Abstract
The COVID-19 pandemic has put the spotlight on the urgent need for integrated nucleic acid tests (NATs) for infectious diseases, especially those that can be used near patient (“point-of-care”, POC), with rapid results and low cost, but without sacrificing sensitivity or specificity of gold standard PCR tests. In the US, the Clinical Laboratory Improvement Amendments Certificate of Waiver (CLIA-waiver) is mandated by the Food and Drug Administration (FDA) and designated to any laboratory testing with high simplicity and low risk for error, suitable for application in the POC. Since the first issuance of CLIA-waiver to Abbot’s ID NOW Influenza A&B in 2015, many more NAT systems have been developed, received the CLIA-waiver in the US or World Health Organization (WHO)’s pre-qualification, and deployed to the front line of infectious disease detection. This review highlights the regulatory process for FDA and WHO in evaluating these NATs and the technology innovation with each of the CLIA-waived system. Understanding the technical advancement and challenges, unmet needs, and the trends of commercialization facilitated through the regulatory processes will help pave the foundation for future development and technology transfer from research to the market place.
Graphical Abstract

Introduction
The field of molecular diagnostics for infectious diseases has emerged as one of the most critical solutions to end the COVID-19 pandemic. Widespread accurate and rapid diagnostics are key to re-opening the economy and social activities while ensuring public safety. Molecular diagnostics has rapidly grown in the last two decades as a specialized branch of in-vitro diagnostics (IVD). These nucleic acid tests (NATs) focus on the detection and identification of nucleic acids (DNA or RNA) from pathogens in human specimens (blood, urine, saliva, vaginal samples, etc.).1 Since PCR was first introduced in 19862, NATs have been gradually replacing immunoassays3, cell culture 4,5, microscopy6,7, and other techniques8 for infectious disease diagnosis. One of the main advantages that NATs offer is the ability to rapidly detect disease targets with high diagnostic sensitivity and specificity, leading to results that give clinicians early and conclusive indication about the disease state of the patient.1 Currently, NATs are considered the gold-standard test for the diagnosis of many infectious diseases.9
NAT Diagnostics as regulated by the US Food and Drug Agency (FDA).
Different regulatory and quality-assurance bodies evaluate NATs before they can enter the market. In the US, the FDA governs the approval or clearance of NATs by the Center for Device and Radiological Health (CDRH) and the Center for Biologics Evaluation and Research (CBER). The FDA classifies medical devices into Classes I, II, and III in the order of increasing potential risks to the patients. For class I and II, and some III devices, the 510(k) premarket notification requires proof of substantial equivalence with a previously marketed predicate device. These diagnostics are categorized by the Clinical Laboratory Improvement Amendments (CLIA) as high complexity, moderate complexity, or exempt or “CLIA-waived”.10 Currently, the FDA lists 510(k) clearance for 302 NAT products for microbials, of which only 14 are CLIA waived.11 This highlights the challenges in simplifying moderately complex tests for ease of operation, while maintaining accuracy and reliability of test results.
CLIA-waived NAT as a subset of point-of-care (POC) NAT diagnostics.
A CLIA-waiver is designated to 510(k)-cleared tests that incorporate methodologies that are easy for an unskilled operator to use and that have low risk of an incorrect result.12 A further proviso is that the patient must not be at critical risk if the test result is incorrect. Sites that perform CLIA-waived tests must obtain the CLIA Certificate of Waiver or Certificate of Compliance, administered by the Center for Medicare & Medicaid Services (CMS). These sites could include hospitals, physician offices, urgent care, outreach clinics, and temporary patient care settings that have appropriately trained personnel to perform the test.13 Besides obtaining the CLIA-waiver, sites performing these tests are recommended to comply with the point-of-care (POC) testing and limited service laboratory checklist put forth by the American College of Pathologists.14
Most NATs require skilled personnel to operate them due to assay, instrument, and/or protocol complexity, even for those intended to be used at the POC, and thus few tests have been granted the CLIA-waiver. A CLIA-waived NAT must integrate the sample processing and amplification components and use hardware/software to operate the test protocol, analyze the raw data and produce a simple, actionable result.15 Test developers have taken many steps to bring POC NATs up to CLIA-waiver criteria or equivalence, such as integration of the sample preparation, amplification, and readout components. These advancements have led to reduction in manual-handling steps while reducing instrumentation costs. Receiving the CLIA-waiver is a milestone towards operating NATs at the POC.16
The CLIA-waiver was issued for the first NAT in 2015 to the then Alere i influenza A & B test, now renamed to the ID NOW Influenza A&B (Abbott Diagnostics, IL, USA).17 Since then, six different NAT systems received CLIA-waiver for 15 assays, often with multiple assays running on one system. These include 4 assays under the Emergency Use Authorization (EUA) for the SARS-CoV-2 diagnostics.
So far, no NAT for blood have achieved the CLIA-waiver due to its unique technical challenges.18–21 Highly infectious bloodborne diseases such as Zika and Ebola have seen regional epidemics with widespread health effects.22–26 Millions of deaths every year are attributed to bloodborne pathogens, specifically HIV (1.1 million), hepatitis (1.3 million), and malaria (438,000).27,28 POC molecular tests for these bloodborne pathogens would have widespread use in addressing existing epidemics and regional outbreaks.29–31
EUA are granted to a subset of CLIA-waived NAT due to the COVID-19 pandemic
Since the outbreak of the COVID-19 pandemic, the requirement for a test to receive the EUA and be performed in the patient care setting has been lowered.32 The FDA has published the “Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency”, which states that the FDA may grant EUA to unapproved medical products and diagnostics for an emerging public health threat such as the SARS-CoV-2.32 In granting these EUAs for molecular diagnostics tests, the FDA evaluates the best available evidence on the validation data for limit of detection, clinical evaluation, inclusivity against publicly available SARS-CoV-2 sequences, and cross-reactivity.32 The FDA weighs associated risk against perceived benefits, and has allowed individual states to assume risk of liability while establishing systems to develop and authorize systems without submitting to the FDA.32 Although authorized through the EUA, some products have received complaints about poor performance compared to marketed claims. For example, Abbott’s ID NOW have been criticized for high false negatives.32 It is also important to note that an EUA is not equivalent to a FDA approval. The EUAs granted are only valid for the duration of the public health emergency. It is unclear what the fate of these tests will be once the declared public health emergency is terminated unless manufacturers follow the appropriate premarket submission pathways to remain on the market long term.33
Reciprocal evaluation systems to the FDA and CLIA outside of the US
Outside of the US, many countries also have a regulatory agency to govern in-country registration for diagnostic tests34, such as CE-IVD marking for EU, the Ministry of Food and Drug Safety (MFDS) for Korea, or the Ministry of Health, Labor and Welfare (MHLW) for Japan. In the low-to-mid income regions (LMIC), the WHO established the pre-qualification (WHO-PQ) guidelines in 2010 for IVDs for high priority diseases with appropriateness to use in resource-limited settings, to help countries make evidence-based purchase selections.35 Despite some challenges36, WHO-PQ has been an effective alternative pathway for global manufacturers to gain market access outside of the US.35,37,38 WHO-PQ is also required when countries seek support from international donors such as the Global Fund, PEPFAR, UNITAID, UNICEF and Médecins Sans Frontières to procure NATs.39
Bringing NATs from research to clinical use requires clearance or approval through the appropriate regulatory pathways before they can be marketed in the targeted regions. When developing diagnostics for specific diseases, it is essential to define the scope of intended use (POC or laboratory), define the intended region for which the tests are developed, to identify the regulatory body (FDA, WHO, or country-specific agencies) and governing process based on the mechanism of action and disease target, and then to satisfy the technical and validation requirements of the particular pathways.
Goals of this review.
The goal of this review is to provide diagnostic developers an understanding of the regulatory process for POC NAT. In addition, we collate the availability of commercially available POC NAT and the technological approaches employed. We analyse the approvals of POC NAT to diagnose specific disease and provide an argument for the need to develop POC NAT for bloodborne diseases.
