Abstract
The gastrointestinal (GI) tract is a vitally important site for the adsorption of nutrients as well as the education of immune cells. Homeostasis of the gut is maintained by the interplay of the intestinal epithelium, immune cells, luminal antigens, and the intestinal microbiota. The wellbeing of the gut is intrinsically linked to the overall health of the host, and perturbations to this homeostasis can have severe impacts on local and systemic health. One factor which causes disruptions in gut homeostasis is age, and recent research has elucidated how critical systems within the gut are altered during the aging process. Intestinal stem cell proliferation, epithelial barrier function, the gut microbiota, and the composition of innate and adaptive immune responses are all altered in advanced age. The aging population continues to expand worldwide, a phenomenon referred to as the “Silver Tsunami,” and every effort must be made to understand how best to prevent and treat age-related maladies. Here, we review recent research about changes observed in the intestinal epithelium, the intestinal immune system, the microbiota, and how the aging gut interacts with and influences other organs such as the liver, lung, and brain. Better understanding these age-related changes and their impact on multi-organ interactions will aid the development of therapies to increase the quality of life for all aged individuals.
Keywords: microbiome, inflammaging, epithelium, inflammation, lung, liver
Graphical Abstract
Summary sentence:
Review of how advanced age alters the gut, the microbiome, and how these alterations impact overall health
Introduction
The global population is aging. According to the World Health Organization, 8.5% of the population is >65 years old, and this proportion is expected to nearly triple by 20501. This massive increase in the aged population will likely coincide with an increased strain on hospitals and assisted living facilities. Gaining a better understanding of how advanced age impacts overall health will allow for targeted therapies to alleviate some of this burden. One arena in which age is appreciated to have a large impact is the health of the gut. The intestine plays a critical role in health and wellbeing2, 3, as well as critical illness4. Advanced age negatively impacts epithelial barriers5. In the gut, this involves exhaustion of intestinal stem cells6 along with aberrant proliferation and differentiation,7 causing an age-dependent delay in the recovery of this organ after injury. Research into non-invasive biomarkers of gut barrier dysfunction8 has allowed investigators to conduct studies in patients utilizing blood and feces to monitor gut barrier integrity and intestinal function9, and develop treatments to restore the barrier10. Intestinal fatty acid binding protein (iFABP) is a gut-specific bio-marker for intestinal epithelial damage that can be readily measured both in the blood and urine following intestinal ischemia11 and necrotizing enterocolitis12–14. Glutathione S-transferases in the blood and urine may also indicate intestinal epithelial damage15 but may also indicate damage to liver and kidneys9. Lipopolysaccharide (LPS) measured in the circulation indicates an increase in microbial translocation, but these tests are highly sensitive and prone to false-positives16. Endotoxin core antibody assays are also used as an indirect measure of increased bacterial translocation17, as is sCD14, which indicates an inflammaging-associated increase in monocyte activation18, 19. iFABP, LPS, and sCD14 are all increased in advanced age in humans20. Pro-inflammatory cytokines, such as IL-6, IL-15, and IL-8, and C-reactive protein, are also increased in advanced age as reviewed elsewhere21. Taken together, these findings describe a correlation between increased age-related inflammation and decreases in intestinal barrier function.
Along with the physical barrier of tight junctions that connect epithelial cells in the intestine, there are also chemical barriers generated by antimicrobial peptides (AMPs)22. These peptides are evolutionarily conserved, natural antibiotics produced by immune and epithelial cells in the gut. One AMP, regenerating islet-derived protein 3-gamma, or Reg3γ, is thought to prevent microbiota from invading intestinal epithelial cells23 by creating spatial segregation of microorganisms within the gut24. Reg3γ carries out its protective, bactericidal functions by binding to peptidoglycan in the cell wall of gram-positive bacteria25 and forming a hexameric membrane-permeabilizing oligomeric pore26. When produced in appropriate amounts by Paneth cells in the intestinal crypts of the small intestine, Reg3γ helps maintain homeostasis of the microbiome23. Interestingly, AMP upregulation is regulated by cytokines and the expression of multiple AMPs is altered with advanced age27. Moreover, evidence in elderly humans and in rodent models of aging show that even in the absence of injury there are dramatic changes in fecal microbiota relative to younger subjects28, 29(discussed in greater detail below). Although there may be significant changes to the microbiota in age, these changes in specific gut microbial populations do not establish causation of disease. These observations reveal that multiple intestinal parameters are altered with age. Despite recent research efforts, our current understanding of the mechanisms behind these changes in the aging gut is still lacking. Here, we review age-related changes to the intestinal epithelium, immune cells within the gut, and how age impacts the interactions between the gut and distant organ systems such as the liver, lung, and brain.
I. Alterations in intestinal epithelial cells in aging
The intestinal epithelium is a single cell layer that serves as a physical barrier separating the microbiota and other luminal contents from the intestinal tissue. Intestinal epithelial cells (IECs) are connected by intercellular tight junction proteins such as occludin and zona occludens-1 (ZO-1), which serve to regulate the migration of materials between the cells30. Maintenance of the intestinal barrier function is vital to the health of the host31. The compromise of this barrier coincides with increased prevalence of damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs)32, and microbe-associated molecular patterns (MAMPs)33 in the intestine, all of which are associated with chronic immune activation, which has broad local and systemic consequences. The compromise of the intestinal barrier is also known as ‘leaky gut34.’ Locally, impairment of the intestinal barrier has been linked to inflammatory bowel disease (IBD)35, colorectal cancer36, celiac disease37, and metabolic disorders like obesity and diabetes38. Recently, advances in available technology, such as intestinal organoids39, 40, have allowed researches to interrogate the effects of aging on the intestinal barrier, as reviewed previously41.
Intestinal stem cells
All subtypes of the intestinal epithelium are derived from intestinal stem cells (ISCs) that reside in intestinal crypts. ISCs reside either in the crypt base as crypt base columnar cells, or at the +4 position from the base of the crypt as +4 label retaining stem cells, which can repopulate the crypt base columnar cells should they be lost due to damage42 (Figure 1). ISCs asymmetrically divide to give rise to two daughter cells, a nascent ISC and a rapidly dividing and maturing cell that occupies the so-called transit amplifying compartment. The ISC and their progeny enable the intestinal epithelium to renew itself every 3–5 days43. As the transit amplifying cells divide and mature, most intestinal epithelial cells travel up the “epithelial conveyer” of the crypt, differentiate, and eventually are sloughed off into the lumen44. As the host ages, however, the capacity for proliferation and self-renewal of ISCs is greatly diminished, as shown by decreased growth of intestinal organoids derived from old mice when compared to young mice6. In addition to decreased growth, ISCs in older mice also highly express a pro-apoptotic gene profile, indicating decreased survival of these cells6. As a consequence of decreased growth and survival, aged mice also exhibit a decreased ability to heal from experimentally induced intestinal damage45.
Figure 1. Alterations in the intestinal epithelium and immune cells with advanced age.
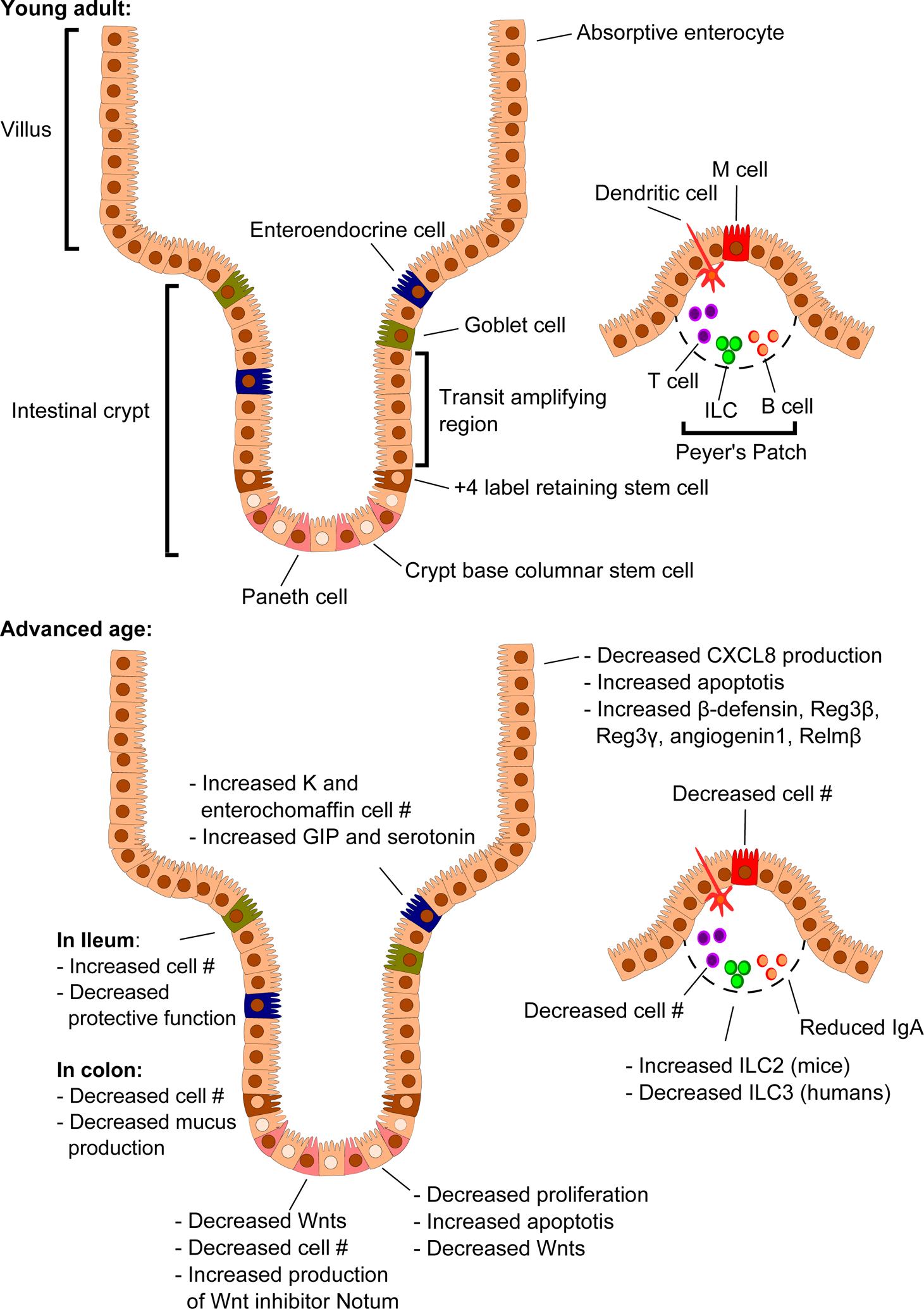
The intestinal epithelium is arranged into crypts and villi. Crypt base columnar stem cells reside at the base of the crypt and asymmetrically divide to give rise to stem cells and rapidly dividing cells that make up the transit amplifying region, which differentiate as they move up the crypt. The +4 label retaining, or “reserve,” stem cells reside at the boundary between the crypt base columnar stem cells and the transit amplifying region and can repopulate the crypt base columnar stem cells if they are lost. As the host enters advanced age, a multitude of changes occur, leading to overall increased inflammation.
