Abstract
Aims
Post-operative pain following cardiac implantable electronic device (CIED) insertion is associated with patient dissatisfaction, emotional distress, and emergency department visits. We sought to identify factors associated with post-operative pain and develop a prediction score for post-operative pain.
Methods and results
All patients from the BRUISE CONTROL-1 and 2 trials were included in this analysis. A validated Visual Analogue Scale (VAS) was used to assess the severity of pain related to CIED implant procedures. Patients were asked to grade the most severe post-operative pain, average post-operative pain, and pain on the day of the first post-operative clinic. Multivariable regression analyses were performed to identify predictors of significant post-operative pain and to develop a pain-prediction score. A total of 1308 patients were included. Multivariable regression analysis found that the presence of post-operative clinically significant haematoma {CSH; P value < 0.001; odds ratio (OR) 3.82 [95% confidence interval (CI): 2.37–6.16]}, de novo CIED implantation [P value < 0.001; OR 1.90 (95% CI: 1.47–2.46)], female sex [P value < 0.001; OR 1.61 (95% CI: 1.22–2.12)], younger age [<65 years; P value < 0.001; OR 1.54 (95% CI: 1.14–2.10)], and lower body mass index [<20 kg/m2; P value < 0.05; OR 2.05 (95% CI: 0.98–4.28)] demonstrated strong and independent associations with increased post-operative pain. An 11-point post-operative pain prediction score was developed using the data.
Conclusion
Our study has identified multiple predictors of post-operative pain after CIED insertion. We have developed a prediction score for post-operative pain that can be used to identify individuals at risk of experiencing significant post-operative pain.
Keywords: Cardiac implantable electronic device implantation, Pacemaker, Implantable cardioverter-defibrillator, Predictors of post-operative pain, Pain prediction score, BRUISE CONTROL trials
What’s new?
Identification of risk factors associated with post-operative pain, specifically related to the cardiac implantable electronic device (CIED) population, from the BRUISE 1 and 2 randomized controlled trial.
Development of a novel post-operative pain prediction score (HeADSS score) to identify subjects at risk of severe post-operative pain following CIED implantation.
Introduction
Cardiac implantable electronic devices (CIED) are being implanted in increasing numbers worldwide for the management of rhythm disorders and congestive heart failure. The complexity of the clinical conditions of patients undergoing CIED implantation has contributed to a number of procedure-related complications that have to be dealt with by treating physicians.1 With an ageing population and increasing life expectancy, we can expect that many patients with CIEDs will have to undergo multiple device replacement procedures.2
Post-operative pain after CIED implantation is associated with patient dis-satisfaction, prolongation of hospital stay, repeat emergency department visits, and emotional distress.3 Understanding the factors contributing to post-operative pain after CIED implantation may help in instituting measures to mitigate this unpleasant complication.3–5
The BRUISE CONTROL-1 (Bridge or continue coumadin for device surgery randomized controlled trial) and BRUISE CONTROL-2 [Continued vs. interrupted direct oral anticoagulants (DOAC) at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events] trials were large, multicentre randomized controlled trials (RCTs) conducted to evaluate the optimal peri-operative anticoagulation strategy [continued vs. interrupted vitamin K antagonists (VKA) or DOAC, respectively] in patients undergoing CIED implantation or replacement.6,7 These large RCTs provided an opportunity to prospectively collect information regarding post-operative pain and determine patient and procedural variables predicting pain.
We hypothesized that multiple demographic and clinical variables, such as age, sex, presence of clinically significant haematoma (CSH), de novo vs. repeat CIED surgery, etc.,8 would predict the severity of post-operative pain in patients undergoing CIED surgery. We also sought to develop a post-operative pain prediction score.
Methods
Study design and patients
This study included all patients from the BRUISE CONTROL-1 and 2 RCTs undergoing CIED implantation. Details of inclusion and exclusion criteria for these trials have been published previously.6,7 Demographic and clinical variables were collected for all included patients. The incidence of primary and secondary outcomes from the two trials was also obtained.
Patient selection and study endpoints
A validated Visual Analogue Scale [VAS; numerical pain rating score (NRS) from 0 to 10, with 0 indicating no pain, and 10 indicating severe pain] was used to assess the severity of pain related to the CIED implant.9 Patients were asked at their first post-operative visit [median 12 (9–14) days post-surgery] to grade their most severe post-operative pain, average post-operative pain, and pain on the day of the clinic visit.
Statistical analyses
Descriptive statistics were reported for all baseline characteristics, operative details, and outcomes. Continuous variables were expressed as means and standard deviations for normally distributed variables or medians with interquartile range (IQR: Q1–Q3) for non-normally distributed variables. Categorical variables were presented as frequencies with percentages. To create a risk score for severe post-operative pain, the continuous variables [age and body mass index (BMI)] in the final prediction model were further categorized using the most meaningful clinical cut-offs. The coefficients for each variable in the final model were calculated. The risk score was then computed for severe post-operative pain by assigning points to each variable in the final model according to their regression coefficients. Two risk scores were developed one with pre-operative variables (e.g. BMI, age, gender, etc.) and the second score with pre- and post-operative variables (i.e. with pocket haematoma that is a post-operative variable; CSH was defined as one that prolonged hospitalization, and/or required interruption of systemic oral anticoagulation, and/or required surgical evacuation). The accuracy of a risk score to predict severe post-operative pain was evaluated by the area under the curve (AUC) and its 95% confidence interval (CI) in a receiver operating characteristic (ROC) curve analysis. The calibration of a risk score was assessed using the Hosmer–Lemeshow χ2 statistics. The sensitivity, specificity, and estimated probability of experiencing severe post-operative pain were also calculated. SAS (version 9.4, SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses and statistical significance was defined as P < 0.05.
