Abstract
BACKGROUND AND PURPOSE: Current MR imaging criteria for multiple sclerosis (MS) do not specify the magnetic field strength. The aim of this study was to investigate whether different MR imaging field strengths, specifically high-field MR imaging, have an impact on the classification of patients with clinically isolated syndromes suggestive of MS, according to MR imaging and diagnostic criteria.
METHODS: In a prospective intraindividual comparative study, we examined 40 patients with clinically isolated syndromes (CIS) consecutively with a 1.5T and 3T MR imaging system, including axial sections of T2 turbo spin-echo, fluid-attenuated inversion recovery, and T1 spin-echo, before and after injection of gadolinium-diethylene-triaminepentaacetic acid. Constant resolution parameters were used for both field strengths. High-signal-intensity white matter lesions with a size of >3 mm were counted and categorized according to their anatomic location in infratentorial, callosal, juxtacortical, periventricular, and other white matter areas. Assessment of the fulfilled Barkhof MR imaging and McDonald diagnostic criteria was made separately for both field strengths in every patient.
RESULTS: Eleven patients fulfilled more MR imaging criteria at 3T. Two of these patients fulfilled the criterion of dissemination in space (DIS) according to the first definition of McDonald criteria, which is based on imaging criteria alone. Another patient had DIS only at 3T, according to the second definition of the McDonald criteria including CSF parameters.
CONCLUSION: MR field strength, specifically high-field MR imaging, has a substantial influence on the classification of patients with CIS according to imaging and a mild influence on the classification according diagnostic criteria for MS, leading to consequences for prognostic classification, imaging guidelines, and clinical trials.
MR imaging plays an important role in the diagnostic criteria for multiple sclerosis (MS) (McDonald criteria).1 Its application in patients with clinically isolated syndromes (CIS) provides an early and specific diagnosis of MS.2–3 For the diagnosis of definite MS, McDonald criteria require the demonstration of lesion dissemination in space and time. To demonstrate dissemination in space (DIS), the panel completely adopted the modified Barkhof MR imaging criteria, in which the diagnostic cutoff point of at least 3 of 4 MR imaging criteria has to be fulfilled.1,4,5 If the CSF is positive for a chronic inflammatory process, the presence of only 2 T2 lesions is sufficient for the demonstration of DIS.1 Recent reviews identified substantial weaknesses of the diagnostic and MR imaging criteria for MS.6–7 One concern is that imaging and diagnostic criteria do not clearly specify sequence and resolution parameters as well as the magnetic field strength.
Since the U.S. Food and Drug Administration established guidelines for a nonsignificant risk for MR field strengths up to 8T, high-field MR imaging is becoming increasingly available in the clinical setting. Higher magnetic field strengths provide a proportional increase of signal-to-noise ratio (SNR) and offer an improved diagnostic accuracy in the diagnosis of central nervous system (CNS) diseases.8,9 Although the data from several studies focusing on magnetic field strength–dependent differences between 0.5T and 1.5T in the diagnosis of MS are inconclusive,10–12 studies comparing 1.5T with high-field MR imaging up to 4T revealed an increased sensitivity in the detection of white matter abnormalities in patients with MS at higher magnetic fields.13,14 To our knowledge, whether higher magnetic field strengths have an influence on the classification of patients with CIS suggestive of MS according to MR imaging and diagnostic criteria has not been investigated so far.
This study focuses on the impact of high-field MR imaging operating at 3T on the classification of patients with CIS according to MR imaging and diagnostic criteria for MS.
Methods
We performed a prospective intraindividual comparative study in patients presenting with a CIS suggestive of MS. The study design was approved by our institutional review board. Written informed consent was obtained from all participants. The inclusion criteria were defined as the following: 1) CIS of the CNS suggestive of MS as defined by the International Panel on MS diagnosis1; 2) patient age at symptom onset between 18 and 59 years; 3) time between onset of CIS and the MR imaging examination less than 3 months; and 4) absence of vascular, other immunologic, malignant, and infectious CNS diseases in the medical history.
All patients were recruited by our Department of Neurology between February 2004 and February 2005. The Expanded Disability Status Scale (EDSS)15 was assessed by a neurologist before the MR imaging examination. CSF examinations included the determination of cellularity, protein level, intrathecal IgG synthesis, and oligoclonal bands by isoelectric focusing.