GIP: Glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide
ILC : Innate lymphoid cell
IgA : Immunoglobulin A
The self-renewal capacity of ISCs is maintained by a gradient of canonical Wnt proteins that increases at the base of the intestinal crypts46, 47. This Wnt gradient is provided by subepithelial mesenchymal cells48, 49 and functions to maintain expression of stem-cell markers in the ISC niche50. The loss of this canonical Wnt gradient at the base of the crypt leads to a complete ablation of intestinal epithelium50, highlighting the vital role of intestinal Wnts. Although the exact mechanisms are not yet known, canonical Wnt proteins, including Wnt3a, are decreased in ISCs, Paneth cells, and subepithelial mesenchymal cells in aged mice when compared to their younger counterparts51. Further, supplementing intestinal organoid cultures derived from aged mice and humans with Wnt3a restored their growth capacity to that of organoids generated from younger subjects51. One explanation for the altered expression of Wnt and Wnt targets is age-related epigenetic changes. One study using murine organoids derived from the small intestine of aged mice found that the stem cell marker lgr5 was epigenetically silenced by histone H3 lysine 27 trimethylation, causing a decrease in cell proliferation within those organoids52. Another study used long term culture of murine colonic organoids to mimic aging and found that the organoids underwent multiple epigenetic changes that led to activated Wnts and mirrored a pro-cancer phenotype53.
As the epithelia proliferate, they migrate to the top of the crypt and become exposed to decreasing concentrations of canonical Wnt and increasing levels of soluble factors called bone-morphogenetic proteins (BMPs)54, 55. BMPs function to inhibit the expression of canonical Wnts, allowing for epithelial cell differentiation and maturation into a number of different cell lineages with important functions in addition to being a physical barrier.
Absorptive enterocytes
Absorptive enterocytes are the most abundant of the IECs. These cells express catabolic enzymes on their luminal surface to digest a variety of molecules, including water, ions, and nutrients, to allow for cell uptake56. Enterocytes also express Toll-like receptors (TLRs) 2, 3, 4, 5, and 957, 58 that, when engaged, initiate production of cytokines and chemokines that activate nearby immune cells57. In aged individuals, however, there is an altered production of cytokines by the intestinal epithelium. Notably, when human epithelial biopsies taken from the ileum of elderly donors were exposed to flagellin, CXCL8 production was decreased59, although there was no notable age-related reduction in TLR5. CXCL8 is an important leukocyte chemoattractant produced by intestinal epithelium in response to bacterial entry60, and its reduction in the aged ileum suggests a decreased ability to respond to bacterial infection. Enterocyte TLR engagement also stimulates production of soluble mediators that exert antimicrobial effects, including iNOS61 and β-defensin62. The production of β-defensin, along with other C-type lectins Reg3β, Reg3γ, angiogenin, and resistin-like molecule beta (Relmβ) are significantly up-regulated in the ileum of aged mice63. Reg3β and Reg3γ are constitutively expressed in the intestines and display antimicrobial activity when upregulated in response to bacterial sensing64–66. Angiogenin, a potent stimulator of angiogenesis, acts within the intestines to promote IEC survival and proliferation67. Relmβ maintains IEC barrier function and regulates expression of Reg3β and Reg3γ68. Increased levels of these C-type lectins in aged mice suggest a constant stress response and an attempt to restore the intestinal barrier. Enterocytes also express MHC class II, allowing the intestinal epithelium to act as nonconventional antigen presenting cells69. Absorptive enterocytes in the duodenum of healthy, elderly human donors were shown to have increased levels of apoptosis and proliferation70, perhaps contributing to an impairment of their usual functions.
Goblet cells
Goblet cells are specialized mucus-secreting IECs that reside in both the small and large intestines71. The mucus layer is composed primarily of Muc2, a highly O-glycosylated protein produced by goblet cells. The mucus layer of the small intestine forms a single, loose layer that extends from the tips of the intestinal villi down to the base of the crypts72. The colon, which houses a much higher density of luminal microbes than the small intestine, has a higher number of goblet cells, resulting in two distinct mucus layers. The inner, firm mucus layer is comprised of polymerized, microbe free Muc2 that is anchored to the epithelium73. Muc2 associated with the inner mucus layer is proteolytically processed by the host and microbiota to produce the outer, loose mucus layer that houses a variety of microbial species. The mucus layer is part of a larger structure called the glycocalyx, which is a filamentous mesh of glycolipids and glycoproteins that forms a barrier between the epithelium and bacteria74. How aging impacts the gut mucus layer appears to differ between spatial regions of the small intestine. The gastric and duodenal mucus layer is not significantly different between young and old human subjects75 but was slightly thicker in the ileum of aged mice, coinciding with an increase in goblet cells76. Despite this increase in goblet cells and mucus production in the ileum of aged mice, there is an increase in epithelial associated bacteria, indicating a decline in the protection offered by the mucus layer77. The most striking difference in mucus production in mice is seen in the colon, where there is a stark decline in the thickness of the mucus layer77, 78 and number of goblet cells77, which paralleled broad changes in the expression of immune cell markers and the composition of the microbiota79. Interestingly, male mice are more susceptible to age-related reductions in colonic mucus thickness than female mice79, perhaps leading to increased susceptibility to dysbiosis and related inflammation.
Enteroendocrine cells
Enteroendocrine cells are another subtype of IECs that secrete a variety of intestinal hormones such as cholecystokinin and glucagon-like peptides (GLP) −1 and −2 that help to control the digestive function of the gut80. These intestinal hormones also regulate the production of the neurotransmitter serotonin80. In addition to these roles, cholecystokinin also regulates differentiation81 and cytokine production of CD4+ T-cells82 and B cells83. In aging mice, there is an increased number and activity of K cells, a specialized enteroendocrine cell population that secretes glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide (GIP), a molecule that increases fat accumulation84. In the ileum of older humans, there is an increase in the number of enterochromaffin cells85, which primarily secrete serotonin80. This increase is theorized to be a compensatory mechanism to attempt to rescue age-related attenuation in afferent nerve sensitivity85.
M cells
Microfold or M cells are specialized epithelial cells in the small intestine that allow for immune cell sampling of luminal content antigens by closely associated antigen-presenting cells. These antigen-presenting cells then deliver acquired luminal antigens to the Peyer’s Patches (PP) in the small intestine and are critical for the induction of antigen-specific immuno-globulin A (IgA) production86. The number of mature M cells in the follicle-associated epithelium in PP of aged mice are significantly reduced, leading to compromised uptake of particulate antigen from the gut lumen87. Additionally, there is a marked reduction in antigen-specific antibody production by intestinal B cells, reduced T-cell proliferation, and reduced antigen specific T-cell cytokine production in aged mice88, which parallels the reduction in M cell numbers.
Paneth cells
Paneth cells are another specialized epithelial cell in the small intestine that produce AMPs and have granules containing IL-17A44, 89 and Wnt346. While Paneth cells may be seen during idiopathic inflammatory bowel disease in the colon, they are generally localized to the small intestine90. Aged mice have decreased small intestinal expression of lysozyme, a Paneth cell marker77, indicating a possible reduction in their numbers. However, Paneth cells from aged mice have increased production of Notum, a Wnt inhibitor that impairs regeneration of the aged intestinal epithelium91.
II. Age associated changes in gut immune cells
As mentioned above, the gut is a vitally important site for the education of immune cells92. Immune cell education within the gut is dependent on the presence of microbes92, a bolus of which neonates receive during vaginal birth93. The microbial community remains relatively unstable in humans until around 2 or 3 years of age94–96. During this period of time, microbial stimulation of immune cells supports the development of PP, mesenteric lymph nodes, and isolated lymphoid follicles97. Further, this microbial stimulation promotes the maturation and recruitment of B and T cells into PP and the lamina propria (LP), a loose connective tissue layer that resides under the epithelium98. In the ileum and colon of aged mice, however, there is a significant reduction in innate and adaptive immune genes, including tlr4, cd3ε, cd4, and cd877, indicating a reduction in overall T cells in the aging intestine. In addition to these alterations, neutrophil recruitment in response to Clostridium difficile infection is markedly reduced in middle-aged mice99, leading to increased mortality. Innate lymphoid cells (ILCs) are also an important cell subset within the intestines of mice and humans and play a critical role in the maintenance of barrier function and the initiation of immune responses against pathogens100. In aged human donors, it was discovered that ILC3s, the ILCs that phenotypically mirror Th17 cells, are decreased in the intestine when compared to young donors101. In aged mice, however, the major difference in intestinal ILCs appears to be an increase in ILC2s102, the ILCs that phenotypically mirror Th2 cells.
Gut microbes induce the development of gut-resident T-regulatory cells (Tregs) in the mesenteric lymph nodes that are vitally important in establishing oral tolerance to ingested food antigens and commensal microbes103. Likewise, antigens from the gut microbiota promote the differentiation of B cells into IgA-producing plasma cells104; secretory IgA promotes gut homeostasis through a number of mechanisms including bacterial disruption, neutralization of bacterial toxins, and blocking the bacterial invasion of the lumen105–107. Aged mice display a drastic reduction in intestinal IgA expression77, contributing to the attenuation of its protective effects.
The microbiota also contains potentially pathogenic bacteria that can contribute to gastrointestinal inflammation and perpetuation of IBD if they become too numerous, or are able to travel into the tissue where they are sensed by the immune system108, perhaps as a consequence of disrupted barrier function in aged individuals. The outgrowth of opportunistic pathogens, or microbes, which may be beneficial in normal abundance that can become pathogenic when overgrown, within the gut microbiota correlates with autoimmune disease outside of the intestines. For example, the outgrowth of Prevotella copri correlates with increased susceptibility to auto-reactive T-cell mediated rheumatoid arthritis109, 110. Further, the gut microbiome is required for the induction and progression of mouse experimental auto-immune encephalomyelitis (EAE)111. The composition of microbiota in the gut modulates the effectiveness of a variety of cancer immunotherapies through regulating the differentiation of T cell subsets112–115.
III. Aging and the gut microbiome
Humans and commensal microbial communities have co-evolved over millions of years, developing a complex and mutually beneficial metabolic dialogue that regulates physiological processes and maintains homeostasis. The importance of this relationship is perhaps most evident in the lower GI tract, where bacteria play roles in nutrient absorption, immune function, synthesis of essential vitamins, drug processing, circadian rhythms, and insulin signaling, among other critical functions116. Impressive numbers help to illustrate the scale of this interdependence: It is generally estimated that the human gut is home to 1013–1014 microorganisms, outnumbering human cells in the entire body. Together, they weigh approximately 1–2 kg, similar to organs like the liver and brain, and contain over 100 times as many genes as in the human genome117, 118.
As humans age, the host-microbiome relationship undergoes changes that have important implications for frailty and disease119. In most cases, these changes are thought to reflect dysbiosis – imbalances in microbial species and a reduction in overall microbial diversity that is detrimental to host fitness. However, given that gut bacteria do not age in the same way as host organs and the numerous variables that influence their relative abundance, a fundamental question about this relationship arises. Do changes in the gut microbiome contribute to phenotypic changes in the host over time, or vice versa? In other words, it is still unclear whether specific age-related changes in microbiota occur independently, as a maladaptive response to other age-related physiologic changes in the host, or as a beneficial, compensatory response120. Answering this question and identifying mechanistic links to disease and overall functional decline are current areas of research focus. While it is difficult to isolate age-dependent effects, longitudinal human studies and animal models utilizing fecal microbiota transplant (FMT) have been useful. Ultimately, this field seeks to identify ways of manipulating the gut microbiome to buffer the normal effects of aging, reduce age-related disease burden, and extend the human lifespan.
Spatial variation of the human gut microbiome in form and function
The gut microbiome is dominated by bacteria, but also includes fungi, viruses, and archaea. Bacteria belonging to the phyla Firmicutes and Bacteroidetes are the most abundant, accounting for 80–90% of the total microbiota94. Proteobacteria, Actinobacteria, Fusobacteria, Cyanobacteria, and Verrucomicrobia are less abundant. At the genus, species, and strain level, significant variation exists between individuals, influenced by several environmental and genetic factors.
