.358 | |||
| Male | 134(56.1) | 68(59.1) | 66(53.2) | |
| Female | 105(43.9) | 47(40.9) | 58(46.8) | |
| Smoking, n (%) | 0.099 | |||
| Yes | 73(30.5) | 41(35.7) | 32(25.8) | |
| No | 166(69.5) | 74(64.3) | 92(74.2) | |
| ECOG, n (%) | 0.424 | |||
| 0–1 | 228(95.4) | 111(96.5) | 117(94.4) | |
| 2 | 11(4.6) | 4(3.5) | 7(5.6) | |
| Extra-metastases, n (%) | 0.846 | |||
| Yes | 92(38.5) | 45(39.1) | 47(37.9) | |
| No | 147(61.5) | 70(60.9) | 77(62.1) | |
| Adjuvant chemotherapy, n (%) | 0.398 | |||
| Yes | 162(67.8) | 81(70.4) | 81(65.3) | |
| No | 77(32.2) | 34(29.6) | 43(34.7) | |
| Gene, n (%) | 0.767 | |||
| EGFR | 131(54.8) | 64(55.7) | 67(54.0) | |
| EGFR19DEL | 76(31.8) | 39(33.9) | 37(29.8) | |
| EGFRL858R | 55(23.0) | 25(21.7) | 30(24.2) | |
| KRAS | 18(7.5) | 11(9.6) | 7(5.6) | |
| WT | 80(33.5) | 36(45.0) | 44(35.5) | |
| NA | 7(2.9) | 3(2.6) | 4(3.2) | |
| ERBB2 | 2(0.8) | 1(0.9) | 1(0.8) | |
| BRAF | 1(0.4) | 0(0) | 1(0.8) | |
| First-line therapy, n(%) | 0.801 | |||
| Chemotherapy | 108(45.2) | 51(44.3) | 57(46.0) | |
| TKI | 131(54.8) | 64(55.7) | 67(54.0) | |
| Stage, n (%) | 0.291 | |||
| rIIIB | 24(10.0) | 14(12.2) | 10(8.1) | |
| rIV | 215(90.0) | 101(87.8) | 114(91.9) | |
| Time to recurrence after surgery, n (%) | 0.084 | |||
| Less than one year | 188(78.7) | 85(73.9) | 103(83.1) | |
| More than one year | 51(21.3) | 30(26.1) | 21(16.9) | |
| Histological subtype, n (%) | <0.001 | |||
| Lepidic predominant | 1(0.4) | 0(0) | 1(0.8) | |
| Acinar predominant | 116(48.5) | 34(29.6) | 82(66.1) | |
| Papillary predominant | 58(24.3) | 17(14.8) | 41(33.1) | |
| Solid predominant | 53(22.2) | 53(46.1) | 0(0) | |
| Micropapillary predominant | 11(4.6) | 11(9.6) | 0(0) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; KRAS, V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; WT, wild type; NA, not measure; ERBB2, Avian Erythroblastic Leukemia Viral Oncogene Homolog 2; BRAF, v-Raf murine sarcoma viral oncogene homolog B.
The median age of the patients was 51 years, and 134 patients were male. The majority of patients were nonsmokers (69.5%) and had an ECOG PS of 0 to 1 (95.4%) and stage IV disease (90.0%). Most of the patients relapsed within one year (78.7%). Moreover, 54.8% (137/239) patients had EGFR mutations and received first-generational EGFR-TKIs treatment, total of 45.2% (108/239) patients received first-line chemotherapies. The baseline clinicopathological characteristics of the two groups are shown in Table 1 and Supplementary Table 1.