All patients initially received corticosteroid therapy (intravenous methylprednisolone, 1 g/day for 3 days) 2–4 weeks before the MR imaging examination. MR imaging at 1.5T and 3T was performed on 2 separate days in a randomized order separated by a time interval of 24–36 hours. Both MR systems (Gyroscan Intera 1.5T and 3T, Philips Medical Systems, Best, the Netherlands) were equipped with gradients with a maximum slew rate of 150 mT/m per millisecond and a maximum strength of 30 mT/m. For the examination at 1.5T, a standard quadrature head coil and, for the examination at 3T, an 8-element phased array head coil were used. Possibly higher SNR values of the 8-element head coil, especially in the anatomic regions close to the central parts of each element, were counterbalanced by doubling the number of signals averaged at 1.5T. This achieves almost constant conditions in terms of SNR for both coil technologies.16 The MR examination was based on a multisequence imaging protocol for both systems, including 24 contiguous axial sections of the following pulse sequences: T2 turbo spin-echo (TSE), fluid-attenuated inversion recovery (FLAIR), and T1 spin-echo (SE) before and after intravenous injection of gadolinium-diethylene-triaminepentaacetic acid (0.1 mmol/kg of body weight). Identical anatomic position and geometric and resolution parameters were used for all sequences at both scanners. Scan orientations and repositioning were performed according to the guidelines of the Consortium of Multiple Sclerosis Centers.17 Detailed sequence parameters are listed in Table 1.
Table 1:
MR imaging sequence parameters
| Pulse Sequence | ||||||
|---|---|---|---|---|---|---|
| System | FLAIR |
T2 TSE |
T1 SE |
|||
| 1.5T | 3T | 1.5T | 3T | 1.5T | 3T | |
| Field of view (mm) | 230 | 230 | 230 | 230 | 230 | 230 |
| Matrix | 256 | 256 | 256 | 256 | 256 | 256 |
| Section thickness (mm) | 5 | 5 | 5 | 5 | 5 | 5 |
| Measured voxel size (mm) | 0.90/0.90/5 | 0.90/0.90/5 | 0.90/0.90/5 | 0.90/0.90/5 | 0.90/0.90/5 | 0.90/0.90/5 |
| Turbo factor | 29 | 38 | 23 | 16 | ||
| Number of signals averaged | 2 | 1 | 2 | 1 | 2 | 1 |
| Repetition time (ms) | 6000 | 12 000 | 3500 | 4100 | 500 | 500 |
| Echo time (ms) | 110 | 140 | 100 | 100 | 12 | 12 |
| Inversion time (ms) | 2000 | 2850 | ||||
| Acquisition time (min:s) | 3:00 | 4:00 | 2:27 | 2:19 | 3:34 | 3:27 |
Note:— FLAIR indicates fluid-attenuated inversion recovery; TSE, turbo spin-echo; SE, spin-echo.
Twenty healthy volunteers (11 women, 9 men; median age, 29 years; range, 22–40 years) were selected for this study and examined at both field strengths to assess the sensitivity in the detection of unspecific white matter abnormalities and to evaluate possible artifacts. The study protocol included FLAIR and T2 TSE pulse sequences as described in Table 1.
All images were transferred to a workstation (Easy Vision, Philips Medical Systems, Best, the Netherlands), and the image analysis of all sequences was performed by 2 radiologists in consensus who were blinded to the clinical presentation and the MR imaging field strength. The images obtained at different field strengths and different pulse sequences were separated and presented in a randomized order. Only high-signal-intensity white matter lesions >3 mm were considered. For all sequences, the total number of lesions was counted and the lesions were categorized according to their location in infratentorial, periventricular, callosal, juxtacortical, and other white matter areas. The MR imaging examinations at both field strengths—each containing FLAIR and T2 TSE as well as gadolinium-enhanced T1 SE—were scored according to the Barkhof MR imaging criteria for each patient. Furthermore, each patient was categorized according to the McDonald criteria for diagnosis of DIS at both field strengths. The diagnosis of DIS included both definitions: 1) fulfilling at least 3 of 4 Barkhof criteria or 2) having at least 2 T2 lesions in combination with a positive CSF.1
To assess the possible conversion to definite MS, we scheduled follow-up visits, including MR examination and neurologic evaluation, 3–4 and 6–7 months after the first clinical event.
Results
Clinical Characteristics
Forty patients (30 women, 10 men) were included in this study. At symptom onset, the median age was 35 years (range, 18–55 years), the median disease duration at MR imaging examination was 34 days (range, 12–67 days), and the median EDSS score was 1.5 (range, 0–3). Twenty-four patients presented with optic neuritis; 7 patients, with brain stem symptoms; 5 patients, with spinal cord symptoms; and 4 patients, with a polysymptomatic CIS. Signs of a chronic inflammatory CNS process in the CSF were detected in 21 patients (52.5%).
