Abstract
BACKGROUND: A model of toe-to-finger transplantation has been used in studying peripheral nerve regeneration and central reorganization. It was found that recovery of sensory perception depends not only on peripheral reinnervation but also on central integrative mechanisms.
OBJECTIVE: Our aim was to investigate functional changes of the brain and somatotopic representation of the transplanted toes after toe-to-finger transplantation.
MATERIALS AND METHODS: Six patients who had toe-to-finger transplantation from 3 to 8 years earlier underwent motor and sensory functional MR imaging studies of transplanted toes and opposite corresponding normal fingers. The motor task was performed by repetitively tapping of the transplanted toe or finger against the thumb, whereas the sensory task was applied by tactilely stimulating the pulp of the transplanted toe or finger.
RESULTS: The main activation areas from both types of stimulations were located in the expected location of the finger homunculus of the primary sensorimotor cortex. In addition, activated volumes from the transplanted toes were significantly greater than those from the opposite fingers (P = .017 for motor task and P = .005 for tactile sensory task, paired samples Student t test).
CONCLUSIONS: Functional recruitment in the primary sensorimotor cortex seemed to have occurred following toe-to-finger transplantation. The transplanted toe was somatotopically represented in the hand area.
Nerve regeneration and functional recovery following nerve repair are important aspects of clinical neurology. Toe-to-finger transplantation appears to be an excellent model to study peripheral nerve regeneration and central reorganization.1, 2 By using this model, researchers have reported neurophysiologic and neuropsychologic changes following nerve reconnection.2–10 These findings reveal that functional recovery of sensibility depends not only on peripheral reinnervation but also on central integrative mechanisms. For example, recovery of simple sensations is better than that of discriminatory and localizing functions.9 Most of the transplanted toes were regarded by patients as their fingers, and such perception usually occurred before the appearance of sensation and electrophysiologic responses,6 In addition, toe-to-finger transplantation is found to facilitate the disappearance of the phantom finger phenomena.8 Therefore, the central nervous system may play an important role in the functional recovery after toe-to-finger transplantation.
The implementation of functional MR imaging (fMRI) by using routine clinical imagers provides a new method for localizing sensory and motor-related activity with an acceptable degree of spatial resolution.11 The observed signal intensity enhancement is due to a decrease in deoxyhemoglobin concentration in the microvasculature, resulting from a local increase in blood oxygenation during cerebral tissue activation, producing an increase in magnetic homogeneity.12,13 With this technique, brain maps of functional representation can be obtained.
The aim of the present study was to investigate functional changes of the brain and somatotopic representation of the transplanted toes after toe-to-finger transplantation by means of motor and sensory fMRI.
Materials and Methods
Subjects
The study subjects consisted of 6 patients whose mean age was 33.3 ± 17.1 years (range, 9–59 years) (Table). Two patients had right-hand injuries and 4 had left-hand injuries. All the patients had sustained hand injuries of an avulsion or crush type, which resulted in finger amputation, often multiple, and the immediate finger-to-finger replantation was not feasible. They later received toe-to-finger transplantation to restore finger and hand functions. The mean interval between hand injury and toe transplantation was 13.0 ± 10.8 months (range, 1–28 months). Transplantation of a single toe was performed in 1 patient and of 2 toes, in 5 patients. None of the patients had neurologic or medical disorders that might interfere with nerve regeneration. The toe-to-finger transplantation was performed by surgeons of the Department of Plastic and Reconstructive Surgery. The microsurgical techniques of the toe-to-finger transplantation consisted of an end-to-end nerve suture, end-to-end arterial and venous anastomosis, fixation of bones, and attachment of tendons. In nerve repair, neuromas at the proximal end of the palmar digital nerves were excised before an end-to-end perineural suture was performed. The mean interval between toe-to-finger transplantation and fMRI study was 61.8 ± 20.8 months (range, 30–93 months), which was sufficiently long for nerve regeneration to be completed.
Patient’s Perception of the Functional Recovery After Transplantation
Each patient was asked how he or she judged the overall functional recovery after transplantation. The evaluation was based primarily on the hand and finger functions in daily activities and daily work as well as on sensation and mobility of the transplanted toes. The patients were particularly concerned with the use of the hand in eating with chopsticks and spoons and in manipulating small tools, as well as writing, dressing, bathing, and cooking. The degree of satisfaction was grouped into 5 categories: excellent, good, moderate, fair, and poor.