In this review, we focus on NATs marketed in the United States with CLIA-waiver as the industry benchmark (Figure 1). We expand to commercial POC systems that have sought WHO-PQ and target POC applications worldwide, especially in the LMIC, in the goal of understanding the technical challenges, unmet needs, and the trends of commercialization. For a broader review of IVD diagnostic or monitoring tests other than the NATs, or in regions other than the US or LMIC, we refer the reader to recent summaries of commercially available POC tests.1,21,40–42
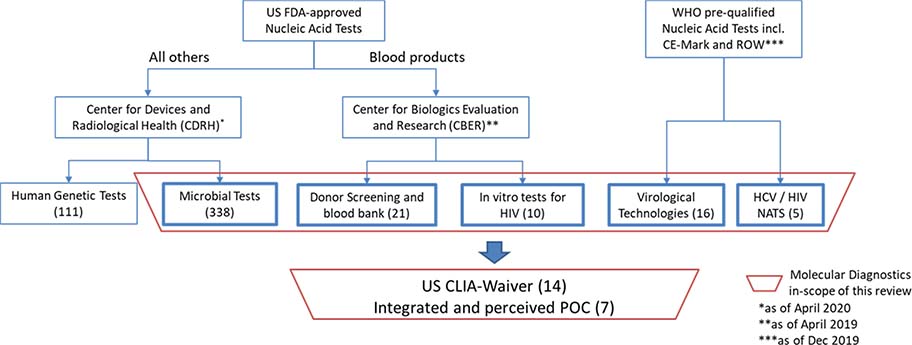
Figure 1.
Molecular Diagnostics as approved or cleared through the FDA regulatory pathway that are in-scope of the paper (in bold boxes)
The FDA regulatory pathways for NATs and the CLIA-waiver
In the US, in vitro diagnostics are regulated by the CDRH through the Premarket Approval Application (PMA) or 510(k) mechanisms11, except tests associated with blood products, donor screening, blood bank practices, as well as HIV diagnostics, which are regulated by the CBER43, primarily through the Biological License Application (BLA).
The 510(k) premarket notification requires proof of substantial equivalence with a previously marketed predicate device, whereas the PMA mechanism is FDA’s regulatory and scientific review process of high-risk class III devices.44 Compared to 510(k), PMA applications have more stringent requirement on clinical data, longer processing time, and higher costs. The majority of CDRH-regulated in vitro diagnostics, including NATs, are cleared through the 510(k) pathways, except for hepatitis, human papilloma virus, and cytomegalovirus diagnostic assays, which have almost always required PMA approval due in insufficient information allowing for test assurance and risk in outcomes associated with incorrect diagnosis.11
Blood bank, donor screening, or HIV NATs that detect bloodborne pathogens, including HIV alone or in combination with HBV / HCV co-infection, are regulated through the BLA pathway and mostly require PMA.45 The review and approval processes typically require the investment of several million dollars before the product can be cleared for market, representing significant barrier most often only afforded by larger corporations.
The CLIA governs standards of quality for laboratory testing, with the goal of ensuring the accuracy, reliability, and timeliness of patient test results.15 The CLIA Statutory Criteria for Waiver entails that a 510(k), PMA, or BLA-cleared diagnostic test must also “employ methodologies that are so simple and accurate as to render the likelihood of erroneous results by the user negligible and pose no unreasonable risk of harm to the patient if performed incorrectly.”15,46 Most POC settings only have the resources to support the handling of such CLIA-waived tests.
Under the approach recommended by the FDA, a first step towards determining if a device is a candidate for the CLIA-waiver including determining whether the test is “simple”, as outlined directly in the Recommendations for Clinical Laboratory Improvement Amendments of 1988 (CLIA) Waiver Applications for Manufacturers of In Vitro Diagnostic Devices - Guidance for Industry and Food and Drug Administration Staff.47
It is important to note that the FDA believes that a test is not “simple” or CLIA-waiver eligible if it requires sample manipulation or intermediate evaluations. 15 For example, centrifugation, complex mixing steps, or evaluation of the sample for conditions such as hemolysis or lipemia by an operator are not considered simple.
The WHO-PQ and ASSURED criteria
For tests outside of the US, it is not necessary to obtain a CLIA-waiver to be performed at the POC, but achieving equivalent performance would improve the likelihood of worldwide adoption. Many manufacturers launched products first outside the US with the WHO-PQ pathway, especially for HIV.
The WHO-PQ it is a quality system that’s recognized by LMIC for adopting diagnostics or IVDs focusing on priority diseases of global importance, such as HIV/AIDS and Hepatitis, and suitability for use in resource-limited settings.35 The assessment for prequalification ensures the safety, quality, and performance of commercially available IVDs for providing procurement guidance to UN agencies and WHO member countries. 35 As of 2019, the WHO has prequalified 89 IVD products for HIV, HCV, HBV, Malaria, HPV, and G6PT enzyme deficiency in the format of rapid diagnostic tests (RDT), enzyme immunoassays (EIA), and NATs.48 Among these, 21 NATs for HIV, HPV, and HCV were prequalified for disease diagnostics and monitoring. Many products gained access to the global market (outside of the US) through this mechanism.
The WHO also advocates for the development of POC diagnostics for resource-limited settings to satisfy the forward-looking ASSURED framework (a wish list criteria for Affordable, Sensitive, Specific, User-friendly, Rapid and robust, Equipment-free and Deliverable).
Table 1 puts the US CLIA criteria for a “simple” test side by side with the WHO ASSURED framework.47 Both frameworks have consistent requirements for readout, operation and maintenance, except that the ASSURED framework has additional requirements for accessibility to deliver to those in need and affordability. In general, both are broad developmental guidelines, which should be adapted for specific diseases, and provide a good starting point for developers to design IVD tests for rapid same-day readout to support immediate clinical decision making. Additional validation is always required to bridge the gap between subjective guidelines and stringent regulatory requirements for obtaining either CLIA-waiver in the US, or WHO-PQ or recommendations for the LMIC. For example, a HIV diagnostic system should satisfy the HIV-specific ASSURED criteria to enable its full utility at the POC as shown in Table 2.49
Table 1.
Comparison between US CLIA-waiver eligibility criteria and the WHO ASSURED framework
| US CLIA criteria for a “simple” test | WHO ASSURED framework |
|---|---|
| Affordable by those at risk of infection. | |
| • Produces results that require no operator calibration, interpretation, or calculation. • Produces results that are easy to determine, such as ‘positive’ or ‘negative,’ a direct readout of numerical values, the clear presence or absence of a line, or obvious color gradations. • Has test performance comparable to a traceable reference method as demonstrated by studies in which intended operators perform the test. |
Sensitive (few false-negative results). Specific (few false-positive results). |
| • Needs only basic, non-technique-dependent specimen manipulation, including any for decontamination. • Needs only basic, non-technique-dependent reagent manipulation, such as “mix reagent A and reagent B.” • Needs no operator intervention during the analysis steps. • Needs no technical or specialized training with respect to troubleshooting or interpretation of multiple or complex error codes. • Provides instructions in the package insert for obtaining and shipping specimens for confirmation testing in cases where such testing is clinically advisable. • Contains a quick reference instruction sheet that is written at no higher than a 7th grade reading level. |
User-friendly (simple to perform by persons with little training). |
| Needs no electronic or mechanical maintenance beyond simple tasks, e.g., changing a battery or power cord. | Rapid treatment at the first visit and robust use without the need for special storage. |
| • Is a fully automated instrument or a unitized or self-contained test. • Uses direct unprocessed specimens, such as capillary blood (fingerstick), venous whole blood, nasal swabs, throat swabs, or urine. |
Equipment-free (that is, no large electricity-dependent instruments needed to perform the test; note that portable handheld battery-operated devices are acceptable, which differs from the criterion of the original authors). |
| Delivered to those who need it |
Table 2.
WHO ASSURED criteria as applied to HIV diagnostics.
| Characteristic | Target Specification (for HIV as an example) |
|---|---|
| Affordable by those at risk of infection | Less than $500 per instrument, less than $10 per test |
| Sensitive with very few false-negatives, Specific with very few false-positives | Lower limit of detection: 500 HIV RNA copies per ml of blood |
| User-friendly tests that are simple to perform and require minimal training | 1–2 days training, easy to use |
| Rapid, to enable treatment at first visit and robust,, for example not requiring refrigerated storage | < 30 minutes for diagnosis; Shelf-life > 1 year at room temperature. |
| Equipment-free | Compact, battery-powered, on-site data analysis, easy disposal, easy sample handling, no cold chain |
| Delivered to those who need it | Portable, hand-held |
CLIA-waived NATs
In this section, we summarize and review details regarding the user steps, diagnostic technologies, resource requirements, and performance of the five CLIA-waived tests as of 2020 (Table 3)16, Roche Cobas Liat, Abbot ID NOW, BioFire FilmArray, Cepheid Xpert Xpress, Mesa Biotech Accula (marketed as Silaris by Sekisui Diagnostics). The summary does not include the Cue that were recently cleared by the EUA to diagnose SARS-COV-2. These tests provide a roadmap for the development of future diagnostic tests aimed at more difficult targets or complex specimens.
