Abstract
SUMMARY: Fetal MR imaging is an increasingly available technique used to evaluate the fetal brain and spine. This is made possible by recent advances in technology, such as rapid pulse sequences, parallel imaging and advances in coil design. This provides a unique opportunity to evaluate processes that cannot be approached by any other current imaging technique and affords a unique opportunity for studying in vivo brain development and early diagnosis of congenital abnormalities inadequately visualized or undetectable by prenatal sonography. This 2-part review summarizes some of the latest developments in MR imaging of the fetal brain and spine and its application to prenatal diagnosis. This first part discusses the utility, safety, and technical aspects of fetal MR imaging, the appearance of normal fetal brain development, and the role of fetal MR imaging in the evaluation of fetal ventriculomegaly. The second part focuses on additional clinical applications of fetal MR imaging, including suspected abnormalities of the corpus callosum, malformations of cortical development, and spine abnormalities.
A valuable complement to prenatal sonography, fetal MR imaging is a powerful technique used to evaluate the fetal brain. Fetal MR imaging has higher contrast resolution than prenatal sonography and allows better differentiation of normal from abnormal tissue. Structural abnormalities such as cerebral malformations and destructive lesions can be sonographically occult on prenatal sonography yet detectable by fetal MR imaging. Moreover, fetal MR imaging is not susceptible to many of the limitations of sonography. In addition, with continued advances in MR techniques, such as diffusion-weighted and parallel imaging, fetal MR imaging offers the promise of contributing to our understanding of normal as well as abnormal brain development.
Fetal MR imaging is used primarily to confirm and characterize brain abnormalities detected by routine prenatal sonography. When a sonographically suspected abnormality is confirmed, fetal MR is also used to identify any additional sonographically occult CNS abnormalities.1,2 Although no formal data exist, it is well accepted that prenatal sonography is limited in its ability to detect many of the destructive and developmental lesions that occur prenatally.3,4 One of the more common sonographically detected brain abnormalities, and therefore referral indications for fetal MR, is ventriculomegaly. Other common indications include sonographically suspected abnormalities of the corpus callosum and cerebellar vermis as well as complications of monochorionic twin pregnancies (see part 2).
Utility and Limitations of Fetal MR Imaging
Fetal MR imaging has several advantages over prenatal sonography. Fetal MR imaging has improved contrast resolution compared with prenatal sonography. Moreover, fetal MR imaging also allows direct visualization of both sides of the fetal brain. This is an advantage over sonography, where the more anterior cerebral hemisphere is often shadowed by the reverberations from the overlying structures, resulting in visualization of only the more posterior cerebral hemisphere. Additional limitations of sonography, resulting from decreased amniotic fluid volume, fetal positioning, and acoustic shadowing from the ossifying calvaria, can also be overcome by fetal MR imaging. Thus, fetal MR imaging allows a more detailed evaluation of the developing brain, including direct visualization and assessment of the developing cortex and sulcation pattern, which is extremely difficult and often impossible with sonography.5–10 Assessment of sulcation is often critical in identifying abnormalities.
Studies have shown that fetal MR imaging can detect sonographically occult abnormalities in up to 50% of cases studied for a variety of indications.8,11–14 These sonographically occult abnormalities include both developmental and destructive lesions, such as agenesis of the corpus callosum, sulcation anomalies, periventricular nodular heterotopia, cerebellar dysplasia, periventricular leukomalacia, porencephaly, multicystic encephalomalacia, germinal matrix hemorrhage, and intraventricular hemorrhage.8,12,15–19 The results of fetal MR imaging, whether verifying absence of abnormality, confirming sonographically detected abnormalities, or discovering additional abnormalities that were not apparent by sonography, have been shown to affect clinical decision-making during pregnancy, both by physicians and parent(s). Indeed, studies have shown that fetal MR imaging affects patient counseling and results in changes in pregnancy management in nearly half of cases.12,13,20 At our institution, most fetal MR imaging is performed during the second trimester.
One of the limitations of fetal MR imaging is that of fetal motion. Because sedation for fetal MR imaging is not used in this country, it was only after the advent of rapid T2-wieghted pulse sequences (where a single image can be acquired in less than 1 second) that fetal MR imaging became embraced as a clinically important imaging technique. Even with rapid image acquisition, however, fetal motion can still affect the quality of the study. In addition to certain clinical measures taken to reduce fetal motion (such as having the mother take nothing by mouth for several hours prior the study, and making sure that she is comfortable during the scan), technical advances that allow interactive scanning have helped to minimize the effects of fetal motion on the study quality. Additional limitations of fetal MR imaging include the small size of the structure being imaged (usually the fetal brain or spine) and the large distance between the fetus (which lies within the uterine cavity) and the receiver coil (which lies on the mother’s abdomen and pelvis). These limitations are currently being overcome with advances in coil design, such as parallel imaging with increasing number of channels, but are still important factors contributing to the inherent limitations of fetal MR imaging with young gestational age fetuses. Maternal claustrophobia and discomfort during the scan are other limitations of fetal MR imaging compared with sonography, though these are typically more problematic with advanced gestational age.
Safety of Fetal MR Imaging
Many studies have been performed to assess the safety of MR imaging in pregnant animals and in animal embryos. However, there is a lack of consensus as to the actual risk, if any, of MR imaging as determined by these studies. Moreover, the studies used MR scanners of differing field strengths and differing scanning conditions, including various scan times, radio-frequencies, and gradient magnetic fields. Thus, it is difficult to directly extrapolate these animal data to clinical human fetal MR examinations. Studies on the safety of MR imaging in pregnant women are even more limited. Several studies failed to show any adverse long-term effects of fetal MR in children who were imaged as fetuses, though these studies are limited somewhat by small sample size.21–25 The American College of Radiology white paper on MR safety published in 2002 states that “Pregnant patients can be accepted to undergo MR images at any stage of pregnancy if, in the determination of a Level Two MR Personnel–designated attending radiologist, the risk-benefit ratio to the patient warrants that the study be performed.”26 However, because of the potential risk of MR imaging to the developing fetus and the current limitations of fetal MR imaging, it is prudent to wait until after the first trimester before performing fetal MR imaging. In fact, it is preferable to wait until at least gestational week 22 to minimize the difficulties created by the small size and excessive motion of younger fetuses. It is also recommended that patients be counseled and sign a consent form at the time of the fetal MR examination. All patients are screened for possible contraindications to MR imaging before the examination. When performing fetal MR imaging, sedation agents are not administered during the examination. Intravenous contrast is also not recommended in fetal MR imaging because of the potential risk to the fetus.
Imaging Techniques
Fetal MR imaging is routinely performed on 1.5T MR scanners. There are several potential limitations to fetal MR imaging, including fetal motion, the small size of the structure being imaged (particularly at younger gestational ages), and the distance between the receiver coil and the structure being imaged. To reduce fetal motion, the mother is kept from consuming anything for 4 hours before the MR examination. For higher quality images, an 8-channel torso phased array coil is used to allow increased coverage of the fetal head and increased signal intensity-to-noise ratio over more standard pelvic phased array coils. The mother lies supine during the course of the examination, which typically lasts 45 minutes. If the mother cannot tolerate lying on her back (eg, because of back pain or compression of the inferior vena cava), then the examination can be performed with the mother lying on her left side.