Imaging Procedures
Experiments were performed on a 1.5T Magnetom Vision MR imaging scanner (Siemens, Erlangen, Germany). Patients and/or families gave written informed consent, and this study was approved by our Institutional Review Board. Before MR imaging, the subject was instructed on the entire experimental procedures and rehearsed the task designs to minimize anxiety and enhance task performance. Following this familiarization, the subject lay supine on the scanning table and was fitted with plastic ear canal molds. The subject’s head was immobilized by a tightly fitting thermally molded plastic facial mask that extended from the hairline to the chin.14 A single-shot T2-weighted gradient echo-planar imaging (EPI) sequence was used for the fMRI scans, with a section thickness of 5 mm; in-plane resolution, 3 × 3 mm; and TR/TE/flip angle, 3000/60 ms/90°. The field of view was 192 × 192 mm, and the acquisition matrix was 64 × 64. Twenty-four contiguous axial sections, paralleling the bicommissural line, were acquired to cover the whole brain. The anatomic MR imaging was acquired by using a spin-echo T1-weighted pulse sequence at the same section locations and thickness as the EPI. The in-plane resolution of the T1-weighted image was 1 × 1 mm2.
Experiment Paradigm
The experimental protocol followed an OFF-ON block design, in which the task was performed during ON condition and stopped during OFF condition. To optimize the detection of the sensorimotor region and minimize the risk of technical failure, we used both motor and tactile sensory tasks. fMRI scans were obtained with the motor task followed by the tactile sensory task on the transplanted side and the normal side, respectively. The experimental finger was the transplanted toe, which was equivalent to the index or middle finger, whereas the control finger was the opposite corresponding normal finger. For the motor task, the subject performed repetitive tapping of the experimental or control finger against the thumb at a frequency of 2 Hz. For the sensory task, the pulp of the experimental or control finger was lightly touched by the finger of the technologist at a frequency of 2 Hz. Care was taken to ensure that neither the technologist nor the patient moved more than necessary during the entire acquisition. Each task contained 4 OFF blocks, which were each followed by 1 ON block. Each of the 8 blocks lasted for 30 seconds, giving a total scanning time of 4 minutes. Every 3 seconds, an entire image volume per run was collected, giving a total of 80 volumes per run.
Data Analysis
We used Matlab (MathWorks, Natick, Mass) and in-house software for image-data processing.15 Each subject’s raw data were spatially smoothed by convolution with a 3D 8-mm full width at half maximum gaussian kernel, and motion was corrected with a 6-parameter rigid-body algorithm by using MEDx (Sensor Systems, Sterling, Va). Skull stripping of the T1-weighted images was done by using Alice Software (Perceptive Systems, Boulder, Colo). These images were then spatially normalized to the Talairach brain atlas16 by using the Convex Hull algorithm.17 Images from the first 6 seconds of each block were excluded from further functional data processing to minimize transit effects of hemodynamic responses. Activation maps were calculated by comparing images acquired during each ON state with those acquired during OFF state, by using a Student group t test with a threshold of 4.6 (P < .05, corrected) for the motor task and 2.4 (P < .01, uncorrected) for the sensory task. A lower threshold and uncorrected P value were used for the sensory task because the activity of the sensory task was so weak that no significant signals would be detected if we used a higher threshold or a corrected P value. Such usage, however, is acceptable and commonly seen in the literature. The cluster threshold for both motor and sensory tasks was 225 mm3.
Like the T1-weighted anatomic images, the activation maps were also spatially normalized into Talairach space by using the Convex Hull algorithm. Regions of interest (ROIs) were chosen in the gray matter area along the central sulcus and enclosed the primary motor and sensory cortex on the basis of the anatomic images. For each condition, the volumes of significantly activated voxels within the ROIs from transplanted and normal sides were compared. The significance of difference between transplanted and control sides was calculated by using the paired sample Student t test. The quantity of cerebral functional change after transplantation was calculated in each patient by using a ratio of active volume of the transplanted toe to that of the normal control finger. Correlation between the satisfaction of functional recovery and quantity of cerebral functional change was calculated by using the Spearman rank correlation.