Image Analysis of the Healthy Volunteers
No unspecific white matter abnormalities could be identified at the 1.5T and 3T examinations. The increasing magnetic field strength did not lead to more artifacts in any anatomic region of the brain that were likely to influence the sensitivity in the detection of inflammatory brain lesions and, therefore, the diagnostic accuracy.
Classification According to MR Imaging and Diagnostic Criteria in Patients with CIS
The number of fulfilled Barkhof criteria was concordant in 29 patients (72.5%). Eleven patients (27.5%) fulfilled 1 additional Barkhof MRI criterion at 3T, compared with 1.5T (Table 2): 4 patients fulfilled the additional criterion of ≥9 T2 lesions; 4 patients, the criterion of ≥1 infratentorial lesion (Fig 1); 2 patients, the criterion of ≥1 juxtacortical lesion (Fig 2); and 1 patient, the criterion of ≥3 periventricular lesions.
Table 2:
Patients with discordant results between 1.5T and 3T according to Barkhof MR imaging criteria
| Patient No. | Clinical Presentation | Number of Fulfilled MR Imaging Criteria |
Additional MR Imaging Criterion | |
|---|---|---|---|---|
| 1.5T | 3T | |||
| 1 | Optic neuritis | 0 | 1 | ≥1 juxtacortical lesion |
| 2 | Optic neuritis | 0 | 1 | ≥1 infratentorial lesion |
| 3 | Brain stem syndrome | 1 | 2 | ≥9 T2 hyperintense lesions |
| 4 | Brain stem syndrome | 1 | 2 | ≥9 T2 hyperintense lesions |
| 5 | Brain stem syndrome | 1 | 2 | ≥1 juxtacortical lesion |
| 6 | Optic neuritis | 1 | 2 | ≥9 T2 hyperintense lesions |
| 7 | Optic neuritis | 1 | 2 | ≥1 infratentorial lesion |
| 8* | Optic neuritis | 2 | 3 | ≥1 infratentorial lesion |
| 9* | Optic neuritis | 2 | 3 | ≥9 T2 hyperintense lesions |
| 10 | Optic neuritis | 3 | 4 | ≥3 periventricular lesions |
| 11 | Spinal cord syndrome | 3 | 4 | ≥1 infratentorial lesion |
Patient fulfilled 3 criteria instead of 2 at 3T, and they were therefore diagnosed of having lesion dissemination in space according to McDonald diagnostic criteria.
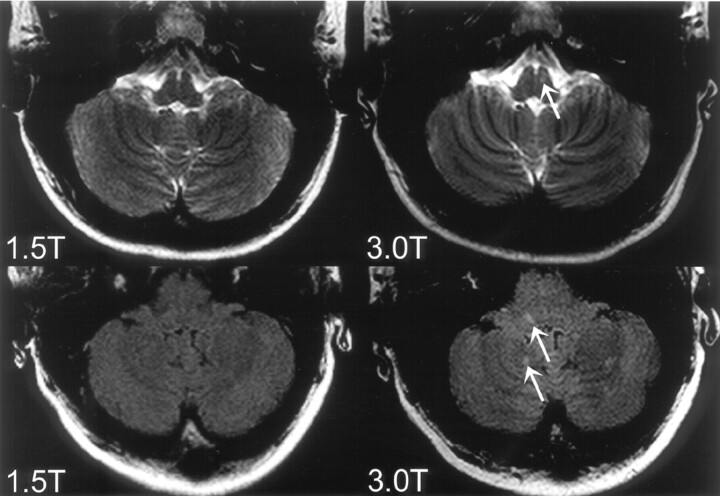
Fig 1.
Top row: T2-weighted images of a 19-year-old patient presenting with unilateral optic neuritis. One infratentorial lesion in the brain stem (arrow) could be identified on the 3T image but not on the corresponding 1.5T image. Bottom row: FLAIR images of a 30-year-old patient presenting with unilateral optic neuritis. The 3T image shows infratentorial lesions in the brain stem and the cerebellum (arrows) which were not prospectively identified on the corresponding 1.5T image.
