Abstract
BACKGROUND AND PURPOSE: Reproducible animal models with appropriate neck size are crucial for preclinical assessment of aneurysm therapies. Our purpose was to determine whether the neck size of elastase-induced aneurysms could be controlled by adjusting the position of the temporary occlusion balloon.
METHODS: Seventy-two elastase-induced aneurysms in rabbits were retrospectively analyzed. Three groups (group 1, n = 35; group 2, n = 32; group 3, n = 5) were defined according to different balloon position (lowest, intermediate, and highest, respectively) related to the origin of right common carotid artery (CCA). Aneurysm sizes in different groups were measured and compared; parent artery dilation was assessed as present or absent. The Wilcoxon rank sum test, the Fisher exact test, and the χ2 test were used for statistics process.
RESULTS: The mean aneurysm neck diameter in group 1 was significantly wider than that in group 2 (P = .0001). The proportion of wide-necked (diameter of neck >4 mm) aneurysms in group 1 was significantly higher than that in group 2 (P = .0011). The mean dome/neck ratio in group 1 was smaller than that of group 2 (P = .0031). Aneurysm width and height and the frequency of parent artery dilation were not different in groups 1 and 2 (P = .43, P = .10, and P = .25). No aneurysms formed in group 3.
CONCLUSION: The neck size of elastase-induced aneurysms can be controlled by adjusting the position of the inflated balloon, with balloon positioning that bridges from the CCA to the subclavian/brachiocephalic arteries yielding narrow-necked aneurysms.
Preclinical assessment of emerging aneurysm therapies is greatly facilitated by reproducible animal models. Numerous species of animals, including rats, rabbits, canines, swine, sheep, and primate species have been proposed as valid model systems.1–5 Both arterial aneurysms and vein pouch aneurysms have been proposed. However, sidewall vein pouch aneurysms fail to reproduce the hemodynamic forces in human intracranial aneurysms, which typically are bifurcation aneurysms or in the curved segment of the artery. Bifurcation-type aneurysms can be created using vein pouches, but such models require substantial microsurgical skill.6 In addition, the creation of a vascular surgical wound also alters the response of the animal to implanted devices, because the open wound and disrupted basement membrane may allow fibroblasts and other cellular elements to migrate into the lumen.
Our group has been working on elastase-induced aneurysm models in the rabbit. A series of investigations validating this model has been reported.2,7–10 The long-term patency of this model has been established for up to 1 year. Further, the aneurysm occurs either at an artery bifurcation or along a vessel curve, similar to the locations typical for berry aneurysm in humans. The model demonstrates hemodynamic forces and geometric, morphologic, and histologic features similar to those of human intracranial aneurysms. Finally, standard catheter systems can be used when embolizing the aneurysms, rendering the model realistic for training physicians or testing devices. This model has been successfully used for testing various endovascular devices, including platinum coils coated with biologically active material.11–16
One potential disadvantage of the elastase-induced aneurysm model is apparent lack of control of neck size, which has relevance for testing of devices aimed at either narrow- or wide-necked aneurysms. Creation of elastase-induced aneurysms involves isolation of the common carotid artery (CCA) lumen by distal ligation and temporary proximal balloon occlusion. Elastase is then incubated above the occlusion balloon, which is placed in the brachiocephalic/subclavian artery junction, across the origin of the right CCA. In this study, we report that the neck size of the aneurysm model can be controlled by adjusting the position of the inflated balloon during elastase incubation.
