Abstract
SUMMARY: A woman aged 68 years who experienced recurrent right hemiparesis caused by hypoglycemia was admitted to our hospital. When she was experiencing a low level of glucose, diffusion-weighted MR imaging showed the presence of hyperintensity lesions in the bilateral internal capsule. Diffusion-weighted MR imaging has been infrequently performed in patients with hypoglycemia. We report the reversible hyperintensity lesions on diffusion-weighted MR imaging in a hypoglycemic period in a patient with reversible hemiparesis. A reduction of apparent diffusion coefficient in a hypoglycemic period was clearly shown.
The effect of severe hypoglycemia on the brain is well known, ranging from alterations of mental status and focal signs of an acute stroke to coma and death. To our knowledge, imaging studies of hypoglycemia in humans are mostly based on patients in hypoglycemic coma. We report a case of recurring focal neurologic deficit due to hypoglycemia, with reversible hyperintensity lesions in the bilateral internal capsules on diffusion-weighted MR imaging. A reduction of apparent diffusion coefficient (ADC) values in hypoglycemia was shown. We discuss the mechanism underlying the reduced ADC values and emphasize the fact that not all hyperintense lesions that appear on diffusion-weighted MR imaging are due to ischemia.
Case Report
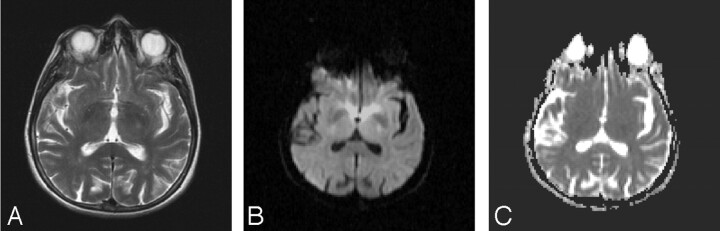
A woman aged 68 years with a 5-day history of right dominant morning weakness, slurred speech, and numbness on her tongue was admitted to our hospital. She noticed that her complaints disappeared 2 to 3 hours after awakening. Her medical history included an endocrine tumor of the cervix 6 years ago, treated with chemotherapy and radiation therapy. She had a regular heart rate and blood pressure, regular respiration, and normal temperature without hypoxia. The findings of the neurologic examination were normal during admission. On checking her status, we considered the possibility of a transient ischemic attack at first and administered heparin. The day after admission, clinical symptoms of right dominant hemiparesis were noticed at 6:00 pm on August 12 and emergent MR imaging was ordered. Findings of MR imaging were interpreted as normal at this time (Fig 1 A–C). However, symptoms worsened in the early morning of August 13, and an MR imaging examination was performed again at 12:00 am. This time diffusion-weighted imaging showed the presence of hyperintense lesions within the bilateral internal capsules.
Fig 1.
A–C, Axial T2 (TR/TE, 3500/111 ms; number of excitations, 2) diffusion-weighted MR images (b = 1000 s/mm2; TE, 97; gradient strength, 24mT/m) and ADC map are interpreted as normal at this time.
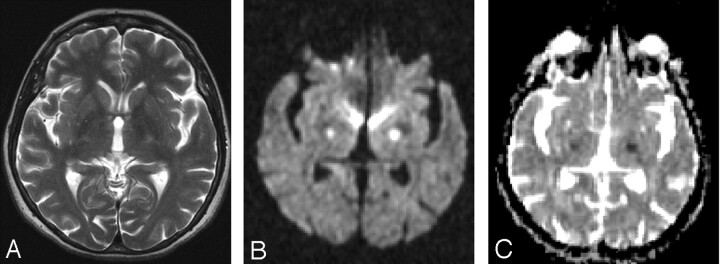
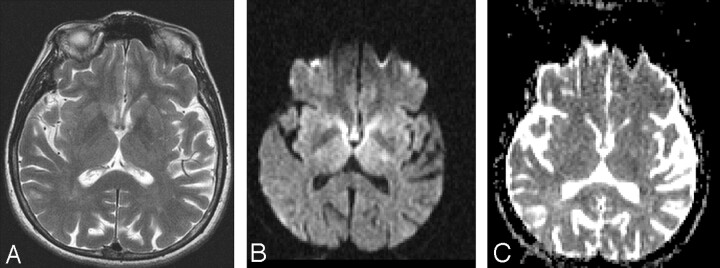
ADC values calculated this time for the left internal capsule were 0.43 min/0.47 max/0.44 avg 10–3 mm2/s and, for the right internal capsule, were 0.50 min/0.55 max/0.52 avg 10–3 mm2/s (Fig 2 A–C). Normal ADC values of internal capsule were given as 0.679 ± 0.052 10–3 mm2/s by DeLano et al.1 Her blood glucose measured 32 mg/dL at 11:00 am. The patient was immediately administered 50 mL of a 50% glucose infusion. Her hemiparesis and slurred speech improved immediately after the glucose infusion, and she recovered completely within hours without neurologic deficit. The glucose level was 80 mg/dL at 6:00 pm; diffusion-weighted MR imaging showed prominent regression of hyperintense lesions within the bilateral internal capsule at 7:00 pm on August 13. ADC values for the left internal capsule were 0.61 min/0.73 max/0.65 avg 10–3 mm2/s and, for the right internal capsule, 0.64 min/0.70 max/0.60 avg 10–3 mm2/s (Fig 3 A–C). After further evaluation of the hypoglycemia, the metastasis of the endocrine tumor of the cervix to the liver was detected by MR imaging, and the biopsy confirmed the diagnosis. The results of blood tests showed an abnormally high insulin level, which was secreted by the tumor and was thought to cause the repetitive hypoglycemic attacks.