Microbiome composition and function also vary based on location in the lower GI tract. Distinct spatial niches are created by proximal-to-distal gradients of oxygen, dietary nutrients, and pH, leading to colonization with bacteria engaging in distinct activities121. The small intestine mainly contains facultative anaerobes that compete with host epithelial transporters for dietary nutrients. The large intestine has far greater microbial diversity, density, and host-microbiome interactions. The majority of colonic bacteria are obligate anaerobes that metabolize insoluble complex carbohydrates, generating absorbable, high-energy short-chain fatty acids (SCFAs), essential amino acids, and vitamins, including B12 and folate, and secondary bile acids122. SCFAs play crucial roles in gut health, as discussed later in this section, but are produced in lower quantities and variable ratios in elderly versus young individuals28, 123. Colonic bacteria also play a role in the activation of satiety pathways124, suggesting that age-related changes may play a role in chronically reduced appetite seen in the elderly.
Age-related dysbiosis: changes in microbiome composition in the elderly
Microbial colonization of the GI tract starts at birth. In infants and children, method of delivery, breastfeeding, and antibiotic exposure can all influence the microbiome, with effects lasting into adulthood125, 126. The gut is initially colonized by facultative anaerobes, which creates a reduced environment appropriate for later colonization with obligate anaerobes126. Microbiome diversity continues to increase until approximately the age of 3 years, after which, it is relatively stable, with variations attributed to environmental factors such as diet or antibiotic exposure94. However, as individuals age, a mirrored process of senescence occurs, leading to declines in microbial diversity and stability127.
The elements that separate age-related dysbiosis from a ‘healthy’ gut microbiome are somewhat controversial, though overarching patterns have been identified by human population studies. Key phenomena include compositional instability, reduced overall diversity, and an increase in pro-inflammatory opportunistic pathogens128, 129, linked to declining immune function,28 and increased frailty in elderly populations119, 130. Specific trends include an increase in facultative anaerobic bacteria and a decrease in SCFA-producers in the colon127. In addition, some phylum and genus-level patterns have emerged in association with health, disease, and lifespan. Dysbiosis and clinical disease have generally been associated with an increase in Proteobacteria129, a phylum containing pathogenic bacteria associated with intestinal inflammation129, 131, and a corresponding decrease in Firmicutes. Pathogenic bacteria are normally kept in check by commensal microbes and AMPs secreted by both commensals and host cells. One such mechanism involves intestinal expression of Reg3β, an AMP that plays a role in maintaining homeostasis of the gut microbiome as described above23, 24, 132. Changes in the relative abundance of Bacteroidetes are much more variable, with some studies reporting higher levels in elderly subjects,119, 128, 133 and others reporting lower levels123, 134–137. Studies that observed an age-dependent increase in Bacteroidetes also noted a corresponding decrease in Firmicutes119, 128, 133.
Investigators have also noted trends in less abundant bacterial populations with age. A seminal study profiled the gut microbiomes of 161 elderly individuals using 16S rDNA sequencing and found that the genera Bacteroides, Alistipes, and Parabacteroides comprised 8–27% of the microbiome in a younger cohort, but more than 50% in those over the age of 65 years119. Of note, there was also relatively greater inter-individual, genus-level variability seen in the elderly gut microbiome. In addition, several studies have shown that the abundance of Bifidobacteria, which are generally believed to exert gut health benefits, decreases in the elderly123, 138–141. As with Bacteroidetes, investigators have reported variable results for bacteria belonging to the genus Lactobacilli. Some groups report increases in old age123, 136, while others report decreases139. These differences can partially be explained by significant inter-individual variations in diet, lifestyle, and environmental exposures. Technical differences in measuring relative microbial abundances between studies may also be a contributing factor127. Ongoing advances in sequencing and metagenomic technology will undoubtedly enhance the accurate and reproducible measurement of microbial species abundance and allow us to gain a better understanding of aging and the microbiome.
One approach used to study connections between age and the microbiome is to examine changes in gut flora at the limits of age. Centenarians and supercentenarians (those who have reached or surpassed the ages of 100 and 110 years, respectively), have been the focus of several investigations attempting to identify factors contributing to extreme longevity, a surrogate measure of health. The lower GI tracts of these individuals appear to be colonized by a disproportionately high number of health-associated bacterial populations, such as SCFA-producers142–144. Biagi et al. examined the fecal microbiome of young adults (22–48 years old), elderly adults (65–75 years old), and semi-supercentenarians (105–109 years old) in an Italian population142. They found that the fecal microbiota in all age groups was dominated by just three families – Bacteroidaceae, Lachnospiraceae, and Ruminococcaceae. However, the relative abundance of bacteria belonging to these families decreased with age. In other words, extreme aging in humans was associated with an increase in certain other bacterial genera, including Bifidobacterium, Akkermansia, and Christensenellaceae. One possible explanation for this shift is that these bacteria may promote healthy aging. Another striking finding was that the overall difference in microbiome composition between elderly individuals and centenarians appear to be greater than that between young adults and elderly adults, even though the latter two groups were separated in age to a greater degree.
Signatures of age-related disease in the microbiome
Many clinical diseases, some of which are strongly associated with advanced age, have distinct gut microbial signatures. Advanced age is an important independent risk factor for type 2 diabetes (T2D)145 and a substantial body of literature describes the role of the gut microbiome in its pathophysiology. Reports somewhat vary with respect to the association between T2D specific taxonomic groups, but Gurung et al. recently reviewed 42 human studies on the topic and identified trends. Commonly-reported findings included a negative association between T2Dand the genera of Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia, and Roseburia, and a positive association with Ruminococcus, Fusobacterium, and Blautia146. A recent cohort study in Northern China involving 16S rRNA sequencing revealed a significant decrease in overall gut microbiota diversity and butyrate-producing bacteria, such as Bifidobacterium and Akkermansia in diabetic patients compared with healthy controls147. Colorectal cancer is another example. On average, it presents in the 6th and 7th decades of life, and the gut microbiome appears to be involved in GI tumorigenesis. Animal models have helped identify associations with Fusobacterium nucleatum, Bacteroides fragilis, Streptococcus bovis, Heliobacter pylori, Enterococcus faecalis, and certain strains of Escherichia coli148–150. Furthermore, recent data suggest that the microbiome composition affects the efficacy and toxicity of cancer immunotherapy, opening the door for synergistic therapies that target gut microbiota151. The association between age-related disease and taxonomic patterns in the gut flora has other implications for therapeutics152. In brief, it is possible that the gut microbiota could be used in the future as biomarkers of specific diseases, and that modulation of the microbiome through FMT or antibiotic, probiotic, or prebiotic therapies could one day be used as a means to prevent or treat disease in individual patients.
In addition to identifying patterns of dysbiosis in association with specific age-related diseases, researchers have found that certain microbial signatures are associated with functional frailty and general ill-health in the elderly28, 153. Even after adjusting for confounding variables like age and medications, trends in microbiota composition correlate with clinical measures of frailty154. Claesson et al. reported that the abundance of bacteria belonging to the Oscillibacter and Alistipes genera and Eubacteriaceae family, as well as the loss of Lactobacillus and Faecalibacterium, is associated with frailty in elderly people28. These individuals also appear to have fewer producers of beneficial SCFAs and polyamines when compared to healthy elderly people155. Some factors common to aging-related dysbiosis, frailty, and disease states include inflammation137 and suboptimal diet156. It has been established that consumption of fiber-rich foods decreases in the elderly157 and that a year-long Mediterranean diet intervention can alter the gut microbiome in older individuals, reduce frailty, and improve overall health status158.
It is important to note that human studies that recognize associations between specific gut microbial populations and aging or disease do not establish causation. It is still largely unclear whether all changes in the microbiome associated with age represent dysbiosis or whether some are simply adaptive responses to other age-related physiologic or behavioral changes. Likewise, it is uncertain whether dysbiosis identified in association with various disease states is a cause or consequence. In an effort to establish mechanistic links, investigators have used animal models of aging with shorter lifecycles and less complex microbiomes than humans, including C. elegans, Drosophila species, zebrafish, and murine strains. Gnotobiotic models, in particular, have helped to elucidate the contributions of specific microbiota components in regulating host metabolism116. A comprehensive review of animal models of aging and host-microbiota interactions is beyond the scope of this paper but can be found elsewhere131, 133, 159.
Regarding mechanism, some investigators have suggested that the host immune system develops an inflammatory response to certain commensal bacteria over time. This is sometimes seen after GI infections, in which microbiota-specific T cells are activated and go on to form memory cells, leading to the loss of mucosal immune tolerance to beneficial commensals160. Chronic inflammation resulting from this process could then lead to intestinal degradation and a higher risk of age-associated diseases161. Another plausible mechanistic link involves insulin-like growth factor 1 (IGF1)162. Yan et al. found that the transfer of pathogen-free microbiota into germ-free mice induces high levels of IGF1 and that supplementation with beneficial SCFAs leads to the upregulation of IGF1162. The growth hormone - IGF1 axis is involved in the activation of canonical aging pathways,163, 164 suggesting a connection between the gut microbiome and aging.
Role of the microbiome in intestinal barrier function and ‘inflammaging’
As noted above, one of the most important functions of the intestinal epithelium is maintaining a barrier to gut luminal content, while allowing for selective absorption of dietary nutrients and innate and adaptive immune response. Commensal gut bacteria help maintain both cellular and junctional components of the barrier and regulate tissue immunity. Hence, one of the key consequences of aging-related dysbiosis is a ‘leaky gut’ and subsequent inflammation, which contributes to further dysbiosis in a feed-forward loop (Figure 2)131, 165, 166.
Figure 2. The ‘leaky gut’ model of aging and inflammation.