Association of High-Grade Patterns with the Outcomes of Chemotherapy
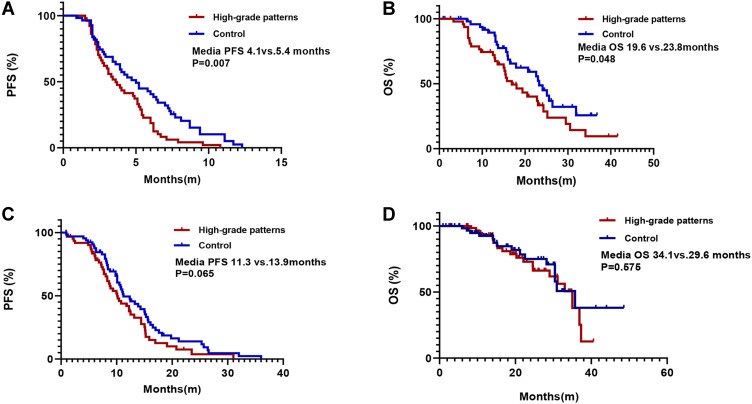
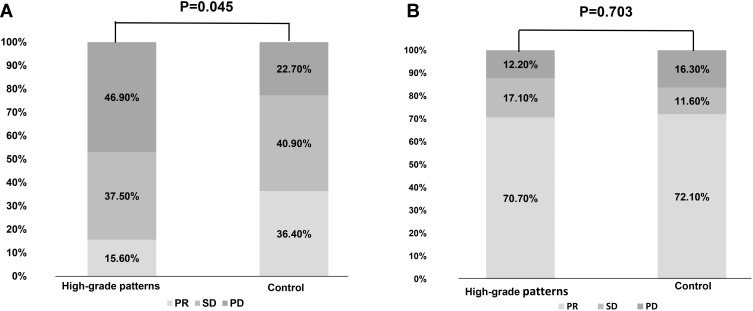
Among the 108 patients who received first-line chemotherapy, there were no significant differences in age, sex, ECOG-PS status, and smoking history between the two groups (Table S2). At the last follow-up of June 15, 2020, 94.4% (102/108) of patients had disease progression. High-grade patterns group had a significant shorter PFS (median 4.1 vs 5.4 months, p=0.007) and OS (median 19.6 vs 23.8m, p=0.048) than the control group (shown in Figure 3A and B). Consistently, multivariate analysis also demonstrated that high-grade patterns were independent factors predicting clinical benefit (HR=1.668, 95% CI 1.096–2.537; p=0.017, Table 2). Meanwhile, high-grade patterns were also associated with a significantly lower ORR (15.60% vs 36.40%, p=0.029, Figure 4A).
Figure 3.
Kaplan-Meier survival curves for patients in different cohorts. (A) Progression-free survival (PFS) of first-line chemotherapy in epidermal growth factor receptor (EGFR) wild-type patients with high-grade patterns and in control subjects; (B) overall survival (OS) of first-line chemotherapy in EGFR wild-type patients with high-grade patterns and control cohorts; (C) PFS curves of first-line EGFR-tyrosine kinase inhibitor (TKI) treatment in EGFR+ patients with high-grade patterns and control cohorts; (D) OS curves of first-line EGFR-TKI treatment in EGFR+ patients with high-grade patterns and control cohorts.
Table 2.
Impact of Predictive Factors on Chemotherapy in Univariate and Multivariate Analyses (n = 108)
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value |
| Age (≥70) | 2.019(1.138–3.580) | 0.016 | 1.734(0.972–3.092) | 0.062 |
| Gender (male) | 1.600(1.027–2.494) | 0.038 | 1.443(0.918–2.268) | 0.112 |
| Smoking status (smoker) | 0.834(0.549–1.267) | 0.395 | ||
| ECOG (2) | 1.391(0.606–3.194) | 0.436 | ||
| Extra metastases (positive) | 1.330(0.808–2.189) | 0.262 | ||
| Histological subtypes (high-grade patterns) | 1.762(1.162–2.672) | 0.008 | 1.668(1.096–2.537) | 0.017 |
| Adjuvant chemotherapy (yes) | 1.066(0.711–1.597) | 0.758 | ||
| First-line chemotherapy (platinum based) | 1.391(0.641–3.017) | 0.404 | ||
| Gene mutation status (WT) | 0.778(0.489–1.238) | 0.220 | ||
| Vascular spread(present) | 0.858(0.476–1.545) | 0.609 | ||
| T stage (T3, T4) | 1.036(0.595–1.804) | 0.899 | ||
| Nodal state (N2, N3) | 1.042(0.662–1.639) | 0.860 | ||
| Pleural involvement(present) | 0.668(0.323–1.382) | 0.227 | ||
| Stage (rIV) | 0.937(0.528–1.663) | 0.958 | ||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; WT, wild type.
