Abstract
BACKGROUND AND PURPOSE: Endovascular treatment of intracranial aneurysms by using detachable coils has become an accepted alternative to surgery. To reduce the rate of aneurysm recanalization after treatment, biologically active polyglycolic/polylactic acid–covered platinum coils have been proposed. A prospective and multicenter registry was conducted in France to evaluate the safety and short-term and long-term efficacy of Matrix detachable coils. This first analysis is focused on the safety and short-term efficacy.
METHODS: Two hundred sixty-one patients having ruptured or unruptured aneurysms treated via endovascular approach were included in this registry. Patients with giant aneurysms or in poor clinical condition (Glasgow Coma Scale < 10) were excluded. Because of various protocol violations, clinical analysis was conducted in 236 patients having 244 aneurysms. Technical and clinical complications were systematically recorded. Angiographic analysis was performed by a core laboratory by using the Raymond Grading Scale on 224 patients having 232 aneurysms.
RESULTS: Complete occlusion was achieved in 102 aneurysms (44.0%); neck remnant, in 58 aneurysms (25.0%); and aneurysm remnant, in 72 aneurysms (31.0%). Technical and clinical complications related to the procedure were encountered in 43 patients (18.2%). Postoperative modification of the clinical status was observed in 12 patients (5.1%). Two patients died (0.8%), 6 had a permanent deficit (2.5%), and 4 had a transient deficit (1.7%). Treatment-related mortality was 0.8% and permanent morbidity was 2.5%.
CONCLUSION: Endovascular treatment of intracranial aneurysms by using Matrix detachable coils is feasible and demonstrated initial angiographic results and overall morbidity and mortality rates that are within the ranges found in the literature in the use of bare platinum coils.
Endovascular treatment of intracranial aneurysms by using bare platinum coils has become an accepted alternative to surgery.1 The most significant limitation of this technique is the incidence of aneurysm recanalization.2–5 Animal models and human studies have shown that the biologic response of intracranial aneurysms to bare coils is complex, requiring stability of the initial occlusion for a significant length of time for treatment to be considered durable.6–11 The modification of the surface of bare platinum coils was proposed in the late 1990s to accelerate the biologic response to coils and subsequently reduce the rate of recanalization.12–14
The first coated coil available for clinical use was coated with a bioactive co-polymer consisting of polyglycolic/polylactic acid (PGLA).15 Despite wide clinical use, no large series has been published with regard to the safety and efficacy of this new device. In 2005, Lubicz et al16 reported a series of 20 patients with intracranial aneurysms treated with PGLA-coated coils, demonstrating good clinical and anatomic results. A prospective multicenter registry was conducted in France from January to October 2004 to evaluate the clinical safety and short- and long-term efficacy of the PGLA-coated coils. The acute clinical and angiographic results of our registry are reported in this article.
Methods
Protocol
A prospective and multicenter registry was conducted in France between January and October 2004 to evaluate the safety and efficacy of Matrix detachable coils (Boston Scientific Neurovascular, Fremont, Calif) in the selective endovascular treatment of intracranial aneurysms. Patients with 1 or more ruptured or unruptured aneurysms considered suitable for endovascular treatment were included. The neurosurgeon and interventional neuroradiologist agreed on the method of treatment. All patients were treated with Matrix detachable coils alone or with a combination of Matrix detachable coils and Gugliemi detachable coils (GDC; Boston Scientific Neurovascular). Giant aneurysms (>25 mm), dissecting and fusiform aneurysms, and aneurysms associated with brain arteriovenous malformations were excluded. Because the final goal of the registry was to evaluate the long-term anatomic results after endovascular treatment with Matrix detachable coils, patients with a Glasgow Coma Scale (GCS) grade <10 were excluded. Patients treated exclusively with GDC were not included, and no control group was available. The protocol was approved by the Ethics Committee of Reims. Informed consent was obtained from all patients.
Patient Population
Two hundred sixty-one patients treated via an endovascular approach (163 women and 98 men; age range, 18–78 years; mean age, 48.5 years) were prospectively included. Patients were not included on a consecutive basis but only if PGLA-coated coils were considered appropriate for the treatment by the treating neuroradiologist. During the period of recruitment in the registry, some patients (not included in the data base) were exclusively treated with bare coils. Because selection for treatment with PGLA-coated coils or bare platinum coils was determined and performed by the treating neuroradiologist in each center, it is not possible to know whether a selection bias existed. In some centers, during the period of recruitment, all aneurysms were treated with PGLA-coated coils or with a combination of PGLA-coated and bare platinum coils. In other centers, few patients were treated with PGLA-coated coils in comparison with the number of patients treated exclusively with bare platinum coils.