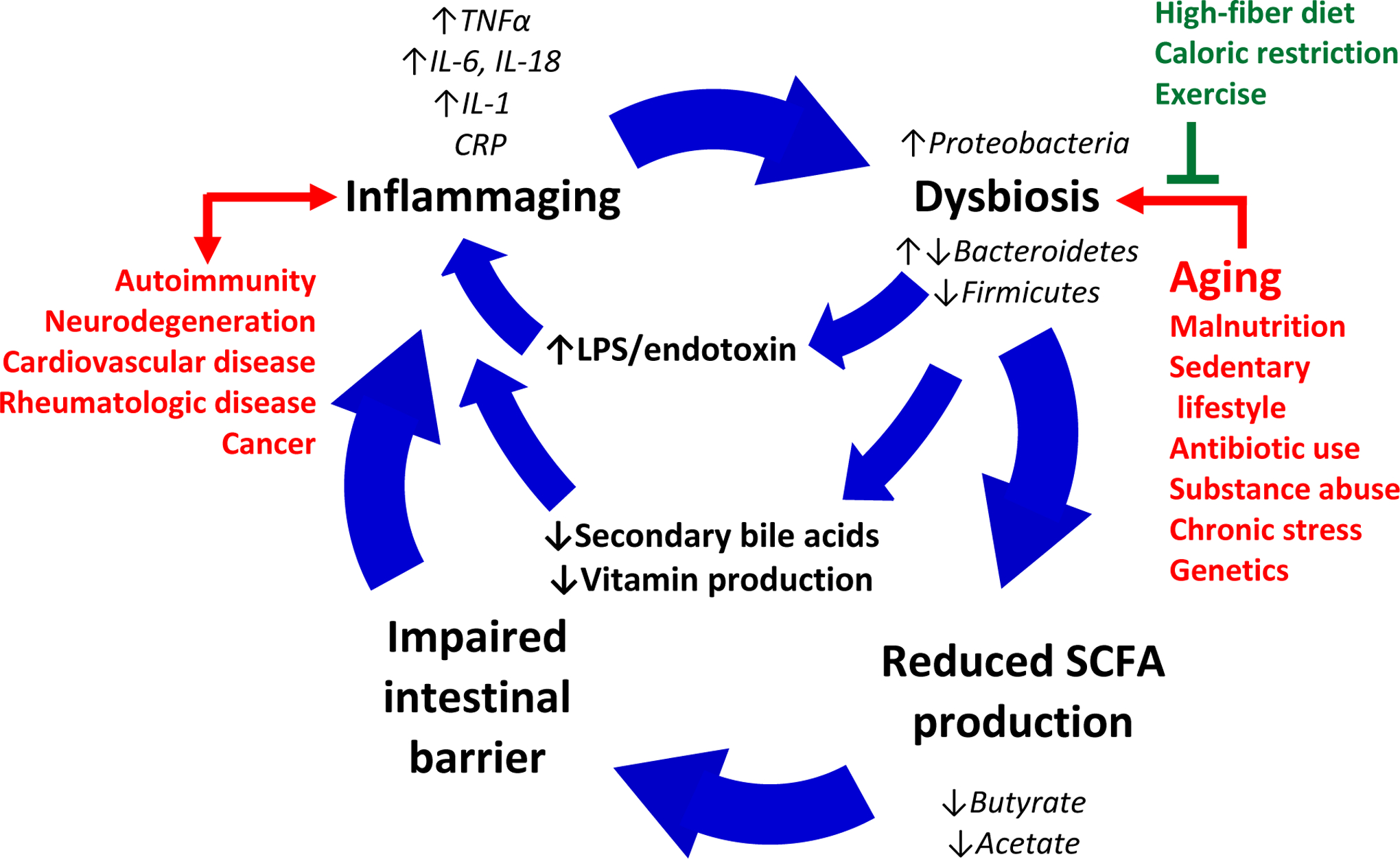
Age-related intestinal microbial dysbiosis leads to reductions in commensal bacteria-derived short-chain fatty acids (SCFAs), increases in lipopolysaccharide (LPS) production, and decreased secondary bile acid production. Loss of SCFAs results in degradation of the intestinal barrier, leakage of bacterial products and other pro-inflammatory luminal and mucosal debris, leading to inflammation and further dysbiosis in a feed-forward cycle.
TNFα: tumor necrosis factor alpha
CRP: C-reactive protein
IL-6, IL-1β, IL-18: Interleukin-1, Interleukin-1 beta, Interleukin-18
The ‘leaky gut’ hypothesis describes a process in which advanced aging, malnutrition, chronic stress, and other harmful factors lead to changes in the microbial populations of the gut. These changes are invariably detrimental and often include reductions in the relative abundance of SCFA-producing bacteria. SCFAs, including butyrate, acetate, and propionate, are generated by bacterial fermentation of dietary fibers and resistant starch. They have roles in immunomodulation, energy balance, and have other far-reaching systemic effects but are also necessary for the maintenance of local gut integrity167–169. For instance, butyrate enhances the intestinal barrier by facilitating tight junction assembly and acting as the primary fuel source for colonocytes170, 171. The main butyrate producing-bacteria in the human gut belong to the phylum Firmicutes, in particular Faecalibacterium prausnitzii, Clostridium leptum, Eubacterium rectale, and Roseburia species171. As the intestinal barrier breaks down, the epithelium allows unregulated passage of luminal content into the bloodstream, including bacterial cells and fragments, such as bacterial endotoxins, and dietary metabolites. This results in immune activation, inflammation, and even autoimmunity171, 172. The contribution of aging to this process is evidenced by studies showing higher levels of intestinal permeability and epithelial pro-inflammatory cytokine levels in older patients compared to younger counterparts59. Ultimately, local inflammation drives further dysbiosis. Beneficial lifestyle factors, such as high-fiber diets, caloric restriction, and exercise, as well as endogenous anti-inflammatory mechanisms, can provide resistance to this feed-forward cycle.
In addition to decreasing levels of SCFAs, age-related dysbiosis can lead to altered levels of other beneficial bacterial metabolites, including secondary bile acids and essential vitamins that are important for immune function. It can also result in higher levels of LPS. In particular, LPS is produced by Enterobacteriaceae, a family of Proteobacteria, whose relative abundance increases in age-related dysbiosis. LPS is recognized by host TLR-4 receptors and results in an increased local expression of inflammatory cytokines173. In combination with a ‘leaky gut,’ this can lead to endotoxemia and systemic inflammation. Finally, AMPs, including α-defensins, lysozyme C, phospholipases, C-type lectin, and Reg3γ, have a critical role in innate immunity and help to keep pathogenic bacteria in check. A subset of these AMPs – bacteriocins – are produced by commensal bacteria, presenting another mechanism driving the feed-forward dysbiosis cycles.
The link between age-dependent changes in gut microbiota and systemic inflammation has been established in animal studies in which germ-free mice co-housed with old, but not young conventionally raised mice, leads to increased intestinal permeability and inflammation174. Gut epithelial barrier integrity has also been shown to decrease as a function of age in C. elegans175 and Drosophila176 models, as well as in humans59.
Implications of age-related dysbiosis for disease and therapy
The connection between aging and gut microbiome has immense implications for human health. Just as enrichment in certain SCFA-producing bacteria is associated with longevity, decreases in overall microbiota richness appear to be a predictor of mortality in older populations177. Other groups have suggested, for example, that the microbial dysbiosis-related inflammation and immune dysfunction, in particular deficits in immune surveillance, may contribute to increased cancer risk in the elderly155. The unregulated leakage of LPS and other bacterial products increases the production of IL-1β, IL-6, TNFα, and interferons systemically, contributing to a chronic pro-inflammatory state in the elderly termed “inflammaging”155, 161, as noted above. In this state, the ability of myeloid cells to remove dysplastic, senescent, apoptotic, or malfunctioning cells is impaired19, creating an environment conducive for various types of cancer. Alterations of the microbiome with age also influence the homeostasis of various gut-systemic axes. For instance, investigators have shown that gut microbiota are key regulators of brain development and neurodegeneration. Mediators of the gut-brain cross-talk include the vagus nerve, gut-derived hormones, gut-derived neurotransmitter precursors (e.g., tryptophan), or neurotransmitters themselves (e.g., dopamine, acetylcholine, and γ-Aminobutyric acid), and SCFAs178–183. Imbalances in these mediators may explain why dysbiosis has been implicated in the pathogenesis of several age-related neuropsychiatric diseases, including inflammation-driven Alzheimer’s disease, schizophrenia, and depression.
Interventions aimed at avoiding or treating the myriad of age-related diseases associated with dysbiosis could take many forms. Fecal microbiota transplant, the transfer of stool or stool-derived microbiota from one individual to another, has been used with significant success in the treatment of opportunistic Clostridium difficile infections and its clinical indications are likely to expand in the future, to include inflammatory and autoimmune diseases184, 185. Barcena et al. showed that in a mouse model of progeria with prominent dysbiosis, FMT from wild type mice enhanced healthspan and overall lifespan and restored secondary bile acids186. A future challenge in this field will be to develop and test mixtures of engineered microbiota that could emulate the microbiome of young, healthy individuals following FMT. Prebiotics, namely fibrous substrates for bacterial SCFA production, probiotics, and selective antibiotics, have also been studied extensively, though with relatively less success. It is worth noting that antibiotics cause significant changes in microbiota composition187, and a study that evaluated 2007–2009 Medicare Part D data found that patients aged ≥65 years used more antimicrobials, at 1.10 per person per year, compared to 0.88 antimicrobials used per person per year in patients aged 0–64 years188.
Caloric restriction and other targeted dietary changes may be the most straightforward approach to mitigate dysbiosis as individuals age189. Avoidance of a ‘western’ high-fat diet, processed foods, and red meat, in favor of a Mediterranean diet, high in whole grains and polyunsaturated fats have shown promise190, 191. Zhang et al. showed that a long-term low fat, 30% calorie restricted-diet in mice led to enrichment in Lactobacillus with age, decreased LPS levels, and prolonged lifespan192. Finally, elderly individuals exhibit a phenomenon called ‘anabolic resistance,’ in which greater amounts of dietary protein are required to stimulate muscle synthesis and maintenance of lean body mass, and the aging microbiome is one of many factors implicated in this. Changes in the gut microbiota can impact the bioavailability of dietary amino acids, and changes in the quantity and type of ingested protein, often seen in the elderly, can alter the relative abundance of bacterial genera, especially protein-fermentors189, 193–195. In particular, plant protein is associated with higher proportions of Bifidobacterium, Lactobacillus, and Roseburia, while animal protein is associated with more Bacteroides, Alistipes, Bilophila and Ruminococcus189, 196. These findings suggest that directed dietary interventions could be used to buffer age-related dysbiosis.
Ultimately, dysbiosis is complex and likely both a result of and a response to inflammation and aging. When developing therapies, it is important to remember that humans are super-organisms comprised of human and microbial cells. Investigations should consider the possibility that not all age-related changes in microbiota are detrimental. Some may reflect adaptations to other drivers of aging, evolved, for instance, to optimize nutrient absorption or adjust the basal metabolic level.
IV. Interactions between aging organ systems
Gut-liver axis
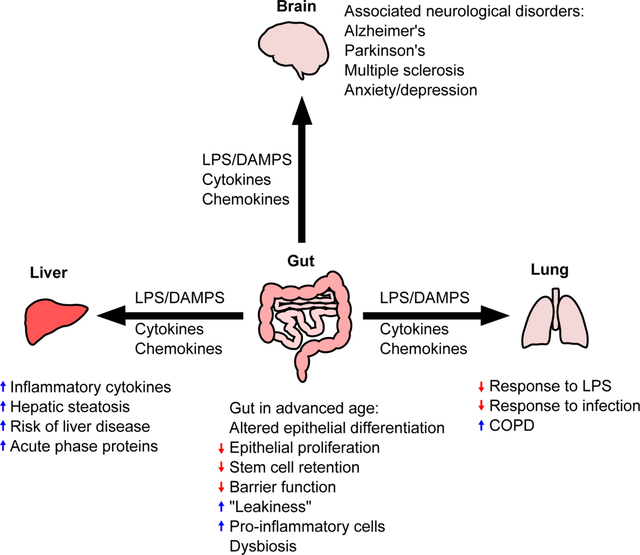
The gut is capable of communicating with a number of organs, including the liver, lung, and brain, and it is likely that age-associated changes in the gut microbiome and intestinal barrier function also affect these organs. The gut-liver axis refers to the bi-directional communication between the gut, including its microbiome, and the liver. This pathway allows for critical communication between the two organs in response to dietary and environmental factors, as reviewed by Tripathi et al197. The liver communicates with the gut by producing and releasing bile acids into the intestine via the biliary tract. In the gut, the intestinal microbiome metabolizes dietary components, bile acids, and other environmental factors that can travel to the liver via the portal tract and regulate a number of metabolic functions. However, when the intestinal barrier is compromised, increased translocation of bacterial derived MAMPs to the liver occurs. MAMPs can then bind to pattern recognition receptors (PRRs) on hepatic non-parenchymal cells, including Kupffer cells and hepatic stellate cells, leading to activation of pro-inflammatory and pro-fibrotic signaling cascades33. Although the liver is a robust organ, a number of physiological changes have been shown to occur with aging, including increases in hepatic expression of the pro-inflammatory cytokines il6, tnfa and il1b, along with increased hepatic steatosis198 and an increase in acute phase proteins199. Interestingly, there is emerging evidence that these age-associated changes in the liver may be a result of impaired gut function. For example, supplementation with the gut brush border enzyme intestinal alkaline phosphatase in aged mice resolves not only aging-induced gut barrier dysfunction but also lowers age-associated increases in liver enzyme levels200. Furthermore, these observations suggest that increased leakage of LPS and other MAMPs from the gut contributes to the aged liver phenotype. Increased endotoxin levels are found in plasma from aged mice, and deletion of the LPS-binding protein (LPB) ameliorates the observed age-related liver inflammation201. Importantly, the aged liver appears to be sensitized to LPS, as LPS-induced up-regulation of the pro-inflammatory IL-1β/inflammasome pathway is increased in the liver of aged rats202. Whether or not impaired gut function and increased translocation of bacterial endotoxins contribute to age-related liver dysfunction in humans remains to be determined but warrants further study.