Fig 2.
MR imaging was performed at 12:00 am on August 13; glucose level was 32 mg/dL at 11:00 am. MR imaging was performed when the patient had hemiparesis. T2-weighted MR imaging (TR/TE, 5810/116 ms; number of excitations, 2) (A) shows suspected hyperintensities within the bilateral internal capsule. Diffusion-weighted imaging (B) (b = 1000 s/mm2; TE, 110; gradient strength, 24 mT/m) shows the presence of hyperintense lesions within the bilateral internal capsule. ADC values (C) calculated this time for the left internal capsule are 0.43 min/0.47 max/0.44 avg 10–3 mm2/s and, for the right internal capsule, are 0.50 min/0.55 max/0.52 avg 10–3 mm2/s.
Fig 3.
MR imaging was performed at 7:00 pm on August 13; glucose level was 80 mg/dL at 6:00 pm. The patient’s hemiparesis improved immediately after the glucose infusion, and she recovered completely within hours without neurologic deficit. T2-weighted MR image (TR/TE, 4000/116 ms; number of excitations, 2) (A) shows no signal intensity changes. Diffusion-weighted MR image (b = 1000 s/mm2; TE, 113; gradient strength, 24 mT/m) (B) after recovery shows prominent regression of hyperintense lesions within the bilateral internal capsule, and ADC values (C) for the left internal capsule are 0.61 min0.73 max/0.65 avg 10–3 mm2/s and, for the right internal capsule, are 0.64 min/0.70 max/0.60 avg 10–3 mm2/s.
Discussion
Low levels of glucose can result from overuse of oral hypoglycemic agents or insulin as well as overproduction of endogenous insulin, which may be the result of a neoplasm. The clinical picture of hypoglycemia has many manifestations but, for educational purposes, can be considered falling into 2 groups: early symptoms due to counter-regulatory hormones cause trembling, clamminess, palpitations, and anxiety. When the brain is deprived of glucose, late onset symptoms, such as confusion, headache, seizures, coma, and ultimately death, may be seen. In the literature, focal signs of an acute stroke, such as hemiplegia, aphasia, and cortical blindness, have been reported with hypoglycemia.2 In our patient, a very rare endocrine tumor of the cervix was responsible for the hypoglycemia.3
Neurochemical changes in hypoglycemia include an arrest of protein synthesis in many regions, incomplete energy failure, and loss of ion homeostasis, cellular calcium influx, intracellular alkalosis, and a release of neuroactive amino acids, especially aspartate, into the extracellular space of the brain. Excessive release of excitatory amino acids, particularly aspartate, results in selective neuronal necrosis,4 predominantly in the cerebral cortex, caudoputamen, and hippocampus. Auer and Siesjo4 showed that the cerebellum, brain stem, and hypothalamus were largely resistant in rats. Neuroimaging of hypoglycemia is not a well-studied issue in humans. Most of the knowledge comes from studies in the literature of patients in hypoglycemic coma, except a case report by Bottcher et al.5
Findings of CT scans of the head on admission were often unremarkable in the reported cases, but in time, some acute-stage and chronic-stage changes have been demonstrated. In the acute stage, the changes varied from subtle decreased attenuation of the basal ganglia to diffuse brain edema, as severity of the hypoglycemia increased. In the chronic stage, diffuse brain atrophy and dilation of the ventricular system have been noted. MR imaging findings are comparable to the CT findings, mainly involving the cortex, internal capsule, basal ganglia, and hippocampus.6-10