Table 3.
CLIA waived molecular diagnostic assays.
| POC System | Manufacturer | Test | Sample | Amplification | Readout | Time to Results | Advantages / Disadvantages |
|---|---|---|---|---|---|---|---|
| Accula (Silaris) | MESA Biotech | Flu A/B | Nasal Swab | RT PCR | Colorimetric | <30 min | colorimetric readout; 97%/94% (Flu A/B), sensitivity; 94%/99% (Flu A/B), specificity |
| SARS-CoV-2 | Throat Swab, Nasal Swab | RT PCR | Colorimetric | <30 min | |||
| Cobas Liat | Roche | Flu A/B | Nasopharyngeal Swab | RT PCR | Fluorescence | 20 min | Lab-in-a-tube rapid readout; 99.6%, 99.3%, and 96.8% sensitivity, and 97.5%, 99.7%, and 98.8% specificity for Influenza A, B, and RSV respectively |
| Flu A/B & RSV | Nasopharyngeal Swab | RT PCR | Fluorescence | 20 min | |||
| Strep A | Throat Swab | RT PCR | Fluorescence | 15 min | |||
| FilmArray | BioFire | Respiratory Panel | Nasopharyngeal Swab | Multiplex nested PCR real time | Fluorescence | 1–2 hr | Panel detection of 20 pathogens at once |
| ID Now | Abbott | Flu A/B | Nasopharyngeal swab | NEAR | Fluorescence | <15 min | Isothermal reactions; rapid readout; high false negatives reported |
| Strep A | Throat Swab | NEAR | Fluorescence | 2–6 min | |||
| RSV | Nasopharyngeal swab | NEAR | Fluorescence | <15 min | |||
| SARS-CoV-2 | Throat Swab, Nasal Swab, Nasopharyngeal Swab | NEAR | Fluorescence | 15 – 21 min | |||
| Xpert Xpress | Cepheid | Flu A/B & RSV | Nasal Swab and Nasopharyngeal Swab | real time RT PCR | Fluorescence | 30 min | Highly accurate: agreement between Xpert Xpress and real-time triplex PCR results achieved 100%, 100%, and 96.7% positive agreement for influenza A, B, and RSV, and 99.7% for negative agreement |
| Flu A/B | Nasal Swab and Nasopharyngeal Swab | real time RT PCR | Fluorescence | 30 min | |||
| Strep A | Throat Swab | real time RT PCR | Fluorescence | <30 min | |||
| SARS-CoV-2 | Nasopharyngeal, Nasal, or Mid-turbinate Swab | real time RT PCR | Fluorescence | < 30 min | |||
| Cue | Cue Health | SARS-CoV-2 | Naso Swab | - | Semi-quantitative nanoampere measurement | 25 min | Connected with readout on mobile platform for potential household rollout |
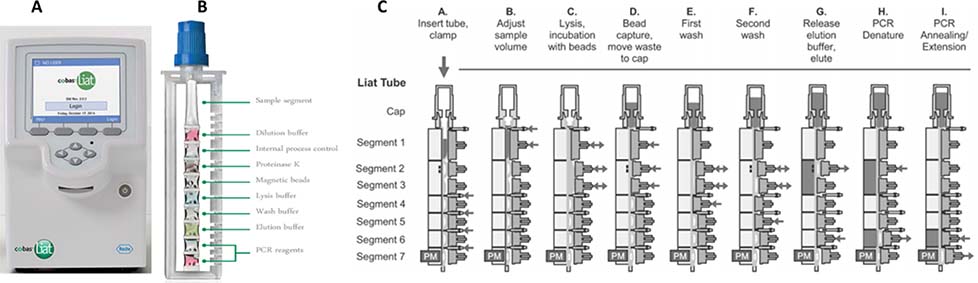
The Cobas Liat assays use a unique “lab-in-a-tube” process to integrate sample preparation, RT-PCR, and readout in a rapid turnaround from sample to result in under 20 minutes.50,51 The system is composed of a pencil-sized reagent tube with breakable seal for pre-packed fluid or dry reagent segments, and an analyzer which automates reagent preparation, sample lysis, nucleic acid extraction, inhibitor removal, amplification, and readout. Figure 2 illustrates the 7-actuator steps.50,52
Figure 2.
A. Roche Liat instrument with B. lab-in-a-tube sample preapration module. C. Liat tube operation steps as through each step from sample prepreation to PCR completion52
The patient sample can be added to the Liat tube using a transfer pipette. In the sample preparation steps (B-G), clams and actuators alternatively apply pressure to the flexible tubule segments, breaking the seal between the tubule segments to facilitate buffer exchanges and reagent mixing. In the nucleic acid purification step (D), silica-coated magnetic beads are well mixed with the lysed sample to capture DNA/RNA in the presence of chaotropic salts and alcohol, while inhibitors are washed away in subsequent wash steps. In the RT-PCR steps (H-I), the heaters (gray shaded) creates different temperature zones as the actuators open and close alternatively to cycle reaction mixture through these segments. The photometer measures real-time readout from fluorescent probes as reaction cycles through. Due to the small sample volume and highly efficient hot start and cycling process, a typical denaturing, annealing, and elongation cycles could occur as quickly as 95°C for 2 seconds, 60°C for 10 seconds, and 72°C for 10 seconds each.50 The additional RT step for RNA virus could occur at 65°C for 10 minutes.
In a multi-center evaluation of 1,361 prospective clinical samples, the Liat system reached sensitivity of 99.6%, 99.3%, and 96.8%, and specificity of 97.5%, 99.7%, and 98.8%, for Influenza A, B, and RSV respectively.53 Overall, the modular and automatic fluidic handling architecture allows gold standard RT-PCR assays to run without any human intervention, making it possible to be adopted by a non-trained user in a minimal-resourced setting.1
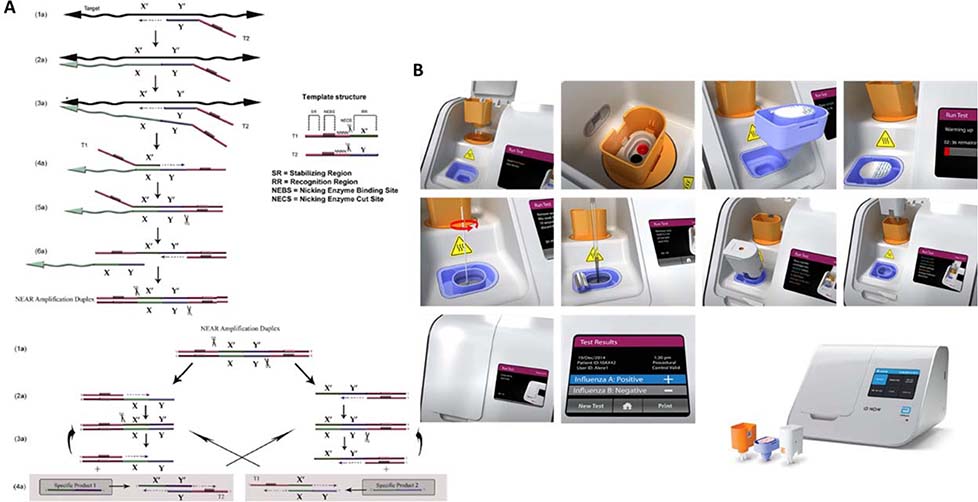
The Abbot ID NOW (previously Alere i) tests for Influenza A&B, Strep A, RSV, and SARS-COV-2 tests. It incorporates two cups for sequential sample preparation and nucleic acid amplification and four user steps. Overall handling time includes menu selection for about one minute, open lid and insert cartridges with confirmation of assay for about 30 seconds, sample lysis and extraction for about 6–7 minutes, and final amplification for about 13 minutes for negative reaction, and 5 minutes for positive reaction. The overall sample-to-result could take as little as 12 minutes. The ID NOW assay uses an isothermal amplification method, Nicking Enzyme Amplification Reaction (NEAR), which eliminates the need for thermal cycling and achieves the robustness, speed, and sensitivity comparable to PCR assays.54 Figure 3 illustrates the amplification method and device.55
Figure 3.