Results
Satisfactory fMRI activation from both motor and sensory tasks was obtained in each subject. The activation from the motor task was always greater than that from tactile sensory task (Table). Activations from the motor and sensory tasks overlapped, with both tasks producing activation straddling the contralateral central sulcus (Fig 1). In some patients, this area was small, with a diameter of 4–6 mm; in other patients, it was broader and more diffuse with a maximal diameter of 20–30 mm. In all cases, the main activation was located in the expected location of the finger homunculus of the primary sensorimotor cortex (approximately two thirds of the distance from the lateral fissure to the interhemispheric fissure).
Fig 1.
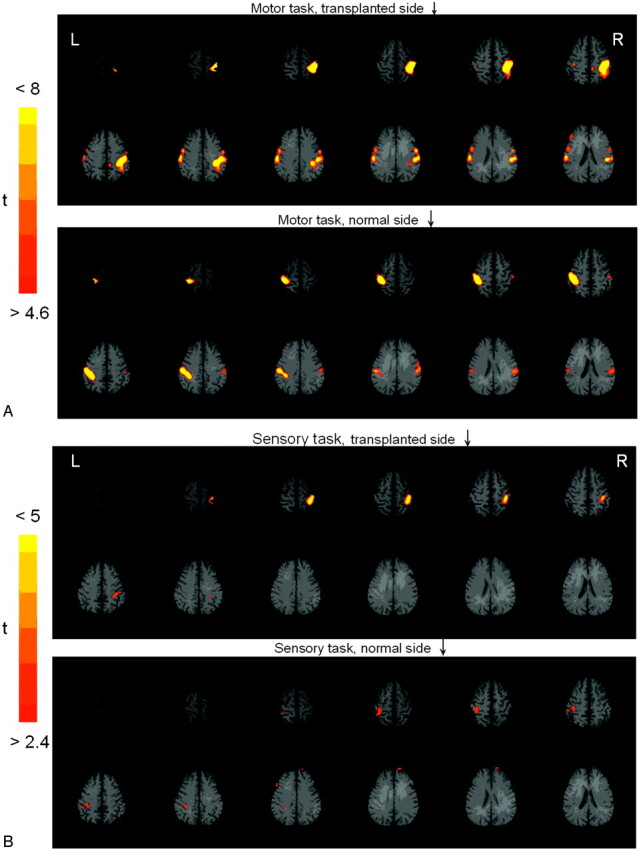
fMRI of the motor task (A) and the tactile sensory task (B) in subject 6. For both motor and sensory tasks, the main activation is located approximately two thirds of the distance from the lateral fissure to the interhemispheric fissure, corresponding to the hand area of the primary sensorimotor cortex. The activated volumes from the transplanted toe are greater than those from the normal finger. During the motor task, less-obvious activation is noted at the supplementary motor area along the midline (arrow in A) and bilateral secondary somatosensory areas along the lateral sulcus (arrowheads in A). Activation of the ipsilateral sensorimotor cortex is also noted during the motor task.
Less-obvious activation was noted at the supplementary motor area along the midline and bilateral secondary somatosensory areas along the lateral sulcus (Fig 1A). Activation of both ipsilateral and contralateral sensorimotor cortex was noted in 4 conditions (of 24 tasks): 2 occurred on the motor task and 2, on the sensory task. Similarly, 2 occurred during activation of the transplanted toe and 2, during activation of the normal finger.
The volumes of activated voxels generated during each task are shown in the Table. On both motor and tactile sensory tasks, the activated volumes from the transplanted toes were significantly greater than those from the normal fingers (P = .017 for the motor task; and P = .005 for the sensory task).
The patient’s satisfaction with finger functional recovery and the quantity of cerebral functional change after transplantation are listed in the Table. One patient graded his recovery as good; 2, as moderate; 2, as fair; and 1, as poor. None of them considered their functional recovery as excellent. No significant correlation was found between the patient’s satisfaction and the quantity of cerebral functional change (for the motor task, the Spearman rank correlation coefficient ρ = −0.383 [− 0.911 to 0.622; P = .419]; for the sensory task ρ = 0.559 [−0.462 to 0.943; P = .297]).
Discussion
To our knowledge, this study represents the first fMRI study to investigate functional changes of the brain after toe-to-finger transplantation. This model of nerve regeneration is unique because the transplanted toe is structurally similar to but functionally different from the finger. Therefore, it would be interesting to explore the topographic representation of the transplanted toes in the primary sensorimotor cortex.
