Abstract
BACKGROUND AND PURPOSE: This paper describes the CT findings that characterize the middle and inner ear anomalies in coloboma, heart defects, choanal atresia, mental retardation, genitourinary, and ear anomalies (CHARGE) syndrome. With this information, neuroradiologists will be better prepared to provide clinically relevant information to their referring physicians regarding this rare syndrome.
MATERIALS AND METHODS: CT studies from 13 patients were reviewed by 2 neuroradiologists with Certificate of Additional Qualification. Each ear was counted separately for a total of 26 ears. Middle and inner ear anomalies associated with CHARGE syndrome were categorized. Investigational review board approval was obtained.
RESULTS: Twenty of 26 (77%) ears demonstrated cochlear aperture atresia. Four of these ears were evaluated with MR imaging and were found to lack a cochlear nerve. Twenty-one of 26 (81%) cochlea had some form of dysplasia. Six of 26 (23%) round windows were aplastic. Three of 26 (12%) round windows were hypoplastic. Twenty-one of 26 (81%) oval windows were atretic or aplastic. Fifteen of 26 (58%) vestibules were hypoplastic or dysplastic. There were 5 of 26 (19%) enlarged vestibular aqueducts. Twelve of 26 (46%) vestibular aqueducts had an anomalous course. All cases demonstrated absent semicircular canals. Twenty-three of 26 (88%) facial nerve canals had an anomalous course. Four of 26 (15%) tympanic segments were prolapsed. Three of 26 (12%) temporal bones had an anomalous emissary vein referred to as a petrosquamosal sinus. Twenty-one of 26 (81%) middle ear cavities were small. Twenty-three of 26 (93%) ossicles were dysplastic with ankylosis. Three of 26 (12%) internal auditory canals were small.
CONCLUSION: The CT findings that correlate to the anomalies of CHARGE syndrome affect conductive as well as sensorineural hearing. Stenosis of the aperture for the cochlear nerve aperture on CT is suggestive of hypoplasia or absence of the cochlear nerve, which has been demonstrated in some cases by MR. Absence of the cochlear nerve would be a contraindication to cochlear implantation.
CHARGE syndrome was first described independently by Hall and Hittner et al in 1979.1,2 CHARGE is an acronym for a complex constellation of anomalies: coloboma, heart defects, choanal atresia, mental retardation, genitourinary hypoplasia, and ear defects.3 Additional anomalies have been reported, including facial palsy or asymmetry, esophageal and laryngeal abnormalities, renal malformations, and facial clefts.4–12
Criteria for CHARGE. Classical diagnostic criteria described by Pagon et al3 in 1981 include: (1) 1 major feature (coloboma/microphthalmia or choanal atresia), and (2) 4 of the 6 features designated in the CHARGE acronym. An updated version of the diagnostic criteria has been published by Blake and is presented in Table 1. A more recent proposed update to the criteria has also been published by Verloes.13
Table 1:
CHARGE syndrome diagnostic criteria53
| Major Criterion | Minor Criterion |
|---|---|
| +Coloboma | +Heart defect |
| +Choanal atresia | −Orofacial cleft |
| +Characteristic ear anomalies | +Genital hypoplasia |
| +Cranial nerve dysfunction (facial palsy, vestibular dysfunction, swallowing difficulties) | +Growth deficiency |
| +Developmental delay | |
| −Tracheo-esophageal fistula | |
| +Distinct facial appearance |
Note:—CHARGE indicates Coloboma, Heart defects, choanal Atresia, mental Retardation, Genitourinary, and Ear anomalies; +, pertinent positive finding; −, pertinent negative finding. A CHARGE diagnosis is indicated by 4 major criteria or 3 major and 3 minor criteria. Exclude other conditions such as velocardiofacial syndrome and DiGeorge sequence using FISH test to exclude 22q11 deletion.
The goal of this article is to provide an analysis of the CT findings of the middle and inner ear in a population of patients with CHARGE syndrome. We will also speculate about the possible causes of CHARGE syndrome. With this information, radiologists can then guide their referring physicians toward the best treatment options for their patients.
Materials and Methods
Investigational review board approval was obtained for a retrospective study. The patient data base was gathered from various institutions over 20 years with a number of different scanner types. Clinical history on these patients was acquired from the radiologists reviewing the cases at the time the cases were clinically relevant. Because the data were gathered retrospectively without a predetermined definition of the type of information desired, some relevant information is lacking in the final analysis.
Materials
CT studies from 13 patients were retrospectively studied to categorize the various middle and inner ear anomalies associated with CHARGE. Inclusion criteria used for the diagnosis of CHARGE syndrome were based on those described by Pagon et al.3 Patient ages ranged from 4 months to 22 years. Most patients were younger than 7 years. Sex distribution was approximately equal. Images from CT scans were obtained using 1-mm axial acquisitions. In 2 patients, T2-weighted MR images were obtained in axial, coronal, and oblique sagittal planes. In some cases, data for only 1 ear were available.
Research Design
Each ear was evaluated separately. Thirteen patients were reviewed by 2 neuroradiologists (with Certificate of Additional Qualification) from our institution. Because of variability in completeness of imaging data, not all structures were visualized equally. This discrepancy was reflected in the final numbers.
Means of Confirming Diagnoses
Criteria for evaluation were as follows: aplasia of the semicircular canals was noted when the canals were completely absent and an isolated vestibule was present. Cochlear anomalies were related to presence and size of the basal and apical turns. Cochlear aperture was also recorded as normal if patent, visible on an MR when available, or trapped if there was a bony bar over the aperture. The criterion for large vestibular aqueduct was based on a diameter greater than 2 mm in the intraosseous portion. Atresia of the oval and/or round window was identified when they were obscured partly or completely by an osseous septum. The middle ear ossicles were evaluated for dysplasia and/or fusion. Dysplasia was noted if the ossicles demonstrated abnormal morphology or size. The criteria for fusion were based on proximity and the appearance of ankylosis to the wall of the epitympanum or interossicular fusion. The size of the middle ear cavity was also taken into consideration as small if there was insufficient air space surrounding the ossicles therefore contributing to ankylosis with the ossicular chain. Abnormal facial nerve canal segment positions were recorded. Abnormal internal auditory canal size was recorded when the internal auditory canal was less than 2 mm in diameter. If an emissary vein was seen emanating from the dorsolateral transverse sinus before the confluence with the superior petrosal sinus, then a petrosquamosal sinus was recorded.