Diffusion-weighted MR imaging detects change in water diffusion with cellular dysfunction and primarily identifies early ischemic changes in stroke. Infarction is the most common cause of such a hyperintense lesion on diffusion-weighted imaging. In hypoglycemia, the incidence of cytotoxic edema, shrinkage of the extracellular space as a result of hypoglycemia, and failure of the ionic pumps cause the hyperintense lesion on diffusion-weighted MR imaging.11 ADC reductions similar to those seen after ischemia occur in hypoglycemia, status epilepticus, spreading depression, and excitotoxic brain injury, pathologic conditions characterized by a significant shrinkage of the extracellular space volume.11 However, status epilepticus and spreading depression in normal brain are conditions that are not associated with energy failure, and the ADC changes are reversible. Glucose deprivation leads to severe brain energy failure and a reduction of cell membrane ionic pump activity, as does anoxia/ischemia, but the topographic and temporal evolution of hypoglycemic brain damage is different from that of anoxia/ischemia.
In the literature, the changes seen in hypoglycemia detected by diffusion-weighted imaging are described as abnormal cortico-subcortical diffusion-weighted MR images, transient hyperintensity lesions in the bilateral internal capsules and corona radiata, and hyperintense signal intensity of the basal ganglia, hippocampus, and cerebral cortex.9,12-16 Three authors also mentioned the ADC mapping of their patients. Aoki et al13 stated that the result in their patient was consistent with the animal study of Hasegawa et al,11 which showed that severe transient hypoglycemia causes reversible change in the ADC of water. Finelli7 mentioned the hypointense signal intensity in the basal ganglia on the MR imaging ADC map but did not give ADC values. Maekawa et al16 reported that ADCs decreased on the bilateral occipital lobes in the early phase, followed by a gradual recovery 14 days after the insult.
Transient hyperintensity lesions on diffusion-weighted MR imaging in the bilateral internal capsules due to hypoglycemic coma were reported by Endo et al14 in the Japanese literature. To our knowledge, our case is the first one that presented only the reversible hyperintensity lesions in bilateral internal capsules without hypoglycemic coma. Recently, Bottcher et al5 reported a case with transient hypoglycemia–induced hemiparesis, in which reversible hyperintensity lesions on diffusion-weighted MR imaging in the bilateral corona radiata and splenium of the corpus callosum were demonstrated. The shared occurrence of reversible reduction of ADC in transient hypoglycemia–induced hemiparesis was evident in our patient and in the patient of Bottcher et al. However, the involvement pattern was different. The bilateral internal capsule was involved in our patient, whereas the corona radiata and splenium of the corpus callosum were involved in the patient of Bottcher et al.
In an animal study, severe transient hypoglycemia caused a reversible change in the ADC of water.11 In our patient, when the full clinical report of right hemiparesis and slurred speech appeared, the ADC values were 0.43 min/0.47 max/0.44 avg 10–3 mm2/s and the blood glucose level was 32 mg/dL. During the asymptomatic period, the ADC values were 0.61 min/0.73 max/0.65 avg 10–3 mm2/s and the glucose level was 80 mg/dL.
Our case is distinct in 2 respects. First, it describes a different pattern of involvement. Second, our patient’s clinical symptoms only involved focal signs like right hemiparesis and slurred speech, unlike the other imaging-verified patients in a hypoglycemic coma. Although infarction and hypoglycemia exhibit similar findings on diffusion-weighted MR imaging, their mechanisms are distinct. It is critical to be confident of the cause of the hyperintensity on diffusion-weighted imaging because the treatment plan would be changed radically. Diffusion-weighted imaging is generally ordered for the investigation of ischemia. Because patients with diabetes are susceptible to both the ischemic assault and the hypoglycemic attacks, one should be aware of the radiologic evaluation of the hyperintensity lesions appearing on diffusion-weighted imaging and the reduced ADC values, as in the case of ischemia, specifically the atypical localization of hyperintensity lesions.