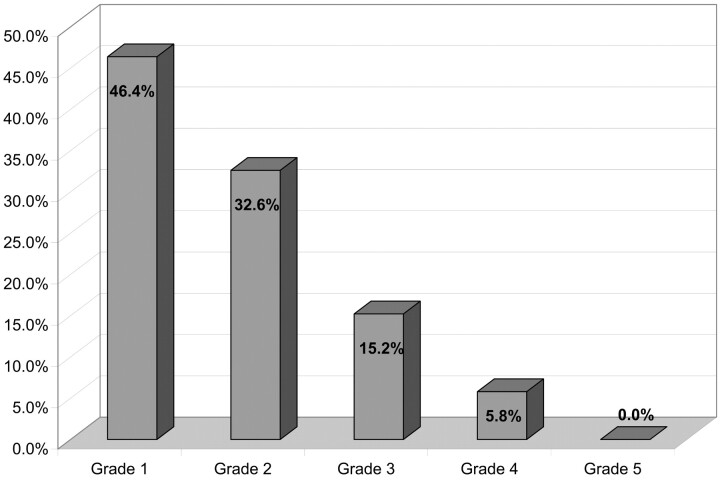
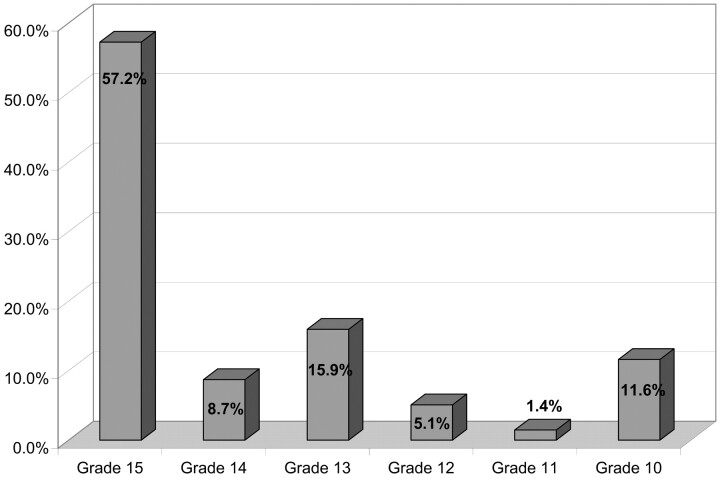
Because of various protocol violations, 25 patients were excluded from analysis: giant (>25 mm) aneurysms in 6 cases, GCS grade <10 in 2 cases, and insufficient clinical and/or anatomic data in 17 cases. Clinical and angiographic analysis was conducted in a total of 236 patients (149 women and 87 men; age range, 21–78; mean age, 48.7) having 244 aneurysms. Clinical presentation was subarachnoid hemorrhage (SAH) in 138 patients (58.5%). Hunt and Hess (HH) scale and GCS grades are presented respectively in Figs 1 and 2.
Fig 1.
HH scale.
Fig 2.
Glasgow Coma Scale grade.
Unruptured aneurysms were present in 98 patients (41.5%). In this group, 19 patients were asymptomatic and 79 had symptoms like headache or vertigo. In a great number of cases, symptoms were not related to the aneurysm. Most aneurysms were asymptomatic. Multiple aneurysms2 were encountered in 3 patients without SAH.
Description of Aneurysms
Aneurysm locations included anterior circulation (carotid siphon, n = 111 [45.5%]; anterior cerebral artery/anterior communicating artery, n = 76 [31.1%]; middle cerebral artery, n = 40 [16.4%]); posterior circulation (posterior cerebral artery, n = 2 [0.8%]; and basilar artery, n = 15 [6.1%]). Aneurysm sizes were 1–5 mm, n = 90 (36.9%); 6–10 mm, n = 115 (47.1%); 11–15 mm, n = 29 (11.9%); and 16–25 mm, n = 10 (4.1%). Aneurysm neck sizes were ≤4 mm, n = 198 (81.1%), and >4 mm, n = 46 (18.9%).
Procedure
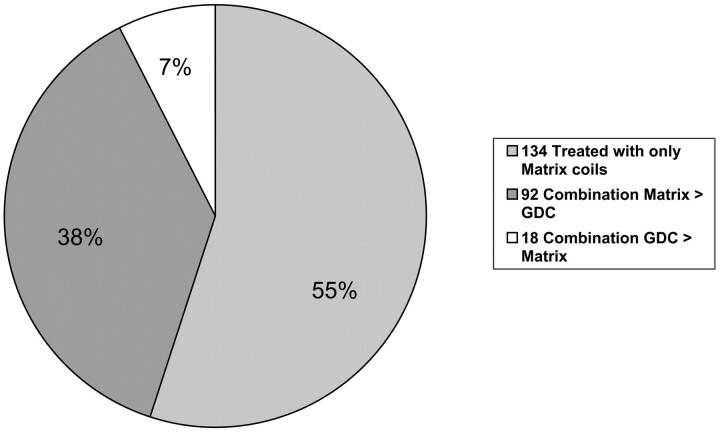
Endovascular procedures were performed with the patient under general anesthesia and systemic heparinization. Anticoagulation therapy was usually continued for 48 hours after treatment. Patients having unruptured aneurysms were placed on antiplatelet medication before or at the beginning of treatment. The aneurysm was catheterized with a 0.14-inch microcatheter with appropriate steam-shaping of the catheter tip. Microcatheter and coil placement was performed by using a subtraction roadmap. All patients were treated with Matrix detachable coils alone or with a combination of Matrix detachable coils and GDC (Fig 3). One hundred thirty-four aneurysms (54.9%) were exclusively treated with Matrix detachable coils. One hundred ten aneurysms (45.1%) were treated with a combination of Matrix detachable coils and GDC. On the basis of the length of coils used, the percentage of Matrix detachable coils was greater than 50% in 92 aneurysms (37.7%) and less than 50% in 18 aneurysms (7.4%). The balloon-remodeling technique was used in 54 patients (22.9%), and an intracranial stent was placed in the parent vessel across the aneurysmal neck in 9 patients (3.8%).