Results
Patients
The BRUISE CONTROL-1 and -2 trials enrolled a total of 1343 patients (681 and 662, respectively), of whom 1308 (661and 647, respectively) underwent CIED implantation and were included in the current analysis. The remaining 35 patients were excluded from the current analysis as they either did not undergo CIED implantation or were lost to follow-up. The baseline demographic, clinical variables, and procedural details are summarized in Table 1. One half of the patients underwent pulse generator replacement, while one-quarter each underwent de novo pacemaker or ICD insertion. The incidence of the primary outcomes in the included patients is summarized in Table 2. The following variables were selected for univariable analysis, based on previously published literature on post-operative pain: age, gender, BMI, diabetes, duration of procedure, de novo or non-de novo surgery, pacemaker vs. implantable cardioverter-defibrillator (ICD) insertion, presence or absence of CSH. Univariable analysis (Table 3) revealed that presence of CSH, de novo surgery, female gender, age <65 years, and BMI <20 showed significant association with post-operative pain and these variables were evaluated in a multivariable logistic regression model (Table 3) for dichotomized pain scores [moderate to severe post-operative pain [pain score ≥ 4; moderate pain (NRS 4–6)—344 (26.8%); and severe pain (NRA 7–10)—342 (26.6%)] subjects vs. mild post-operative pain [pain score 0–3; mild pain (NRS 1–3)—599 (46.6%)] based on accepted visual pain score classification schemes.10 Only the variables that remained significant were included into the final prediction models for dichotomized pain scores. The mean average, most severe, and post-operative pain scores, and results of univariable and multivariable analyses are summarized in Tables3–5.
Table 1.
Baseline characteristics of patients enrolled in BRUISE CONTROL-1 and -2 trials
| Characteristics | All patients in Bruise CONTROL-1 and -2 trials that underwent CIED implantation (N = 1308, 661, and 647, respectively) |
|---|---|
| Age (years ± SD) | 72.7 ± 9.7 |
| Male sex | 950 (72.6%) |
| Body mass indexa | 28.5 ± 5.6 |
| Stroke | 186 (14.2%) |
| Transient ischaemic attack | 172 (13.2%) |
| Peripheral embolus | 36 (2.8%) |
| Systemic hypertension | 951 (72.7%) |
| Diabetes mellitus | 474 (36.2%) |
| Cardiomyopathy | 744 (56.9%) |
| Warfarina | 661 (50.5%) |
| Bridging heparin | 326 (24.9%) |
| Continued coumadin | 335 (25.6%) |
| Interrupted DOAC | 328 (25.1%) |
| Continued DOAC | 319 (24.4%) |
| Aspirin | 371 (28.4%) |
| New implant of a pacemaker | N = 341 |
| Single | 156 (45.8%) |
| Dual | 163 (47.8%) |
| Cardiac resynchronization | 22 (6.5%) |
| New implant of an implantable cardioverter-defibrillator | N = 315 |
| Single | 142 (45.1%) |
| Dual | 61 (19.4%) |
| Cardiac resynchronization | 112 (35.6%) |
| Device replacement or revision | N = 652 |
| Pulse generator change only | 229 (35.1%) |
| Pulse generator change with additional interventionsa | 177 (27.2%) |
| Other | 9 (1.4%) |
| Duration of procedure (min), median (IQR) | 45 (28–70) |
Data are expressed as N (%), median (IQR), mean ± standard deviation (SD), or n/N (%).
CIED, cardiac implantable electronic device; DOAC, direct oral anticoagulant.
Only patients enrolled in Bruise CONTROL-1 trial were on Warfarin. These variables were available only for patients enrolled in the BRUISE CONTROL-2 trial.
Table 2.
Primary and secondary outcomes in patients enrolled in BRUISE CONTROL-1 and -2 trials
| Trial outcomes | Total subjects = 1308 (BC-1: 661 and BC-2: 647) |
|---|---|
| Clinically significant haematoma (CSH) | 80/1308 (6.1%) |
| CSH prolonged hospitalization | 23/1308 (1.8%) |
| CSH requiring interruption of anti-coagulation | 73/1308 (5.6%) |
| CSH requiring re-operation | 14/1308 (1.1%) |
| Non-clinically significant haematomaa | 21/647 (3.3%) |
| Any haematomaa | 34/647 (5.3%) |
| All-cause mortality | 7/1308 (0.5%) |
Data are expressed as n/N (%).
These variables were available only for patients enrolled in the BRUISE CONTROL-2 trial.
Table 3.