Because fetal MR imaging is performed without maternal or fetal sedation, image acquisition is susceptible to fetal motion; therefore, fetal MR imaging is performed primarily using ultrafast MR imaging techniques known as single-shot, fast spin-echo (SS-FSE) or half-Fourier acquired single-shot turbo spin-echo (HASTE). Using these rapid pulse sequences, a single T2-weighted image can be acquired in less than 1 second, reducing the likelihood of fetal motion during image acquisition. Because each image is acquired separately, fetal motion typically affects only the particular image that was acquired while the fetus moved.
An initial localizer is obtained in 3 orthogonal planes with respect to the mother, using 6–8-mm SS-FSE T2-weighted sections with a 1–2-mm gap and large field of view. The localizer is useful for visualizing the position of the fetus and determining fetal sidedness. The localizer is also used to ensure that maximal signal intensity is obtained from the area of interest. In certain cases, such as imaging of twins and fetuses with myelomeningoceles, the coil may need to be repositioned in the middle of the examination (eg, when switching from one twin to the other, or from the fetal brain to the spine). From this localizer, the SS-FSE images of the fetal brain are prescribed. Each subsequent image set is prescribed at a plane orthogonal to the preceding set. In this manner, axial, sagittal, and coronal image sets of the fetal brain are obtained. We routinely obtain at least 2 good quality image sets in each plane.
When evaluating the fetal brain, images are obtained with a section thickness of 3 mm with no gap. For the fetal spine, a section thickness of 2 mm is used. Images are acquired during free maternal breathing, though we have found respiratory triggering useful in decreasing motion. Field of view is typically small, though should be adjusted for increased fetal and/or maternal size, or when aliasing artifact is problematic. Imaging parameters include echo time (TE)eff = 90 ms, repetition time (TR) = 4000 ms, bandwidth = 25 kHz, matrix = 192 × 160, field of view = 24 cm, and number of excitations = 0.5. To reduce potential signal intensity loss due to cross-talk between sections, images are acquired in an interleaved manner. Depending upon the MR platform being used, a pause of 1 second may need to be programmed to allow sequential scanning in an interleaved manner.
Some manufacturers have, or are developing, interactive scanning programs that can be applied to fetal MR imaging. These programs allow adjustment of scanning parameters, such as section angle and orientation, in “real-time.”27 Thus, the technologist can adjust the angle of a prescribed axial image when the fetus moves without having to exit and reprogram a new SS-FSE T2 sequence. Using this technique, nonoblique axial, sagittal, and coronal planes can be obtained more easily and rapidly. This is particularly critical when interpreting midline structures. Moreover, in cases of aliasing artifact, the field of view can be increased and/or the phase encoding and frequency encoding directions can be changed during image acquisition. Thus, overall image quality is improved, and scan time is reduced.
The utility of T1-weighed imaging to fetal MR imaging is more limited. Fast multiplanar gradient recalled-echo techniques, such as FMPSPGR (fast multiplanar spoiled gradient-recalled acquisition in the steady state), are primarily used to detect hemorrhage or calcification, both seen as hyperintensity compared with the fetal brain. T1-weighted images are of lower signal intensity-to-noise ratio and require longer acquisition times (18 seconds), with consequent increased susceptibility to both maternal and fetal motion. Images are acquired during a single maternal breath hold. Scanning parameters include TR = 120 ms, TE = min, flip angle = 70°, field of view = 24 cm, matrix = 256 × 160, number of excitations = 1, section thickness = 5 mm, intersection gap = 1 mm, and bandwidth = 31.25 kHz, which yields 8 sections in the axial plane. Alternative T1-weighted techniques are being explored but are still subject to the limitations resulting from the long T1 relaxation time of the fetal brain (poor tissue contrast and either long acquisition time or low signal intensity-to-noise).28 Gradient echo echo-planar T2* weighted images can be useful in detecting hemorrhage.
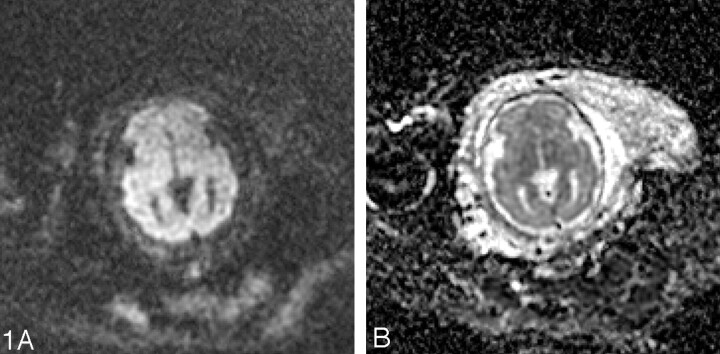
Advanced MR techniques, such as diffusion-weighted imaging, spectroscopy, and parallel imaging, have also recently been successfully applied to fetal MR imaging, though their development is still in the early stages. Diffusion-weighted imaging provides quantitative information about water motion and tissue microstructure. Fetal diffusion-weighted imaging has applications for both developmental and destructive brain processes. Single-shot, echo-planar, diffusion-weighted images are acquired in 18 seconds during a single maternal breath hold (Fig 1). Scanning parameters include TR = 4500 ms, TE = minimum, field of view = 32 cm, matrix = 128 × 128, section thickness = 5 mm, intersection gap = 2 mm, and bandwidth = 167 kHz. Gradients are applied in 3 orthogonal directions using a b-value of 0 and 600 seconds/mm2. Because of the relatively long scan time, images are susceptible to both fetal and maternal motion. With increasing gestational age and engagement of fetal head in the pelvis, the amount of motion is decreased and the quality of the studies improves. Diffusion-tensor imaging (DTI) has recently been applied to fetal autopsy specimens.29 Although its application to living fetuses is currently not feasible because of long acquisition times, progress is being made in this direction.30 Because of the long acquisition time (4.5 minutes) and size of the voxel relative to the fetal brain size, MR spectroscopy is limited to application in the third trimester when the fetal head is relatively large and engaged in the pelvis. Shorter acquisition times can be used but are limited by signal intensity-to-noise ratio.31 Normal metabolites such as N-acetylaspartate, creatine, and choline can be detected.32,33 Parallel imaging can also be applied to fetal MR imaging to decrease the scan time, increase image resolution, or decrease specific absorption rate.
Fig 1.
A, Diffusion-weighted image at the level of the deep gray nuclei in a 29-week-old fetus is free of motion artifact.
B, Corresponding apparent diffusion coefficient map shows lower apparent diffusion coefficient (ADC) values in the developing cortex compared with the developing white matter.