Fig 3.
Relative ratio matrix/GDC (length of coils).
For ruptured aneurysms, the policy was to treat as soon as possible to prevent aneurysmal re-bleeding. In this series, endovascular treatment was performed from 0 to 35 days after bleeding (mean, 3.4 ± 5.2 days). The longest delays were generally related to a delay between the first symptom and the consultation or to the fact that the diagnosis was initially not suspected. In patients with SAH with multiple aneurysms, treatment was performed in 1 session in 3 cases and in 2 separate sessions in 1 case.
For unruptured aneurysms, treatment took place after the patient and/or his or her family received complete information and gave informed consent. In patients with multiple aneurysms, treatment was performed in 1 session in all cases.
Data Analysis
Clinical and procedural data were collected and entered via an electronic Website (Kika-Medical, Nancy, France) and reviewed anonymously by a core laboratory (A.B., S.B., X.L., L.P.). Each time the core laboratory believed that the data reported by investigators were insufficient or imprecise, medical records were sought and evaluated. Statistical analysis was independently conducted by Kika-Medical.
All reported adverse events related to the treatment were analyzed blindly by a neuroradiologist (L.P.) and are described in this series. A global rate of adverse events was calculated on the basis of all abnormal events reported by the investigators. Clinical complications were evaluated through systematic neurologic examination conducted postoperatively and at discharge by a neurologist, neurosurgeon, neuroanesthesiologist, or neuroradiologist.
The permanent morbidity of the treatment was calculated, including all permanent neurologic events related to the treatment, even if the neurologic modification was mild. Treatment-related mortality was also calculated. Morbidity and mortality were determined at discharge. The degree of aneurysmal occlusion was assessed by the treating neuroradiologist, and a second blinded analysis was conducted by the core laboratory on 224 patients having 232 aneurysms. The analysis was initially conducted independently by 2 neuroradiologists. In case of disagreement, a third neuroradiologist was involved. The degree of aneurysmal occlusion was defined by using the simplified 3-point Jean Raymond classification scale5: complete occlusion, neck remnant, and aneurysm remnant.
Results
Anatomic Results
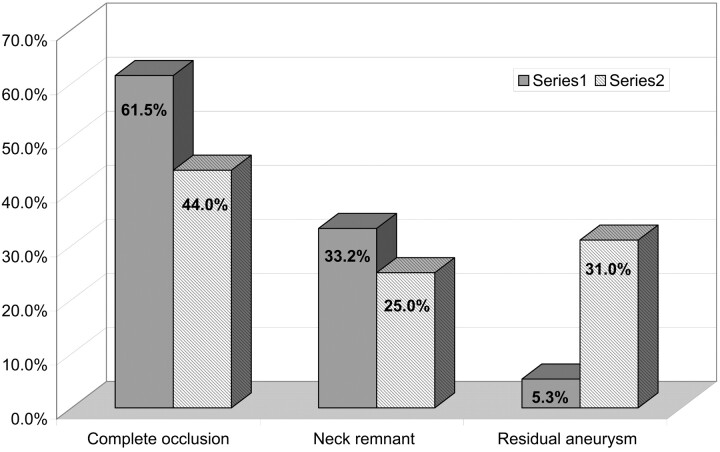
According to the first analysis conducted by the treating neuroradiologist, the postoperative aneurysmal occlusions (Fig 4) were the following: complete occlusion, 150 aneurysms (61.5%); neck remnant, 81 aneurysms (33.2%); aneurysm remnant, 13 aneurysms (5.3%). Results obtained after the independent analysis conducted by the core laboratory were the following (Fig 4): complete occlusion, 102 aneurysms (44%); neck remnant, 58 aneurysms (25%); and aneurysm remnant, 72 aneurysms (31%).
Fig 4.
Acute anatomic analysis (number of aneurysms).
Clinical Results
No early bleeding or re-bleeding was reported in the month following treatment.