Methods
Aneurysm Creation
Elastase-induced saccular aneurysms were created in 72 New Zealand white rabbits. All procedures were approved by the Institutional Animal Care and Use Committee at our institution. Detailed procedures for aneurysm creation have been described.2,7 In brief, New Zealand white rabbits (3–4 kg) were anesthetized with intramuscular injection of ketamine, xylazine, and acepromazine (75, 5, and 1 mg/kg, respectively). Using sterile technique, the right common carotid artery (RCCA) was exposed and ligated distally. A 5F sheath (Cordis Endovascular, Miami Lakes, Fla) was advanced retrograde in the RCCA to a point approximately 3 cm cephalad to the origin of RCCA. A roadmap image was obtained by injection of contrast through the sheath retrograde in the RCCA, to identify the junction between the RCCA and the subclavian and brachiocephalic arteries (Advantx; General Electric, Milwaukee, Wis). Through the indwelling sheath, a 3F Fogarty balloon (Baxter Healthcare Corporation, Irvine, Calif) was advanced to the origin of the RCCA at its junction with the right subclavian artery. The balloon was inflated with just enough iodinated contrast material to achieve flow arrest in the RCCA. Porcine elastase (5.23 U/mgP, 40.1 mgP/mL, approximately 200 U/mL; Worthington Biochemical, Lakewood, NJ) mixed with iodinated contrast material was incubated in the dead space of the RCCA, above the inflated balloon, through a microcatheter (Tracker 10; Target Therapeutics, Fremont, Calif). Balloon position and shape were documented with the use of fluoroscopic spot imaging, which was saved and printed. After incubation of the elastase solution, the balloon and sheath were removed, and the RCCA was ligated below the sheath entry site.
Follow-Up Angiography
Intra-arterial digital subtraction angiography (IADSA) was performed at least 3 weeks after creation. The animals were anesthetized as described above. Using a sterile technique, surgical exposure of the right common femoral artery was performed, and a 5F vascular sheath was placed. Heparin (100 U/kg) was administered intravenously. A 5F catheter (Envoy; Cordis Endovascular) was advanced into the brachiocephalic artery, and digital subtraction angiography (DSA) was performed. An external sizing device was in place during IADSA. Two observers (Y.H.D. and M.A.D.) measured the sizes of the aneurysms, which were determined in reference to the external sizing device. Presence or absence of parent artery dilation was also noted. Aneurysm formation was considered to have been achieved when the maximum diameter of the RCCA exceeded 2.5 mm. The necks of aneurysms were characterized as narrow (neck diameter ≤ 4 mm) or wide (neck diameter > 4 mm). Continuous variables were compared with the use of the Wilcoxon rank sum test. Proportions were compared with the use of the χ2 or Fisher exact test (J.N.M. and Y.H.D.).
Definition of Experimental Groups
A single experienced observer viewed the spot films of balloon position obtained during aneurysm creation surgery to determine group assignment. We defined the study sample based on height of the balloon relative to the CCA. Group 1 had lowest balloon position; the inflated balloon was located completely in the brachiocephalic artery and right subclavian artery across the origin of RCCA, without herniation of the balloon into the RCCA (Fig 1). Group 2 had an intermediate balloon position; the inflated balloon was located not only in the brachiocephalic/subclavian arteries but also within the proximal RCCA (Fig 2). Group 3 had highest balloon position; most or all of the inflated balloon was in the proximal common carotid artery (Fig 3).
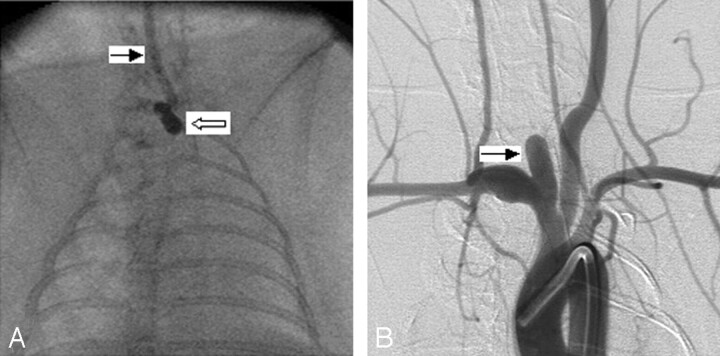
Fig 1.
Group 1 subject (low balloon position).
A, Anteroposterior (AP) spot film obtained during aneurysm creation surgery. The right common carotid artery (RCCA) is opacified with the elastase/iodinated contrast mixture. The RCCA is denoted by the black arrow. Note that the inflated balloon is completely in the right subclavian artery and brachiocephalic artery (white arrow).
B, Same subject as in A. Intra-arterial digital subtraction angiogram, right anterior oblique view, demonstrating a wide-neck aneurysm (black arrow). Neck size is 5.3 mm.