A. Principle of operation for NEAR, B. Abbot ID NOW operation steps including sample prepration modules55
The key component in the assay is the nicking enzyme (endonuclease), which selectively binds and nicks at the recognition sites along the template strand.55 A DNA polymerase extends at the nicking site of the NEAR amplification duplex and displaces the single strands. Repeated recognition, nicking, polymerization, and strand displacement achieves rapid exponential production of short strands of oligonucleotides (18–20 bp) from the template sequence.55 Driven by two templates (primer set) and three enzymes (DNA polymerase, reverse transcriptase, and a thermostable nicking endonuclease), the NEAR process enables detection of low concentration targets within minutes.56–58
In a post-market study of 360 frozen influenza A and B samples from the 2012 and 2013 flu season, the ID NOW assay showed sensitivities of 87.2%, 92.5%, 25.0%, and 97.4% for influenza virus A-1, A-3, A-u, and B, respectively, after discordant resolution, and 100% specificity for all influenza A/B subtypes.55 All results were obtained within 15 min.
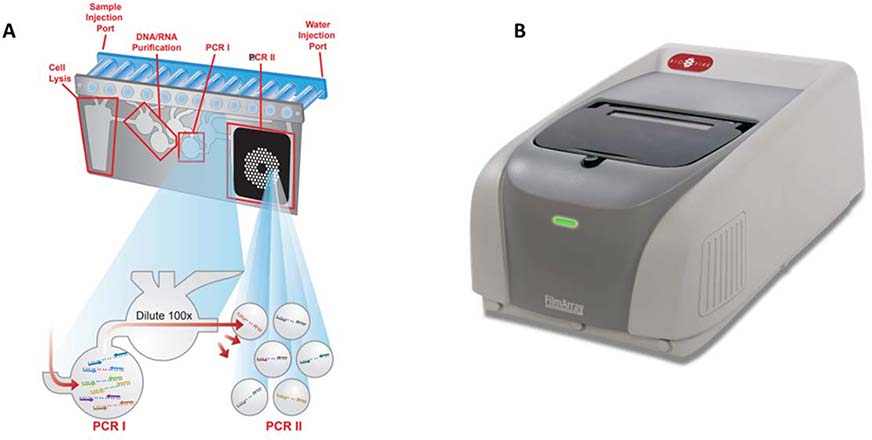
The BioFire FilmArray Respiratory Panel EZ (RP EZ) leverages multiplexed PCR for detecting 20 respiratory pathogens at once using a nested PCR technique.59 Fluidic movement for sample preparation is achieved by three individual pneumatic pistons compressing the reagents into the fluid pouches as shown in figure 4.60 Subsequent inflation of silicone bladders over the fluidic pouches results in flow across the blisters. Nested PCR in the FilmArray assay achieves highly specific detection.
Figure 4.
A. FilmArray pouch and B. instrument 59
The principle of nested PCR lies in a 2-staged amplification of the target sequence. A pair of “outer” primers in the first stage results in the first amplicons, which are diluted and amplified again in the second stage using primers located within the sequence of first amplicons.59,60 The nested process increase specificity due to the requirement to match all 4 primers on the template.59 In multiplexing the assay, the FilmArray RP EZ pouch includes lyophilized PCR reagents specific for each of the pathogens in separated wells. The end-point fluorescence signal from specific reagent wells would indicate existence of specific pathogens.60
To start the Filmarray assay, the FilmArray RP EZ pouch is inserted in the loading block, where vacuum draws a set volume of the hydration buffer and patient sample in the sample buffer into the pouch. The system requires an average of two minutes of hands-on time, 300 μL of nasopharyngeal swab (NPS) sample volume, and one hour of runtime. In a multi-center evaluation of 1,612 prospectively collected NPS samples, the FilmArray RP2 and the comparator testing had 99.2% agreement. The RP2 also demonstrated a positive percent agreement of ≥91.7% for detection of all but three analytes, and a negative percent agreement of ≥93.8% for all analytes.61
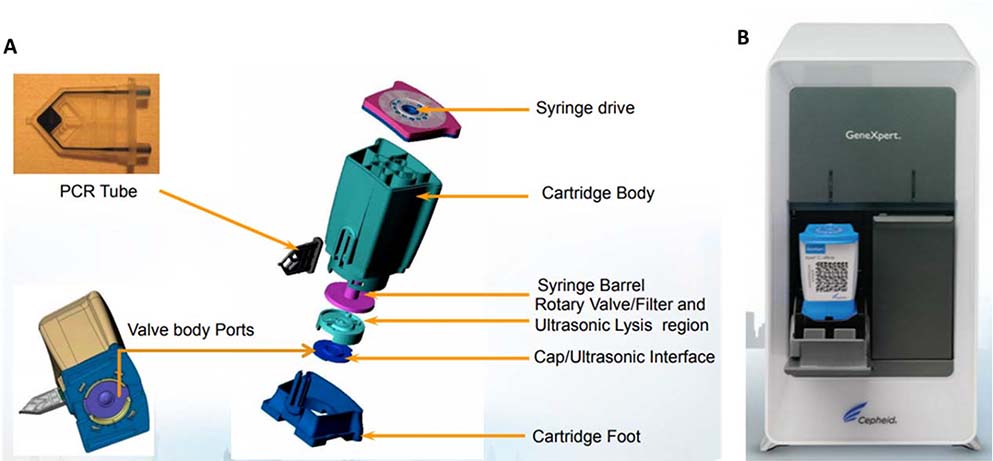
The Cepheid Xpert Xpress Flu/RSV assay relies on several unique features of the GeneXpert cartridge to perform integrated sample preparation and amplification: an ultrasonic horn for lysis, a rotary plunger system for sequential fluidic transfer, and a honeycomb PCR tube to define fluidic path for multiplex microwell nucleic acid amplification.62–64 (Figure 5)
Figure 5.
Cepheid GeneXpert Xpress A. cartridge and B. reader 62,63,92
The first step of sample introduction to the Xpert Xpress is done by pipetting the sample into the sample area of the cartridge. After the lid is closed on the cartridge, a plunger is activated to move up and down to draw the sample into the lysis region. In the lysis region, an ultrasonic transducer is coupled with an interface cap at a vibration frequency that generates pressure waves through the interface to disrupt the cellular or viral membrane.64 Lysed samples release nucleic acids, which are pulled through a porous membrane by the rotary plunger to flow towards the amplification honeycomb tube.62 On the honeycomb are hundreds of wells, each capable of fluidic capacity of between a few nanoliters to hundreds of nanoliters, depending on the assay volume needs.63 Sample preparation, enzyme, and target specific reagent beads are pre-filled and retained in their respective reaction chambers, and can be hydrated with liquid reagents. Each well can then perform an individual PCR reaction for multiplexed targets (if necessary) with rapid thermal cycling.62
In a post-market study with 172 clinical respiratory samples, agreement between Xpert Xpress and real-time triplex PCR results achieved 100%, 100%, and 96.7% positive agreement for influenza A, B, and RSV, and 99.7% for negative agreement.65
The Accula is a rapid PCR test combines a disposable cartridge for sample preparation, fluidic control, and the downstream Oscillating PCR Amplification Reaction (OPCRar) as shown in Figure 6.66–68 Accula has FDA cleared tests for Influenza A &B, RSV, and most recently a SARS-COV-2 test available under COVID-19 EUA.
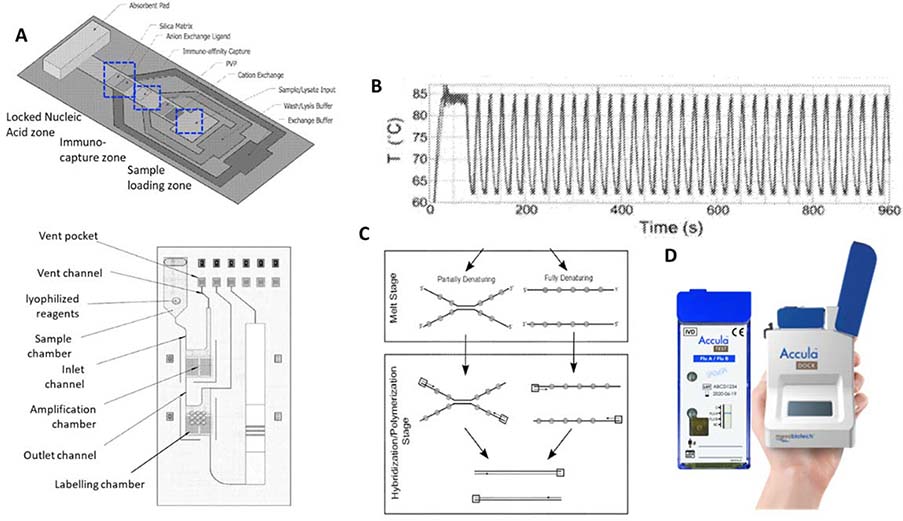
Figure 6.