Adverse Events Related to the Technique
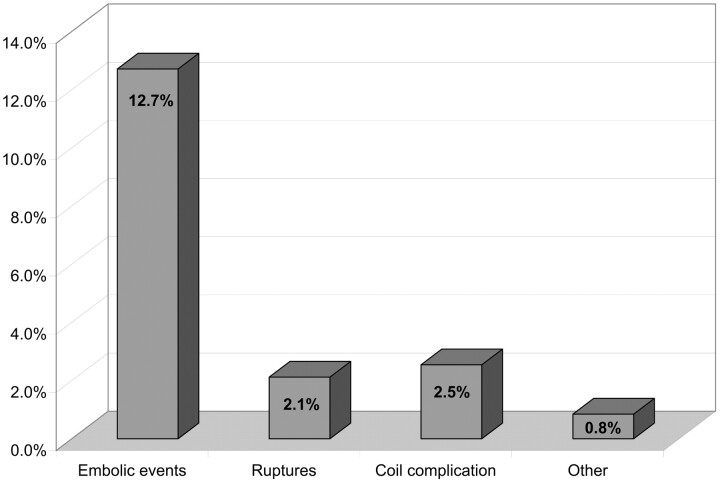
Overall, treatment-related adverse events (Fig 5) were encountered in 43 patients (18.2%). Thirty were thromboembolic, 5 of which were directly related to coil migration or stretching. Intraprocedural aneurysmal rupture occurred in 5 cases. Coil-related complications without an associated thromboembolic event occurred in 6 cases (coil stretching, coil detachment, etc). Other complications included brain edema a few hours after treatment in 1 patient and severe vasospasm induced by the treatment in another patient. All perioperative thromboembolic events were angiographically depicted by the performing investigator and are reported in this series: clotting near the neck of the aneurysm, clotting in the distal branches, and parent vessel occlusion. Postoperative thromboembolic events were depicted in case of sudden neurologic modification by performing MR imaging and/or digital subtraction angiography (DSA).
Fig 5.
Treatment-related events (number of patients). Percentages are related to the total number of patients.
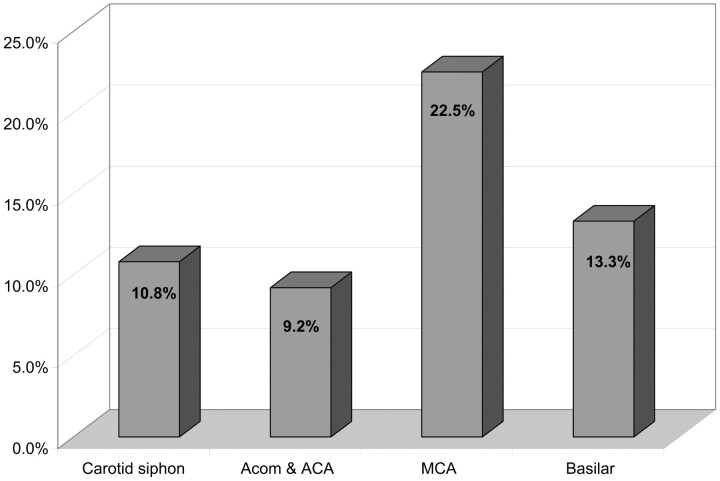
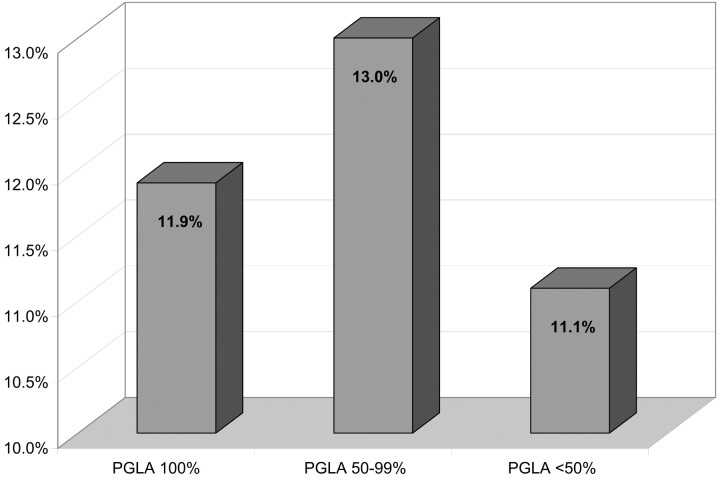
In 5 cases, thromboembolic events were directly related to a problem with a coil (rupture, stretching, or migration of the coil). In 2 other patients, the thromboembolic complication was related to coil protrusion outside the aneurysm. Thromboembolic events relative to the localizations of aneurysms and the percentage of PGLA coils used are reported in Figs 6 and 7. There was no significant difference in the percentage of thomboembolic complications regarding the aneurysmal location or the ratio of PGLA-coated coils GDC.
Fig 6.
Thromboembolic events related to aneurysm localization (percentages). Percentages are related to the total number of aneurysms for each localization. CI: carotid siphon, 6.3–18.0%; anterior communicating artery and anterior cerebral artery, 4.5–17.8%; middle cerebral artery, 12.3–37.5%; basilar artery, 3.7–37.9%.
Fig 7.
Thromboembolic events related to the percentage of PGLA. Percentages are calculated in each group of patients defined according to the relative ratio matrix/GDC (Fig 3). CI: PGLA 100%, 7.5%–18.5%; PGLA 50%–99%, 7.6%–21.4%; PGLA <50%, 3.1%–32.8%.