Odds ratio for experiencing significant pain in the univariable and multivariable model, dichotomized by primary outcome variable, for average post-operative pain, most severe post-operative pain, and post-operative pain on day of follow-up
| Average post-operative pain |
Most severe post-operative pain |
Post-operative pain on day of follow-up |
||||
|---|---|---|---|---|---|---|
| Variables | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value |
| Univariable analysis | ||||||
| CSH | 3.70 (2.32–5.90) | <0.0001 | 2.87 (1.69–4.88) | 0.0001 | 1.96 (1.14–2.03) | 0.02 |
| De novo surgery | 1.90 (1.47–2.42) | <0.0001 | 1.61 (1.29–2.00) | <0.0001 | 1.47 (1.06–2.03) | 0.02 |
| Female sex | 1.63 (1.25–2.12) | 0.0003 | 1.33 (1.04–1.70) | 0.02 | 1.78 (1.28–2.49) | 0.0007 |
| Age <65 years | 1.55 (1.15–2.08) | 0.004 | 1.95 (1.46–2.61) | <0.0001 | 1.08 (0.72–1.61) | NS |
| BMI <20 kg/m2 | 2.06 (1.02–4.20) | 0.05 | 1.69 (0.81–3.53) | 0.15 | 1.45 (0.60–3.66) | NS |
| Pacemaker vs. ICD | 0.81 (0.49–1.34) | NS | 0.70 (0.44–1.12) | NS | 1.13 (0.60–2.16) | NS |
| Type II DM | 0.87 (0.67–1.12) | NS | 0.91 (0.73–1.15) | NS | 1.18 (0.85–1.64) | NS |
| Procedure duration | 1.00 (1.00–1.01) | NS | 1.00 (1.00–1.01) | NS | 1.00 (1.00–1.01) | NS |
| Multivariable analysis | ||||||
| CSH | 3.82 (2.37–6.16) | <0.0001 | 2.85 (1.67–4.87) | 0.0001 | 2.02 (1.16–3.54) | 0.01 |
| De novo surgery | 1.90 (1.47–2.46) | <0.0001 | 1.60 (1.27–2.00) | <0.0001 | 1.44 (1.04–1.99) | 0.03 |
| Female sex | 1.61 (1.22–2.12) | 0.0007 | 1.31 (1.02–1.69) | 0.04 | 1.78 (1.28–2.49) | 0.0007 |
| Age <65 years | 1.54 (1.14–2.10) | 0.006 | 1.94 (1.44–2.61) | <0.0001 | NS | NS |
| BMI <20 kg/m2 | 2.05 (0.98–4.28) | 0.05 | 1.72 (0.81–3.67) | 0.15 | NS | NS |
The models are dichotomized for moderate to severe post-operative pain (>4) vs. mild post-operative pain (0–3).
BMI, body mass index; CSH, clinically significant haematoma; ICD, implantable cardioverter-defibrillator; NS, not significant.
Table 4.
Results of multivariable analysis with comparison of mean pain scores between groups with and without the presence of pre and post-operative variable
| Variables | Mean average post-operative pain score (R2 = 0.08) | Standardized beta (SE) | P value | Mean most severe post-operative pain score (R2 = 0.08) | Standardized beta (SE) | P value | Mean post-operative pain score on day of follow-up (R2 = 0.04) | Standardized beta | P value |
|---|---|---|---|---|---|---|---|---|---|
| Haematoma | 3.8 ± 2.2 | 0.15 (0.24) | < 0.0001 | 6.1 ± 3.1 | 0.16 (0.34) | <0.0001 | 2.4 ± 2.2 | 0.11 (0.23) | 0.0001 |
| No haematoma | 2.4 ± 2.1 | 4.0 ± 2.9 | 1.4 ± 2.0 | ||||||
| De novo surgery | 2.7 ± 2.2 | 0.14 (0.11) | <0.0001 | 4.5 ± 3.0 | 0.13 (0.16) | <0.0001 | 1.7 ± 2.0 | 0.11 (0.11) | <0.0001 |
| Non-de novo surgery | 2.1 ± 2.1 | 3.7 ± 2.9 | 1.3 ± 2.0 | ||||||
| Male | 2.3 ± 2.0 | −0.12 (0.13) | 0.0005 | 4.0 ± 2.9 | −0.09 (0.18) | 0.0009 | 1.4 ± 1.9 | −0.10 (0.12) | 0.0002 |
| Female | 2.8 ± 2.3 | 4.5 ± 3.1 | 1.8 ± 2.3 | ||||||
| Age | |||||||||
| <50 | 3.0 ± 1.8 | −0.15 (0.06) | < 0.0001 | 5.9 ± 2.7 | −0.16 (0.08) | <0.0001 | 1.7 ± 1.5 | −0.07 (0.06) | 0.0159 |
| 50–59 | 3.2 ± 2.2 | 5.2 ± 2.8 | 1.8 ± 1.9 | ||||||
| 60–69 | 2.6 ± 2.2 | 4.3 ± 3.0 | 1.6 ± 2.0 | ||||||
| 70–79 | 2.4 ± 2.1 | 4.0 ± 2.9 | 1.5 ± 2.1 | ||||||
| ≥80 | 2.1 ± 2.1 | 3.6 ± 3.0 | 1.3 ± 2.0 | ||||||
| BMI | |||||||||
| <20 | 3.2 ± 2.4 | −0.07 (0.06) | 0.013 | 5.2 ± 3.4 | −0.06 (0.08) | 0.0203 | NA | NA | NA |
| 20–24 | 2.6 ± 2.2 | 4.3 ± 3.0 | NA | ||||||
| 25–29 | 2.3 ± 2.1 | 4.0 ± 2.9 | NA | ||||||
| 30–34 | 2.4 ± 2.2 | 4.1 ± 3.1 | NA | ||||||
| 35–39 | 2.4 ± 2.0 | 4.1 ± 3.0 | NA |
The models are dichotomized for moderate to severe post-operative pain (>4) vs. mild post-operative pain (0–3).