Figure 4.
Response to different therapies in different cohorts. (A) Response to first-line chemotherapy in high-grade patterns and control cohorts. (B) Response to first-generation tyrosine kinase inhibitors (TKIs) in high-grade patterns and control cohorts.
Abbreviations: ORR, objective response rate; PR, partial response; SD, stable disease; PD, progressive disease.
Association of High-Grade Patterns with the Outcomes of EGFR-TKIs
Among the 131 patients who received first-line EGFR-TKIs, 48.9% were in the high-grade pattern group and 51.1% were in the control group. Of these patients, 58.8% (77/131) received gefitinib, 27.5% (36/131) received erlotinib, and 13.7% (18/131) received icotinib (Table S3). Patients in the high-grade pattern group had similar ORR (70.7% vs 72.1%, P=0.703, Figure 4B), similar PFS (median 11.3 vs 13.9 months, P=0.065, Figure 3C) and similar OS (median 34.1 vs 29.6 months, P=0.575, Figure 3D) as that of the control group. Consistently, Cox regression analysis demonstrated that smoking status was the only independent factor that could predict the outcomes of EGFR-TKIs between the two groups (HR, 1.882; 95% CI, 1.168–3.033; P=0.009, Table 3).
Table 3.
Impact of Predictive Factors on EGFR-TKIs in Univariate and Multivariate Analyses (n = 131)
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value |
| Age (≥70) | 0.877(0.519–1.483) | 0.625 | ||
| Sex (female) | 1.052(0.708–1.563) | 0.801 | ||
| Smoking status (smoker) | 1.947(1.209–3.138) | 0.006 | 1.882(1.168–3.033) | 0.009 |
| ECOG (2) | 1.491(0.546–4.070) | 0.436 | ||
| Extra-metastases (positive) | 0.859(0.575–1.282) | 0.447 | ||
| Histological subtypes (high-grade patterns) | 1.450(0.974–2.159) | 0.067 | 1.395(0.936–2.080) | 0.102 |
| EGFR mutations (19DEL) | 1.183(0.792–1.768) | 0.412 | ||
| Targeted therapy (Gefitinib) | 1.198(0.962–1.491) | 0.106 | ||
| Vascular spread(positive) | 1.066(0.661–1.710) | 0.794 | ||
| T stage (T3, T4) | 1.317(0.795–2.182) | 0.285 | ||
| Nodal state (N2, N3) | 1.166(0.751–1.810) | 0.494 | ||
| Pleural involvement(present) | 0.570(0.278–1.171) | 0126 | ||
| Stage (rIV) | 1.025(0.496–2.118) | 0.946 | ||
Abbreviations: ECOG, Eastern Cooperative Oncology Group; 19DEL, exon 19 deletion.
Discussion
To the best of our knowledge, this is the first study to investigate the distribution of adenocarcinoma with high-grade patterns and its association with the efficacy of first-line treatment in patients with NSCLC. We found a high incidence of 32.4% and 10.9% for solid pattern and micropapillary pattern, respectively, in patients with relapsed NSCLC (Figure 2). More importantly, we found that high-grade patterns have a significantly lower ORR and shorter PFS and OS in patients receiving first-line chemotherapy than the control group, while the difference was not observed in these patients who received first-line EGFR-TKIs.