Normal Fetal Brain Development
Interpreting fetal MR images requires a comprehensive understanding of both embryology and pediatric neuroradiology. A complete discussion of normal brain development is beyond the scope of this article, and the reader is referred to several excellent references on brain development34–38 and its appearance on fetal MR imaging.5,7,9,39–44 In brief, MR imaging of the fetal cerebrum is characterized initially by the presence of multiple layers that disappear as the brain matures and the sulci form. Knowledge of the timing and appearance of these layers and sulci are very important in the proper interpretation of fetal brain MR imaging studies.
The ventricular zone, or germinal matrix, is the innermost layer of the fetal cerebral hemisphere; it forms a smooth, dark band of low T2 and high T1 signal intensity lining the lateral ventricles from early gestation. The germinal matrix gradually regresses during the third trimester, persisting in the roof of the temporal horn and in the lateral wall of the occipital horn until approximately gestational week 33 and in the caudothalamic groove until several months after birth. The germinal matrix is the source of neuroectodermal elements that constitute the brain parenchyma, giving rise to both neuronal and non-neuronal cells.
The first group of neurons that migrate outward from the germinal matrices form the primitive cerebral cortex (generally called the preplate). A second group of migrating cells subsequently splits the preplate into the marginal zone (future layer I) and the transient subplate (sometimes called layer VII). The remainder of the cortical plate (layers II–VI) then develops as neurons arrive in an inside-out pattern, with newer arriving cells migrating beyond the previously formed layers and then stopping before the marginal zone in response to chemical signals from Cajal-Retzius (layer I) cells. In humans, neuronal migration begins by approximately gestational week 7; most migration to the cortex is complete by approximately 24 weeks. Like the germinal matrix, the cortical plate appears as a dark band on the T2-weighted images and a bright band on T1-weighted images.
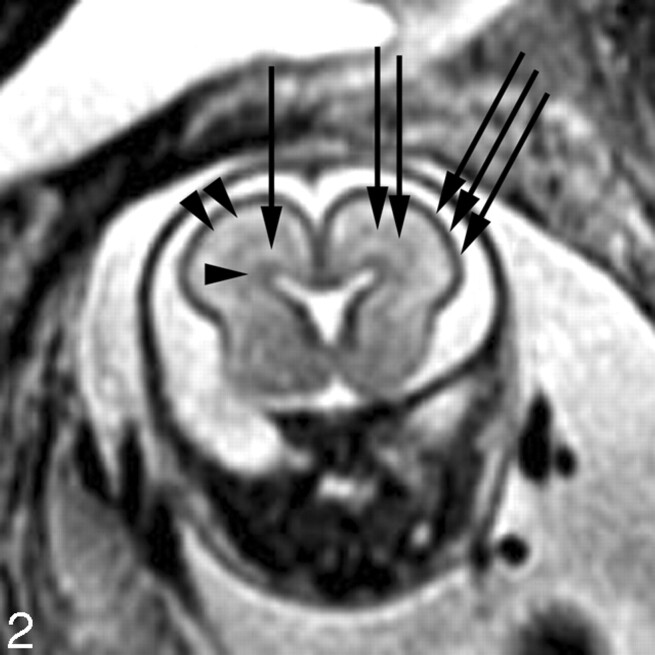
The intervening parenchyma appears relatively bright on the T2-weighted images, and dark on the T1-weighted images, especially before approximately gestational week 20. Girard and Raybaud initially described a multilayered pattern on T1-weighted images beginning at approximately 20 weeks and persisting until approximately 28 weeks.7,45 It is characterized by 3 bands of alternating T1 signal intensity between the germinal matrix and developing cortex. An excellent study by Kostovic et al42 demonstrated that the different layers visible on the fetal MR images represent the different developing layers of the fetal brain. In particular, the first layer superficial to the germinal matrix represents the periventricular zone (dark on T1-weighted images); the next layer represents both the subventricular and intermediate zones (bright on T1-weighted images); and the next layer, which is just deep to the developing cortex, represents the subplate (dark on T1-weighted images). A similar multilayered pattern is seen on T2-weighted images in the normally developing fetus (Fig 2).46,47
Fig 2.
Coronal SS-FSE T2-weighted image at gestational week 23 demonstrates a multilayered pattern. The deepest layer is low in signal intensity and represents the germinal matrix (arrowhead). Immediately superficial to the germinal matrix is a hyperintense layer that represents the periventricular zone (arrow). Immediately superficial to the periventricular zone is a hypointense layer that represents the subventricular and intermediate zones (double arrows). Superficial to this hypointense layer is a band of high signal intensity that represents the subplate (double arrowheads). The most superficial layer of the developing brain represents the developing cortex and marginal zone and is isointense to the germinal matrix (triple arrows).
Sulcation is another important aspect of brain development that can be visualized by fetal MR imaging. The cerebral sulci appear in an organized manner, with increasing development of primary followed by secondary and tertiary sulci with increasing gestational age. Pathologists consider brain sulcation as the most accurate way to date a pregnancy.35 Thus, knowledge of the gestational age is critical when assessing the sulcation pattern. In general, when a primary sulcus first develops, it appears as a shallow infolding on the surface of the brain and then becomes progressively deeper. Sulcation of the fetal brain on MR imaging lags behind that observed on fetal autopsy specimens. Levine and Barnes41 showed a mean lag of 1.9 ± 2.2 weeks in the appearance of a sulcus on MR imaging compared with the published pathology literature. Garel et al9 showed a mean lag of 1 week in the appearance of sulci on fetal MR imaging compared with neuropathology reports. The delay in the appearance of sulci on fetal MR imaging compared with autopsy is probably due to limitations of MR resolution; difficulty obtaining true axial, sagittal, and coronal planes; limitations in section thickness; and fetal motion. In addition, Garel et al9 observed a 2-week lag between when a sulcus was first identified in a particular gestational age group and when it was present in 75% of fetuses of the same gestational age. Thus, there is some variation in the normal appearance of primary sulci on fetal MR imaging (Table 1). Sulcation has been observed to be delayed in twin gestations and to have some right to left asymmetry.48
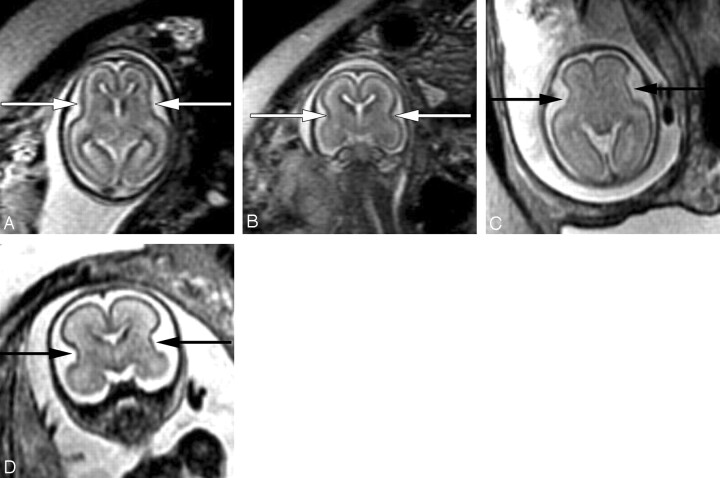
The sylvian fissures are visible on fetal MR imaging before gestational week 18. They initially appear as smooth, curved, wide infoldings on the surface of the brain. At gestational week 23, they appear more angular and less smooth as a result of increased formation of the anterior and posterior operculum (Fig 3). The shape of the sylvian fissures in early phases of development is best assessed on coronal and axial images. Late maturation of the sylvian fissure is a sign of abnormal development.