In our report, we demonstrate hypoglycemia-induced reversible hyperintensity lesions located at the bilateral internal capsule on diffusion-weighted MR imaging in a patient with right hemiparesis and slurred speech. A reduction of ADC in hypoglycemia is shown as well. In conclusion, we emphasize that every hyperintensity lesion appearing on MR imaging is not caused by ischemia, and one must not forget hypoglycemia, which may cause such a change on diffusion-weighted MR imaging.
References
- 1.DeLano MC, Cooper TG, Siebert JE, et al. High-b-value diffusion-weighted MR imaging of adult brain: image contrast and apparent diffusion map features. AJNR Am J Neuroradiol 2000;21:1830–36 [PMC free article] [PubMed] [Google Scholar]
- 2.Kossoff EH, Ichord RN, Bergin AM. Recurrent hypoglycemic hemiparesis and aphasia in an adolescent patient. Pediatric Neurol 2001;24:385–86 [DOI] [PubMed] [Google Scholar]
- 3.Kuzuya K, Nakanishi T. Endocrine tumor of the uterine cervix [in Japanese]. Nippon Rinsho 2004;62:903–06 [PubMed] [Google Scholar]
- 4.Auer RN, Siesjo BK. Hypoglycaemia: brain neurochemistry and neuropathology. Baillieres Clin Endocrinol Metab. 1993;7:611–25 [DOI] [PubMed] [Google Scholar]
- 5.Bottcher J, Kunze A, Kurrat C, S et al. Localized reversible reduction of apparent diffusion coefficient in transient hypoglycemia-induced hemiparesis. Stroke 2005;36:20–22. Epub 2005 Feb 3 [DOI] [PubMed] [Google Scholar]
- 6.Boeve BF, Bell DG, Noseworthy JH. Bilateral temporal lobe MRI changes in uncomplicated hypoglycemic coma. Can J Neurol Sci 1995;22:56–58 [DOI] [PubMed] [Google Scholar]
- 7.Finelli PF. Diffusion-weighted MR in hypoglycemic coma. Neurology 2001;57:933. [DOI] [PubMed] [Google Scholar]
- 8.Fujioka M, Okuchi K, Hiramatsu KI, et al. Specific changes in human brain after hypoglycemic injury. Stroke 1997;28:584–87 [DOI] [PubMed] [Google Scholar]
- 9.Garambois K, Grand S, Jaillard A, et al. Diffusion-weighted magnetic resonance imaging in hypoglycemic coma [in French]. Rev Neurol (Paris) 2004;160(5 Pt 1):575–78 [DOI] [PubMed] [Google Scholar]
- 10.Cubo E, Andres MT, Rojo A, et al. Neuroimaging of hypoglycemia [in Spanish]. Rev Neurol 1998;26:774–76 [PubMed] [Google Scholar]
- 11.Hasegawa Y, Formato JE, Latour LL, et al. Severe transient hypoglycemia causes reversible change in the apparent diffusion coefficient of water. Stroke 1996;27:1648–55 [DOI] [PubMed] [Google Scholar]
- 12.Chan R, Erbay S, Oljeski S, et al. Case report: hypoglycemia and diffusion-weighted imaging. J Comput Assist Tomogr 2003;27:420–23 [DOI] [PubMed] [Google Scholar]
- 13.Aoki T, Sato T, Hasegawa K, et al. Reversible hyperintensity lesions on diffusion-weighted MRI in hypoglycemic coma. Neurology 2004;63:392–93 [DOI] [PubMed] [Google Scholar]
- 14.Endo H, Shimizu H, Tominaga T, et al. Transient hyperintensity lesions on diffusion-weighted MRI in the bilateral internal capsules due to hypoglycemic coma [in Japanese]. No To Shinkei 2003;55:174–75 [PubMed] [Google Scholar]
- 15.Shirayama H, Ohshiro Y, Kinjo Y, et al. Acute brain injury in hypoglycaemia-induced hemiplegia. Diabet Med 2004;21:623–24 [DOI] [PubMed] [Google Scholar]
- 16.Maekawa S, Aibiki M, Kikuchi K, et al. Time-related changes in reversible MRI findings after prolonged hypoglycemia. Clin Neurol Neurosurg 2006;108:511–13 [DOI] [PubMed] [Google Scholar]