Pulmonary adenocarcinoma classification and grading were found to be associated with prognosis and showed the potent to guide systemic therapy.11,14,15,19,24–29 This study included the largest cohort of patients with pulmonary adenocarcinoma who relapsed after surgical resection. We found a higher incidence of solid and micropapillary predominant adenocarcinoma compared to that in the open-source data.14,30 Meanwhile, patients with lepidic-predominant lung adenocarcinoma were lower in our study than in previous reports,10,31 reiterating adenocarcinoma with high-grade patterns had a higher risk of recurrence than those with other adenocarcinoma subgroups.11
Due to its aggressive nature,32 the current NCCN guidelines recommend adjuvant chemotherapy for patients with stage IB adenocarcinoma with high-grade patterns. However, few studies have investigated the efficacy of first-line treatment of micropapillary or solid components. Our study found that adenocarcinoma with high-grade patterns (any tumor with more than 20% solid, micropapillary, or complex glandular patterns) had a significantly lower ORR and shorter median PFS and OS in patients who received first-line chemotherapy when compared with the control group. Similar to our studies, Cruz et al found that the presence of the solid subtype was related to poor PFS and OS.27 Notably, Campos-Parra et al reported that patients with micropapillary/solid/papillary-predominant adenocarcinoma have a better response to chemotherapy;33 however, a subgroup analysis showed that patients with high-grade patterns were associated with shorter PFS and OS. Taken together, these findings confirmed the benefit of adjuvant chemotherapy in patients with adenocarcinoma with high-grade patterns and stage IB. We are still looking forward to the results of several ongoing randomized trials in this setting.
Currently, targeted therapy has been the standard of care in patients with advanced NSCLC and EGFR, ALK, ROS1, BRAF and other mutations.34 As for adjuvant target therapy, both the ADJUVANT and EVAN studies demonstrated that gefitinib or erlotinib had longer disease-free survival than chemotherapy in patients with stage II to IIIA.35,36 Recently, in the ADURA study, osimertinib was associated with significantly longer disease-free survival, even in EGFR-mutant patients with stage IB NSCLC as adjuvant treatment.37 Our study investigated the efficacy of EGFR-TKIs according to different histological subtypes and observed similar ORR, PFS, and OS, which supports the potency of adjuvant EGFR-TKIs rather than chemotherapy for patients with stage IB with EGFR mutation and high-grade pattern adenocarcinoma. Moreover, Cox regression analysis demonstrated smoking status was the only independent factor for the outcomes of EGFR-TKIs. However, EGFR-TKIs have a significant effect in slowing down disease’s progression in elderly patients with advanced NSCLC in another study.38 The inconsistence might be due to the limited sample size in our study.
There are several limitations to this study. Firstly, this was a single institutional retrospective study with a limited number of patients, targeted group did not collect sufficient sample size for subgroup analysis for rare mutations other than the common EGFR mutations, which inevitably results in selection bias, and large-scale studies, especially prospective studies, are needed. Secondly, only patients who received first-generation EGFR-TKIs were included, and the results could not be generalized to patients receiving 2nd/3rd generation EGFR-TKIs. Thirdly, we did not perform rebiopsy after recurrence. However, due to a high proportion (78.7%) of patients relapsed within one year, the pathological classification from the resection samples could represent the relapse situation in the majority of patients. Forthly, we did not detect other biomarkers that might affect the efficacy. For example, Chabon et al found that ctDNA levels were associated with radiologic appearance in lung adenocarcinomas.8
Conclusion
In conclusion, an adenocarcinoma with high-grade patterns is more likely to relapse after resection. Importantly, it is associated with inferior outcomes to first-line chemotherapy in patients with relapsed lung cancer. However, no difference in efficacy was observed after treatment with EGFR-TKIs, which might shed light on the future direction of adjuvant anti-cancer treatment in patients with early-stage adenocarcinoma with high-grade patterns.
Acknowledgments
I thank the members of my laboratory for helpful discussions. Xiaofei Yu and Zhengwei Dong share first authorship.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (No. 81772467 to S.R., No. 81972167 to S.R.), Shanghai Pujiang Program (No. 2019PJD048 to S.R.), Shanghai Shenkang Hospital Development Center (No. SHDC12019133 to S.R.) and the Backbone Program of Shanghai Pulmonary Hospital (NO. FKGG1802).
Abbreviations
ECOG, Eastern Cooperative Oncology Group; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; KRAS, V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; WT, wild type; NA, not measure; ERBB2, Avian Erythroblastic Leukemia Viral Oncogene Homolog 2; BRAF, v-Raf murine sarcoma viral oncogene homolog B; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; ORR, objective response rate; CR, complete response; PR, partial response; PFS, progression-free survival; OS, overall survival.
Ethics Approval and Consent to Participate
The experiments were approved by the Shanghai Pulmonary Hospital, Tongji University School of Medicine. Written informed consent was obtained from all patients.