BMI, body mass index; CSH, clinically significant haematoma; ICD, implantable cardioverter-defibrillator; NS, not significant.
Table 5.
Mean pain scores in subjects enrolled in BRUISE CONTROL 1 and 2 trials
| Average post-operative pain |
Most severe post-operative pain |
Post-operative pain on day of follow-up |
||||
|---|---|---|---|---|---|---|
| Variables | Mean (± SD) | P value (comparison with absence) | Mean ( ± SD) | P value (comparison with absence) | Mean (± SD) | P value (comparison with absence) |
| CSH | 3.8 ± 2.2 | <0.0001 | 6.1 ± 3.1 | <0.0001 | 2.4 ± 2.2 | 0.0001 |
| De novo surgery | 2.7 ± 2.2 | <0.0001 | 4.5 ± 3.0 | <0.0001 | 1.7 ± 2.0 | <0.0001 |
| Female sex | 2.8 ± 2.3 | 0.0005 | 4.5 ± 3.1 | 0.0009 | 1.8 ± 2.3 | 0.0002 |
| Age <65 years | 3.2 ± 2.2 | <0.0001 | 5.2 ± 2.8 | <0.0001 | 1.8 ± 1.9 | 0.02 |
| BMI <20 kg/m2 | 3.2 ± 2.4 | 0.01 | 5.2 ± 3.4 | 0.02 | Not associateda | Not associateda |
| Overall pain score for all 1308 patients in BC-1 and BC-2 (mean ± SD) | 2.4 ± 2.1 | 4.1 ± 3 | 1.5 ± 2.0 | |||
The models are dichotomized for moderate to severe post-operative pain (>4) vs. mild post-operative pain (0–3).
BMI, body mass index; CSH, clinically significant haematoma; ICD, implantable cardioverter-defibrillator; NS, not significant.
Post-operative pain on day of follow-up was not significantly different between subjects with BMI <20 kg/m2 and those with BMI ≥20 kg/m2 and are not being presented.
Pain assessment
Patients completed the visual analogue scale at their first post op clinic visit [median 12 (9–14) days post-surgery].6,7 The mean (±standard deviation) scores of the most severe, average, and pain on the day of the clinic follow-up visit were analysed for all enrolled patients. All variables that met the selection criterion (P value < 0.25) were entered into the multivariable model for test of inclusion. In the final risk prediction model, statistically significant predictors of increased post-operative pain were: CSH, de novo surgery, female sex, age <65 years, and BMI <20 kg/m2 (Tables 3–5).
BRUISE-CONTROL HeADSS post-operative pain risk score development
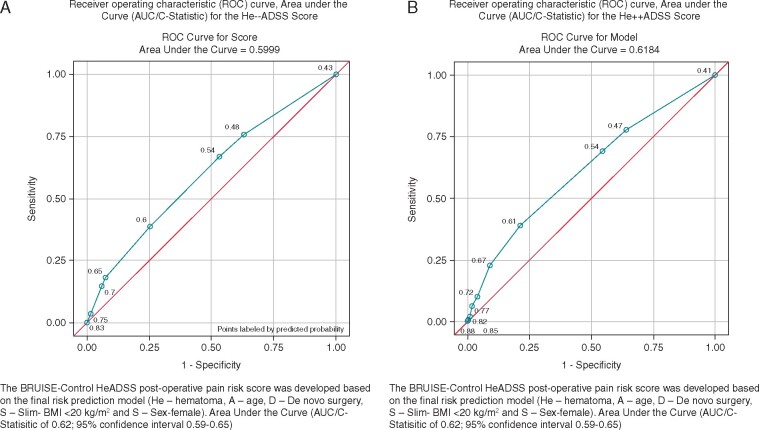
Multivariable logistic regression analyses were performed for dichotomized pain scores including pre-operative variables (without CSH; Table 6) and both pre- and post-operative variables (with CSH; Table 7). The variables that remained significant were included into the final prediction models for creating dichotomized pain scores [The BRUISE-CONTROL post-operative pain risk score, HeADSS: He: clinically significant haematoma (CSH); A, age; D, de novo surgery; S, slim—BMI <20 kg/m2; and S, sex: female; Tables 8 and9; Figure 1A and B]. To create a risk score for severe post-operative pain, the continuous variables (age and BMI) in the final prediction model were further categorized using the most meaningful clinical cut-offs. The coefficients for each variable in the final model were calculated. The risk score was then computed for severe post-operative pain by assigning points to each variable in the final model according to their regression coefficients. The accuracy of a risk score to predict severe post-operative pain was evaluated by the area under the curve (AUC) and its 95% CI in a ROC curve analysis (Figure 1A and B). The calibration of risk scores with and without CSH (He++ADSS and He−−ADSS scores) were assessed using the Hosmer–Lemeshow χ2 statistic. The sensitivity, specificity, and estimated probability of experiencing severe post-operative pain were also calculated (AUC of 0.62; 95% CI 0.59–0.65; Tables6–9 and Figure 1). We chose female sex as the reference group as it had the lowest risk. The risk score was calculated for each patient by summing the point assigned to each predictor: CSH (4 points), age (2 points age <65 years), de novo surgery (2 points), slim individuals (2 points for BMI < 20 kg/m2), and sex (1 point for female sex). The minimum risk score was 0 for patients without any risk factors and the maximum risk score was 11. The sensitivities, specificities, and estimated probabilities of experiencing moderate to severe post-operative pain for He++ADSS and He−−ADSS scores are summarized in Tables 10 and 11.