Results
CT data for the 13 patients were somewhat variable, with some limitations in the quality of the images and in the portions of the axial and coronal images that were saved for evaluation. As a result, the enumerated findings provided in each of the following sections reflect the variability in the data. When a patient did not have images that corresponded to the anatomy of interest, the data that could not be assessed were also recorded. The data were tabulated for reference as indicated.
Internal Auditory Canal
Three of 26 internal auditory canals were small. The remainder of the internal auditory canals were normal (Table 2).
Table 2:
Summary of data for internal auditory canal, cochlea, and labyrinth
| Patient No./Age (mo/sex) | Side | IAC | Cochlea |
Vest | VA | VA angle | Semi-circular canals |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aperture | Basal | Apical | LSCC | PSCC | SSCC | ||||||
| 1/UTI/UTI | R | Normal | Trapped | Normal | Normal | Dys | Present | Reversed | Absent | Absent | Absent |
| L | Normal | Trapped | Normal | Normal | Normal | Present | Normal | Absent | Absent | Absent | |
| 2/UTI/UTI | R | Normal | UTI | Normal | Hypo | Hypo | UTI | UTI | Absent | Absent | Absent |
| L | Normal | UTI | Normal | Hypo | Dys | UTI | UTI | Absent | Absent | Absent | |
| 3/4/F | R | Normal | Trapped | Normal | Hypo | Hypo | Present | Reversed | Absent | Absent | Absent |
| L | Normal | Trapped | Normal | Hypo | Hypo | Present | Reversed | Absent | Absent | Absent | |
| 4 | R | Normal | Trapped | Normal | Hypo | Dys | Present | Reversed | Absent | Absent | Absent |
| L | Normal | Trapped | Normal | Hypo | Dys | Present | Normal | Absent | Absent | Absent | |
| 5/UTI/UTI | R | Normal | Trapped | Normal | Normal | Dys | Large | Normal | Absent | Absent | Absent |
| L | Normal | Trapped | Normal dys | Normal | Normal | Present | Normal | Absent | Absent | Absent | |
| 6/264/F | R | Normal | Trapped | Modio dys | Hypo | Normal | Present | Normal | Absent | Absent | Absent |
| L | Small | Trapped | Modio | Hypo | Normal | Large | Normal | Absent | Absent | Absent | |
| 7/65/UTI | R | Normal | UTI | UTI | UTI | UTI | UTI | UTI | UTI | UTI | UTI |
| L | Normal | UTI | Enlarged | Enlarged | Dys | UTI | UTI | Absent | Absent | Absent | |
| 8/UTI/UTI | R | Normal | UTI | Enlarged | Enlarged | UTI | UTI | UTI | Absent | Absent | Absent |
| L | Normal | UTI | Enlarged | Enlarged | UTI | UTI | UTI | Absent | Absent | Absent | |
| 9/3/M | R | Small | Trapped | Normal | Hypo | Hypo | Present | Reversed | Absent | Absent | Absent |
| L | Small | Trapped | Normal | Hypo | Hypo | Present | Reversed | Absent | Absent | Absent | |
| 10/4/M | R | Normal | Trapped | Normal | Hypo | Hypo | Large | Reversed | Absent | Absent | Absent |
| L | Normal | Trapped | Normal | Hypo | Hypo | Present | Reversed | Absent | Absent | Absent | |
| 11/16/F | R | Normal | Trapped | Normal | Hypo | Hypo | Present | Reversed | Absent | Absent | Absent |
| L | Normal | Trapped | Normal | Hypo | Hypo | Present | Reversed | Absent | Absent | Absent | |
| 12/60/M | R | Normal | Trapped | Normal | Hypo | Normal | Large | Reversed | Absent | Absent | Absent |
| L | Normal | Trapped | Normal | Hypo | Normal | Present | Reversed | Absent | Absent | Absent | |
| 13/72/M | R | Normal | Trapped | Normal | Hypo | Normal | Large | Normal | Absent | Absent | Absent |
| L | Normal | Trapped | Normal | Hypo | Normal | Present | Normal | Absent | Absent | Absent | |
Note:—IAC indicates internal auditory canal; Vest, vestibule; VA, vestibular aqueduct; LSCC, lateral semicircular canal; SSCC, superior semicircular canal; PSCC, posterior semicircular canal; UTI, unable to identify; Dys, dysplastic; Modio, modiolus; Hypo, hypoplastic.
Cochlea
The findings described below are summarized in Table 2.
Cochlear Aperture.
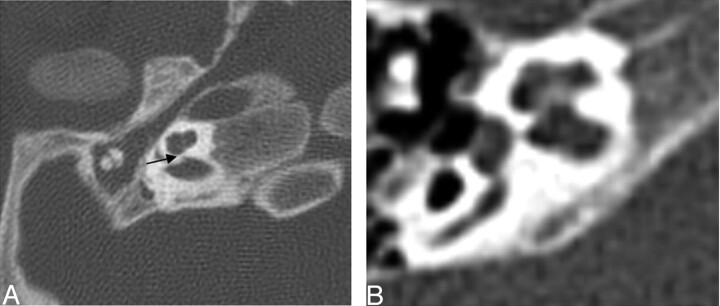
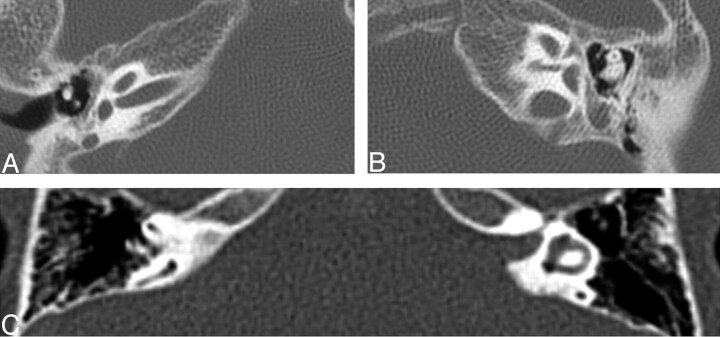
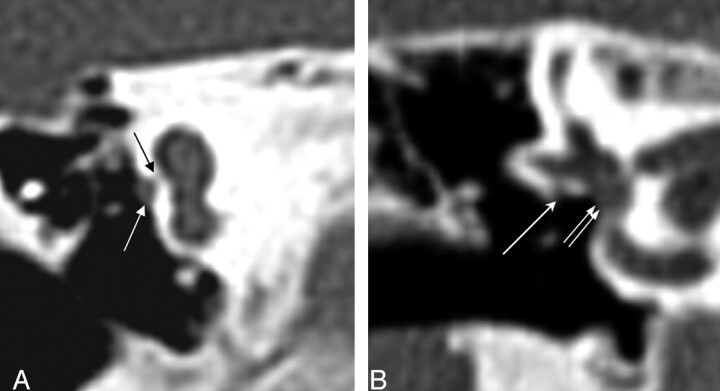
Of the cochlea evaluated, 20 of 26 demonstrated an abnormally thickened bony covering over the aperture for the cochlear nerve, giving the cochlea an isolated appearance referred to as a “trapped cochlea.” Six of the apertures could not be evaluated because of the limitations of the datasets. An example of a trapped cochlea is provided in Fig 1.