Author Contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest or financial interest in this work.
References
- 1.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 2.von der Thüsen JH, Tham YS, Pattenden H, et al. Prognostic significance of predominant histologic pattern and nuclear grade in resected adenocarcinoma of the lung: potential parameters for a grading system. J Thorac Oncol. 2013;8(1):37–44. doi: 10.1097/JTO.0b013e318276274e [DOI] [PubMed] [Google Scholar]
- 3.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25(8):1117–1127. doi: 10.1038/modpathol.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadota K, Nitadori JI, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage i lung adenocarcinomas. J Thorac Oncol. 2015;10(5):806–814. doi: 10.1097/JTO.0000000000000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadota K, Kushida Y, Kagawa S, et al. Cribriform subtype is an independent predictor of recurrence and survival after adjustment for the eighth edition of TNM staging system in patients with resected lung adenocarcinoma. J Thorac Oncol. 2019;14(2):245–254. doi: 10.1016/j.jtho.2018.09.028 [DOI] [PubMed] [Google Scholar]
- 6.Moreira AL, Ocampo PSS, Xia Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the international association for the study of lung cancer pathology committee. J Thorac Oncol. 2020;15(10):1599–1610. doi: 10.1016/j.jtho.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J, Li R, Tiselius E, et al. Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent. Cochrane Database Syst Rev. 2017;12(12):Cd011300. doi: 10.1002/14651858.CD011300.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohsenzadegan M, Peng RW, Roudi R. Dendritic cell/cytokine-induced killer cell-based immunotherapy in lung cancer: what we know and future landscape. J Cell Physiol. 2020;235(1):74–86. doi: 10.1002/jcp.28977 [DOI] [PubMed] [Google Scholar]
- 9.Tartarone A, Roviello G, Lerose R, Roudi R, Aieta M, Zoppoli P. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: a meta-analysis. Future Oncol. 2019;15(20):2423–2433. doi: 10.2217/fon-2018-0868 [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Wang R, Shen X, et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol. 2016;23(6):2099–2105. doi: 10.1245/s10434-015-5043-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsao MS, Marguet S, Le Teuff G, et al. Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection. J Clin Oncol. 2015;33(30):3439–3446. doi: 10.1200/JCO.2014.58.8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki M, Yokose T, Nakayama H. Prognostic contribution of non-predominant solid and micropapillary components in lung adenocarcinomas. J Thorac Dis. 2017;9(3):504–506. doi: 10.21037/jtd.2017.03.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saruwatari K, Ikemura S, Sekihara K, et al. Aggressive tumor microenvironment of solid predominant lung adenocarcinoma subtype harboring with epidermal growth factor receptor mutations. Lung Cancer. 2016;91:7–14. doi: 10.1016/j.lungcan.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 14.Pyo JS, Kim JH. Clinicopathological significance of micropapillary pattern in lung adenocarcinoma. Pathol Oncol Res. 2018;24(3):547–555. doi: 10.1007/s12253-017-0274-7 [DOI] [PubMed] [Google Scholar]
- 15.Ma M, She Y, Ren Y, et al. Micropapillary or solid pattern predicts recurrence free survival benefit from adjuvant chemotherapy in patients with stage IB lung adenocarcinoma. J Thorac Dis. 2018;10(9):5384–5393. doi: 10.21037/jtd.2018.08.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuang M, Shen X, Yuan C, et al. Clinical significance of complex glandular patterns in lung adenocarcinoma: clinicopathologic and molecular study in a large series of cases. Am J Clin Pathol. 2018;150(1):65–73. doi: 10.1093/ajcp/aqy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol. 2014;27(5):690–700. doi: 10.1038/modpathol.2013.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19.Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6(9):1496–1504. doi: 10.1097/JTO.0b013e318221f701 [DOI] [PubMed] [Google Scholar]