Table 6.
Logistic prediction model for moderate to severe post-operative pain prediction score excluding clinically significant haematoma (He−−ADSS score) after CIED insertion (the most severe post-operative pain scores were used to perform the above analyses)
| Variables | Beta (SE) | Odds ratio (95% CI) | P value |
|---|---|---|---|
| Age <65 | 0.68 (0.15) | 1.97 (1.46–2.64) | <0.0001 |
| De novo surgery | 0.47 (0.11) | 1.60 (1.28–2.00) | <0.0001 |
| BMI <20 kg/ m2 | 0.55(0.38) | 1.73 (0.82–3.67) | 0.15 |
| Female sex | 0.26 (0.13) | 1.29 (1.01–1.67) | 0.046 |
AUC of 0.60; 95% confidence interval 0.57–0.63. The BRUISE-Control He−−ADSS and He++ADSS scores post-operative pain risk score were developed based on the final risk prediction model (He—CSH; A, age; D, de novo surgery; S, slim—BMI <20 kg/m2; and S, sex—female).
AUC, area under the curve; BMI, body mass index; CSH, clinically significant haematoma; CIED, cardiac implantable electronic device.
Table 7.
Logistic prediction model for moderate to severe post-operative pain prediction score including clinically significant haematoma (He++ADSS score) after CIED insertion (the most severe post-operative pain scores were used to perform the above analyses)
| Variables | Beta (SE) | Odds ratio (95% CI) | P value |
|---|---|---|---|
| CSH | 1.05 (0.27) | 2.85 (1.67–4.87) | 0.0001 |
| Age <65 years | 0.66 (0.15) | 1.94 (1.44–2.61) | <0.0001 |
| De novo surgery | 0.47 (0.12) | 1.60 (1.27–2.00) | <0.0001 |
| BMI <20 kg/m2 | 0.55 (0.39) | 1.72 (0.81–3.67) | 0.15 |
| Female sex | 0.27 (0.13) | 1.31 (1.02–1.69) | 0.04 |
AUC of 0.62; 95% confidence interval 0.59–0.65. The BRUISE-Control He−−ADSS and He++ADSS scores post-operative pain risk score were developed based on the final risk prediction model (He—CSH; A, age; D, de novo surgery; S, slim—BMI <20 kg/m2; and S, sex—female).
AUC, area under the curve; BMI, body mass index; CSH, clinically significant haematoma; CIED, cardiac implantable electronic device.
Table 8.
He−−ADSS (clinically significant haematoma excluded) post-operative pain prediction score from the BRUISE-Control Trials (the most severe post-operative pain scores were used to perform the above analyses)
| Variables | Beta | Beta after inflationa | Points |
|---|---|---|---|
| Age <65 years | 0.68 | 2.6 | 3 |
| De novo surgery | 0.47 | 1.82 | 2 |
| BMI <20 kg/m2 | 0.55 | 2.13 | 2 |
| Female sex | 0.26 | 1 | 1 |
BMI, body mass index; CSH, clinically significant haematoma.
Beta coefficient was inflated by dividing the smallest beta coefficient (0.27), and then points were derived by rounding to the nearest integer. The BRUISE-Control He−−ADSS and He++ADSS scores post-operative pain risk score were developed based on the final risk prediction model (He, CSH; A, age; D, de novo surgery; S, slim—BMI <20 kg/m2; and S, sex—female).
Table 9.
He++ADSS (clinically significant haematoma included) post-operative pain prediction score from the BRUISE-Control Trials
| Variables | Beta | Beta after inflationa | Points |
|---|---|---|---|
| CSH | 1.05 | 3.84 | 4 |
| Age <65 years | 0.66 | 2.44 | 2 |
| De novo surgery | 0.47 | 1.71 | 2 |
| BMI <20 kg/ m2 | 0.55 | 2 | 2 |
| Female sex | 0.27 | 1 | 1 |
BMI, body mass index; CSH, clinically significant haematoma.
Beta coefficient was inflated by dividing the smallest beta coefficient (0.27), and then points were derived by rounding to the nearest integer. The BRUISE-Control He−−ADSS and He++ADSS scores post-operative pain risk score were developed based on the final risk prediction model (He, CSH; A, age; D, de novo surgery; S, slim—BMI <20 kg/m2; and S, sex—female).
Figure 1.
ROC curve and AUC (C-statistic) for the He−−ADSS score (A). ROC curve and AUC (C-statistic) for the He++ADSS score (B). AUC, area under the curve; ROC, receiver operating characteristic.
Table 10.