Fig. 1.
Axial CT at the level of the cochlea demonstrates cochlear aperture atresia or trapped cochlea. The modiolus is dysplastic as well. Left, there is ossification over the cochlear aperture (black arrow), which is normally widely patent and occupied by the cochlear nerve (right).
Basal Turn.
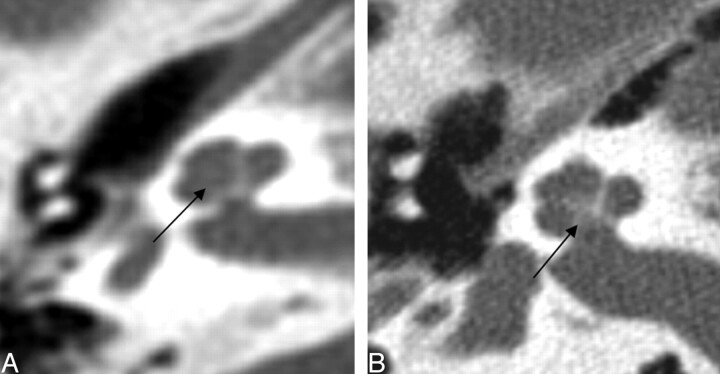
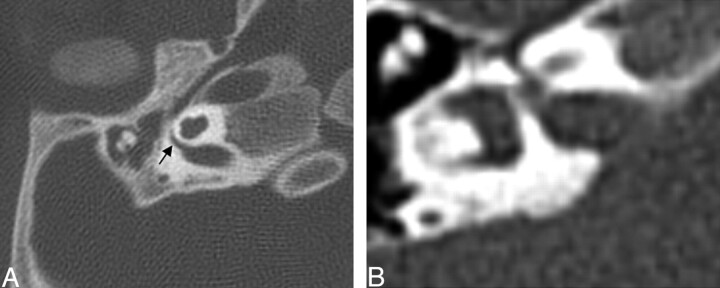
Two of 26 cochlea had a dysplastic modiolus that was either incompletely formed with less of the ossific and crown-like structure or had more ossification in a smudgy pattern (Fig 2). Three of 26 basal turns were dysplastic. They were enlarged, rounded, or featureless in configuration (Fig 3). One basal turn could not be classified because it was not included.
Fig. 2.
Axial CT of the cochlea. Left: Dysplastic posterior strut of the modiolus (black arrows) with lack of the normally ossified crown-like structure seen on the right.
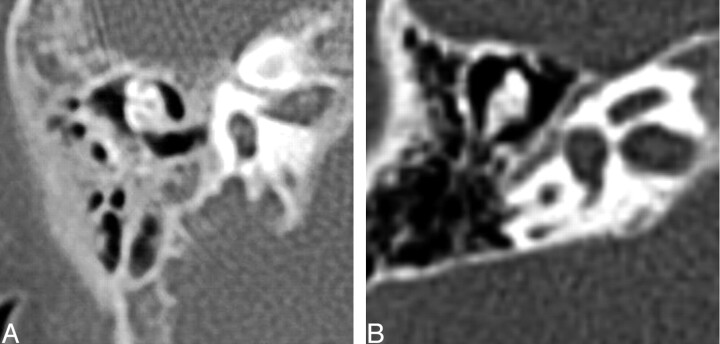
Fig. 3.
Three axial CT images at the level of the cochlea demonstrating examples of cochlea that are amorphous and lack definable turns, internal septation, or a modiolus. The lower 2 cases demonstrate gross hypoplasia.
Apical Turn.
Eighteen of 26 cochlea evaluated were classified as having a hypoplastic apical turn. Three of 26 were classified as enlarged. Those with enlarged apical turns were the same as those with enlarged basal turns because the entire cochlea was a single enlarged anomaly. One apical turn could not be classified because it was not included. Figure 4 shows apical turn hypoplasia. Basal and middle turns are normal.
Fig. 4.
Axial CT of the cochlea. Left, Apical turn hypoplasia. Right: Normal cochlea.
Labyrinth
The findings described below are summarized in Table 2.
Lateral, Superior, and Posterior Semicircular Canals.
Twenty-five of 26 lateral, superior, and posterior semicircular canals were absent. One set of semicircular canals was not included for evaluation (Table 2). Figure 5 demonstrates absent semicircular canals.
Fig. 5.
Axial images at the level of the vestibule. Top, Bilateral absent semicircular canals with isolated vestibules. Bottom, Normally formed semicircular canals.
Vestibule.
Nine of 26 vestibules were hypoplastic. Six of 26 vestibules were dysplastic (Fig 6), because they were either enlarged or had irregular margins. Three could not be evaluated, one because the entire side was not included and the others because images of the vestibule were not included.
Fig. 6.
Axial CT at the level of the vestibule. The black arrow points to a dysplastic vestibule. The middle ear cavity is small and nonpneumatized, and the head of the malleus is ankylosed to the anterior epitympanic wall.
Vestibular Aqueduct.
Five of 26 vestibular aqueducts were enlarged. Twelve had a reversed angle, indicating a more posteromedial angulation rather than posterolateral. Six could not be evaluated.
Middle Ear
Findings described below are summarized in Table 3.