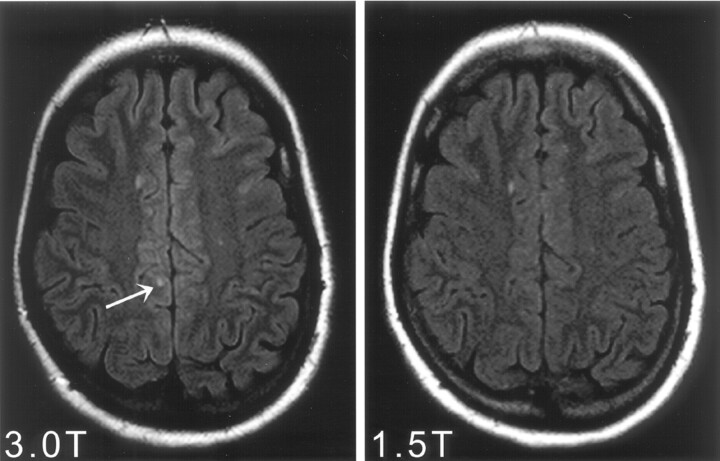
Fig 2.
Identical axial sections of FLAIR images within the supratentorial brain obtained at 3T (left) and 1.5T (right) MR imaging. One more juxtacortical lesion (arrow) could be identified on the 3T FLAIR images in comparison with the corresponding 1.5T examination.
Fourteen patients fulfilled at least 3 MR imaging criteria at 1.5T, leading to the diagnosis of DIS according to the first definition of the McDonald diagnostic criteria, whereas 2 more patients (16 patients) met 3 of 4 Barkhof MR imaging criteria at 3T.
If both definitions of DIS according to McDonald criteria were considered, DIS was diagnosed in 22 patients at 1.5T and in 23 patients at 3T. In this additional patient, with oligoclonal bands in the CSF, the 3T examination visualized 1 juxtacortical and 1 deep white matter lesion, whereas on the 1.5T images only 1 single juxtacortical lesion was detected. Therefore, this patient fulfilled only 1 MR imaging criterion at both field strengths. According to the second definition of the McDonald criteria, which includes positive CSF findings, only the 3T MR imaging study allowed the diagnosis of DIS.
The 2 patients (patient 8 and 9, Table 2) diagnosed with DIS at 3T according to MR imaging criteria alone also had oligoclonal bands in the CSF. Thus, both patients were diagnosed as having DIS according to the second definition, regardless of the used field strength.
Conversion to Definite MS
Of the group of patients with more fulfilled MR imaging criteria at 3T, 10 patients completed both follow-up visits until 7 months after the CIS. Among those 10 patients, 3 patients (33%) developed definite MS. One patient (fulfilling 4 MR imaging criteria at 3T instead of 3 criteria at 1.5T) had a new gadolinium-enhancing lesion 4 months after the CIS, 1 patient (fulfilling 4 MR imaging criteria at 3T instead of 3 criteria at 1.5T) developed a second clinical event 6 months later, and another patient (fulfilling 3 MR imaging criteria at 3T instead of 2 criteria at 1.5T) presented with new T2-hyperintense lesions 7 months after the CIS.
Among the 29 patients with concordant MR imaging criteria at both field strengths, follow-up results were available in 26 patients. Ten patients (38%) developed definite MS after 7 months. Five patients developed a second clinical attack, thus converting to clinical definite MS. Four patients presented with a new gadolinium-enhancing lesions after 3–4 months, and 1 patient presented with new gadolinium-enhancing and consequently new T2-hyperintense lesions after 7 months.
Discussion
Several technical aspects of MR imaging, such as pulse sequences and parameters related to spatial resolution, are likely to influence the sensitivity in the detection of inflammatory brain lesions.18–20 This study focuses on the influence of a higher magnetic field strength (specifically 3T MR imaging) on the classification of patients with CIS suggestive of MS according to Barkhof MR imaging and McDonald diagnostic criteria. The studies validating different MR imaging criteria are based on MR imaging technologies by using magnetic field strengths ranging from 0.5T to 1.5T.4,5 Compared with lower field strengths, MR imaging at 3T provides a higher SNR, leading to a higher detection rate of inflammatory brain lesions.14
The higher lesion-load measurements of white matter lesions at 3T in our study resulted in an increased number of fulfilled MR imaging criteria in 27.5% of our study patients when compared with the corresponding 1.5T examinations. Accordingly, the risk assessment for the conversion to clinically definite MS might vary in those patients, depending on the magnetic field strength with which the MR examination was performed. However, our short-term follow-up of 7 months did show a prognostic relevance in terms of the conversion to definite MS between the group of patients with more fulfilled MR imaging criteria at 3T and the group of patients with concordant results at both field strengths.