The sensitivity, specificity, and estimated probability of having severe post-operative pain for He−−ADSS score (the most severe post-operative pain scores were used to perform the above analyses)
| He−−ADSS score | Number | Sensitivity | Specificity | Estimated probability of having moderate to severe post-operative pain |
|---|---|---|---|---|
| 0 | 396 | 1 | 0 | 0.43 |
| 1 | 122 | 0.76 | 0.37 | 0.48 |
| 2 | 361 | 0.67 | 0.47 | 0.54 |
| 3 | 253 | 0.39 | 0.75 | 0.60 |
| 4 | 35 | 0.18 | 0.93 | 0.65 |
| 5 | 106 | 0.15 | 0.94 | 0.70 |
| 6 | 33 | 0.04 | 0.98 | 0.75 |
| 8 | 1 | 0.002 | 1 | 0.83 |
The combined BC-1 and BC-2 patient cohort did not have patients with a score of 7, when CSH was excluded. The BRUISE-Control He−−ADSS and He++ADSS scores post-operative pain risk score were developed based on the final risk prediction model (He, CSH; A, age; D, de novo surgery; S, slim—BMI <20 kg/m2; and S, sex—female).
BC-1, BRUISE CONTROL Trial 1; BC-2, BRUISE CONTROL Trial 2; BMI, body mass index; CSH, clinically significant haematoma.
Table 11.
The sensitivity, specificity, and estimated probability of having severe post-operative pain for He++ADSS score (the most severe post-operative pain scores were used to perform the above analyses)
| He++ADSS score | Number | Sensitivity | Specificity | Estimated probability of having moderate to severe post-operative pain |
|---|---|---|---|---|
| 0 | 374 | 1 | 0 | 0.41 |
| 1 | 117 | 0.78 | 0.36 | 0.47 |
| 2 | 412 | 0.69 | 0.46 | 0.54 |
| 3 | 187 | 0.39 | 0.79 | 0.61 |
| 4 | 124 | 0.23 | 0.91 | 0.67 |
| 5 | 39 | 0.10 | 0.96 | 0.72 |
| 6 | 34 | 0.06 | 0.98 | 0.77 |
| 7 | 11 | 0.02 | 0.99 | 0.82 |
| 8 | 5 | 0.01 | 0.99 | 0.85 |
| >9 | 4 | 0.004 | 0.99 | 0.88 |
The BRUISE-Control He−−ADSS and He++ADSS scores post-operative pain risk score were developed based on the final risk prediction model (He, CSH; A, age; D, de novo surgery; S, slim—BMI <20 kg/m2; and S, sex—female).
BC-1, BRUISE CONTROL Trial 1; BC-2, BRUISE CONTROL Trial 2; BMI, body mass index; CSH, clinically significant haematoma.
Discussion
This is the largest cohort study of patients undergoing de novo or replacement CIED implantation that have been prospectively evaluated for factors contributing to post-operative pain. We found that the presence of post-operative CSH, de novo CIED implantation, female sex, younger age (<65 years), and lower BMI (< 20 kg/m2) were associated with increased post-operative pain. Identifying peri-operative factors associated with post-operative pain intensity in patients undergoing CIED implantation is important for developing interventions to effectively manage post-operative pain.11 This is important as post-operative pain can result in significant patient dis-satisfaction and increased health care resource ulitlization.12,13
The only published prospective study on post-operative pain in patients undergoing CIED implantation collected information on post-operative pain every 2 h, for a period of 24 h on a numeric rating scale.12 In that study, 39% of 102 patients reported moderate to severe (numeric rating scale score > 3) post-operative pain after CIED insertion. Multivariate analysis identified female sex as the only demographic or clinical variable associated with increased post-operative pain (P = 0.046). Women have been shown to have higher pain scores in multiple clinical conditions including osteoarthritis, headache syndromes, fibromyalgia, etc.13 Post-operative and procedural pain have also been shown to be severe in women compared with men, although the association is not as strong as for the previously mentioned conditions.5 In addition, women are more likely to perceive greater intensity of pain in experimentally induced pain such as intra-muscular injection of algesic substances.14 Lastly, there is evidence to suggest that a variety of social and psychological processes are likely to influence the differences in pain perception between women and men.15
In addition to female sex, our study identified four other independent risk factors associated with post-operative pain: the presence of CSH, de novo CIED implantation, younger age (<65 years), and lower BMI (<20 kg/m2). The development of CSH and resultant pain due to stretching of pectoral tissue might be similar to pain experienced by women undergoing mastectomy with immediate breast reconstruction using tissue expanders.16 A study evaluating predictors of CSH formation, in 2500 subjects receiving ICD implantation in the Shockless Implant Evaluation (SIMPLE) trial, identified heparin bridging, sub-pectoral implantation, upgrade from a pre-existing CIED, previous stroke, and older age as independent predictors of CSH on multivariable analysis.17 Clinically significant haematoma developing after CIED implantation was associated with increased post-operative pain in our analyses, and many of the independent factors associated with development of CSH also are associated with post-operative pain.