Gut-lung axis
The bi-directional link between the gut and lung has recently become more appreciated. The effects of the gut microbiome on lung immunity have been reviewed elsewhere203–205. The gut and lung share many similar physiological characteristics at their mucosal surfaces, and it has been shown that immune cells readily traffic between these sites206. Further, the lung contains its own unique microbiome that affects many aspects of host immunity207, 208, and there is microbial continuity along the aerodigestive tract209. Studies carried out in germ-free, or antibiotic-treated animals have shown that these animals have increased susceptibility and aberrant immune responses following infection with various respiratory pathogens as reviewed by Dumas et al.210 Additionally, the function of alveolar macrophages, the tissue-resident phagocytes of the lung, is impaired in the absence of a gut microbiota211. Interestingly, one study found that the antibacterial function of murine alveolar macrophages can be restored to basal levels through the addition of NOD-like receptor agonists via the GI tract211. Furthermore, there is inflammatory cross-talk between the gut and lungs in diseases that primarily affect one or the other organs, such as Crohn’s disease (gut) and chronic obstructive pulmonary disease (COPD; lung)212, 213.
In advanced age, heightened bacterial translocation believed to result from a ‘leaky gut,’ contributes to persistent inflammation and organ dysfunction, including pulmonary dysfunction. Lungs from aged mice and cells from elderly human donors showed sustained basal p38 MAPK activation and a delayed inflammatory response to LPS, which may contribute to age-related inflammatory lung injury214. In addition, various chronic pulmonary conditions disproportionately affect the elderly, including COPD. COPD is the 3rd leading cause of global death, with an estimated 3 million yearly mortalities. 92% of these fatalities are in people over 60 years of age, and 73% are in those over 70 years of age215. Indeed, advanced age has become recognized as a clinical risk factor that may contribute to gut dysbiosis and the development of COPD (reviewed in216). A proposed intervention to target the gut-lung axis and improve health outcomes associated with COPD is to increase dietary fiber216. Given the importance of the gut-lung axis in health and disease, further studies that target the gut microbiome to treat chronic and acute respiratory conditions in elderly subjects are indeed merited.
Gut-brain axis
The gut-brain axis refers to the cross-talk between the central and enteric nervous systems, primarily dominated by signaling from the brain to the gut microbiota and vice versa, as reviewed elsewhere217, 218. Importantly, the presence of gut microbiota is vital for the development and maturation of both the enteric and central nervous systems. For example, germ-free mice have impaired sensorimotor function219 and memory formation220. Normal communication from the gut microbiota to the brain occurs through the vagus nerve, and severing the vagus nerve attenuates altered emotional behavior seen in mice infected with Lactobacillus rhamnosus178. In addition to this normal cross-talk, mounting evidence from the past ten years has linked dysfunction in the intestinal epithelial barrier to a variety of neurological disorders. It is enticing to speculate that age-related increases in intestinal inflammation and permeability contribute to the pathogenesis of these neurological disorders. Occludin, an important intestinal tight-junctional protein, is reduced, and the normal distribution of zona occludens protein-1 is disrupted in the colonic epithelium of human patients with Parkinson’s Disease221. Further, markers of intestinal inflammation and permeability, calprotectin, and alpha-1-antitrypsin/zonulin, respectively, were found to be increased in the fecal material of Parkinson’s patients222, further implicating intestinal permeability in the pathogenesis of the disease. Gut leakiness has also been implicated in the pathogenesis of Alzheimer’s disease as previously reviewed by Köhler et al223, and multiple sclerosis as previously reviewed by Camara-Lemarroy et al.224. Finally, intestinal permeability is linked to depression and anxiety. Plasma markers of intestinal barrier dysfunction, zonulin, and iFABP, are increased in the plasma of those with depression and anxiety, which correlated with dysbiosis225. Whether these neurological disorders driven by alterations in gut permeability are further exacerbated by age warrants further study.
V. Possible systemic and/or clinical implications
Nutrient absorption, drug metabolism and efficacy
A common health condition that negatively impacts health and predicts premature mortality in the elderly is malnutrition226, 227. While aging does not inevitably accompany malnutrition, malabsorption of nutrients does contribute to deficiencies in vitamin D, vitamin B12, folate and anemia228. Clinical manifestations of malabsorption in the elderly, can be mild and the issue often goes undiagnosed229. The digestion and absorption of nutrients is largely controlled by the enteric nervous system (ENS), an integrated network of 200–600 million neurons within the myenteric and submucosal plexuses230. Importantly, the ENS modulates both the contractility and transit of material throughout the gut. Multiple studies have found enteric nerve fibers are negatively impacted with age and contribute to constipation and diarrhea229, 231. Age-related changes of the ENS include neuronal loss232 as well as neurodegeneration of nerve fibers233, 234. Importantly, decreased innervation as well as degeneration of villi are thought to play predominate roles in malabsorption of material in the GI tract228.
An added consequence of aging is altered drug metabolism. The GI tract is a key component of first-pass metabolism and several studies have reported that drug clearance occurring via phase I metabolism typically decreases by 30–40% in individuals ≥65 years, while phase II metabolism is largely unaffected235, 236. Collectively, altered metabolism can lead to prolonged drug half-lives and reduced drug clearance, but other confounding factors, including nutritional status and genetics, can also impact drug efficacy in the aged. Moreover, renal drug metabolism is highly impacted, leading to an approximate 50% reduction in drug clearance in the majority of the geriatric population236. Overall, adverse drug reactions are more frequent and have serious clinical implications in the aged237. Common prescriptions used in the elderly population include, but are not limited to, antibiotics, anticoagulants, digoxin, diuretics, hypoglycemics agents, antineoplastic agents, and non-steroidal anti-inflammatory drugs (NSAIDs) which collectively contribute to 60% of adverse drug reactions (ADR) leading to hospital admission and 70% of ADRs occurring post-admission237. Importantly, combination therapies may synergistically increase toxicity. For example, using NSAIDs increases the risk for peptic ulcers by 10% in aged individuals, while combination therapy of corticosteroids and NSAIDs increase the risk of peptic ulcers by 15 fold238. While drug metabolism largely takes place in the liver, the small intestine does express CYP450s, including CYP3A4, that eliminate a large proportion of drugs prior to reaching systemic circulation239, 240. Intestinal expression of CYP3A4 does not appear to change with age in rodents241; however, data are lacking on the expression CYP450 isoforms in the intestine of humans. As the gut microbiota are becoming increasingly recognized as an important contributor to xenobiotic metabolism242, 243, and since the microbiome changes with age, it will be important for future studies to examine the complex interactions between the host, microbiota, and aging, and their relative contributions to drug metabolism.
Concluding remarks
As the global population continues to age, we are presented with an opportunity to conduct impactful research into treatments that will increase not only the length but the quality of life. Aging comes with a host of associated diseases that can impact every organ system, and recent scientific advancements have highlighted the aging gut as a focal point that interacts with and influences other organ systems. More focus should be placed on a better understanding of age-related alterations in the gut and how those changes can negatively impact overall health. However, the benefits of these studies would not be limited to increasing the quality of life in the aging population. Perturbations in gut barrier function can occur due to traumatic injury244, alcohol consumption245, combination burn injury and alcohol consumption246, 247, and autoimmune diseases such as IBD. Lessons learned from the aging gut may yield novel therapies to treat or prevent systemic diseases linked to gut dysfunction in humans of all ages.
Acknowledgments
The work herein was supposed in part by US National Institutes of Health AG018859 (EJK), GM131831 (EJK), AA026295 (EJK), AA027687 (HJH), GM134185 (JPI), and AA025386 (RLM), and the Veterans Administration 1 I01 BX004335 (EJK).
List of abbreviations:
- AMP
Antimicrobial peptide
- BMP
Bone-morphogenetic protein
- DAMP
Damage-associated molecular pattern
- EAE
Experimental auto-immune encephalomyelitis
- FMT
Fecal microbiota transplant
- GI
Gastrointestinal
- GIP
Glucose-dependent insulinotropic polypeptide/gastric inhibitory polypeptide
- GLP-1/2
Glucagon-like peptide 1/2
- IBD
Inflammatory bowel disease
- IEC
Intestinal epithelial cell
- iFABP
Intestinal fatty acid binding protein
- IgA
Immunoglobulin A
- IGF1
Insulin-like growth factor 1
- ILC
Innate lymphoid cell
- ISC
Intestinal stem cell
- LP
Lamina propria
- LPS
Lipopolysaccharide
- MAMP
Microbe-associated molecular pattern
- NSAID
Non-steroidal anti-inflammatory drug
- PAMP
Pathogen-associated molecular pattern
- PP
Peyer’s patch
- Reg3γ
Regenerating islet-derived protein 3-gamma
- SCFA
Short-chain fatty acid
- T2D
Type-2 diabetes
- TLR
Toll-like receptor
- TNFα
Tumor necrosis factor alpha
- Treg
T-regulatory cell
- ZO-1
Zona occludens-1
Footnotes
Conflict-of-Interest Disclosure
The authors declare no conflict of interest.