A. Accula sample preparation cartridge including sample preapration layer (top) connected through internal capillary flow channel (not shown) to the ampfliciation layer (bottom)67; B. OPCRar amplification thermal cycles with reduced temperature range and rapid speed68; C. OPCRar principle of operation with partial denaturation instead of full denaturation68; D. the Accular cartridge and analyzer93
The sample preparation is achieved through lateral flow immuno-capture and passive buffer exchange in a porous nitrocellulose substrate which leverage channel width differential to control fluid flow sequences.66 As sample flows over the lateral immuno-capture zone, influenza virus in the swab samples are first captured by immobilized antibodies while undesired constituents are washed away. As sample depletes towards the subsequent Locked Nucleic Acid (LNA) zone, passive exchange between the sample buffer and lysis reagent results in disruption of the captured virus and liberation of RNA for hybridization-based affinity capture.66 Captured RNA is interfaced with the intake chamber for amplification. In Figure 6, the fluidic cartridge is composed of laminated plastics to accommodate sample intake, amplification, and labelling.67 Resistive heating elements and temperature sensors are part of the disposable cartridge beneath the amplification chamber, and interfaces with heating control circuitry in the benchtop unit.67
Compared to conventional PCR, where double helix is denatured at 95°C and annealing occurs at 50–65°C, OPCRar drastically reduces the temperature differential between the denaturation and annealing temperatures from more than 30°C to less than 20°C.68 OPCRar achieves this through two key components in the amplification reagents: 1) double strand destabilizing agents such as high concentrations of DMSO (between 10% and 15%), which lower the reaction melting temperature, and 2) oligonucleotide primers of unusually long length (35–55 nt) and high GC content to raise the annealing temperature to 70–80°C in a given thermal cycle.68 In the lowered denaturation temperature, double stranded targets may be partially denatured. OPCRar leverages selected DNA polymerase to displace partially denatured strands, thus making complete denaturing unnecessary.68 By minimizing the temperature differentials encountered during thermal cycling, OPCRar combines the speed and reliability of PCR with the lowered instrumentation requirements of isothermal amplification methodologies.69
In supporting regulatory filing with the FDA for Accula Flu A/B, Mesa Biotech claimed substantial equivalence to Abbott’s ID NOW, with 97% (Flu A), 94% (Flu B) sensitivity, and 94% (Flu A), 99% (Flu B) specificity.
Each of these CLIA-waived systems target respiratory infections. The focus on respiratory is historically driven by US market demand for influenza testing as well as the ease of sample collection and processing as respiratory specimens are non-invasively collected using a nasal swab or nasopharyngeal swab.70,71 Compared to blood, these samples have fewer confounding components which could lead to matrix-based interference with downstream processes (e.g. amplification), and therefore are relatively easier to process.72–74 The urgent need for testing for the COVID-19 pandemic has driven companies to seek emergency authorization of their platforms for testing the novel coronavirus.75 The recent episodes of globally spread influenza seasons also triggered larger investments and willingness to reimburse by insurance companies in the US to seek more efficient and cost-effective means to detect and monitor respiratory diseases.76
These are all turnkey systems which include integrated sample preparation, amplification, and readout steps, resulting in drastically simplified operation steps and reduced handling errors. Time-to-results are all within an hour (with the exception of Biofire) while patients wait. The time-to-results have been effectively reduced in these systems with either reduced reagent volume (Liat) or through rapid isothermal amplification which doesn’t require multiple thermal cycling steps (ID NOW). These CLIA waived systems are comparable and highly sensitive and specific in detecting the target pathogens in comparison to reference laboratory products.53,55,59,69,77–80
Selected Non-CLIA-waived NATs
Some NATs target difficult-to-diagnose bloodborne diseases and that have been designed to use at the POC, but are not CLIA-waived or marketed in the US as shown in Figure 4.
SAMBA II
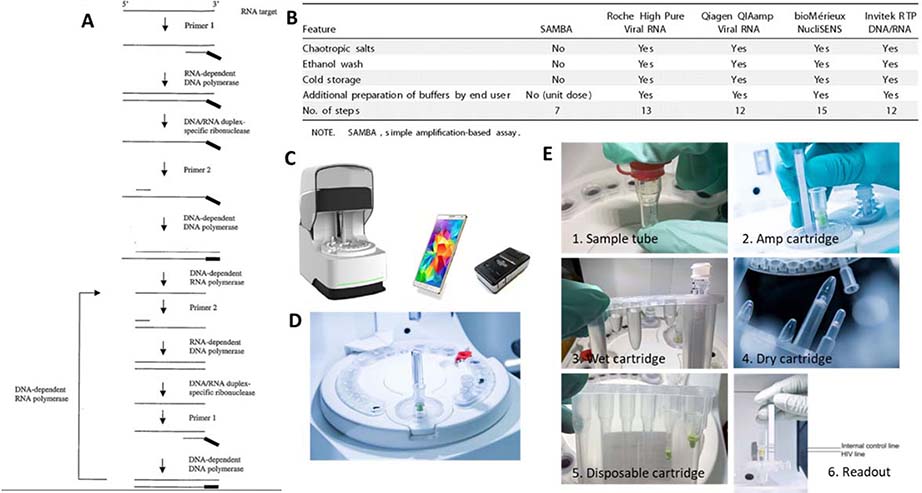
(Diagnostics for the Real World) is being piloted in Uganda and Malawi by Doctors Without Borders. The SAMBA I and II instruments and SAMBA I HIV viral load test for plasma samples are approved with CE mark. The SAMBA II instrument is a lower throughput POC version of the SAMBA I instrument, and the HIV Semi-Q whole blood test is a semi-quantitative test of HIV viral load in whole blood, an improvement over the earlier version test in plasma.81
The sample preparation of the SAMBA is unknown; however, evidence suggests that it is an aqueous-based extraction method that does not require cold storage or addition of addition of carrier RNA or ethanol, nontoxic to handle or dispose, and uses an aqueous wash buffer compatible with downstream amplification.82 The unit dose packaging allows automation and integration with the amplification.
SAMBA relies on a self-sustained isothermal amplification method, as shown in Figure 7.83 In this method, RNA amplicons are produced from an RNA template with RNA dependent RNA polymerase. cDNA-RNA duplexes are then formed, and rapidly denatured and amplified to additional RNA amplificons in an isothermal condition. Multiple enzymes are critical for the reaction, which mimics an in vivo retroviral replication: reverse transcriptase (RNA-dependent DNA polymerase), RNase H (DNA/RNA duplex-specific ribonuclease), DNA polymerase, and a DNA-dependent RNA polymerase.83 Similar transcription-based amplification methods include Nucleic Acid Sequence-based Amplification (NASBA, BioMerieux)84, Transcription-mediated amplification (TMA, Gen-Probe / Hologic) 85, and self-sustained sequence replication (3SR).86 The amplification and readout of the amplification product on a dipstick is carried out in a sealed disposable cartridge that contains all the required reagents and components. This integration allows containment of the amplification products and prevents carry-over of amplicons.81 A laboratory evaluation of the POC-ready SAMBA II II, yielded an LOD of 433 copies/ml (95% CI, 341–525 copies/ml) and 100% specificity when testing HIV-1 seroconversion panels diluted in in HIV-negative whole blood. In a performance evaluation study of the SAMBA semi-Q in London, Malawi, and Uganda, diluted HIV-1 subtype C patient samples and undiluted samples yielded 99% (95% confidence interval [CI], 93.8 to 99.9%) and 96.9% (95% CI 94.9 to 98.3%) concordance compared with the gold standard COBAS AmpliPrep/COBAS TaqMan HIV-1 test, v2.0.87
Figure 7.