The present data demonstrate that similar to the fingers, both motor and sensory tasks of the transplanted toes consistently produced activation in the expected location of the finger homunculus of the primary sensorimotor cortex. Previous fMRI and positron-emission tomography studies have also found that arm and hand movements produced indistinguishable areas of increased activation approximately two thirds of the distance from the lateral fissure to the interhemispheric fissure.11,18,19 In contrast, toe movement resulted in a medial area of activation from the interhemispheric fissure to the dorsolateral surface that did not overlap with that of hand or arm movement.11 Therefore, the transplanted toes were represented in the hand area of the primary sensorimotor cortex. Such representation may partly explain why most the patients regarded the transplanted toes as their fingers, but not their toes.6
The present study further demonstrates that there was functional recruitment in the primary sensorimotor cortex after toe-to-finger transplantation. Both motor and sensory tasks of the transplanted toes evoked stronger activation in the sensorimotor cortex than those of the controlled fingers. For the motor task, the enhanced fMRI activity might be related to the incomplete nerve regeneration after toe-to-finger transplantation.20,21 Patients thus have to “think harder” to move their transplanted toes because of the incomplete nerve transduction. This “compensation hypothesis” is supported by the study that showed that in patients with amyotrophic lateral sclerosis, a larger volume of activation was seen in the motor cortex during the motor task when compared with that of the controls.22 Unfortunately, our data (correlation coefficient ρ = −0.383; P = .419) did not show significance for this notion. Part of the reason may be the too small sample size of our study. For the sensory task, the increased fMRI activity might be related to persistence of the enlargement of sensory cortical representation after nerve injury, not to the incomplete nerve regeneration. With incomplete nerve regeneration, there should be a decrease in the sensory input to the posterior central gyrus and thus a decrease in the volume of fMRI activity. The increased fMRI activity during sensory stimulation could not be explained by the theory of incomplete nerve regeneration.
Cortical representations of the re-innervated skin regions are recovered via a sequential process entailing at least 2 stages: The first stage occurs as a direct result of the transection injury and involves a loss of the cortical representations of denervated skin surfaces and a corresponding enlargement of the representation of adjacent skin surfaces into the cortical regions that previously represented the denervated skin.23 The secondary stage occurs as a result of subsequent nerve regeneration and involves new cortical representations of the re-innervated skin that displaced the enlarged representations that had been established after injury.23 The transition from the first to the second stage depends on the interaction of injury- and regeneration-related inputs. Hypersensitive reactions to tactile stimulation are seen in both stages. In the denervated stage, the hypersensitivity is evoked from skin areas adjacent to the denervated zone, whereas following reinnervation, it is evoked from re-innervated skin, as well as from adjacent skin areas. The former may be related to the enlarged cortical representations of adjacent skin regions. The later may be related to the persistence of these enlargements.23
As in previous studies, we found that cortical activations from the motor and sensory tasks overlapped, with both tasks producing activations spanning the central sulcus.24,25 Some explanations can be offered for the overlap: 1) It might be that the subjects moved their hands unconsciously when the tactile stimulation was applied, 2) A different in-plane resolution (3 × 3 mm for functional images and 1 × 1 mm for anatomic images) or a mismatch between the anatomic and functional images could also allocate the activation from the central sulcus to the anterior face of the postcentral gyrus, and 3) sensory and motor areas may not be strictly separated. The last notion was suggested by some intraoperative stimulation results indicating that a direct stimulation of the motor cortex in humans sometimes evokes sensory experiences and a stimulation of the sensory cortex may evoke motor responses.26
In some of our subjects, activation of both the ipsilateral and contralateral sensorimotor cortex was noted during motor or sensory stimulation. It has long been recognized that the motor cortex has some influence on the ipsilateral muscles. High-field fMRI has demonstrated that signal intensity related to ipsilateral hand movements was present in the primary motor cortex, but was 20 times smaller than the contralateral signal intensity.27 The degree of ipsilateral activation may be related to the proportion of uncrossed fibers in the lateral corticospinal tracts, which normally is 10%–15%.28,29 In this study, however, the incidence of bilateral activation was found equally between transplanted and normal sides, suggesting that toe-to-finger transplantation does not affect such activation.