References
- 1.Dey AB. World report on ageing and health. Indian J Med Res 2017; 145(1): 150–151. [Google Scholar]
- 2.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9(11): 799–809. [DOI] [PubMed] [Google Scholar]
- 3.von Martels JZH, Sadaghian Sadabad M, Bourgonje AR, Blokzijl T, Dijkstra G, Faber KN et al. The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. Anaerobe 2017; 44: 3–12. [DOI] [PubMed] [Google Scholar]
- 4.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med 2014; 20(4): 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parrish AR. The impact of aging on epithelial barriers. Tissue Barriers 2017; 5(4): e1343172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moorefield EC, Andres SF, Blue RE, Van Landeghem L, Mah AT, Santoro MA et al. Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging (Albany NY) 2017; 9(8): 1898–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunther C, Buchen B, Neurath MF, Becker C. Regulation and pathophysiological role of epithelial turnover in the gut. Semin Cell Dev Biol 2014; 35: 40–50. [DOI] [PubMed] [Google Scholar]
- 8.Derikx JP, Luyer MD, Heineman E, Buurman WA. Non-invasive markers of gut wall integrity in health and disease. World J Gastroenterol 2010; 16(42): 5272–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grootjans J, Thuijls G, Verdam F, Derikx JP, Lenaerts K, Buurman WA. Non-invasive assessment of barrier integrity and function of the human gut. World J Gastrointest Surg 2010; 2(3): 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 2017; 14(1): 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y et al. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology 1996; 110(2): 339–343. [DOI] [PubMed] [Google Scholar]
- 12.Derikx JP, Evennett NJ, Degraeuwe PL, Mulder TL, van Bijnen AA, van Heurn LW et al. Urine based detection of intestinal mucosal cell damage in neonates with suspected necrotising enterocolitis. Gut 2007; 56(10): 1473–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelson MB, Sonnino RE, Bagwell CE, Lieberman JM, Marks WH, Rozycki HJ. Plasma intestinal fatty acid binding protein in neonates with necrotizing enterocolitis: a pilot study. Journal of pediatric surgery 1999; 34(10): 1453–1457. [DOI] [PubMed] [Google Scholar]
- 14.Guthmann F, Börchers T, Wolfrum C, Wustrack T, Bartholomäus S, Spener F. Plasma concentration of intestinal- and liver-FABP in neonates suffering from necrotizing enterocolitis and in healthy preterm neonates. Molecular and cellular biochemistry 2002; 239(1–2): 227–234. [PubMed] [Google Scholar]
- 15.Delaney CP, O’Neill S, Manning F, Fitzpatrick JM, Gorey TF. Plasma concentrations of glutathione S-transferase isoenzyme are raised in patients with intestinal ischaemia. The British journal of surgery 1999; 86(10): 1349–1353. [DOI] [PubMed] [Google Scholar]
- 16.Hurley JC. Endotoxemia: methods of detection and clinical correlates. Clinical microbiology reviews 1995; 8(2): 268–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barclay GR, Scott BB, Wright IH, Rogers PN, Smith DG, Poxton IR. Changes in anti-endotoxin-IgG antibody and endotoxaemia in three cases of gram-negative septic shock. Circulatory shock 1989; 29(2): 93–106. [PubMed] [Google Scholar]
- 18.Reiner AP, Lange EM, Jenny NS, Chaves PH, Ellis J, Li J et al. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arteriosclerosis, thrombosis, and vascular biology 2013; 33(1): 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012; 11(5): 867–875. [DOI] [PubMed] [Google Scholar]
- 20.Steele AK, Lee EJ, Vestal B, Hecht D, Dong Z, Rapaport E et al. Contribution of intestinal barrier damage, microbial translocation and HIV-1 infection status to an inflammaging signature. PloS one 2014; 9(5): e97171–e97171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 2007; 128(1): 92–105. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee S, Vaishnava S, Hooper LV. Multi-layered regulation of intestinal antimicrobial defense. Cell Mol Life Sci 2008; 65(19): 3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science (New York, NY) 2011; 334(6053): 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci U S A 2008; 105(52): 20858–20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehotzky RE, Partch CL, Mukherjee S, Cash HL, Goldman WE, Gardner KH et al. Molecular basis for peptidoglycan recognition by a bactericidal lectin. Proceedings of the National Academy of Sciences of the United States of America 2010; 107(17): 7722–7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee S, Zheng H, Derebe MG, Callenberg KM, Partch CL, Rollins D et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2014; 505(7481): 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castaneda-Delgado JE, Frausto-Lujan I, Gonzalez-Curiel I, Montoya-Rosales A, Serrano CJ, Torres-Juarez F et al. Differences in Cytokine Production during Aging and Its Relationship with Antimicrobial Peptides Production. Immunol Invest 2017; 46(1): 48–58. [DOI] [PubMed] [Google Scholar]
- 28.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488(7410): 178–184. [DOI] [PubMed] [Google Scholar]
- 29.Langille MG, Meehan CJ, Koenig JE, Dhanani AS, Rose RA, Howlett SE et al. Microbial shifts in the aging mouse gut. Microbiome 2014; 2(1): 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol 2006; 169(6): 1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi W, Yeruva S, Turner JR. Contributions of intestinal epithelial barriers to health and disease. Experimental Cell Research 2017; 358(1): 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nanini HF, Bernardazzi C, Castro F, de Souza HSP. Damage-associated molecular patterns in inflammatory bowel disease: From biomarkers to therapeutic targets. World J Gastroenterol 2018; 24(41): 4622–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol 2012; 590(3): 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camilleri M Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 2019; 68(8): 1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Assche GV et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. The American Journal of Gastroenterology 2002; 97(8): 2000–2004. [DOI] [PubMed] [Google Scholar]
- 36.He C, Yu T, Shi Y, Ma C, Yang W, Fang L et al. MicroRNA 301A Promotes Intestinal Inflammation and Colitis-Associated Cancer Development by Inhibiting BTG1. Gastroenterology 2017; 152(6): 1434–1448.e1415. [DOI] [PubMed] [Google Scholar]
- 37.Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. The Lancet 2000; 355(9214): 1518–1519. [DOI] [PubMed] [Google Scholar]
- 38.Araújo JR, Tomas J, Brenner C, Sansonetti PJ. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 2017; 141: 97–106. [DOI] [PubMed] [Google Scholar]
- 39.Sato T, Clevers H. Growing Self-Organizing Mini-Guts from a Single Intestinal Stem Cell: Mechanism and Applications. Science (New York, NY) 2013; 340(6137): 1190. [DOI] [PubMed] [Google Scholar]
- 40.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009; 459(7244): 262–265. [DOI] [PubMed] [Google Scholar]
- 41.Branca JJV, Gulisano M, Nicoletti C. Intestinal epithelial barrier functions in ageing. Ageing research reviews 2019; 54: 100938. [DOI] [PubMed] [Google Scholar]
- 42.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 1974; 141(4): 537–561. [DOI] [PubMed] [Google Scholar]
- 43.Eastwood GL. Gastrointestinal epithelial renewal. Gastroenterology 1977; 72(5 Pt 1): 962–975. [PubMed] [Google Scholar]
- 44.Wright NA. Epithelial stem cell repertoire in the gut: clues to the origin of cell lineages, proliferative units and cancer. International journal of experimental pathology 2000; 81(2): 117–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi J, Rakhilin N, Gadamsetty P, Joe DJ, Tabrizian T, Lipkin SM et al. Intestinal crypts recover rapidly from focal damage with coordinated motion of stem cells that is impaired by aging. Scientific Reports 2018; 8(1): 10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farin HF, Jordens I, Mosa MH, Basak O, Korving J, Tauriello DV et al. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 2016; 530(7590): 340–343. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development (Cambridge, England) 1999; 126(6): 1211–1223. [DOI] [PubMed] [Google Scholar]
- 48.Degirmenci B, Valenta T, Dimitrieva S, Hausmann G, Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018; 558(7710): 449–453. [DOI] [PubMed] [Google Scholar]
- 49.Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB et al. Wnt Ligands Secreted by Subepithelial Mesenchymal Cells Are Essential for the Survival of Intestinal Stem Cells and Gut Homeostasis. Cell reports 2016; 15(5): 911–918. [DOI] [PubMed] [Google Scholar]
- 50.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 2007; 27(21): 7551–7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nalapareddy K, Nattamai KJ, Kumar RS, Karns R, Wikenheiser-Brokamp KA, Sampson LL et al. Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells. Cell reports 2017; 18(11): 2608–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchida R, Saito Y, Nogami K, Kajiyama Y, Suzuki Y, Kawase Y et al. Epigenetic silencing of Lgr5 induces senescence of intestinal epithelial organoids during the process of aging. npj Aging and Mechanisms of Disease 2018; 4(1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao Y, Kang B, Petkovich DA, Bhandari YR, In J, Stein-O’Brien G et al. Aging-like Spontaneous Epigenetic Silencing Facilitates Wnt Activation, Stemness, and Braf(V600E)-Induced Tumorigenesis. Cancer Cell 2019; 35(2): 315–328.e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nature genetics 2004; 36(10): 1117–1121. [DOI] [PubMed] [Google Scholar]
- 55.Batts LE, Polk DB, Dubois RN, Kulessa H. Bmp signaling is required for intestinal growth and morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists 2006; 235(6): 1563–1570. [DOI] [PubMed] [Google Scholar]
- 56.Ross MH, Kaye GI, Pawlina W. Histology : a text and atlas : with cell and molecular biology. Lippincott Williams Wilkins: Philadelphia, PA, 2003. [Google Scholar]
- 57.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 2010; 10(2): 131–144. [DOI] [PubMed] [Google Scholar]
- 58.McClure R, Massari P. TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Frontiers in immunology 2014; 5: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Man AL, Bertelli E, Rentini S, Regoli M, Briars G, Marini M et al. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clinical science (London, England : 1979) 2015; 129(7): 515–527. [DOI] [PubMed] [Google Scholar]
- 60.Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infection and immunity 1993; 61(11): 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lotz M, Konig T, Menard S, Gutle D, Bogdan C, Hornef MW. Cytokine-mediated control of lipopolysaccharide-induced activation of small intestinal epithelial cells. Immunology 2007; 122(3): 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infection and immunity 2007; 75(5): 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tremblay S, Côté NML, Grenier G, Duclos-Lasnier G, Fortier LC, Ilangumaran S et al. Ileal antimicrobial peptide expression is dysregulated in old age. Immunity & ageing : I & A 2017; 14: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keilbaugh SA, Shin ME, Banchereau RF, McVay LD, Boyko N, Artis D et al. Activation of RegIIIbeta/gamma and interferon gamma expression in the intestinal tract of SCID mice: an innate response to bacterial colonisation of the gut. Gut 2005; 54(5): 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Ampting MTJ, Loonen LMP, Schonewille AJ, Konings I, Vink C, Iovanna J et al. Intestinally secreted C-type lectin Reg3b attenuates salmonellosis but not listeriosis in mice. Infection and immunity 2012; 80(3): 1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science (New York, NY) 2006; 313(5790): 1126–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bai R, Sun D, Chen M, Shi X, Luo L, Yao Z et al. Myeloid cells protect intestinal epithelial barrier integrity through the angiogenin/plexin-B2 axis. The EMBO journal 2020; 39(13): e103325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML et al. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J Allergy Clin Immunol 2006; 118(1): 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biton M, Haber AL, Rogel N, Burgin G, Beyaz S, Schnell A et al. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 2018; 175(5): 1307–1320 e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ciccocioppo R, Di Sabatino A, Luinetti O, Rossi M, Cifone MG, Corazza GR. Small bowel enterocyte apoptosis and proliferation are increased in the elderly. Gerontology 2002; 48(4): 204–208. [DOI] [PubMed] [Google Scholar]
- 71.Schofield G The argentaffin and mucous cells of the small and large intestines of the mouse. Acta anatomica 1952; 16(1–2): 1–15. [DOI] [PubMed] [Google Scholar]
- 72.Ermund A, Schütte A, Johansson ME, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol 2013; 305(5): G341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 2008; 105(39): 15064–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med 2017; 49(5): e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Newton JL, Jordan N, Pearson J, Williams GV, Allen A, James OFW. The Adherent Gastric Antral and Duodenal Mucus Gel Layer Thins with Advancing Age in Subjects Infected with Helicobacter pylori. Gerontology 2000; 46(3): 153–157. [DOI] [PubMed] [Google Scholar]