Table 3:
Middle ear findings
| Patient No. | Middle Ear Findings |
Tegmen | Round Window | Over Window | |||
|---|---|---|---|---|---|---|---|
| Cavity Size | Ossicles | Ankylosis | |||||
| 1 | R | Small | Dys | Yes | Dehiscent | Normal | Aplasia |
| L | Small | Dys | Yes | Dehiscent | Normal | Aplasia | |
| 2 | R | Small | Dys | Yes | Normal | Normal | Aplasia |
| L | Small | Dys | Yes | Normal | Normal | Aplasia | |
| 3 | R | Small | Dys | Yes | Normal | Normal | Aplasia |
| L | Small | Dys | Yes | Normal | Normal | Aplasia | |
| 4 | R | Small | Dys | Yes | Normal | Normal | Aplasia |
| L | Small | Dys | Yes | Normal | Normal | Aplasia | |
| 5 | R | Small | Dys | Yes | Normal | Normal | Aplasia |
| L | Small | Dys | Yes | Normal | Normal | Aplasia | |
| 6 | R | Normal | Normal | No | Normal | Small | Aplasia |
| L | Small | Normal | No | Normal | Small | Aplasia | |
| 7 | R | Normal | UTI | UTI | UTI | UTI | UTI |
| L | Normal | Dys | Yes | Normal | Normal | Aplasia | |
| 8 | R | Normal | Dys | Yes | Normal | UTI | Normal |
| L | Normal | Dys | Yes | Normal | UTI | Normal | |
| 9 | R | Small | Dys | Yes | Normal | UTI | UTI |
| L | Small | Dys | Yes | Normal | UTI | UTI | |
| 10 | R | Small | Dys | Yes | Normal | Aplasia | Aplasia |
| L | Small | Dys | Yes | Normal | Small | Aplasia | |
| 11 | R | Small | Dys | Yes | Normal | Aplasia | Aplasia |
| L | Small | Dys | Yes | Normal | Aplasia | Aplasia | |
| 12 | R | Small | Dys | Yes | Normal | Aplasia | Aplasia |
| L | Small | Dys | Yes | Normal | Aplasia | Aplasia | |
| 13 | R | Small | Dys | Yes | Normal | Normal | Aplasia |
| L | Small | Dys | Yes | Normal | Normal | Aplasia | |
Note:—UTI indicates unable to identify; Dys, dysplastic.
Middle Ear Cavity.
Twenty-one of 26 evaluated middle ear cavities were small (Fig 7). The remaining 5 were normal. Twenty-three of 26 evaluated middle ear ossicles were dysplastic and ankylosed to the anterior epitympanic wall evidenced by the proximity and lack of visualized air between the head of the malleus and the anterior epitympanic wall. Two middle ear cavities on the same patient demonstrated dehiscent tegmen tympani. Of the 2 remaining middle ear ossicles, 2 were normal (from the same patient) and 1 could not be assessed. Figures 6 and 7 demonstrate the small middle ear cavity, dysplastic ossicles, and ankylosis of the malleus with the anterior epitympanic wall.
Fig. 7.
Axial CT images of the middle ear. Left, Small middle ear cavity in patient with CHARGE syndrome with ankylosis of the dysplastic ossicles to each other and to the epitympanic wall. Right, Normal middle ear cavity.
Round Window.
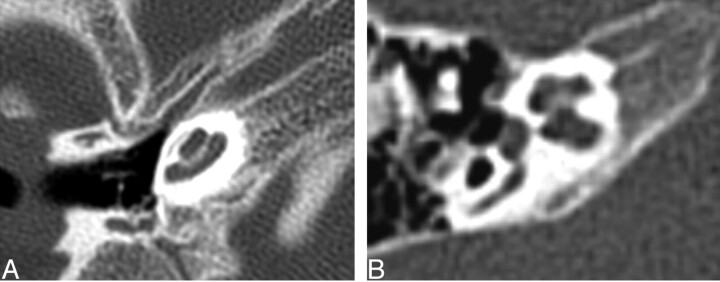
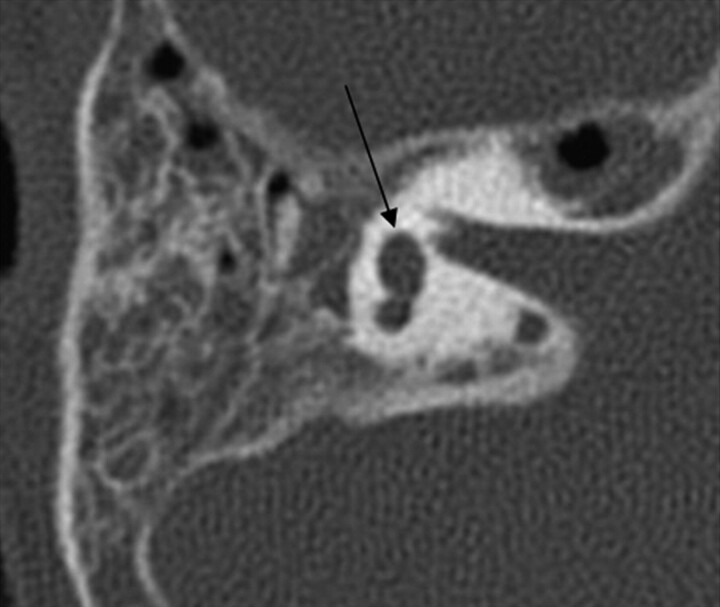
There were 6 of 26 round windows that were classified as aplastic. Three of 26 were hypoplastic. Five round windows could not be classified (Fig 8).
Fig. 8.
Axial CT at the level of the round window. Left, The black arrow points to the small round window. The middle ear cavity is also hypoplastic. Right, Normal round window.
Oval Window.
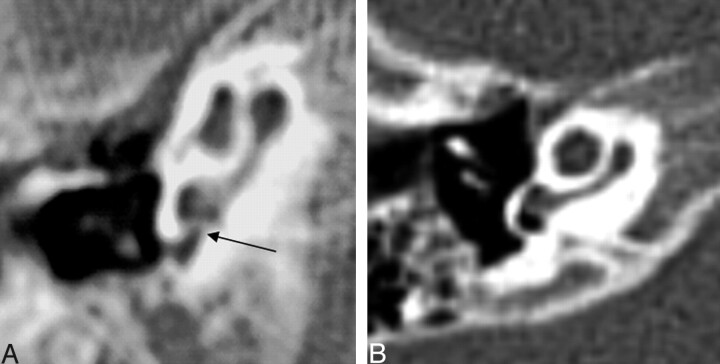
Twenty-one of 26 oval windows were classified as aplastic or atretic. Three oval windows could not be classified. Figure 9 demonstrates oval window atresia.
Fig. 9.
Coronal CT at the oval window. Left, The black arrow points to a bony bar at the expected location of the normal membranous oval window. There is a prolapsed tympanic segment of the facial nerve (white arrow). Right, Normal oval window (double white arrow) and tympanic segment of the facial nerve (white arrow).
Facial Nerve
The findings described below are summarized in Table 4.
Table 4.