SAMBA semi-Q system. A: The self-sustained isothermal amplification method. B: the sample preparation features of SAMBA semi-Q compared with gold standard methods. C. SAMBA II instruments. D. SAMBA II cartridge. E. SAMBAII process flow including from sample-to-results 82,83,94,95.
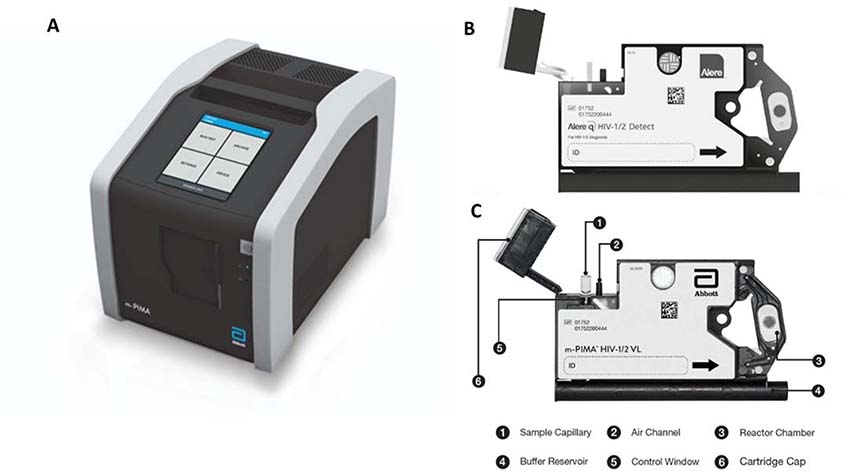
Abbott m-PIMA
Analyzer is designed to be compatible with the m-PIMA HIV-1/2 VL and Alere q HIV-1/2 sample preparation cartridges (Figure 8). The m-PIMA HIV-1/2 VL requires 50 μL of plasma per test run, whereas the Alere q is the juvenile / infant focused product which requires a very small amount of sample - 25 μL from a finger / heel prick to measure HIV 1/2 as early as birth of the infant.88–90 The cartridge utilizes solid phase extraction and the analyzer runs rapid RT-PCR with multiplexing capabilities, providing results in under an hour.88
Figure 8.
A. The m-PIMA Analyzer, compatible with both B. Alere q HIV-1/2 Detect and C. m-PIMA HIV-1/2 VL sample preparation cartridges.88,89
Specifically, after chaotropic agents lyse the virion, the released HIV-1 or HIV-2 viral genome hybridizes with biotin-oligonucleotides, resulting in capturing of the target sequences onto a surface of streptavidin-sepharose particles through biotin-streptavidin binding. Subsequent washing of the streptavidin-sepharose particles remove all contaminants bound non-specifically, leaving the sample ready for RT-PCR.88 The m-PIMA system is now being sold in selected countries, and have received CE-IVD and WHO-PQ. We are not aware of any published performance data for the m-PIMA HIV −1/2.
In a study with 443 participants in a primary care setting in Mozambique, the Alere q with 25 μL of whole blood input sample had sensitivity and specificity of 96.8% and 47.8% for a viral load cutoff of 1,000 copies/mL, which improved to 84.0% and 90.3% at a cutoff of 10,000 copies/mL.90
Testing for bloodborne pathogens as an unmet need
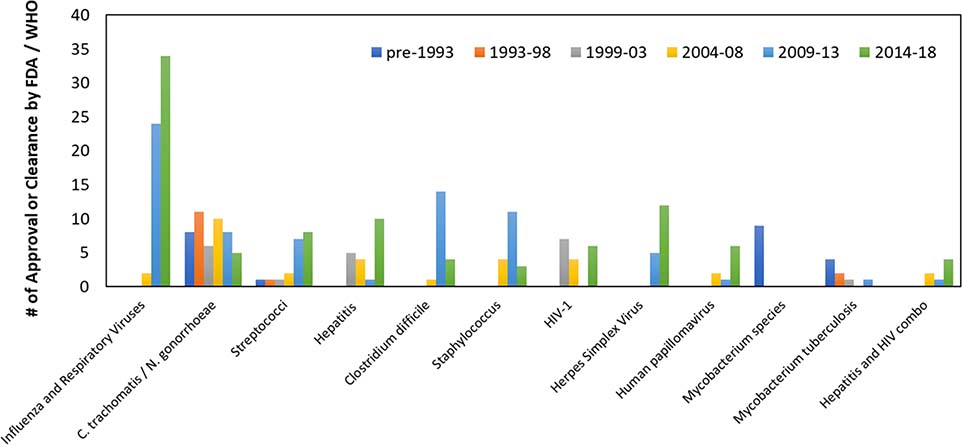
Figure 9 summarizes the number and variety of FDA- or WHO-endorsed NATs over time by major diseases for which the most number of tests have been developed. The total number of clearance or approvals continue to increase over time, with a greater variety of targeted pathogens, including respiratory, STD, and bloodborne diseases. Despite this increase, bloodborne pathogen NATs remain a significant gap without any CLIA-waived (or equivalence) test. Although Abbott, Cepheid, Roche, and DRW all have developed POC bloodborne pathogen NATs intended for the POC, greater efforts are required to simplify these NATs before they can be CLIA-waived.
Figure 9.
Trend of number FDA-cleared or WHO-prequalified nucleic acid tests over time for major disease areas. Cleared NA tests continue to increase over time with a greater variety of pathogens included.
Human blood is a useful sample for molecular testing due to the number of infectious microorganisms that can be found suspended in plasma or residing in host cells. Blood is a complex colloidal suspension comprised of mainly plasma, with a large percentage of its volume made up of red blood cells (40% to 45%) that can clog fluidic pathways and foul diagnostic devices. Plasma carries bloodborne pathogens and is the preferred sample for diagnostic operations due to the absence of cumbersome blood cells. The primary roadblock for the development of simple blood-based POC NATs is sample preparation. Blood is also rich with salts, proteins, and other biomolecules that complicate diagnostic operations, such as nucleic acid purification and downstream amplification assays.72 Perhaps one of the most troublesome aspects of using blood for diagnosing viral diseases is the activity if endogenous RNases which can rapidly degrade viral RNA targets.18 Chaotropic salt based solid phase extraction, that is the backbone of PCR sample prep for NATs, simultaneously performs lysis, protein denaturation, RNase deactivation, and RNA purification making it a potent sample prep method; however, it is has significant penalties in cost, complexity, and toxicity that hampers the development and of POC blood based NATs. We and others have been developing alternative sample preparation chemistry and devices to avoid the use of solid phase extraction.18,19,21
A simple, CLIA-waived, POC NAT must not require preliminary sample manipulation, so if plasma separation is used, it must be automated in the device. For example, the gold standard for HIV viral load is defined as HIV RNA copies in plasma, while what can be collected directly from the patients is whole blood. As a result, blood sample preparation will require automated metered whole blood collection, blood cell removal, viral lysis, and viral RNA extraction. Each step of processing should eliminate the risk of sample loss to dead space or undesired non-specific adhesion to the apparatus, without adding chemicals which could digest the target NA or inhibit the downstream amplification.
Summary
There are significant commercial NAT products and research pipeline targeting POC NATs. This review provided a technical overview of the currently available CLIA-waived and WHO-PQ products, and landscape review of potential hurdles and opportunities for further development towards simpler-to-use products that can be adopted at the low-resource POC. Furthermore, the overall number of patients with chronic infectious diseases such as HIV and HBV are growing due to the advancement of treatment and longer survival.27 Continuous monitoring and follow-ups at the POC for these chronic diseases become the critical unmet needs not satisfied with current NATs which require central lab practices.1 In addition, the latest eruption of infectious disease pandemic of COVID-19 and Ebola demands faster, simpler, and reliable diagnostic methods at the onset of infection for disease surveillance, public policy, re-opening the economy, and building safe workplace to reduce the risk of spreading.31 Current commercial systems, despite being “simple”, still require capital investment, maintenance and service plans, as well as regular follow-ups post-deployment for long-term benefits to the low resource settings.91 The business model and commercial value proposition is yet to justify the long development horizon and capital investment for some of the highest unmet needs in the lowest resource locations. Fostering POC NAT innovation requires alignment between the unmet needs, research efforts, and commercial collaboration between the manufacturer, R&D researchers, and local staff and government. With recognized opportunity and joint efforts, more patients will benefit from POC NAT.
Table 4.