Fig 3.
Axial (A) and coronal (B) SS-FSE T2-weighted image demonstrates smooth shallow appearance of the Sylvian fissures at gestational week 20. At gestational week 23, the Sylvian fissures (arrows) appear more angled on both axial (C) and coronal (D) images.
The corpus callosum develops between gestational weeks 8 and 20 as axons from the developing cerebral hemispheres navigate to and through the hemispheres and interhemispheric fissure. It can be seen best on midline sagittal T2 images by gestational week 20 as a band of low signal intensity superior to the fornix. It can also be seen on coronal T2 images. It has morphology similar to that of neonates39 with fairly uniform thickness.
The development of the cerebellum begins early in embryogenesis and continues postnatally.49,50 The fetal cerebellum appears relatively small compared with the cerebrum, by comparison with the brain of the term neonate and, especially, the mature brain. The cerebellar vermis is best assessed on direct midline sagittal images, and coronal images; measurements can be made and compared with established norms.40 The cerebellar hemispheres are best assessed on nonoblique axial and coronal views.
The size of the lateral ventricles is best assessed by sonographic measurements of the atria, because both the technique of measurement and the normative values are well established. In particular, the measurement must be made through the posterior aspect of the glomus of the choroid plexus on an axial plane obtained through the thalami, which is easy to obtain with sonography.51 Fetal MR imaging can be helpful in assessing the shape of the entire ventricular system, and in assessing the walls of the lateral ventricles, which should be smooth throughout gestation. It should also be noted that fetal subarachnoid spaces are prominent, particularly before gestational week 30, relative to a term neonate.
Clinical Applications of Fetal MR Imaging
Ventriculomegaly
Ventriculomegaly is the most common central nervous system (CNS) abnormality identified on prenatal sonography.52 It is defined as atrial width greater than 10 mm on sonogram, measured at the posterior margin of the glomus of the choroid plexus on an axial plane through the thalami.51 Although the atrial diameter is relatively constant from gestational weeks 15–35, the relative size of the lateral ventricles decreases with increasing gestational age causing the ventricles to appear larger early in gestation.51,53 Fetal MR measurement of atrial diameter on axial images is usually within 2 mm of sonographic measurements54 even when the MR examination and sonogram are performed on the same day (unpublished personal observations).
The causes of ventriculomegaly are very heterogeneous and include developmental, destructive, and obstructive processes. As many as 80% of fetuses with ventriculomegaly have additional abnormalities that are detected by prenatal sonography and/or by postnatal evaluation.52,5565 Additional abnormalities include chromosomal, extra-CNS and CNS anomalies, such as neural tube defects, agenesis of the corpus callosum, Dandy-Walker complex, lissencephaly, holoprosencephaly, periventricular nodular heterotopia, polymicrogyria, intraventricular hemorrhage, subependymal hemorrhage, and porencephaly. It is noteworthy that sonography can be limited in its ability to detect additional abnormalities in the setting of ventriculomegaly.52,62,66–69
The neurodevelopmental outcome of fetal ventriculomegaly depends, at least in part, on the presence of additional abnormalities identified either in utero or at birth.52,58,59,61,64,66–75 In a large study of sonographically isolated ventriculomegaly, Gupta et al70 reported that the incidence of developmental delay was 37% in children with isolated ventriculomegaly, compared with 84% in children in whom additional abnormalities were identified at birth. Neurodevelopmental disabilities can occur in anywhere from 0% to 36% of children diagnosed prenatally with isolated mild ventriculomegaly (defined as atrial width between 10–15 mm and no additional abnormalities).64,67–71,74 Several studies have found that the risk of developmental delay is lower if the atrial diameter is less than 12 mm and if the fetus is male.64,67,68,71 Several factors likely contribute to differences in the reported outcome of isolated mild ventriculomegaly, including variable duration of clinical follow-up, different methods of assessing neurodevelopment, and differing definitions of ventriculomegaly. The variability in reported neurodevelopmental outcome of fetal isolated mild ventriculomegaly makes counseling these patients extremely challenging.
Because the prognosis of fetal ventriculomegaly is related to the presence of additional abnormalities, the prenatal detection of such abnormalities is critical. Although sonography is the principal technique for screening the fetal brain, it can be limited in detecting abnormalities of the brain parenchyma. Studies have shown that fetal MR imaging can detect additional sonographically occult CNS abnormalities in up to 40%–50% of cases of fetal ventriculomegaly.8,16 Sonographically occult findings include developmental abnormalities, such as agenesis of the corpus callosum, cortical malformations, periventricular nodular heterotopia, cerebellar dysplasia, partial agenesis of the septum pellucidum, Walker-Warburg syndrome, and pontocerebellar dysplasia, and destructive abnormalities, such as periventricular leukomalacia, porencephaly, multicystic encephalomalacia, intraventricular hemorrhage, and subependymal hemorrhage.8,12,13,16,18,76
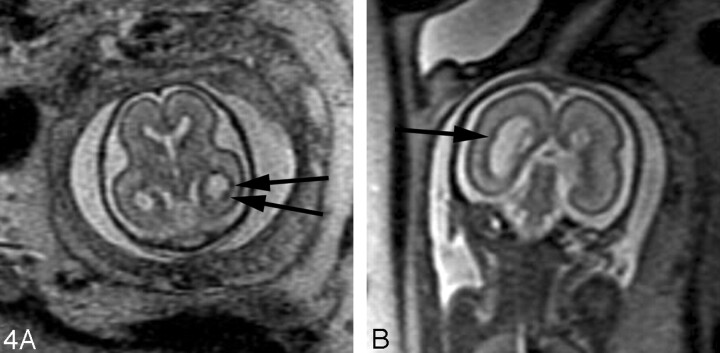
In our experience, fetal MR imaging is especially useful in detecting malformations of cortical development in fetuses with sonographically isolated ventriculomegaly. When interpreting fetal MR images in this clinical setting, the margins of the lateral ventricles should be carefully scrutinized for any areas of nodularity that may represent periventricular nodular heterotopia. Such nodules are isointense to the germinal matrix (Fig 4). Periventricular nodular heterotopia, however, are indistinguishable from the subependymal nodules present in tuberous sclerosis, and other manifestations of tuberous sclerosis, such as transmantle dysplasias, cortical tubers, and cardiac rhabdomyoma, should be sought when ventricular nodularity is identified (Fig 5). Cortical malformations, such as polymicrogyria, can be identified by noting alteration of the normal sulcation pattern for a fetus’ particular gestational age (Fig 6A); they may be identified as too many sulci in a less mature fetus or as too few or abnormally deep or abnormally located sulci in a more mature fetus. The identification of malformations of cortical development in the setting of ventriculomegaly should raise the possibility of a genetic (including metabolic disturbances) or an infectious cause of the ventriculomegaly.