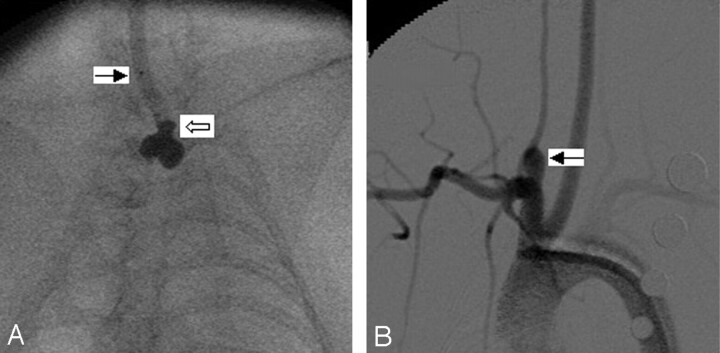
Fig 2.
Group 2 subject (intermediate balloon position).
A, Anteroposterior (AP) spot film obtained during aneurysm creation surgery. The right common carotid artery (RCCA) is opacified with the elastase/iodinated contrast mixture. The RCCA is denoted by the black arrow. Note that a small portion of the inflated balloon has herniated into the proximal RCCA (white arrow).
B, Same subject as in A. Intra-arterial digital subtraction angiogram, right anterior oblique view, demonstrating a narrow-neck aneurysm (black arrow). Neck size is 2.5 mm.
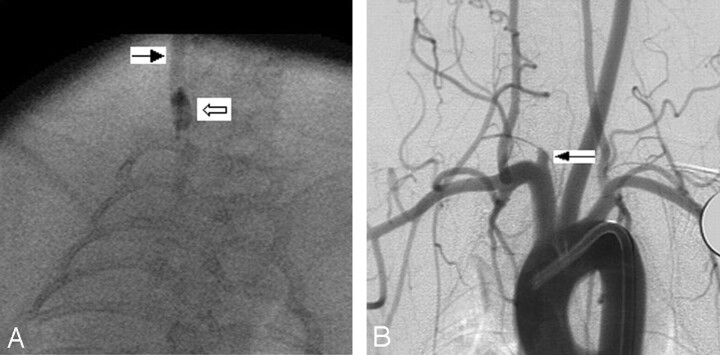
Fig 3.
Group 3 subject (high balloon position).
A, Anteroposterior (AP) spot film obtained during aneurysm creation surgery. The right common carotid artery (RCCA) is opacified with the elastase/iodinated contrast mixture. The RCCA is denoted by the black arrow. Note that the inflated balloon is completely in RCCA (white arrow).
B, Same subject as in A. Intraarterial digital subtraction angiogram, right anterior oblique view, demonstrating a remnant of RCCA (black arrow). The maximum diameter of RCCA is 1.5 mm.
Interobserver variability was measured with the use of intraclass correlation (ICC). ICC values < 0.4 indicate poor reproducibility, 0.4–0.75 indicate fair to good reproducibility, and ≥ 0.75 indicate excellent reproducibility.
Results
Aneurysm sizes are shown in Table 1. Representative images are shown in Figs 1–3.
Aneurysm sizes in groups 1 and 2
| Group 1 (n = 35) | Group 2 (n = 32) | P Value | |
|---|---|---|---|
| Aneurysm width, mm (mean ± SD) | 4.12 ± 1.02 | 3.85 ± 0.80 | .43 |
| Aneurysm height, mm (mean ± SD) | 8.64 ± 2.26 | 8.08 ± 1.92 | .1 |
| Neck width, mm (mean ± SD) | 3.93 ± 0.91 | 2.89 ± 0.76 | < .0001 |
| Dome:neck ratio (mean ± SD) | 1.32 ± 0.40 | 1.79 ± 0.99 | .0031 |
| Parent artery dilation present | 18 | 12 | .25 |
Interobserver Reproducibility
Interobserver reproducibility was excellent for all measurements (neck ICC, 0.89; aneurysm width ICC, 0.85; aneurysm height ICC, 0.95).
Formation of Aneurysm
Aneurysm formation was seen in 35 (100%) of 35 subjects in group 1, 32 (100%) of 32 subjects in group 2, and 0 (0%) of 5 subjects in group 3. Thus, group 3 was excluded from further analysis.