Commercially available POC systems that are not CLIA-waived
| POC System | Test | Manufacturer | Source for inclusion | Integrated Sample Prep | Integrated Amplification |
|---|---|---|---|---|---|
| Alere q | HIV-1/2 | Abbott | WHO-PQ | Yes | Yes |
| Cobas Liat | C.diff | Roche | CLIA Moderate, 1 | Yes | Yes |
| Idylla | Respiratory Panel (IFV-RSV) | Janssen / Biocartis | CLIA Moderate, 1 | Yes | Yes |
| SAMBA II | HIV-1 | DRW | Approval in Kenya, Malawi and Uganda, CE Mark | Yes | Yes |
| Xpert Xpress | HPV, HCV, HIV-1 | Cepheid | WHO-PQ, 1 | Yes | Yes |
Acknowledgements
We thank A. Olanrewaju, B. Sullivan, Marta Suárez, and L. Lillis for graciously reviewing and providing feedback on the manuscript. This work was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R01EB022630 and R01EB022630-03S1. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.Niemz A, Ferguson TM and Boyle DS, Trends Biotechnol, 2011, 29, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Patent, US4683195A, Process for amplifying, detecting, and/or-cloning nucleic acid sequences, 1987.
- 3.Yolken RH, in Methods in Enzymology, eds. Wilchek M and Bayer EA, Academic Press, 1990, vol. 184, pp. 529–537.2388587 [Google Scholar]
- 4.Forward KR, Haldane D, Webster D, Mills C, Brine C and Aylward D, Can. J. Infect. Dis. Med. Microbiol, 2006, 17, 221–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leland DS and Ginocchio CC, Clin. Microbiol. Rev, 2007, 20, 49–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazelton PR, Gelderblom HR. Electron Microscopy for Rapid Diagnosis of Emerging Infectious Agents. Emerging Infectious Diseases. 2003;9(3):294–303. doi: 10.3201/eid0903.020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey & Scott’s Diagnostic Microbiology, 14th Edition - 9780323354820.
- 8.Hazen Kevin C., Non-Nucleic Acid–Based Identification Methods for Infectious Disease - Infectious Diseases, 2019. [Google Scholar]
- 9.Olano JP and Walker DH, Arch. Pathol. Lab. Med, 2011, 135, 83–91. [DOI] [PubMed] [Google Scholar]
- 10.Van Norman GA, JACC Basic Transl. Sci, 2016, 1, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center for Devices and Radiological Health, U.S. Food and Drug Administration, Nucleic Acid Based Tests, 2020 [Google Scholar]
- 12.CLIA | HIV Testing in Non-Clinical Settings | HIV Testing | HIV/AIDS | CDC, 2018. [Google Scholar]
- 13.Recommendations for Clinical Laboratory Improvement Amendments of 1988 (CLIA) Waiver Applications for Manufacturers of In Vitro Diagnostic Devices - Guidance for Industry and Food and Drug Administration Staff, U.S. Department of Health and Human Services, Food and Drug Administration, Docket number FDA-2017-D-5570, February 26, 2020. [Google Scholar]
- 14.Point-of-Care-Testing Checklist (Master), CAP Accreditation Program, College of American Pathologists, August 8, 2017. [Google Scholar]
- 15.Center for Devices and Radiological Health, U.S. Food and Drug Administration, Center for Devices and Radiological, Recommendations for Clinical Laboratory Improvement Amendments of 1988 (CLIA) Waiver Applications for Manufacturers of In Vitro Diagnostic Devices - Guidance for Industry and Food and Drug Administration Staff, 2003 [Google Scholar]
- 16.Centers for Disease Control, CLIA Test Complexities, 2019.
- 17.Press Announcements - FDA grants first CLIA waiver for nucleic acid-based flu diagnostic test, Alere i Influenza A & B Test (Direct Nasal swab only), Document Number CW140008. [Google Scholar]
- 18.Bender AT, Borysiak MD, Levenson AM, Lillis L, Boyle DS and Posner JD, Anal. Chem, 2018, 90, 7221–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bender AT, Sullivan BP, Lillis L and Posner JD, J. Mol. Diagn, 2020, 22, 1030–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan BP, Bender AT, Ngyuen DN, Zhang JY and Posner JD, J. Chromatogr. B, 2021, 1163, 122494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drain PK, Dorward J, Bender A, Lillis L, Marinucci F, Sacks J, Bershteyn A, Boyle DS, Posner JD and Garrett N, Clin. Microbiol. Rev, 2019, 32, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansuy J, Mengelle C, Pasquier C, et al. Zika Virus Infection and Prolonged Viremia in Whole-Blood Specimens. Emerging Infectious Diseases. 2017;23(5):863–865. doi: 10.3201/eid2305.161631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control, Health Effects & Risks | Zika Virus | CDC, 2019. [Google Scholar]
- 24.Schieffelin JS, Shaffer JG, Goba A, Gbakie M, Gire SK, Colubri A, Sealfon RSG, Kanneh L, Moigboi A, Momoh M, Fullah M, Moses LM, Brown BL, Andersen KG, Winnicki S, Schaffner SF, Park DJ, Yozwiak NL, Jiang P-P, Kargbo D, Jalloh S, Fonnie M, Sinnah V, French I, Kovoma A, Kamara FK, Tucker V, Konuwa E, Sellu J, Mustapha I, Foday M, Yillah M, Kanneh F, Saffa S, Massally JLB, Boisen ML, Branco LM, Vandi MA, Grant DS, Happi C, Gevao SM, Fletcher TE, Fowler RA, Bausch DG, Sabeti PC, Khan SH, Garry RF, KGH Lassa Fever Program, Viral Hemorrhagic Fever Consortium, and WHO Clinical Response Team, N. Engl. J. Med, 2014, 371, 2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The World Health Organization, Ebola Situation Report - 15 July 2015 | Ebola, 2018. [Google Scholar]
- 26.Office of the Commissioner, U.S. Food and Drug Administration, Zika Virus response Updates from FDA, 2020. [Google Scholar]
- 27.Our World in Data, HIV / AIDS, 2019. [Google Scholar]
- 28.WHO | Viral hepatitis: a hidden killer gains visibility, 2019. [Google Scholar]
- 29.Ren P, Ortiz DA, Terzian ACB, Colombo TE, Nogueira ML, Vasilakis N and Loeffelholz MJ, J. Clin. Microbiol, 2017, 55, 2198–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song J, Mauk MG, Hackett BA, Cherry S, Bau HH and Liu C, Anal. Chem, 2016, 88, 7289–7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broadhurst MJ, Brooks TJG and Pollock NR, Clin. Microbiol. Rev, 2016, 29, 773–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Center for Devices and Radiological Health, U.S. Food and Drug Administration, _Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency (Revised), docket number FDA-2020-D-0987. [Google Scholar]
- 33.Center for Devices and Radiological Health, U.S. Food and Drug Administration, Emergency Use Authorization of Medical Products and Related Authorities, OMB Control No. 0910–059 [Google Scholar]
- 34.WHO | WHO Global Atlas of medical devices, The World Health Organization, 2017. [Google Scholar]
- 35.Overview of the WHO Prequalification of in Vitro Diagnostics Assessment - Prequalification of in Vitro Diagnostics, The World Health Organization, 2018. [Google Scholar]
- 36.Morin S, Bazarova N, Jacon P and Vella S, Clin. Infect. Dis, 2018, 66, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO | Prequalification of in vitro diagnostics, The World Health Organization, 2019. [Google Scholar]
- 38.WHO | Guidance for procurement of in vitro diagnostics and related laboratory items and equipment, The World Health Organization, 2017, ISBN: 978–92-4–151255-8 [Google Scholar]
- 39.WHO | In lead-up to World AIDS Day, WHO prequalifies second HIV self-test, The World Health Organization, 2019. [Google Scholar]
- 40.Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai W, Rodriguez W and Bassett IV, Lancet Infect. Dis, 2014, 14, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hänscheid T, Rebelo M and Grobusch MP, Lancet Infect. Dis, 2014, 14, 922. [DOI] [PubMed] [Google Scholar]
- 42.Azar MM and Landry ML, J. Clin. Microbiol, 2018, 56, e00367–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Office of the Commissioner, Classification and Jurisdictional Information - Intercenter Agreement Between the Center for Biologics Evaluation and Research and the Center for Devices and Radiological Health, U.S. Food and Drug Administration, 2019.