It is possible that individuals undergoing de novo CIED implantation and younger individuals may experience more post-operative pain as they are unlikely to have been pre-conditioned by pain related to chronic degenerative illnesses (e.g. Osteoarthritis) or prior invasive procedures.11,18 Patients with CSH and those with minimal subcutaneous adipose tissue (patients with BMI < 20 kg/m2) are likely to experience greater stretching of the skin over the incision resulting and this may contribute to higher post-operative pain scores.11
Post-operative pain has been widely studied in other surgeries and procedures. There is wide variability in pain perception and analgesic requirements in patients undergoing surgical procedures.11,19 Post-surgical pain assessment has focused on two main pain variables: pain perception measured by pain intensity scores and pain behaviour displayed by patterns of self-administered analgesia. Studies have identified demographic, procedural, and psychological factors that can predict increased post-operative pain perception. Some of the known factors associated with post-operative pain include female sex, type of surgery (laparoscopic vs. open incisional, site of surgery, tissue plane, etc.), and psychological factors (pre-existing depression, affective, and anxiety disorders).5,8,11,13,19
The He++ADSS score might be able to assist implanting physicians in estimating the probability of moderate to severe post-operative pain in subjects undergoing CIED implantation. For instance, a CIED implant patient with a He++ADSS score of 8 (CSH, receiving a de novo device and with a BMI < 20 kg/m2) has an 85% probability of experiencing moderate to severe post-operative pain with a 99% specificity for this prediction to be accurate. Patients identified to have high probability of post-operative pain can be prescribed enhanced pain management regimens or provided with patient-controlled analgesia to reduce the intensity of post-operative pain, shorten recovery from CIED surgery, and improve satisfaction.11,19
One of the challenges with the He++ADSS score is that CSH may develop only in a proportion of patients after CIED surgery. The score predicting pain for an individual patient can be calculated accurately only if the patient develops a CSH after the procedure, thereby delaying the institution of aggressive pain relief measures. To provide the clinician with an a priori score to predict post-operative pain in patients undergoing CIED surgery we have also provided the He−−ADSS score that does not include CSH (Figures 1 and 2). Using this score an individual with a He−−ADSS score of 4 (de novo surgery and BMI < 20 kg/m2) has a 65% probability and an individual with a score of 6 (age < 65 years, de novo surgery, and BMI < 20 kg/m2) has a 75% probability of experiencing moderate to severe post-operative pain, with a specificity of 93% and 98%, respectively. Patients with He−−ADSS score of ≥4, especially if they have established risk factors for developing CSH (patients on systemic oral anticoagulation, those on combined antiplatelet and anticoagulant agents and patients with diabetes mellitus)6,7 may be selected for aggressive pain relief measures.
Limitations
We were not able to assess psychological factors, such as pre-existing depression or affective and anxiety disorders and their impact on the severity of post-operative pain this study. We also did not quantify post-operative analgesic requirement in our study patients to determine if the factors associated with increased post-operative pain also predicted post-operative analgesic requirement. We have not included post-operative analgesic regimens used by the participating institutions in our analyses. Institutional variations in analgesic regimens could have impacted post-operative pain scores introducing a source of bias in this analysis. Another limitation of the predictive score is that CSH develops only after the procedure and hence, this component of the score cannot be used to predict increased post-operative pain prior to the procedure. However, in subjects with high likelihood of developing post-operative CSH (upgrade from existing CIED, older age, sub-pectoral implant) can be identified for counselling and enhanced post-operative analgesic treatment including pectoral nerve blocks prior to the procedure.20 It is possible that institutional variation in peri-operative pain management protocols might have influenced the intensity of pain reported by patients. Lastly, the VAS was administered on the first post-operative visit [12 (range 4–20) days] and this could have introduced a recall bias in patients reporting pain scores.
Conclusions
We have identified five independent predictors to predict moderate to severe post-operative pain and have developed a user-friendly post-operative pain score (the HeADSS score) to identify CIED implant patients who should be targeted for additional pain management. This pain prediction score will need prospective validation using other cohorts of patients undergoing CIED implantation.
Acknowledgements
The authors thank Karen MacDonald for central study co-ordination and Keri O’Reilly for data entry. We thank staff at the UOHI CVS Research Methods Center for database and statistical support/analysis: Lily Chen, My-Linh Tran, Jordan Bernick, and Liz Yetisir.
Funding
Bruise CONTROL-1 was supported by an operating grant from the Canadian Institutes of Health Research (CIHR), a CIHR Clinician Scientist Award (V.E.), and an Innovations grant from the University of Ottawa Heart Institute Academic Medical Organization Alternate Funding Program (funded by the Ministry of Health of Ontario). Bruise CONTROL-2 was supported by a grant from the Heart and Stroke Foundation of Canada (grant number G-14-0005725), a Fonds de recherché du Quebec-Santé (FRQS) Clinical Research Scholar Award (V.E.) and grants from Boehringer Ingelheim, Germany; Bayer HealthCare AG, Leverkusen, Germany; Pfizer and Bristol-Myers Squibb, New York, NY, USA.
Conflict of interest: V.E. reports personal fees from Bayer, Boehringer Ingelheim, BMS Pfizer, and Servier, during the conduct of the study (Modest). J.S.H. reports Research grants and speaking fees from Bristol-Meyers-Squibb and Pfizer. Speaking fees from Servier; research grants and speaking fees from Medtronic and Boston Scientific (Modest). A.V. reports grants from Bayer, Medtronic, and Biosense Webster, outside the submitted work (Modest). B.C. reports personal fees from Bayer, outside the submitted work (Modest). Eikelboom reports honoraria and grant support from Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb/Pfizer, Daiichi Sankyo, Glaxo Smith Kline, Janssen, Sanofi Aventis, and Eli Lilly as well as a personnel award from the Heart and Stroke Foundation (Modest). B.T. reports personal fees and other from Medtronic, personal fees and other from Abbott (Modest). D.H.B. reports grants from Boehringer Ingelheim, Germany, grants from Pfizer and Bristol-Myers Squibb, New York, during the conduct of the study (Modest). The other authors have nothing to disclose.