Aneurysm Width
Mean widths for Groups 1 and 2 were 4.12 ± 1.02 (range, 2.9 to 7.6 mm) and 3.85 ± 0.80 (range, 2.6 to 5.8 mm), respectively (Wilcoxon rank sum test, P value = 0.43).
Aneurysm Neck Size
Mean neck sizes for groups 1 and 2 were 3.93 ± 0.91 (range, 2.2 to 7.1 mm) and 2.89 ± 0.76 (range, 1.3 to 4.7 mm), respectively (Wilcoxon rank sum test, P value < 0.0001). Sixteen (46%) of 35 aneurysms in group 1 were wide-necked; in group 2, 3 (9%) of 32 aneurysms were wide-necked (Fisher exact test, P value = 0.0011).
Aneurysm Dome-Neck Ratio
Mean dome-neck ratio for groups 1 and 2 were 1.32 ± 0.40 (range, 0.6 to 2.2), and 1.79 ± 0.99 (range, 0.8 to 3.5), respectively (Wilcoxon rank sum test, P value = 0.0031).
Aneurysm Height
Mean heights for group 1 and 2, were 8.64 ± 2.26 (range, 2.6 to 12.5 mm), and 8.08 ± 1.92 (range, 5.0 to 13.3 mm), respectively (Wilcoxon rank sum test, P value = 0.10).
Frequency of Parent Artery Dilation
Frequencies of parent artery dilation for groups 1 and 2 were 51% (18 of 35) and 38% (12 of 32) respectively. No difference between the 2 groups was statistically significant (χ2 test, P value = 0.25).
Discussion
Elastase-induced aneurysms in rabbits represent an important advance in aneurysm model research. However, unlike surgical models, in which the size of the aneurysm cavity and neck can be controlled by choice of vein pouch and suture technique, control of elastase-induced aneurysm morphology has not previously been reported. In the current study, we tested the hypothesis that, depending upon position of the occlusion balloon during elastase incubation, the degree of elastase exposure of the neck would vary. As a result, we hypothesized that lower balloon position, as seen in group 1, would yield relatively wider-necked experimental aneurysms.
The results presented here demonstrate that balloon position during elastase incubation represents an important determinant of resultant aneurysm morphology. In particular, if a small part of the inflated balloon herniated cephalad into the proximal RCCA and presumably protected the neck region from elastase injury, then the neck of the resultant aneurysm would be relatively narrow. Conversely, when the inflated balloon was placed entirely in the brachiocephalic and right subclavian artery, without herniation into the proximal RCCA, the neck size tended to be relatively wide. These observations of wider-necked aneurysms with lower balloon position suggest that elastase injury was extended down to the neck region. It is noteworthy that even though neck diameter was changed as a function of balloon position, the aneurysm sizes were similar between groups. Thus, variable dome/neck ratios could be obtained, with higher dome/neck ratios seen with higher balloon position. These results should prove useful to investigators who use the rabbit elastase-induced aneurysm model for preclinical testing of aneurysm occlusion devices.
Parent artery dilation was noted in both group 1 and group 2. Compared with group 1, part of the inflated balloon was located in the proximal end of RCCA in group 2, which theoretically should have decreased the chance of the parent artery being exposed to elastase solution. However, our data demonstrate that the difference in the frequency of parent artery dilation between group 1 and group 2 was not significant. The precise mechanism of parent artery dilation remains unknown.10 In other work, we have noted increased expression of matrix metalloproteinase 9 within the wall of the parent artery (unpublished data), which may lead to matrix degradation and vessel dilation even without direct elastase injury.
During this study, we found that appropriate position of the inflated balloon in the proximal RCCA is critical for aneurysm formation. If the position of the inflated balloon in the RCCA was too high, effectively protecting a wide length of the proximal RCCA from elastase injury, then aneurysms did not form. We encountered 5 cases (group 3) in which the whole inflated balloon was placed entirely in the proximal end of the RCCA during creation. DSA images after 3 weeks of creation showed no aneurysm formation. Thus, care should be taken to optimize balloon positioning when creating elastase-induced aneurysms.