Fig 4.
A, 22-week old fetus with several nodular areas of low signal intensity along the margin of the left lateral ventricle (arrows) on axial SS-FSE T2-weighted image. This was confirmed on coronal SS-FSE T2-weighted images (not shown) and is consistent with periventricular nodular heterotopia; and was confirmed at autopsy.
B, 23-week-old fetus with smaller nodular area of low signal intensity along the atrium of the right lateral ventricle (arrow) on coronal SS-FSE T2-weighted image. Finding was confirmed on axial image (not shown). Findings are also consistent with periventricular nodular heterotopia in this fetus with a family history of periventricular nodular heterotopia.
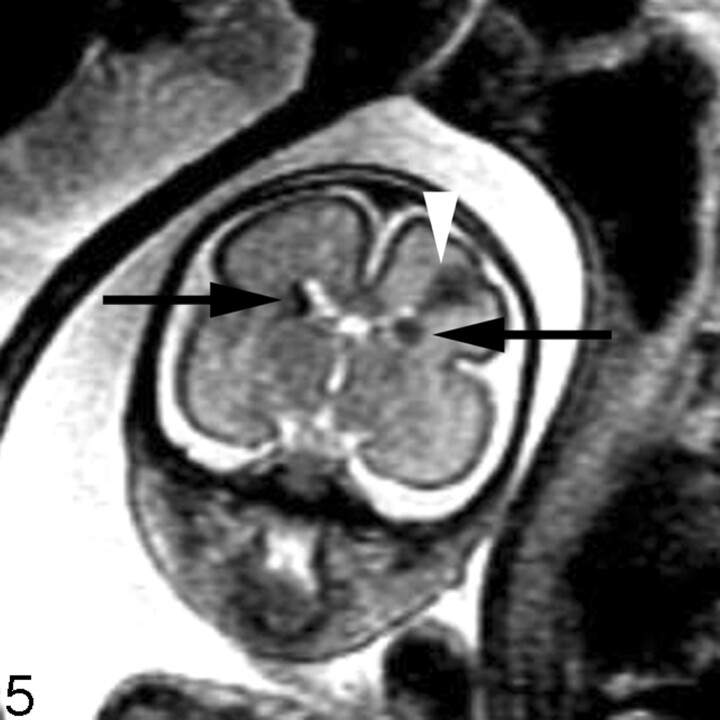
Fig 5.
Coronal SS-FSE T2-weighted image of a 26-week-old fetus demonstrates several hypointense nodules along the margins of both lateral ventricles (arrows). The nodules are somewhat similar to those in Fig 3 (though much larger in this example). A hypointense wedge-shaped area is also seen extending from the margin of the left lateral ventricle to the developing cortex (arrowhead), consistent with transmantle dysplasia. The fetus also had a cardiac rhabdomyoma (not shown). Findings are consistent with tuberous sclerosis.
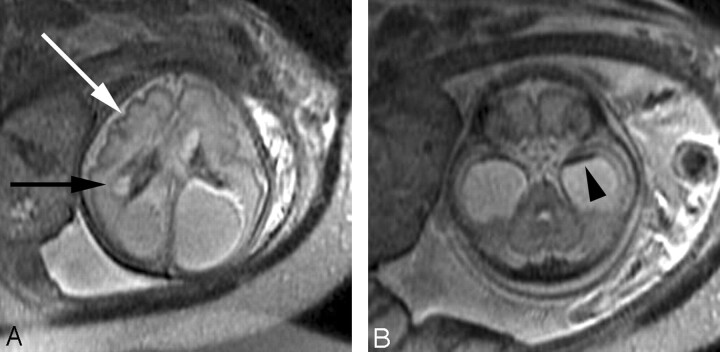
Fig 6.
A, Axial SS-FSE T2-weighted image in a fetus at gestational week 27 demonstrates multiple abnormal infoldings of the developing cortex (white arrow) for expected gestational age, consistent with polymicrogyria. Areas of cystic encephalomalacia with hemorrhage (black arrow) are also seen.
B, Low signal intensity consistent with intraventricular hemorrhage is also seen layering in the temporal horns bilaterally (arrowhead). Fetus was referred for ventriculomegaly and choroid plexus cysts detected on prenatal sonogram.
Destructive lesions generally appear as small periventricular areas of T2 hyperintensity, focal defects or irregularities in the germinal matrix, or larger areas of abnormal signal intensity (with or without volume loss) involving the developing white matter and overlying cortex (Fig 7). Subtle irregularities of the ventricular margin may also be an indication of injury to the adjacent germinal matrix and/or overlying developing white matter. Hemorrhage usually appears as a hypointense area on T2-weighted images and hyperintense area on T1-weighted images, though the signal intensity can vary depending on the stage of hemorrhage. Intraventricular hemorrhage can appear as debris layering in the dependent portion of the ventricle or as a focal hematoma (Fig 6B). The detection of small subependymal hemorrhages is more difficult, partly because the normal germinal matrix has similar signal intensity to blood (hypointense on T2-weighted images and hyperintense on T1-weighted images) because of its high cellularity (Fig 8). T2* weighted gradient-echo sequences may be useful to help to confirm the presence of blood, because the hemorrhage appears more hypointense than the germinal matrix.
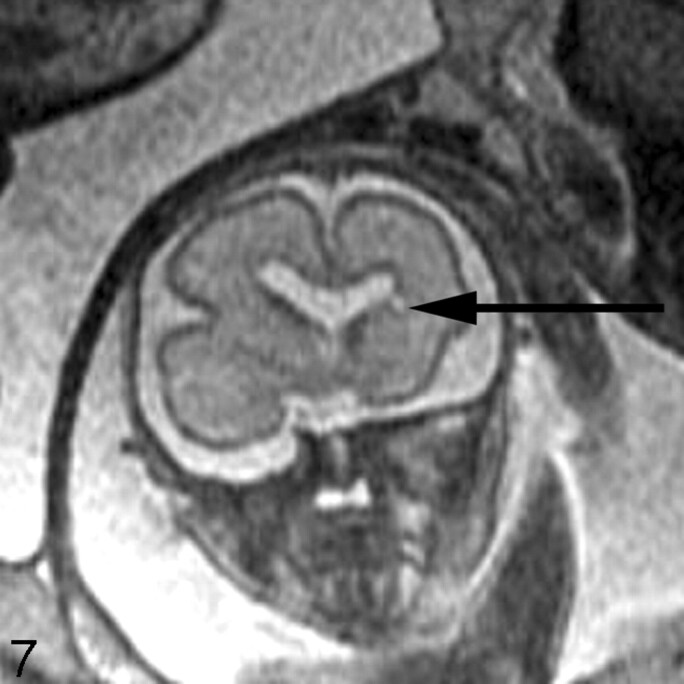
Fig 7.