Treatment of thromboembolic complications was tailored to the specific situation of each patient. In 11 patients, no specific treatment of the thromboembolic event was performed. In 10 patients, medical treatment was modified during the procedure and included an increase of the systemic heparinization or the administration of intravenous antiplatelet therapy. In 9 patients, intra-arterial fibrinolysis was performed by using mechanical or chemical tools (intra-arterial administration of antiplatelet medication or fibrinolytics). In 21 of 30 patients, no clinical modification was observed. Four patients had a transient deficit, 4 patients had a permanent deficit, and 1 patient died. Then, the global rate of clinically relevant thromboembolic complications was 3.8%.
Intraprocedural aneurysmal rupture was observed in 5 patients. Aneurysmal rupture was depicted by the issue of the tip of the coil or the microcatheter outside the limit of the aneurysmal sac and/or extravasation of contrast media. In 4 patients, no clinical modification was observed after the perioperative rupture. In the final patient, with SAH, a severe vasospasm was observed 5 days after the perioperative rupture and was responsible for a neurologic deterioration with a permanent neurologic deficit.
In 6 patients, complications occurred as a direct result of the device used (stretching of the coil or a problem of detachment), but no clinical modification was observed. In 1 patient, a severe brain edema occurred a few hours after treatment and the patient died. In 1 patient, severe vasospasm was observed as a result of treatment and resulted in a postoperative permanent deficit.
Ruptured and Unruptured Aneurysms
The global rate of adverse events was 21% in the ruptured group (confidence interval [CI], 15.1%–28.6%); and 14.3% in the unruptured group (CI, 8.7%–22.6%). The percentage of thromboembolic complications was 13.8% (CI, 9%–20.5%) in patients presenting with SAH and 11.2% (CI, 6.4%–19%) in patients treated for unruptured aneurysms. These differences were not statistically significant.
Clinical consequences of thromboembolic events were 1 death, 3 permanent deficits, 2 transient deficits in the ruptured group, and 1 permanent deficit and 1 transient deficit in the unruptured group. In all except 1 patient, peroperative rupture occurred in ruptured aneurysms. The rate of peroperative rupture was 1% in the unruptured group versus 2.9% in the ruptured group (not significant).
Morbidity and Mortality of the Treatment
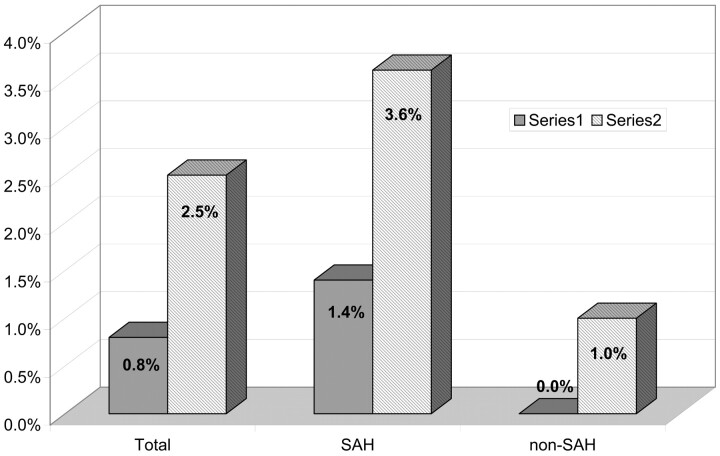
Of the 43 patients (18.2%) who experienced perioperative complications, postoperative modification of the clinical status was observed in 12 patients (5.1%). Two patients died (0.8%), 6 patients had a permanent deficit (2.5%), and 4 patients had a transient deficit (1.7%). Mortality related to treatment was 0.8% and permanent morbidity was 2.5% (Fig 8). In patients with SAH, mortality and permanent morbidity was 1.4% and 3.6%, respectively. In patients with unruptured aneurysms, mortality was 0% and permanent morbidity was 1%. The differences between ruptured and unruptured groups were not significant.
Fig 8.
Permanent morbidity and mortality related to the treatment.
Overall Mortality and Morbidity
Because of the evolution of the SAH, 7 patients died and 3 patients had a permanent deficit. The global mortality and permanent morbidity of this series was 3.8% and 3.8%, respectively.
Discussion
Selective endovascular treatment (EVT) of intracranial aneurysms by using standard bare platinum detachable coils was introduced in 1991.17–18 Since then, the role of EVT has rapidly evolved and the International Subarachnoid Aneurysms Trial (ISAT)1 has verified the clinical importance of endovascular treatment of ruptured aneurysms. Although the safety and efficacy of EVT by using bare platinum coils has been demonstrated, some concern remains with regard to the long-term evolution of aneurysms treated with coils.
Long-Term Follow-Up of Aneurysms Selectively Treated with Coils
The long-term stability of aneurysmal occlusion obtained with coils remains the most significant limitation of this technique. According to the most important series published in the literature, the rate of recanalization after selective EVT of intracranial aneurysms is between 13% and 34%.2–5 Several clinical, technical, and anatomic factors are implicated in the rate of recanalization observed after EVT of intracranial aneurysms.