- 20.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 21.Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 22.Wu C, Zhao C, Yang Y, et al. High discrepancy of driver mutations in patients with NSCLC and synchronous multiple lung ground-glass nodules. J Thorac Oncol. 2015;10(5):778–783. doi: 10.1097/JTO.0000000000000487 [DOI] [PubMed] [Google Scholar]
- 23.Li W, Ren S, Li J, et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patients. Lung Cancer. 2014;84(3):295–300. doi: 10.1016/j.lungcan.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 24.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riely GJ, Travis WD. Can IASLC/ATS/ERS subtype help predict response to chemotherapy in small biopsies of advanced lung adenocarcinoma? Eur Respir J. 2014;43(5):1240–1242. doi: 10.1183/09031936.00048814 [DOI] [PubMed] [Google Scholar]
- 26.Kuo SW, Chen JS, Huang PM, Hsu HH, Lai HS, Lee JM. Prognostic significance of histologic differentiation, carcinoembryonic antigen value, and lymphovascular invasion in stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014;148(4):1200–1207.e1203. doi: 10.1016/j.jtcvs.2014.04.038 [DOI] [PubMed] [Google Scholar]
- 27.Da Cruz V, Yvorel V, Casteillo F, et al. Histopathological subtyping is a prognostic factor in stage IV lung adenocarcinoma. Lung Cancer. 2020;147:77–82. doi: 10.1016/j.lungcan.2020.07.010 [DOI] [PubMed] [Google Scholar]
- 28.Casteillo F, Guy JB, Dal-Col P, et al. Pathologic subtypes of lung adenocarcinoma brain metastasis is a strong predictor of survival after resection. Am J Surg Pathol. 2018;42(12):1701–1707. doi: 10.1097/PAS.0000000000001161 [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Hu Z, Zhao J, et al. Both the presence of a micropapillary component and the micropapillary predominant subtype predict poor prognosis after lung adenocarcinoma resection: a meta-analysis. J Cardiothorac Surg. 2020;15(1):154. doi: 10.1186/s13019-020-01199-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong ZY, Zhang C, Li YF, et al. Genetic and immune profiles of solid predominant lung adenocarcinoma reveal potential immunotherapeutic strategies. J Thorac Oncol. 2018;13(1):85–96. doi: 10.1016/j.jtho.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 31.Yaldiz D, Ors Kaya S, Ceylan KC, et al. Prognostic affect of predominant histologic subtypes in resected pulmonary adenocarcinomas. Balkan Med J. 2019. doi: 10.4274/balkanmedj.galenos.2019.2019.1.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang C, Sun X, Zhao W, et al. Minor components of micropapillary and solid subtypes in lung invasive adenocarcinoma (≤ 3 cm): PET/CT findings and correlations with lymph node metastasis. Radiol Med. 2020;125(3):257–264. doi: 10.1007/s11547-019-01112-x [DOI] [PubMed] [Google Scholar]
- 33.Campos-Parra AD, Avilés A, Contreras-Reyes S, et al. Relevance of the novel IASLC/ATS/ERS classification of lung adenocarcinoma in advanced disease. Eur Respir J. 2014;43(5):1439–1447. doi: 10.1183/09031936.00138813 [DOI] [PubMed] [Google Scholar]
- 34.Fathi Z, Syn NL, Zhou JG, Roudi R. Molecular epidemiology of lung cancer in Iran: implications for drug development and cancer prevention. J Hum Genet. 2018;63(7):783–794. doi: 10.1038/s10038-018-0450-y [DOI] [PubMed] [Google Scholar]
- 35.Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a Randomised, Open-Label, Phase 3 Study. Lancet Oncol. 2018;19(1):139–148. doi: 10.1016/S1470-2045(17)30729-5 [DOI] [PubMed] [Google Scholar]
- 36.Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, Phase 2 trial. Lancet Respir Med. 2018;6(11):863–873. doi: 10.1016/S2213-2600(18)30277-7 [DOI] [PubMed] [Google Scholar]
- 37.Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N Engl J Med. 2020;383(18):1711–1723. doi: 10.1056/NEJMoa2027071 [DOI] [PubMed] [Google Scholar]
- 38.Roviello G, Zanotti L, Cappelletti MR, et al. Are EGFR tyrosine kinase inhibitors effective in elderly patients with EGFR-mutated non-small cell lung cancer? Clin Exp Med. 2018;18(1):15–20. doi: 10.1007/s10238-017-0460-7 [DOI] [PubMed] [Google Scholar]