Coronal ssFSE T2-weighted image in a 25-week old fetus demonstrates a focal area of T2 hyperintensity adjacent to the frontal horn of the left lateral ventricle (arrow). This was also confirmed on axial image (not shown) and is consistent with an area of parenchymal injury. The lateral ventricles are mildly dilated (measuring 12–13 mm on sonography).
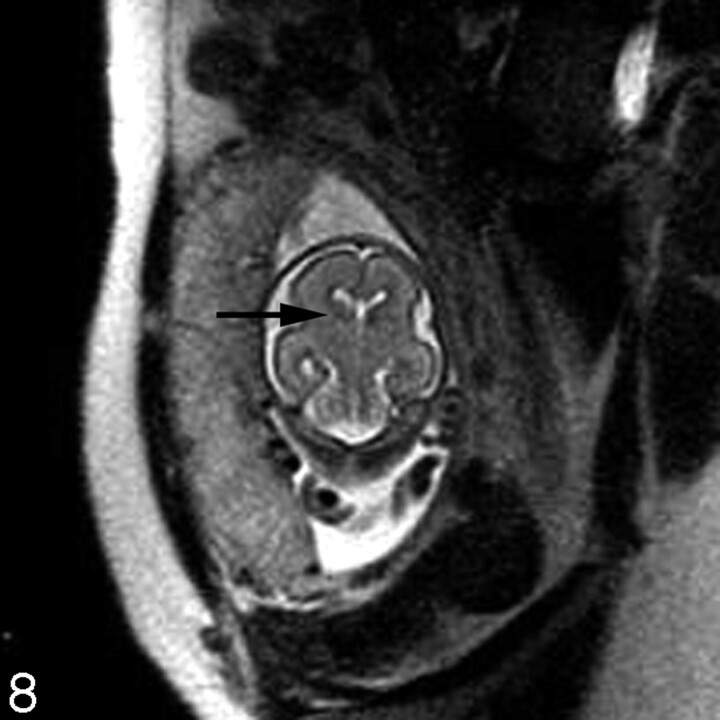
Fig 8.
Coronal ssFSE T2-weighted image in a fetus at gestational week 23 demonstrates prominent hypointensity in the right caudothalamic groove (arrow) consistent with a germinal matrix hemorrhage.
Conclusion
Technical advances in fetal MR imaging have made it an increasingly important tool in the prenatal evaluation of central nervous system abnormalities. In particular, the development of ultrafast T2-weighted technique has made it clinically practical and easy to image the fetus without maternal or fetal sedation. Additional technical advances, such as the recent application of diffusion-weighted imaging to the fetal brain also allow quantitative analysis of different aspects of brain development. This has promising applications for the study of specific brain disorders, as well as for the study of normal in utero brain development. Although future improvements in both MR hardware and software will improve the quality of fetal MR imaging, continued multidisciplinary collaboration with obstetricians, perinatologists, sonographers, child neurologists, and pediatric neurosurgeons is critical to ensure maximal growth in the field of fetal neuroimaging.
Appearance of sulci by gestational age*
| Sulcus | Detectable by Pathology in 25–50% of Fetuses48 (wk) | Detectable by Fetal MR Imaging in 75% of Fetuses9 (wk) |
|---|---|---|
| Parietooccipital | 16 | 22–23 |
| Calcarine | 16 | 24–25 |
| Callosal | 14 | 22–23 |
| Cingulate | 18 | 24–25 |
| Central | 20 | 27 |
| Precentral | 24 | 27 |
| Postcentral | 25 | 28 |
| Superior temporal | 23 | 27 |
| Superior frontal | 25 | 29 |
| Inferior frontal | 28 | 29 |
| Inferior temporal | 30 | 33 |
Modified from Garel et al9.
Acknowledgments
This work was supported in part by a NINDS institute K23 NS52506 award to O.A.G.
References
- 1.Raybaud C, Levrier O, Brunel H, et al. MR imaging of fetal brain malformations. Childs Nerv Syst 2003;19:455–70 [DOI] [PubMed] [Google Scholar]
- 2.Coakley FV, Glenn O, Qayyum A, et al. Fetal MRI: A developing technique for the developing patient. AJR Am J Roentgenol 2004;182:243–52 [DOI] [PubMed] [Google Scholar]
- 3.Filly RA, Goldstein RB, Callen PW. Fetal ventricle: importance in routine obstetric sonography. Radiology 1991;181:1–7 [DOI] [PubMed] [Google Scholar]
- 4.Aubry MC, Aubry JP, Dommergues M. Sonographic prenatal diagnosis of central nervous system abnormalities. Childs Nerv Syst 2003;19:391–402 [DOI] [PubMed] [Google Scholar]
- 5.Girard N, Raybaud C, Dercole C, et al. In vivo MRI of the fetal brain. Neuroradiology 1993;35:431–36 [DOI] [PubMed] [Google Scholar]
- 6.Girard N, Raybaud C, Poncet M. In vivo MR study of brain maturation in normal fetuses. AJNR Am J Neuroradiol 1995;16:407–13 [PMC free article] [PubMed] [Google Scholar]
- 7.Brisse H, Fallet C, Sebag G, et al. Supratentorial parenchyma in the developing fetal brain: in vitro MR study with histologic comparison. AJNR Am J Neuroradiol 1997;18:1491–97 [PMC free article] [PubMed] [Google Scholar]
- 8.Levine D, Barnes PD, Madsen JR, et al. Central nervous system abnormalities assessed with prenatal magnetic resonance imaging. Obstet Gynecol 1999;94:1011–19 [DOI] [PubMed] [Google Scholar]
- 9.Garel C, Chantrel E, Brisse H, et al. Fetal cerebral cotex: Normal gestational landmarks indentified using prenatal MR imaging. AJNR Am J Neuroradiol 2001;22:184–89 [PMC free article] [PubMed] [Google Scholar]
- 10.Garel C, Chantrel E, Elmaleh M, et al. Fetal MRI: normal gestational landmarks for cerebral biometry, gyration and myelination. Child’s Nerv Syst 2003;19:422–25 [DOI] [PubMed] [Google Scholar]
- 11.Levine D, Barnes PD, Madsen JR, et al. Fetal central nervous system anomalies: MR imaging augments sonographic diagnosis. Radiology 1997;204:635–42 [DOI] [PubMed] [Google Scholar]
- 12.Simon EM, Goldstein RB, Coakley FV, et al. Fast MR imaging of fetal CNS anomalies in utero. AJNR Am J Neuroradiol 2000;21:1688–98 [PMC free article] [PubMed] [Google Scholar]
- 13.Coakley FV, Hricak H, Filly RA, et al. Complex fetal disorders: effect of MR imaging on management - preliminary clinical experience. Radiology 1999;213:691–96 [DOI] [PubMed] [Google Scholar]
- 14.Glenn O, Goldstein R, Li K, et al. Magnetic resonance imaging of the fetal brain in the evaluation of sonographically suspected abnormalities of the corpus callosum. In: Proceedings of the American Society of Neuroradiology 41st Annual Meeting; Apr 26–May 2,2003; Washington, DC.