To evaluate the long-term stability of aneurysmal occlusion obtained with coils, one needs a scale to classify the rate of occlusion of an aneurysm. Several classifications of the degree of angiographic occlusion have been used. The 3-point classification scale of Raymond et al5 was used in our registry: complete occlusion, neck remnant, and aneurysm remnant. In our registry, the evaluation provided by the performing clinician and by the core laboratory was often different. Any persistent opacification inside the aneurysmal sac was classified by the core laboratory as an aneurysm remnant and was often considered by the performing investigator as complete occlusion. On the basis of our series experience, classification of aneurysm occlusion is difficult and subjective. Independent evaluation should, therefore, be considered for all scientific studies and prospective multicenter registries involving anatomic results of EVT of intracranial aneurysms. For this reason, it will be difficult to directly compare our results with those from previous series.
Matrix Detachable Coils
Matrix detachable coils were the first coated coils introduced into clinical practice. Matrix detachable coils use PGLA, which covers a standard 0.010-inch bare platinum coil. The PGLA biopolymer degrades slowly, producing a mild inflammatory reaction.
Histologic results in porcine models show that the molecular and histologic responses elicited by these materials accelerate clot maturation at 14 days.15 At 3 and 6 months, both GDC- and Matrix detachable coil–treated aneurysms were completely occluded with fibrous connective tissue.15 Thicker neointima and neoendothelial coverage of the neck of experimental aneurysms in swine has also been demonstrated at 14 days and 3 months with Matrix detachable coils versus GDCs, but no significant difference was observed at month 6.15
In the rabbit elastase-induced aneurysm model, enhanced tissue response was also demonstrated with the Matrix detachable coils, but angiographic outcome was not improved because of coil compaction.19
Finally, in different animal models, a clear difference was observed between Matrix detachable coils and platinum coils regarding tissue deposition across the aneurysm neck and inflammation around coil surfaces. However, the clinical significance of these tissue reactions is unclear because coil compaction was observed in some cases.
How to Evaluate a New Coil
In a recent editorial, Raymond et al20 discussed the evaluation of new endovascular devices, with particular attention given to coated coils. They concluded that a randomized prospective controlled trial comparing coated coils with standard bare platinum coils is the only way to validate the safety and effectiveness of coated coils, but they recognized the high cost of producing such a trial.
Results of our registry partially confirmed the point of view of Raymond et al.20 Effectively, a precise knowledge of technical and clinical complications, thromboembolic events, and permanent morbidity and mortality related to the treatment with PGLA-coated coils can be obtained from a prospective multicenter registry. Unfortunately, our results are difficult to compare with the existing literature on bare platinum coils for several reasons: 1) Most of the results regarding bare coils were obtained in single-center retrospective studies. The results of single-center series are partly related to the skill of practitioners. In retrospective series, only clinically relevant complications or major events are generally reported, leading to a lower rate of adverse events. 2) Moreover, the methodology used to evaluate complications is not consistent throughout the different series. 3) Finally, series in the literature are different regarding patients and aneurysms treated (ruptured/unruptured aneurysms, size of aneurysms, size of the neck, etc). For these reasons, very different results are reported from 1 series to another regarding complications and morbidity and mortality associated with treatment with bare coils.
At this point, a direct comparison between PGLA-coated coils and bare platinum coils may be necessary to assess the respective safety and efficacy of these devices. However, well-designed registries probably represent a good alternative to initially evaluate a new device, but a precise methodology is certainly needed (multicentric study, prospective inclusion with “intention to treat,” independent clinical monitoring, or independent analysis of clinical and anatomic results). Most of these variables are recognized and included in the design and management of our registry.
We also have to keep in mind that evaluation of new devices is difficult due to the fact that they will rapidly evolve and that their use will follow a learning curve. To overcome the second limitation, a clinical trial should start with a “running-in phase” that will assure that the centers master the use of the device and that results obtained with the very first patients are excluded, if necessary, from final analysis.
Safety of the Matrix Detachable Coils
Because our registry did not include a bare platinum control group, our results can only be interpreted in the light of the available literature on platinum coils. The analysis of the rate of complications and technical problems in previous series is difficult. From 1 series to another, it is difficult to know if operators of approximately similar expertise were using similar techniques to treat a similar selection of patients, if they used the same criteria and applied the same rigor in the detection of complications, or if they adopted identical perioperative protocols as to medication and diagnostic imaging techniques used in the detection of events. For example, case selection vastly differs between North American and European series. In our registry, some exclusion criteria may affect our results, such as exclusion of patients with GCS <10 in case of ruptured aneurysms or of patients having giant aneurysms.