- 76.Tremblay S, Côté NML, Grenier G, Duclos-Lasnier G, Fortier L-C, Ilangumaran S et al. Ileal antimicrobial peptide expression is dysregulated in old age. Immunity & Ageing 2017; 14(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sovran B, Hugenholtz F, Elderman M, Van Beek AA, Graversen K, Huijskes M et al. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Scientific Reports 2019; 9(1): 1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Beek AA, Sovran B, Hugenholtz F, Meijer B, Hoogerland JA, Mihailova V et al. Supplementation with Lactobacillus plantarum WCFS1 Prevents Decline of Mucus Barrier in Colon of Accelerated Aging Ercc1−/Δ7 Mice. Frontiers in immunology 2016; 7: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elderman M, Sovran B, Hugenholtz F, Graversen K, Huijskes M, Houtsma E et al. The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLOS ONE 2017; 12(9): e0184274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Worthington JJ. The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem Soc T 2015; 43(4): 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Q, Han D, Cong B, Shan B, Zhang J, Chen H et al. Cholecystokinin octapeptide significantly suppresses collagen-induced arthritis in mice by inhibiting Th17 polarization primed by dendritic cells. Cell Immunol 2011; 272(1): 53–60. [DOI] [PubMed] [Google Scholar]
- 82.Zhang JG, Liu JX, Jia XX, Geng J, Yu F, Cong B. Cholecystokinin octapeptide regulates the differentiation and effector cytokine production of CD4(+) T cells in vitro. Int Immunopharmacol 2014; 20(2): 307–315. [DOI] [PubMed] [Google Scholar]
- 83.Zhang JG, Cong B, Li QX, Chen HY, Qin J, Fu LH. Cholecystokinin octapeptide regulates lipopolysaccharide-activated B cells co-stimulatory molecule expression and cytokines production in vitro. Immunopharmacology and immunotoxicology 2011; 33(1): 157–163. [DOI] [PubMed] [Google Scholar]
- 84.Ikeguchi E, Harada N, Kanemaru Y, Sankoda A, Yamane S, Iwasaki K et al. Transcriptional factor Pdx1 is involved in age-related GIP hypersecretion in mice. Am J Physiol Gastrointest Liver Physiol 2018; 315(2): G272–g282. [DOI] [PubMed] [Google Scholar]
- 85.Yu Y, Daly DM, Adam IJ, Kitsanta P, Hill CJ, Wild J et al. Interplay between mast cells, enterochromaffin cells, and sensory signaling in the aging human bowel. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 2016; 28(10): 1465–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol 2013; 6(4): 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi A, Donaldson DS, Erridge C, Kanaya T, Williams IR, Ohno H et al. The functional maturation of M cells is dramatically reduced in the Peyer’s patches of aged mice. Mucosal Immunology 2013; 6(5): 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koga T, McGhee JR, Kato H, Kato R, Kiyono H, Fujihashi K. Evidence for early aging in the mucosal immune system. Journal of immunology (Baltimore, Md : 1950) 2000; 165(9): 5352–5359. [DOI] [PubMed] [Google Scholar]
- 89.Umar S Intestinal stem cells. Curr Gastroenterol Rep 2010; 12(5): 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tanaka M, Saito H, Kusumi T, Fukuda S, Shimoyama T, Sasaki Y et al. Spatial distribution and histogenesis of colorectal Paneth cell metaplasia in idiopathic inflammatory bowel disease. Journal of gastroenterology and hepatology 2001; 16(12): 1353–1359. [DOI] [PubMed] [Google Scholar]
- 91.Pentinmikko N, Iqbal S, Mana M, Andersson S, Cognetta AB 3rd, Suciu RM et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 2019; 571(7765): 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Q, Elson CO. Adaptive immune education by gut microbiota antigens. Immunology 2018; 154(1): 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010; 107(26): 11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402): 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 2011; 108 Suppl 1: 4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012; 489(7415): 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol 2009; 2(6): 478–485. [DOI] [PubMed] [Google Scholar]
- 98.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012; 489(7415): 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peniche AG, Spinler JK, Boonma P, Savidge TC, Dann SM. Aging impairs protective host defenses against Clostridioides (Clostridium) difficile infection in mice by suppressing neutrophil and IL-22 mediated immunity. Anaerobe 2018; 54: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ganal-Vonarburg SC, Duerr CU. The interaction of intestinal microbiota and innate lymphoid cells in health and disease throughout life. Immunology 2020; 159(1): 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yudanin NA, Schmitz F, Flamar AL, Thome JJC, Tait Wojno E, Moeller JB et al. Spatial and Temporal Mapping of Human Innate Lymphoid Cells Reveals Elements of Tissue Specificity. Immunity 2019; 50(2): 505–519 e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D’Souza SS, Shen X, Fung ITH, Ye L, Kuentzel M, Chittur SV et al. Compartmentalized effects of aging on group 2 innate lymphoid cell development and function. Aging Cell 2019; 18(6): e13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nutsch K, Chai JN, Ai TL, Russler-Germain E, Feehley T, Nagler CR et al. Rapid and Efficient Generation of Regulatory T Cells to Commensal Antigens in the Periphery. Cell reports 2016; 17(1): 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim M, Qie Y, Park J, Kim CH. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell host & microbe 2016; 20(2): 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A 2009; 106(46): 19256–19261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hendrickx AP, Top J, Bayjanov JR, Kemperman H, Rogers MR, Paganelli FL et al. Antibiotic-Driven Dysbiosis Mediates Intraluminal Agglutination and Alternative Segregation of Enterococcus faecium from the Intestinal Epithelium. mBio 2015; 6(6): e01346–01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Johnson S, Sypura WD, Gerding DN, Ewing SL, Janoff EN. Selective neutralization of a bacterial enterotoxin by serum immunoglobulin A in response to mucosal disease. Infection and immunity 1995; 63(8): 3166–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev 2005; 206(1): 260–276. [DOI] [PubMed] [Google Scholar]
- 109.Pianta A, Arvikar S, Strle K, Drouin EE, Wang Q, Costello CE et al. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients With Rheumatoid Arthritis. Arthritis & rheumatology (Hoboken, NJ) 2017; 69(5): 964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013; 2: e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011; 479(7374): 538–541. [DOI] [PubMed] [Google Scholar]
- 112.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science (New York, NY) 2015; 350(6264): 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, NY) 2015; 350(6264): 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science (New York, NY) 2018; 359(6371): 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (New York, NY) 2018; 359(6371): 91–97. [DOI] [PubMed] [Google Scholar]
- 116.Seidel J, Valenzano DR. The role of the gut microbiome during host ageing. F1000Res 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav 2014; 13(1): 69–86. [DOI] [PubMed] [Google Scholar]
- 118.Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016; 164(3): 337–340. [DOI] [PubMed] [Google Scholar]
- 119.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A 2011; 108 Suppl 1: 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Toole PW, Jeffery IB. Gut microbiota and aging. Science (New York, NY) 2015; 350(6265): 1214–1215. [DOI] [PubMed] [Google Scholar]
- 121.Tropini C, Earle KA, Huang KC, Sonnenburg JL. The Gut Microbiome: Connecting Spatial Organization to Function. Cell host & microbe 2017; 21(4): 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Donia MS, Fischbach MA. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science (New York, NY) 2015; 349(6246): 1254766–1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Salazar N, Lopez P, Valdes L, Margolles A, Suarez A, Patterson AM et al. Microbial targets for the development of functional foods accordingly with nutritional and immune parameters altered in the elderly. J Am Coll Nutr 2013; 32(6): 399–406. [DOI] [PubMed] [Google Scholar]
- 124.Breton J, Tennoune N, Lucas N, Francois M, Legrand R, Jacquemot J et al. Gut Commensal E. coli Proteins Activate Host Satiety Pathways following Nutrient-Induced Bacterial Growth. Cell metabolism 2016; 23(2): 324–334. [DOI] [PubMed] [Google Scholar]
- 125.Goedert JJ, Hua X, Yu G, Shi J. Diversity and composition of the adult fecal microbiome associated with history of cesarean birth or appendectomy: Analysis of the American Gut Project. EBioMedicine 2014; 1(2–3): 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 2003; 91(441): 48–55. [DOI] [PubMed] [Google Scholar]
- 127.Salazar N, Valdes-Varela L, Gonzalez S, Gueimonde M, de Los Reyes-Gavilan CG. Nutrition and the gut microbiome in the elderly. Gut Microbes 2017; 8(2): 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 2016; 16: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015; 33(9): 496–503. [DOI] [PubMed] [Google Scholar]
- 130.Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE et al. Signatures of early frailty in the gut microbiota. Genome Med 2016; 8(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maynard C, Weinkove D. The Gut Microbiota and Ageing. Sub-cellular biochemistry 2018; 90: 351–371. [DOI] [PubMed] [Google Scholar]
- 132.Wang L, Fouts DE, Stärkel P, Hartmann P, Chen P, Llorente C et al. Intestinal REG3 Lectins Protect against Alcoholic Steatohepatitis by Reducing Mucosa-Associated Microbiota and Preventing Bacterial Translocation. Cell host & microbe 2016; 19(2): 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kundu P, Blacher E, Elinav E, Pettersson S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017; 171(7): 1481–1493. [DOI] [PubMed] [Google Scholar]
- 134.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007; 104(34): 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hayashi H, Sakamoto M, Kitahara M, Benno Y. Molecular analysis of fecal microbiota in elderly individuals using 16S rDNA library and T-RFLP. Microbiol Immunol 2003; 47(8): 557–570. [DOI] [PubMed] [Google Scholar]
- 136.Tiihonen K, Ouwehand AC, Rautonen N. Human intestinal microbiota and healthy ageing. Ageing research reviews 2010; 9(2): 107–116. [DOI] [PubMed] [Google Scholar]
- 137.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 2010; 5(5): e10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mitsuoka T Intestinal flora and aging. Nutr Rev 1992; 50(12): 438–446. [DOI] [PubMed] [Google Scholar]
- 139.Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 2001; 48(2): 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Biagi E, Candela M, Fairweather-Tait S, Franceschi C, Brigidi P. Aging of the human metaorganism: the microbial counterpart. Age (Dordr) 2012; 34(1): 247–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woodmansey EJ, McMurdo ME, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol 2004; 70(10): 6113–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S et al. Gut Microbiota and Extreme Longevity. Curr Biol 2016; 26(11): 1480–1485. [DOI] [PubMed] [Google Scholar]
- 143.Biagi E, Rampelli S, Turroni S, Quercia S, Candela M, Brigidi P. The gut microbiota of centenarians: Signatures of longevity in the gut microbiota profile. Mech Ageing Dev 2017; 165(Pt B): 180–184. [DOI] [PubMed] [Google Scholar]
- 144.Santoro A, Ostan R, Candela M, Biagi E, Brigidi P, Capri M et al. Gut microbiota changes in the extreme decades of human life: a focus on centenarians. Cell Mol Life Sci 2018; 75(1): 129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Selvin E, Parrinello CM. Age-related differences in glycaemic control in diabetes. Diabetologia 2013; 56(12): 2549–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020; 51: 102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Q, Chang Y, Zhang K, Chen H, Tao S, Zhang Z. Implication of the gut microbiome composition of type 2 diabetic patients from northern China. Sci Rep 2020; 10(1): 5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brennan CA, Garrett WS. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu Rev Microbiol 2016; 70: 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gagniere J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol 2016; 22(2): 501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol 2019; 16(11): 690–704. [DOI] [PubMed] [Google Scholar]
- 151.Gori S, Inno A, Belluomini L, Bocus P, Bisoffi Z, Russo A et al. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit Rev Oncol Hematol 2019; 143: 139–147. [DOI] [PubMed] [Google Scholar]
- 152.Kim S, Jazwinski SM. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018; 64(6): 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J 2016; 10(1): 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Toole PW, Jeffery IB. Microbiome-health interactions in older people. Cell Mol Life Sci 2018; 75(1): 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Biragyn A, Ferrucci L. Gut dysbiosis: a potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol 2018; 19(6): e295–e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 2017; 5(1): 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Donini LM, Savina C, Cannella C. Nutrition in the elderly: role of fiber. Arch Gerontol Geriatr 2009; 49 Suppl 1: 61–69. [DOI] [PubMed] [Google Scholar]
- 158.Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kostic AD, Howitt MR, Garrett WS. Exploring host-microbiota interactions in animal models and humans. Genes Dev 2013; 27(7): 701–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science (New York, NY) 2012; 337(6101): 1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 2014; 69 Suppl 1: S4–9. [DOI] [PubMed] [Google Scholar]