- 15.Lacey DJ. Agenesis of the corpus callosum—clinical features in 40 children. Am J Dis Child 1985;139:953–55 [DOI] [PubMed] [Google Scholar]
- 16.Wagenvoort AM, Bekker MN, Go AT, et al. Ultrafast scan magnetic resonance in prenatal diagnosis. Fetal Diagn Ther 2000;15:364–72 [DOI] [PubMed] [Google Scholar]
- 17.Glenn O, Goldstein R, Li K, et al. Fetal MRI in the evaluation of fetuses referred for sonographically suspected abnormalities of the corpus callosum. J Ultrasound Med 2005;24:791–804 [DOI] [PubMed] [Google Scholar]
- 18.de Laveaucoupet J, Audibert F, Guis F, et al. Fetal magnetic resonance imaging (MRI) of ischemic brain injury. Prenat Diagn 2001;21:729–36 [DOI] [PubMed] [Google Scholar]
- 19.Whitby EH, Paley MNJ, Sprigg A, et al. Comparison of ultrasound and magnetic resonance imaging in 100 singleton pregnancies with suspected brain abnormalities. BJOG 2004;111:784–92 [DOI] [PubMed] [Google Scholar]
- 20.Levine D, Barnes PD, Robertson RR, et al. Fast MR imaging of fetal central nervous system abnormalities. Radiology 2003;229:51–61 [DOI] [PubMed] [Google Scholar]
- 21.Baker P, Johnson I, Harvey R, et al. A three-year follow-up of children imaged in utero with echo-planar magnetic resonance. Am J Obstet Gynecol 1994;170:32–33 [DOI] [PubMed] [Google Scholar]
- 22.Glover P, J Hykin, Gowland PA, et al. An assessment of the intrauterine sound intensity level during obstetric echo-planar magnetic resonance imaging. Br J Radiol 1995;68:1090–94 [DOI] [PubMed] [Google Scholar]
- 23.Myers C, Duncan KR, Gowland PA, et al. Failure to detect intrauterine growth restriction following in utero exposure to MRI. Br J Radiol 1998;71:549–51 [DOI] [PubMed] [Google Scholar]
- 24.Clements H, Duncan KR, Fielding K, et al. Infants exposed to MRI in utero have a normal paediatric assessment at 9 months of age. Br J Radiol 2000;73:190–94 [DOI] [PubMed] [Google Scholar]
- 25.Kok RD, de Vries MM, Heerschap A, et al. Absence of harmful effects of magnetic resonance exposure at 1.5T in utero during the third trimester of pregnancy: a follow-up study. Magn Reson Imaging 2004;22:851–54 [DOI] [PubMed] [Google Scholar]
- 26.Kanal E, Borgstede JP, Barkovich AJ, et al. American College of Radiology white paper on MR safety. AJR Am J Roentgenol 2002;178:1335–47 [DOI] [PubMed] [Google Scholar]
- 27.Busse R, Carrillo A, Brittain J, et al. On-demand real-time imaging: interactive multislice acquisition applied to prostate and fetal imaging. In: Proceedings of the Tenth Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine; May 18–24,2002; Honolulu, Hawaii.
- 28.Busse R, Zaharchuk G, Glenn O. A rapid two-shot T1-measurement and its applicability to oxygen partial pressure measurements in fluid. In: Proceedings of the Thirteenth Scientific Meeting and Exhibition of the Society for Magnetic Resonance in Medicine; May 7–13,2005; Miami Beach, Fla
- 29.Gupta RK, Hasan KM, Trivedi R, et al. Diffusion tensor imaging of the developing human cerebrum. J Neurosci Res 2005;81:172–78 [DOI] [PubMed] [Google Scholar]
- 30.Scifo P, Baldoli C, Righini A, et al. Diffusion tensor imaging using SENSE in prenatal studies. In: Proceedings of the Twelfth Scientific Meeting and Exhibition of the International Society for Magnetic Resonance in Medicine; May 15–21,2004; Kyoto, Japan.
- 31.Fenton BW, Lin C, Macedonia C, et al. The fetus at term: in utero volume-selected proton MR spectroscopy with a breath-hold technique–a feasibility study. Radiology 2001;219:563–66 [DOI] [PubMed] [Google Scholar]
- 32.Kok RD, van den Berg PP, van den Bergh AJ, et al. Maturation of the human fetal brain as observed by 1H MR spectroscopy. Magn Reson Med 2002;48:611–16 [DOI] [PubMed] [Google Scholar]
- 33.Heerschap A, Kok RD, and van den Berg PP. Antenatal proton MR spectroscopy of the human brain in vivo. Childs Nerv Syst 2003;19:418–21 [DOI] [PubMed] [Google Scholar]
- 34.Marin-Padilla M. Origin, formation, and prenatal maturation of the human cerebral cortex: an overview. J Craniofac Genet Dev Biol 1990;10:137–46 [PubMed] [Google Scholar]
- 35.Larroche JC, Encha-Razavi F, de Vries F. Central nervous system. In: Gilbert-Barnes E, ed. Potter’s Pathology of the Fetus and Infant. St. Louis: Mosby;1997. :1028–1150
- 36.Rakic P. Evolving concepts of cortical radial and areal specification. Progr Brain Res 2002;136:265–80 [DOI] [PubMed] [Google Scholar]
- 37.Hatten ME. New directions in neuronal migration. Science 2002;297:1660–63. [DOI] [PubMed] [Google Scholar]
- 38.Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci 2003;26:441–83 [DOI] [PubMed] [Google Scholar]
- 39.Barkovich AJ. Pediatric Neuroimaging, 4th ed. Philadelphia: Lippincott Williams & Wilkins;2005. .
- 40.Garel C. MRI of the Fetal Brain: Normal Development and Cerebral Pathologies. Berlin: Springer-Verlag;2004. .