Facial nerve course and venous anomalies
| Patient No. | Side | Labyrinth | Facial Nerve |
Venous Anomaly | Juglar | ||
|---|---|---|---|---|---|---|---|
| Frist Genu | Tympanic | Mastoid | |||||
| 1 | R | Posterior | Posterior | Prolapsed | Normal | ||
| L | Posterior | Posterior | Prolapsed | Normal | PSS | Diverticulum | |
| 2 | R | Normal | Normal | UTI | UTI | ||
| L | Normal | Normal | UTI | UTI | |||
| 3 | R | Posterior | Posterior | Prolapsed | Normal | ||
| L | Posterior | Posterior | Prolapsed | Normal | |||
| 4 | R | Posterior | Posterior | Normal | Normal | ||
| L | Posterior | Posterior | Normal | Normal | |||
| 5 | R | Posterior | Posterior | Normal | Normal | ||
| L | Posterior | Posterior | Normal | Normal | |||
| 6 | R | Posterior | Posterior | Normal | Normal | ||
| L | Posterior | Posterior | Normal | Normal | |||
| 7 | R | UTI | UTI | UTI | UTI | ||
| L | Posterior | Posterior | Normal | Normal | |||
| 8 | R | Posterior | Posterior | Normal | Normal | ||
| L | Posterior | Posterior | Normal | Normal | |||
| 9 | R | Posterior | Posterior | Normal | Normal | ||
| L | Posterior | Posterior | Normal | Normal | |||
| 10 | R | Posterior | Posterior | Normal | Normal | ||
| L | Posterior | Posterior | Normal | Normal | PSS | ||
| 11 | R | Posterior | Posterior | Normal | Normal | ||
| L | Posterior | Posterior | Normal | Normal | |||
| 12 | R | Posterior | Posterior | Normal | Normal | ||
| L | Posterior | Posterior | Normal | Normal | |||
| 13 | R | Posterior | Posterior | Normal | Normal | ||
| L | Posterior | Posterior | Normal | Normal | PSS | Diverticulum | |
Note:—PSS indicates petrosquamosal sinus; UTI, unable to identify.
The course of the facial nerve canal was recorded and separated into labyrinthine, first genu, tympanic, and mastoid segments. Twenty-three of 26 labyrinthine segments were posteriorly displaced relative to the cochlea (Fig 10). In general, the labyrinthine segment overlies the posterior cochlea on axial views. Twenty-three of 26 first genu segments were also posteriorly displaced, as would be expected because the labyrinthine segment forms the proximal segment of the genu. Four of 26 tympanic segments were prolapsed or found within the oval window niche (Fig 9). The remaining segments were normal or could not be assessed.
Fig. 10.
Axial CT at the level of the internal auditory canal. Abnormal course of facial nerve labyrinthine segment. Left, Arrow shows posteriorly bowing labyrinthine segment of the facial nerve. Right, Normal labyrinthine segment is straight and overlies a portion of the cochlea.
Venous Anomalies
The findings described below are summarized in Table 4.
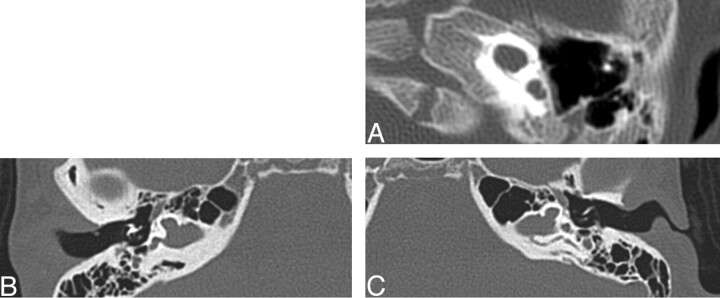
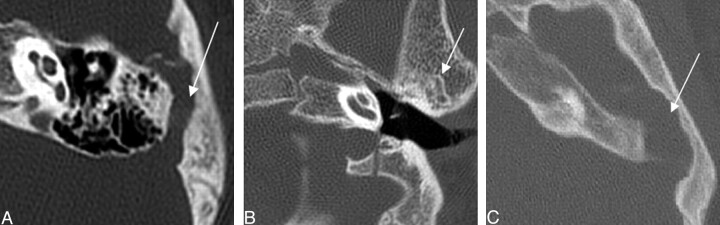
Three of 26 temporal bones demonstrated some form of venous anomaly. All 3 of these were described as a petrosquamosal sinus based on the criteria from Marsot-Dupuch (Fig 11). There were 2 jugular diverticula.
Fig. 11.
Three axial CT images demonstrating examples of petrosquamosal sinuses (white arrows), a form of emissary vein anomaly.
Discussion
Although several authors14–16 have described various ear anomalies associated with CHARGE syndrome, Lemmerling et al17 provided one of the most comprehensive descriptions of the inner and middle ear anomalies based on CT. Their findings were similar to ours, as will be delineated below. Stapedius muscle aplasia was a finding they described that was not evaluated in our population. Wright et al18 also described absent stapedius muscles in all cases. This is not surprising given that the ossicular abnormalities in the CHARGE syndrome (generally of the long process of the incus and the stapes) seem to result primarily from a defective development of the second branchial arch, from which the stapedius muscle also arises. Tellier19 also described anomalies of the cochlea, including Mondini20 malformations, vestibular dysplasias, enlarged vestibular aqueducts, oval window atresia, hypoplastic incus, small middle ear cavity, and also stapedial tendon anomalies.
Vestibular dysfunction evidenced by delayed postural development and abnormal vestibular function tests (otolith and canal vestibulo-ocular responses) were found in 94% of patients with CHARGE syndrome with confirmed absence of their semicircular canals on CT scans.21
Others have suggested that the temporal bone anomalies seen in patients with CHARGE syndrome are more consistent and therefore a more sensitive finding in the diagnosis.22 Absence of the semicircular canals, oval window atresia, and cochlear dysplasias in that order were some of the most sensitive findings in another study.23 Associated anomalous emissary veins within the temporal bone have also been reported.24 Some of these are referred to as petrosquamosal sinuses.
Hypothesized Cause
One of the original works on congenital anomalies of the inner ear was written by Jackler et al.25 Semicircular canal aplasia in CHARGE syndrome is not included in their original classification. Jackler et al’s hypothesis for semicircular aplasia is based on an arrest of embryogenesis in the 6th week of gestation. During this time, the semicircular canals begin as evaginations of the vestibular appendage. The superior semicircular canal forms first followed by the posterior and then lateral semicircular canals. Jackler et al’s theories of arrested embryogenesis provide an explanation for a wide range of developmental anomalies including labyrinthine aplasia, cochlear aplasia, common cavity, cystic cochleovestibular anomaly, semicircular canal dysplasia, and large endolymphatic sac anomalies. The hypothesis of developmental arrest, however, does not address the anomalies of the middle ear that are seen in patients with CHARGE syndrome, and it does not address the multitude of other anomalies.