This study suffers from several limitations. First, and most important, the study was retrospective in nature. Thus, we cannot predict with certainty that aneurysm neck size can be customized based on balloon position. We are at the early stages of a prospective study to confirm the findings of the current work. Further, exact aneurysm cavity and neck dimensions are subject to interpretation error. We used 2 readers in this study to diminish such errors.
Conclusion
Neck size of elastase-induced aneurysm models in rabbits can be controlled by adjusting the position of the inflated balloon, which makes this model more favorable for testing of neurointerventional devices.
Footnotes
This study was supported by National Institutes of Health grant NS42646.
References
- 1.Hashimoto N, Handa H, Hazama F. Experimentally induced cerebral aneurysms in rats: part III. Pathology. Surg Neurol 1979;11:299–304 [PubMed] [Google Scholar]
- 2.Cloft HJ, Altes TA, Marx WF, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology 1999;213:223–28 [DOI] [PubMed] [Google Scholar]
- 3.Sorteberg A, Sorteberg W, Rappe A, et al. Effect of Guglielmi detachable coils on intraaneurysmal flow: experimental study in canines. AJNR Am J Neuroradiol 2000;23:288–94 [PMC free article] [PubMed] [Google Scholar]
- 4.Guglielmi G, Ji C, Massoud TF, et al. Experimental saccular aneurysms. II. A new model in swine. Neuroradiology 1994;36:547–50 [DOI] [PubMed] [Google Scholar]
- 5.Stehbens WE. Histological changes in chronic experimental aneurysms surgically fashioned in sheep. Pathology 1997;29:374–79 [DOI] [PubMed] [Google Scholar]
- 6.Reul J, Weis J, Spetzger U, et al. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. AJNR Am J Neuroradiol 1997;18:35–42 [PMC free article] [PubMed] [Google Scholar]
- 7.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award: creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices: American Roentgen Ray Society. AJR Am J Roentgenol 2000;174:349–54 [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara NH, Cloft HJ, Marx WF, et al. Serial angiography in an elastase-induced aneurysm model in rabbits: evidence for progressive aneurysm enlargement after creation. AJNR Am J Neuroradiol 2001;22:698–703 [PMC free article] [PubMed] [Google Scholar]
- 9.Short JG, Fujiwara NH, Marx WF, et al. Elastase-induced saccular aneurysms in rabbits: comparison of geometric features with those of human aneurysms. AJNR Am J Neuroradiol 2001;22:1833–37 [PMC free article] [PubMed] [Google Scholar]
- 10.Kallmes DF, Fujiwara NH, Berr SS, et al. Elastase-induced saccular aneurysms in rabbits: a dose-escalation study. AJNR Am J Neuroradiol 2002;23:295–98 [PMC free article] [PubMed] [Google Scholar]
- 11.Kallmes DF, Helm GA, Hudson SB, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology 1999;213:217–22 [DOI] [PubMed] [Google Scholar]
- 12.Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. AJNR Am J Neuroradiol 2002;23:1580–88 [PMC free article] [PubMed] [Google Scholar]
- 13.Kallmes DF, Fujiwara NH, Yuen D, et al. A collagen-based coil for embolization of saccular aneurysms in a New Zealand white rabbit model. AJNR Am J Neuroradiol 2003;24:591–96 [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara NH, Kallmes DF. Healing response in elastase-induced rabbit aneurysms after embolization with a new platinum coil system. AJNR Am J Neuroradiol 2002;23:1137–44 [PMC free article] [PubMed] [Google Scholar]
- 15.Marx WF, Cloft HJ, Helm GA, et al. Endovascular treatment of experimental aneurysms by use of biologically modified embolic devices: coil-mediated intraaneurysmal delivery of fibroblast tissue allografts. AJNR Am J Neuroradiol 2001;22:323–33 [PMC free article] [PubMed] [Google Scholar]
- 16.De Gast AN, Altes TA, Marx WF, et al. Transforming growth factor beta-coated platinum coils for endovascular treatment of aneurysms: an animal study. Neurosurgery 2001;49:690–96 [DOI] [PubMed] [Google Scholar]