- 162.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A 2016; 113(47): E7554–E7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Altintas O, Park S, Lee SJ. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep 2016; 49(2): 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vitale G, Pellegrino G, Vollery M, Hofland LJ. ROLE of IGF-1 System in the Modulation of Longevity: Controversies and New Insights From a Centenarians’ Perspective. Front Endocrinol (Lausanne) 2019; 10: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Clark RI, Walker DW. Role of gut microbiota in aging-related health decline: insights from invertebrate models. Cell Mol Life Sci 2018; 75(1): 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol 2013; 14(7): 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 2020; 11: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wenzel TJ, Gates EJ, Ranger AL, Klegeris A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol Cell Neurosci 2020; 105: 103493. [DOI] [PubMed] [Google Scholar]
- 169.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015; 11(10): 577–591. [DOI] [PubMed] [Google Scholar]
- 170.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 2009; 139(9): 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in immunology 2019; 10: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ahmad R, Sorrell MF, Batra SK, Dhawan P, Singh AB. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol 2017; 10(2): 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 2013; 45: e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell host & microbe 2017; 21(4): 455–466 e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McGee MD, Weber D, Day N, Vitelli C, Crippen D, Herndon LA et al. Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell 2011; 10(4): 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rera M, Clark RI, Walker DW. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci U S A 2012; 109(52): 21528–21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA et al. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep 2017; 7(1): 11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011; 108(38): 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lyte M Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 2014; 5(3): 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol 2014; 817: 221–239. [DOI] [PubMed] [Google Scholar]
- 181.Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol 2011; 2: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.El Aidy S, Dinan TG, Cryan JF. Gut Microbiota: The Conductor in the Orchestra of Immune-Neuroendocrine Communication. Clin Ther 2015; 37(5): 954–967. [DOI] [PubMed] [Google Scholar]
- 183.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016; 165(6): 1332–1345. [DOI] [PubMed] [Google Scholar]
- 184.Sunkara T, Rawla P, Ofosu A, Gaduputi V. Fecal microbiota transplant - a new frontier in inflammatory bowel disease. J Inflamm Res 2018; 11: 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ et al. Fecal Microbiota Transplantation in Neurological Disorders. Front Cell Infect Microbiol 2020; 10: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Barcena C, Valdes-Mas R, Mayoral P, Garabaya C, Durand S, Rodriguez F et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med 2019; 25(8): 1234–1242. [DOI] [PubMed] [Google Scholar]
- 187.Perez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 2013; 62(11): 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Giarratano A, Green SE, Nicolau DP. Review of antimicrobial use and considerations in the elderly population. Clinical interventions in aging 2018; 13: 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505(7484): 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016; 65(11): 1812–1821. [DOI] [PubMed] [Google Scholar]
- 191.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016; 529(7585): 212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang C, Li S, Yang L, Huang P, Li W, Wang S et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun 2013; 4: 2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Beaumont M, Portune KJ, Steuer N, Lan A, Cerrudo V, Audebert M et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am J Clin Nutr 2017; 106(4): 1005–1019. [DOI] [PubMed] [Google Scholar]
- 194.Butteiger DN, Hibberd AA, McGraw NJ, Napawan N, Hall-Porter JM, Krul ES. Soy Protein Compared with Milk Protein in a Western Diet Increases Gut Microbial Diversity and Reduces Serum Lipids in Golden Syrian Hamsters. J Nutr 2016; 146(4): 697–705. [DOI] [PubMed] [Google Scholar]
- 195.Li Q, Lauber CL, Czarnecki-Maulden G, Pan Y, Hannah SS. Effects of the Dietary Protein and Carbohydrate Ratio on Gut Microbiomes in Dogs of Different Body Conditions. mBio 2017; 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017; 15(1): 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B et al. The gut–liver axis and the intersection with the microbiome. Nature Reviews Gastroenterology & Hepatology 2018; 15(7): 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hunt NJ, Kang SW, Lockwood GP, Le Couteur DG, Cogger VC. Hallmarks of Aging in the Liver. Computational and Structural Biotechnology Journal 2019; 17: 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Post DJ, Carter KC, Papaconstantinou J. The effect of aging on constitutive mRNA levels and lipopolysaccharide inducibility of acute phase genes. Annals of the New York Academy of Sciences 1991; 621: 66–77. [PubMed] [Google Scholar]
- 200.Kühn F, Adiliaghdam F, Cavallaro PM, Hamarneh SR, Tsurumi A, Hoda RS et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight 2020; 5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jin CJ, Baumann A, Brandt A, Engstler AJ, Nier A, Hege M et al. Aging-related liver degeneration is associated with increased bacterial endotoxin and lipopolysaccharide binding protein levels. American Journal of Physiology-Gastrointestinal and Liver Physiology 2020; 318(4): G736–G747. [DOI] [PubMed] [Google Scholar]
- 202.Chung KW, Lee EK, Kim DH, An HJ, Kim ND, Im DS et al. Age-related sensitivity to endotoxin-induced liver inflammation: Implication of inflammasome/IL-1β for steatohepatitis. Aging Cell 2015; 14(4): 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Frontiers in Microbiology 2015; 6(1085). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dang AT, Marsland BJ. Microbes, metabolites, and the gut–lung axis. Mucosal Immunology 2019; 12(4): 843–850. [DOI] [PubMed] [Google Scholar]
- 205.Wypych TP, Wickramasinghe LC, Marsland BJ. The influence of the microbiome on respiratory health. Nature Immunology 2019; 20(10): 1279–1290. [DOI] [PubMed] [Google Scholar]
- 206.Tomasi TB, Jr. Mechanisms of Immune Regulation at Mucosal Surfaces. Reviews of Infectious Diseases 1983; 5(Supplement_4): S784–S792. [DOI] [PubMed] [Google Scholar]
- 207.Cui L, Morris A, Huang L, Beck JM, Twigg HL 3rd, von Mutius E et al. The microbiome and the lung. Ann Am Thorac Soc 2014; 11 Suppl 4(Suppl 4): S227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B et al. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Frontiers in Cellular and Infection Microbiology 2020; 10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015; 6(2): e00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol 2018; 20(12): e12966. [DOI] [PubMed] [Google Scholar]
- 211.Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infection and immunity 2014; 82(11): 4596–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol 2012; 5(1): 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Keely S, Hansbro PM. Lung-gut cross talk: a potential mechanism for intestinal dysfunction in patients with COPD. Chest 2014; 145(2): 199–200. [DOI] [PubMed] [Google Scholar]
- 214.Ren X, Du H, Li Y, Yao X, Huang J, Li Z et al. Age-related activation of MKK/p38/NF-κB signaling pathway in lung: from mouse to human. Experimental gerontology 2014; 57: 29–40. [DOI] [PubMed] [Google Scholar]
- 215.Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. https://www.who.int/healthinfo/global_burden_disease/estimates/en/, 2018, Accessed Date Accessed 2018 Accessed.
- 216.Vaughan A, Frazer ZA, Hansbro PM, Yang IA. COPD and the gut-lung axis: the therapeutic potential of fibre. J Thorac Dis 2019; 11(Suppl 17): S2173–s2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015; 28(2): 203–209. [PMC free article] [PubMed] [Google Scholar]
- 218.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009; 6(5): 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R et al. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol 2005; 100(11): 2560–2568. [DOI] [PubMed] [Google Scholar]
- 220.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011; 60(3): 307–317. [DOI] [PubMed] [Google Scholar]
- 221.Clairembault T, Leclair-Visonneau L, Coron E, Bourreille A, Le Dily S, Vavasseur F et al. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta neuropathologica communications 2015; 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Bürmann J, Faßbender K et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism & related disorders 2018; 50: 104–107. [DOI] [PubMed] [Google Scholar]
- 223.Köhler CA, Maes M, Slyepchenko A, Berk M, Solmi M, Lanctôt KL et al. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Current pharmaceutical design 2016; 22(40): 6152–6166. [DOI] [PubMed] [Google Scholar]
- 224.Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain : a journal of neurology 2018; 141(7): 1900–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018; 67(8): 1555–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Remond D, Shahar DR, Gille D, Pinto P, Kachal J, Peyron MA et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015; 6(16): 13858–13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Soderstrom L, Rosenblad A, Adolfsson ET, Saletti A, Bergkvist L. Nutritional status predicts preterm death in older people: a prospective cohort study. Clin Nutr 2014; 33(2): 354–359. [DOI] [PubMed] [Google Scholar]
- 228.Soenen S, Rayner CK, Jones KL, Horowitz M. The ageing gastrointestinal tract. Current opinion in clinical nutrition and metabolic care 2016; 19(1): 12–18. [DOI] [PubMed] [Google Scholar]
- 229.Holt PR. Intestinal malabsorption in the elderly. Dig Dis 2007; 25(2): 144–150. [DOI] [PubMed] [Google Scholar]
- 230.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 2014; 817: 39–71. [DOI] [PubMed] [Google Scholar]
- 231.Saffrey MJ. Aging of the mammalian gastrointestinal tract: a complex organ system. Age (Dordr) 2014; 36(3): 9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, Lurken MS, Schmalz PF, Roeder JL et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 2009; 21(7): 746–e746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gamage PP, Ranson RN, Patel BA, Yeoman MS, Saffrey MJ. Myenteric neuron numbers are maintained in aging mouse distal colon. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 2013; 25(7): e495–e505. [DOI] [PubMed] [Google Scholar]
- 234.Phillips RJ, Billingsley CN, Powley TL. Macrophages are unsuccessful in clearing aggregated alpha-synuclein from the gastrointestinal tract of healthy aged Fischer 344 rats. Anatomical record (Hoboken, NJ : 2007) 2013; 296(4): 654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kinirons MT, O’Mahony MS. Drug metabolism and ageing. Br J Clin Pharmacol 2004; 57(5): 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 2009; 41(2): 67–76. [DOI] [PubMed] [Google Scholar]
- 237.Brahma DK, Wahlang JB, Marak MD, Ch Sangma M. Adverse drug reactions in the elderly. J Pharmacol Pharmacother 2013; 4(2): 91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med 1991; 114(9): 735–740. [DOI] [PubMed] [Google Scholar]
- 239.Iswandana R, Irianti MI, Oosterhuis D, Hofker HS, Merema MT, de Jager MH et al. Regional Differences in Human Intestinal Drug Metabolism. Drug Metab Dispos 2018; 46(12): 1879–1885. [DOI] [PubMed] [Google Scholar]
- 240.Ding X, Kaminsky LS. Human Extrahepatic Cytochromes P450: Function in Xenobiotic Metabolism and Tissue-Selective Chemical Toxicity in the Respiratory and Gastrointestinal Tracts. Annual Review of Pharmacology and Toxicology 2003; 43(1): 149–173. [DOI] [PubMed] [Google Scholar]
- 241.Warrington JS, Greenblatt DJ, von Moltke LL. Age-related differences in CYP3A expression and activity in the rat liver, intestine, and kidney. J Pharmacol Exp Ther 2004; 309(2): 720–729. [DOI] [PubMed] [Google Scholar]
- 242.Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res 2017; 179: 204–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019; 570(7762): 462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.de Haan JJ, Lubbers T, Derikx JP, Relja B, Henrich D, Greve JW et al. Rapid development of intestinal cell damage following severe trauma: a prospective observational cohort study. Critical care 2009; 13(3): R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol 1999; 276(4): G965–974. [DOI] [PubMed] [Google Scholar]
- 246.Chen MM, Zahs A, Brown MM, Ramirez L, Turner JR, Choudhry MA et al. An alteration of the gut-liver axis drives pulmonary inflammation after intoxication and burn injury in mice. Am J Physiol Gastrointest Liver Physiol 2014; 307(7): G711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zahs A, Bird MD, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. Am J Physiol Gastrointest Liver Physiol 2012; 303(6): G705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]