There are some limitations of this study. First, although we tried to standardize the frequency or intensity of the tasks by rehearsing the tasks before the experiment, variation in the stimulus would be inevitable. This variation might sometimes affect the activated voxels. However, this effect should be random, not systematic. Furthermore, the chosen threshold, which maximized the number of positives and minimized variation, would also help to eliminate this effect. Second, we calculated only the activation that occurred in the primary sensorimotor cortex, not including the adjacent cortices such as the supplementary motor cortex or secondary somatosensory area. This may result in an incomplete evaluation of the functional changes of the brain in response to toe-to-finger transplantation. However, different experimental designs with more sophisticated motor and sensory tasks will be needed to better demonstrate the functional changes in these areas. This will be the subject of our continuing study.
In conclusion, motor and sensory tasks from the transplanted toes evoked enhanced activation in the hand area of the primary sensorimotor cortex. These findings suggest that after toe-to-finger transplantation, a cortical recruitment has occurred in response to the re-innervated process of the transplanted toes and that the transplanted toes are represented in the hand area. Such representation may partly explain patients’ perception of transplanted toes as their fingers.
Clinical data of patients and ratio of activated voxels generated during motor and sensory tasks
| Patient No./Sex | Ages (y) at Inj/Op/MRI | Transplanted sites | Quantity of Motor Functional Change (Transpl./normal) | Quantity of Sensory Functional Change (Transpl./normal) | Satisfaction for Recovery |
|---|---|---|---|---|---|
| 1/M | 8/9/14 | L2, L3 | 1.58 (19 305/12 240) | 11.20 (2520/225) | Good |
| 2/M | 33/35/42 | L3, L4 | 1.29 (8235/6390) | 3.98 (9315/2340) | Fair |
| 3/M | 29/29/35 | L2, L3 | 1.18 (3915/3320) | 5.00 (4950/990) | Moderate |
| 4/M | 1/4/9 | R3 | 11.61 (3645/314) | 3.56 (2565/720) | Poor |
| 5/F | 51/51/59 | R2, R3 | 1.23 (13 680/11 115) | 3.52 (9990/2835) | Moderate |
| 6/M | 20/20/23 | L3, L4 | 1.42 (19 710/13 905) | 1.42 (7200/2610) | Fair |
Note:—In, indicates injury; Op, operation. The activated volumes from the transplanted toes were significantly greater than those from the normal fingers on both motor and sensory tasks (P = .017 motor task; P = .005 sensory task; paired sample Student t-test). Patient satisfaction of the functional recovery after transplantation was grouped into 5 categories: excellent, good, moderate, fair, and poor.
Acknowledgments
We thank J.C. Huang, H.L. Hsu, and Y.C. Tseng for technical assistance.
Footnotes
This study was supported by a research grant from the Chang Gung Memorial Hospital and grants (NSC 93-2314-B214-007; NSC 93-2314-B214-008) from the National Science Council, Taiwan.
References
- 1.Dellon AL. Sensory recovery in replanted digits and transplanted toes: a review. J Reconstr Microsurg 1986;2:123–29 [DOI] [PubMed] [Google Scholar]
- 2.Chu NS. Toe-to-finger transplantation: peripheral regeneration, functional transformation, and central perception. Crit Rev Phys Rehabil Med 1998;10:303–17 [Google Scholar]
- 3.Chu NS. Recovery of sympathetic skin responses following digit-to-digit replantation and toe-to-digit transplantation in humans. Ann Neurol 1996;40:67–74 [DOI] [PubMed] [Google Scholar]
- 4.Chu NS, Chu EC, Yu JM. Conduction study of digital nerve function recovery following toe-to-digit transplantation and a comparison with digit-to-digit replantation. Muscle Nerve 1995;18:1257–64 [DOI] [PubMed] [Google Scholar]
- 5.Chu NS, Wei FC. Recovery of sensation and somatosensory evoked potentials following toe-to-digit transplantation in man. Muscle Nerve 1995;18:859–66 [DOI] [PubMed] [Google Scholar]
- 6.Chu NS. Perception of transplanted toes following toe-to-finger transplantation. J Clin Exp Neuropsychol 1998;20:599–602 [DOI] [PubMed] [Google Scholar]
- 7.Chu NS. Current perception threshold in toe-to-digit transplantation and digit-to-digit replantation. Muscle Nerve 1996;19:183–86 [DOI] [PubMed] [Google Scholar]