In the most important multicenter results involving GDCs,21–22 complications related to the technique were encountered in 11.2% and 9.18%, respectively. However, in the largest series,21 only clinically relevant complications were reported. In our series, the rate of clinically relevant events was 5.1%. In the review performed by Brilstra et al in 1999,23 the rate of complications was 12% and 15.4% in high-quality studies. In the largest single-center series reporting results of EVT of intracranial aneurysms,24 the rate of complications was reported in 17.45% of aneurysms, but various techniques of treatment were used, including parent artery occlusion and injection of ethylene-vinyl alcohol co-polymer (Onyx; MTI, San Leandro, Calif) in some cases. In the recent series published by Park et al,25 complications were observed in 17.6% of patients. In the end, the reported rate of complications from these most prominent published series ranges between 9% and 18%, and in some series, only clinically relevant complications were reported. The complication rate reported in our series is within this range, though at the upper limit. This may be due to the design of our prospective registry, because all clinical and technical complications were systematically reported. Comparison of the rate of clinically relevant complications in large series of the literature and in our series confirms this hypothesis.
The percentage of thromboembolic events is also difficult to assess from 1 series to another. Effectively, the occurrence of a thromboembolic event is dependant on several factors such as the technique used to treat the aneurysm or the medication administered before, during, and after treatment. These parameters are not always reported in the series and may dramatically influence the rate of thromboembolic complications. Moreover, the detection of a thromboembolic event can be made by various techniques: angiography, MR imaging including diffusion-weighted images, and transcranial Doppler sonography. On the basis of the technique used, the percentage of thromboembolic events is highly variable and ranges from 1%–28%. In our series, all thromboembolic events were depicted via perioperative angiography or by MR imaging or DSA in case of postoperative clinical modification. On perioperative angiography, all thromboembolic events were described, including small clots close to the neck of the aneurysm, small silent distal emboli, and occlusion of the parent vessel. This may explain the high rate of 12.7%, even if most events were clinically silent. In the series of Gallas et al,21 clinically relevant thromboembolic complications were reported in 6% of cases as compared with 3.8% in our series. In the review of Brilstra et al,23 ischemic complications were observed in 8.5% of patients treated with standard bare platinum coils. In the series of Cognard et al,26 Henkes et al,24 and Park et al,25 the reported thromboembolic complication rates were 11%, 9.1%, and 10.4%, respectively. Finally, the rate of thromboembolic events and related clinical complications in our series is in the same range as in the other series.
Procedure-related morbidity and mortality rates as reported in most published series are between 1.5% and 4%.4,24–26 The mortality rate observed in our series (0.8%) is not higher than that reported in patients treated with bare coils, though some bias may be responsible for the difference. Morbidity rates4,24–26 also appear to be in the same range in the bare coils series (4%–22%) and in our group (2.5%). Finally, no increase of procedure-related morbidity or mortality can be observed in our series.
Immediate Efficacy of the PGLA Coil
The comparison of the immediate efficacy of coiling between case series is difficult. Anatomic or clinical parameters can be used for this purpose. As was shown previously, the anatomic result of EVT is in fact difficult to evaluate. The degree of angiographic occlusion can be measured in different ways. Moreover, in most series, the degree of angiographic occlusion is not evaluated independently. As is shown in our registry, the degree of angiographic occlusion is frequently overestimated by the practitioner treating the patient. Accordingly, our results are difficult to compare with those of previous series, and an independent analysis of the angiographic results is recommended for future studies and publications.
With regard to clinical efficacy, the main parameter is the protection against bleeding or re-bleeding provided by the treatment. In our series, no early bleeding or re-bleeding was reported in the month following treatment.
Conclusion
Endovascular treatment of intracranial aneurysms by using Matrix detachable coils is feasible and demonstrated initial angiographic results and overall morbidity and mortality rates that are within the ranges found in the literature on the use of bare platinum coils. Durability of aneurysm occlusion with PGLA-coated coils in the long-term is still unknown and will be the focus of a future report from our series. A trial with a control group may be necessary to precisely assess the efficacy of this new device.
Appendix
Centers and investigators participating in this study were the following:
CHU Larrey, Angers, France; Anne Pasco.
CHU Pellegrin, Bordeaux, France; Xavier Barreau, Jérôme Berge.
CHU de la Côte de Nacre, Caen, France; Patrick Courthé oux.
CHU Gabriel Montpied, Clermont Ferrand, France; Emmanuel Chabert, Jean Gabrillargues.
CHU Roger Salengro, Lille, France; Xavier Leclerc.
CH Neurologique, Lyon, France; Francis Turjman.
CHU Gui de Chauliac, Montpellier, France; Alain Bonafé.
CHU Hôpital Neurologique, Nancy, France; Serge Bracard.
CHU G. & R. Laënnec, Nantes, France; Hubert Desal.
CHU Saint-Roch, Nice, France; Mustapha Dib, Jacques Sedat.
CH La Pitié Salpêtrière, Paris, France; Alessandra Biondi, Nader Sourour.
CH Lariboisière, Paris, France; Emmanuel Houdart.
CHU de la Milétrie, Poitiers, France; Jacques Drouineau.
CHU Maison Blanche, Reims, France; Laurent Pierot.
CHU Purpan, Toulouse, France; Christophe Cognard.
CH Bretonneau, Tours, France; Denis Herbreteau.