An arrest during the 7th week results in 1 to 1.5 turns, or the classic Mondini deformity. Based on prior work on quail chick embryology, the otic capsule is thought to originate from 3 sources: neural crest, cephalic mesoderm, and somatic mesoderm.26 Therefore, the cochlear developmental anomaly is consistent with the hypothesis of an anomaly of neural crest cell derivatives in CHARGE syndrome.
Higher mean paternal age at conception,27 concordance in monozygotic twins,28 and the existence of rare recurrence in families,29 support the possibility of genetic etiologies of CHARGE syndrome, such as de novo mutation of a dosage-sensitive gene or a submicroscopic chromosome rearrangement effecting contiguous genes. The phenotypic heterogeneity of this disorder, along with the inconsistent chromosomal rearrangements in rare patients, however, limits genetic localization of this disorder.
A genetic description for the development of the vertebrate inner ear has recently been published that provides detailed accounting of the growth factors, gene expression, and induction as it was understood at that time.30
Studies in mice genetics in Netrin-1, Otx1, and Otx2 genes have revealed defects in the inner ear associated with various combinations; however, none resulted in the combined findings seen in patients with CHARGE syndrome. Other genetic influences have been studied in mice as well.31–37 Several investigators have looked into systemic microdeletions, as well as PAX2 and PITX2, without finding a common mutation.38–40 Vissers et al41 used array comparative genomic hybridization to identify a 2.3-megabase pair de novo overlapping microdeletion on chromosome 8q12 of the CHD7 gene in 2 patients with CHARGE syndrome. Ten heterozygous mutations in the gene CHD7 were identified: 7 stop-codon, 2 missense, and 1 intron-exon boundary mutation. CHD7 has a putative role as a general controller of developmental gene expression as well as mesodermal patterning. Still others have proposed that CHARGE syndrome is due to a disruption of mesenchymal-epithelial interaction (“epithelial” includes ectoderm and endoderm).42 It is also hypothesized by the authors that disruption of more than 1 gene is necessary to generate the CHARGE phenotype, as has been proposed for the holoprosencephaly sequence because critical developmental pathways must be robust and redundant to minimize errors.
Middle Ear Anomalies
A high percentage of patients, 81% (21 of 26), had small middle ear cavities as well as dysplastic middle ear ossicles ankylosed to the anterior epitympanic wall, 93% (23 of 26), providing evidence of a clinically significant finding, especially in combination with the oval window atresia for conductive hearing loss.
Overall, there was a high number (21 of 26, 81%) of oval window aplasia or atresia. A significant number of patients with this finding was also described by Lemmerling et al.17 There was, however, a lower number of round window abnormalities than was seen by Lemmerling et al, who described only soft tissue obliteration of the round window. Most of their patients, however, demonstrated middle ear cavity opacification that may have mimicked soft tissue obliteration, probably from fluid or infection. Oval window atresia is associated with conductive hearing loss.43
Inner Ear Anomalies
Internal auditory canal hypoplasia and cochlear development can be an indirect indication of inner ear nerve aplasia.44 Of the 3 internal auditory canals that were classified as small, only 2 had a corresponding MR image that confirmed cochlear and vestibular nerve aplasia. Two other ears from the same patient confirmed cochlear nerve aplasia with a corresponding normal internal auditory canal size. The remaining cases did not have a corresponding MR imaging.
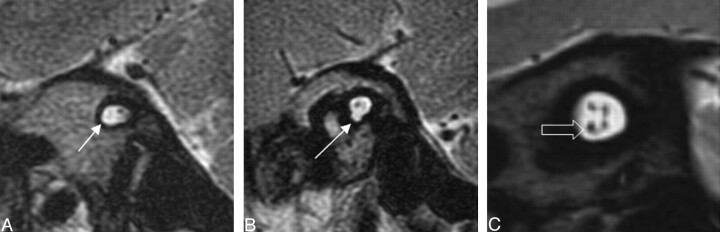
Our study found 20 of 26 patients who were investigated had a trapped cochlea. Lemmerling et al17 did not describe this finding. Trapped cochleae have been found in association with sensorineural hearing loss in combination with other potential causes, such as a dysplastic modiolus.45 Three patients with bilateral trapped cochleae had associated MR imaging findings of cochlear nerve aplasia. Two patients had bilateral vestibular nerve aplasia as well. An MR imaging example of a diminutive cochlear nerve corresponding to cochlear aperture atresia is provided in Fig 12.
Fig. 12.
Oblique sagittal T2-weighted images that demonstrate lack of a normal-appearing cochlear nerve (white arrow) in the 2 left images. The third image demonstrates a normal-appearing 4-nerve bundle of facial, cochlear (open arrow), and vestibular nerves.
Five of 26 vestibular aqueducts were enlarged. Patients with large endolymphatic sacs clinically present with progressive sensorineural hearing loss. Large vestibular aqueducts or large endolymphatic sacs can be evaluated by CT or MR imaging, respectively, with better delineation of the soft tissues on MR imaging.46 All enlarged vestibular aqueducts were associated with a trapped cochlea. One enlarged vestibular aqueduct was associated with a dysplastic modiolus, and 3 were associated with a hypoplastic apical turn. An enlarged vestibular aqueduct is known to have an association with cochlear dysplasia.
Twelve of 26 patients had a reversed angle to the vestibular aqueduct with a posteromedial orientation instead of the normal posterolateral orientation. An abnormal vestibular aqueduct orientation has not been reported in the radiologic literature so far. This anomaly is hypothesized to be the result of semicircular aplasia and the associated displacement of normal surrounding structures.
Semicircular canals were universally absent. Semicircular canal aplasia alone is not believed to be the cause but probably plays a role in developmental delay of motor skills along with visual deficits and environmental factors.47,48
When the entire course of the facial nerve is included, 23 of 26 labyrinthine segments were posteriorly displaced, hypothetically secondary to aplasia of the semicircular canals and resulting displacement of the surrounding structures. Each of the 4 tympanic segments classified as prolapsed were associated with oval window atresia, a known association. Some reports suggest that facial palsy may be a reliable predictor for sensorineural hearing loss.49 Given the high percentage of patients with CHARGE syndrome who have an anomalous facial nerve course, it is essential for the radiologist to accurately describe the entire course of the facial nerve canal, especially if cochlear implantation is going to be performed, to minimize the possibility of iatrogenic facial nerve damage. Round window atresia and hypoplastic cochleae are also problematic for implantation and should be mentioned as well.