- 8.Chu NS. Phantom finger phenomena and the effects of toe-to-finger transplantation. Neurorehabil Neural Repair 2000;14:277–85 [DOI] [PubMed] [Google Scholar]
- 9.Chu NS, Wei FC, Lin CH, et al. Sensory recovery after toe-to-finger transplantation: a three-year observation with quantitative sensory and functional sensibility tests. Acta Neurol Taiwan 2000;11:70–84 [Google Scholar]
- 10.Hsieh SC, Chu NS. Immunohistochemical study of skin nerve regeneration after toe-to-finger transplantation: correlations with clinical, quantitative sensory, and electrophysiological evaluations. Acta Neurol Taiwan 2004;13:178–85 [PubMed] [Google Scholar]
- 11.Rao SM, Binder JR, Hammeke TA, et al. Somatotopic mapping of the human primary motor cortex with functional magnetic resonance imaging. Neurology 1995;45:919–24 [DOI] [PubMed] [Google Scholar]
- 12.Ogawa S, Lee TM. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn Reson Med 1990;16:9–18 [DOI] [PubMed] [Google Scholar]
- 13.Frostig RD, Lieke EE, Ts’o DY, et al. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A 1990;87:6082–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox PT, Perlmutter JS, Raichle ME. A stereotactic method of anatomical localization for positron emission tomography. J Comput Assist Tomogr 1985;9:141–53 [DOI] [PubMed] [Google Scholar]
- 15.Xiong J, Gao JH, Lancaster JL, et al. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp 1995;3:287–301 [Google Scholar]
- 16.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. New York: Theime Medical;1988
- 17.Lancaster JL, Fox PT, Downs H, et al. Global spatial normalization of human brain using convex hulls. J Nucl Med 1999;40:942–55 [PubMed] [Google Scholar]
- 18.Colebatch JG, Deiber MP, Passingham RE, et al. Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J Neurophysiol 1991;65:1392–401 [DOI] [PubMed] [Google Scholar]
- 19.Grafton ST, Woods RP, Mazziotta JC. Within-arm somatotopy in human motor areas determined by positron emission tomography imaging of cerebral blood flow. Exp Brain Res 1993;95:172–76 [DOI] [PubMed] [Google Scholar]
- 20.Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, et al. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain 1993;116:511–25 [DOI] [PubMed] [Google Scholar]
- 21.Dettmers C, Adler T, Rzanny R, et al. Increased excitability in the primary motor cortex and supplementary motor area in patients with phantom limb pain after upper limb amputation. Neurosci Lett 2001;307:109–12 [DOI] [PubMed] [Google Scholar]
- 22.Konrad C, Henningsen H, Bremer J, et al. Pattern of cortical reorganization in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Exp Brain Res 2002;143:51–56 [DOI] [PubMed] [Google Scholar]
- 23.Wall JT, Kaas JH, Sur M, et al. Functional reorganization in somatosensory cortical areas 3b and 1 of adult monkeys after median nerve repair: possible relationship to sensory recovery in humans. J Neurosci 1986;6:218–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yetkin FZ, McAuliffe TL, Cox R, et al. Test-retest precision of functional MR in sensory and motor task activation. AJNR Am J Neuroradiol 1996;17:95–98 [PMC free article] [PubMed] [Google Scholar]
- 25.Yousry TA, Schmid UD, Jassoy AG, et al. Topography of the cortical motor hand area: prospective study with functional MR imaging and direct motor mapping at surgery. Radiology 1995;195:23–29 [DOI] [PubMed] [Google Scholar]
- 26.Yetkin FZ, Mueller WM, Morris GL, et al. Functional MR activation correlated with intraoperative cortical mapping. AJNR Am J Neuroradiol 1997;18:1311–15 [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SG, Ashe J, Georgopoulous AP, et al. Functional imaging of human motor cortex at high magnetic field. J Neurophysiol 1993;69:297–302 [DOI] [PubMed] [Google Scholar]
- 28.Li A, Yetkin FZ, Cox R, et al. Ipsilateral hemisphere activation during motor and sensory tasks. AJNR Am J Neuroradiol 1996;17:651–55 [PMC free article] [PubMed] [Google Scholar]
- 29.Nyberg-Hansen R, Rinvik E. Some comments on the pyramidal tract with special reference to its individual variation. Acta Neurol Scand 1963;39:1–30 [Google Scholar]