Footnotes
The registry was financially supported by Boston Scientific. France. L. Pierot is consultant to Boston Scientific France.
References
- 1.Molyneux A, Kerr R, Stratton I, et al; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet 2002;360:1262–74 [DOI] [PubMed] [Google Scholar]
- 2.Byrne JV, Sohn MJ, Molyneux A. Five-year experience in using coil embolization for ruptured intracranial aneurysm: outcomes and incidence of late rebleeding. J Neurosurg 1999;90:656–63 [DOI] [PubMed] [Google Scholar]
- 3.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 1999;212:348–56 [DOI] [PubMed] [Google Scholar]
- 4.Murayama Y, Nien LN, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg 2003;98:959–66 [DOI] [PubMed] [Google Scholar]
- 5.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003;34:1398–403 [DOI] [PubMed] [Google Scholar]
- 6.Mawad ME, Mawad JK, Cartwright JJ, et al. Long-term histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1995;16:7–13 [PMC free article] [PubMed] [Google Scholar]
- 7.Mizoi K, Yoshimoto T, Takahashi A, et al. A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation: technical case report. Neurosurgery 1996;39:165–69 [DOI] [PubMed] [Google Scholar]
- 8.Horowitz MB, Purdy PD, Burns D, et al. Scanning electron microscopic findings in a basilar tip aneurysm embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1997;18:688–90 [PMC free article] [PubMed] [Google Scholar]
- 9.Stiver S, Porter PJ, Willinsky RA, et al. Acute human histopathology of an intracranial aneurysm treated using Guglielmi detachable coils; case report and review of the literature. Neurosurgery 1998;43:1203–07 [DOI] [PubMed] [Google Scholar]
- 10.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic hispathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284–93 [DOI] [PubMed] [Google Scholar]
- 11.Ishihara S, Mawad ME, Ogata K, et al. Histopathologic findings in human cerebral aneurysms embolized with platinum coils: report of two cases and review of the literature. AJNR Am J Neuroradiolol 2002;23:970–74 [PMC free article] [PubMed] [Google Scholar]
- 12.Kallmes DF, Borland MK, Cloft HJ, et al. In vitro proliferation and adhesion of basic fibroblast growth factor-producing fibroblasts on platinum. Radiology 1998;206:237–43 [DOI] [PubMed] [Google Scholar]
- 13.Raymond J, Desfaits AC, Roy D. Fibrinogen and vascular smooth muscle cell grafts promote healing of experimental aneurysms treated by embolization. Stroke 1999;30:1657–64 [DOI] [PubMed] [Google Scholar]
- 14.Murayama Y, Vinuela F, Tateshima S, et al. Bioabsorbable polymeric material coils for embolization of intracranial aneurysms: a preliminary experimental study. J Neurosurg 2001;94:454–63 [DOI] [PubMed] [Google Scholar]
- 15.Murayama Y, Tateshima S, Gonzalez NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke 2003;34:2031–37 [DOI] [PubMed] [Google Scholar]
- 16.Lubicz B, Leclerc X, Gauvrit JY, et al. Endovascular treatment of intracranial aneurysms with matrix coils: a preliminary study of immediate post-treatment results. AJNR Am J Neuroradiol 2005;26:373–75 [PMC free article] [PubMed] [Google Scholar]
- 17.Guglielmi G, Vinuela F, Sepetka I, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1. Electrochemical basis, technique, and experimental results. J Neurosurg 1991;71:1–7 [DOI] [PubMed] [Google Scholar]
- 18.Guglielmi G, Vinuela F, Dion J, et al. Electrothrombosis of saccular aneurysms via endovascular approach. J Neurosurg 1991;75:8–14 [DOI] [PubMed] [Google Scholar]
- 19.Ding YH, Dai D, Lewis DA, et al. Angiographic and histologic analysis of experimental aneurysms embolized with platinum coils, Matrix and HydroCoil. AJNR Am J Neuroradiol 2005;26:1757–63 [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond J, Guilbert F, Weill A, et al. Safety, science, and sales: a request for valid clinical trials to assess new devices for endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2004;25:1128–30 [PMC free article] [PubMed] [Google Scholar]
- 21.Gallas S, Pasco A, Cottier JP, et al.. A multicenter study of 705 ruptured intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol 2005;26:1723–31 [PMC free article] [PubMed] [Google Scholar]
- 22.Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: preoperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475–82 [DOI] [PubMed] [Google Scholar]
- 23.Brilstra EH, Rinkel GJE, Van der Graaf T, et al. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke 1999;30:470–76 [DOI] [PubMed] [Google Scholar]
- 24.Henkes H, Fischer S, Weber W, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery 2004;54:268–85 [DOI] [PubMed] [Google Scholar]
- 25.Park HK, Horowitz M, Jungreis C, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol 2005;26:506–14 [PMC free article] [PubMed] [Google Scholar]
- 26.Cognard C, Weill A, Castaings L, et al. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology 1998;206:499–510 [DOI] [PubMed] [Google Scholar]