In comparison with the substantial influence of high-field MR imaging on the classification according to imaging criteria, the influence on the classification according to the diagnostic criteria in terms of DIS is rather mild. Applying both definitions of DIS according to the International Panel criteria, which includes CSF parameters, only 1 additional patient was diagnosed of having DIS at 3T, but not at 1.5T.
Because high-field MR imaging systems have become increasingly available in the clinical setting, a major concern of high-field MR imaging is a possible increase of the sensitivity for the detection of rather unspecific discrete white matter abnormalities, which might lead to false-positive results.7 This issue is, of course, not only limited to higher magnetic field strengths and may be observed on high-resolution images at lower field strengths as well. To investigate whether 3T MR imaging might detect more unspecific white or gray matter changes that possibly mimic chronic inflammatory lesions, we examined 20 healthy volunteers at both field strengths. In these volunteers, the higher magnetic field strengths did not lead to false-positive white or gray matter abnormalities. Moreover, to overcome the risk of counting unspecific white matter changes in the patient group with CIS, we only considered sharply delineated high-signal-intensity white matter lesions with a minimal size of >3 mm, according to the image analysis of major treatment trials.21
A limitation of our study is the use of an imaging protocol for both field strengths, which was not optimized in terms of spatial resolution. Current imaging guidelines such as those from the Consortium of Multiple Sclerosis Centers recommend a section thickness of 3 mm.17 However, according to study imaging protocols from which the current imaging and diagnostic criteria were derived,3,4 as well as in a previously performed multicenter treatment study,22,23 our sequence protocol included axial sections of 5-mm thickness.
Another important point concerning the interpretation of our results is the timing of the initial MR examinations. The initial MR examination in patients with the first clinical attack suggestive of MS should be performed during the clinical episode to assess acute inflammatory activity in terms of contrast-enhancing brain lesions. In studies comparing different field-strengths or MR scanners, a time interval of at least 24 hours between both gadolinium injections is needed to establish comparable conditions in terms of contrast-enhancement. During an acute demyelinating episode, withholding treatment with high-dose corticosteroids for at least 24 hours is a major ethical concern. Additionally, the inflammatory activity varies during 24 hours, thus possibly leading to different detection rates, especially of gadolinium-enhancing lesions.24 Therefore, to achieve a more stable disease activity between both MR examinations, we obtained both scans after the completion of the high-dose corticosteroid treatment, though this might lead to a decreased sensitivity in the detection of gadolinium-enhancing lesions.
Conclusion
In conclusion, MR field strength, specifically high-field, has a substantial influence on the classification of patients with CIS according to imaging criteria and a mild influence on the classification according to diagnostic criteria. This influence of high-field MR imaging operating at 3T in terms of the classification according to MR imaging and diagnostic criteria has far-reaching consequences for the diagnostic work-up and clinical trials. This is of particular interest in MR imaging studies assessing and monitoring disease activity and lesion load in response to immune-modulating therapy. Therefore, it is important to include clear definitions of MR imaging field strengths into the imaging and diagnostic criteria for MS to establish standardized MR imaging guidelines and avoid different classifications of patients with the first demyelinating episode and suspected MS.
Acknowledgments
We thank Frank Träber, PhD, for statistical advice; Hanno Schimikowski, for help establishing the figures; and Renate Blömer and Barbara Mosen, for the technical assistance.
Footnotes
This work was presented at the 43rd annual meeting of the American Society of Neuroradiology, Toronto, Canada, April-May 2005; and at the 21st Congress of the European Committee of Treatment and Research in Multiple Sclerosis (ECTRIMS) and the 10th annual meeting of the Americas Committee of Treatment and Research in Multiple Sclerosis (ACTRIMS), Thessaloniki, Greece, September-October, 2005.