Three rare emissary veins met the criteria for classification as a petrosquamosal sinus. These anomalous venous sinuses should be described for the referring clinicians for surgical planning and to observe pathways for spread of infection or thrombosis.
Conclusion
This retrospective study of 13 patients with CHARGE syndrome chronicles CT findings for the practicing radiologist that will help guide the clinical management of their patients. Because surgery, in the form of cochlear or brain stem implantation, is currently one of the only treatment options for these patients, knowledge of aberrant facial nerve course, round window atresia, cochlear hypoplasia, and the status of the cochlear nerve are vital to successful treatment.
Our early research indicates that cochlear aperture atresia has a potential association with hypoplastic or aplastic cochlear nerves. Other studies have also demonstrated a relationship between either small internal auditory canals or cochlear aperture atresia and abnormal brain stem vestibulocochlear responses.50,51 A recent article describes the benefit of MR imaging over CT in evaluation of cochlear aperture patency and presence of the cochlear nerve for cochlear implantation planning. The authors do not establish direct correlations between cochlear aperture atresia seen on CT and cochlear nerve aplasia seen on MR imaging.52 They do, however, establish that 50% of nonpatent cochlear apertures were not diagnosed by CT compared with MR. For this reason, we recommend that all patients with CHARGE syndrome obtain high resolution sagittal T2-weighted MR imaging to evaluate for cochlear aperture atresia and establish the existence of the cochlear nerve, especially if they have concomitant cochlear aperture atresia.
Footnotes
Abstract presented at the 43rd Annual Meeting of the American Society of Neuroradiology; May 21–27, 2005; Toronto, Ontario, Canada.
References
- 1.Hall BD. Choanal atresia and associated multiple anomalies. J Pediatr 1979;95:395–98 [DOI] [PubMed] [Google Scholar]
- 2.Hittner HM, Hirsch NJ, Kreh GM, et al. Colobomatous microphthalmia, heart disease, hearing loss, and mental retardation–a syndrome. J Pediatr Ophthalmol Strabismus 1979;16:122–28 [DOI] [PubMed] [Google Scholar]
- 3.Pagon RA, Graham J, Zonana J, et al. Coloboma, congenital heart disease and choanal atresia with multiples anomalies: CHARGE association. J Pediatr 1981;99:223–27 [DOI] [PubMed] [Google Scholar]
- 4.August GP, Rosenbaum KN, Friendly D. Hypopituitarism and the CHARGE association. J Pediatr 1983;103:424–25 [DOI] [PubMed] [Google Scholar]
- 5.Meinecke P, Polke A, Schmiegelow P. Limb anomalies in the CHARGE association. J Med Genet 1989;26:202–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asher BJ, Mc Gill TJI, Kaplan L, et al. Airway complications in CHARGE association. Arch Otolaryngol Head Neck Surg 1990;116:594–95 [DOI] [PubMed] [Google Scholar]
- 7.Lin AE, Siebert JR, Graham JM Jr. Central nervous system malformation in the CHARGE association. Am J Med Genet 1990;37:304–10 [DOI] [PubMed] [Google Scholar]
- 8.Marin JF, Garcia B, Quintana A, et al. The CHARGE association and athyreosis. J Med Genet 1991;28:207–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutiyanawala M, Wyse RK, Brereton RJ, et al. The CHARGE association and oesophagial atresia. J Pediatr Surg 1992;27:558–60 [DOI] [PubMed] [Google Scholar]
- 10.Kushnick T, Wiley JE, Palmer. Agonadism in a 46, XY patient with CHARGE association. Am J Med Genet 1992;42:96–99 [DOI] [PubMed] [Google Scholar]
- 11.Byerli KA, Pauli RM. Cranial nerve abnormalities in CHARGE association. Am J Med Genet 1993;45:751–57 [DOI] [PubMed] [Google Scholar]
- 12.Lacombe D. Facial palsy and cranial abnormalities in CHARGE association. Am J Med Genet 1994;49:351–52 [DOI] [PubMed] [Google Scholar]
- 13.Verloes A. Updated diagnostic criteria for CHARGE syndrome: a proposal. Am J Med Genet A 2005;133:306–08 [DOI] [PubMed] [Google Scholar]
- 14.Davenport SLH, Hefner MA, Thelin JW. CHARGE syndrome. I. External ear anomalies. Int J Ped Otorhinolaryngol 1986;12:137–43 [DOI] [PubMed] [Google Scholar]
- 15.Morgan D, Bailey M, Phelps P, et al. Ear-nose-throat abnormalities in the CHARGE association. Arch Otolaryngol Head Neck Surg 1993;119:49–54 [DOI] [PubMed] [Google Scholar]
- 16.Murofushi T, Ouvrier RA, Parker GD, et al. Vestibular abnormalities in CHARGE association. Ann Otol Rhinol Laryngol 1997;106:129–34 [DOI] [PubMed] [Google Scholar]
- 17.Lemmerling M, Dhooge I, Mollet P, et al. CT of the temporal bone in the CHARGE association. Neuroradiology 1998;40:462–65 [DOI] [PubMed] [Google Scholar]
- 18.Wright CG, Brown OE, Meyerhoff WL, et al. Auditory and temporal bone abnormalities in CHARGE association. Ann Otol Rhinol Laryngol 1986;95:480–86 [DOI] [PubMed] [Google Scholar]
- 19.Tellier AL, Cormier-Daire V, Abadie V, et al. CHARGE syndrome: report of 47 cases and review. Am J Med Genet 1998;76:402–09 [DOI] [PubMed] [Google Scholar]
- 20.Mondini C. Minor works of Carlo Mondini: the anatomical section of a boy born deaf. Am J Otol 1997;18:288–93 [PubMed] [Google Scholar]
- 21.Abadie V, Wiener-Vacher S, Morisseau-Durand MP, et al. Vestibular anomalies in CHARGE syndrome: investigations on and consequences for postural development. Eur J Pediatr 2000;159:569–74 [DOI] [PubMed] [Google Scholar]
- 22.Amiel J, Attie-Bitach T, Marianowski R, et al. Temporal bone anomaly proposed as a major criteria for diagnosis of CHARGE syndrome. Am J Med Genet 2001;99:124–27 [DOI] [PubMed] [Google Scholar]