- 44.Center for Devices and Radiological Health, U.S. Food and Drug Administration, Premarket Approval (PMA), 2019. [Google Scholar]
- 45.U.S. Food and Drug Administration, Guidance In the Manufacture and Clinical Evaluation of In Vitro Tests to Detect Nucleic Acid Sequences of HIV Types 1 and 2, 1999 [Google Scholar]
- 46.Tobin P, Clinical Laboratory Improvement Amendments of 1998 (CLIA) Waiver Applications Draft Guidances, 1998. [Google Scholar]
- 47.Urdea M, Penny LA, Olmsted SS, Giovanni MY, Kaspar P, Shepherd A, Wilson P, Dahl CA, Buchsbaum S, Moeller G and Hay Burgess DC, Nature, 2006, 444 Suppl 1, 73–79. [DOI] [PubMed] [Google Scholar]
- 48.WHO | WHO list of prequalified in vitro diagnostic products, 2018. [Google Scholar]
- 49.Wu G and Zaman MH, Bull. World Health Organ, 2012, 90, 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen S, Lemieux B, Wang Z, Kopczynski KR, Chen L. Sample Processing PAT:US7718421.
- 51.Melchers WJG, Kuijpers J, Sickler JJ and Rahamat-Langendoen J, J. Med. Virol, 2017, 89, 1382–1386. [DOI] [PubMed] [Google Scholar]
- 52.Tanriverdi S, Chen L and Chen S, J. Infect. Dis, 2010, 201 Suppl 1, S52–58. [DOI] [PubMed] [Google Scholar]
- 53.Gibson J, Schechter-Perkins EM, Mitchell P, Mace S, Tian Y, Williams K, Luo R and Yen-Lieberman B, J. Clin. Virol, 2017, 95, 5–9. [DOI] [PubMed] [Google Scholar]
- 54.Ness JV, Ness LKV and Galas DJ, Proc. Natl. Acad. Sci, 2003, 100, 4504–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nie S, Roth RB, Stiles J, Mikhlina A, Lu X, Tang Y-W and Babady NE, J. Clin. Microbiol, 2014, 52, 3339–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore N, Alere i Isothermal Amplification, 2013. [Google Scholar]
- 57.St John A and Price CP, Clin Biochem Rev, 2014, 35, 155–167. [PMC free article] [PubMed] [Google Scholar]
- 58.Van Ness J, Van Ness LK and Galas DJ, Proc. Natl. Acad. Sci, 2003, 100, 4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poritz MA, Blaschke AJ, Byington CL, Meyers L, Nilsson K, Jones DE, Thatcher SA, Robbins T, Lingenfelter B, Amiott E, Herbener A, Daly J, Dobrowolski SF, Teng DH-F and Ririe KM, PLOS ONE, 2011, 6, e26047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karsten H, Manfred E, Detlev R. Process for the determination of in vitro amplified nucleic acids. PAT:US5871908.
- 61.Leber AL, Everhart K, Daly JA, Hopper A, Harrington A, Schreckenberger P, McKinley K, Jones M, Holmberg K and Kensinger B, J. Clin. Microbiol,, DOI: 10.1128/JCM.01945-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soundiram I, GeneXpert Technology, 21. [Google Scholar]
- 63.Nagai H, Murakami Y, Morita Y, Yokoyama K and Tamiya E, Anal. Chem, 2001, 73, 1043–1047. [DOI] [PubMed] [Google Scholar]
- 64.Taylor MT. Apparatus and method for rapid disruption of cells or viruses. PAT:US6739531.
- 65.Ho YII, Wong AH and Lai RWM, J. Med. Microbiol, 2018, 67, 1576–1580. [DOI] [PubMed] [Google Scholar]
- 66.Cary RB. Highly simplified lateral flow-based nucleic acid sample preparation and passive fluid flow control. PAT:US 9207236.
- 67.DeJohn M, Cary RB, Cobb NJ. Integrated device for nucleic acid detection and identification. PAT:CA2853615.
- 68.Cai H and Cobb NJ. Oscillating amplification reaction for nucleic acids. PAT:US10316358.
- 69.Mesa Biotec Inc. Influenza A and Influenza B Multiplex Nucleic Acid Assay. FDA#K171641. [Google Scholar]
- 70.Information on Collection of Respiratory Specimens for Influenza Virus Testing | CDC, August 24, 2020. [Google Scholar]
- 71.Unexplained Respiratory Outbreaks | Specimen Collection Guidelines| URDO | CDC, August 24, 2020. [Google Scholar]
- 72.Sidstedt M, Hedman J, Romsos EL, Waitara L, Wadsö L, Steffen CR, Vallone PM and Rådström P, Anal. Bioanal. Chem, 2018, 410, 2569–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Soud WA and Rådström P, J. Clin. Microbiol, 2001, 39, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schrader C, Schielke A, Ellerbroek L and Johne R, J. Appl. Microbiol, 2012, 113, 1014–1026. [DOI] [PubMed] [Google Scholar]
- 75.Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices, FDA, August 9, 2020. [Google Scholar]
- 76.Kumar S and Henrickson KJ, Clin. Microbiol. Rev, 2012, 25, 344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.BioFire Diagnostics FilmArray, January 9, 2019. [Google Scholar]
- 78.Buccambuso M, Cook C, Hoge J, Bishop B, Andjelic C, Amiott B, Holmberg K and Lingenfelter B, 1. [Google Scholar]
- 79.Gibson J, Schechter-Perkins EM, Mitchell P, Mace S, Tian Y, Williams K, Luo R and Yen-Lieberman B, J. Clin. Virol, 2017, 95, 5–9. [DOI] [PubMed] [Google Scholar]
- 80.Cepheid. Group A, C and G Beta-Hemolytic Streptococcus Nucleic Acid Amplification System. FDA#K172126. [Google Scholar]
- 81.HIV viral load and early infant diagnosis selection and procurement informaiton tool. Sourcing and Management of Health Products. The Global Fund August 24, 2020. [Google Scholar]
- 82.Lee HH, Dineva MA, Chua YL, Ritchie A, Ushiro-Lumb I and Wisniewski CA, J. Infect. Dis, 2010, 201, S65–S71. [DOI] [PubMed] [Google Scholar]
- 83.Lee HH, Dineva MA, Fletcher-Brown FFS. Nucleic acid amplification and testing. PAT:US20100136542.
- 84.Compton J, Nature, 1991, 350, 91–92. [DOI] [PubMed] [Google Scholar]
- 85.Kacian DL, and Fultz TJ. Nucleic acid sequence amplification methods. PAT:US5399491.
- 86.Guatelli JC, Whitfield KM, Kwoh DY, Barringer KJ, Richman DD and Gingeras TR, Proc. Natl. Acad. Sci. U. S. A, 1990, 87, 1874–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ritchie AV, Ushiro-Lumb I, Edemaga D, Joshi HA, De Ruiter A, Szumilin E, Jendrulek I, McGuire M, Goel N, Sharma PI, Allain J-P and Lee HH, J. Clin. Microbiol, 2014, 52, 3377–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.WHO Prequalification of In Vitro diagnostics public report: AlereTM q HIV-1/2 Detect. WHO reference number: PQDx 0226–032-00. June 2016. [Google Scholar]
- 89.m-PIMATM HIV-1/2 Detect, Abbott. August 24, 2020. [Google Scholar]
- 90.Jani IV, Meggi B, Vubil A, Sitoe NE, Bhatt N, Tobaiwa O, Quevedo JI, Loquiha O, Lehe JD, Vojnov L and Peter TF, J. Clin. Microbiol, 2016, 54, 2104–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Drain PK, Hyle EP, Noubary F, Freedberg KA, Wilson D, Bishai WR, Rodriguez W and Bassett IV, Lancet Infect. Dis, 2014, 14, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor MT. Apparatus and method for rapid disruption of cells or viruses. PAT:US6739531.
- 93.Accula System. Mesabiotech August 24, 2020. [Google Scholar]
- 94.Ritchie AV, Goel N, Sembongi H, Lehga J, Farleigh LE, Edemaga D, Wisniewski CA and Lee HH, J. Virol. Methods, 2016, 237, 143–149. [DOI] [PubMed] [Google Scholar]
- 95.Ritchie AV, Ushiro-Lumb I, Edemaga D, Joshi HA, De Ruiter A, Szumilin E, Jendrulek I, McGuire M, Goel N, Sharma PI, Allain J-P and Lee HH, J. Clin. Microbiol, 2014, 52, 3377–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]