- 41.Levine D, Barnes PD. Cortical maturation in normal and abnormal fetuses as assessed with prenatal MR imaging. Radiology 1999;210:751–58 [DOI] [PubMed] [Google Scholar]
- 42.Kostovic I, Judas M, Rados M, et al. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 2002;12:536–44 [DOI] [PubMed] [Google Scholar]
- 43.Stazzone MM, Hubbard AM, Bilaniuk LT, et al. Ultrafast MR imaging of the normal posterior fossa in fetuses. AJR Am J Roentgenol 2000;175:835–39 [DOI] [PubMed] [Google Scholar]
- 44.Fogliarini C, Chaumoitre K, Chapon F, et al. Assessment of cortical maturation with prenatal MRI. Part I: normal cortical maturation. Eur Radiol 2005;15:1671–85 [DOI] [PubMed] [Google Scholar]
- 45.Girard N, Raybaud C. In vivo MRI of fetal brain cellular migration. J Comput Assist Tomogr 1992;16:265–67 [DOI] [PubMed] [Google Scholar]
- 46.Sbarbati A, Pizzini F, Fabene PF, et al. Cerebral cortex three-dimensional profiling in human fetuses by magnetic resonance imaging. J Anat 2004;204:465–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Judas M, Rados M, Jovanov-Milosevic N, et al. Structural, immunocytochemical, and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol 2005;26:2671–84 [PMC free article] [PubMed] [Google Scholar]
- 48.Chi J, Dooling E, Gilles F. Gyral development of the human brain. Ann Neurol 1977. :86–93 [DOI] [PubMed]
- 49.Ten Donkelaar HJ, Lammens M, Wesseling P, et al. Development and developmental disorders of the human cerebellum. J Neurol 2003;250:1025–36 [DOI] [PubMed] [Google Scholar]
- 50.Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Progr Neurobiol 2004;72:295–339 [DOI] [PubMed] [Google Scholar]
- 51.Cardoza JD, Goldstein RB, Filly RA. Exclusion of fetal ventriculomegaly with a single measurement: the width of the lateral ventricular atrium. Radiology 1988;169:711–14 [DOI] [PubMed] [Google Scholar]
- 52.Goldstein RB, La Pidus AS, Filly RA, et al. Mild lateral cerebral ventricular dilatation in utero: clinical significance and prognosis. Radiology 1990;176:237–42 [DOI] [PubMed] [Google Scholar]
- 53.Filly RA, Goldstein RB. The fetal ventricular atrium. Radiology 1994;193:315–17 [DOI] [PubMed] [Google Scholar]
- 54.Levine D, Trop I, Mehta T, et al. MR imaging appearance of fetal cerebral ventricular morphology. Radiology 2002;223:652–60 [DOI] [PubMed] [Google Scholar]
- 55.Cochrane DD, Myles ST, Nimrod C, et al. Intrauterine hydrocephalus and ventriculomegaly: associated abnormalities and fetal outcome. Can J Neurol Sci 1985;12:51–59 [DOI] [PubMed] [Google Scholar]
- 56.Chervenak FA, Duncan C, Ment LR, et al. Outcome of fetal ventriculomegaly. The Lancet 1984;2(8396):179–81 [DOI] [PubMed] [Google Scholar]
- 57.Pretorius DH, Davis K, Manco-Johnson ML, et al. Clinical course of fetal hydrocephalus: 40 cases. AJR Am J Roentgenol 1985;144:827–31 [DOI] [PubMed] [Google Scholar]
- 58.Nyberg DA, Mack LA, J Hirsch, et al. Fetal hydrocephalus: sonographic detection and clinical significance of associated anomalies. Radiology 1987;163:187–91 [DOI] [PubMed] [Google Scholar]
- 59.Nicolaides KH, Berry S, Snijders RJM, et al. Fetal lateral cerebral ventriculomegaly: associated malformations and chromosomal defects. Fetal Diagn Ther 1990;5:5–14 [DOI] [PubMed] [Google Scholar]
- 60.Hudgins RJ, Edwards MS, Goldstein R, et al. Natural history of fetal ventriculomegaly. Pediatrics 1988;82:692–97 [PubMed] [Google Scholar]
- 61.Bromley B, Frigoletto FD, Benacerraf BR. Mild fetal lateral cerebral ventriculomegaly: clinical course and outcome. Am J Obstet Gynecol 1991;164:863–67 [DOI] [PubMed] [Google Scholar]
- 62.Twining P, Jaspan T, Zuccollo J. The outcome of fetal ventriculomegaly. Br J Radiol 1994;67:26–31 [DOI] [PubMed] [Google Scholar]
- 63.Vintzileos AM, Campbell WA, Weinbaum PJ, et al. Perinatal management and outcome of fetal ventriculomegaly. Obstet Gynecol 1987;69:5–11 [PubMed] [Google Scholar]
- 64.Gaglioti P, Danelon D, Bontempo S, et al. Fetal cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet Gynecol 2005;25:372–77 [DOI] [PubMed] [Google Scholar]
- 65.den Hollander NS, Vinkesteijn A, Schmitz-van Splunder P, et al. Prenatally diagnosed fetal ventriculomegaly; prognosis and outcome. Prenat Diagn 1998;18:557–66 [DOI] [PubMed] [Google Scholar]
- 66.Greco P, Laforgia N, Vimercati A, et al. Mild ventriculomegaly as a counselling challenge. Fetal Diagn Ther 2001;16:398–401 [DOI] [PubMed] [Google Scholar]
- 67.Patel MD, Filly RA, Hersch DR, et al. Isolated mild fetal cerebral ventriculomegaly: clinical course and outcome. Radiology 1994;192:759–64 [DOI] [PubMed] [Google Scholar]
- 68.Pilu G, Falco P, Gabrielli S, et al. The clinical significance of fetal isolated cerebral borderline ventriculomegaly: report of 31 cases and review of the literature. Ultrasound Obstet Gynecol 1999;14:320–26 [DOI] [PubMed] [Google Scholar]
- 69.Mercier A, Eurin D, Mercier PY, et al. Isolated mild fetal cerebral ventriculomegaly: a retrospective analysis of 26 cases. Prenat Diagn 2001;21:589–95 [DOI] [PubMed] [Google Scholar]
- 70.Gupta JK, Bryce FC, Lilford RJ. Management of apparently isolated fetal ventriculomegaly. Obstet Gynecol Surv 1994;49:716–21 [DOI] [PubMed] [Google Scholar]
- 71.Vergani P, Locatelli A, Strobelt N, et al. Clinical outcome of mild fetal ventriculomegaly. Am J Obstet Gynecol 1998;178:218–22 [DOI] [PubMed] [Google Scholar]
- 72.Mahony BS, Nyberg DA, Hirsch JH, et al. Mild idiopathic lateral cerebral ventricular dilatation in utero: sonographic evaluation. Radiology 1988;169:715–21 [DOI] [PubMed] [Google Scholar]
- 73.Drugan A, Krause B, Canady A, et al. The natural history of prenatally diagnosed cerebral ventriculomegaly. JAMA 1989;261:1785–88 [PubMed] [Google Scholar]
- 74.Bloom SL, Bloom DD, Dellanebbia C, et al. The developmental outcome of children with antenatal mild isolated ventriculomegaly. Obstet Gynecol 1997;90:93–97 [DOI] [PubMed] [Google Scholar]
- 75.Breeze A, Dey P, Lees C, et al. Obstetric and neonatal outcomes in apparently isolated mild fetal ventriculomegaly. J Perinat Med 2005;33:236–40 [DOI] [PubMed] [Google Scholar]
- 76.D’Ercole CD, Girard N, Boubli L, et al. Prenatal diagnosis of fetal cerebral abnormalities by ultrasonography and magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol 1993;50:177–84 [DOI] [PubMed] [Google Scholar]