- 23.Satar B, Mukherji SK, Telian SA. Congenital aplasia of the semicircular canals. Otol Neurotol 2003;24:437–46 [DOI] [PubMed] [Google Scholar]
- 24.Marsot-Dupuch K, Gayet-Delacroix M, Elmaleh-Bergès M, et al. The petrosquamosal sinus: CT and MR findings of a rare emissary vein. AJNR Am J Neuroradiol 2001;22:1186–93 [PMC free article] [PubMed] [Google Scholar]
- 25.Jackler RK, Luxford WM, House WF. Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope 1987;97(Suppl 40):2–14 [DOI] [PubMed] [Google Scholar]
- 26.Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 1993;117:409–29 [DOI] [PubMed] [Google Scholar]
- 27.Tellier AL, Lyonnet S, Cormier-Daire V, et al. Increased paternal age in CHARGE association. Clin Genet 1996;50:548–50 [DOI] [PubMed] [Google Scholar]
- 28.Blake KD, Ratcliffe JM, Wyse RK. CHARGE association in two monozygous triplets. Int J Cardiol 1989;25:339–41 [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JA, Giangiacomo J, Hefner MA, et al. Dominant CHARGE association. Ophthalmic Paediatr Genet 1985;6:271–76 [PubMed] [Google Scholar]
- 30.Rinkwitz S, Bober E, Baker R. Development of the vertebrate inner ear. Ann N Y Acad Sci 2001;942:1–14 [DOI] [PubMed] [Google Scholar]
- 31.Salminen M, Meyer BI, Bober E. Netrin 1 is required for semicircular canal formation in the mouse inner ear. Development 2000;127:13–22 [DOI] [PubMed] [Google Scholar]
- 32.Morsli H, Tuorto F, Choo D, et al. Otx1 and Otx2 activities are required for normal development of the mouse inner ear. Development 1999;126:2335–43 [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Chan EK, Baron S, et al. Hmx2 homeobox gene control of murine vestibular morphogenesis. Development 2001;128:5017–29 [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Van De Water T, Lufkin T. Inner ear and maternal reproductive defects in mice lacking the Hmx3 homeobox gene. Development 1998;125:621–34 [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Xiang J, Duan C. Insulin-like growth factor-binding protein-3 plays an important role in regulating pharyngeal skeleton and inner ear formation and differentiation. J Biol Chem 2005;280:3613–20 [DOI] [PubMed] [Google Scholar]
- 36.Merlo GR, Paleari L, Mantero S, et al. The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway. Dev Biol 2002;248:157–69 [DOI] [PubMed] [Google Scholar]
- 37.Ozaki H, Nakamura K, Funahashi J, et al. Six1controls patterning of the mouse otic vesicle. Development 2003;131:551–62 [DOI] [PubMed] [Google Scholar]
- 38.Lalani SR, Stockton DW, Bacino C, et al. Toward a genetic etiology of charge syndrome: I. A systematic scan for submicroscopic deletions. Am J Med Genet 2003;118A:260–66 [DOI] [PubMed] [Google Scholar]
- 39.Tellier AL, Amiel J, Delezoide AL, et al. Expression of the PAX2 gene in human embryos and exclusion in the CHARGE syndrome. Am J Med Genet 2000;93:85–88 [DOI] [PubMed] [Google Scholar]
- 40.Martin DM, Probst FJ, Fox SE, et al. Exclusion of PITX2 mutations as a major cause of CHARGE association. Am J Med Genet 2002;111:27–30 [DOI] [PubMed] [Google Scholar]
- 41.Vissers LELM, van Ravenswaaij CMA, Admiraal R, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet 2004;36:955–57 [DOI] [PubMed] [Google Scholar]
- 42.Williams MS. Speculations on the pathogenesis of CHARGE syndrome. Am J Med Genet A 2005;133:318–25 [DOI] [PubMed] [Google Scholar]
- 43.Lambert PR. Congenital absence of the oval window. Laryngoscope 1990;100:37–40 [DOI] [PubMed] [Google Scholar]
- 44.Shelton C, Luxford WM, Tonokawa LL, et al. The narrow internal auditory canal in children: a contraindication to cochlear implants. Otolaryngol Head Neck Surg 1989;100:227–31 [DOI] [PubMed] [Google Scholar]
- 45.Glastonbury CM, Davidson HC, Harnsberger HR, et al. Imaging findings of cochlear nerve deficiency. AJNR Am J Neuroradiol 2002;23:635–43 [PMC free article] [PubMed] [Google Scholar]
- 46.Dahlen RT, Harnsberger HR, Gray SD, et al. Overlapping thin-section fast spin-echo MR of the large vestibular aqueduct syndrome. AJNR Am J Neuroradiol 1997;18:67–75 [PMC free article] [PubMed] [Google Scholar]
- 47.Wiener-Vacher SR, Amanou L, Denise P, et al. Vestibular function in children with the CHARGE association. Arch Otolaryngol Head Neck Surg 1999;125:342–47 [DOI] [PubMed] [Google Scholar]
- 48.Abadie V, Wiener-Vacher S, Morisseau-Durand MP, et al. Vestibular anomalies in CHARGE syndrome: investigations on and consequences for postural development. Eur J Pediatr 2000;159:569–74 [DOI] [PubMed] [Google Scholar]
- 49.Edwards BM, Van Riper LA, Kileny PR. Clinical manifestations of CHARGE Association. Int J Pediatr Otorhinolaryngol 1995;33:23–42 [DOI] [PubMed] [Google Scholar]
- 50.Ito K, Suzuki S, Murofushi T, et al. Neuro-otologic findings in unilateral isolated narrow internal auditory meatus. Otol Neurotol 2005;26:767–72 [DOI] [PubMed] [Google Scholar]
- 51.Papsin BC. Cochlear implantation in children with anomalous cochleovestibular anatomy. Laryngoscope 2005;115(Suppl 106):1–26 [DOI] [PubMed] [Google Scholar]
- 52.Parry DA, Booth T, Roland PS. Advantages of magnetic resonance imaging over computed tomography in preoperative evaluation of pediatric cochlear implant candidates. Otol Neurotol 2005;26:976–82 [DOI] [PubMed] [Google Scholar]
- 53.Blake KD, Davenport SLH, Hall BD, et al. CHARGE association: an update and review for the primary pediatrician. Clin Pediatr 1998;37:159–74 [DOI] [PubMed] [Google Scholar]