References
- 1.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–27 [DOI] [PubMed] [Google Scholar]
- 2.Dalton CM, Brex PA, Miszkiel KA, et al. Application of the new McDonald criteria to patients with clinically isolated syndromes suggestive of multiple sclerosis. Ann Neurol 2002;52:47–53 [DOI] [PubMed] [Google Scholar]
- 3.Tintoré M, Rovira A, Río J, et al. New diagnostic criteria for multiple sclerosis: application in first demyelinating episode. Neurology 2003;60:27–30 [DOI] [PubMed] [Google Scholar]
- 4.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997;120:2059–69 [DOI] [PubMed] [Google Scholar]
- 5.Tintoré M, Rovira A, Martinez MJ, et al. Isolated demyelinating syndromes: comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. AJNR Am J Neuroradiol 2000;21:702–06 [PMC free article] [PubMed] [Google Scholar]
- 6.Frohman EM, Goodin DS, Calabresi PA, et al. The utility of MRI in suspected MS: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2003;61:602–11 [DOI] [PubMed] [Google Scholar]
- 7.Miller DH, Filippi M, Fazekas F, et al. Role of magnetic resonance imaging within diagnostic criteria for multiple sclerosis. Ann Neurol 2004;56:273–78 [DOI] [PubMed] [Google Scholar]
- 8.Frayne R, Goodyear BG, Dickhoff P, et al. Magnetic resonance imaging at 3.0 Tesla: challenges and advantages in clinical neurological imaging. Invest Radiol 2003;38:385–402 [DOI] [PubMed] [Google Scholar]
- 9.Norris DG. High field human imaging. J Magn Reson Imaging 2003;18:519–29 [DOI] [PubMed] [Google Scholar]
- 10.Schima W, Wimberger D, Schneider B, et al. The importance of magnetic field strength in the MR diagnosis of multiple sclerosis: a comparison of 0.5T and 1.5T. RoFo 1993;158:368–71 [DOI] [PubMed] [Google Scholar]
- 11.Filippi M, van Waesberghe JH, Horsfield MA, et al. Interscanner variation in brain MRI lesion load measurements in MS. Neurology 1997;49:371–77 [DOI] [PubMed] [Google Scholar]
- 12.Lee DH, Vellet AD, Eliasziw M, et al. MR imaging field: prospective evaluation of the diagnostic accuracy of MR for diagnosis of multiple sclerosis at 0.5 and 1.5T. Radiology 1995;194:257–62 [DOI] [PubMed] [Google Scholar]
- 13.Keiper MD, Grossmann RI, Hirsch JA, et al. MR identification of white matter abnormalities in multiple sclerosis: a comparison between 1.5T and 4T. AJNR Am J Neuroradiol 1998;19:1489–93 [PMC free article] [PubMed] [Google Scholar]
- 14.Sicotte NL, Voskuhl RR, Bouvier S, et al. Comparison of multiple sclerosis lesions at 1.5 and 3.0T. Invest Radiol 2003;38:423–27 [DOI] [PubMed] [Google Scholar]
- 15.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–52 [DOI] [PubMed] [Google Scholar]
- 16.de Zwart JA, Ledden PJ, Kellman P, et al. Design of a SENSE-optimized high-sensitivity MRI receive coil for brain imaging. Magn Reson Med 2002;47:1218–27 [DOI] [PubMed] [Google Scholar]
- 17.Simon JH, Li D, Traboulsee A, et al. Standarized MR imaging protocol for multiple sclerosis: consortium of MS centers consensus guidelines. AJNR Am J Neuroradiol 2006;27:455–61 [PMC free article] [PubMed] [Google Scholar]
- 18.Bakshi R, Ariyaratana S, Benedict RH, et al. Fluid-attenuated inversion recovery magnetic resonance imaging detects cortical and juxtacortical multiple sclerosis lesions. Arch Neurol 2001;58:742–48 [DOI] [PubMed] [Google Scholar]
- 19.Molyneux PD, Tubridy N, Parker GJ, et al. The effect of section thickness on MR lesion detection and quantification in multiple sclerosis. AJNR Am J Neuroradiol 1998;19:1715–20 [PMC free article] [PubMed] [Google Scholar]
- 20.Filippi M, Horsfield MA, Campi A, et al. Resolution-dependent estimates of lesion volumes in magnetic resonance imaging studies of the brain in multiple sclerosis. Ann Neurol 1995;38:749–54 [DOI] [PubMed] [Google Scholar]
- 21.Jacobs DL, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med 2000;343:898–904 [DOI] [PubMed] [Google Scholar]
- 22.Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomized study. Lancet 2001;357:1576–82 [DOI] [PubMed] [Google Scholar]
- 23.Barkhof F, Rocca M, Francis G, et al. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon β1a. Ann Neurol 2003;53:718–24 [DOI] [PubMed] [Google Scholar]
- 24.Stone LA, Albert PS, Smith ME, et al. Changes in the amount of diseased white matter over time in patients with relapsing-remitting multiple sclerosis. Neurology 1995;45:1808–14 [DOI] [PubMed] [Google Scholar]