Data availability
The data underlying this article is stored at the University of Ottawa Heart Institute Clinical Trials Methodology centre under the stewardship of Drs David H. Birnie and George A. Wells and can be shared on reasonable request to the corresponding author.
References
- 1. Bibas L, Levi M, Touchette J, Mardigyan V, Bernier M, Essebag V. et al. Implications of frailty in elderly patients with electrophysiological conditions. JACC Clin Electrophysiol 2016;2:288–94. [DOI] [PubMed] [Google Scholar]
- 2. Greenspon AJ, Patel JD, Lau E, Ochoa JA, Frisch DR, Ho RT. et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol 2012;60:1540–5. [DOI] [PubMed] [Google Scholar]
- 3. Biocic M, Vidosevic D, Boric M, Boric T, Giunio L, Fabijanic D. et al. Anesthesia and perioperative pain management during cardiac electronic device implantation. J Pain Res 2017;10:927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stone ME, Salter B, Fischer A.. Perioperative management of patients with cardiac implantable electronic devices. Br J Anaesth 2011;107:i16–26. [DOI] [PubMed] [Google Scholar]
- 5. Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD. et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013;368:2084–93. [DOI] [PubMed] [Google Scholar]
- 7. Birnie DH, Healey JS, Wells GA, Ayala-Paredes F, Coutu B, Sumner GL. et al. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur Heart J 2018;39:3973–9. [DOI] [PubMed] [Google Scholar]
- 8. Mattila K, Toivonen J, Janhunen L, Rosenberg PH, Hynynen M.. Postdischarge symptoms after ambulatory surgery: first-week incidence, intensity, and risk factors. Anesth Analg 2005;101:1643–50. [DOI] [PubMed] [Google Scholar]
- 9. Price DD, McGrath PA, Rafii A, Buckingham B.. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17:45–56. [DOI] [PubMed] [Google Scholar]
- 10. Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB.. The faces pain scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain 1990;41:139–50. [DOI] [PubMed] [Google Scholar]
- 11. Ip HY, Abrishami A, Peng PW, Wong J, Chung F.. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 2009;111:657–77. [DOI] [PubMed] [Google Scholar]
- 12. Bode K, Breithardt OA, Kreuzhuber M, Mende M, Sommer P, Richter S. et al. Patient discomfort following catheter ablation and rhythm device surgery. Europace 2015;17:1129–35. [DOI] [PubMed] [Google Scholar]
- 13. Smith G, Dunbar SB, Valderrama AL, Viswanathan B.. Gender differences in implantable cardioverter-defibrillator patients at the time of insertion. Prog Cardiovasc Nurs 2006;21:76–82. [DOI] [PubMed] [Google Scholar]
- 14. Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD. et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain 2005;6:116–24. [DOI] [PubMed] [Google Scholar]
- 15. Robinson ME, Dannecker EA, George SZ, Otis J, Atchison JW, Fillingim RB.. Sex differences in the associations among psychological factors and pain report: a novel psychophysical study of patients with chronic low back pain. J Pain 2005;6:463–70. [DOI] [PubMed] [Google Scholar]
- 16. Legeby M, Segerdahl M, Sandelin K, Wickman M, Ostman K, Olofsson C.. Immediate reconstruction in breast cancer surgery requires intensive post-operative pain treatment but the effects of axillary dissection may be more predictive of chronic pain. Breast 2002;11:156–62. [DOI] [PubMed] [Google Scholar]
- 17. Masiero S, Connolly SJ, Birnie D, Neuzner J, Hohnloser SH, Vinolas X. et al. Wound haematoma following defibrillator implantation: incidence and predictors in the Shockless Implant Evaluation (SIMPLE) trial. Europace 2017;19:1002–6. [DOI] [PubMed] [Google Scholar]
- 18. Chia YY, Chow LH, Hung CC, Liu K, Ger LP, Wang PN.. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled iv analgesia: a prospective survey of 2,298 Chinese patients. Can J Anesth 2002;49:249–55. [DOI] [PubMed] [Google Scholar]
- 19. Chanthong P, Abrishami A, Wong J, Herrera F, Chung F.. Systematic review of questionnaires measuring patient satisfaction in ambulatory anesthesia. Anesthesiology 2009;110:1061–7. [DOI] [PubMed] [Google Scholar]
- 20. Tripathy S, Mandal I, Rao PB, Panda A, Mishra T, Kar M.. Opioid-free anesthesia for breast cancer surgery: a comparison of ultrasound guided paravertebral and pectoral nerve blocks. A randomized controlled trial. J Anaesthesiol Clin Pharmacol 2019;35:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article is stored at the University of Ottawa Heart Institute Clinical Trials Methodology centre under the stewardship of Drs David H. Birnie and George A. Wells and can be shared on reasonable request to the corresponding author.